Abstract
The management of wasting in Ethiopia is heavily reliant on the Community‐based Management of Acute Malnutrition (CMAM) programme that has been implemented in more than 18,000 service delivery points scattered across the country. Despite the full‐scale implementation of the CMAM, the number of child death averted, and the cost per child death averted remains unknown. This study aimed to estimate the cost and the number of child death averted by the CMAM programme between 2008 and 2020. Using data from routine monitoring of the CMAM programme, we estimated the excess mortality averted by the programme and estimated the cost per averted child death based on supply and labour. Over the past 13 years between 2008 and 2020, 3.6 million children under 5 years were admitted to the Ethiopian CMAM programme. The yearly average admission of 317,228 was achieved since 2011. On average, ~34,000 child deaths were averted yearly. The CMAM programme was estimated to have saved 437,654 (95% confidence interval [CI]: 320,161; 469,932) child deaths between 2008 and 2020, approximately 12% of the admitted cases. The average cost of the programme per adverted death was estimated at US$762/child death averted (95% CI = 639; 1001). The CMAM programme in Ethiopia is cost‐effective and has continued to avert a significant number of child death. Given the high short‐ and long‐term economic and health consequences of child wasting, concerted multi‐sectoral efforts are needed to accelerate progress not only in its treatment but also in its prevention.
Keywords: averted death, cost‐effective, malnutrition, prevention, treatment, wasting
Following a 95% confidence interval, the estimate is that between 320,161 and 469,932 children were saved from death.
Key messages
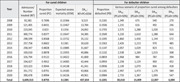
A total of 3.6 million children were admitted between 2008 and 2020, increasing annually from 92,000 in 2008 to 390,000 in 2020 due to improvement in case findings and referral.
Death rate in Community‐based Management of Acute Malnutrition (CMAM) programmes has decreased by sixfold between 2008 and 2020 from 1.23% to 0.22%
During 13 years of implementation, the Ethiopian CMAM programme averted the death of >400 thousand children under five.
1. INTRODUCTION
Globally, 13.6 million young lives are in jeopardy due to severe wasting (WHO, 2021). Out of those affected, more than 22.1% are from Africa. Linked to almost a 10‐fold increased chance of dying severe acute malnutrition (SAM) (severe wasting) is ranked as one of the top deadly diseases worldwide (Tirore et al., 2017). Despite some remarkable progress, Ethiopia still has one of the highest rates of child undernutrition in Sub‐Saharan Africa, and child wasting including the severe form has remained stable throughout the years. Since 2005, the prevalence of severe wasting in the country has been steady around 2% (Ethiopian Public Health Institute EPHI, 2016), translating to more than three million children facing moderate or severe forms of wasting. If the prevalence of global wasting (moderate and severe forms) remains at the current level, the economic burden of wasting is estimated to be between US$152 and 225 million annually (Laillou et al., 2020).
To meet the Sustainable Development Goal (SDG) wasting targets of less than 5% prevalence by 2030, a much faster reduction of the wasting prevalence will be required (Laillou et al., 2020). In response to this, the Government of Ethiopia has developed multi‐sectoral Food and Nutrition Policies over the last two decades; hence, providing the much‐needed policy framework and policy directions to combat malnutrition and related problems. Increasing coverage of and access to the Community‐based Management of Acute Malnutrition (CMAM) is, for example, an essential strategy for realizing the Growth and Transformation Plan of the country (the Federal Democratic Republic of Ethiopia, 2016). Ethiopia was, in fact, one of the first countries where the CMAM model, a normative guidance, was piloted in the early 2000s (2000/2002). Since the first pilots in Wollo (first ever pilot in CMAM history) and Southern Ethiopia, the country has embarked on a massive roll‐out of the CMAM Programme in the Health Extension Package (HEP) (Lemma et al., 2012), and the CMAM programme is now being delivered in over 18,000 facilities. The community HEP has enabled Ethiopia to achieve significant improvements in maternal and child health and prevent child death through the timely admission and treatment of severe wasting (Assefa et al., 2019).
In 2010, the World Bank ranked the treatment of severe wasting with ready‐to‐use therapeutic food (RUTF) as one of several proven interventions to improve nutrition and prevent child mortality (Horton et al., 2010). Nevertheless, the effectiveness of such a programme at saving the lives of children at high risk of death (Collins et al., 2006), the high treatment cost per child (around US$100–150) which, for example, in Ethiopia, corresponds to more than US$50 million every year to achieve only 40% coverage, has led to its questioning. Particularly when competition for public and bilateral donors funding has grown, the funding is now shared by various nutrition‐specific interventions. For example, according to the 2019 analysis by the Federal Ministry of Health, nutrition expenditures nearly doubled between the fiscal year 2013 and 2016, but this increase was not proportionally reflected for nutrition‐specific activities as most of the increase was observed for nutrition‐sensitive interventions.
Given the persistent concern about the costs of CMAM, and the limited quantified estimates on the effects of such programmes (Bulti et al., 2015), it is critical to estimate the number of child deaths averted during its full‐scale implementation in Ethiopia. The averted deaths calculation is particularly important given that the programme cost and implementation efficiency might change over time. Therefore, this study aimed to estimate cost and the number of child death averted by the CMAM programme between 2008 and 2020. This can support the prioritization of programmes for funding and implementation.
2. METHODS
2.1. Averted death among cured children
Estimating the number of deaths averted requires data from the routine programme collected through routine system. The number of deaths averted by the CMAM programme is valued by the number of severe wasting cases treated by the programme (NT), the proportion of treated children with SAM cases cured (PC), and the expected excess mortality (EEM) in untreated SAM cases with similar severity of wasting as those treated by the programme. Thus, the number of deaths averted from cured children (DAcc) by the CMAM programme is evaluated as follows (Equation 1):
| (1) |
The EEM was estimated from background mortality (BM) and the case fatality rate (CFR) in untreated severe wasting cases (Equation 2).
| (2) |
From 2008 to 2019, the BM was extracted from the under‐five mortality rate (U5MR) for Ethiopia from United Nations Children's Funds (UNICEFs) database. However, for the year 2020 for which mortality data are not available yet, we have estimated the U5MR using the time series data analysis method, comprising annual estimates of U5MR. Using U5MR data from UNICEF covering 1966–2019, we applied the Box‐Jenkins (ARIMA) time‐series model. The 2020 mortality rate was estimated to be 48.73/1000 live births. Details of the methods used to estimate U5MR is presented as a supplement. According to several paper published, the EEM also used different linear interpolation (Table 1) (Briend & Zimicki, 1986; Briend et al., 1987; Vella et al., 1993, 1994). The mean of the EEM was calculated according to Equation 3:
| (3) |
Table 1.
Linear interpolation process used to estimate expected excess mortality associated with untreated SAM at the admission MUAC level of (115 mm)
| Source | MUAC (mm) | Mortality per year/1000 | Slop (B) | Expected excess mortality/year/1000 | |
|---|---|---|---|---|---|
| Briend et al. (1987) | 110 | 178 |
|
178−12.4 × 5−13.48 = 102.52 | |
| 120 | 54 | ||||
| Briend et al. (1987) | 110 | 199 |
|
199−12.9 × 5−13.48 = 121.32 | |
| 120 | 70 | ||||
| Vella et al. (1993) | 115 | 188 | 188−13.48 = 174.52 | ||
| Vella et al. (1994) | 115 | 187 | 187−13.48 = 173.52 |
Abbreviation: MUAC, mid‐upper arm circumference.
The estimated excess mortality rate extracted from Table 1 was 138.52 over the year.
2.2. Averted death among defaulter children
Based the number of severely wasted children treated by the programme (NT), the calculated EEM, the proportion of children defaulting from the program (PD), and the proportion of cured among defaulted (PCaD), we have estimated the number of deaths averted from defaulter children using different scenario (Equation 4)1:
| (4) |
Defaulters were defined using the standard definition of the World health Organization (WHO) and national guidelines that refers to beneficiaries that is absent for two consecutive weighing. Given that not all defaulters die, but also the lack of accurate estimates of cure rate among defaulters, we used different levels (10%–50%) of PCaD in our model.
2.3. Cost per averted child death
Based on the number of children admitted (NT), we calculated the the annual estimated cost due to treatment (AET) according to workforce and supplies needed, as follows:
| (5) |
With
10% of the complicated severe wasting children will go through a stabilization centre (SC) (UNICEF Global and Ethiopia estimates).
Cost, including supply,2 training, and monitoring have been assessed from 2013 to 2021 and the costs were from US$76–99/child for a severely wasted child (SAM) in out‐patient treatment (OPT), US$122–168/child for a severe wasting in a SC and then OTP (Table S1).
Cost of labour: to estimate the cost of the health extension worker (HEW) during the subsequent visits, we have estimated that he works 196 h/month and receives a salary of US$150/month. Therefore, the cost per hour of work is equal to US$0.76. A similar calculation was done for a health worker (HW) who works 196 h/month and receives a salary of US$198/month. His cost per hour will be equal to US$1.01 USD. If we consider that each contact with a wasted child is 20 min, a severely wasted child (OTP) with 12 visits3 will each cost US$2.7, while a severely wasted child (SC + OTP) with 19 visits will costs US$4.9. The cost for screening, case findings, and referral have not been included an should be considered as a limitation of the exercise.
3. RESULTS
Over the 13 years between 2008 and 2020, 3.6 million children under 5 years have been admitted within the Ethiopian CMAM programme, a yearly average of 277,639. Almost 9 out of 10 children are discharged as cured, according to retrospective data. As described in Table 2, on average 956 children are dying yearly, even though the death rate has decreased by sixfold between 2008 and 2020 from 1.2% to 0.2%, while defaulter rate declined by twofold. While approximately 34,000 children's deaths are averted on a yearly basis (Table 3), the number of deaths averted by the Ethiopian CMAM programme is estimated to be 437,654 children (without considering the potential through defaulted children). Following a 95% confidence interval, the estimate is that between 320,161 and 469,932 children were saved from death. Considering the proportion of defaulters self‐discharged as cured lay towards 20% of them (referred as PCaD within Equation 4 of the methodology), an additional 12,000 adverted death could be estimated (Table 3). In this study, the deaths averted are dependent on the expected CFR in severe wasting cases that do not receive the CMAM intervention (Figure 1). According to our analyses, between 2008 and 2020, over US$340 million was dedicated to the CMAM programme of which 97% was allocated for supplies. Therefore, the average cost of the programme per adverted deaths (Table S2) was estimated at US$762/child death averted (95% CI = US$639; US$1,001).
Table 2.
Smmary of admission, cure, death, and default rate from the CMAM programme by year
| Year | Admission | Cure | Death | Default | |||
|---|---|---|---|---|---|---|---|
| Frequency | Frequency | % | Frequency | % | Frequency | % | |
| 2008 | 92,581 | 71,247 | 76.96 | 1140 | 1.23 | 20,194 | 21.81 |
| 2009 | 121,405 | 102,359 | 84.31 | 762 | 0.63 | 18,284 | 15.06 |
| 2010 | 223,045 | 183,576 | 82.30 | 1478 | 0.66 | 37,991 | 17.03 |
| 2011 | 300,774 | 251,969 | 83.77 | 1612 | 0.54 | 47,193 | 15.69 |
| 2012 | 298,599 | 255,834 | 85.68 | 1120 | 0.38 | 41,645 | 13.95 |
| 2013 | 257,758 | 221,730 | 86.02 | 899 | 0.35 | 35,129 | 13.63 |
| 2014 | 258,347 | 228,059 | 88.28 | 665 | 0.26 | 29,623 | 11.47 |
| 2015 | 330,010 | 292,969 | 88.78 | 665 | 0.20 | 36,376 | 11.02 |
| 2016 | 326,794 | 298,087 | 91.22 | 621 | 0.19 | 28,086 | 8.59 |
| 2017 | 334,427 | 301,400 | 90.12 | 955 | 0.29 | 32,072 | 9.59 |
| 2018 | 323,323 | 292,414 | 90.44 | 659 | 0.20 | 30,250 | 9.36 |
| 2019 | 351,772 | 309,243 | 87.91 | 984 | 0.28 | 41,545 | 11.81 |
| 2020 | 390,478 | 351,178 | 89.94 | 878 | 0.22 | 38,422 | 9.84 |
| Total | 3,609,313 | 3,160,065 | 87.55 | 12,438 | 0.34 | 436,810 | 12.10 |
Table 3.
The number of deaths averted within cured children and through various proportion of cured among defaulted
| Year | For cured children | For defaulter children | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Admission/number treated (NT) | Proportion cured (PC) | Expected excess mortality (EEM) | DAcc: NT × PC × EEM | Proportion defaulted (PD) | Various scenario of proportion cured among defaulters | ||||
| DADC (PCaD = 50%) | DADC (PCaD = 25%) | DADC (PCaD = 20%) | DADC (PCaD = 10%) | ||||||
| 2008 | 92,581 | 0.7696 | 0.13366 | 9,523 | 0.2181 | 1,349 | 675 | 540 | 270 |
| 2009 | 121,405 | 0.8431 | 0.13467 | 13,784 | 0.1506 | 1,231 | 616 | 492 | 246 |
| 2010 | 223,045 | 0.823 | 0.1356 | 24,892 | 0.1703 | 2,575 | 1,288 | 1,030 | 515 |
| 2011 | 300,774 | 0.8377 | 0.13647 | 34,385 | 0.1569 | 3,220 | 1,610 | 1,288 | 644 |
| 2012 | 298,599 | 0.8568 | 0.13732 | 35,132 | 0.1395 | 2,860 | 1,430 | 1,144 | 572 |
| 2013 | 257,758 | 0.8602 | 0.1381 | 30,620 | 0.1363 | 2,426 | 1,213 | 970 | 485 |
| 2014 | 258,347 | 0.8828 | 0.13885 | 31,667 | 0.1147 | 2,057 | 1,029 | 823 | 411 |
| 2015 | 330,010 | 0.8878 | 0.13955 | 40,886 | 0.1102 | 2,538 | 1,269 | 1,015 | 508 |
| 2016 | 326,794 | 0.9122 | 0.14022 | 41,800 | 0.0859 | 1,968 | 984 | 787 | 394 |
| 2017 | 334,427 | 0.9012 | 0.14083 | 42,444 | 0.0959 | 2,258 | 1,129 | 903 | 452 |
| 2018 | 323,323 | 0.9044 | 0.14138 | 41,341 | 0.0936 | 2,139 | 1,070 | 856 | 428 |
| 2019 | 351,772 | 0.8791 | 0.14185 | 43,866 | 0.1181 | 2,947 | 1,473 | 1,179 | 589 |
| 2020 | 390,478 | 0.8994 | 0.14226 | 49,961 | 0.0984 | 2,733 | 1,367 | 1,093 | 547 |
| Total | 3,609,313 | 0.8755 | 0.1385 | 437,654 | 0.1201 | 30,018 | 15,009 | 12,007 | 6,004 |
Figure 1.
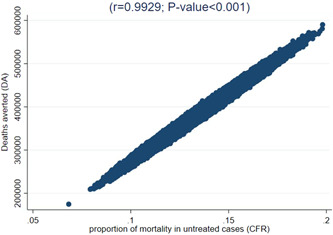
Sensitivity analysis
4. DISCUSSION
We evaluated the indirect coverage of CMAM during the 13 years of implementation in Ethiopia and estimated its effect on child mortality. Due to recurrent emergency episodes in Ethiopia, the CMAM programme is estimated to have saved ~450,000 child deaths between 2008 and 2020, which is approximately 12% of the admitted case. Our cost estimates also indicate that the fee of treatment has decreased with time and is below previous estimates.
Clearly, our findings show that the Ethiopian CMAM programme averted a significant number of excessive child death (~450,000) that would have occurred without the programme. These estimates are based on figures from various published literature linked to coverage data and a statistical model used to create counterfactuals. Nevertheless, the 12% reduction in mortality among admitted cases used in our model is much lower when compared with the 20% observed in Yemen (Al‐Dheeb et al., 2018). Averting an even higher number of excessive deaths could be possible by identifying the most vulnerable children and more at risk of dying. There is no definition of a threshold below which an intervention is considered cost‐effective. However, it is worth considering that our figure of child death averted was achieved at a much lower cost per child death averted (US$700) than those (US$1365–1760) considered to be cost‐effective in previous studies (Bachmann, 2009; Wilford et al., 2012). The lower cost in Ethiopia can be partly explained by the reduction in the cost of supply over the years, the lower labour costs, and the effective integration of the programme into the health system.
Previous cost estimates of the treatment of child wasting seem to have been overestimated. The estimates using the Lives Saved Tool (LiST) model was, for example, 15 times higher (US$10,000) (Shekar et al., 2017), than our estimated cost. This might have contributed to the exclusion of this programme from recent cost‐effectiveness estimates in Ethiopia and beyond. Worth noting is also the possible long‐term effects of child wasting on neurocognitive development and child linear growth. Indeed, long‐term effects like limited growth and neurocognitive development among children recovering from wasting have been documented (Grantham‐McGregor et al., 2007; Martins et al., 2011). Consequently, the cost‐effectiveness of this intervention should not only be considered from the point of view of averting child deaths but also preventing stunting and cognitive deficits. Such estimates are not available yet but will clearly suggest that the CMAM programme is more cost‐effective than most other prioritized interventions.
Nevertheless, more could be done to increase coverage and further reduce costs. For example, increased detection through family mid‐upper arm circumference screening can allow timely detection and treatment of affected children, further increasing the intervention's effectiveness. The more recent findings by Mertens et al. (2020) suggest that a significant proportion of children are experiencing their first episodes of wasting as early as from birth to the age of 3 months, underscoring the need for timely and early action. Further reducing the cost of the CMAM programme should be possible and can also increase coverage. This high coverage could be achieved, for example, by optimizing the dosage, as shown in recent studies that evaluated the effectiveness of reduced dosage of RUTF. Future studies should thus evaluate the effectiveness of such simplified approaches of wasting treatment in Ethiopia.
Several limitations should be considered when interpreting our findings. First, most of the data used are secondary data collected through monitoring systems at health facilities, and interpretations of our results should thus consider the uncertainties in such a data set. Therefore, uncaptured data from health facilities could not be included in the analysis. Second, our costing results should be interpreted carefully as we did not fully capture all CMAM‐related expenditures such as storage, equipment, and related cost other for screening, case findings and referral. Therefore, the margin of error is likely to be significant, and the figures should instead be considered as an order of magnitude. The estimated excess mortality rate come from older evidence before CMAM was implemented and hence may not fully reflect (i.e., overestimate) current EEM, which cannot be calculated given the widespread CMAM implementation.
Notwithstanding the above limitations, this analysis can support programmes and policy discussions and the prioritization of interventions. The pace of investment for referral, treatment, and prevention of child wasting should be accelerated and prioritized to avert as many deaths as possible. Based on our findings, we call all partners for more efforts to increase cost‐effectiveness through timely detection and treatment of wasting and programme modalities like the simplified approach for treatment of wasting that can further reduce cost and increase efficiency. Given the high short‐ and long‐term economic and health consequences of child wasting, concerted multi‐sectoral efforts are needed to accelerate progress not only in its treatment but also in its prevention.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Arnaud Laillou, Kaleab Baye, and Stanley Chitekwe designed the scope of the paper. Arnaud Laillou, Kaleab Baye conducted the analysis. Arnaud Laillou and Kaleab Baye wrote the first draft of the paper. Arnaud Laillou, Kaleab Baye, Saul Ignacio Guerrero Oteyza, Tewoldeberha Daniel, and Stanley Chitekwe reviewed the manuscript. All authors read and approved the final manuscript.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGEMENT
This study was funded by FCDO Ethiopia.
Laillou, A. , Baye, K. , Guerrero Oteyza, S. I. , Abebe, F. , Daniel, T. , Getahun, B. , & Chitekwe, S. (2024). Estimating the number of deaths averted from 2008 to 2020 within the Ethiopian CMAM programme. Maternal & Child Nutrition, 20(S5), e13349. 10.1111/mcn.13349
Footnotes
Due to missing on admission profile of defaulters and time spent in the programme, all defaulters are considered similarly even though early defaulters (i.e., 1 week after admission) and a late defaulter (e.g., 9 weeks after admission) are obviously at different risk of mortality.
Including supplies (RUTF, F75, F100, printed materials, funding for the caretakers for food during hospitalization) and its logistics.
It is an average stay, however the maximum is 4 months (16 weeks).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Al‐Dheeb, N. , Ziolkovska, A. , & Chitekwe, S. (2018). Experiences of implementing CMAM in Yemen and number of deaths averted. Field Exchange, 58, 64. www.ennonline.net/fex/58/cmamyemenaverteddeaths [Google Scholar]
- Assefa, Y. , Gelaw, Y. A. , Hill, P. S. , Taye, B. W. , & Van Damme, W. (2019). Community health extension program of Ethiopia, 2003–2018: Successes and challenges toward universal coverage for primary healthcare services. Globalization and Health, 15, 24. 10.1186/s12992-019-0470-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann, M. O. (2009). Cost effectiveness of community‐based therapeutic care for children with severe acute malnutrition in Zambia: Decision tree model. Cost Effectiveness and Resource Allocation, 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briend, A. , Wojtyniak, B. , & Rowland, M. M. (1987). Arm circumference and other factors in children at high risk of death in rural Bangladesh. The Lancet, 330(8561), 725–728. [DOI] [PubMed] [Google Scholar]
- Briend, A. , & Zimicki, S. (1986). Validation of arm circumference as an indicator of risk of death in one to four year old children. Nutrition Research, 6(3), 249–261. [Google Scholar]
- Bulti, A. , Chitekwe, S. , Puett, C. , & Myatt, M. (2015). How many lives do our CMAM programmes save? A sampling‐based approach to estimating the number of deaths averted by the Nigerian CMAM programme. Field Exchange, 50. https://www.ennonline.net/fex/50/deathsavertedcmamnigeria [Google Scholar]
- Collins, S. , Dent, N. , Binns, P. , Bahwere, P. , Sadler, K. , & Hallam, A. (2006). Management of severe acute malnutrition in children. The Lancet, 368, 1992–2000. [DOI] [PubMed] [Google Scholar]
- Ethiopian Public Health Institute (EPHI) . (2016). ICF. Ethiopia Demographic and Health Survey; Ministry of Health Addis Ababa: Addis Ababa, Ethiopia; The DHS Program ICF: Rockville, MD, 2016.
- Federal Democratic Republic of Ethiopia . (2016). Growth and Transformation Plan II (GTP II). Addis Ababa, Ethiopia. Retrieved August 15, 2020.
- Grantham‐McGregor, S. , Cheung, Y. B. , Cueto, S. , Glewwe, P. , Richter, L. , Strupp, B. , & International Child Development Steering, G. (2007). Developmental potential in the first 5 years for children in developing countries. The Lancet, 369, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, S. , Shekar, M. , McDonald, C. , Mahal, A. , & Brooks, J. K. (2010). Scaling up Nutrition: What will it Cost? World Bank. [Google Scholar]
- Laillou, A. , Baye, K. , Meseret, Z. , Darsene, H. , Rashid, A. , & Chitekwe, S. (2020). Wasted children and wasted time: A challenge to meeting the nutrition sustainable development goals with a high economic impact to Ethiopia. Nutrients, 12(12), 3698. 10.3390/nu12123698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemma, F. , Daniel, T. , Fekadu, H. , & Mates, E. (2012). CMAM rollout in Ethiopia: The ‘way in’ to scale up nutrition. Field Exchange, 43, 15–20. [Google Scholar]
- Martins, V. J. , Toledo Florêncio, T. M. , Grillo, L. P. , do Carmo P Franco, M. , Martins, P. A. , Clemente, A. P. , Santos, C. D. , de Fatima A Vieira, M. , & Sawaya, A. L. (2011). Long‐lasting effects of undernutrition. International Journal of Environmental Research and Public Health, 8, 1817–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens, A. , Benjamin‐Chung, J. , Colford, J. M., Jr. , Hubbard, A. E. , van der Laan, M. J. , Coyle, J. , Sofrygin, O. , Cai, W. , Jilek, W. , Rosete, S. , Nguyen, A. , Pokpongkiat, N. N. , Djajadi, S. , Seth, A. , Jung, E. , Chung, E. O. , Malenica, I. , Hejazi, N. , Li, H. , … Arnold, B. F. (2020). Child wasting and concurrent stunting in low‐ and middle‐income countries. medRxiv. 10.1101/2020.06.09.20126979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekar, M. , Kakietek, J. , Eberwein, J. D. , & Walters, D. (2017). An investment framework for nutrition: Reaching the global targets for stunting, anemia, breastfeeding, and wasting. World Bank. 10.1596/978-1-4648-1010-7 [DOI] [Google Scholar]
- Tirore, M. G. , Atey, T. M. , & Mezgebe, H. B. (2017). Survival status and factors associated with treatment outcome of severely malnourished children admitted to Ayder referral hospital: A cross‐sectional study. BMC Nutrition, 3(1), 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella, V. , Tomkins, A. , Borghesi, A. , Migliori, G. B. , Ndiku, J. , & Adriko, B. C. (1993). Anthropometry and childhood mortality in northwest and southwest Uganda. American Journal of Public Health, 83(11), 1616–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella, V. , Tomkins, A. , Ndiku, J. , Marshal, T. , & Cortinovis, I. (1994). Anthropometry as a predictor for mortality among Ugandan children, allowing for socio‐economic variables. European Journal of Clinical Nutrition, 48(3), 189–197. [PubMed] [Google Scholar]
- WHO . (2021). Levels and trends in child malnutrition: UNICEF/WHO/The World Bank Group joint child malnutrition estimates: Key findings of the 2021 edition. In Levels and trends in child malnutrition: UNICEF/WHO/The World Bank Group joint child malnutrition estimates: Key findings of the 2021 edition. Retrieved August 15, 2020.
- Wilford, R. , Golden, K. , & Walker, D. G. (2012). Cost‐effectiveness of community‐based management of acute malnutrition in Malawi. Health Policy and Planning, 27(2), 127–137. 10.1093/heapol/czr017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


