Abstract
Background:
The safety and efficacy of mycophenolate mofetil (MMF) for lupus nephritis (LN) treatment is established in adults and in some children. MMF is rapidly converted to the biologically active metabolite mycophenolic acid (MPA) whose pharmacokinetics (PK) is characterized by large inter- and intra-individual variability.
Methods/Design:
This randomized, double-blind, active comparator, controlled clinical trial of pediatric subjects with proliferative LN compares pharmacokinetically-guided precision-dosing of MMF (MMFPK, i.e. the dose is adjusted to the target area under the concentration-time curve (AUC0–12h) of MPA ≥ 60–70 mg*h/L) and MMF dosed per body surface area (MMFBSA, i.e. MMF dosed 600 mg/m2 body surface area), with MMF dosage taken about 12 hours apart. At baseline, subjects are randomized 1:1 to receive blinded treatment with MMFPK or MMFBSA for up to 53 weeks. The primary outcome is partial clinical remission of LN (partial renal response, PRR) at week 26, and the major secondary outcome is complete renal response (CRR) at week 26. Subjects in the MMFBSA arm with PRR at week 26 will receive MMFPK from week 26 onwards, while subjects with CRR will continue MMFBSA or MMFPK treatment until week 53. Subjects who achieve PRR at week 26 are discontinued from study intervention.
Discussion:
The Pediatric Lupus Nephritis Mycophenolate Mofetil (PLUMM) study will provide a thorough evaluation of the PK of MMF in pediatric LN patients, yielding a head-to-head comparison of MMFBSA and MMFPK for both safety and efficacy. This study has the potential to change current treatment recommendations for pediatric LN, thereby significantly impacting childhood-onset SLE (cSLE) disease prognosis and current clinical practice.
Keywords: Pediatric, Lupus, Childhood-onset, SLE, Lupus nephritis, Mycophenolate mofetil, Pharmacokinetics
INTRODUCTION
Systemic lupus erythematosus (SLE) is a multi-organ autoimmune disease with relatively increasing mortality that often targets young women and children of U.S. minorities [1–3]. Childhood-onset SLE (cSLE) has similar manifestations as lupus in adults, but childhood-onset disease is accompanied by more severe multi-organ involvement, including LN in up to 80% of affected children [4–6].
Although the use of cyclophosphamide (CYC) has long been regarded the standard of care in proliferative LN [7], MMF is similarly accepted as a cornerstone of LN therapy [8], with a more favorable side-effect profile compared with CYC [9]. Off-label use for the treatment of LN in Europe and the U.S. is supported by recommendations of the American College of Rheumatology (ACR) [10] and the European League against Rheumatism (EULAR) [11]. The safety and efficacy of MMF in LN have been tested in adults and in some pediatric subjects. However, since the optimal MMF dosing to achieve improvement in LN in cSLE is not well-established [12], the pediatric renal transplant dosing regimen of MMF based on weight or body surface area (BSA) has been adopted instead.
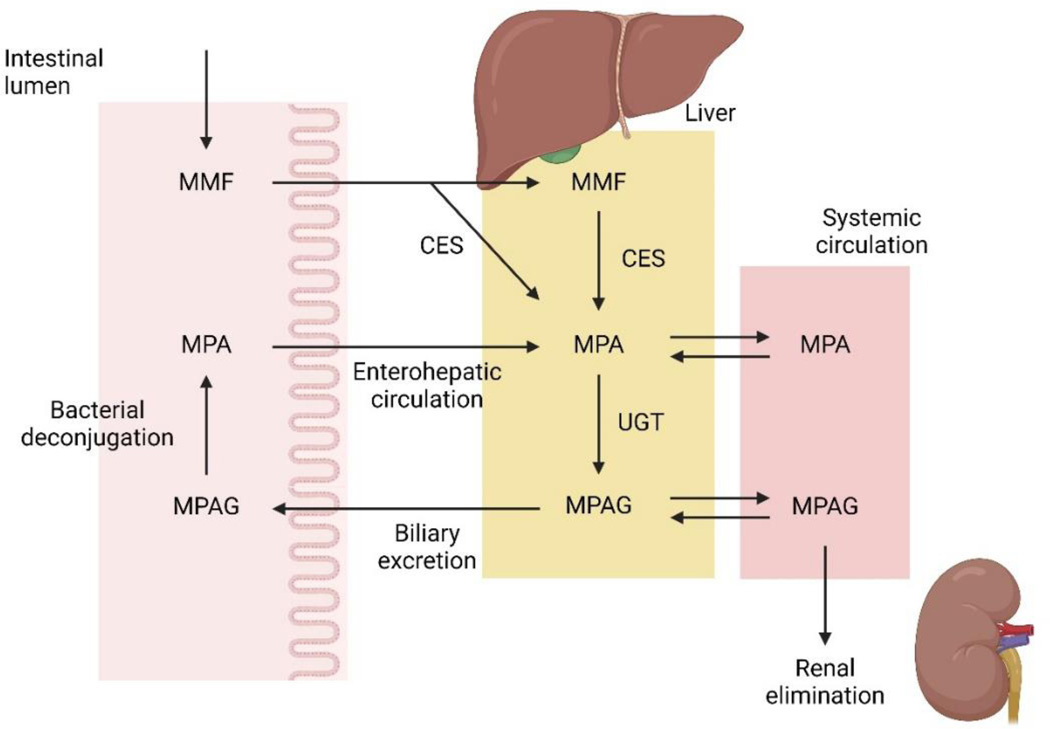
MMF, an ester prodrug, is rapidly converted to the biologically active moiety mycophenolic acid (MPA, Figure 1). Exposure to MPA is associated with MMF’s immunosuppressive and anti-inflammatory effect and the patient’s clinical response. However, the PK of MPA is characterized by large inter-individual and intra-individual variability. Factors contributing to this variability include differences in albumin concentration, corticosteroid use, impaired renal function, altered hepatic function, and genetic polymorphisms in drug metabolizing enzymes and drug transporters. Our previous work and that of other researchers [12–15], have shown that there is only a weak correlation between the dose of MMF (either based on weight or BSA) and the area under the time-concentration curve of MPA (MPA-AUC0–12). The apparent disconnect between MMF dosing based on body weight or BSA and MPA exposure stems from MPA’s complex metabolism which is influenced by concurrent therapies, the subject’s pharmacogenetic make-up, and presence of systemic disease including liver and renal function, which have all been extensively investigated by our group [12,16–20]. However, it remains uncertain whether higher MPA exposure with MMF dosing based on PK is well-tolerated, safe, and produces better renal outcomes compared to MMF dosing based on BSA when used for the treatment of pediatric LN. This underscores the need for therapeutic drug monitoring to optimize the use of MMF in pediatric LN. In this current study, our group aims to yield high-quality evidence for the superiority of pharmacokinetically-guided precision dosing over BSA-based dosing of MMF in pediatric LN.
Figure 1:
Pharmacokinetic pathway of MMF and MPA. Note: MMF-mycophenolate mofetil; MPA-mycophenolic acid; MPAG-MPA-glucuronide; CES-carboxylesterases; UGT-uridine 5’-diphospho-glucuronosyltransferases.
METHODOLOGY
Design
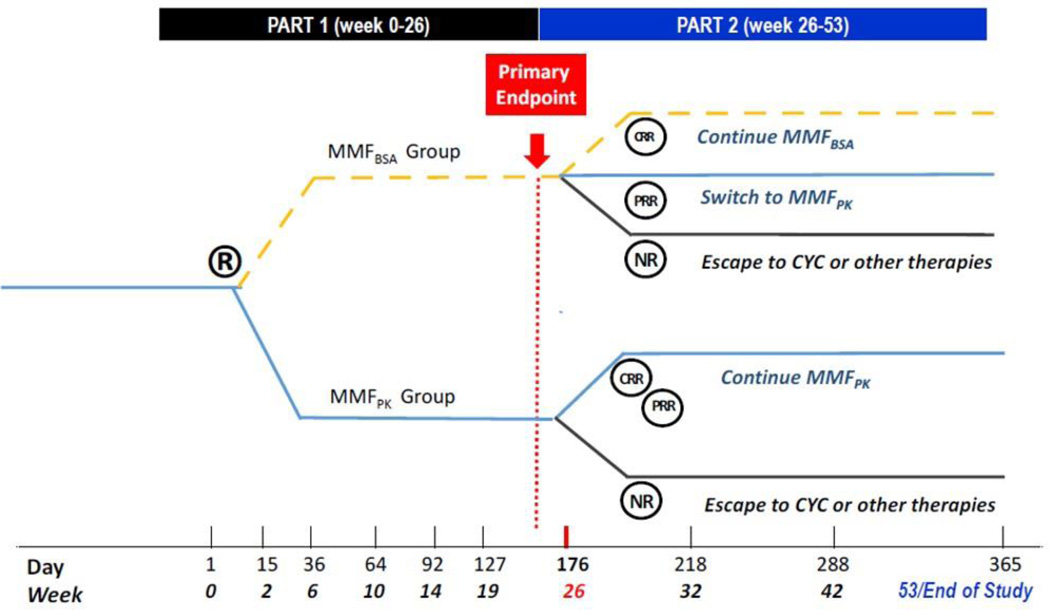
This is a randomized double-blind active comparator clinical trial of pediatric subjects (8 to<18 years of age) with proliferative LN as per the International Society of Nephrology/ Renal Pathology Society (ISN/PRS) classification criteria [21]. All eligible subjects are randomized at baseline to one of 2 arms: the current standard of clinical care (MMFBSA) or pharmacokinetically-guided precision dosing (MMFPK). Subjects in the MMFBSA arm who achieve PRR at week 26 will be switched to the MMFPK arm. At the end of Part 1, subjects in the MMFPK arm with CRR or PRR or MMFBSA arm subjects with CRR will continue their respective treatment arms during Part 2 of the study. At week 26 (end of Part 1) treatment non-responders, i.e. subjects who did not achieve PRR at week 26, will be discontinued from active medication management in the study but will still be monitored for a total of 53 weeks. The MMF starting dose for both treatment arms will be the recommended MMF dose for pediatric LN at 600 mg/m2 twice daily, about 12 hours apart [22]. Only in the MMFPK arm will the MMF dose be adjusted for a target MPA-AUC0–12 of 60 mg*h/L, with initial Bayesian PK-profiling performed after drug steady state is achieved. A schema of the study design is shown in Figure 2.
Figure 2:
The PLUMM Study design. Note: R-randomization; MMFBSA-treatment arm of MMF dosed at 600 mg/m2 body surface area about 12 hours apart; MMFPK-treatment arm of MMF dosed twice daily to achieve an area under the concentration-time curve (AUC0–12h) of MPA ≥ 60–70 mg*h/L, CRR-complete renal response; PRR-partial renal response; NR-non-responder; CYC-cyclophosphamide.
The study is overseen by a Centralized Coordinating Center (CCC), a study steering committee which includes a representative of the Lupus Foundation of America, and an independent Data Safety Committee that was selected by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
Setting
The study will be conducted at 19 U.S. sites, all located at major pediatric academic centers. Site investigators are listed in ClinicalTrials.gov (NCT05538208). Site investigators are either board-certified pediatric rheumatologists or pediatric nephrologists.
Population
Study participation is not restricted by patient sex, race, or ethnicity. Eligible patients fulfill all inclusion and no exclusion criteria. Given the potential use of immunosuppressants other than MMF for the treatment of proliferative LN, the decision to choose MMF is made as part of clinical care. The complete listing of the key eligibility criteria is provided in Table 1, while Supplementary Table 1 provides a full listing. Information about prohibited concurrent medications is shown in Supplementary Table 2.
Table 1:
Key eligibility criteria.
| Key inclusion criteria | Key exclusion criteria |
|---|---|
| 1. Age 8-<18 years at the time of enrollment | 1. Presence of features (from SLE or other chronic disease) that a-priori suggests that the subject benefits from other therapies than that suggested or allowable by the study protocol. |
| 2. New diagnosis with proliferative LN as per the ISN/RPS classification criteria [21], based on kidney biopsy done within 60 days of enrollment. For study inclusion, the kidney biopsy needs to be newly interpreted as one of the following classes: class 3, class 3/5, class 4, or class 4/5 | 2. History of significant kidney disease prior to the diagnosis of SLE |
| 3. Diagnosis with cSLE [47,48], per the classification criteria of the ACR [49]/EULAR [50] (i.e., SLE with diagnosis prior to or at age 18 years) | 3. Estimated GFR <40 mL/min/1.73 m2 using the modified Schwartz equation [55]. |
| 4. Tolerates MMF as per the treating physician | 4. Need for renal replacement therapy at the time of enrollment |
| 5. SLEDAI-R [25,51–54], score>0 | 5. CYC within 12 weeks of enrollment |
| 6. Anti-CD20 monoclonal antibody treatment within 6 months, except if CD19/20+ counts are normal by flow cytometry analysis) | |
| 7. Specific blood dyscrasias |
Note: LN-lupus nephritis; ISN/RPS-International Society of Nephrology/Renal Pathology Society; cSLE-childhood-onset systemic lupus erythematosus; ACR-American College of Rheumatology; EULAR-European League Against Rheumatism; MMF-mycophenolate mofetil; SLEDAI-R-renal domain score of Systemic Lupus Erythematosus Disease Activity Index; GFR-glomerular filtration rate; CYC-cyclophosphamide.
Outcome measures and safety and efficacy endpoints
The primary efficacy outcome of the study is the improvement of proliferative LN as measured by the presence of at least PRR at the end of Part 1 of the study (week 26). The major secondary outcome is the achievement of CRR at the end of Part 1 of the study. Other secondary outcomes are the achievement of CRR at the end of Part 2 of the study (week 53) and frequency of adverse events defined as grade 3 or higher during all parts of the study using the National Cancer Institute Common Terminology Criteria for Adverse Events.
Exploratory outcomes include: change in Renal Activity Index for Lupus (RAIL) score (baseline visit to week 53) [23,24], rate of LN flares, time to PRR and CRR during the study (baseline visit to week 53), mean time-adjusted score of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) [25], considering extra-renal domain items during the study (baseline visit to week 53), cumulative dose of oral corticosteroids (CS; in prednisone-equivalents) and cumulative exposure to intravenous CS during the study, frequency of patients fulfilling discontinuation criteria during the study, change in health-related quality of life as measured by the Pediatric Quality of Life Inventory (PedsQL) Generic Core Scale [26], during the study, percentage of patients with intake of ≥ 80% of prescribed MMF doses as per blister-pack count during the study, and presence of MPA-levels<0.1 mg/L from random testing or in-clinic testing during the study. The MPA primary and secondary outcome evaluations will be performed by independent blinded evaluators at the CCC, according to validated criteria. Outcome definitions are provided in Table 2.
Table 2:
Definition of key efficacy measures.
| Outcome measure | Definition | References |
|---|---|---|
| Partial renal remission | PRR is defined as relevant improvement of 2 LN-RV* with the remaining LN-RV being at least stable. | [22,34] |
| Complete renal remission | CRR is the presence of all 3 LN-RVs within normal range. | [22,34] |
| Lupus nephritis flare | A LN flare is the reproducible+ presence of 1 or more of the following (a-d) that is at least partially due to renal inflammation from LN per the treating physician. a. Newly abnormal GFR plus increase in hematuria by at least 2 categories (normal: 0 to 5 RBC/HPF (normal), 6 to 10 RBC/HPF, 11 to 25 RBC/HPF, 26 to 50 RBC/HPF, >50 RBC/HPF) OR new gross hematuria (>50 RBC/HPF). b. Abnormal GFR that decreased by >10% plus increase in hematuria by at least 2 categories (normal: 0 to 5 RBC/HPF (normal), 6 to 10 RBC/HPF, 11 to 25 RBC/HPF, 26 to 50 RBC/HPF, >50 RBC/HPF) OR new gross hematuria (>50 RBC/HPF). c. Persistent increase of UPCR to >0.5, after CRR d. Persistent doubling of UPCR with values >1.0, after PRR |
[22,34] |
Note: PRR-partial renal response; LN-RV-lupus nephritis core response variables; CRR-complete renal response; GFR-glomerular filtration rate; RBC/HPF-red blood cell per high-power field; UPCR-urine protein to creatinine ratio.
LN-RVs are the upper limit of normal (ULN) or lower limit of normal (LLN) of the following:
Urine protein to creatinine ratio (ULN: 0.2), estimated GFR (LLN: 95 ml/min/1.73m2) and glomerular hematuria (ULN:0)
Reproducibility means presence on >2 subsequent time points >1 week apart.
Randomization
Eligible subjects enrolled in the study will be randomized (1:1) at baseline to receive either MMFPK or MMFBSA. Randomization will be stratified by LN class (class 3 or 3/5 vs. class 4 or 4/5) as per the ISN/RPS and extrarenal manifestations of cSLE (extrarenal SLEDAI score<10 vs. ≥ 10). Race (white or non-white) and belimumab treatment (yes/no), as well as other important factors which have a known association with long-term outcomes of LN will be considered as potential confounders during the analyses. We will use variable block sizes to ensure balance and to minimize risk for unmasking. Thus, we will be using block sizes of 4 and 2 in random order.
Blinding
Subjects and research personnel at the CCC and sites will remain blinded to the study intervention (MMFPK or MMFBSA) until the end of the study. The following team members will remain unblinded: research pharmacist, unblinded study physicians, and the PK analysis team. During the study, masking will be maintained by using placebo capsules which have the same appearance as MMF. Genentech will supply MMF to the central pharmacy at Cincinnati Children’s Hospital Medical Center (CCHMC) Investigational Drug Service (IDS).
MMF tablets (500 mg/tab) and/or MMF capsules (250 mg/cap) and/or identical placebo capsules will be used to ensure double-blinding of the study treatment. Over-encapsulated MMF 250 mg and placebo capsules will be prepared by the IDS. Blister packs containing combinations of MMF 500 mg tablets, over-encapsulated MMF 250 mg, and/or placebo capsules are produced by the IDS to reflect the desired MMF dosage, based on the treatment arm. Each subject is instructed to take medication in the morning and evening from the appropriately labelled blister card containing 10 medication blisters. The appearance of study medication is changed after every study visit and study month, regardless of the treatment arm. After the final data lock, the site investigators will be informed of the treatment allocation.
Investigational and reference therapy
MMF in tablet and/or capsule form will be taken twice daily by mouth, approximately 12 hours apart. The investigational therapy will be individualized using MMFPK or MMFBSA. Based on PK assessment (as detailed in the next section), MMF dosage of the MMFPK arm will be adjusted in 250 mg increments to achieve a target MPA-AUC0–12>60–70 mg*h/L. For the MMFBSA arm, the amount of MMF prescribed will be around 600 mg/m2 BSA per dose, up to the recommended maximum of 3 grams per day. The dosage of MMF will be kept at the level prescribed on day 1 unless the patient’s weight changes. Necessary dose adjustments will be assessed whenever there is a weight change that exceeds 10 lbs. (or 4.5 kg) since the last dose. We expect trough levels of MPA to be>1–3.5 mg/L in both treatment arms.
Pharmacokinetic assessment and Bayesian estimation
The study team developed a novel assay to measure MPA levels utilizing Volumetric Absorptive Microsampling (VAMS) devices from Mitra® [27,28]. This will be used to measure MPA levels randomly or serially to assess MPA exposure and adherence to MMF. Capillary blood will be collected just prior to the next MMF intake (trough) and 20 minutes, 1 hour, and 3 hours after dose intake, which will then be sent to CCHMC for liquid chromatography tandem mass spectrometry (LC-MS/MS) drug assay and PK analysis. This method of PK assessment offers the convenient option of performing blood sampling for random MPA level measurement in the subject’s home setting.
The PLUMM study will use pharmacokinetic assessment at the following 5 patient visits during the study to adjust the dose of the MMFPK arm subjects: baseline visit, week 3, week 10, week 26, and week 32. Dosing for this arm will be based on individual abbreviated AUC0–12h estimates. These AUC0–12h estimates will be generated with a well-established PK model-based Bayesian approach using MPA concentration results equivalent to plasma concentrations derived from capillary whole blood concentration results.
Two prospective studies were conducted at CCHMC and the University of Cincinnati (Dr. Rita Alloway, PI; CCHMC IRB: 2022–0416) in preparation for the PLUMM study. These studies were used to validate the methodology of the PLUMM study using the VAMS device for PK-profiling and to ensure the study team can reliably convert capillary whole blood concentrations (as obtained with the Mitra VAMS device) to plasma concentrations. Individual PK parameters and dose to achieve the target AUC0–12h will be estimated with Bayesian estimation using clinical precision dosing software MwPharm++ (Mediware, Prague, Czech Republic). The Bayesian estimation method and the sparse sampling strategy (at 0 h, 20 min, 1 h, and 3 h) for MPA have been developed and validated in various patient populations including pediatric lupus patients by Dr. Pierre Marquet and colleagues at the Limoges University Hospital Laboratory of Pharmacology [29–32].
Standardized steroid regimen
Corticosteroids (CS), mainly oral prednisone, are an integral part of treatment for patients with LN, but there is large variability of CS dosing among treating physicians [33]. Since CS have a strong anti-inflammatory effect that could influence the comparison of the two treatment arms and thereby affect the primary outcome of the study, site investigators are strongly encouraged to use the dose recommendations from the CCC which reflect a standardized steroid regimen as previously published by Chalhoub et al. [34].
Establishing a CS dosing algorithm was necessary to control the use of CS during the PLUMM study. Planning for the CS dosing was initiated with a retrospective chart review to document current use of CS at 15 sites. The chart review enabled the research team to build patient profiles to support consensus formation science using the Delphi methodology. The dosing parameters were developed based on the consensus ratings of 103 reviewing pediatric nephrologists and rheumatologists who rated 5056 patient profiles that outlined the disease course of LN patients. After the initial review, validation of the steroid dosing regimen was conducted with 60 raters on 1838 patient profiles. The resulting Standardized Steroid Regimen (SSR) has been published by Chalhoub et al. [34]. For the PLUMM study, the CCC will provide steroid dose recommendations based on data points from sites, using a calculator developed by one of the authors (BH).
Phone application and other adherence tools
To alert subjects to random MPA testing and to remind of twice daily medication intake, the PLUMM phone application was developed which will be loaded onto the subjects’ device or parents’/caretaker’s device. We expect communication to occur primarily with the parent/caretaker. Random MPA measurements between study visits will be obtained if with suspected non-adherence or failure to log medication intake. An MPA level of<0.1 mg/L will be considered to reflect non-adherence.
Sample size and power estimation
The PLUMM study is the largest clinical trial in pediatric LN, to the best of our knowledge, which is adequately powered. Sample size was determined from a combination of prevalence data and precedents in the literature [13,14,35–43]. A sample size of 45 subjects per group with proliferative LN is anticipated to provide a power of 80% for the primary aim of detecting a significant difference in the rate of PRR between the MMFPK and MMFBSA arms at the end of Part 1, at a 2-sided 5% significance level by a two-group Chi-square test, assuming the rate of PRR is 55% in MMFBSA and 83% in MMFPK. The sample size of 90 will ensure 81% of power to detect a statistically significantly difference in the rate of CRR between the two study arms, assuming a smaller proportion of 14% and a larger proportion of 43%. Based on the above presented power analyses, we will plan to enroll 105 subjects, to allow for up to 14.3% attrition rate during Part 1 of the study.
Statistical analysis
The primary outcome, superiority of MMFPK over MMFBSA for the percentage of subjects with clinical remission of LN (PRR, CRR) at the end of Part 1, will be tested using the analytical approach for the binomial outcome, such as Chi-square test, Fisher’s exact test, or a Bayesian approach with non-informative Beta prior for the binary probability parameter.
The choice of approach will be determined by the distribution of the data. Subjects who discontinue the treatment due to any reason will be considered as being non-responders. All secondary and exploratory efficacy outcomes will be analyzed by treatment group. For the binary secondary or exploratory outcomes, the analytical approach for the binary outcome, as used for the primary analysis, will be performed.
For the continuous exploratory endpoints, including change from baseline in PROMIS scores or RAIL scores, a mixed-effect model with repeated measures will be applied. The Kaplan-Meier plots will be generated for the exploratory endpoints of time to CRR and time to PRR. Since no measures are collected from the discontinued subject, the analyses will take an Inverse Propensity Weighting (IPW) approach where the propensity for subjects to be discontinued from each arm are estimated using logistic regression modeling or the covariate balance propensity score method.
The IPW approach will create pseudo subject samples that represent the original randomized subject cohort at the beginning of the study, and therefore addresses the missing data issue due to subject discontinuation.
Descriptive/summary statistics for all endpoints, with 95% confidence interval for treatment difference, will be provided. Baseline demographic characteristics, primary and major secondary outcomes will also be summarized overall and by age (<12 years vs. ≥12 years), gender and race (white vs. non-white). Safety analysis will be performed on all subjects who received at least one dose of study drug. Safety data will be subject to clinical review and summarized by appropriate descriptive statistics.
DISCUSSION
The safety and efficacy of MMF in the treatment of LN have been tested in adults and in some pediatric subjects. However, data in both adults and children suggest that MMFBSA does not reliably correlate with exposure to and subsequent immunological and clinical response to its biologically active metabolite, MPA [44]. Because of this weak correlation between MMFBSA and MPA exposure, therapeutic drug monitoring presents a potential approach to optimize the use of MMF in pediatric LN.
The PLUMM study will provide high-level evidence that MMFPK yields higher renal remission rates compared with MMFBSA when used in the treatment of proliferative LN in cSLE. Currently, there are no controlled studies which provide a blinded, controlled head-to-head comparison of MMFPK and MMFBSA in either adult or pediatric LN. To the best of our knowledge, the PLUMM study is the first treatment trial in pediatric LN which is adequately powered.
We will collect data and biospecimens from 105 well-characterized pediatric LN patients, which will offer an unprecedented potential for collaborative ancillary studies. This will also allow for biobanking of DNA, RNA, plasma, and urine which will be used for supplementary studies of the pharmacogenetics (PG) of MPA. Future supplementary studies on MPA PG and the relationship of MPA with the pharmacology of hydroxychloroquine are anticipated to be successful, given the breadth of this study.
Additional strengths of the design of this study include a thorough evaluation of the pharmacokinetics of MMF in pediatric LN patients who are on concurrent medications, additional validation of a novel LN biomarker panel, and the testing of a novel SSR [34].
Notably, the SSR has been specifically developed for the PLUMM study in an international effort that used state-of-the art consensus methodology, statistical modeling, and true disease courses of children with LN. In brief, the SSR considers the time since LN diagnosis, the course of LN and extrarenal SLE, as well as the prior CS dosage since the preceding clinical assessment of the subject to suggest the dose of oral prednisone or IV methylprednisolone. Standardization of CS use is important, as CS are potent effect modifiers that require careful dosing in any randomized clinical trial of an inflammatory disease and has been identified to be one of the significant factors impacting the success of clinical trials in lupus [45,46]. This SSR is anticipated to manage and reduce variability of CS dosage using international consensus and real-life cSLE patient data.
The study design has some limitations. The feasibility of recruitment may be affected by the need in some patients for therapy with IV cyclophosphamide and/or B-cell depletion therapy (rituximab or belimumab). If such is deemed necessary for the treatment of a patient leading to their discontinuation of the study, their clinical outcome will still be monitored longitudinally. Concurrent use of calcineurin inhibitors may also affect MPA PK, so a subanalysis may be performed in those patients receiving this. Given the complex clinical course of cSLE patients, variability in CS dosing may persist despite SSR use, leading to potential deviation from the proposed standardized steroid regimen. If this was to be the case, then we will correct analyses for variation in CS dose and consider Bayesian analysis with causal interference strategies to correct for the CS effect. Since high doses of CS decrease exposure to MPA, we will repeat MPA PK measurement if a patient’s CS dose has changed by>50% or>20 mg since the last MPA PK profile. MPA PK may also be affected by concurrent medications. However, these will be kept stable during the study, other than CS.
CONCLUSION
The PLUMM study is the first study of its kind to conduct a head-to-head comparison of pharmacokinetically-guided precision-dosing of MMF versus MMF dosed on body surface area, with respect to the achievement of renal disease remission in pediatric proliferative lupus nephritis. This study will yield high-quality evidence that will provide a foundation for leveraging therapeutic drug monitoring of MMF, thus leading to improved clinical outcomes in pediatric lupus nephritis.
Supplementary Material
ACKNOWLEDGEMENT
This clinical trial benefited from the invaluable contributions of the following individuals: Joy Buie, Catherine Robben, Kenneth Setchell, Junfang Zhao, Denise Lagory, and Alex Herman. The authors and the study team are grateful for the endorsement of the PLUMM Study by the Childhood Arthritis and Rheumatology Research Alliance (CARRA) and the Pediatric Nephrology Research Consortium.
FINANCIAL SUPPORT
This clinical trial is supported by the NIAMS (R01AR079124, P30AR076316; P30P30AR070549; R34AR071651), the Cincinnati Center for Clinical and Translational Science Research and Training (UL1TR001425). Study medication is provided free of charge by GENENTECH, Inc.
Footnotes
Trial registration number:
TRIAL STATUS
Recruitment is currently active in 11 centers. Patient enrollment and data capture is expected to be completed by March 2026.
REFERENCES
- 1.Mina R, Brunner HI. Update on differences between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Res Ther. 2013;15:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasan B, Fike A, Hasni S. Health disparities in systemic lupus erythematosus—A narrative review. Clin Rheumatol. 2022;41(11):3299–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yen EY, Shaheen M, Woo JM, Mercer N, Li N, McCurdy DK, et al. 46-year trends in systemic lupus erythematosus mortality in the United States, 1968 to 2013: A nationwide population-based study. Ann Intern Med. 2017;167(11):777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mina R, Brunner HI. Pediatric lupus—are there differences in presentation, genetics, response to therapy, and damage accrual compared with adult lupus?. Rheum Dis Clin North Am. 2010;36(1):53–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinheiro SV, Dias RF, Fabiano RC, Araujo SD. Pediatric lupus nephritis. J Bras Nefrol. 2018;41:252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oni L, Wright RD, Marks S, Beresford MW, Tullus K. Kidney outcomes for children with lupus nephritis. Pediatr Nephrol. 2021;36:1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg AD, Steinberg SC. Long-term preservation of renal function in patients with lupus nephritis receiving treatment that includes cyclophosphamide versus those treated with prednisone only. Arthritis Rheum. 1991;34(8):945–950. [DOI] [PubMed] [Google Scholar]
- 8.Walsh M, James M, Jayne D, Tonelli M, Manns BJ, Hemmelgarn BR. Mycophenolate mofetil for induction therapy of lupus nephritis: A systematic review and meta-analysis. Clin J Am Soc Nephrol. 2007;2(5):968–975. [DOI] [PubMed] [Google Scholar]
- 9.Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–2228. [DOI] [PubMed] [Google Scholar]
- 10.Liang MH, Schur PH, Fortin P. The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria. Arthritis Rheum. 2006;54(2):421–432. [DOI] [PubMed] [Google Scholar]
- 11.Gordon C, Jayne D, Pusey C, Adu D, Amoura Z, Aringer M, et al. European consensus statement on the terminology used in the management of lupus glomerulonephritis. Lupus. 2009;18(3):257–263. [DOI] [PubMed] [Google Scholar]
- 12.Sagcal-Gironella AC, Fukuda T, Wiers K, Cox S, Nelson S, Dina B, et al. Pharmacokinetics and pharmacodynamics of mycophenolic acid and their relation to response to therapy of childhood-onset systemic lupus erythematosus. Semin Arthritis Rheum. 2011;40(4):307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daleboudt GM, Reinders ME, Hartigh JD, Huizinga TW, Rabelink AJ, De Fijter JW, et al. Concentration-controlled treatment of lupus nephritis with mycophenolate mofetil. Lupus. 2013;22(2):171–179. [DOI] [PubMed] [Google Scholar]
- 14.Kittanamongkolchai W, Rukrung C, Supasiri T, Lertjirachai I, Somparn P, Chariyavilaskul P, et al. Therapeutic drug monitoring of mycophenolate mofetil for the treatment of severely active lupus nephritis. Lupus. 2013;22(7):727–732. [DOI] [PubMed] [Google Scholar]
- 15.Bergan S, Brunet M, Hesselink DA, Johnson-Davis KL, Kunicki PK, Lemaitre F, et al. Personalized therapy for mycophenolate: consensus report by the international association of therapeutic drug monitoring and clinical toxicology. Ther Drug Monit. 2021;43(2):150–200. [DOI] [PubMed] [Google Scholar]
- 16.Sherwin CM, Sagcal-Gironella AC, Fukuda T, Brunner HI, Vinks AA. Development of population PK model with enterohepatic circulation for mycophenolic acid in patients with childhood-onset systemic lupus erythematosus. Br J Clin Pharmacol. 2012;73(5):727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda T, Brunner HI, Sagcal-Gironella AC, Vinks AA. Nonsteroidal anti-inflammatory drugs may reduce enterohepatic recirculation of mycophenolic acid in patients with childhood-onset systemic lupus erythematosus. Ther Drug Monit. 2011;33(5):658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda T, Goebel J, Cox S, Maseck D, Zhang K, Sherbotie JR, et al. UGT1A9, UGT2B7, and MRP2 genotypes can predict mycophenolic acid pharmacokinetic variability in pediatric kidney transplant recipients. Ther Drug Monit. 2012;34(6):671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagcal-Gironella AC, Fukuda T, Klein-Gitelman MS, Vinks AA, Brunner HI. A156: Pharmacokinetics and Pharmacogenetics of mycophenolic acid and response to therapy in childhood-onset systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:202. [Google Scholar]
- 20.Yang X, Sherwin CM, Yu T, Yellepeddi VK, Brunner HI, Vinks AA. Pharmacokinetic modeling of therapies for systemic lupus erythematosus. Expert Rev Clin Pharmacol. 2015;8(5):587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weening JJ, D’agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65(2):521–530. [DOI] [PubMed] [Google Scholar]
- 22.Mina R, Von Scheven E, Ardoin SP, Eberhard BA, Punaro M, Ilowite N, et al. Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res. 2012;64(3):375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunner HI, Bennett MR, Abulaban K, Klein-Gitelman MS, O’Neil KM, Tucker L, et al. Development of a novel renal activity index of lupus nephritis in children and young adults. Arthritis Care Res. 2016;68(7):1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunner HI, Bennett MR, Gulati G, Abulaban K, Klein-Gitelman MS, Ardoin SP, et al. Urine biomarkers to predict response to lupus nephritis therapy in children and young adults. J Rheumatol. 2017;44(8):1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunner HI, Feldman BM, Bombardier C, Silverman ED. Sensitivity of the systemic lupus erythematosus disease activity index, British Isles lupus assessment group index, and systemic lupus activity measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1999;42(7):1354–1360. [DOI] [PubMed] [Google Scholar]
- 26.Brunner HI, Higgins GC, Wiers K, Lapidus SK, Olson JC, Onel K, et al. Health-related quality of life and its relationship to patient disease course in childhood-onset systemic lupus erythematosus. J Rheumatol. 2009;36(7):1536–1545. [DOI] [PubMed] [Google Scholar]
- 27.Andersen IK, Rosting C, Gjelstad A, Halvorsen TG. Volumetric absorptive MicroSampling vs. other blood sampling materials in LC–MS-based protein analysis–preliminary investigations. J Pharm Biomed Anal. 2018;156:239–246. [DOI] [PubMed] [Google Scholar]
- 28.Protti M, Mandrioli R, Mercolini L. Tutorial: Volumetric absorptive microsampling (VAMS). Anal Chim Acta. 2019;1046:32–47. [DOI] [PubMed] [Google Scholar]
- 29.Le Guellec C, Bourgoin H, Büchler M, Le Meur Y, Lebranchu Y, Marquet P, et al. Population pharmacokinetics and Bayesian estimation of mycophenolic acid concentrations in stable renal transplant patients. Clin Pharmacokinet. 2004;43:253–266. [DOI] [PubMed] [Google Scholar]
- 30.Marquet P. Clinical application of population pharmacokinetic methods developed for immunosuppressive drugs. Ther Drug Monit. 2005;27(6):727–732. [DOI] [PubMed] [Google Scholar]
- 31.Saint-Marcoux F, Guigonis V, Decramer S, Gandia P, Ranchin B, Parant F, et al. Development of a Bayesian estimator for the therapeutic drug monitoring of mycophenolate mofetil in children with idiopathic nephrotic syndrome. Pharmacol Res. 2011;63(5):423–431. [DOI] [PubMed] [Google Scholar]
- 32.Woillard JB, Bader-Meunier B, Salomon R, Ranchin B, Decramer S, Fischbach M, et al. Pharmacokinetics of mycophenolate mofetil in children with lupus and clinical findings in favour of therapeutic drug monitoring. Br J Clin Pharmacol. 2014;78(4):867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunner HI, Klein-Gitelman MS, Ying J, Tucker LB, Silverman ED. Corticosteroid use in childhood-onset systemic lupus erythematosus-practice patterns at four pediatric rheumatology centers. Clin Exp Rheumatol. 2009;27(1):155–162. [PubMed] [Google Scholar]
- 34.Chalhoub NE, Wenderfer SE, Levy DM, Rouster-Stevens K, Aggarwal A, Savani SI, et al. International consensus for the dosing of corticosteroids in childhood-onset systemic lupus erythematosus with proliferative lupus nephritis. Arthritis Rheumatol. 2022;74(2):263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith EM, Al-Abadi E, Armon K, Bailey K, Ciurtin C, Davidson J, et al. Outcomes following mycophenolate mofetil versus cyclophosphamide induction treatment for proliferative juvenile-onset lupus nephritis. Lupus. 2019;28(5):613–620. [DOI] [PubMed] [Google Scholar]
- 36.Smits TA, Cox S, Fukuda T, Sherbotie JR, Ward RM, Goebel J, et al. Effects of unbound mycophenolic acid on inosine monophosphate dehydrogenase inhibition in pediatric kidney transplant patients. Ther Drug Monit. 2014;36(6):716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varnell CD, Fukuda T, Kirby CL, Martin LJ, Warshaw BL, Patel HP, et al. Mycophenolate mofetil-related leukopenia in children and young adults following kidney transplantation: Influence of genes and drugs. Pediatr Transplant. 2017;21(7):13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tönshoff B, David-Neto E, Ettenger R, Filler G, van Gelder T, Goebel J, et al. Pediatric aspects of therapeutic drug monitoring of mycophenolic acid in renal transplantation. Transplant Rev. 2011;25(2):78–89. [DOI] [PubMed] [Google Scholar]
- 39.Tett SE, Saint-Marcoux F, Staatz CE, Brunet M, Vinks AA, Miura M, et al. Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure. Transplant Rev. 2011. Apr 1;25(2):47–57. [DOI] [PubMed] [Google Scholar]
- 40.Palmer SC, Tunnicliffe DJ, Singh-Grewal D, Mavridis D, Tonelli M, Johnson DW, et al. Induction and maintenance immunosuppression treatment of proliferative lupus nephritis: A network meta-analysis of randomized trials. Am J Kidney Dis. 2017;70(3):324–336. [DOI] [PubMed] [Google Scholar]
- 41.Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J, Vinks AA. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: A pilot study. Clin Transplant. 2008;22(3):281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prausa SE, Fukuda T, Maseck D, Curtsinger KL, Liu C, Zhang K, et al. UGT genotype may contribute to adverse events following medication with mycophenolate mofetil in pediatric kidney transplant recipients. Clin Pharmacol Ther. 2009;85(5):495–500. [DOI] [PubMed] [Google Scholar]
- 43.Filler G, Vinks AA, Huang SH, Jevnikar A, Muirhead N. Similar MPA exposure on modified release and regular tacrolimus. Ther Drug Monit. 2014;36(3):353–357. [DOI] [PubMed] [Google Scholar]
- 44.Balevic SJ, Sagcal-Gironella AC. Precision medicine: Towards individualized dosing in pediatric rheumatology. Rheum Dis Clin North Am. 2022;48(1):305–330. [DOI] [PubMed] [Google Scholar]
- 45.Kalunian KC, Kim M, Xie X, Baskaran A, Daly RP, Merrill JT. Impact of standard of care treatments and disease variables on outcomes in systemic lupus erythematosus trials: analysis from the Lupus Foundation of America Collective Data Analysis Initiative. Eur J Rheumatol. 2016;3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merrill JT, Manzi S, Aranow C, Askenase A, Bruce I, Chakravarty E, et al. Lupus community panel proposals for optimising clinical trials: 2018. Lupus Sci Med. 2018;5(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva CA, Avcin T, Brunner HI. Taxonomy for systemic lupus erythematosus with onset before adulthood. Arthritis Care Res. 2012;64(12):1787–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fonseca AR, Rodrigues MC, Sztajnbok FR, Land MG, Oliveira SK. Comparison among ACR1997, SLICC and the new EULAR/ACR classification criteria in childhood-onset systemic lupus erythematosus. Adv Rheumatol. 2019;59:20. [DOI] [PubMed] [Google Scholar]
- 49.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 50.Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–291. [PubMed] [Google Scholar]
- 52.Mina R, Abulaban K, Klein-Gitelman MS, Eberhard BA, Ardoin SP, Singer N, et al. Validation of the lupus nephritis clinical indices in childhood-onset systemic lupus erythematosus. Arthritis Care Res. 2016;68(2):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunner HI, Silverman ED, To T, Bombardier C, Feldman BM. Risk factors for damage in childhood-onset systemic lupus erythematosus: Cumulative disease activity and medication use predict disease damage. Arthritis Rheum. 2002;46(2):436–444. [DOI] [PubMed] [Google Scholar]
- 54.Mina R, Klein-Gitelman MS, Ravelli A, Beresford MW, Avcin T, Espada G, et al. Inactive disease and remission in childhood-onset systemic lupus erythematosus. Arthritis Care Res. 2012;64(5):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pierrat A, Gravier E, Saunders C, Caira MV, Aït-Djafer Z, Legras B, et al. Predicting GFR in children and adults: a comparison of the Cockcroft-Gault, Schwartz, and modification of diet in renal disease formulas. Kidney Int. 2003;64(4):1425–1436. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz GJ, Mun A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.