Abstract
Oncogenesis is associated with intestinal dysbiosis, and stool shotgun metagenomic sequencing in individuals with this condition might constitute a non-invasive approach for the early diagnosis of several cancer types. The prognostic relevance of antibiotic intake and gut microbiota composition urged investigators to develop tools for the detection of intestinal dysbiosis to enable patient stratification and microbiota-centred clinical interventions. Moreover, since the advent of immune-checkpoint inhibitors (ICIs) in oncology, the identification of biomarkers for predicting their efficacy before starting treatment has been an unmet medical need. Many previous studies addressing this question, including a meta-analysis described herein, have led to the description of Gut OncoMicrobiome Signatures (GOMS). In this Review, we discuss how patients with cancer across various subtypes share several GOMS with individuals with seemingly unrelated chronic inflammatory disorders who, in turn, tend to have GOMS different from those of healthy individuals. We discuss findings from the aforementioned meta-analysis of GOMS patterns associated with clinical benefit from or resistance to ICIs across different cancer types (in 808 patients), with a focus on metabolic and immunological surrogate markers of intestinal dysbiosis, and propose practical guidelines to incorporate GOMS in decision-making for prospective clinical trials in immuno-oncology.
Introduction
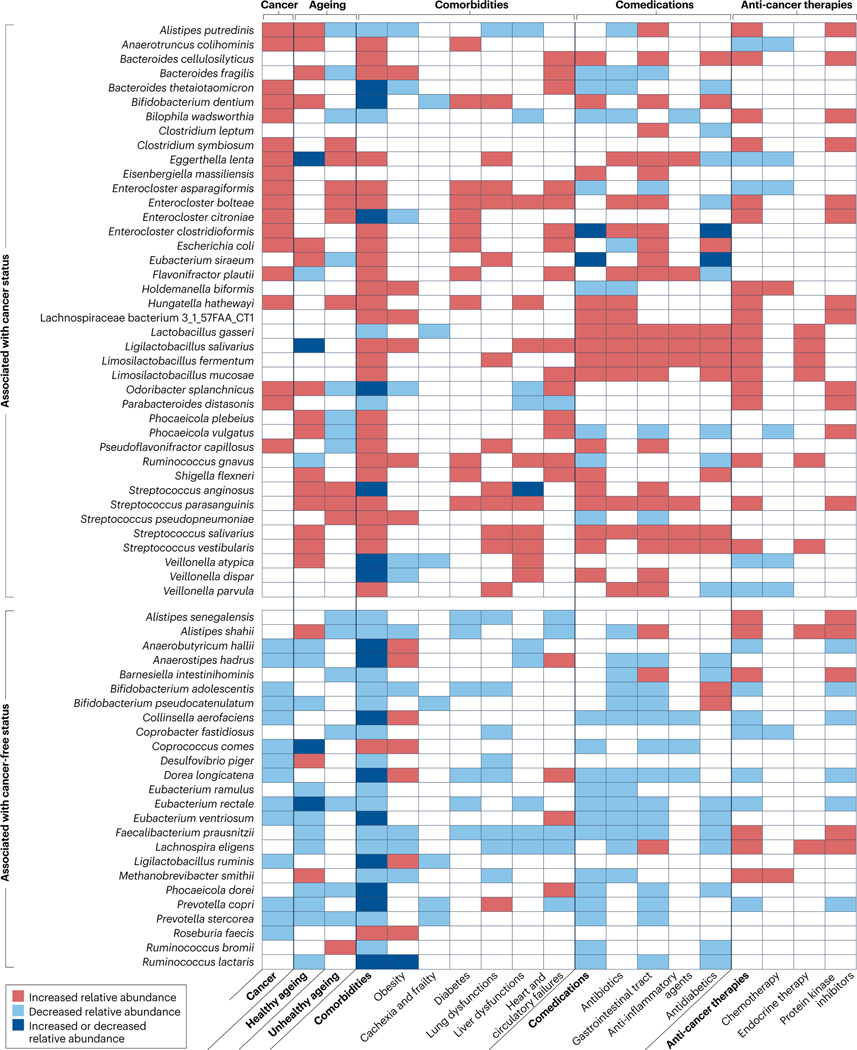
Carcinogenesis is a complex progressive cell-autonomous process driven by (epi)genetically unstable cells that have the potential to acquire phenotypic features that enable them to corrupt local tissue barriers and generate a chronic inflammatory process, which culminate in the group of diseases referred to as cancer1. A more ‘ecological’ view of cancers encompasses their malicious exploitation of local and distant systems, including the autophagic machinery2, senescence3, metabolism, immunity, clonal haematopoiesis, endocrine and neurological networks, and the local and intestinal microbiota1,4,5. In fact, many pathophysiological disorders associated with cancer, referred to as comorbidities (namely, systemic inflammation6–8, obesity, lung and liver dysfunction, ageing-related abnormalities9,10, cachexia11,12 and heart and circulatory failures), as well as specific antitumour therapies and their comedications, can directly or indirectly converge to alter intestinal barrier integrity and the taxonomic composition of the gut microbial ecosystem with a feedforward loop13 (Fig. 1). In this Review, we describe the compositional deviations of the gut microbiota observed in patients with advanced-stage cancer compared with healthy individuals (referred to here as Gut OncoMicrobiome Signatures (GOMS)) and summarize the current knowledge on their effect on cancer-related states as well as their utility as biomarkers during cancer immunotherapy, with a focus on immune-checkpoint inhibitors (ICIs).
Fig. 1 |. Cancer-associated confounding factors (ageing, comorbidities and comedications) associated with or causatively link to intestinal dysbiosis.
PubMed-related search as of October 2022 of shotgun metagenomic sequencing-defined taxonomic composition of stool in cancer in relation to various confounding factors9,22,23,25–27,45,65,97,192–216. The assessment of the effect of immune-checkpoint inhibitors on the intestinal taxonomic composition is currently underway and therefore cannot be provided in this table217,218.
Microbiota in ageing and cancer
The fact that cancer incidence increases with age has been well described. Studies have focused on microbiome features in individuals from the general population have revealed that variations in gut microbiota patterns associated with ageing might influence life expectancy10,14. Hence, ‘unhealthy ageing’ (defined as a status with increased risk of all-cause mortality, independent of age, BMI, clinical site, self-perceived health and/or diagnosis of congestive heart failure) is characterized by an increased presence of certain taxa (such as those from the Bacteroides genus), reduced sharing of unique gut microbiome signatures (that is, higher diversity between individuals) and the presence of gut microorganism-derived xenobiotic metabolites (for example, toxic phenylalanine and tyrosine fermentation products)9,10. By contrast, ‘healthy ageing’ continues to develop along a distinct metagenomics-based trajectory that originates in adulthood, and is accompanied by a rise in specific plasma microbial metabolites, culminating in extended longevity. Hence, healthy ageing correlates with an increased presence of certain indoles, which are gut bacterial degradation products of tryptophan that mediate immune homeostasis upon binding to aryl hydrocarbon receptors and IL-10 receptors15–17.
The chronic inflammatory process associated with oncogenesis is a feature of unhealthy ageing4. Many patients with advanced-stage cancer present with cachexia, a process involving but not limited to frailty, sarcopenia and fat loss, which lead to systemic inflammation. In mice, increased gut permeability precedes similar processes18. Members of the Enterobacteriaceae family, including the typically pro-inflammatory Gammaproteobacteria class and Veillonella genus, tend to be more abundant among patients with cancer-associated cachexia12. Faecal levels of short-chain fatty acids (SCFAs), more specifically acetate, are associated with health-related stool taxonomic composition, and have a inverse correlation with inflammation-related calprotectin12 but tend to be reduced in patients with cancer-associated cachexia12. Other gut microbiota-transformed metabolites, such as biliary salts involved in the differentiation of intestinal regulatory T (Treg) and Tr17 cells, have a protective role against distinct cancers19–21. In fact, specific secondary bile acids (including isoallolithocholic acid) produced by distinct gut species (such as Alistipes putredinis and Odoribacter splanchnicus) are more prevalent in centenarian individuals and reportedly contribute to intestinal homeostasis22.
An ‘unhealthy’ gut microbiome signature will be determined by both the associated pathological state and the medication used to treat it. Comedications often prescribed to patients with cancer, such as proton pump inhibitors (PPIs), antibiotics, anti-inflammatory agents, osmotic laxatives and biguanide antidiabetic drugs, strongly affect the taxonomic composition of the intestinal microbial ecosystem23. For example, in various patient cohorts receiving either PPIs or antibiotics these medications are associated with significantly lower microbial diversity (P < 0.05), over-representation of supraglottic commensals (such as Streptococcaceae family members in those receiving PPIs24) and an increased abundance of gut commensals considered immunosuppressive (such as Hungatella and Enterocloster genera in those receiving antibiotics25,26). Lastly, polychemotherapy and endocrine therapy (such as androgen deprivation) can modulate the alpha and beta diversities of the microbiota, which have been proposed to affect treatment-related adverse events27,28 as well as drug metabolism and efficacy29,30. Altogether, these pathological and iatrogenic factors can converge to deviate the healthy repertoire of the intestinal ecosystem, a process referred to here as ‘intestinal dysbiosis’, in patients diagnosed with localized or locally advanced intestinal and extra-intestinal malignancies.
Likewise, tumours can drive compositional shifts of the microbiota to their own benefit (Supplementary Fig. 1). Indeed, as shown in the early 1960s, certain malignancies can cause jejunal and ileal mucosa atrophy25,31,32. Results from studies in mouse xenografts show that cancer promotes ileal mucosa atrophy (monitored measuring the villous–crypt height ratio) characterized by increased crypt apoptosis, defects in proliferation, activation of endoplasmic reticulum stress responses and induction of autophagy in villus enterocytes11,25. Tumour inoculation in mice also disturbs the secretory components of ileal crypts, including the enteroendocrine cells that accumulate beyond the crypts and express tyrosine 3-monooxygenase, leading to synthesis of the amino acid L-DOPA and accumulation of catecholamine end products25. Moreover, imbalances between catecholamine and cholinergic signalling are observed in the ileal mucosa of mice harbouring tumours25 (Supplementary Fig. 1). Ileal atrophy also leads to a transient increase in intestinal permeability, paving the way to an overt and protracted dysbiosis25. The prototypic dysbiosis process accompanying tumour growth is dominated by Gram-positive Clostridium species (belonging to the Enterocloster genus, including but not limited to E. bolteae, E. clostridioformis, E. asparagiformis and E. citroniae) and Flavonifractor plautii, with under-representation of Eubacterium and Lactobacillus spp.25. Further experiments revealed a cause–effect relationship between this stress ileopathy and carcinogenesis. In preclinical studies, strategies including pharmacological blockade of β-adrenergic receptors, Adrb2 deficiency, administration of vancomycin or co-housing of the tumour-bearing mice with tumour-free littermates all prevented cancer-induced ileopathy, eventually slowing tumour growth kinetics25. Importantly, patients with cancer can also have hallmarks of stress ileopathy. Indeed, in patients diagnosed with gastrointestinal (including colon adenocarcinoma and neuroendocrine intestinal tumours) or genitourinary malignancies, crypt apoptosis is correlated with ectopic enteroendocrine cells25. In addition, the faecal microbiota composition in these patients substantially differs from that in individuals without cancer, with the former having an over-representation of the Enterocloster genus and loss of Lachnospiraceae or Oscillospiraceae family members (including Eubacterium, Dorea and Faecalibacterium spp.). Hence, stress ileopathy is a corollary disease of intestinal and extra-intestinal malignancies culminating in protracted intestinal dysbiosis. Of note, this stress ileopathy is not cancerspecific. For example, Stanley et al.33 showed that ischaemic brain injuries can also cause gut barrier dysfunction with increased permeability, enabling the translocation of ileal bacteria to peripheral organs34. In summary, patients with cancer can develop intestinal dysbiosis that might be harnessed to stratify patient cohorts owing to its clinical relevance, which we discuss in later sections.
Gut OncoMicrobiome Signatures
The intestinal microbiota is modulated during the course of many different diseases, most specifically inflammatory bowel diseases (IBD), metabolic syndrome, autoimmune disorders and cancer13,35. However, universal signatures of a healthy or unhealthy gut microbiome are difficult to identify because of intra-individual and inter-individual variability of the microbiota, methodological issues, geographical variance and many other confounding factors, including genetics, the exposome, lifestyle and diet. As explained, the cancer-associated stress ileopathy caused by the chronic inflammatory process might pave the way to a distinct taxonomic composition of stool showing deviation from the healthy status, or GOMS.
The Dutch Microbiome Project (DMP) within Lifelines, a three-generation prospective population cohort study comprising >8,000 individuals from northern Netherlands who provided faecal and blood samples, analysed associations between stool taxonomic composition and certain well-defined phenotypes (on the basis of health-related, demographic and lifestyle factors)23. The DMP identified nine core species (Subdoligranulum sp., Alistipes onderdonkii, A. putredinis, Alistipes shahii, Bacteroides uniformis, Bacteroides vulgatus, Eubacterium rectale, Faecalibacterium prausnitzii and Oscillibacter sp.) that form central nodes in microbial co-abundance networks in >95% of individuals23. Surprisingly, a common gut microbiome signature was shared between individuals with seemingly unrelated diseases (such as cancer, cardiovascular diseases, metabolic disorders, gastrointestinal syndromes and mental health disorders). Therefore, this signature independent of comorbidities was referred to as the DMP gut microbiome signature of health. The signature shared 86% of the genera and species identified in the Gut Microbiome Health Index, another broad study (involving ~2,600 and ~1,700 healthy and unhealthy individuals, respectively)36. In addition, the DMP identified other health-related microbiome patterns, comprising Butyrivibrio, Akkermansia and Prevotella genera. In brief, the DMP gut microbiome signature of health mainly consists of decreases in the relative abundances of Enterocloster, Flavonifractor, Eggerthella, Streptococcus, Hungatella and Veillonella genera, and increases in the relative abundances of members of several families (Prevotellaceae, Lachnospiraceae and Oscillospiraceae) and distinct immunogenic representatives of the Bacteroidales order (Barnesiella intestinihominis, A. shahii and Alistipes senegalensis) (Supplementary Table 1). Gut microbiota-associated functional pathways shared across unrelated diseases consist mainly of increased biosynthesis of L-ornithine, ubiquinol, menaquinol and enterobacterial common antigens, as well as decreased biosynthesis of amino acids, deoxyribonucleosides and nucleotides, and reduced fermentation of SCFAs (mainly butanoate)23. These findings are in line with the aforementioned study showing that intestinal dysbiosis is a corollary syndrome of cancer25. Indeed, we confirmed this consistency when we admixed pan-cancer metagenome data from a prospective cohort of 1,426 patients diagnosed with eight different malignancies (including colon, kidney, breast, lung, ovarian or prostate cancer, melanoma or chronic leukaemia) at various stages with those from 705 patients with colon cancer referenced in publicly available databases, and compared them with those from >5,570 individuals without cancer. As found in other studies23,36, statistically significant differences in microbiota taxonomic profiles distinguished individuals with and without cancer. Selected bacteria taxa (Prevotellaceae and Lachnospiraceae family members, and Bifidobacterium spp.) were over-represented in individuals without cancer, whereas Gram-positive Enterocloster and Clostridium spp., Eisenbergiella spp., as well as Gammaproteobacteria and Deltaproteobacteria were relatively dominant across six of eight cancer types25 (Supplementary Table 1).
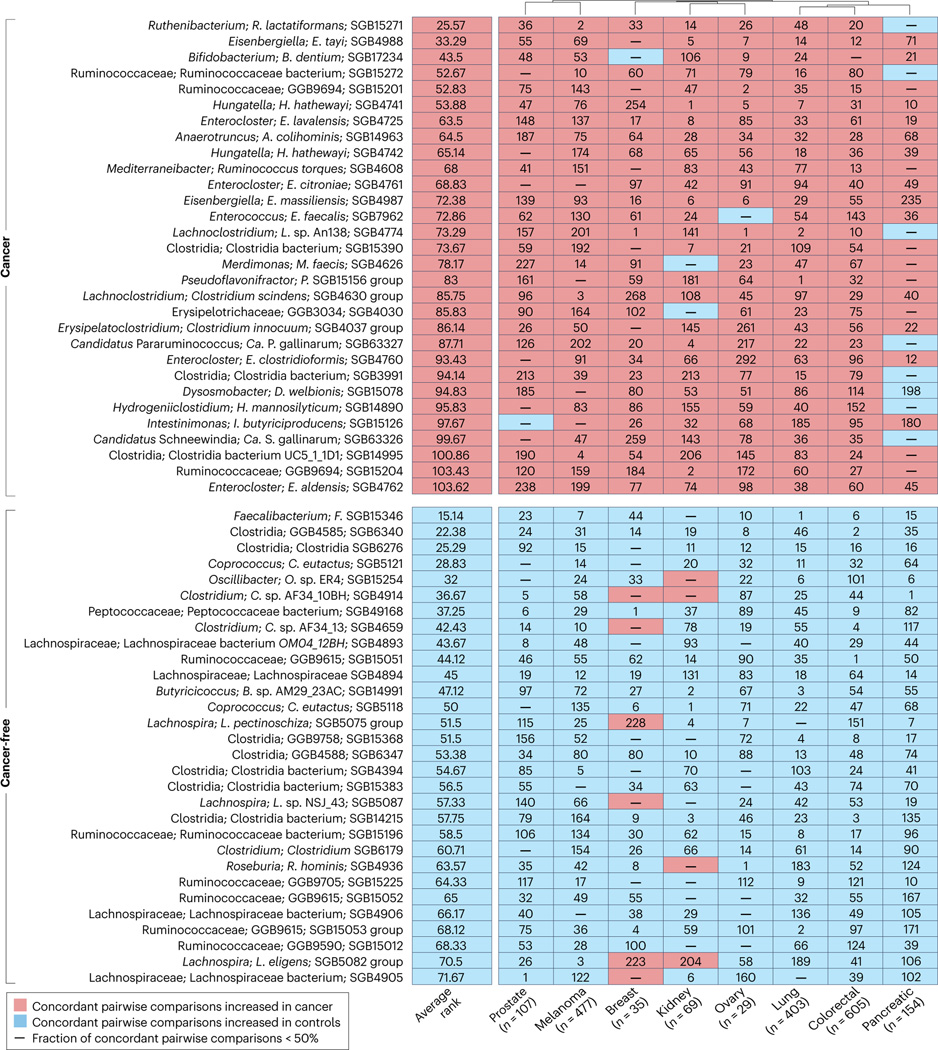
To refine the definition of these GOMS related to pan-cancer-associated intestinal dysbiosis, we performed a meta-analysis. We used MetaPhlAn 4 (ref. 37) for microbiome taxonomic profiling of stool samples from 1,879 adults across eight different cancer types (included in 30 cohorts from 23 published studies)25–29,38–55 and from a control group comprising 5,341 individuals without cancer (included in 17 cohorts from 14 published studies), all spanning diverse geographical locations48,56–68 (Supplementary Table 2, Supplementary Methods). With the exception of colorectal cancer (CRC), the cancer datasets typically included only patients with cancer and not individuals without cancer because the aim of the studies was to study responses to treatment, and matched control individuals were not meant to be recruited. Therefore, a random-effects meta-analysis was performed on pair-wise comparisons between all possible cohorts of patients with and without cancer for all the detected species-level genome bins (SGBs).
By computing the ranking of statistically significant associations for each SGB with the cancer or non-cancer condition, we identified several species consistently associated with cancer, both across distinct cohorts for the same cancer type and across different cancer types (Fig. 2, Supplementary Tables 2,3). Specifically, stools from individuals across the eight cancer types were consistently enriched for the Enterocloster genus, Hungatella and Clostridium spp., Pseudoflavonifractor and species from the genus Eisenbergiella, and had a decrease abundance of members of several families such as Lachnospiraceae and Oscillospiraceae (including Faecalibacterium spp.), all relative to individuals without cancer (Fig. 2, Supplementary Tables 1,3). Notably, many such biomarkers belong to yet-to-be-characterized species represented by SGBs solely defined on the basis of metagenome-assembled genomes, lacking any cultivated representative, and assignable only at high taxonomic levels (for example, families), highlighting once again the need for further microbiology studies using cultures.
Fig. 2 |. Pan-cancer GOMS compared with healthy metagenomic profiles.
Top 30 ranked species-level genome bins (SGBs) in individuals with or without cancer. Ranks were determined by ordering P values calculated using a random effects meta-analysis of all possible cancer and control cohort pairs. Only SGBs with 95% credible intervals of >0 or <0 in >50% of pairwise comparisons were considered for the ranking. GOMS, Gut OncoMicrobiome Signatures.
GOMS and prognostic value
While pan-cancer GOMS could indirectly reflect a systemic chronic inflammatory process, certain deviations of the gut microbiota repertoire might be more specifically attributable to distinct tumour types. Here, we only focus on results derived from large biobanks (containing ≥100 specimens) that revealed important and robust specific features, and discuss their potential clinical relevance (Supplementary Table 4).
Breast cancer
To define a breast cancer GOMS, we used samples from an analysis of faecal compositional differences in women between those with and without breast cancer performed in France27. The breast cancer GOMS comprised families from the Bacteroidales order, and Tannerellaceae, Rikenellaceae, Prevotellaceae and Odoribacteraceae families as well as viruses (C2 like virus unclassified, Lactococcus phage 936, enterobacteria phage JL1, E. coli phage phAPEC8 and Sodalis phage SO1)27 (Supplementary Table 4). The Enterocloster genus, which has a reported association with pathophysiological disorders23,25, was also over-represented in the breast cancer GOMS.
Importantly, this GOMS had prognostic significance, in the sense that women with stage II–III breast cancer at diagnosis tended to harbour this signature whereas those with stage I breast cancer instead had non-malignant-like metagenomic stool profiles (Supplementary Table 4). Reinforcing this notion, microorganisms within the breast cancer GOMS were also enriched in patients with resistance to neoadjuvant chemotherapy27. Overall, B. uniformis, Parabacteroides merdae and Enterocloster spp. were over-represented in stools from women presenting with stage II–III breast cancer (compared with stage I breast cancers), and Bacteroides thetaiotaomicron, E. bolteae and E. clostridioformis were associated with resistance to cytotoxic agents. In fact, chemotherapy was associated with a substantial shift in the alpha and beta diversity of stool composition in women with breast cancer towards an anti-inflammatory pattern27, entailing a relative under-representation of distinct species commonly found across several pathological disorders (Veillonella parvula, Veillonella atypica, Eggerthella lenta and E. asparagiformis)23. Finally, many breast cancer GOMS species (B. uniformis, B. thetaiotaomicron and many members of the Enterocloster genus) were also substantially associated with adjuvant chemotherapy-related neurotoxicity27. Interestingly, many of these breast cancer GOMS components (V. parvula, B. uniformis and E. clostridioformis, among others) had previously been identified in an analysis of the breast cancer microbiome comprising >9,000 species after filtering for artefactual contaminants69. We eagerly await the results of future studies seeking to identify links between gut microbiome patterns and breast cancer incidence, their relevance with breast cancer-related oncogene or tumour-suppressor gene profiles (for example, those related to HER2, p53 or PI3K–PTEN signalling), and resistance to endocrine therapies as well as anti-PD-1 or anti-PD-L1 (from here onwards referred to as anti-PD-(L)1 antibodies).
Pancreatic ductal carcinoma
Across various studies taking into account all known possible cofounding factors relevant to pancreatic ductal adenocarcinoma (PDAC), the faecal microbiome composition in patients with this devastating disease differed from that in individuals without cancer45,54. Researchers used shotgun metagenomic profiling to analyse stool and salivary samples from individuals in three cohorts from Asia and Europe45, and identified a shared PDAC GOMS in which the pro-inflammatory Streptococcus and Veillonella spp.70 and 58 of their bacteriophages were substantially enriched and SCFA producers (such as F. prausnitzii, E. rectale and Ruminococcus bicirculans) were depleted45 (Supplementary Table 4). These PDAC GOMS matched gut microbiome signatures observed in individuals taking PPIs24. Given the epidemiological association between PPI use and risk of PDAC71, we consider that further studies addressing whether PPIs accelerate the development and/or progression of PDAC are warranted.
Prospective follow-up revealed correlations between the presence of the PDAC GOMS and PDAC-related mortality. An abundance of SCFA producers in the gut relative to that of Ruminococcus torques, Haemophilus parainfluenzae and Neisseria bacilliformis was associated with prolonged overall survival (OS). Functional analysis of GOMS in these three cohorts also revealed a substantial increase in the biosynthesis of C5 isoprenoid from the mevalonate pathway and of ADP-L-glycero-D-manno-heptose in PDAC45. Isoprenoids are required for the activity of GTPases, including oncogenic KRAS, which is involved in the initiation and maintenance of PDAC45. ADP-L-glycero-D-manno-heptose is a precursor of the pro-inflammatory molecule lipopolysaccharide, culminating in procarcinogenic NF-κB activation45.
Shotgun metagenomic sequencing data from two case–control studies (one performed in Spain and the other in Germany) were used to describe a microbiota-based classification model with an area under the receiver operating characteristic curve (AUC) of up to 0.84 for a set of 27 microbial species specific for a PDAC GOMS. This signature was highly disease-specific when validated against 25 publicly available metagenomic datasets derived from various health conditions (encompassing >5,000 individuals)54. Albeit similar to those of the previous study45, the findings of this study were complementary. Firstly, new taxa (not listed in the pan-disease GOMS so far (Supplementary Table 1)) such as Romboutsia timonensis and the Methanobrevibacter smithii archaea, stood out in the PDAC GOMS as negatively and positively associated with the diagnosis of this malignancy, respectively. Secondly, molecular traits of specific gut microorganisms (from Akkermansia spp., Lactobacillus spp., Bifidobacterium spp., Veillonella spp., Bacteroides spp. and Streptococcus spp.) were detectable in pancreatic tissues using 16S ribosomal RNA sequencing and fluorescence in situ hybridization (FISH) assays with genus-specific probes. Thirdly, comparison of faecal and saliva samples revealed that distinct strains of faecal PDAC-associated microorganisms could originate from the oral cavity, owing to oral–intestinal transmission. Altogether, these studies demonstrated the feasibility of developing a global, specific and reproducible predictive model based on non-invasive gut microbiome profiling to screen for early stage I–II PDAC54.
Colorectal cancer
The gut microbiome in patients with CRC has been extensively studied and provides arguably the most paradigmatic example of the role of the gut microbiome in cancer. Initial studies in single cohorts helped to elucidate the potential of gut microbiomes as diagnostic tools to detect CRC46,52,72. These results have been validated in multiple geographically distinct cohorts, identifying reproducible gut micro biome signatures49,50,73. This signature, identified using both supervised machine learning and a meta-analysis, included the bacterial species Solobacterium moorei, Fusobacterium nucleatum, Parvimonas micra, Peptostreptococcus stomatis, Peptostreptococcus anaerobius and Gemella morbillorum (Supplementary Table 4). Some members of this signature, such as F. nucleatum and P. anaerobius, have been shown to promote colorectal carcinogenesis and modulate tumour immunity74,75 and, along with P. stomatis and P. micra, are commonly found in the oral cavity in the general population. The enrichment of bacterial species from the oral cavity is characteristic of the CRC GOMS50,76–79, along with an enrichment in invasive polymicrobial bacterial biofilms in patients with right-sided tumours76,80,81.
Thus far, most studies have focused on studying CRC GOMS in patients who were diagnosed at a later age (median 68 years in men and 72 years in women); however, incidence rates of CRC in patients <50 years old, referred to as early onset CRC, are on the rise82. Compared with patients of ≥50 years of age, those with early onset CRC usually present with more advanced disease, and have different pathological features and CRC GOMS83,84. The latter is characterized by an enrichment of F. plautii and increased metabolism of tryptophan, bile acids and choline. Advances in microbiome research coupled with the availability of large publicly available cohorts have enabled the discovery of additional biomarkers from the often overshadowed, but equally important, non-bacterial members of gut microbial communities85. Studies have discovered specific virome79 and mycobiome86,87 signatures for CRC GOMS, and included in vitro and in vivo experiments showing that the fungus Aspergillus rambellii can promote CRC cell growth86.
GOMS and prediction of response to ICIs
ICIs have revolutionized cancer therapy, either as monotherapy or as part of combination regimens, across multiple tumour types88,89. However, only a minority of patients derive prolonged clinical benefit from ICIs. In addition, owing to their unique mode of action as blockers of inhibitory signalling pathways in immune cells, ICIs generate immune-related adverse events that can be life threatening and, thus, can limit their broad use (in particular, in patients with early stage disease) in the absence of robust biomarkers of response. The validation of such biomarkers has become a priority in order to improve the therapeutic index of ICIs and facilitate the clinical management of patients receiving them, specifically in the adjuvant or neoadjuvant settings. Currently, only three biomarkers (PD-L1, tumour mutational burden (TMB), microsatellite instability and DNA mismatch repair (MMR) deficiency) have been approved by the FDA, with some limited performance (Table 1).
Table 1 |.
Potential role of biomarkers associated with host–microbiota interactions in immuno-oncology
| Biomarker | Rationale | Role in routine oncology |
|---|---|---|
| Standard use | ||
| PD-L1 | Tumour PD-L1 expression assessed by IHC positively correlates with CD8+ T cell infiltrates and ICI efficacy in cancer174 | FDA approval of PD-L1 IHC as a companion diagnostic biomarker for anti-PD(L)1 antibodies, in metastatic or advanced-stage NSCLC, cervical cancer, HNSCC, ESCC, TNBC and urothelial carcinoma175 |
| MMR | MMR deficiency (assessed by tumour DNA sequencing) associated with TMB, CD8+ TILs and clinical efficacy176 | FDA approval for endometrial carcinoma and solid tumours175,176 |
| TMB | High TMB (≥10 mut/Mb) associated with improved survival in NSCLC, melanoma and HNSCC, but not in ESCC, gastric cancer, CRC, urothelial carcinoma, brain cancer, unknown primary tumours or other tumours after stratification based on MMR and polymerase δ status177–179 | FDA approval, available in solid tumours, for example with FoundationOne CDx175,180 |
| LIPI | Association between the neutrophil-to-lymphocyte ratio and LDH in blood181; association with gut dysbiosis (alpha and beta diversity)182 | Investigator choice for routine implementation |
| IL-8 | Plasma, PBMC and tumour IL-8 levels at baseline or on treatment are surrogates for intratumoural high densities of myeloid infiltrates and neutrophils that predict reduced efficacy (when high or increased) of ICIs in metastatic melanoma, NSCLC, urothelial carcinoma and RCC in retrospective phase III trials183,184 | Plasma IL-8 (≥23 pg/ml) determined by ELISA, at baseline and on treatment in metastatic melanoma, NSCLC, urothelial carcinoma and RCC; prospective validation ongoing |
| Gut-derived biomarkers (from microbiota and epithelia) | ||
| Microbiome | Baseline faecal bacteria and archaea alpha diversity, richness and specific taxonomic composition associated with ICI clinical outcome26,38–40,42,44,91,96,97,100,105,125,126, as assessed by 16S rRNA sequencing, MGS or targeted-PCR on specific taxa | Shown in retrospective studies of CD19-targeted CAR T cells, and of ICIs in neoadjuvant and metastatic cancers; prospective validation pending (NCT04567446, ANR-21-RHUS-0017) |
| sCD14, sST2, LBP and sMAdCAM-1 | Serum markers of leaky gut associated with cancer, and stress ileopathy25,26,42, and dysbiosis21 | Biological and clinical relevance of ICI resistance shown in retrospective studies in NSCLC, RCC and urothelial carcinoma for sMAdCAM-1 only21 |
| Exfoliome | Mammalian DNA contamination in stool MGS and/or non-invasive stool transcriptomics as surrogate markers of pathogenic host–commensal crosstalk40 | Association with endotoxaemia and dysbiosis40 |
| Microbiota-derived metabolites | ||
| SCFAs | High faecal levels of acetate, propionate, butyrate and valerate, and high blood levels of isovalerate associated with improved outcomes in ICI-treated patients144 | Controversial findings145; prospective validation required; might support the recommendation of high-fibre-based diet to increase ICI efficacy53 |
| Inosine | During ICI or CpG immunostimulation, inosine exhibited a T cell intrinsic IL-12Rb-dependent stimulatory capacity167 | Translation of these findings in mouse models to humans awaited |
| Kyn to Trp ratio, I3A and 3-IAA | Higher plasmatic Kyn to Trp ratio measured by LC–MS is associated with early progression and reduced OS to ICIs in several cancer types151,152; enrichment of Trp pathway in five gut-associated phyla154; 3-IAA associated with response to chemotherapy in PDAC185 | Controversial findings186; might support Trp-enriched diet, or I3A or 3-IAA-based prebiotics in combination with ICIs155,185 |
| L-Arg | Low baseline blood L-Arg levels associated with worse clinical outcomes in ICI-treated patients159,160 | To be validated in prospective cohorts |
| Bile acids | Preclinical studies associated bile acids and gut microbiota composition with cancer immunosurveillance19 and ICI efficacy162; modulation of bile acid composition after FMT or after anti-PD-1 antibody therapy in responders128 | Further studies required to assess the clinical relevance of bile acids during immunostimulation |
| TMAO | TMAO induces a type I interferon fingerprint in tumoural macrophages; CutC-encoding commensals associated with response to anti-PD-1 antibodies in melanoma170 | Prospective validation required to address whether choline-enriched diet promotes ICI efficacy170 |
| Immune responses directed against commensals or pathobionts | ||
| Bacteria-specific memory T cells | Reactivities towards commensals, such as Enterococcus hirae, Bacteroides fragilis, Akkermansia muciniphila or faecal bacteria, predict response to chemotherapy126, ICIs42,125 or FMT128 | Prospective validation required |
| IgA and IgG titres against commensals | Blood or faecal IgA and IgG bound to bacteria (detected by flow cytometry) are associated with gut microbiota composition and ICI response113,116,118,187 | Prospective validation of clinical relevance needed |
| TLS and TFH | Association between intratumoural Escherichia coli, maturation of TLS, on-treatment increase in serum CXCL13, E. coli-specific IgG titres and response to neoadjuvant ICI in urothelial carcinoma119,120 | Cause–effect relationship between tumoural bacteria and TLS not established |
| Intratumoural infection by pathobionts, antigen mimicry and adjuvanticity | Bacterial MHC class I and class II epitopes presented at tumour membrane activate TILs188; molecular mimicry between oncogenes or tumour antigens, and phage or bacterial peptides123,124; immunogenicity of outer membrane vesicles and genetically modified bacteria123,189–191 | Ongoing clinical trials (NCT04116658, NCT04187404) |
16S rRNA, 16S ribosomal RNA; 3-IAA, indole-3-acetic acid; L-Arg, L-Arginine; CAR, chimeric antigen receptor; CRC, colorectal cancer; ELISA, enzyme-linked immunosorbent assay; ESCC, oesophageal squamous cell cancer; FMT, faecal microbiota transplant; HNSCC, head and neck squamous cell carcinoma; I3A, indole-3-aldehyde; ICI, immune-checkpoint inhibitor; IHC, immunohistochemistry, Kyn, kynurenine; LBP, LPS-binding protein; LC–MS, liquid chromatography–mass spectrometry; LDH, lactate dehydrogenase; LIPI, Lung Immune Prognostic Score; MGS, shotgun metagenomics sequencing; MMR, DNA mismatch repair; NSCLC, non-small-cell lung cancer; OS, overall survival; PBMC, peripheral blood mononuclear cell; PDAC, pancreatic ductal adenocarcinoma; RCC, renal cell carcinoma; SCFA, short-chain fatty acid; TFH, T follicular helper cell; TIL, tumour-infiltrating lymphocyte; TLS, tertiary lymphoid structure; TMAO, trimethylamine N-oxide; TMB, tumour mutational burden; Trp, tryptophan; sCD14, soluble CD14; sMAdCAM-1; soluble mucosal addressin cell adhesion molecule 1; sST2, soluble IL-1 receptor-like 1; TNBC, triple-negative breast cancer.
Over the past decade, following the epidemiological demonstration of the negative effect of antibiotics on the efficacy of ICIs13,42,90, specific microbial taxa have been associated with response or resistance to ICIs across distinct cancer types26,38–40,42,91–100. Some overlap between beneficial or harmful bacterial taxa within a specific cancer type has been found across studies, although it was modest and could not be simply explained by methodological considerations39,40,97. Beyond confounding factors that could affect the robustness of ICI-response GOMS for each cancer type, each study included in our metagenome-based meta-analysis used different end points (such as objective response rate (ORR), best response, progression-free survival (PFS) or OS). Here, we first summarize findings obtained mostly in sizeable cohorts (comprising ≥100 patients) diagnosed with non-small-cell lung cancer (NSCLC) or melanoma, describing robust GOMS associated with clinical outcome. Secondly, we provide the first meta-analysis across all patients with cancer treated with anti-PD-(L)1 antibodies with or without anti-CTLA4 antibodies across various geographical locations, unveiling common GOMS associated with response or resistance to ICIs in terms of ORR only (Figs. 3,4, Supplementary Tables 5–7).
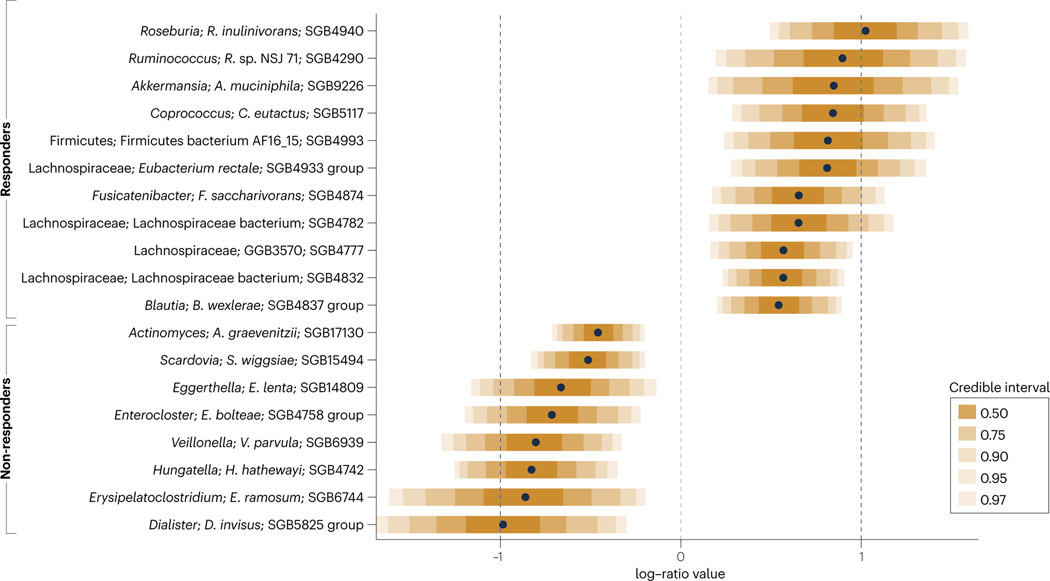
Fig. 3 |. GOMS related to response or lack of response to immune checkpoint inhibitors identified by mega-analysis.
Results of a mega-analysis using pibble models on centred log ratio-transformed species-level genome bin (SGB)-level relative abundances. Pibble models also included age, sex and cohort (Supplementary Table 2) as covariates. SGBs whose 95% credible interval is >0.2 or <0.2 are shown. SGBs with a prevalence >5% and their corresponding 95% credible intervals are reported in Supplementary Table 6. Responders had a complete or partial response, and non-responders had stable or progressive disease. GOMS, Gut OncoMicrobiome Signatures.
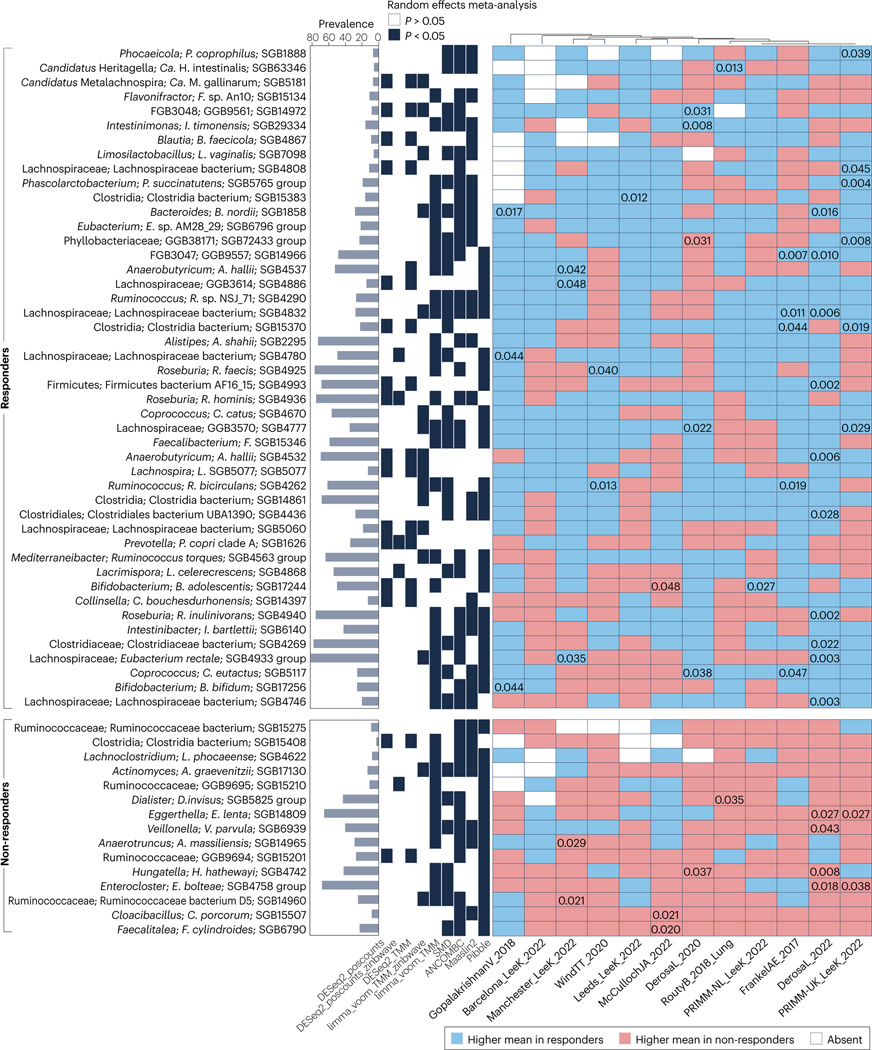
Fig. 4 |. GOMS related to response or lack of response to immune checkpoint inhibitors identified by meta-analysis.
The meta-analysis was performed on 12 cohorts from 8 published studies26,38–44 depicted in the x-axis labels. Species-level genome bins (SGBs) associated with objective response rates (ORRs) and identified by a meta-analysis using different differential abundance methods and adjusting for age and sex. The SGBs shown have random-effects model P values <0.05 by at least three methods. The values included inside the cells refer to unadjusted P values <0.05 obtained by two-tailed Wilcoxon tests on differences in relative abundance between responders and non-responders. Additional information is provided in Supplementary Table 7. Responders had a complete or partial response, and non-responders had stable or progressive disease. GOMS, Gut OncoMicrobiome Signatures.
Non-small-cell lung cancer
Akkermansia muciniphila is a Gram-negative homeostatic commensal bacteria that influences host metabolism to maintain glucose tolerance and intestinal barrier function. In preclinical and clinical studies, this species, which can both produce and degrade mucin, mitigated intestinal dysbiosis through the prevention of increased gut permeability and liver inflammation101–103. A. muciniphila activates intestinal immune responses under homeostatic or immunostimulatory conditions by recruiting local follicular T helper (TFH) cells104 or systemic TH1 cells in an IL-12-dependent manner, respectively42. We performed shotgun metagenomic sequencing of stool samples from a prospective cohort of 493 patients with advanced-stage NSCLC before they started treatment with anti-PD-(L)1 antibodies. In this cohort, the prevalence of A. muciniphila (in particular of SGB9226, the main SGB, which was detected in 39% of patients) was associated with an inflamed tumour microenvironment (TME) and favourable clinical outcomes (in terms of ORR and OS), specifically in the 30% of patients in whom its relative abundance did not exceed 4.8% of the whole metagenome41. Within this range, abundance of Akkermansia spp. is a surrogate marker of a healthy microbiome signature, characterized by the dominance of Lachnospiraceae and Oscillospiraceae family members41. An enrichment of SGB9226 (or SGB9228, another species of Akkermansia) beyond the 4.8% threshold (in 9% of patients), which is typically caused by antibiotic resistance and thus coincides with a dominance of the immunosuppressive Enterocloster genus and Clostridium species23,25, predicted resistance to anti-PD-(L)1 antibodies41. This trichotomic distribution of Akkermansia spp. (absence, <4.8% and ≥4.8%) enables an accurate prediction of clinical benefit in patients with advanced-stage NSCLC receiving first-line or second-line anti-PD-(L)1 antibodies, independently of other clinical prognostic factors41,42.
The clinical significance of the prevalence and relative abundance of intestinal Akkermansia spp. was extended with findings from two studies in which patients with NSCLC received ICIs in the neoadjuvant setting93,105. In a cohort of patients with renal cell carcinoma (RCC) receiving second-line treatment with the anti-PD-1 antibody nivolumab, the prevalence of this species was significantly associated with PFS durations of >12 months (P < 0.01)26. Together, these findings validate those obtained in preclinical avatar models involving the oral transfer of patient-derived stool samples to mice harbouring hepatocarcinomas103, sarcomas, or kidney, lung or prostate tumours28,41.
Melanoma
Studies in mouse models have provided compelling evidence that gut microbiota modifications resulting from faecal microbiota transplantation (FMT) improve ICI response rates38,42,91; however, the magnitude of these antitumour responses depends of factors such as the mouse strain or provider, mouse sex and individual experimental conditions106. Differences have also been observed in the human setting. Indeed, single studies in patients with melanoma receiving ICIs identified an enrichment of species such as F. prausnitzii, Bifidobacterium longum and Bacteroides caccae in samples from patients with a response (‘responders’)38,44,91, although the concordance of these microbiome characteristics with treatment response across studies was low97. To overcome these limitations, in the past few years, researchers have analysed multiple cohorts and used meta-analytical approaches39,40,107,108. They identified a panel of species, including Bifidobacterium pseudocatenulatum, Roseburia spp. and A. muciniphila, enriched in responders, whereas V. parvula, Bacteroides spp., Clostridium spp., Parasutterella excrementihominis, Scardovia wiggsiae, Oribacterium sinus and Megasphaera micronuciformis, among others, were enriched in samples from patients without a response (‘non-responders’); however, no single species was reproducibly shown as a fully consistent biomarker of response across studies. To solve this problem, McCulloch et al.40 evaluated data from a new cohort of patients and four previously published datasets, and found that baseline microbiota composition was associated with 1-year PFS. A meta-analysis of the combined data confirmed that the presence of bacteria from the Lachnospiraceae and Bifidobacteriaceae families (including many Ruminococcus, Mediterraneibacter and Blautia spp.) is associated with a favourable response and proposed that two opposite gut microbiome signatures enriched in either Lachnospiraceae or Streptococcaceae family members could predict a favourable and unfavourable outcome, respectively, after treatment with ICIs40. In addition, they found that an enrichment in Gram-negative bacteria was associated with an inflammatory host intestinal gene signature and a subsequent rise in the blood neutrophil-to-lymphocyte ratio, which were associated with unfavourable outcomes (specifically 1-year PFS). Despite between-cohort heterogeneity, optimized leave-one-dataset-out (also known as all-minus-one) supervised learning algorithms trained on batch-corrected microbiome data consistently predicted outcomes of treatment with anti-PD-(L)1 antibodies across all five cohorts40.
Cohort effects can depend on many factors, including methodological choices in sample processing and analysis, and their consideration could reconcile differences in melanoma responder versus non-responder GOMS across cohorts. Geographical differences can also have a major role in human gut microbiota variation109 and have been shown to influence these GOMS40,110. Indeed, cross-validation identified unfavourable bacteria of the Bacteroidetes phylum as predictive for most cohorts, while favourable bacteria of the Clostridium genus were predictive only for certain subsets of cohorts40. Dietary-driven gut microbiota variation is a factor involved in cohort effects; indeed, higher dietary fibre contents have been linked with a favourable response to ICIs in patients with melanoma53, possibly through effects on gut microbiome assembly patterns110.
Response across cancer types
To decipher whether responder and non-responder GOMS were significantly different across cohorts, cancer histotypes and geographical sites, we performed a meta-analysis and mega-analysis of stools taxonomically profiled with MetaPhlAn 4 (ref. 37) and from patients enrolled in 12 cohorts from eight published studies26,38–44 across three cancer types, and classified as responders (n = 248) or non-responders (n = 513) based on RECIST1.1 criteria response rates — responders had a partial or complete response and non-responders had stable or progressive disease (Supplementary Table 5). Using the mega-analysis approach at the single SGB level (Fig. 3, Supplementary Methods), non-responder GOMS comprised microorganisms already described as immunosuppressive (such as the Enterocloster and Anaerotruncus genera, Eisenbergiella tayi, Hungatella hathewayi and Clostridium symbiosum) or pro-inflammatory (such as Veillonellaceae, Eggerthellaceae, Enterobacteriaceae and Erysipelotrichaceae family members) and oral genera (Streptococcus and Actinomyces) shared across various pathological disorders9,23,41,97 (Fig. 2). In fact, many taxa from the non-responder GOMS were already included within the GOMS enriched in individuals with cancer compared with those without25 (Fig. 2, Supplementary Tables 1–3). By contrast, responder GOMS encompassed genera enriched in the healthy GOMS and SCFA producers (such as Lachnospiraceae, including Roseburia spp., Coprococcus spp., Blautia spp., Eubacterium spp. and Dorea spp.), Ruminococcaceae family members and the propionate-producing A. muciniphila SGB9226. Moreover, a number of organisms involved in the fermentation of dietary fibre and accounting for a major fraction of the butyrate-producing species (including F. prausnitzii, E. rectale, Roseburia spp., Fusicatenibacter saccharivorans and Anaerobutyricum hallii) are included in the responder GOMS (Fig. 3, Supplementary Table 6). The meta-analysis approach (Fig. 4, Supplementary Tables 1,7, Supplementary Methods) unveiled additional taxa associated with ORR, including immunogenic commensals (such as A. shahii111, Bifidobacterium bifidum and Bifidobacterium adolescentis), and Prevotella copri SGB1626 (which was also part of the healthy GOMS)23,40. Distinct species included in the responder GOMS (such as Faecalibacterium SGB15346, Clostridia bacterium SGB15383 and Roseburia hominis SGB4936) had been described as being part of the healthy GOMS25 (Fig. 1).
A random forest model applied in a leave-one-dataset-out setting on a total of 761 patients with cancer receiving ICIs (for whom sex and age data were available) built to differentiate responders from non-responders had a moderate and rather inconsistent predictive power across left-out datasets and a modest predictive power when merging all datasets for a single cross-validation evaluation (AUC 0.71) (Supplementary Table 5). These results are consistent with individual reports that responder and non-responder GOMS are compositionally distinguishable but with limited overlap of biomarkers across studies. Of note, responder GOMS for patients with NSCLC (AUCs of 0.78 and 0.70) and RCC (AUC 0.65) had higher predictive values than melanoma responder GOMS (AUCs of 0.49, 0.53, 0.58, 0.62, 0.66 and 0.73).
In summary, the need for GOMS that enable prediction of response to ICIs is increasing as the number of patients receiving these agents grows. Of note, GOMS from individuals without cancer and responders might have some degree of overlap, and a similar situation occurs between GOMS from those with cancer and non-responders. Overall, increasing the sample size of single cohorts, providing long-term follow up (1-year PFS or OS data), standardizing protocols across cohorts and using more systematic integrative analysis together with data sharing are necessary to identify biomarkers of response to ICIs.
GOMS and immunity
T cell responses against commensals are commonly detected in healthy individuals, suggesting that these cells participate in intestinal homeostasis by producing barrier-protective cytokines, and might constitute a sizeable pool of pathobiont-reactive T cells112. In a survey of healthy volunteers, enteric bacteria-reactive CD4+ T cells were present at precursor frequencies of 40–500 cells per 106 circulating CD4+ T cells for almost all enteric bacteria analysed112; the frequency of bacteria-reactive T cells in gut tissue was threefold to eightfold that in circulation. Microbiota-reactive T cells produced IL-2 and TNF, and co-expressed CCR7, CCR4, CD161 and CCR6 in various combinations, some of which also included integrin β7 and CCR9. When comparing CD4+ T cells reactive against gut microbiota (such as E. coli, Bifidobacterium animalis, F. prausnitzii, R. hominis or Ruminococcus obeum) with those reactive against non-enteric microorganisms (including Streptococcus aureus, Mycobacterium tuberculosis and the fungus Candida albicans), the former were partially enriched only in CCR4 expression. Compared with non-enteric microbiota-reactive T cells, gut-resident bacteria-reactive T cells produced high amounts of IFNγ, IL-17A and IL-2, and low amounts of IL-22, GMCSF and IL-4 (ref. 112). Interestingly, T cells in the lamina propria expressed higher levels of IL-17A and secreted lower levels of IFNγ relative to circulating T cells with similar specificity. Microbiota-reactive memory T cells frequently co-expressed IL-17A, IL-22 and IFNγ and, in addition, a subset of CD4+ T cells reactive to F. prausnitzii, Lactobacillus acidophilus or B. animalis produced the immunoregulatory cytokine IL-10. This pattern of cytokine release might have biological relevance, as suggested by the results of in vitro experiments showing that triple blockade of IFNγ, IL-17A and TNF inhibits the capacity of bacteria-reactive T cells to activate non-haematopoietic intestinal stromal and epithelial cells112. In pathological circumstances of mucosal barrier dysfunction, such as IBD, circulating and gut-resident microbiota-reactive CD4+ T cells secreted increased levels of IL-17A compared with those from healthy volunteers112. IBD results in excessive translocation of luminal antigens, eliciting both mucosal and systemic immune responses. The humoral immune response against gut microorganisms encompasses T cell-dependent and T cell-independent memory B cell responses and can be monitored by serological reactivities against prevalent intestinal bacteria113–115. This response has clinical significance in IBD as it might exacerbate intestinal inflammation113,116. Investigators have compared the repertoire of IgA-tagged and IgG-tagged commensals in individuals with and without IBD. A variety of humoral immune responses are directed against the majority of the main faecal genera in the former group, although with a large overlap between the two groups. Anti-IgG-based flow cytometry of faecal samples from patients with IBD revealed that 13–77% (mean 38%) of bacteria were IgG-coated after incubation with autologous serum113. In particular, serum IgGs targeted microorganisms typically found in the small intestine (such as Streptococcus, Coprococcus, Dorea, Ruminococcus gnavus-like bacteria, Lactobacillus, Dialister, Veillonella and Turicibacter), whereas the presence of IgGs targeting colonic anaerobic bacterial genera (such as Faecalibacterium, Roseburia and Blautia) was low. IgG-coated bacterial genera and oral bacteria have been consistently reported as being increased in abundance in stools from patients with IBD116. The observed enrichment of several types of Lachnospiraceae and species from the Enterocloster genus (such as E. bolteae and E. clostridioformis) or R. gnavus after IgG coating could be explained by the fact that these bacteria have flagella or flagellins, which have been reported to be highly immunogenic proteins and dominant antigens in the context of IBD114,115. The highly immunogenic properties of flagellin would be related to the main function of bacterial flagella, which is to enhance contact with a disrupted epithelial barrier and facilitate bacterial transport across the epithelial mucus layer.
A number of lines of evidence indicate the potential clinical relevance of humoral responses against microbial antigens in patients with cancer (Table 1). As previously outlined, a compromised ileal mucosa is an eventual feature of some malignancies, leading to increased serum levels of soluble CD14 and soluble IL-1 receptor-like 1 (also known as sST2), both surrogate markers of intestinal barrier leakage25. As demonstrated in mouse models of colon cancer, immune responses directed against gut microorganisms participate in cancer immunosurveillance117. Moreover, B cell proliferative responses to several commensals, mostly Bacteroides ovatus, Hafnia spp., V. atypica and C. albicans, were increased in patients with CRC compared with healthy volunteers118.
In a study reported in 2022, Meylan et al.119 unveiled a new mechanism mediating immunosurveillance in RCC. Analysis of tumour tissue from three cohorts of patients who had received ICIs revealed that, in those harbouring tertiary lymphoid structures (TLS), these structures favoured in situ B cell maturation and differentiation into IgG-secreting plasma cells. They also detected tumour cell labelling with IgG and RCC apoptosis in these samples. Patients with TLS+ tumours and IgG-labelled RCC cells had higher ORR and prolonged PFS than those with TLS+ tumours and no IgG labelling119. Intriguingly, an analysis of samples derived from patients with locally advanced and metastatic muscle-invasive bladder cancer (MIBC) provided evidence that intratumoural bacteria infecting the uroepithelial layer of such cancers might constitute a prominent target for TFH cells and B cells within TLS, linking innate and cognate CD8+ T cell memory responses120. Moreover, memory TFH cell responses against uropathogenic E. coli (UPEC), a colibactin-encoding strain of E. coli, had clinical significance in patients treated with anti-PD-1 antibodies120. Circulating central memory TFH cells accumulated in tumours, where they differentiated into CXCL13-producing effector memory TFH cells. CXCL13 plasma levels increased in pembrolizumab-treated responders compared to baseline, but not in non-responders. CXCL13-producing effector memory TFH cells were reactive against UPEC residing in tumour and myeloid cells within the TME. Baseline immune responses mediated by E. coli-restricted CXCL13-producing effector memory TFH cells were mostly detectable in responders to neoadjuvant pembrolizumab120 (Table 1). Importantly, the presence of IgG targeting UPEC (but not the other urinary commensals) in serum was associated with favourable clinical responses (2-year relapse-free survival (RFS)) in three independent cohorts of stage III–IV MIBC treated with ICIs120. Finally, the MHC class II-dependent reactivation of bacteria-reactive TFH cells and antibody-secreting cells within MIBCs exposed to UPEC led to the release of CCL19 and CCL21, two prototypic chemokines involved in TLS formation120. Given that urinary commensals and pathobionts might originate from the gut121,122, intestinal dysbiosis might have important consequences at remote sites, including the TME from tumours in other tissue types. Further prospective validation of the clinical relevance of the UPEC and GOMS signatures in patients with MIBC treated with ICIs is warranted and might be generalized to any neoplasia developing at portals of entry, where local or intestinal microbiota could be harnessed for optimal ICI efficacy.
Beyond the immunogenicity of intratumoural bacteria that will be processed and presented by tumour-exposed MHC molecules, other molecular cues (including molecular mimicry between commensals and cancer antigens, or the intrinsic adjuvanticity of intestinal microorganisms) could account for T cell reactivation in the TME. Bacteria-reactive T cells might act as bystander T helper cells or T cells endowed with cross-reactive T cell receptors (TCRs), recognizing epitopes shared between the intestinal ecosystem and cancer antigens123,124. Several independent studies in patients with cancer found associations between cellular immune responses against selected intestinal commensals and clinical outcome of anticancer therapies, including chemotherapy and ICIs, across various regions worldwide42,125–128. These include TH1 immune responses directed at B. fragilis and A. muciniphila were associated with responses to the anti-CTLA4 antibody ipilimumab in patients with melanoma125 and to anti-PD-1 antibodies in patients with RCC or NSCLC, respectively42 (Table 1). The presence of E. hirae-specific memory TH1 cells correlated with clinical benefit from chemotherapy in patients with advanced-stage NSCLC126 and favourable prognosis in patients with hepatitis C virus-induced hepatocarcinoma in China127 (Table 1). In patients with RCC and NSCLC receiving anti-PD-1 antibodies, the presence of an antigenic Siphoviridae enterophage that lysogenized E. hirae within stools at baseline was associated with prolonged OS124. Finally, IgG responses directed at the allogeneic microflora after FMT correlated with efficient colonization of the exogenous ecosystem, and clinical benefit from the reintroduction of ICIs after primary resistance128.
GOMS and metabolism
Gut microbiota functions have been monitored using several methods. Using metagenomic data, we annotated organism-specific gene hits according to the Kyoto Encyclopedia of Genes and Genomes Orthology (Table 1). On the basis of these annotations, reads from each sample were reconstructed into metabolic pathways using the MetaCyc hierarchy of pathway classifications. Investigators have used the linear discriminant analysis effect size to compare hits between responders and non-responders, with contrasting results38,40,98,129. Here, we summarize the findings of serial metabolomics analyses of faecal material and/or plasma in distinct cohorts and of non-invasive transcriptomic analyses of the exfoliome, which enables the identification of components of inflammatory pathways expressed in dendritic cells, monocytes, macrophages and neutrophils, as well as enterocytes and goblet cells130.
Short-chain fatty acids
SCFAs are major end-product metabolites produced by the gut microbiota that act at various levels of host physiology131. SCFAs, which include acetate, propionate and butyrate, are water-soluble and diffusible gut-microbiota-derived metabolites with concentrations that peak in the caecum and then decrease from the proximal to the distal colon132. SCFAs have context-dependent functions: they can promote the expansion of Treg cells and also improve effector T cell functions133–136. The presence of butyrate is associated with protection from acute inflammatory processes, such as autoimmune diseases and graft-versus-host disease in mice137,138. Moreover, butyrate also promotes the memory potential and antiviral cytotoxic effector functions of CD8+ T cells in animals139,140. Similarly, pentanoate (also known as valerate) is a bacterial metabolite generated by commensals found at low abundance, such as Megasphaera massiliensis141,142. A report published in 2021 showed that ex vivo culture of cytotoxic T lymphocytes, derived either from the endogenous repertoire or through genetic engineering with a TCR or chimeric antigen receptor (CAR), in the presence of pentanoate or butyrate could promote a TH1 phenotype characterized by cytokine release and antitumour reactivity through a mechanism dependent on mTOR-mediated inhibition of histone deacetylases143.
Very few translational research studies have analysed the predictive role of SCFAs in patients with cancer receiving ICIs (Table 1). In a study performed in Japan and involving 52 patients with solid tumours receiving anti-PD-1 antibodies, stool SCFAs were associated with favourable clinical outcomes144. High concentrations of faecal acetate, propionate, butyrate and valerate, and of serum isovalerate were associated with prolonged PFS144. By contrast, Coutzac et al.145 found that high serum levels of butyrate and propionate are associated with resistance to anti-CTLA4 antibodies and increased numbers of Treg cells in patients with melanoma. Given that preclinical tumour models also revealed negative effects of SCFAs on the efficacy of anti-CTLA4 antibodies or radiotherapy145,146, further studies are needed to validate the predictive value of SCFAs in large cohorts at different geographical sites before leveraging any of them, in blood or faeces, as biomarkers of ICI efficacy.
Tryptophan pathway
Indoleamine 2,3-dioxygenase (IDO) is a key enzyme catalysing the first and rate-limiting step along the kynurenine pathway of tryptophan metabolism outside the liver147. Therefore, IDO is viewed as an immune checkpoint involved in peripheral immune tolerance owing to the fact that, in the absence of tryptophan, T cell proliferation is inhibited and T cell apoptosis contributes to immunosuppression148. Compared with the general population, patients with NSCLC have increased tryptophan catabolism, which leads to higher kynurenine serum concentrations and has been associated with advanced stage disease at diagnosis, an unfavourable prognosis and resistance to chemotherapy148,149. Preclinical evidence suggests that IDO activity can also be involved in resistance to ICIs150. Two subsequent studies investigated the clinical significance of the kynurenine to tryptophan (Kyn to Trp) ratio in patients with advanced-stage cancer receiving ICIs (Table 1). In the first one, Botticelli et al.151 assessed baseline serum levels of tryptophan and kynurenine in 26 patients with stage IV NSCLC treated with second-line nivolumab (who met the eligibility criteria from the CheckMate 017 and CheckMate 057 trials). Patients with early disease progression (within 3 months) had significantly higher Kyn to Trp ratios than responders in univariate and multivariate analyses (P = 0.017)151. In the second study, Li et al.152 used liquid chromatography–mass spectrometry to profile >100 metabolites in pretreatment and multiple on-treatment blood samples from patients involved in three independent trials (CA209–038, CA209–009 and CheckMate 025), which involved 78 patients with advanced-stage melanoma, 91 patients with metastatic RCC receiving nivolumab, 394 patients with RCC receiving nivolumab and 349 patients with RCC receiving everolimus. In a subset of patients with melanoma and RCC receiving nivolumab, increases in the Kyn to Trp ratio (by >50%) 4–6 weeks after starting treatment robustly correlated with shorter median OS (P ≤ 0.026). Given the relevance of metabolic adaptations in cancer immunotherapy and the lack of efficacy of ICIs in combination with selective IDO1 inhibitors in unselected patients with melanoma (in the ECHO-301/KEYNOTE-252 phase III trial)153, these findings question not only the need for patient stratification based on monitoring of serum Kyn to Trp ratios in future trials but also the analysis of the dynamic modulation of the gut microbiota during ICI therapy. Indeed, tryptophan is not only catabolized by tumour and myeloid cells but also catabolized by distinct taxa from the gut microbiota into indole and indole derivatives, including indole acetic acid, indole-3-aldehyde (I3A) and indole propionic acid, capable of binding aryl hydrocarbon receptors154. Indeed, when Lactobacillus reuteri is administered in combination with ICIs to mice, it can translocate from the ileum to melanoma where it releases I3A that engages TCR signalling in CD8+ T cells to promote IFNγ release and antitumour effects. The clinical relevance of these experimental findings has been addressed in a study involving patients with melanoma treated with ICIs, paving the way for tryptophan-based diet interventions in patients with cancer155.
In distinct circumstances, such as in individuals with obesity and/or those who consume a high-fat diet, increased IDO activity can shift tryptophan metabolism from the generation of indole derivatives towards kynurenine production156. These findings are reminiscent of the clinical relevance of the tryptophan–kynurenine–anthranilate pathway, which is activated in patients with cancer and severe COVID-19, in whom it is associated with exacerbated lymphopenia and reduced patient survival157,158.
L-Arginine
Not only tryptophan but also intracellular L-Arginine concentrations directly affect both the metabolic fitness and survival of T cells and, thus, their ability to mediate effective antitumour immune responses in mouse models159. Elevated L-Arginine levels induced global metabolic changes, including a shift from glycolysis to oxidative phosphorylation in activated T cells, and promoted the generation of central memory-like T cells160. The correlation between L-Arginine levels and clinical ICI activity was found in a study with results published in 2022 assessing plasma pretreatment L-Arginine concentrations in a discovery cohort of anti-PD-1 antibody-treated patients with advanced-stage cancer from two independent trials (NCT02534649, n = 77; NCT03984318, n = 296) and a validation cohort from a phase I first-in-human study of the anti-PD-1 antibody budigalimab in patients with various solid tumour types (NCT03000257)159 (Table 1). In both the discovery and validation cohorts, low serum L-Arginine levels (<42 μM) were significantly (P = 0.004) and independently associated with a reduced clinical benefit rate, PFS and OS. In addition, low serum L-Arginine levels were associated with increased expression of PD-L1 in myeloid cells. Hence, plasma L-Arginine monitoring could constitute a suitable predictive biomarker of ICI efficacy.
Synthetic biological approaches leveraging engineered microbial platforms have been developed to deliver high local concentrations of L-Arginine as a unique means of local metabolic modulation of the TME161. While dietary L-Arginine supplements must be administered daily in high doses, in mouse models L-Arginine-producing bacteria colonized and persisted in tumours, continuously releasing this amino acid. The synergy between this bacterial-based therapy and anti-PD-L1 antibodies leads to potent T cell-dependent, long-term antitumour immunity161.
Other metabolic pathways
Other metabolic pathways mediated by the gut microbiota and with crucial biological functions relevant to cancer biology or immunosurveillance include: biliary salts involved in lipid metabolism and Treg homeostasis128,162; vitamins163,164; polyamines165,166; inosine167; urolithins162; hypoxanthine and histidine168; and iron bioavailability40. The following metabolites and their associated pathways could be relevant to clinical responses to ICIs; prospective studies are awaited to validate these hypotheses.
Inosine.
Mager et al.167 identified the first immunostimulatory oncometabolite produced in the gut by Bifidobacterium spp. (such as B. pseudolongum) among other commensals. Local and distant inosine improved the efficacy of anticancer immunotherapy in colon cancer-bearing mice. Inosine produced by bacteria bound adenosine receptor A2A in tumour-infiltrating CD8+ T cells, triggering the T cell-intrinsic IL-12Rβ signalling pathway167.
Biliary acids.
In mouse models, liver cancer immunosurveillance depends on natural killer T (NKT) cell activation19. Importantly, liver sinusoidal endothelium can release CXCL16 following stimulation with primary biliary acids (such as chenodeoxycholic acid and tauro β-muricholic acid), which attracts and activates the tumoricidal functions of CCR6-expressing NKT cells19. However, primary biliary acids are transformed by many Clostridium spp. (such as C. scindens) into secondary biliary acids (such as glycoconjugated lithocholic acid) that downregulate CXCL16, dampening NKT cell-mediated antitumour effects in the liver19. Elimination of these Gram-positive bacteria by vancomycin restored liver cancer immunosurveillance19. Moreover, in patients with liver cancers, the hepatic levels of primary chenodeoxycholic acid correlated with CXCL16 expression in tumour-free hepatic tissues, whereas an inverse correlation was observed with glycolithocholate19. In another study in mice, distinct biliary acids (mostly lithocholic acid) or ileal prevalence of Clostridium spp. (such as C. clostridioformis) were correlated with downregulation of ileal Madcam1. The decrease in this ileal addressin on high endothelial venules led to the migration of enterotropic α4β7+ Tr17 cells towards distant tumour lesions, culminating in cancer progression and resistance to anti-PD-1 antibodies21. Finally, in patients with melanomas resistant to first-line therapy with anti-PD-1 antibodies and receiving FMT, the serum levels of biliary acids were increased in responders compared with non-responders, with more efficient transformation of primary to secondary biliary acids in the former group128.
TMAO.
Dietary choline is converted to trimethylamine (TMA) by the gut bacterial enzyme choline TMA lyase, comprising CutC and CutD. TMA is oxidized to TMA N-oxide (TMAO) in the liver. TMAO can induce inflammation and immune activation while decreasing anti-inflammatory mediators such as IL-10 (ref. 169). In an orthotopic PDAC mouse model, TMAO rendered tumours responsive to anti-PD-1 and anti-TIM3 antibodies170. Metronidazole, an antibiotic that kills CutC-containing bacteria (such as Clostridia, Bacilli, Desulfovibrionia and Gammaproteobacteria), dramatically reduced the levels of choline-dependent TMAO and TMA in serum and diminished the tumour-reducing effects of choline supplementation170. TMAO reprogrammed the TME into a contexture rich in M1 macrophages directly increasing levels of regulators of the type I interferon response (such as IRF7, IFNβ, STING1 and STAT1) in tumour-associated macrophages, while mitigating immunosuppressive signalling pathways and extracellular matrix remodelling170. Moreover, in several cohorts of patients with melanoma, the clinical response to anti-PD-1 antibodies correlated with the expression of the CUTC gene170 in the faecal microbiome40,91,128.
Exfoliome
In a study involving patients with advanced-stage melanoma treated with anti-PD-1 antibodies alone or in combination with type I interferon, McCulloch et al.40 identified host genes differentially expressed between responders and non-responders. Genes encoding pro-inflammatory cytokines (such as IL-1β and CXCL8), transcription factors (including NF-κB inhibitors ζ and α and TNF-induced protein 3) and superoxide dismutase were overexpressed in non-responders, coinciding with the over-representation of Gram-negative bacteria in faeces, and suggesting that lipopolysaccharide could be a major contributor to a pro-inflammatory gene signature in these patients40 (Table 1). These findings are reminiscent of the first clinical trial using FMT to revert primary resistance to ICIs in patients with melanoma. In this study, plasma CXCL8 stood out as a marker of lack of response to anti-PD-1 antibodies despite compositional changes in microbiota128.
Circulating and intratumoural bacteria
In the past few years, tumour microbiomes have been characterized in many different cancer types using a multiplexed bacterial 16S ribosomal DNA PCR sequencing technique, FISH and culturomics to gain species-level resolution69. These studies have helped to appreciate the relevant bacterial functional traits of each kind of TME as well as bacteria intracellular localization in both cancer and immune cells. Whether tumour microbiota have a causal role in tumorigenesis or in the metastatic programme, or whether intratumoural bacteria instead reflect local immunosuppression and superinfections of established neoplasia remain an open conundrum. To determine the clinical relevance of intratumoural microbial signatures in patients receiving ICIs, this study included a bacterial atlas of 29 responders and 48 non-responders among a cohort of patients with metastatic melanoma69. While the overall bacterial load was similar in responders and non-responders, multiple bacterial taxa had significantly different representation, notably Veillonellaceae and Gammaproteobacteria were associated with resistance, as already identified in GOMS.
Moreover, an independent study investigating biomarkers of response to neoadjuvant pembrolizumab, another anti-PD-1 antibody, concluded that UPEC has immunological and clinical relevance in patients with locally advanced MIBC120. Pre-existing circulating IgGs and cellular memory TFH CD4+ T cells reactive against E. coli predicted 2-year RFS to anti-PD-1 antibodies that were associated with the presence of TLS in cystectomy-derived samples120. Finally, the cancer mycobiome171 as well as circulating microbial DNA172, might have a role in prognosis or early diagnosis of cancer, respectively, that would also influence response to ICIs, and therefore should be another area of prospective research.
Conclusions
In this Review, we have aimed at detailing why and how the microbiome has earned its credentials as a predictor of immunotherapy efficacy in contemporary oncology. Research efforts have led to the description of several GOMS that could become prognostic and predictive markers across several cancer types. GOMS might help to predict the efficacy of anti-PD-1 antibodies in patients with advanced-stage NSCLC, even in those with PD-L1-negative disease41. Early evidence suggests that patients with cancer likely to respond to immunotherapy harbour a gut microbiota sharing features of the prototypic healthy GOMS; the biological and clinical relevance of these findings needs to be validated. Confounding factors (such as age, sex, comorbidities, comedications or ECOG performance status) should be carefully taken into consideration. The predictive power of GOMS will probably be improved by combining them with other biomarkers (such as PD-L1, DNA MMR, TMB, IL-8 or certain metabolites, among others) that independently modulate ICI efficacy. Multivariate analyses incorporating these predictors are being performed in meta-cohorts and prospective studies.
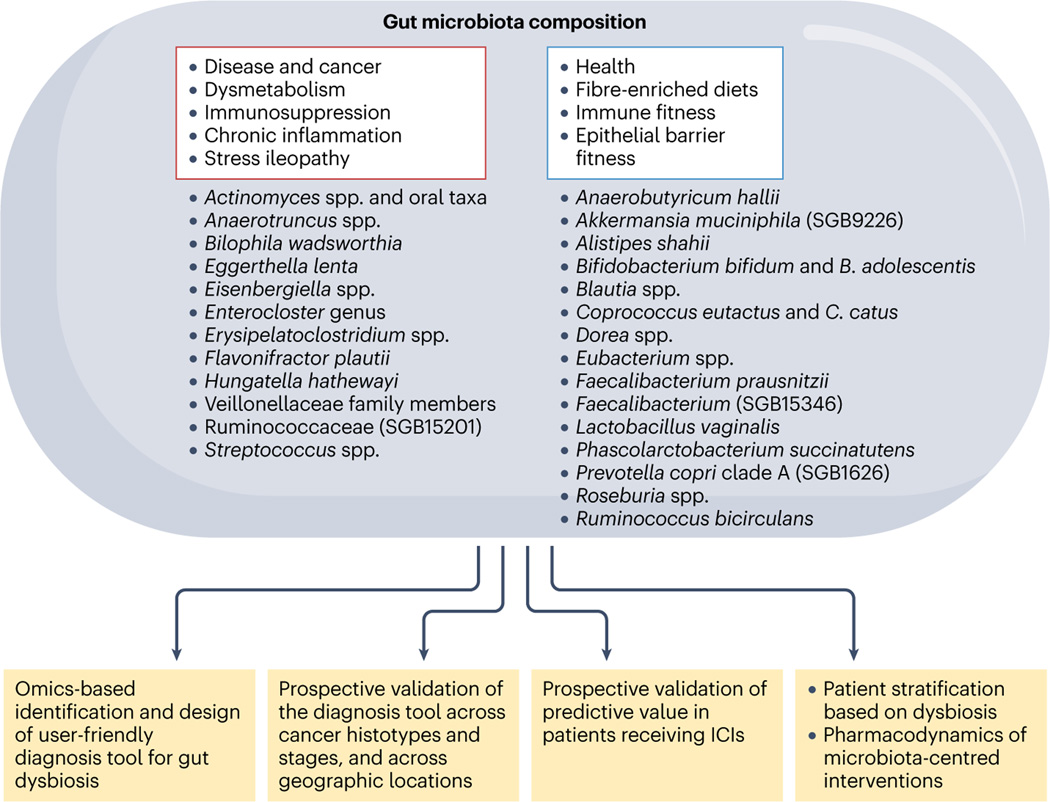
On the basis of the effect of the gut taxonomic composition on the clinical outcome, at least in response to ICIs and potentially CAR T cell therapy129,173, prospective validation of the robustness of GOMS patterns is necessary in various cancer types, disease stages and lines of therapy. The next step includes computing a score encompassing the necessary and sufficient taxa discriminating responders from non-responders, and designing user-friendly and actionable devices to monitor their prevalence and/or relative abundance using PCR. Efforts should also be devoted to developing surrogate markers of a healthy or disturbed microbiota, such as metabolic or microbiota-specific humoral and cellular immune response classifiers. These diagnostic tools should enable the prospective stratification of patients based on response to ICIs, and the dynamics of faecal taxonomic composition to be followed during cancer treatment and comedications (Figs. 5,6, Supplementary Table 8). Such a strategy could help to identify patients requiring microbiota-centred interventions to improve cancer immunosurveillance and hence guide future clinical trials combining such interventions with ICIs.
Fig. 5 |. Challenges in using microbiota-related biomarkers in oncology.
Shotgun metagenomic sequencing is currently the state-of-the-art method for analysing the taxonomic composition of the stools or ileal content using machine learning in cohorts of patients with cancer. Challenges in this field include designing user-friendly diagnosis devices and scores that need to be validated prospectively across different cancer types, staging, geographical regions, lifestyles, treatments and comorbidities. This score will be instrumental to stratify patients according to their degree of intestinal dysbiosis for future microbiota-centred interventions or interceptive measures. ICI, immune-checkpoint inhibitor; SGB, species-level genome bin.
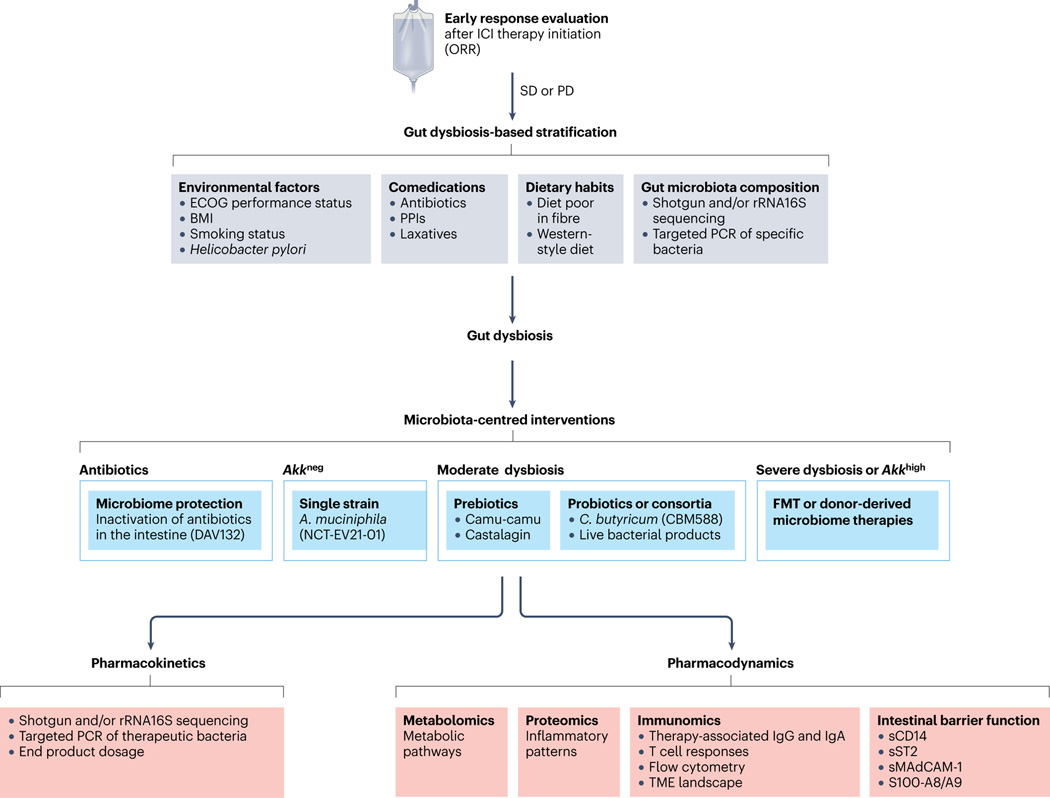
Fig. 6 |. Proposed pathway to define GOMS-based clinical guidelines.
Summary of the proposed clinical management of patients with cancer who could receive an approved immune-checkpoint inhibitor considering gut microbiota composition. Faecal samples from patients with a history of antibiotic treatment (any kind except vancomycin) taken between 60 days before and 42 days after the first administration of anti-PD-1 antibodies (alone or combined with anti-CTLA4 antibodies) or comedications known to alter microbiota composition (such as proton pump inhibitors (PPIs) or laxatives) should be investigated using shotgun metagenomic sequencing or targeted PCR at diagnosis to monitor taxonomic composition. Depending on the relative abundances of Akkermansia spp. as well as other family members or genera or species (listed as harmful or beneficial in Gut OncoMicrobiome Signatures (GOMS)) (Figs. 3 and 4), different putative scenarios are described with their respective clinical management as for a potential microbiota-centred intervention to compensate for intestinal dysbiosis. The overabundance of Akkermansia spp. (SGB9226, SGB9228 or others) is defined as a relative abundance of >4.8% among all species using MetaphLAN or MetaO-MineR algorithms (defined as Akkhigh, as opposed to Akkneg). Several trials are evaluating interventions involving antibiotics (NCT02176005, NCT02917200), prebiotics (NCT05303493), Clostridium butyricum (NCT05122546, NCT03829111), live bacterial products (NCT03637803, NCT03775850, NCT04208958) and donor-derived therapies (NCT04116775, NCT05273255, NCT04758507, NCT05251389, NCT04577729, NCT04988841, NCT04975217, NCT05286294, NCT04924374, NCT04729322, NCT04264975, NCT04130763, NCT04521075, NCT04951583, NCT05502913, NCT03686202, NCT03817125). The pharmacodynamics and pharmacokinetics of microbiota-centred interventions should be assessed to monitor their effects on the host metabolic, immunological or haematological functions or inflammatory tonus, and the gut microbiota composition or deviation from baseline. FMT, faecal microbiota transplantation; ICI, immune-checkpoint inhibitor; ORR, objective response rate; PD, progressive disease; sCD14, soluble CD14; SD, stable disease; sMAdCAM-1; soluble mucosal addressin cell adhesion molecule 1; sST2, soluble IL-1 receptor-like 1; TME, tumour microenvironment.
Supplementary Material
Key points.
Oncogenesis can cause a stress ileopathy (characterized by an ectopic accumulation of enteroendocrine cells and an imbalance between sympathetic and cholinergic signalling) associated with an intestinal dysbiosis.
Studies of the links between intestinal dysbiosis and microbial tissue colonization or infection could provide novel insights relevant to the aetiology, prevention and treatment of various cancer types, such as pancreatic adenocarcinomas and urothelial carcinomas.
Patients with cancer share gut microbiome signatures with individuals with seemingly unrelated disorders characterized by an imbalance between health-related and chronic inflammatory disease-related commensals.
In the past decade, investigators have identified Gut OncoMicrobiome signatures (GOMS) that share profile commonalities across cancer histotypes.
GOMS might constitute a promising, non-invasive and cost-effective approach for early diagnosis of various different cancer types.
GOMS are candidate predictors of resistance to immune-checkpoint inhibitors.
Acknowledgements
M.F. has received funding from the Seerave Foundation. B.R. has received support from the Canadian Institute for Health Research (CIHR), Fonds de la Recherche Québec-Santé (FRQS), the Seerave Foundation, the Terry Fox Marathon of Hope Program and the Weston Foundation. J.A.W. receives support from the American Association for Cancer Research Stand Up To Cancer (SU2C-AACR-IRG-19-17), MD Anderson Cancer Center’s Melanoma Moon Shots Program, Melanoma Research Alliance (4022024) and the National Institutes of Health (NIH) (1 R01 CA219896-01A1). N.S. has received support from the European Union Horizon 2020 programme (ONCOBIOME-825410 project, MASTER-818368 project and IHMCSA-964590), European Research Council (ERC-STG project MetaPG-716575 and ERC-CoG microTOUCH-101045015), the National Cancer Institute of the NIH (1U01CA230551) and Premio Internazionale Lombardia e Ricerca 2019. L.Z. has received support from ANR grant–French-German Ileobiome 19-CE15-0029-01, European Union Horizon 2020 research and innovation programme under grant agreement number 825410 (project acronym ONCOBIOME, project title Gut OncoMicrobiome Signatures (GOMS) associated with cancer incidence, prognosis, and prediction of treatment response), European Union Horizon Europe research and innovation programme under grant agreement number 101095604-GAP-101095604 (project acronym PREVALUNG-EU, project title Personalized lung cancer risk assessment leading to stratified Interception), RHU5 “ANR-21-RHUS-0017” IMMUNOLIFE, SIGN’IT ARC foundation and SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE). L.Z. and G.K. have received donations from Elior and the Seerave Foundation; and.support from ANR projets blancs, Badinter Philantropia, Cancéopole Ile-de-France; Dassault, FHU CARE, Fondation pour la Recherche Médicale (FRM), Inserm (HTE), Institut National du Cancer (INCa), Institut Universitaire de France, LabEx Immuno-Oncology and Ligue contre le Cancer (Equipe labelisée).
Competing interests
A.M.T. is a new employee of Microbiotica. B.R. has received grants from AstraZeneca, Bristol Myers Squibb, Davoltera, Kaleido, Merck and Vedanta outside the submitted work; and has a patent pending (G17004-00006-AD, use of castalagin or analogues thereof for anticancer efficacy and to increase the response to immune-checkpoint inhibitors). G.K. has held research contracts with Daiichi Sankyo, Elior, Kaleido, Lytix Pharma, PharmaMar, Samsara, Sanofi, Tollys, Vascage and Vasculox/Tioma; is on the Board of Directors of the Bristol Myers Squibb Foundation in France; is a scientific co-founder of EverImmune, Osasona Therapeutics, Samsara Therapeutics and Therafast Bio; and is an inventor in patents covering therapeutic targeting of ageing, cancer, cystic fibrosis and metabolic disorders. J.A.W. is an inventor on a US patent application (PCT/US17/53.717) submitted by the University of Texas MD Anderson Cancer Center, which covers methods to enhance immune-checkpoint inhibitor responses by modulating the microbiome; reports compensation for speaker’s bureau and honoraria from Bristol Myers Squibb, Dava Oncology, Exelixis, Gilead, Illumina, Imedex, MedImmune, Omniprex, PeerView and Physician Education Resource; has served as a consultant or advisory board member for AstraZeneca, Bristol Myers Squibb, EverImmune, GlaxoSmithKline, Merck Novartis, Roche/Genentech, Micronoma and OSE therapeutics; and receives stock options from Micronoma and OSE therapeutics. L.Z. is the scientific cofounder and President of the scientific advisory board of EverImmune, a company devoted to the use of commensal microorganisms (Oncobax) for the treatment of cancers; has received research grants from 9 meters, Daichi Sankyo, EverImmune and Pileje; is a former member of the board of directors of Transgene; and is a former consulting expert for Bristol Myers Squibb, GlaxoSmithKline, Lytix biotherapeutics and Tusk. M.F. and N.S. declare no competing interests.
Glossary
- 16S ribosomal RNA sequencing
Targeted, high-throughput method that consists of amplifying and sequencing the small-subunit ribosomal 16S RNA (rRNA) gene present in a sample (for example, a microbial community in stools). Sequences are generally the result of targeted amplification of one or more variable regions within the 16S rRNA gene, and can be used to profile the taxonomic composition of both archaeal and eubacterial members of the microbial community. This method provides lower taxonomic resolution than shotgun metagenomics but is generally less computationally intensive. Nevertheless, a variety of processing steps can introduce bias and limit the ability to combine data from different studies
- Alpha and beta diversities
Alpha diversity or richness measures the variability of species within a faecal sample while beta diversity accounts for the differences in composition between individuals
- Dysbiosis
Imbalance of microbiota that involves changes in composition and/or functions
- Exfoliome
Shed intestinal luminal cells contained within faecal samples
- Exposome
Encompasses the large number of individual exposures from various origins, such as chemical, physical, biological or psychological stimuli, and also takes into account the time dimension of the exposure (short or long, early or late, punctual or repeated). The exposome has an impact on individual physiology and, to a larger extent, overall health
- Leave-one-dataset-out
Analytical approach in which data from one cohort are set aside as an external validation set whereas data from the remaining cohorts are pooled together as a single training set, iterating along all the cohorts
- Linear discriminant analysis effect size
Determines the features (organisms, clades, operational taxonomic units, genes or functions) most likely to explain differences between classes by coupling standard tests for statistical significance with additional tests encoding biological consistency and effect relevance
- Mega-analysis
Analysis that gathers raw data across multiple studies
- Random forest model
Supervised machine learning algorithm used to solve classification and regression problems that averages multiple decision trees
- Short-chain fatty acids
Metabolic by-products derived from the fermentation of carbohydrates by anaerobic bacteria in the gut
- Shotgun metagenomic sequencing
Non-targeted, high-throughput method that consists of sequencing all DNA present in a sample (for example, a microbial community). These DNA sequences are computationally analysed and can be used to profile the taxonomic composition of members of the microbial community, including bacteria, fungi and viruses
- Species-level genome bins
Large-scale metagenomic analyses have uncovered a myriad of bacteria never before described. The species-level genome bin (SGB) nomenclature enables the annotation of bacterial sequences to the species level
- Stress ileopathy
Ileal mucosa atrophy associated with dominance of sympathetic over cholinergic signalling provoked by intra-intestinal and extra-intestinal malignancies or chronic inflammatory disorders (inflammatory bowel disease, stroke)
Footnotes
Additional information
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41571-023-00785-8.
References
- 1.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Cassidy LD et al. Temporal inhibition of autophagy reveals segmental reversal of ageing with increased cancer risk. Nat. Commun. 11, 307 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demaria M et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Otín C & Kroemer G. Hallmarks of health. Cell 184, 33–63 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Derosa L et al. The immuno-oncological challenge of COVID-19. Nat. Cancer 1, 946–964 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Rutkowski MR et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 27, 27–40 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee AM et al. Antibiotic-induced disturbances of the gut microbiota result in accelerated breast tumor growth. iScience 24, 103012 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchta Rosean C et al. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor-positive breast cancer. Cancer Res. 79, 3662–3675 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh TS, Das M, Jeffery IB & O’Toole PW. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. eLife 9, e50240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilmanski T et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 3, 274–286 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bindels LB et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget 9, 18224–18238 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ubachs J et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J. Cachexia Sarcopenia Muscle 12, 2007–2021 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zitvogel L, Ma Y, Raoult D, Kroemer G & Gajewski TF. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science 359, 1366–1370 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Claesson MJ et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl Acad. Sci. USA 108, 4586–4591 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roager HM & Licht TR. Microbial tryptophan catabolites in health and disease. Nat. Commun. 9, 3294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonowal R et al. Indoles from commensal bacteria extend healthspan. Proc. Natl Acad. Sci. USA 114, E7506–E7515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexeev EE et al. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am. J. Pathol. 188, 1183–1194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bindels LB et al. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 10, 1456–1470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma C et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song X et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fidelle M et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 380, eabo2296 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Sato Y et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 599, 458–464 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Gacesa R et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 604, 732–739 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Jackson MA et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 65, 749–756 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonekura S et al. Cancer induces a stress ileopathy depending on β-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov. 12, 1128–1151 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Derosa L et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 78, 195–206 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Terrisse S et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 28, 2778–2796 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terrisse S et al. Immune system and intestinal microbiota determine efficacy of androgen deprivation therapy against prostate cancer. J. Immunother. Cancer 10, e004191 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pernigoni N et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science 374, 216–224 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Geller LT et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins JR. Small intestinal mucosal damage with villous atrophy: a review of the literature. Am. J. Clin. Pathol. 44, 36–44 (1965). [DOI] [PubMed] [Google Scholar]
- 32.Gilat T, Fischel B, Danon J & Loewenthal M. Morphology of small bowel mucosa in malignancy. Digestion 7, 147–155 (1972). [DOI] [PubMed] [Google Scholar]
- 33.Stanley D et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 22, 1277–1284 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Singh V et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch SV & Pedersen O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Gupta VK et al. A predictive index for health status using species-level gut microbiome profiling. Nat. Commun. 11, 4635 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco-Míguez A et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nat. Biotechnol. 10.1038/s41587-023-01688-w (2023) [DOI] [PMC free article] [PubMed]
- 38.Gopalakrishnan V et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KA et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 28, 535–544 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCulloch JA et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 28, 545–556 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derosa L et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 28, 315–324 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Routy B et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Wind TT et al. Gut microbial species and metabolic pathways associated with response to treatment with immune checkpoint inhibitors in metastatic melanoma. Melanoma Res. 30, 235–246 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Frankel AE et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 19, 848–855 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagata N et al. Metagenomic identification of microbial signatures predicting pancreatic cancer from a multinational study. Gastroenterology 163, 222–238 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Yu J et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 66, 70–78 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Feng Q et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 6, 6528 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Yachida S et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25, 968–976 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Wirbel J et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 25, 679–689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas AM et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 25, 667–678 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogtmann E et al. Colorectal cancer and the human gut microbiome: reproducibility with whole-genome shotgun sequencing. PLoS ONE 11, e0155362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeller G et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 10, 766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spencer CN et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632–1640 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kartal E et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut 71, 1359–1372 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peters BA et al. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. 11, 61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asnicar F et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 27, 321–332 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Filippis F et al. Distinct genetic and functional traits of human intestinal Prevotella copri strains are associated with different habitual diets. Cell Host Microbe 25, 444–453.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Dhakan DB et al. The unique composition of Indian gut microbiome, gene catalogue, and associated fecal metabolome deciphered using multi-omics approaches. Gigascience 8, giz004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keohane DM et al. Microbiome and health implications for ethnic minorities after enforced lifestyle changes. Nat. Med. 26, 1089–1095 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Zhernakova A et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352, 565–569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vieira-Silva S et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 581, 310–315 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Nielsen HB et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat. Biotechnol. 32, 822–828 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Qin J et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Qin N et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Schirmer M et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167, 1897 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Xie H et al. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst. 3, 572–584.e3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeevi D et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Nejman D et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bogert B, van den Meijerink, M., Zoetendal EG, Wells JM & Kleerebezem M. Immunomodulatory properties of Streptococcus and Veillonella isolates from the human small intestine microbiota. PLoS ONE 9, e114277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong H-E, Kim A-S, Kim M-R, Ko H-J & Jung MK. Does the use of proton pump inhibitors increase the risk of pancreatic cancer? A systematic review and meta-analysis of epidemiologic studies. Cancers 12, 2220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zackular JP, Rogers MAM, Ruffin MT & Schloss PD. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 7, 1112–1121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beghini F et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife 10, e65088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rubinstein MR et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long X et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 4, 2319–2330 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Drewes JL et al. High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes 3, 34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flemer B et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 67, 1454–1463 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt TS et al. Extensive transmission of microbes along the gastrointestinal tract. Elife 8, e42693 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakatsu G et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 155, 529–541.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Tomkovich S et al. Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J. Clin. Invest. 129, 1699–1712 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dejea CM et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl Acad. Sci. USA 111, 18321–18326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lui RN et al. Global increasing incidence of young-onset colorectal cancer across 5 continents: a joinpoint regression analysis of 1,922,167 cases. Cancer Epidemiol. Biomark. Prev. 28, 1275–1282 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Yang Y et al. Dysbiosis of human gut microbiome in young-onset colorectal cancer. Nat. Commun. 12, 6757 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kong C et al. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut 10.1136/gutjnl-2022-327156 (2022). [DOI] [PubMed]
- 85.Thomas AM & Segata N. Multiple levels of the unknown in microbiome research. BMC Biol. 17, 48 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin Y et al. Altered mycobiota signatures and enriched pathogenic Aspergillus rambellii are associated with colorectal cancer based on multicohort fecal metagenomic analyses. Gastroenterology 163, 908–921 (2022). [DOI] [PubMed] [Google Scholar]
- 87.Liu N-N et al. Multi-kingdom microbiota analyses identify bacterial-fungal interactions and biomarkers of colorectal cancer across cohorts. Nat. Microbiol. 7, 238–250 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Topalian SL, Taube JM & Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367, eaax0182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matson V, Chervin CS & Gajewski TF. Cancer and the microbiome–influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology 160, 600–613 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Derosa L et al. Microbiota-centered interventions: the next breakthrough in immuno-oncology? Cancer Discov. 11, 2396–2412 (2021). [DOI] [PubMed] [Google Scholar]
- 91.Matson V et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hakozaki T et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol. Res. 8, 1243–1250 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Cascone T et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat. Med. 27, 504–514 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salgia NJ et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur. Urol. 78, 498–502 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Jin Y et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in chinese patients with NSCLC. J. Thorac. Oncol. 14, 1378–1389 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Newsome RC et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 14, 35 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park EM et al. Targeting the gut and tumor microbiota in cancer. Nat. Med. 28, 690–703 (2022). [DOI] [PubMed] [Google Scholar]
- 98.Mao J et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer 9, e003334 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peng Z et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol. Res. 8, 1251–1261 (2020). [DOI] [PubMed] [Google Scholar]
- 100.Zheng Y et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 7, 193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Depommier C et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Vos WM, Tilg H, Van Hul M & Cani PD. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schneider KM et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat. Commun. 13, 3964 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ansaldo E et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 364, 1179–1184 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cascone T et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat. Med. 29, 593–604 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shaikh FY et al. Murine fecal microbiota transfer models selectively colonize human microbes and reveal transcriptional programs associated with response to neoadjuvant checkpoint inhibitors. Cancer Immunol. Immunother. 71, 2405–2420 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shaikh FY et al. A uniform computational approach improved on existing pipelines to reveal microbiome biomarkers of nonresponse to immune checkpoint inhibitors. Clin. Cancer Res. 27, 2571–2583 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Limeta A, Ji B, Levin M, Gatto F & Nielsen J. Meta-analysis of the gut microbiota in predicting response to cancer immunotherapy in metastatic melanoma. JCI Insight 5, 140940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He Y et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 24, 1532–1535 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Simpson RC et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 10.1038/s41591-022-01965-2 (2022). [DOI] [PubMed]
- 111.Iida N et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hegazy AN et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology 153, 1320–1337.e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bourgonje AR et al. Patients with inflammatory bowel disease show IgG immune responses towards specific intestinal bacterial genera. Front. Immunol. 13, 842911 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lodes MJ et al. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 113, 1296–1306 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lloyd-Price J et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rengarajan S et al. Dynamic immunoglobulin responses to gut bacteria during inflammatory bowel disease. Gut Microbes 11, 405–420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Overacre-Delgoffe AE et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity 54, 2812–2824.e4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Noble A et al. Altered immunity to microbiota, B cell activation and depleted γδ/resident memory T cells in colorectal cancer. Cancer Immunol. Immunother. 10.1007/s00262-021-03135-8 (2022). [DOI] [PMC free article] [PubMed]
- 119.Meylan M et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541.e5 (2022). [DOI] [PubMed] [Google Scholar]
- 120.Goubet A-G et al. Escherichia coli-specific CXCL13-producing TFH are associated with clinical efficacy of neoadjuvant PD-1 blockade against muscle-invasive bladder cancer. Cancer Discov. 10.1158/2159-8290.CD-22-0201 (2022). [DOI] [PubMed]
- 121.Wu J et al. A highly polarized TH2 bladder response to infection promotes epithelial repair at the expense of preventing new infections. Nat. Immunol. 21, 671–683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yacouba A, Tidjani Alou M, Lagier J-C, Dubourg G & Raoult D. Urinary microbiota and bladder cancer: a systematic review and a focus on uropathogens. Semin. Cancer Biol. 10.1016/j.semcancer.2021.12.010 (2022). [DOI] [PubMed]
- 123.Zitvogel L & Kroemer G. Cross-reactivity between microbial and tumor antigens. Curr. Opin. Immunol. 75, 102171 (2022). [DOI] [PubMed] [Google Scholar]
- 124.Fluckiger A et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020). [DOI] [PubMed] [Google Scholar]
- 125.Vétizou M et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Daillère R et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45, 931–943 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Rong Y et al. Reactivity toward Bifidobacterium longum and Enterococcus hirae demonstrate robust CD8+ T cell response and better prognosis in HBV-related hepatocellular carcinoma. Exp. Cell Res. 358, 352–359 (2017). [DOI] [PubMed] [Google Scholar]
- 128.Davar D et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595–602 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Smith M et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 28, 713–723 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Whitfield-Cargile CM et al. The non-invasive exfoliated transcriptome (exfoliome) reflects the tissue-level transcriptome in a mouse model of NSAID enteropathy. Sci. Rep. 7, 14687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Koh A, De Vadder F, Kovatcheva-Datchary P & Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Haenen D et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J. Nutr. 143, 274–283 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Arpaia N et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith PM et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park J, Goergen CJ, HogenEsch H & Kim CH. Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis. J. Immunol. 196, 2388–2400 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kespohl M et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4+ T cells. Front. Immunol. 8, 1036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mariño E et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 18, 552–562 (2017). [DOI] [PubMed] [Google Scholar]
- 138.Mathewson ND et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505–513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trompette A et al. Dietary fiber confers protection against flu by shaping Ly6c− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 48, 992–1005.e8 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Bachem A et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 51, 285–297.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 141.Luu M et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 10, 760 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yuille S, Reichardt N, Panda S, Dunbar H & Mulder IE. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 13, e0201073 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luu M et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 12, 4077 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nomura M et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw. Open 3, e202895 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Coutzac C et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 11, 2168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Uribe-Herranz M et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Invest. 130, 466–479 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Munn DH et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Uyttenhove C et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 9, 1269–1274 (2003). [DOI] [PubMed] [Google Scholar]
- 149.Wang Y, Hu G-F & Wang Z-H. The status of immunosuppression in patients with stage IIIB or IV non-small-cell lung cancer correlates with the clinical characteristics and response to chemotherapy. Onco Targets Ther. 10, 3557–3566 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD & Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 210, 1389–1402 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Botticelli A et al. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J. Transl. Med. 16, 219 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li H et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat. Commun. 10, 4346 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Long GV et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 20, 1083–1097 (2019). [DOI] [PubMed] [Google Scholar]
- 154.Kaur H, Bose C & Mande SS. Tryptophan metabolism by gut microbiome and gut-brain-axis: an in silico analysis. Front. Neurosci. 13, 1365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bender MJ et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 186, 1846–1862.e26 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Laurans L et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat. Med. 24, 1113–1120 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Goubet A-G et al. Prolonged SARS-CoV-2 RNA virus shedding and lymphopenia are hallmarks of COVID-19 in cancer patients with poor prognosis. Cell Death Differ. 28, 3297–3315 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Danlos F-X et al. Metabolomic analyses of COVID-19 patients unravel stage-dependent and prognostic biomarkers. Cell Death Dis. 12, 258 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Peyraud F et al. Circulating L-Arginine predicts the survival of cancer patients treated with immune checkpoint inhibitors. Ann. Oncol. 33, 1041–1051 (2022). [DOI] [PubMed] [Google Scholar]
- 160.Geiger R et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Canale FP et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598, 662–666 (2021). [DOI] [PubMed] [Google Scholar]
- 162.Messaoudene M et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 12, 1070–1087 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Grajeda-Iglesias C et al. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging 13, 6375–6405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Montégut L, de Cabo R, Zitvogel L & Kroemer G. Science-driven nutritional interventions for the prevention and treatment of cancer. Cancer Discov. 10.1158/2159-8290.CD-22-0504 (2022). [DOI] [PMC free article] [PubMed]
- 165.Pietrocola F et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bourgin M et al. Circulating acetylated polyamines correlate with Covid-19 severity in cancer patients. Aging 13, 20860–20885 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mager LF et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489 (2020). [DOI] [PubMed] [Google Scholar]
- 168.Nie X et al. Serum metabolite biomarkers predictive of response to PD-1 blockade therapy in non-small cell lung cancer. Front. Mol. Biosci. 8, 678753 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu K et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood 136, 501–515 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mirji G et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 7, eabn0704 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Narunsky-Haziza L et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 185, 3789–3806.e17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Poore GD et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stein-Thoeringer CK et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 29, 906–916 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mok T et al. Phase 3 KEYNOTE-042 trial of pembrolizumab (MK-3475) versus platinum doublet chemotherapy in treatment-naive patients (pts) with PD-L1–positive advanced non-small cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 33 (Suppl. 15), TPS8105 (2015). [Google Scholar]
- 175.Center for Devices and Radiological Health. List of cleared or approved companion diagnostic devices (in vitro and imaging tools) (FDA, 2023). [Google Scholar]
- 176.Marabelle A et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Snyder A et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rizvi H et al. Molecular determinants of response to anti-programmed cell death (PD)−1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J. Clin. Oncol. 36, 633–641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rousseau B et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N. Engl. J. Med. 384, 1168–1170 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hellmann MD et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378, 2093–2104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mezquita L et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 4, 351–357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoon H-Y et al. Association between neutrophil-to-lymphocyte ratio and gut microbiota in a large population: a retrospective cross-sectional study. Sci. Rep. 8, 16031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schalper KA et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 26, 688–692 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yuen KC et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 26, 693–698 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tintelnot J et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 615, 168–174 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hezaveh K et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 55, 324–340.e8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moor K et al. Analysis of bacterial-surface-specific antibodies in body fluids using bacterial flow cytometry. Nat. Protoc. 11, 1531–1553 (2016). [DOI] [PubMed] [Google Scholar]
- 188.Kalaora S et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 592, 138–143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mizukoshi E et al. Peptide vaccine-treated, long-term surviving cancer patients harbor self-renewing tumor-specific CD8+ T cells. Nat. Commun. 13, 3123 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liang J et al. Personalized cancer vaccines from bacteria-derived outer membrane vesicles with antibody-mediated persistent uptake by dendritic cells. Fundam. Res. 2, 23–36 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cheng K et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology. Nat. Commun. 12, 2041 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li C et al. Deep insights into the gut microbial community of extreme longevity in south Chinese centenarians by ultra-deep metagenomics and large-scale culturomics. NPJ Biofilms Microbiomes 8, 28 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rampelli S et al. Shotgun metagenomics of gut microbiota in humans with up to extreme longevity and the increasing role of xenobiotic degradation. mSystems 5, e00124–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang J et al. The landscape in the gut microbiome of long-lived families reveals new insights on longevity and aging – relevant neural and immune function. Gut Microbes 14, 2107288 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Luan Z et al. Metagenomics study reveals changes in gut microbiota in centenarians: a cohort study of Hainan centenarians. Front. Microbiol. 11, 1474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang X et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat. Aging 1, 87–100 (2021). [DOI] [PubMed] [Google Scholar]
- 197.Liu R et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 859–868 (2017). [DOI] [PubMed] [Google Scholar]
- 198.Wang Q et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol. 18, 114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cui X et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 8, 635 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Crovesy L, Masterson D & Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur. J. Clin. Nutr. 74, 1251–1262 (2020). [DOI] [PubMed] [Google Scholar]
- 201.Bowerman KL et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 11, 5886 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Calderón-Pérez L et al. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci. Rep. 10, 6436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ni Y et al. Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. ISME J. 15, 3207–3220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jiao N et al. Alterations in bile acid metabolizing gut microbiota and specific bile acid genes as a precision medicine to subclassify NAFLD. Physiol. Genom. 53, 336–348 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Behary J et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 12, 187 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Solé C et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: relationship with acute-on-chronic liver failure and prognosis. Gastroenterology 160, 206–218.e13 (2021). [DOI] [PubMed] [Google Scholar]
- 207.Nakai M et al. Essential hypertension is associated with changes in gut microbial metabolic pathways: a multisite analysis of ambulatory blood pressure. Hypertension 78, 804–815 (2021). [DOI] [PubMed] [Google Scholar]
- 208.Aasmets O, Krigul KL, Lüll K, Metspalu A & Org E. Gut metagenome associations with extensive digital health data in a volunteer-based Estonian microbiome cohort. Nat. Commun. 13, 869 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Clooney AG et al. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment. Pharmacol. Ther. 43, 974–984 (2016). [DOI] [PubMed] [Google Scholar]
- 210.Imhann F et al. Proton pump inhibitors affect the gut microbiome. Gut 65, 740–748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lin Y-T et al. Anti-acid drug treatment induces changes in the gut microbiome composition of hemodialysis patients. Microorganisms 9, 286 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Palleja A et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 3, 1255–1265 (2018). [DOI] [PubMed] [Google Scholar]
- 213.Parker EPK et al. Changes in the intestinal microbiota following the administration of azithromycin in a randomised placebo-controlled trial among infants in south India. Sci. Rep. 7, 9168 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Singh G et al. The effect of gastric acid suppression on probiotic colonization in a double blinded randomized clinical trial. Clin. Nutr. ESPEN 47, 70–77 (2022). [DOI] [PubMed] [Google Scholar]
- 215.Vich Vila A et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 11, 362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li JKM et al. A cross-sectional study on gut microbiota in prostate cancer patients with prostatectomy or androgen deprivation therapy. Prostate Cancer Prostatic Dis. 24, 1063–1072 (2021). [DOI] [PubMed] [Google Scholar]
- 217.Thompson NA et al. The MITRE trial protocol: a study to evaluate the microbiome as a biomarker of efficacy and toxicity in cancer patients receiving immune checkpoint inhibitor therapy. BMC Cancer 22, 99 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shoji F et al. Chronological analysis of the gut microbiome for efficacy of atezolizumab-based immunotherapy in non-small cell lung cancer: protocol for a multicenter prospective observational study. Thorac. Cancer 13, 2829–2833 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.