Abstract
Background
The proximal humerus is a common site for primary malignant and benign aggressive bone tumors, necessitating wide resection and subsequent skeletal defect reconstruction. Various reconstruction options include osteoarticular allografts, autografts, endoprosthesis, nail-cement spacer, reverse shoulder arthroplasty, and allograft-prosthesis composites. However, there is no consensus on the optimal reconstruction method. This study aims to compare functional outcomes and complications between these two methods.
Methods
A total of 40 patients with proximal humerus tumors who underwent endoprosthesis or nail-cement spacer reconstruction between March 2012 and December 2020 were included. The mean follow-up in the study was 31.37 +/− 12 months. Demographic and clinical data were collected, and functional outcomes were assessed using the Musculoskeletal Tumor Society 93 scoring system and the Disabilities of the Arm, Shoulder, and Hand questionnaire. Complications and oncological outcomes were recorded.
Results
Both groups were similar in terms of demographic and clinical variables. Endoprosthesis reconstruction demonstrated significantly better active shoulder forward flexion compared to nail-cement spacer (45.8 vs. 25.2 degrees) (P = .015). Endoprosthesis group also exhibited greater active shoulder internal rotation (68.25 vs. 63.25 degrees) (P = .004). No statistically significant differences were observed in overall functional outcomes. Complications, including radial nerve palsy and infection, were comparable between groups, with one case of spacer loosening.
Conclusion
Both endoprosthesis and nail-cement spacer reconstruction provide comparable functional outcomes and complication rates following proximal humerus tumor resection. Nail-cement spacer offers a cost-effective alternative for patients in resource-constrained settings.
Keywords: Proximal humerus tumors, Nail-cement spacer reconstruction, Endoprosthesis reconstruction, Limb salvage surgery, Oncologic resections, Functional outcomes
The proximal humerus is a common site for primary malignant as well as benign aggressive bone tumors. Surgical management commonly involves wide resection followed by reconstruction of the skeletal defect. Various reconstruction options include osteoarticular allografts, autografts, endoprosthesis, reverse shoulder arthroplasty, and graft-prosthesis composites.4,5,20,21,23
Resection often involves the sacrifice of the soft tissue attachments to the proximal humerus including the rotator cuff muscles and deltoid to a variable extent to obtain safe margins, while other muscular insertions including pectoralis major and latissimus dorsi can be released from their proximal humeral attachment without the need for resection. Also, the axillary nerve often needs to be sacrificed as it lies in direct contact with the proximal humerus. This leads to functional deficit with restricted range of motion (ROM) at the shoulder as well as loss of structural restraints. This can lead to instability in the reconstruction.3,16,22
While endoprosthetic reconstruction is often the preferred reconstruction modality, it is limited by cost and availability. In some situations, with extensive soft tissue resection and inadequate soft tissue coverage to the endoprosthesis, flap coverage, either rotational or free tissue transfer, can be used if available to provide soft tissue coverage to defects after resection to help restore function, improve cosmesis, and mitigate risk of infection. Nail cement space may provide equal functional outcomes with good implant survival and can be used as an alternative in developing countries as a reconstruction method for proximal humerus tumors.15 There is no consensus in existing literature regarding the best reconstruction method following proximal humerus tumor resection. The present study aims to compare the functional outcomes of two reconstruction methods, namely endoprosthesis and nail-cement spacer with the following research questions:
-
•
Does nail-cement spacer reconstruction differ from endoprosthesis in terms of functional outcomes and complications?
-
•
Does the shoulder ROM differ between the two groups?
Materials and methods
The study was carried out after approval by the institutional review board and included patients with tumors of the proximal humerus who underwent resection followed by reconstruction with endoprosthesis or nail-cement spacer. We obtained demographic and clinical information on these individuals from a bone tumor database, including age, sex, side of involvement, primary or recurrent tumor, prior surgical therapy, stage of the tumor, length of resection, and neo-adjuvant/adjuvant chemotherapy/radiation therapy received. All patients underwent an initial assessment using plain radiographs and magnetic resonance imaging of the arm to determine the extent of the bony lesion, extra-osseous component, and relationship to the neurovascular structures and to facilitate surgical planning, including margins and level of resection. A core-needle biopsy was performed, and a histological diagnosis was obtained for all the patients before definitive treatment.
A total number of 67 patients underwent surgery for proximal humerus tumors between March 2012 and December 2020. Seven patients underwent amputation, while four patients underwent reconstruction using other modalities. Twelve patients died of metastatic disease, and four patients were lost to follow-up. Forty patients who underwent reconstruction by either endoprosthesis (Fig. 1) or nail cement spacer (Fig. 2) and completed a minimum follow-up of 24 months were included in the study. These included 22 females and 18 males, with a mean age of 24.87 ± 10.2 years (range 5-66 years). The mean follow-up in the study was 31.37 ± 12 months (range 24-95 months). Osteosarcoma was the most common tumor seen in 37.5% of the patients, followed by Ewing’s sarcoma in 27.5%, giant cell tumor in 15%, chondrosarcoma in 12.5% of the cases, and 1 case (2.5%) each of malignant mesenchymal tumor, multiple myeloma, and metastatic tumor. The mean resection length (measured from the top of humeral head proximally to the osteotomy distally) was found to be 9.6 ± 1.8 cm (range 6-14.5 cm) in endoprosthesis group and 10.9 ± 2.5 cm (range 7.5-16 cm) in nail-cement spacer group.
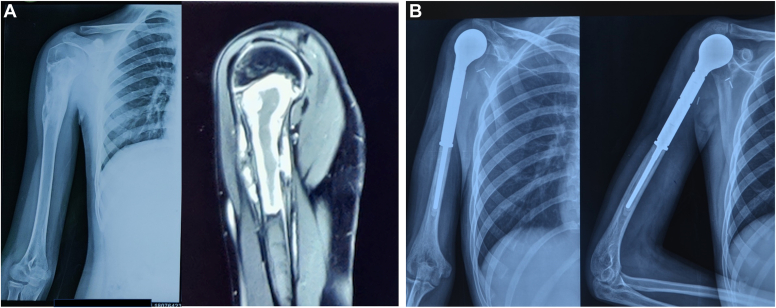
Figure 1.
(A) Radiological images of 13-year-old male with osteosarcoma right proximal humerus. (B) Postoperative x-rays following resection and endoprosthesis reconstruction at 32-month follow-up.
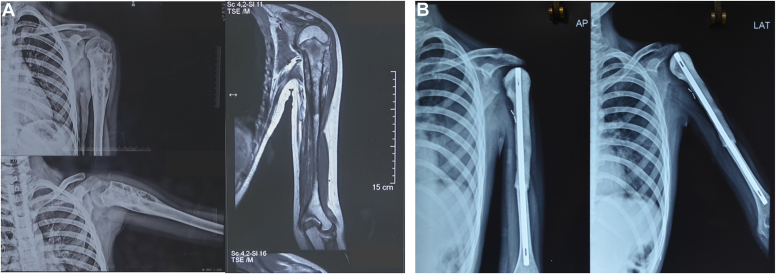
Figure 2.
(A) Radiological images of 11-year-old female with Ewing’s sarcoma left proximal humerus. (B) Postoperative x-rays following resection and nail-cement spacer reconstruction at 36-month follow-up.
Reconstruction technique
During endoprosthetic reconstruction, special attention was paid to the humeral component positioning, retroversion of the head, and the height of the implant chosen to reproduce the native humeral length by measuring precisely the resected part of the humerus. A prolene mesh was sutured to the remaining capsule and the glenoid labrum using Ethibond No. 5 sutures (J&J MedTech, New Brunswick, NJ, USA). The implanted endoprosthesis humeral head was then positioned in the glenoid and stabilized by wrapping the prolene mesh around the prosthesis and securing it with sutures (Fig. 3).12
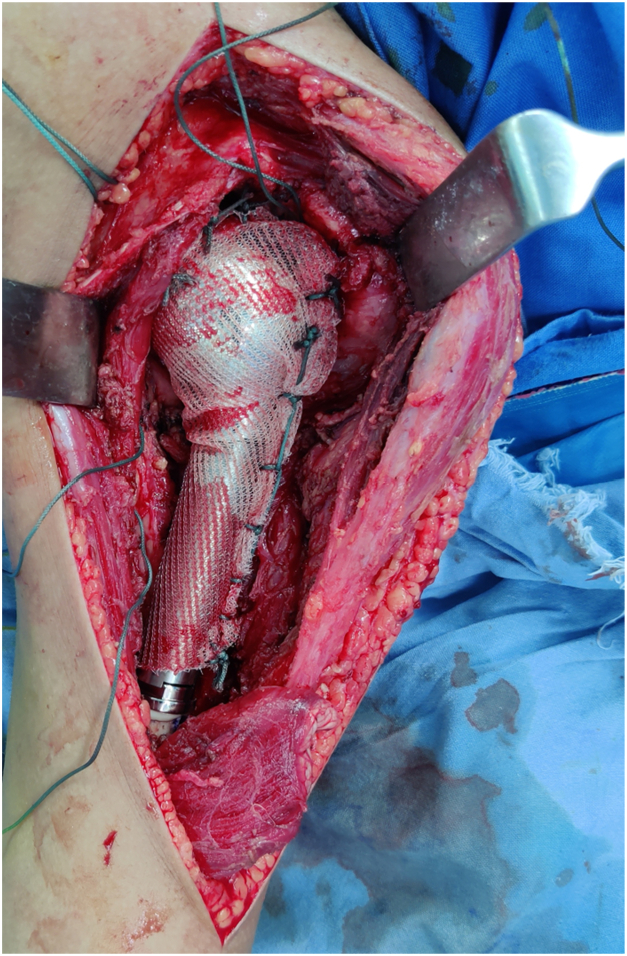
Figure 3.
Use of prolene mesh for anchoring proximal humeral endoprosthesis to the glenoid following resection of tumor of proximal humerus.
The nail-cement spacer reconstruction was performed using one or two Kuntscher nails (K-nails) introduced in the reamed humeral canal. A sleeve of prolene mesh was passed through the eye of the nail and secured with sutures. Cement was contoured over the proximal extramedullary part of the nail (Fig. 4). The proximal part of the construct is then anchored to the glenoid by suturing the mesh sleeve using Ethibond No. 5 sutures.
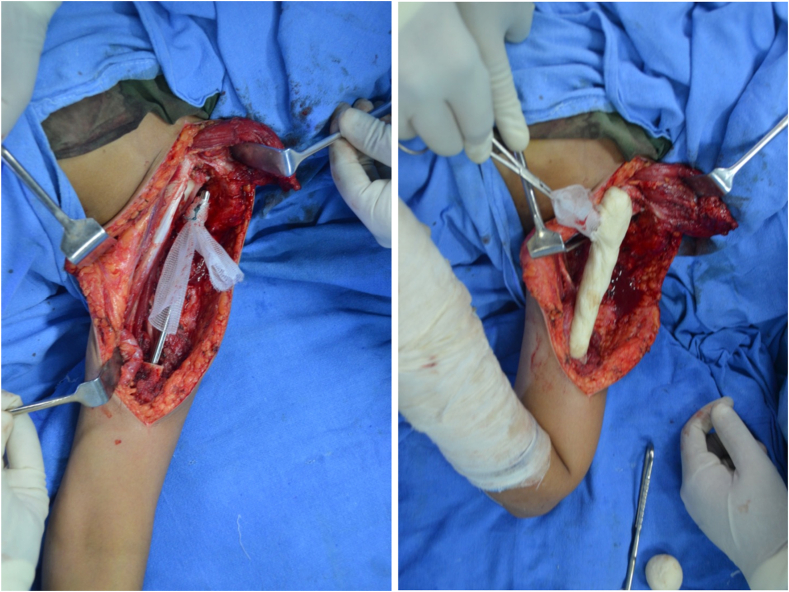
Figure 4.
Nail-cement spacer reconstruction using a sleeve of prolene mesh passed through the eye of the nail to anchor the construct to the glenoid.
The constructs were covered with the preserved muscles, and the surgical incision was closed following anatomical plans. Postoperatively, the constructs were protected in a plaster U-slab for 3 weeks. Following the removal of the splint, gentle range-of-motion exercises were permitted after 3 weeks. Patients were then followed up every 3 months for the first 2 years and biannually thereafter. Functional outcome was assessed using the Musculoskeletal Tumor Society 93 (MSTS 93) scoring system and the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire. MSTS 93 is based on six parameters, including pain, functional activities, emotional acceptance, hand positioning, dexterity, and lifting ability. A numerical value was assigned for each of the parameters (0 to 5), with a higher value indicating a better function. These values are added to give a total score with a maximum of 30.7
The DASH questionnaire is an outcome measure designed to assess patient-reported disability with musculoskeletal disorders of the upper limb. It is a 30-item questionnaire in which the response options are presented as 5-point Likert scales. The total score ranges from 0 (no disability) to 100 (the most severe disability).11
Any local complications, as well as oncological outcomes with respect to local recurrences and distant metastases were also recorded.
Statistical analysis
The data analysis was done using the SPSS Version 20.0 (IBM Corp., Armonk, NY, USA) and STATA version 15 (StataCorp, College Station, TX, USA). The categorical data were compared using either chi-square or Fisher’s exact tests, while the continuous data were compared using either the student’s t-test or Mann-Whitney U test. All the observations were considered to be significant when the P value was <.05.
Results
The demographic details including age, gender, histopathological diagnosis, chemotherapy received and follow-up duration of both groups are tabulated in Table I. Both the groups were similar with respect to the forementioned variables.
Table I.
Comparison of variables between the two groups.
| Variables | Nail-cement spacer (n = 20) | Endoprosthesis (n = 20) | P value |
|---|---|---|---|
| Mean age in years (range) | 27.5 (5-60) | 26 (10-66) | .639 |
| Sex | .209 | ||
| Male (n = 18) | 11 | 7 | |
| Female (n = 22) | 9 | 13 | |
| Diagnosis | .187 | ||
| Osteosarcoma (n = 15) | 5 (25%) | 10 (50)% | |
| Ewing’s sarcoma (n = 11) | 7 (35%) | 4 (20%) | |
| Chondrosarcoma (n = 5) | 3 (15%) | 2 (10%) | |
| GCTB (n = 6) | 3 (15%) | 3 (15%) | |
| Others (n = 3) | 2 (10%) | 1 (5%) | |
| Chemotherapy received | .617 | ||
| Yes (n = 29) | 14 | 15 | |
| No (n = 11) | 6 | 5 | |
| Follow-up in months (range) | 29.4 (12-95) | 33.35 (14-95) | .432 |
GCTB, giant cell tumor of bone.
The ROM of the shoulder and functional score of DASH and MSTS93 in each group are mentioned in Table II. Mean active shoulder forward flexion of 45.8° in endoprosthesis group was noted to be significantly better than 25.2° in nail-cement spacer group (P = .015) (Fig. 5). A statistically significant difference was also noted in the mean active shoulder internal rotation (P = .0045), with endoprosthesis group having more internal rotation (68.25°) than nail-cement spacer (63.25°). However, there were no statistically significant differences in the functional outcomes in both the groups.
Table II.
Comparison of range of motion at shoulder, DASH score, and MSTS-93 between the two groups.
| Variables | Nail-cement spacer (n = 20) | Endoprosthesis (n = 20) | P value |
|---|---|---|---|
| Mean flexion | 25.2° (15-40°) | 45.8° (20-60°) | .015 |
| Mean extension | 20.75° (10-30°) | 22.5° (10-40°) | .515 |
| Mean abduction | 29.25° (5-40°) | 40.5° (10-50°) | .393 |
| Mean adduction | 4.25° (0-20°) | 5.75° (0-15°) | .440 |
| Mean external rotation | 28° (0-75°) | 36.75° (0-110°) | .529 |
| Mean internal rotation | 63.25° (35-90°) | 68.25° (50-80°) | .004 |
| DASH score | 22.86 (11.7-35.8) | 24.3 (12.5-30) | .736 |
| MSTS-93 | 75.33 (66.66-83.33) | 77.496 (66.66-90) | .239 |
DASH, Disabilities of the Arm, Shoulder, and Hand; MSTS-93, Musculoskeletal Tumor Society 93.
The bold value in Table II highlight P-values < .05, which denote statistically significant differences in the indicated variables among the 2 groups.
Figure 5.
Active forward flexion in (A) nail-cement spacer and (B) endoprosthesis groups.
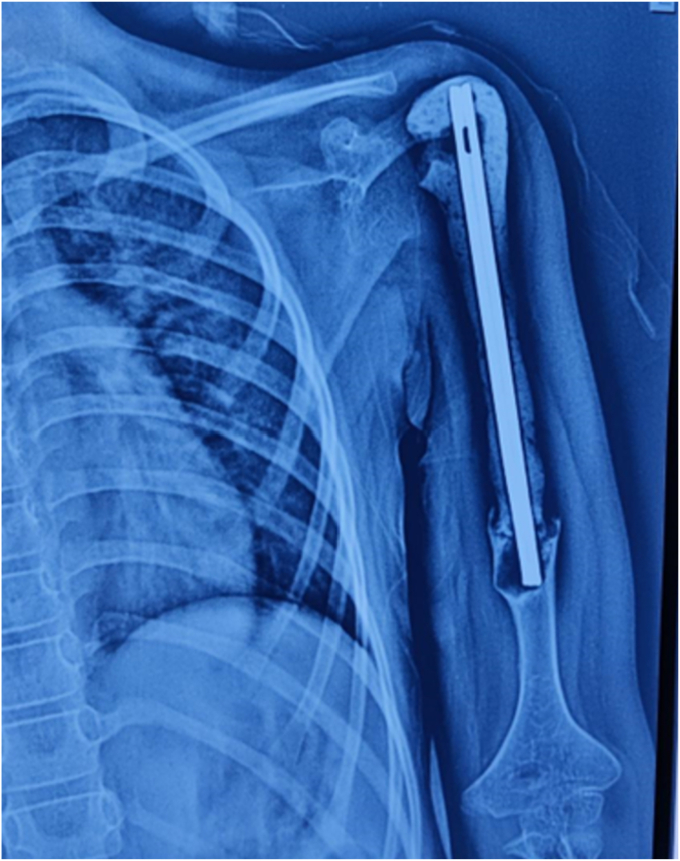
Two patients developed radial nerve palsy, one in each group. One patient in the endoprosthesis group developed a deep infection and underwent débridement, removal of endoprosthesis, and the replacement with cement spacer. Another patient in the spacer group underwent débridement and spacer revision for managing deep infection. One patient in the spacer group developed a superficial infection, which was managed with wound débridement and antibiotics. The same patient developed loosening of the implant during follow-up (Fig. 6). No instability was reported in both the groups.
Figure 6.
Implant loosening in a patient in nail-cement spacer group.
Local recurrence occurred in 3 patients (7.5%): 1 in the endoprosthesis group (chondrosarcoma), and 2 in the spacer group (osteosarcoma and Ewing’s sarcoma). The endoprosthesis group had 3 patients (15%) with complications, while the spacer group had 5 patients (25%) with complications (P = .879).
Discussion
The proximal humerus is the fourth most frequent location for primary bone cancers, accounting for 10%-15% of all osteosarcomas and 10% of Ewing’s sarcomas.2,19,28 In our study, the most common tumor was osteosarcoma (37.5%), followed by Ewing’s sarcoma (27.5%). Whereas giant cell tumor was seen in 15% and chondrosarcoma was diagnosed in 12.5% of the cases.
There are various reconstruction methods described in the literature for the reconstruction of the skeletal defect after resection of the proximal humerus tumors. The goal of any reconstruction method following proximal humerus resection is to retain the function of the hand, wrist, and elbow and restore the maximum possible stable mobility of the shoulder with limited complications. A number of factors play an important role in deciding the reconstruction method: age of the patient, extent of tumor involvement, tumor characteristics, and the patient's response. In most malignant cases, it's difficult to preserve the deltoid or axillary nerve, which contraindicates the use of reverse shoulder arthroplasty. In this study, we analyzed the functional outcome and related complications of two reconstruction methods: endoprosthesis and nail-cement spacer.
Loss of soft tissue support to the surrounding joint after tumor resection may lead to subluxation or dislocation of the prosthesis and functional deficit. Among the surgical choices, endoprosthetic replacement is probably the most widely used because of its availability, relatively low complication rate, high implant survival, and comparable functional results to those of other approaches.20,29 However, endoprosthetic replacement of the proximal humerus is associated with limitation of motion of the shoulder because of difficulty in reattaching the rotator cuff and tendons to the prosthesis, which can lead to shoulder instability and dislocation.26 Ross et al used endoprosthetic reconstruction after resection of the proximal humerus and did not make any attempt to reattach the soft tissues to the prosthesis. They reported an incidence of subluxation or dislocation of the prosthesis in 66.7% of the patients.24
Prolene mesh is a useful method to reconstruct such soft tissue defects. It is readily available, reliable, and provides reproducible results with no added risk of wound complications.12 The use of prolene mesh augmentation of the joint capsule helps in the stability of the joint and reduces subluxation of the endoprosthesis.27 In this study, we used the prolene mesh in both groups for the anchorage of the construct. We did not observe any cases of subluxation or dislocation in either of the groups after stabilization of the nail cement spacer or endoprosthesis to the glenoid or remnant capsule using prolene mesh.
Christopher reported the results of endoprosthetic reconstruction in 41 surviving patients during a one-year follow-up.3 The remaining rotator cuff was sutured to the prosthesis using either Dacron tape or a braided polyester suture. They observed a mean MSTS score of 63%, with mean active abduction of 41 degrees, and mean active forward flexion of 42 degrees. Kumar et al reported functional outcomes of 47 endoprosthesis reconstructions following proximal humerus tumor resection, where the prosthesis was retained with polyester knitted nonabsorbable undyed mesh that was sutured to the labrum and around the humeral head in order to contain the prosthesis.14 They reported mean MSTS score of 79% and abduction of the shoulder of 45° in their patients.
Tang et al compared the results of endoprosthetic proximal humerus reconstruction with and without synthetic mesh and concluded that synthetic mesh had better shoulder function and ROM.26 The mean MSTS scores for patients with synthetic mesh reconstruction were 79% and those without synthetic mesh reconstruction were 66%. They also reported better mean active forward flexion (P = .020), abduction (P = .001), and external rotation (P = .001) for patients with synthetic mesh reconstruction than patients without synthetic mesh reconstruction. In our endoprosthetic group, we observed a comparable MSTS93 of 77.49%, mean abduction of 40.5°, forward flexion of 45.8°, and mean internal rotation of 68.25°. Internal rotation is helpful in using the back pocket, managing the toilet, washing the back, and washing the opposite shoulder and axilla,13 while forward flexion is important in various activities of daily living: shampooing, combing hair, eating, touching mouth/nose, touching/scratching chest and contralateral axilla, genital hygiene, and perineal care.17
A nail cement spacer is a simple and cost-effective method of reconstruction after proximal humerus tumor resection and can be considered as an alternative to endoprosthetic reconstruction. The rationale for the use of the nail cement spacer is that, in the absence of active abduction, even a proximal humerus endoprosthesis functions like a spacer. Kundu et al reported reconstruction using nail cement spacer after tumor resection.15 They used braided, nonabsorbable sutures passed through holes drilled into the cement before it set and solidified to attach the remaining muscles to the nail spacer. They reported an MSTS of 63% in 11 patients at a mean follow-up of 30 months. Gulia et al used a mesh sleeve passed through the eye of the nail or the proximal screw hole of the plate and anchored it in the glenoid with nonabsorbable braided polyester sutures.10 They reported an MSTS of 71% in 40 patients with a median follow-up of 34 months. In our group of nail-cement spacer reconstruction, we observed MSTS 93 of 75.33% and a DASH score of 22.86 in 20 patients with a mean follow-up of 29.4 months.
Rafalla et al compared functional outcomes of endoprosthetic replacement vs. cement spacer in reconstruction of proximal humerus after tumor resection. They reported that functional outcome was almost comparable in both types of reconstruction, with a mean function of 65%.21 Another retrospective comparative study was conducted by Ebeid et al including 58 patients with proximal humerus tumors who had undergone tumor resection and reconstruction with modular endoprosthesis (humeral hemiarthroplasties) or cement spacer.5 They reported comparable functional outcome with a mean MSTS score of 24.8 ± 1.1 in the endoprosthesis group vs. 23.9 ± 1.4 in the spacer group (P = .018), along with comparable complications rate of 26.3% in the endoprosthesis group and 28.2% in the spacer group (P = .879). They concluded that both endoprosthesis and cement spacers are durable reconstructions with almost equal functional outcomes with no added advantage of the expensive endoprosthesis. Our results are consistent with the results of both the aforementioned studies, as we did not observe any statistically significant differences in the overall functional outcomes. However, both the studies failed to provide a comparison of the ROM at the reconstructed shoulder. A comparison of functional ROM in our study showed that endoprosthesis reconstruction demonstrated significantly better active shoulder forward flexion compared to nail-cement spacer (45.8 vs. 25.2 degrees) along with greater active shoulder internal rotation (68.25 vs. 63.25 degrees).
Local recurrence rate of 7.5% was observed in our patients, which is comparable to the recurrence rate of 5%-11% described in other series.6,9,18 Bickel et al1 reported 13 transient nerve palsies in a series of 134 patients who underwent limb-sparing resection for tumors around the shoulder girdle. We observed two radial nerve palsy in 40 patients in our study.
Getty et al reported one infection in 16 patients undergoing surgery using osteoarticular allograft after intra-articular resection of the proximal humerus.8 Whereas Schmolder reported only one case of periprosthetic joint infection in their series of proximal humerus endoprosthetic reconstructions.25 Deep infection was seen in three patients in our series, of whom two were managed with débridement only while one required implant removal. Our reoperation rate of 13% is comparable to reoperation rates of 10.47% documented in a systematic review of various methods of reconstruction after proximal humerus resection by Dubina et al.4
We saw one case of a nail cement spacer developing a loosening spacer. The probable reason for that could be the triangular cross-section of the distal humerus. If the nail is not snuggly fit, then it can cause rotational instability followed by loosening of the implant.
The study has its limitations due to its inherent retrospective study design. The patients were not randomly allocated into the two groups. The choice of reconstruction was based on the age of the patient, the surgeon's preference, and affordability. Also, the results in the study are obtained from a short to intermediate follow-up only. Long-term results need to be evaluated by future prospective studies. We acknowledge that a prospective randomized controlled trial with longer follow-up would have been ideal to establish the findings of this study.
Conclusion
Our study results conclude that both endoprosthesis and nail-cement spacer reconstruction following proximal humerus tumor resection provide comparable outcomes, with none of the methods reigning supreme over the other. Nail cement spacer is a simple and cost-effective option for patients with tumors of the proximal humerus.
Disclaimers:
Funding: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflicts of interest: The authors, their immediate families, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
This study was granted approval by the Institute Ethics Committee of All India Institute of Medical Sciences, New Delhi (Ref No. IEC-784/07.08.2020) and was performed in line with the principles of the Declaration of Helsinki.
Informed consent was obtained from all individual participants included in the study including consent for publication of the images.
References
- 1.Bickels J., Wittig J.C., Kollender Y., Kellar-Graney K., Meller I., Malawer M.M. Limb-sparing resections of the shoulder girdle. J Am Coll Surg. 2002;194:422–435. doi: 10.1016/S1072-7515(02)01124-9. [DOI] [PubMed] [Google Scholar]
- 2.Bielack S.S., Kempf-Bielack B., Delling G., Exner G.U., Flege S., Helmke K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/jco.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 3.Cannon C.P., Paraliticci G.U., Lin P.P., Lewis V.O., Yasko A.W. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg. 2009;18:705–710. doi: 10.1016/j.jse.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Dubina A., Shiu B., Gilotra M., Hasan S.A., Lerman D., Ng V.Y. What is the optimal reconstruction option after the resection of proximal humeral tumors? A systematic review. Open Orthop J. 2017;11:203. doi: 10.2174/1874325001711010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebeid W.A., Eldaw S., Badr I.T., Mesregah M.K., Hasan B.Z. Outcomes of modular endoprosthesis reconstruction versus cement spacer reconstruction following resection of proximal humeral tumors. BMC Musculoskelet Disord. 2022;23:484. doi: 10.1186/s12891-022-05432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckardt J.J., Eilber F.R., Rosen G., Mirra J.M., Dorey F.J., Ward W.G., et al. Endoprosthetic replacement for stage IIB osteosarcoma. Clin Orthop Relat Res. 1991:202–213. [PubMed] [Google Scholar]
- 7.Gerrand C.H., Rankin K. Classic papers in orthopaedics. Springer; Berlin, Germany: 2014. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system; pp. 489–490. [Google Scholar]
- 8.Getty P.J., Peabody T.D. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81:1138–1146. doi: 10.2106/00004623-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Grimer R.J., Sommerville S., Warnock D., Carter S., Tillman R., Abudu A., et al. Management and outcome after local recurrence of osteosarcoma. Eur J Cancer. 2005;41:578–583. doi: 10.1016/j.ejca.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Gulia A., BK A.R., Gupta S., Patil A., Puri A. Implant cement spacer-a cost-effective solution for reconstruction of proximal humerus defects after tumor resection. J Clin Orthop Trauma. 2021;22 doi: 10.1016/j.jcot.2021.101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jester A., Harth A., Wind G., Germann G., Sauerbier M. Disabilities of the arm, shoulder and hand (DASH) questionnaire: determining functional activity profiles in patients with upper extremity disorders. J Hand Surg. 2005;30:23–28. doi: 10.1016/J.JHSB.2004.08. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor L., Banjara R., Ragase A., Majeed A., Kumar V.S., Khan S.A. Outcomes of major musculoskeletal oncological reconstructions using prolene mesh-a retrospective analysis from a tertiary referral centre. J Clin Orthop Trauma. 2021;16:195–201. doi: 10.1016/j.jcot.2020.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M.S., Jeong H.Y., Kim J.D., Ro K.H., Rhee S.M., Rhee Y.G. Difficulty in performing activities of daily living associated with internal rotation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2020;29:86–94. doi: 10.1016/j.jse.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Kumar D., Grimer R.J., Abudu A., Carter S.R., Tillman R.M. Endoprosthetic replacement of the proximal humerus: long-term results. J Bone Joint Surg Br. 2003;85:717–722. doi: 10.1302/0301-620X.85B5.13838. [DOI] [PubMed] [Google Scholar]
- 15.Kundu Z.S., Gogna P., Gupta V., Kamboj P., Singla R., Sangwan S.S. Proximal humeral reconstruction using nail cement spacer in primary and metastatic tumours of proximal humerus. Strategies Trauma Limb Reconstr. 2013;8:149–154. doi: 10.1007/s11751-013-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moran M., Stalley P.D. Reconstruction of the proximal humerus with a composite of extracorporeally irradiated bone and endoprosthesis following excision of high-grade primary bone sarcomas. Arch Orthop Trauma Surg. 2009;129:1339–1345. doi: 10.1007/s00402-008-0752-1. [DOI] [PubMed] [Google Scholar]
- 17.Oosterwijk A.M., Nieuwenhuis M.K., Van der Schans C.P., Mouton L.J. Shoulder and elbow range of motion for the performance of activities of daily living: a systematic review. Physiother Theory Pract. 2018;34:505–528. doi: 10.1080/09593985.2017.1422206. [DOI] [PubMed] [Google Scholar]
- 18.Picci P., Sangiorgi L., Rougraff B.T., Neff J.R., Casadei R., Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 19.Piccioli A., Maccauro G., Rossi B., Scaramuzzo L., Frenos F., Capanna R. Surgical treatment of pathologic fractures of humerus. Injury. 2010;41:1112–1116. doi: 10.1016/j.injury.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Potter B.K., Adams S.C., Pitcher J.D., Malinin T.I., Temple H.T. Proximal humerus reconstructions for tumors. Clin Orthop Relat Res. 2009;467:1035–1041. doi: 10.1007/s11999-008-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafalla A.A., Abdullah E.S. Endoprosthetic replacement versus cement spacer in reconstruction of proximal humerus after tumor resection: cost and benefits. J Orthop Surg. 2017;25 doi: 10.1177/2309499017713937. [DOI] [PubMed] [Google Scholar]
- 22.Raiss P., Kinkel S., Sauter U., Bruckner T., Lehner B. Replacement of the proximal humerus with MUTARS tumor endoprostheses. Eur J Surg Oncol. 2010;36:371–377. doi: 10.1016/j.ejso.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Rodl R.W., Gosheger G., Gebert C., Lindner N., Ozaki T., Winkelmann W. Reconstruction of the proximal humerus after wide resection of tumours. J Bone Joint Surg Br. 2002;84:1004–1008. doi: 10.1302/0301-620X.84B7.0841004. [DOI] [PubMed] [Google Scholar]
- 24.Ross A.C., Wilson J.N., Scales J.T. Endoprosthetic replacement of the proximal humerus. J Bone Joint Surg Br. 1987;69:656–661. doi: 10.1302/0301-620X.69B4.3611177. [DOI] [PubMed] [Google Scholar]
- 25.Schmolders J., Koob S., Schepers P., Kehrer M., Frey S.P., Wirtz D.C. Silver-coated endoprosthetic replacement of the proximal humerus in case of tumour—is there an increased risk of periprosthetic infection by using a trevira tube? Int Orthop. 2017;41:423–428. doi: 10.1007/s00264-016-3329-6. [DOI] [PubMed] [Google Scholar]
- 26.Tang X., Guo W., Yang R., Tang S., Ji T. Synthetic mesh improves shoulder function after intraarticular resection and prosthetic replacement of proximal humerus. Clin Orthop Relat Res. 2015;473:1464–1471. doi: 10.1007/s11999-015-4139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B., Wu Q., Liu J., Yang S., Shao Z. Endoprosthetic reconstruction of the proximal humerus after tumour resection with polypropylene mesh. Int Orthop. 2015;39:501–506. doi: 10.1007/s00264-014-2597-2. [DOI] [PubMed] [Google Scholar]
- 28.Wittig J.C., Bickels J., Kellar-Graney K.L., Kim F.H., Malawer M.M. Osteosarcoma of the proximal humerus: long-term results with limb-sparing surgery. Clin Orthop Relat Res. 2002;397:156–176. doi: 10.1097/00003086-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Van de Sande M.A., Dijkstra P.S., Taminiau A.H. Proximal humerus reconstruction after tumour resection: biological versus endoprosthetic reconstruction. Int Orthop. 2011;35:1375–1380. doi: 10.1007/s00264-010-1152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]