Abstract
Background
The use of stemless shoulder arthroplasty for osteoarthritis has grown substantially over the past decades. The goal of this study is to evaluate the clinical and radiological outcomes of the Lima SMR stemless anatomic and reverse total shoulder arthroplasty.
Methods
Seventy-three implants in 73 patients (61 anatomic total shoulder arthroplasties [aTSAs] and 12 reverse shoulder arthroplasties [RSAs]) were analyzed with a minimum follow-up of 2 years. The average age in the aTSA group was 65.8 ± 8.7 and 78.3 ± 4.8 in the RSA group. Primary osteoarthritis was the indication in most cases (aTSA 93.7%, RSA 67%). Patients were evaluated preoperatively, at 4, 12, and 24 months postoperatively using the Constant score, the ASES, Oxford Shoulder Score, EuroQol 5 Dimensions 5 Levels questionnaire, range of motion scores, and radiographically. Statistical significance was evaluated using the paired t-test (P < .5).
Results
At 2-year follow-up, the overall average Constant score significantly improved from 40.0 ± 16.7 to 80.9 ± 21.4 (P < .001). Improvement of the ASES (from 31.7 ± 15.6 to 82.5 ± 19.4) and Oxford Shoulder Score (from 19.1 ± 7.4 to 41.9 ± 7.9) was also significant (P < .001). In the aTSA group, all range of motion scores improved significantly (P < .001). In the RSA group, all range of motion scores improved but only active forward flexion and external rotation in abduction improved significantly (P < .05). Most patients were satisfied or completely satisfied at 24 months (aTSA 93.9%, RSA 100%). Two humeral implants in the RSA configuration showed loosening on the first postoperative day related to excessive forces exerted on the shoulder, both requiring revision to a stemmed implant. In the aTSA group, no signs of radiolucencies, osteolysis, gradual loosening, or migration of the components were seen at the final follow-up. In the RSA group, one case had radiolucent lines with subsidence of the humeral core at 12 months, which had not progressed at 24 months and was asymptomatic. All other RSA cases showed no radiolucent lines, migration, scapular notching, or osteolysis. Three anatomic implants were converted with retention of the glenoid baseplate and humeral core to a reverse arthroplasty due to atraumatic cuff failure (N = 2) and traumatic cuff failure (N = 1). After these procedures, patients were satisfied with their results. There were no other complications.
Conclusion
The 2-year results presented in this study show good functional and radiological outcomes using the SMR stemless system.
Keywords: Shoulder arthroplasty, Stemless reverse total shoulder, Stemless anatomic total shoulder, Prospective case series, Outcomes, Complications
Over the past two decades, the use of stemless shoulder arthroplasty implants has gained much popularity due to the advantages of avoiding stem-related problems, such as intraoperative and postoperative periprosthetic humeral fractures, proximal humeral bone loss due to stress shielding, and difficulty explanting a well-fixed stem in the case of revision.5,6,8 Another advantage is the ability of the surgeon to replicate the patient’s anatomy, regardless of the relation of the humeral head to the shaft, for instance, in fracture malunion cases.2 Stemless arthroplasty should be distinguished from resurfacing arthroplasty as it requires a standard osteotomy through the anatomical neck, facilitating a better access to the glenoid reducing the chance of glenoid implant malpositioning. It also avoids the frequently observed problem of overstuffing in resurfacing arthroplasty.8 The stemless humeral implant relies solely on metaphyseal fixation, leaving the medullary canal undisturbed. Recent long-term studies have shown this method of fixation stands the test of time in both anatomic and reverse stemless arthroplasty.3,10,11 All developed stemless implants use cementless fixation, either press fit or screw fixation of the coated and often fenestrated humeral core. Since the first introduction in 2004, over 10 stemless prostheses have been designed, one of them being the SMR stemless prosthesis in 2014 (LimaCorporate S.p.A., Udine, Italy). It is the first convertible platform stemless system which uses a press-fit three-dimensional-printed humeral core enhanced with Trabecular Titanium which promotes bony ingrowth.
The aim of this prospective multicenter clinical follow-up study was to evaluate the clinical, radiographic, and patient-reported results of the SMR stemless anatomic total shoulder arthroplasty (aTSA) and reverse total shoulder arthroplasty (RSA) after a minimum of two-year follow-up. The secondary aim was to define the survival of the implant and to identify possible risk factors that may lead to failure.
Materials and methods
A prospective, multicenter study of 78 consecutive patients with primary or secondary osteoarthritis, inflammatory arthritis, or cuff arthropathy requiring an anatomic or reverse total shoulder replacement was performed. The choice of implant was at the treating surgeon’s discretion with respect to their normal arthroplasty practice. All patients had exhausted nonoperative treatment (eg, physiotherapy, non-steroidal anti-inflammatory drugs, steroid injections) before the decision to proceed with surgery was made. Patients were recruited at four different hospitals (Germany, United Kingdom, the Netherlands) and treated by four shoulder specialists between March 2016 and October 2019. The participants at each center obtained approval for the study from their individual institutional review board. All patients met the inclusion criteria and consented to participate in the study. See Table 1 for inclusion and exclusion criteria.
Table I.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age ≥ 18 years old; Exhausted nonsurgical treatment prior to surgery; Clinical indication for total shoulder replacement due to one or more of the following diagnoses:
Willing and able to complete scheduled follow-up evaluations; Written informed consent was obtained. |
Patient requiring revision shoulder arthroplasty; Osteoporosis with a history of nontraumatic fractures; Steroid injections within the previous 3 months; Contralateral shoulder replacement within the previous 3 months; Lack of sufficient quality bone to support the implant Post-traumatic tuberosity nonunion; Ongoing septicemia; Significant proven or suspicious infection of the target shoulder or any serious infectious disease before the study; Neuromuscular compromise of the shoulder Nonfunctional deltoid muscle; Hypersensitivity to any of the implant materials; Medical history of systemic immune disorders; Treatment for malignancy in previous 2 years; Previous organ transplant; Any intercurrent chronic disease or condition and any significant finding that may interfere with the completion of the 5-year follow-up; Inability to comply with study protocol. Alcohol or drug abuse. Participation in any experimental drug/device study within the 6 months prior to the preoperative visit; Women of childbearing potential who are pregnant, nursing, or planning to become pregnant. |
MORES, male osteoporosis risk estimation score; SCORE, simple calculated osteoporosis risk estimation.
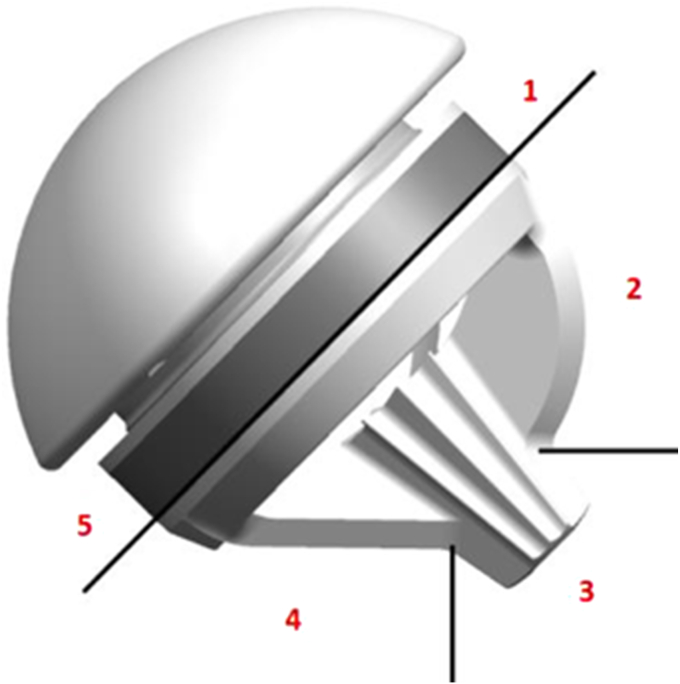
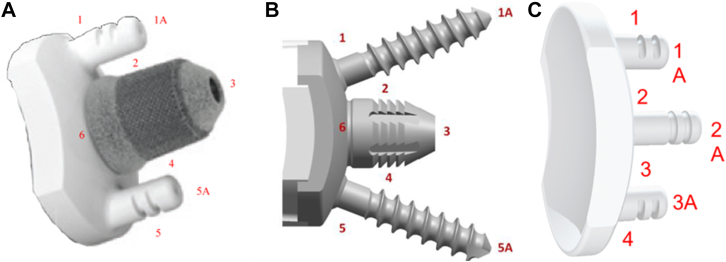
Implant description
The SMR stemless humeral prosthesis is designed to be implanted press-fit into the proximal humeral metaphysis. The three-dimensional-printed humeral core features a trabecular titanium proximal ring to promote osseointegration into the metaphysis. The three-fin configuration with a central post facilitates primary fixation and implant stability in metaphyseal bone. The core has a taper connection which allows coupling with anatomical heads or reverse liners, which makes it the only platform stemless system on the market at this moment.
Surgical technique
All procedures were performed by an orthopedic shoulder arthroplasty specialist. In all cases, surgery was performed under general anesthesia with additional interscalene block for adequate intraoperative and postoperative pain relief. A deltopectoral approach was used in all patients in both groups, with tenotomy of subscapularis if intact. An osteotomy was performed through the anatomical neck in native retroversion and the metaphyseal bone was analyzed visually and by palpation of the cut surface. In cases of significant metaphyseal bone cysts or poor-quality bone indicated by impaction with gentle thumb pressure, the bone was deemed insufficient for stemless core implantation and the decision was made to implant a stemmed humeral component. Either a cemented all polyethylene pegged glenoid, an uncemented metal back component coupled to an ultra-high molecular weight polyethylene liner or a hybrid glenoid with an uncemented central peg and cemented peripheral pegs was used in aTSA cases. The same metal back component was used in all RSA cases with an ultra-high molecular weight polyethylene glenosphere in combination with a humeral metal reverse liner. Following implantation, the subscapularis tendon was repaired end to end in all aTSA cases and in RSA cases when present.
Postoperatively, all patients followed a standardized rehabilitation protocol consisting of physiotherapy sessions including active and passive exercise mobilization techniques to increase power and range of motion (ROM) and prevent muscular deficit or imbalance. Aggressive ROM and subcapularis stretching exercises were in any case avoided during the first 6 weeks. Physiotherapy was initiated on the ward on the day of surgery and continued for a minimum of 12 weeks after surgery.
Evaluation
Patients were evaluated preoperatively at 4 months, 1 year, and 2 years after the operation using the Constant-Murley score, American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form (ASES), Oxford Shoulder Score (OSS), EuroQol 5 Dimensions 5 Levels questionnaire (EQ-5D-5L), and by measuring active and passive ROM.
The preoperative radiological assessment was performed using standardized radiographs in 2 planes: true anteroposterior (AP) view with arm at side in neutral rotation (Grashey’s view) and axillary view with arm held actively in 60 degrees of abduction. Cross-sectional imaging was used as per the surgeon’s usual practice either computed tomography or magnetic resonance imaging to evaluate humeral or glenoid defects, bone quality and glenoid morphology using the modified Walch classification4 and to estimate integrity or fatty infiltration of the rotator cuff.
The postoperative radiological evaluation was performed with radiographs in the same two planes on the first postoperative day and at 6 weeks, 1 year, and 2 years of follow-up. These radiographs were assessed for implant positioning, presence or progression of radiolucent lines, and evidence of subluxation, dislocation, subsidence, or migration of components. The implant-bone interface of the humeral component was divided into 5 different zones and the glenoid component was divided into 6 zones in AP view for measurement of periprosthetic radiolucency (Figs. 1 and 2).
Figure 1.
Zones for radiolucent lines evaluation of the humeral component (with permission LimaCorporate S.p.A.).
Figure 2.
(A-C) Zones for radiolucent lines evaluation of the glenoid components (with permission LimaCorporate S.p.A.).
In RSA cases, presence of a scapular notch and its progression were analyzed on the AP views according to the Nerot classification.19
Statistical analysis
The statistical analysis was performed with SAS, Version 9.4 (Cary, NC, USA) program and 0.05 was adopted as the level of statistical significance for all statistical analyses. Baseline and follow-up data are reported using descriptive statistics: mean, standard deviation, and range were used to summarize continuous variables; otherwise, absolute and relative frequencies are used to characterize categorical distributions. Paired t-tests were used to determine the statistical significance of the change from baseline to 24 months for Constant score, ASES score, EQ-5D-5L, OSS, and ROM. For the Constant score, both the total score and the age-/sex-adjusted score were assessed.
Results
Of the 78 enrolled patients, one withdrew consent. Of the 62 anatomic implants, 61 were available for a 2-year follow-up. Of 15 implanted RSAs, 12 were available for a 2-year follow-up. In the aTSA group, 1 patient died due to bilateral pulmonary emboli. In the RSA group, 1 patient died due to COVID-19 and 2 patients were revised to a stemmed implant due to failed initial fixation within the immediate postoperative phase. None of the patients recruited for this study needed a conversion to a stemmed implant at the time of surgery. Demographic, preoperative, and intraoperative data are described in Tables II and III.
Table II.
Demographic and preoperative data of anatomic and reverse groups.
| Patient characteristics | Overall | Anatomic | Reverse |
|---|---|---|---|
| Age (y) | 68.1 ± 9.54 (77) (46.0-87.0) |
65.7 ± 8.73 (62) (46.0-87.0) |
78.3 ± 4.82 (15) (68.0-86.0) |
| Weight (kg) | 86.6 ± 17.21 (77) (55.1-124.0) |
88.8 ± 17.25 (62) (55.1-124.0) |
77.5 ± 14.23 (15) (60.0-110.0) |
| Height (cm) | 171.2 ± 10.37 (77) (140.0-195.0) |
172.2 ± 10.42 (62) (140.0-195.0) |
166.9 ± 9.32 (15) (155.0-181.0) |
| Body mass index (kg/m2) | 29.5 ± 5.03 (77) (20.5-41.7) |
29.9 ± 5.24 (62) (20.5-41.7) |
27.7 ± 3.66 (15) (23.6-37.2) |
| Gender | |||
| Male | 40 (51.9%) | 34 (54.8%) | 9 (60%) |
| Female | 37 (48.1%) | 28 (45.2%) | 6 (40%) |
| Activity level | |||
| Normal | 55 (71.4%) | 42 (67.7%) | 13 (86.7%) |
| Sedentary | 13 (16.9%) | 11 (17.7%) | 2 (13.3%) |
| Intense | 8 (10.4%) | 8 (12.9%) | - |
| n/a | 1 (1.3%) | 1 (1.6%) | - |
| Affected side | |||
| Left | 42 (54.5%) | 37 (59.7%) | 5 (33.3%) |
| Right | 35 (45.5%) | 25 (40.3%) | 10 (66.7%) |
| Diagnosis | |||
| Primary OA | 68 (88.3%) | 58 (93.5%) | 10 (66.7%) |
| Cuff tear arthropathy | 5 (6.5%) | - | 5 (33.3%) |
| Secondary OA | 3 (3.9%) | 3 (4.8%) | - |
| Inflammatory arthropathy | 1 (1.3%) | 1 (1.6%) | - |
| Glenoid morphology∗ | |||
| A1 | 14 (18.2%) | 9 (14.5%) | 5 (33.3%) |
| A2 | 27 (35.1%) | 20 (32.3%) | 7 (46.6%) |
| B1 | 19 (24.7%) | 18 (29.0%) | 1 (6.7%) |
| B2 | 10 (13.0%) | 9 (14.5%) | 1 (6.7%) |
| B3 | 5 (6.5%) | 4 (6.5%) | 1 (6.77%) |
| D | 2 (2.6%) | 2 (3.2%) | 0 (0%) |
OA, osteoarthritis.
Continuous variables are summarized as mean ± standard deviation (n) | (range).
Categorical variables are summarized as number of patients in each category (percentage).
Modified Walch classification.4
Table III.
Intraoperative data.
| Patient characteristics | Overall | Anatomic | Reverse |
|---|---|---|---|
| Surgery time (min) | 91.3 ± 18.73 (76) (37.0-152.0) |
94.0 ± 17.40 (61) (61.0-152.0) |
80.4 ± 20.58 (15) (37.0-110.0) |
| Blood loss (mL) | 167.0 ± 105.78 (77) (20.0-500.0) |
173.2 ± 112.76 (62) (20.0-500.0) |
141.3 ± 66.85 (15) (50.0-300.0) |
| Humerus bone quality | |||
| Good | 70/77 (90.9%) | 55/62 (88.7%) | 15/15 (100%) |
| Osteoporotic | 5/77 (6.5%) | 5/62 (8.1%) | - |
| Sclerotic | 2/77 (2.6%) | 2/62 (3.2%) | - |
| Status cuff | |||
| Intact | 56/77 (72.7%) | 56/62 (91.9%) | - |
| Massive tear (>5 cm) | 9/77 (11.7%) | - | 9/15 (60%) |
| Attenuated | 7/77 (9.1%) | 5/62 (8.1%) | 2/15 (13.3%) |
| Minor tear (<5 cm) | 5/77 (6.5%) | - | 4/15 (26.7%) |
| Type glenoid implant | |||
| Metal back | 55/62 (88.7%) | 15 (100%) | |
| Hybrid | 6/62 (9.7%) | ||
| Glenoid | 1/62 (1.6) |
Continuous variables are summarized as mean ± standard deviation (n) | (range).
Clinical results
At 2-year follow-up, all patients showed significant improvements in the absolute and adjusted Constant, OSS, ASES, and EQ-5D-5L scores (P < .05). The mean overall Constant score improved from 40.0 ± 16.73 to 80.9 ± 21.46 at the final follow-up (P < .001). The mean overall Constant score adjusted for age and sex improved from 46.5 ± 19.66 to 94.2 ± 25.05 (P < .001). The mean overall ASES score improved from 31.7 ± 15.66 at baseline to 82.5 ± 19.46 at the final follow-up (P < .001). The mean overall OSS score improved from 19.1 ± 7.44 to 41.9 ± 7.90 points (P < .001) (Tables IV and V).
Table IV.
All outcome data of the aTSA group.
| aTSA | Baseline | 4 mo | 1 y | 2 y | Change 2 y from baseline | 2 y vs. baseline P value |
|---|---|---|---|---|---|---|
| Absolute Constant score | 42.4 ± 16.76 (62) | 72.8 ± 18.61 (61) | 82.6 ± 19.43 (60) | 84.5 ± 17.85 (57) | 42.1 | <.001 |
| Adjusted Constant score | 49.2 ± 19.96 (62) | 84.6 ± 21.98 (61) | 95.6 ± 22.61 (60) | 98.0 ± 21.12 (57) | 48.8 | <.001 |
| OSS | 19.2 ± 7.69 (62) | 38.1 ± 8.75 (62) | 43.4 ± 6.81 (60) | 42.9 ± 7.28 (59) | 23.7 | <.001 |
| ASES | 33.1 ± 16.12 (60) | 75.9 ± 18.31 (60) | 87.7 ± 15.81 (59) | 85.3 ± 18.77 (58) | 52.2 | <.001 |
| Satisfied + very satisfied | - | 93.2% | 88.7% | 93.9% | - | |
| Active forward flexion | 99.2 ± 34.52 (62) | 126.9 ± 38.84 (61) | 151.4 ± 33.18 (58) | 156.9 ± 27.36 (54) | 57.7 | <.001 |
| Active external rotation, arm side | 22.3 ± 16.15 (56) | 50.8 ± 25.32 (60) | 60.1 ± 19.83 (58) | 62.7 ± 21.64 (54) | 40.4 | <.001 |
| Active external rotation in abd | 29.8 ± 26.75 (59) | 62.2 ± 22.08 (61) | 77.7 ± 28.56 (58) | 77.5 ± 15.81 (54) | 47.7 | <.001 |
| Active internal rotation > SI joint∗ | 52% | 87% | 93% | 94% | 42% | <.001 |
| Passive forward flexion | 104.3 ± 35.00 (62) | 137.0 ± 31.19 (61) | 156.1 ± 25.45 (58) | 156.3 ± 28.68 (54) | 52 | <.001 |
| Passive external rotation, arm side | 26.9 ± 18.25 (57) | 55.0 ± 22.94 (61) | 60.7 ± 19.25 (58) | 63.3 ± 20.67 (54) | 36.4 | <.001 |
| Passive external rotation in abd | 33.7 ± 28.38 (59) | 65.5 ± 20.74 (61) | 79.2 ± 27.28 (58) | 79.1 ± 14.11 (54) | 45.4 | <.001 |
| Passive internal rotation > SI joint∗ | 55% | 87% | 93% | 89% | 34% | <.001 |
aTSA, anatomic total shoulder arthroplasty; OSS, Oxford Shoulder Score; ASES, American Shoulder and Elbow Surgeons; abd, abduction; SD, standard deviation.
Values given as average ± SD (n).
Percentage of patients reaching the lumbosacral junction or higher.
Table V.
All outcome data of the RSA group.
| RSA | Baseline | 4 mo | 1 y | 2 y | Change 2 y from baseline | 2 y vs. baseline P value |
|---|---|---|---|---|---|---|
| Absolute Constant score | 30.0 ± 12.76 (15) | 57.6 ± 13.97 (13) | 66.3 ± 16.41 (12) | 60.7 ± 29.35 (10) | 30.7 | .017 |
| Adjusted Constant score | 35.5 ± 14.22 (15) | 69.1 ± 15.72 (13) | 79.8 ± 20.10 (12) | 72.7 ± 34.90 (10) | 37.2 | .015 |
| OSS | 18.6 ± 6.50 (15) | 37.2 ± 6.82 (13) | 37.2 ± 7.87 (13) | 36.6 ± 9.00 (12) | 18 | <.001 |
| ASES | 25.8 ± 12.42 (15) | 69.2 ± 15.91 (13) | 69.1 ± 15.61 (13) | 69.0 ± 17.55 (12) | 43.2 | <.001 |
| Satisfied + very satisfied | - | 100% | 100% | 100% | - | |
| Active forward flexion | 71.4 ± 37.89 (15) | 108.8 ± 20.73 (13) | 129.6 ± 26.98 (12) | 115.5 ± 34.17 (11) | 44.1 | .006 |
| Active external rotation, arm side | 25.9 ± 19.84 (14) | 28.5 ± 17.13 (13) | 24.0 ± 22.55 (12) | 30.0 ± 22.80 (11) | 4.1 | .58 |
| Active external rotation in abd | 9.6 ± 20.42 (14) | 49.2 ± 22.90 (13) | 62.8 ± 27.99 (12) | 54.1 ± 27.82 (11) | 44.5 | .002 |
| Active internal rotation > SI joint∗ | 67% | 62% | 69% | 92% | 25% | - |
| Passive forward flexion | 93.3 ± 41.56 (15) | 115.4 ± 21.45 (13) | 133.1 ± 26.08 (12) | 120.0 ± 32.32 (10) | 26.7 | .24 |
| Passive external rotation, arm side | 32.9 ± 24.53 (15) | 31.9 ± 18.77 (13) | 33.7 ± 22.41 (12) | 35.0 ± 20.68 (10) | 2.1 | .65 |
| Passive external rotation in abd | 26.8 ± 33.60 (14) | 52.3 ± 21.66 (13) | 68.8 ± 27.20 (12) | 61.0 ± 23.66 (10) | 34.2 | .08 |
| Active internal rotation > SI joint∗ | 73% | 61% | 69% | 83% | 10% | - |
RSA, reverse shoulder arthroplasty; OSS, Oxford Shoulder Score; ASES, American Shoulder and Elbow Surgeons; abd, abduction; SI, sacroiliac joint; SD, standard deviation.
Values given as average ± SD (n).
Percentage of patients reaching the lumbosacral junction or higher.
Most patients were satisfied or completely satisfied at 24 months (aTSA 93.9%, RSA 100%).
Active and passive ROM improved significantly in all aTSA cases (P < .001). In RSA cases, active forward elevation (FE) and external rotation (ER) in abduction improved significantly (P < .05). Other ROM scores in the RSA group did improve from baseline, although due to the small sample size, these improvements did not reach statistical significance (P > .05) (Tables III and IV).
Radiologic results
No radiolucent lines, osteolysis, humeral dislocation, migration, subsidence, or heterotopic ossification were seen at the final follow-up in the aTSA group. In the same group, 2 cases (3.4%) showed subluxation less than 25% and 2 cases (3.4%) showed 25-50% subluxation at the final follow-up. The four subluxation cases showed significantly lower ASES scores (59.3 ± 19.7 vs. 82.4 ± 18.8) compared to the non-subluxed group (P = .019). No significant difference was seen in the Constant and OSS scores. Preoperatively, 3 cases showed a Walch type A1 glenoid and one a type B1. The patient with the B1 glenoid showed 25% of subluxation. All four patients had an intact cuff.
In the RSA group, one case had radiolucent lines with subsidence of the humeral core at 12 months, which had not progressed at 24 months and was asymptomatic. All other cases showed no radiolucent lines. No scapular notching and no osteolysis was seen. Three cases (27%) showed presumed heterotopic ossifications in the axillary recess. In a subgroup analysis, presence of HO did not cause a statistically significant difference in PROMS.
Complications
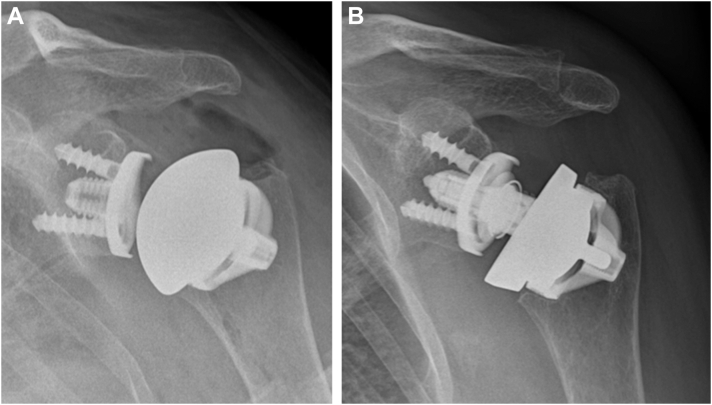
o intraoperative complications occurred. Two patients died for causes unrelated to the surgery or implant. Two RSA cases were revised to a stemmed implant due to early humeral loosening, which both occurred immediately postoperatively following uneventful primary surgery. The first case was a 78-year-old male patient with a stable intraoperative ROM that showed a dislocation the following day for unknown reasons. An attempt at closed reduction under general anesthetic was difficult during which the humeral core was displaced requiring open surgery. Implant revision to stemmed was performed with no further complications. The second case was also a 78-year-old male patient who became delirious during the night following surgery and removed his sling immobilization. He became uncooperative and during his agitation displaced the humeral core of the implant into varus. An early revision to a stemmed implant was undertaken. These events contributed to a revision rate in the RSA group of 13% due to the small sample size. Three aTSA cases were revised to RSA with retention of the humeral core due to rotator cuff failure. The radiographs of one of these cases are shown in Fig. 3 A and B. Two patients sustained an atraumatic supraspinatus tendon tear leading to inferior results at 9 and 12 months of follow-up. The third patient initially went well after surgery but sustained a subscapularis tear while lifting heavy objects as a demolition man resulting in subluxation and a dissociated polyethylene liner. In these patients, the glenoid was metal backed. This results in a revision rate of 4.6% in the aTSA group. One aTSA case had a traumatic cuff tear which was successfully repaired without the need for a revision. No other complications occurred.
Figure 3.
(A and B) An example of an aTSA case converted into an RSA case due to cuff failure. aTSA, anatomic total shoulder arthroplasty; RSA, reverse shoulder arthroplasty.
Discussion
The 2-year results of the SMR stemless system presented in this study show good clinical and radiological outcomes with high patient satisfaction rates for both aTSA and RSA groups. This is the first study to date that presents both aTSA and RSA cases using the same stemless SMR platform system. It also demonstrates the advantage of core retention in rotator cuff deficient aTSA cases.
In both groups, the Constant, ASES, OSS, and EQ-5D-5L scores improved significantly (P < .05). The Constant and ASES scores reached the minimal clinically important difference (MCID) threshold.7,17 This was also the case for active FE and ER in both groups.7,17 The MCID for the OSS was also reached for the aTSA group.12 For reverse arthroplasty, the MCID for the OSS has not been defined to date. The ASES and Constant score improvements also reached the threshold for the substantial clinical benefit in both groups.18 This was also the case for active FE in both groups and active ER in the aTSA group.18 Active ER in the RSA group maintained a positive trend despite not reaching a statistical significance. For the other ROM scores and subjective scores, the MCID and substantial clinical benefit have not yet been defined.
The results of both groups are similar to comparable studies with 2-year follow-up. Albers et al analyzed the SMR stemless for 49 aTSA cases.1 They found a similar rate of satisfaction (97% satisfied or very satisfied), improvement in ASES and OSS scores (89 and 46 points at final follow-up, respectively), and improvement in ROM (FE 149 and ER 44 at final follow-up). They reported two revisions due to cuff failure; in both cases, the humeral core did not need replacement. Schoch et al reported a series of 56 SMR stemless RSA cases. They found a mean improvement of 39.5 points with the Constant scores, which is higher than the improvement seen in this series (30,7).15 Active FE increased with 51 degrees and active ER increased with 13 degrees. In our series, FE increased with 44.1 degrees and ER did not significantly increase. It should be noted that a comparison is unreliable due to the small sample size in our study. However, a possible reason for this difference in outcome may be the difference in age. The mean age was 61.2 compared to 78.3 years in our study. The subjective and clinical outcomes in this series are similar when compared to stemmed aTSA and RSA.9,13,14,16 This is also the case when comparing with other stemless implants.20 In the study by Schoch et al, one case of gradual aseptic loosening was reported due to an incomplete seating of the humeral core during surgery.15 In our series, there were 2 RSA cases with early loosening, but no cases of gradual loosening were seen. One case of subsidence was seen which stabilized over time. In both cases with loosening, the humeral core endured excessive forces in the early postoperative phase in elderly patients with possible lower bone quality which combined could have resulted in impaired primary implant stability.
This study has some limitations. The design is a prospective clinical non-randomized outcome study. Future studies should compare the results of this stemless system with traditional stemmed implants. Another limitation is created by the rather small RSA group creating less reliable results. A third limitation is caused by the three different glenoid implants used in the aTSA group, which creates possible heterogeneity.
Conclusion
The SMR stemless system shows good clinical, radiographic and patient reported results in the short term in the aTSA and RSA group. To our knowledge, this is the first publication showing the possible advantage of the convertibility of the SMR stemless from aTSA to RSA. Further randomized controlled research should be done with larger groups and longer follow-up to draw definitive conclusions and to validate these outcomes.
Disclaimers:
Funding: Funding was received for this study from Lima Corp., Udine, Italy, which is related to the subject of this study. Data collection was performed at each site, and analysis was done by the authors. The preparation and editing of the manuscript was performed by the authors.
Conflicts of interest: Mark Crowther participates as faculty during courses from Lima Corporate, Udine, Italy, which is related to the subject of this work. Arthur van Noort received funding from Lima Corp., Udine, Italy, for this study. He also has a consultant agreement with this company, which is related to the subject of this work. The other authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
The following ethical committees approved this study with the following approval codes: ZorgSaam, Terneuzen, the Netherlands: 19/117-LdB/grvh; St. Anna, Germany: 2015-742-f-S; Spaarne Gasthuis, the Netherlands: M015-024; Bristol, United Kingdom: 16/WS/0078, ID 193744.
References
- 1.Albers C.G.M., Chatindiara I., Moreno G., Poon P.C. Good clinical and radiologic outcomes with the SMR Stemless anatomic TSA after a minimum of 2 years’ followup. Semin Arthroplasty: JSES. 2021;31:563–570. doi: 10.1053/j.sart.2021.03.006. [DOI] [Google Scholar]
- 2.Ballas R., Teissier P., Teissier J. Stemless shoulder prosthesis for treatment of proximal humeral malunion does not require tuberosity osteotomy. Int Orthop. 2016;40:1473–1479. doi: 10.1007/s00264-016-3138-y. [DOI] [PubMed] [Google Scholar]
- 3.Beck S., Patsalis T., Busch A., Dittrich F., Dudda M., Jäger M., et al. Long-term results of the reverse total Evolutive shoulder system (TESS) Arch Orthop Trauma Surg. 2019;139:1039–1044. doi: 10.1007/s00402-019-03135-5. [DOI] [PubMed] [Google Scholar]
- 4.Bercik M.J., Kruse K., Yalizis M., Gauci M.O., Chaoui J., Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25:1601–1606. doi: 10.1016/j.jse.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Bohsali K.I., Bois A.J., Wirth M.A. Complications of shoulder arthroplasty. J Bone Joint Surg Am. 2017;99:256–269. doi: 10.2106/JBJS.16.00935. [DOI] [PubMed] [Google Scholar]
- 6.Chin P.Y.K., Sperling J.W., Cofield R.H., Schleck C. Complications of total shoulder arthroplasty: are they fewer or different? J Shoulder Elbow Surg. 2006;15:19–22. doi: 10.1016/j.jse.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Copay A.G., Chung A.S., Eyberg B., Olmscheid N., Chutkan N., Spangehl M.J. Minimum clinically important difference: Current trends in the Orthopaedic Literature, Part I: Upper Extremity: a systematic review. JBJS Rev. 2018;6 doi: 10.2106/JBJS.RVW.17.00159. [DOI] [PubMed] [Google Scholar]
- 8.Farng E., Zingmond D., Krenek L., SooHoo N.F. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20:557–563. doi: 10.1016/j.jse.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Khan A., Bunker T.D., Kitson J.B. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91:1594–1600. doi: 10.1302/0301-620X.91B12.22139. [DOI] [PubMed] [Google Scholar]
- 10.Magosch P., Lichtenberg S., Habermeyer P. Survival of stemless humeral head replacement in anatomic shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2021;30:e343–e355. doi: 10.1016/j.jse.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Märtens N., Heinze M., Awiszus F., Bertrand J., Lohmann C.H., Berth A. Long-term survival and failure analysis of anatomical stemmed and stemless shoulder arthroplasties. Bone Jt J. 2021;103 B:1292–1300. doi: 10.1302/0301-620X.103B7.BJJ-2020-0915.R3. [DOI] [PubMed] [Google Scholar]
- 12.Nyring M.R.K., Olsen B.S., Amundsen A., Rasmussen J.V. Minimal clinically important differences (MCID) for the Western Ontario osteoarthritis of the shoulder Index (WOOS) and the Oxford shoulder score (OSS) Patient Relat Outcome Meas. 2021;12:299–306. doi: 10.2147/prom.s316920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrillo S., Longo U.G., Papalia R., Denaro V. Reverse shoulder arthroplasty for massive irreparable rotator cuff tears and cuff tear arthropathy: a systematic review. Musculoskelet Surg. 2017;101:105–112. doi: 10.1007/s12306-017-0474-z. [DOI] [PubMed] [Google Scholar]
- 14.Raiss P., Schmitt M., Bruckner T., Kasten P., Pape G., Loew M., et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94:1–10. doi: 10.2106/JBJS.K.00580. [DOI] [PubMed] [Google Scholar]
- 15.Schoch C., Plath J.E., Ambros L., Geyer M., Dittrich M. Clinical and radiological outcomes of a stemless reverse shoulder implant: a two-year follow-up in 56 patients. JSES Int. 2021;5:1042–1048. doi: 10.1016/j.jseint.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevivas N., Ferreira N., Andrade R., Moreira P., Portugal R., Alves D., et al. Reverse shoulder arthroplasty for irreparable massive rotator cuff tears: a systematic review with meta-analysis and meta-regression. J Shoulder Elbow Surg. 2017;26:e265–e277. doi: 10.1016/j.jse.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Simovitch R., Flurin P.H., Wright T., Zuckerman J.D., Roche C.P. Quantifying success after total shoulder arthroplasty: the minimal clinically important difference. J Shoulder Elbow Surg. 2018;27:298–305. doi: 10.1016/j.jse.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Simovitch R., Flurin P.H., Wright T., Zuckerman J.D., Roche C.P. Quantifying success after total shoulder arthroplasty: the substantial clinical benefit. J Shoulder Elbow Surg. 2018;27:903–911. doi: 10.1016/j.jse.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Valenti P., Boutens D., Nérot C. In: Sauramps Médical. Paris, France: Sau- ramps Médical. Walch G., Boileau P., Molé D., editors. 2001. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long term results; pp. 253–259. [Google Scholar]
- 20.Willems J.I.P., Hoffmann J., Sierevelt I.N., va den Bekerom M.P.J., Alta T.D.W., Noort A van. Results of stemless shoulder arthroplasty: a systematic review and meta-analysis. EFORT Open Rev. 2021;6:35–49. doi: 10.1302/2058-5241.6.200067. [DOI] [PMC free article] [PubMed] [Google Scholar]