Abstract
Neuroblastoma is a cancer of the sympathetic nervous system that develops in young children, either as low-risk or high-risk disease. The tumor microenvironment (TME) is now recognized as an important player of the tumor ecosystem that may promote drug resistance and immune escape. Targeting the TME in combination with therapies directly targeting tumor cells therefore represents an interesting strategy to prevent the emergence of resistance in cancer and improve patient’s outcome. The development of such strategies however requires an in-depth understanding of the TME landscape, due to its high complexity and intra and inter-tumoral heterogeneity. Various approaches have been used in the last years to characterize the immune and non-immune cell populations present in tumors of neuroblastoma patients, both quantitatively and qualitatively, in particular with the use of single-cell transcriptomics. It is anticipated that in the near future, both genomic and TME information in tumors will contribute to a precise approach to therapy in neuroblastoma. Deciphering the mechanisms of interaction between neuroblastoma cells and stromal or immune cells in the TME is key to identify novel therapeutic combinations. Over the last decade, numerous in vitro studies and in vivo pre-clinical experiments in immune-competent and immune-deficient models have identified therapeutic approaches to circumvent drug resistance and immune escape. Some of these studies have formed the basis for early phase I and II clinical trials in children with recurrent and refractory high-risk neuroblastoma. This review summarizes recently published data on the characterization of the TME landscape in neuroblastoma and novel strategies targeting various TME cellular components, molecules and pathways activated as a result of the tumor-host interactions.
Keywords: Neuroblastoma, Tumor Microenvironment, Heterogeneity, Clinical Trials, Pediatric Cancer
1. Introduction
Neuroblastoma is a pediatric cancer that arises from the sympathetic nervous system [1]. Whereas some patients present with localized tumors that are mostly associated with a favorable outcome (low-risk cases), half of them are affected by a metastatic disease at diagnosis, that is associated with a severe outcome (high-risk cases). One of the hallmarks of neuroblastoma is heterogeneity, that is observed at various levels. Tumor heterogeneity has not only been described between patients (inter-tumor heterogeneity) but also within tumors (intra-tumor heterogeneity). Importantly, intra-tumor heterogeneity does not only concern the features of tumor cells but also the composition of the tumor microenvironment (TME) that includes a variety of immune and non-immune cells. Over the last 2 decades, substantial improvement has been achieved in the treatment of high-risk neuroblastoma with myeloablative chemotherapy, radiation therapy, surgery, bone marrow transplantation and immunotherapy with anti-GD2 monoclonal antibodies (mAb) like Dinutuximab. However, resistance to therapy remains a major cause of failure to control or cure this cancer. Whereas much attention has been paid to intrinsic mechanisms of resistance like multidrug resistant proteins and anti-apoptotic mechanisms developed by neuroblastoma cells, less attention has been paid to extrinsic mechanisms of therapeutic resistance. There is now clear evidence that the TME plays an important role in promoting drug resistance and immune escape in cancer and this aspect has been the subject of increased attention in neuroblastoma. This review first reports recently published data that characterize the TME landscape in neuroblastoma and then discusses novel approaches targeting various TME components, in the frame of pre-clinical and clinical studies. Since a number of excellent reviews have already been published on the characterization of the neuroblastoma TME in the last years [2–4], for this manuscript we selected papers published between 2020 and 2023, using the following keywords present in the title and abstract of non-review papers: Neuroblastoma AND tumor microenvironment; Neuroblastoma AND TME; Neuroblastoma AND immunotherapy and Neuroblastoma microenvironment.
2. The TME landscape in neuroblastoma
Three main approaches have been used in the last years to understand the TME landscape and characterize various cell populations of neuroblastoma: the use of in silico and deconvolution methods applied to various datasets of bulk transcriptomic data (obtained by microarrays or RNA-seq); the more recent single-cell transcriptomic approaches; the isolation of populations from neuroblastoma samples relying on the expression of specific proteins at the membrane and their further characterization. Whereas in silico approaches on bulk expression datasets allow to explore large cohorts of neuroblastoma patients, their efficacy to detect different cell populations is limited by the complexity of the cell populations in the tumor ecosystem and relatively low abundance of certain subsets. Single-cell transcriptomic analyses allow to provide a complete characterization of the TME without any prior assumptions on surface markers and can document the transcriptomic profile of small cell populations yet with a limited number of genes detected in each subset and possible bias in the detection of different cell types. The isolation of specific populations by FACS relies on the previous identification of markers of interest and subsequent in vitro analyses. Combining these different approaches with standard techniques such as immunohistochemistry or immunofluorescence and functional validation is critical to obtain robust conclusions. Below, we report recent results obtained using various strategies to better decipher the neuroblastoma TME.
2.1. TME composition and survival
Several papers have explored how different cell populations of the TME relate to survival in neuroblastoma patients. Using CIBERSORTx, an established RNA deconvolution algorithm [5] 22 immune cell populations were imputed from a cohort of 153 primary tumors selected from the TARGET cohort of the NCI [6]. Not surprisingly, the proportions of immune cells varied between individuals. M2 macrophages were the most abundant cell type. Lower levels of monocytes, CD4+ naïve T cells, and CD4+ activated memory T cells were associated with reduced overall survival in the analyzed cohort. Another group [7] used 11 neuroblastoma datasets and a method called BASE [8–10], measuring the similarity between patient gene expression profile and profile of specific immune cell types, to infer the abundance of six common cell types (naïve B cells, memory B cells, natural killer (NK) cells, CD4+ T cells, CD8+ T cells and monocytes). This approach revealed that infiltration of naïve B cells, NK cells and CD8+ cells was associated with improved patient prognosis, with naïve B cells being the most significant factor, in all evaluated datasets using univariate analysis. The observed effect was independent of MYCN amplification and clinical variables such as age and tumor stage. Patients with high B cell infiltration exhibited high co-infiltration of other immune cell types, including memory B cells, CD8+ T cells and NK cells. Complementary experiments are still required to address the precise role of B cells in neuroblastoma. On the same line, Feng et al. developed an approach in which they first generated a self-curated gene list from single-cell transcriptomic data (see below) and then characterized three prognostic TME-subgroups associated with specific Gene-Subgroups [11]. This study revealed a TME-subgroup/Gene-Subgroup3, associated with a lack of immune cell infiltration and low scores of immune pathways as well as high scores of MYCN- and ALK-related signatures. Not surprisingly, this subgroup exhibited the worst overall survival prognosis. Using a variety of scores such as Stromal Score and Immune Score, that infer the fraction of stromal cells and immune cells, respectively, and ESTIMATE Score, that infers tumor purity in tumor tissue [12], one study concluded that the low-risk group of neuroblastoma had a better immune status compared to the high-risk group [13]. Employing the same approach as well as multiple machine learning algorithms, Jin et al. also reported two categories of neuroblastoma, with a high or low level of immune characteristics. The high immunity group exhibited higher infiltration of stromal and immune cells. Furthermore, an immune signature of nine genes (SOCS1, MARCO, KLRK1, IRF7, UNC93B1, IGHV3–20, IGKV1–16, AMH and SCTM1) was defined and shown to more accurately identify neuroblastoma patients with poor prognosis than common clinical features [14].
Another study [15] reported that high CD3E expression correlates with an enrichment of immune-stimulatory cytokines and chemokines in a large cohort of 498 primary neuroblastomas of all stages analyzed by RNA-seq [16]. As these chemokines may be involved in the recruitment of dendritic cells (DCs) and NK cells, these two populations were further explored and their relationship with tumor-infiltrating lymphocytes (TILs) was analyzed at the transcript and protein levels. This work revealed that both intra-tumoral DCs and NK cells correlate with T-cell infiltration and survival and that DC and NK signatures predict favorable clinical outcome in neuroblastoma. Among various functions, intra-tumoral DCs have been shown to play a role in the accumulation and activation of tumor-infiltrating CD8+ cells [17], consistent with the observed correlation between the abundance of TILs and DCs [18]. The mechanisms of crosstalk between DCs and NKs have been previously described, demonstrating both contact-dependent as well as contact-independent mechanisms, as well as a role of NK cells in DCs maturation [19]. Altogether these data suggest a DC-NK axis associated with good prognosis that may serve as a clinical biomarker to improve risk stratification of neuroblastoma patients.
The impact of infiltrating T cells on the behavior of neuroblastoma has also been addressed relying on the transcriptional program of T cells [20], after the observation by immunohistochemistry that tumor-infiltrating T cells have a prognostic value in neuroblastoma and that a higher number of proliferating T cells is associated with good prognosis [21]. The use of a previously defined T cell-inflamed gene expression signature from various cancer types [22] allowed to identify three subtypes of patients in two independent cohorts (TARGET from the NCI and GMKF (Gabriella Miller Kids First)), defined as T cell-inflamed, non-T cell-inflamed and intermediate. The specific analysis of high-risk patients revealed that patients with T cell-inflamed tumors had significantly better overall survival compared with those with non-T cell-inflamed tumors. In COX multivariate models including age, MYCN status and ploidy, the T cell-inflamed signature remained of independent statistical significance. For patients of the TARGET cohort for which whole exome sequencing data of matched tumor/normal DNA were available, a high neoantigen load was shown to be significantly associated with better event-free survival (EFS) and overall survival (OS). Neoantigens are antigens that are only expressed by tumor cells and neoantigen-derived epitopes can be recognized by antigen-specific CD8+ T cells contributing to antitumor immunity [23]. In this study, no difference in neoantigen load was observed between non-T cell-inflamed and T cell-inflamed tumors. It should be highlighted here that, although tumor mutation burden (or neoantigen load) and T cell-inflamed expression have been described as both prognostic in several cancer types they have little correlation [24]. In addition, in neuroblastoma, the number of neoantigens is particularly low, with a threshold of 5 used in the Bao study to discriminate tumors with a low versus a high neoantigen load [20]. The link between T cell-inflamed expression signature, neoantigen load and prognosis remains to be understood.
2.2. Global description of the TME landscape by single-cell transcriptomics
A number of research teams have now taken advantage of single-cell transcriptomics to decipher the TME landscape of neuroblastoma. Dong et al. first analyzed 16 tumors from the adrenal gland obtained from treatment-naïve patients, including 14 neuroblastomas and 2 ganglioneuroblastomas [25]. Several populations were identified in addition to tumor cells, including Schwann cells, fibroblasts, endothelial cells (ECs) and immune cells (Table 1). Among them, T and B cells, myeloid cells and plasmacytoid DCs were observed but no functional characterization was reported. Another study recently reported the global immune cell landscape of human neuroblastoma by single-cell transcriptomic approach on 17 neuroblastoma patients with a protocol to enrich immune cells [26]. The main immune subtypes included myeloid, B, T, and NK cell lineages. Although the proportions of the different populations varied between patients, T cells and myeloid cells appeared as the most frequent immune cells in this cohort. In the same line, the characterization of a cohort of 10 patient tumors, obtained either at diagnosis or at relapse, unraveled T and B cells, NK, DCs, and a complex content in myeloid cells [27]. Endothelial cells and fibroblasts were also detected in this study. Various cell types were reported in the analysis by Liu and colleagues of a cohort of 17 neuroblastic tumors including 11 neuroblastomas, 3 ganglioneuroblastomas and 3 ganglioneuromas from untreated patients as well as three peritumoral adrenal tissues (Table 1) [28]. In the neuroblastoma group, the ratio of T and myeloid cells decreased gradually from stages I/II, stage IV without MYCN amplification and to stage IV with MYCN amplification. Using in silico pseudotime trajectory analyses, the authors suggested a possible differentiation process from a subgroup of neuroendocrine tumor cells with genetic alterations towards a subcluster of benign fibroblasts. This differentiation is proposed as a mechanism of neuroblastoma spontaneous regression. Experimental validation will be required to confirm this hypothesis and explain how genetic alterations could be lost during such a differentiation. Recently, Wienke et al. published a paper reporting the identification of 17 distinct immune subsets infiltrating neuroblastoma, based on the analysis of 24 tumors (10 pre- and 14 post-chemotherapy cases, including 5 pairs) (Table 1). In agreement with previous reports, they documented that tumors are infiltrated by NK, T and B cells, and immunosuppressive myeloid populations. NK cells showed reduced cytotoxicity and T cells exhibited a dysfunctional profile (see below) [29].
Table 1.
Different players of the neuroblastoma TME identified by single-cell transcriptomics.
| Analyzed datasets | T lineage | B lineage | NK cell lineage | Myeloid lineage | Dendritic cells | Endothelial cells | Fibroblasts | Publication (PMID) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 14 NB and 2 GGNB, treatment-naïve | T cells | B cells | ND | Myeloid cells / 3 subtypes | pDCs | Endothelial cells | Fibroblasts | 32946775 |
| 17 treatment-naïve or pre-treated NB | T cytotoxic /4 subtypes | Active B cells | Active NK | Monocytes / 2 subtypes | Myeloid DCs / 3 subtypes | ND | ND | 35688160 |
| Th17 | Memory B cells | CD56dim | Macrophages / 4 subtypes | |||||
| Tregs | Germinal Centre B cells | CD56bright | ||||||
| NKT Naïve T cells Proliferating T cells | Plasma B cells | ILC3 | ||||||
| 10 treatment-naïve or at relapse NB | CD4+ | B cells | NK cells | Monocytes | cDCs | Endothelial cells | CAF-S1 and CAF-S4 | 36054452 |
| CD8+ | Non-classical monocytes Macrophages / 4 subtypes MDSCs | |||||||
| 11 NB, 3 GGNB and 3 GN, treatment-naïve | Naïve T cells | Naïve B cells | ND | Monocytes | cDCs and pDCs | Endothelial cells | 4 subtypes | 36157484 |
| T helper cells | Plasma cells | Non-classical monocytes | ||||||
| CD8+ | Proliferative B cells | Proliferative macrophages | ||||||
| CD8+ proliferating | Proliferative plasma cells | M1-like macrophages | ||||||
| Tregs | M2-like macrophages | |||||||
| 10 treatment-naïve and 14 post-chemotherapy samples | CD4+/2 clusters | B cells | NK cells | Mast cells | cDCs and pDCs | Endothelial cells | 4 subtypes | 38181797 |
| CD8+ | Plasma B cells | Macrophages / 4 subtypes | ||||||
| Tregs | Undifferentiated monocytes | |||||||
| Naïve T cells γδT cells | ||||||||
cDCs: conventional dendritic cells
pDCs: plasmacytoid dendritic cells
CAFs: Cancer-Associated Fibroblasts
MDSCs: Myeloid-Derived suppressor Cells
NB: neuroblastoma
GGNB: Ganglioneuroblastoma
GN: Ganglioneuroma (benign)
ND: not detected
The studies mentioned above have been performed using single-cell RNA-seq, which requires fast handling of fresh samples. Another option is the use of single-nucleus RNA-seq (snRNA-seq) allowing to profile nuclei isolated from frozen tumors. Yet, one challenge of this technique is that nuclei contain lower amounts of mRNA compared to cells. Interestingly, a few neuroblastoma samples have been studied by scRNA-seq and/or snRNA-seq experiments [30]. Importantly this work documented that both techniques recovered the same cell types but at different proportions.
2.3. T cell features
Characterizing the features of T cells present in neuroblastoma tumors is key to setup therapeutic strategies based on immune checkpoint blockade or strategies relying on CAR-T cells. It is now well described that immunosuppressive cells and molecules in the TME lead to T-cell dysfunction, resulting in T-cell exhaustion [31]. In neuroblastoma, Verhoeven et al. reported four cytotoxic T lymphocytes (CTL populations) but did not find any differences for both cytotoxicity and exhaustion score [26]. Yet, in two studies, T cells from neuroblastoma patients were characterized by features of exhaustion, with the expression of several inhibitory receptors and low expression of effector cytokines [27,29]. The Costa et al. paper reported that CD8+T cells mostly expressed LAG3 and TIGIT [27] whereas Wienke et al. documented significantly increased levels of PDCD1 (encoding PD-1) and LAG3 compared to other T/NK cell clusters [29]. Clusters of CD4+ FOXP3+ regulatory T cells (Tregs), described to exhibit enhanced suppressive capacity, and naive(-like) T cells were identified in 3 independent studies [26,28,29], with Tregs expressing high levels of the CTLA4 and TIGIT checkpoint inhibitors [29].
Besides these data obtained from transcriptomic analyses of neuroblastoma samples, TILs characterization after in vitro expansion from 30 patient tumors obtained from surgical resection or biopsies revealed heterogeneity of their phenotypes and functions [32]. A mixed population of both helper and cytotoxic T cells was observed but also a high proportion of CD3 cells staining negatively for both CD4 and CD8. High numbers of γδ T cells, mostly Vδ1 and non-Vδ1 Vδ2, and natural killer T cells (NKT), consistent with the phenotype of non-classical type 2 NKT cells were characterized within this population, suggesting that such cells might play a role in neuroblastoma. In addition, expanded TILs became more differentiated and expressed modest level of PD-1.
2.4. Macrophages
Macrophages are an important component of the neuroblastoma TME and several subsets have now been identified by single-cell transcriptomics [26–29]. Costa et al. reported that different clusters, expressing the classical macrophage markers CD68 and apolipoprotein-E (APOE), were positive for a M2 signature which is in favor of a pro-tumoral activity [27]. Consistently, in the Verhoven paper four macrophage clusters had high expression of the CD68 marker. One of these clusters had a significantly higher M2 score than the other macrophage populations, and all these clusters exhibited a higher M2 score compared with the monocytes detected in this analysis [26]. Four subtypes of macrophages were also reported in the paper by Wienke et al., expressing a M2-like signature [29]. At that stage, without a common integration of these different datasets, it remains difficult to decipher whereas the different clusters of these different studies represent the same entities.
In silico analysis of ligand/receptor pairs highlighted a number of interactions involving tumor cells and macrophages [26,28]. Of note, high expression of the CD24-SIGLEC10 pair, with CD24 being specifically expressed on tumor cells and SIGLEC10 expressed by macrophages, has been associated with a better prognosis in neuroblastoma samples [26]. Interestingly CD24 has been described as a dominant innate immune checkpoint providing a “do not eat me” signal that hampers the phagocytic activity of macrophages upon binding on its receptor, particularly in ovarian and breast cancers [33]. The role of this “do not eat me” signal in neuroblastoma remains to be explored. Another innate immune checkpoint, i.e., CD47-SIRPα has been shown to be of high interest in the context of neuroblastoma (see below).
2.5. Tumor-associated neutrophils (TANs)
A population of TANs has been identified and characterized by single-cell transcriptomics performed on fresh biopsies of neuroblastoma patients at diagnosis or at relapse [27] (Table 1). These TANs highly expressed S100A8, S100A9 and FCGR3B, as well as a neutrophil signature and a Polymorphonuclear Neutrophil-MDSC (PMN-MDSC) signature. Myeloid-Derived Suppressive cells (MDSCs) constitute a heterogeneous population of myeloid cells that are pathologically activated and have immunosuppressive properties. Based on their phenotypes, two major subsets have been described, being either granulocytic-MDSCs (G-MDSC, also called PMN-MDSCs) or monocytic-MDSCs (M-MDSCs) [34]. PMN-MDSCs and TANs refer to the same population. In their recent publication, Wienke et al. also reported a cluster of undifferentiated monocytes highly expressing the S100A8 and S100A9 transcripts, likely corresponding to this MDSCs/TANs population in their cohort of pre- and post-treatment tumors [29]. Of note, neutrophils detected by immunohistochemistry using neutrophil elastase (NE) as marker in the Verhoeven cohort were absent in the scRNA-seq data presented in the same study, likely due to the sample preparation protocol [26].
Interestingly the MYCN-driven mouse neuroblastoma model was shown to exhibit a low content in T cells, several phenotypes of macrophages and a population of MDSCs [27]. These cells exhibited immunosuppressive properties, impairing the proliferation of T lymphocytes in a functional ex vivo assay. Interestingly, the comparison of cell populations between the mouse model and human tumors revealed striking commonalities between the transcriptomic profiles, in particular for the MDSCs/TANs population, suggesting that such cells may also be involved in immunosuppression of the TME of patients [27]. Immunofluorescence experiments confirmed the presence of MDSCs/TANs expressing S100A8 but not HLA-DRB1 in a subset of patients.
2.6. Non-immune cells of the TME
Regarding non-immune cells, several insights have been obtained recently on stromal cells in the neuroblastoma TME. Cancer-associated fibroblasts (CAFs) heterogeneity with CAF-S1, previously associated with immunosuppressive function and CAF-S4, associated with pro-metastatic function subsets [35,36] was reported in human neuroblastoma and upregulated genes were identified in each cluster. Interestingly, CAFs-S1 express several cytokines, including CXCL12, CSF1 and CCL2 [27]. CAFs are related to mesenchymal stromal cells (MSCs) from which they derive and with which they share phenotypic and functional characteristics. MSCs have been isolated from neuroblastoma tumor biopsies and from bone marrow of patients with neuroblastoma [37,38]. They express several markers seen in normal MSC, such as CD105/Endoglin, CD90/THY1, CD73/NT5E, CD29/ITGB1 and CD146/MCAM. However, information on transcriptional signatures for CAFs-MSCs is presently missing. Functional analysis documented that a non-senescent type of these cells after short time culture are resistant to the cytolytic activity of isolated NK cells [38]. Interestingly, inhibitors of soluble immunosuppressive factors like prostaglandin E2 and L-kynurenine are able to counteract these effects suggesting that the mechanisms of interactions between MSC and NK are cell-cell contact-independent. These data indicate that CAFs-MSCs contribute to the immunosuppressive environment of high-risk neuroblastoma.
2.7. The TME landscape of metastasis
Although much focus is presently placed at understanding the TME landscape of primary - untreated and recurrent/relapse - neuroblastoma tumors, much less is known of the TME landscape of metastasis in neuroblastoma, especially the liver and the bone marrow which are rarely the subject of tissue sampling. The TME of the metastatic bone marrow however has begun to be elucidated. The presence of a subtype of these MSCs (CD146+/CD271−) was detected only in the bone marrow of patients with metastatic neuroblastoma, decreased during therapy and was detected again at relapse [39]. A recent report documented that neuroblastoma tumor cells interact mostly with CD14+ CD16+ myeloid cells in the bone marrow niche through the MIF/CD44/CD74/CXCR4 axis and MK/LRP/NCL axis [40].
3. Targeting the TME in neuroblastoma therapy: pre-clinical studies and current and future clinical strategies
Various cell populations of the neuroblastoma TME have now been suggested to promote resistance to chemotherapy, radiation therapy and immunotherapy through contact-independent and contact-dependent mechanisms of cell-cell interaction. Soluble growth factors, cytokines, chemokines, and extracellular vesicles produced by neuroblastoma cells and TME cells create dynamic contact-independent interactions in the TME recruiting non-resident cells and activating signaling pathways in neuroblastoma cells leading toward therapeutic resistance. Contact-dependent interactions between molecules expressed at the surface of neuroblastoma cells and molecules present in immune cells in the TME modulate immune recognition (Fig. 1). Consequently, targeting the TME is now considered in combination with therapies directly targeting tumor cells to prevent the emergence of resistance. Whereas much of the work in this area is still at the pre-clinical level, several clinical trials targeting the TME in neuroblastoma have been initiated over the last 5 years (Table 2). In this section, we summarize pre-clinical data and ongoing clinical trials recently (last update 2019 and later) listed as initiated or completed in clinicaltrials.gov that test drugs and biologicals specifically targeting mechanisms of interaction between neuroblastoma cells and stromal cells in the TME. We have organized the review of these recent studies based on specific approaches.
Fig. 1. : Diagram summarizing the therapeutic strategies used to target the TME in neuroblastoma described in section 3.
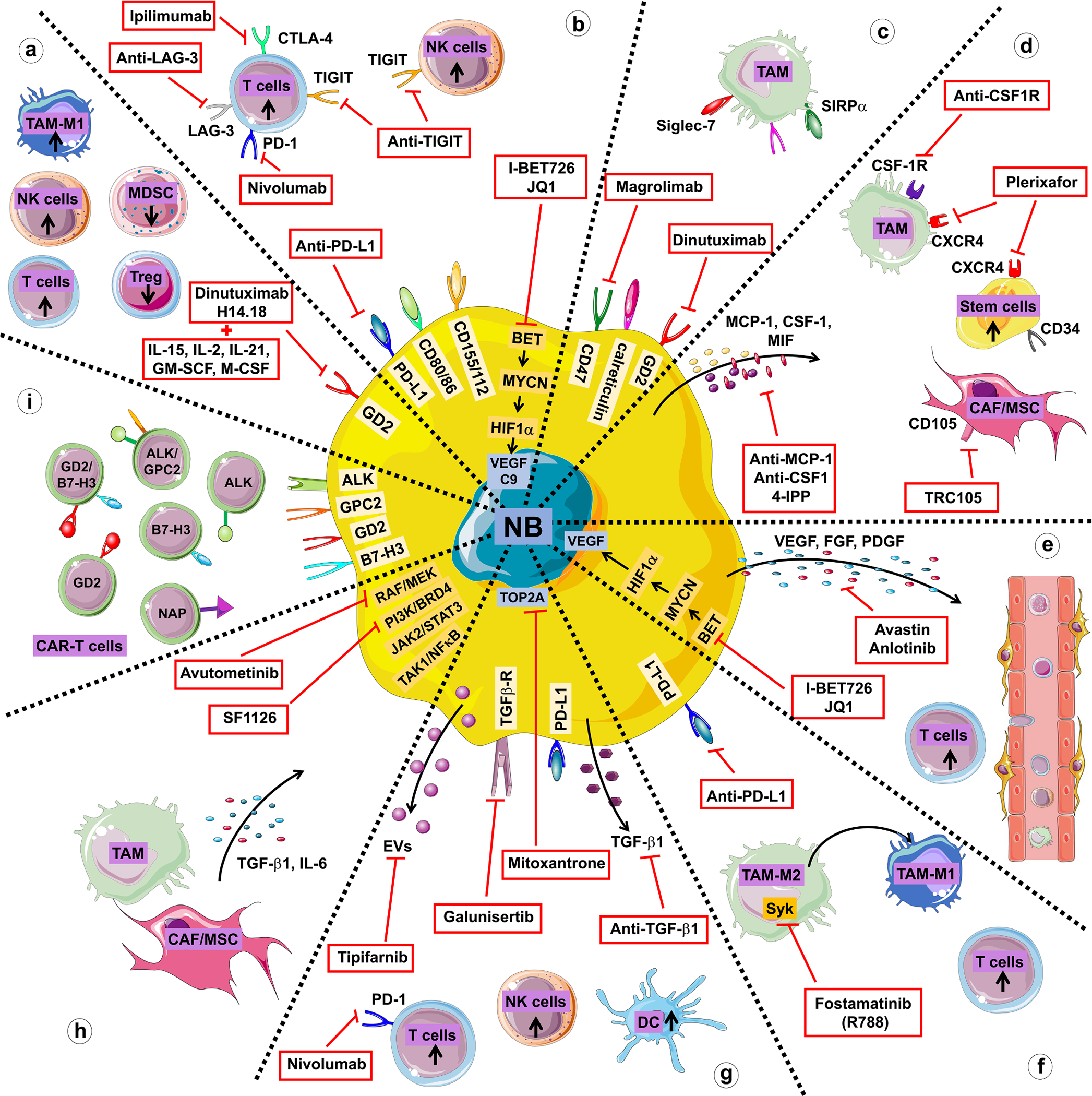
a. Approaches promoting the recruitment of immune cells with anti-tumor activity. b. Approaches preventing the suppression of anti-tumor activity through immune checkpoint blockade. c. Approaches promoting tumor cell phagocytosis. d. Approaches targeting macrophages and MSCs/CAFs. e. Approaches normalizing the tumor vasculature. f. Approaches reprogramming the TME. g. Approaches targeting immune-suppressive cytokines and extracellular vesicles (EVs). h. Approaches targeting pathways activated in neuroblastoma by TME cells. i. Approaches targeting cells with chimeric antigen receptor (CAR)-T cells.
Table 2. Ongoing clinical trials targeting the TME in neuroblastoma.
The table lists all the phase I, II and III clinical trials in patients with neuroblastoma (and solid tumors including neuroblastoma) that tested drugs and biologicals targeting the tumor microenvironment listed in clinicaltrials.gov and initiated between 2001 and 2023 with last update in 2019 or thereafter. The search included neuroblastoma, tumor microenvironment and the name of a specific drug/biological mentioned in Section 3.
| Therapy | Target | Population | NCT No | Sponsor/Name | Phase | Start date | Statut | Last update | Publication (PMID) |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Dinutuximab +Isotretinoin + cytokines | GD2 | High risk NB | NCT00026312 | National Cancer Institute (NCI) | III | 2001/10/18 | active, not recruiting | 2023/11/21 | 33504555★ |
| Hu14.18K322A (GD2 mAb) + cyclophosphamide + topotecan | GD2 | High risk NB | NCT01857934 | St. Jude Children’s Research Hospital | II | 2019/11/05 | active, not recruiting | 2023/08/25 | 34871104★ |
| Dinutuximab + Vincristine + Carboplatin + Etoposide + Cyclophosphamide | GD2 | High risk NB | NCT04221035 | Gustave Roussy, Cancer Campus, Grand Paris / SIOPEN | III | 2001/10/20 | recruiting | 2023/02/13 | |
| (GD2 mAb) + IL-2 | GD2 | Relapsed or refractory NB | NCT02258815 | University Children’s Hospital Tuebingen | II | 2010/08/01 | completed | 2023/12/08 | 36854071★ |
| BMS-986158 / BMS-986378 (CC-90010) | BET | Pediatric tumor | NCT03936465 | Dana-Farber Cancer Institute | I | 2019/09/27 | recruiting | 2023/07/27 | |
| Nivolumab + cyclophosphamide | PD-1 | Relapsed or refractory NB | NCT02813135 | Gustave Roussy, Cancer Campus, Grand Paris / ESMART (Arm G) | II | 2016/08/03 | recruiting | 2023/05/30 | 33892407★ |
| Nivolumab + Dinutuximab | PD-1/GD2 | Relapsed or refractory NB | NCT02914405 | University Hospital Southampton NHS Foundation Trust / Minivan | I | 2018/05/24 | recruiting | 2023/08/02 | 35464627 |
| Nivolumab + cyclophosphamide + Vinblastine ± Capecitabin | PD-1 | Relapsed or refractory NB | NCT03585465 | Centre Oscar Lambret | I/II | 2019/03/26 | recruiting | 2023/07/03 | 36097618 |
| Ipilimumab | CTLA-4 | Refractory Pediatric tumor | NCT01445379 | National Cancer Institute (NCI) | I | 2007/10/01 | Completed | 2019/12/17 | |
| Ipilimumab + Nivolumab | CTLA-4/PD-1 | Relapsed or refractory Pediatric tumor | NCT04500548 | National Cancer Institute (NCI) | Ib | 2021/01/28 | withdrawn | 2022/09/10 | |
| Ipilimumab + Nivolumab & Cryoablation | CTLA-4/PD-1 | Relapsed or refractory Pediatric tumor | NCT05302921 | Children’s National Research Insitute | II | 2022/02/18 | active, not recruiting | 2023/09/05 | |
| Ipilimumab + Nivolumab | CTLA-4/PD-1 | Relapsed or refractory Pediatric tumor | NCT02304458 | National Cancer Institute (NCI) | II | 2015/03/30 | Completed | 2023/10/17 | |
| Hu5F9-G4 (Magrolimab) + Dinutuximab | CD47 / GD2 | Relapsed or refractory NB | NCT04751383 | National Cancer Institute (NCI) | I | 2021/08/31 | suspended | 2023/12/06 | 35027753 |
| Plerixafor | CXCR4 | Pediatric tumor | NCT01288573 | Genzyme, a Sanofi Company | I/II | 2014/03/03 | completed | 2021/05/16 | 32127657★ |
| Anlotinib + Temozolomide + Irinotecan | VEGF, PDGF, | Relapsed or refractory NB | NCT04842526 | Tianjin medical university | II | 2021/04/12 | ongoing | 2022/04/13 | 34844980 |
| Bevacizumab + Temozolomide ± Irinotecan | VEGF | Relapsed or refractory NB | NCT02308527 | University of Birmingham / BEACON | IIb | 2013/07 | ongoing | 2023/05/10 | 36990507 |
| Bevacizumab + Temozolomide ± Irinotecan | VEGF | Relapsed or refractory NB | NCT01114555 | Memorial Sloan Kettering Cancer Center | II | 2010/04/29 | completed | 2019/11/21 | 36990507 |
| SF1126 | PI3K/BRD4 | Relapsed or refractory NB | NCT02337309 | New Approaches to Neuroblastoma Therapy Consortium (NANT) | I | 2015/07/09 | terminated | 2019/08/20 | 37477144 |
| Avutometinib | RAF/MEK | Relapsed or refractory NB | NCT06104488 | Memorial Sloan Kettering Cancer Center | I | 2023/10/20 | recruiting | 2023/10/27 | |
clinical paper
3.1. Approaches promoting the recruitment of immune cells with anti-tumor activity
This approach has been extensively tested with the administration of hu14.18, the chimeric anti-GD2 antibody, to recruit immune cells and increase antibody-dependent cell-mediated cytotoxicity (ADCC). A long-term follow-up study of patients who received Dinutuximab in combination with IL-2 and GM-CSF revealed a 56% response compared with 46.1% in the control group (NCT00026312) [41]. M-CSF and G-CSF have been used in Japanese patients post induction-consolidation with equal efficacy [42]. GM-CSF and IL-2 have also been used with hu14.18 in children with newly diagnosed neuroblastoma with improved end of induction response rate (NCT01857934, NCT02258815) [43,44]. The International Society for Paediatric Oncology Europe Neuroblastoma Group (SIOPEN) conducted a large analysis in patients with high-risk Neuroblastoma (HR-NBL1 trial) that demonstrated the important role of Dinutuximab-based immunotherapy. They compared the outcome of patients in the pre-Dinutuximab era treated with Isotretinoin alone in maintenance with patients in the Dinutuximab era treated with Dinutuximab (with or without IL-2) and Isotretinoin and demonstrated a superior two-year EFS and OS for patients receiving immunotherapy (57% and 64%, respectively) compared with patients receiving Isotretinoin only (42% and 50%) [45]. A multivariable analysis also identified the absence of immunotherapy as a risk factor for relapse and progression. In pre-clinical models, the combination of hu14.18 with IL-15 or IL-21, showed an increase anti-tumor activity in xenotransplanted mice and an increase in CD8+ T cells and anti-tumor macrophages (M1) and decreased regulatory T cells and MDSCs in the TME [46]. Recently, the combination of Dinutuximab with GM-CSF and chemotherapy (Irinotecan and Temozolomide) has been tested in patients with relapsed neuroblastoma with 50% showing an effective response [47].
3.2. Approaches preventing the suppression of anti-tumor activity through immune checkpoint blockade (ICB)
The use of ICB agents to circumvent immune escape in neuroblastoma has only begun to be investigated with some encouraging preclinical data, and several clinical trials initiated. Yet, therapeutic strategies to block immune checkpoint-mediated signaling alone have shown promising outcomes only in a subset of patients. However, combining immune checkpoint blockade with other therapies such as immunotherapy with Dinutuximab, chemotherapy, radio-immunotherapy, tumor vaccines, or cellular therapies, has shown encouraging results in enhancing anti-tumor immunity in the preclinical settings [48]. Shirinbak et al. have shown that in a murine model of minimal disease, single and dual immune checkpoint blockade (anti-PD-1 and/or anti-CTLA-4) promoted tumor rejection, improved survival, and established immune memory with long-term anti-tumor immunity against tumor re-challenge [49]. However, in a model of an established tumor, only dual immune checkpoint blockade (anti-PD-1 and anti-CTLA-4) showed efficacy [48]. Interestingly, dual immune checkpoint therapy distinctly influenced adaptive and innate immune responses, with significant increase in PD-1+ T cells and inflammatory macrophages in tumor-draining lymph nodes. Adding chemotherapy before immunotherapy also provided significant survival benefit in mice with established tumors receiving anti-PD-1 or dual immune checkpoint blockade. Although ADCC by Dinutuximab promoted the activation of NK cells leading to an effective tumor cell lysis, it was associated with an induction of PD-L1 in neuroblastoma cells and of TIGIT and PD-1 in effector cells contributing to immune escape. Adding anti-TIGIT or anti-PD-L1 treatments to Dinutuximab in tumor-bearing mice effectively inhibited tumor growth and improved survival. The combination of Dinutuximab with double immune checkpoint blockade resulted in an almost complete eradication of the tumors and the highest overall survival in mice [29,50]. Nivolumab (anti-PD-1 mAb) in combination with chemotherapy (Cyclophosphamide or Vinblastine) or high dose radiation therapy increased mouse survival and tumor infiltration with CD3+/CD8+ T cells in an immunocompetent mouse model and decreased tumor growth [51,52].
Another interesting approach has been the use of a combination of MYCN inhibition and ICB. MYCN contributes to immune escape by several mechanisms including the down regulation of the major histocompatibility complex. MYCN inhibition is thus associated with an increase in antigen recognition. Targeting MYCN in cancer cells is challenging but treatment with bromodomain inhibitors that inhibit MYCN such as I-BET726 and JQ1 resulted in cell cycle arrest in neuroblastoma cells and induced cell immunogenicity. Combining vaccination with MYCN inhibition and IBC induced a robust anti-tumor immunity in mouse neuroblastoma tumors [53]. Accordingly, in the TH-MYCN mouse model, anti-PD-1 monotherapy did not affect tumor growth, but a combination therapy with JQ1 and IBC significantly decreased tumor growth and improved the therapeutic efficacy of anti-PD-1 [54]. MYCN being an inducer of HIF-1α, BET inhibition in the TH-MYCN model inhibited HIF-1α and the expression of downstream genes like CA9 and vascular endothelial cell growth factor (VEGF). As a result, decreasing hypoxia by JQ1 was associated with improved blood vessel quality and integrity. Such a combination represents an interesting and potentially powerful approach targeting both tumor cells and TME. Although JQ1 had no major impact on infiltrating immune cells into the TME, it significantly increased the percentage of CD8+ PD-1+, conventional CD4+ PD-1+, and Treg PD-1+ cells [49]. Clinical trials combining BET inhibitors with ICB in neuroblastoma have not been initiated yet, but some BET inhibitors (BMS-986158 and BMS-986378) are presently tested for tolerability and toxicity in a phase I trial in pediatric cancers (NCT03936465).
Several clinical trials testing ICB in combination with chemotherapy have been initiated (Table 2). A limited phase II study (NCT02813135, Arm G of AcSé-ESMART, France) testing a combination of ICB and metronomic Cyclophosphamide in 13 pediatric patients with cancer including neuroblastoma, revealed that the combination was well tolerated but had limited activity in this pediatric setting [55]. Metronomic Cyclophosphamide did not affect the limited T-cell infiltration and the immunosuppressive TME found in these tumors. Recently, the combination of Dinutuximab with Nivolumab led to a complete and a very good partial remission in two patients with relapsed/refractory neuroblastoma [56]. Nivolumab and Dinutuximab in combination are tested in a phase I clinical study for patients with recurrent or refractory neuroblastoma by the University of Southampton (UK, NCT02914405) and Nivolumab in combination with metronomic chemotherapy is tested in a phase I and II clinical trial for recurrent or refractory neuroblastoma by the Oscar Lambert Center (France, NCT03585465) (Table 2). More recently, some clinical trials combining Nivolumab and Ipilimumab (anti-CTLA-4) have been tested in phase I/II for patients with recurrent and refractory pediatrics cancers with a good tolerance (NCT01445379, NCT04500548, NCT05302921, NCT02304458) [57]. Whereas treatments targeting PD-1 and CTLA-4 immune checkpoints have been explored in neuroblastoma patients with limited effects, targeting other immune checkpoints, like TIGIT or LAG-3 may provide better results in the future [27,29].
3.3. Approaches promoting tumor cell phagocytosis
As described above, macrophages are an important component of the neuroblastoma TME and several subsets have now been identified by single-cell transcriptomics [26–29]. Importantly, the phagocytic activity of macrophages against tumor cells is controlled by a balance of pro-phagocytic and anti-phagocytic surface molecules, like calreticulin (pro-phagocytic) and CD47 “do not eat me” signal (anti-phagocytic). Anti-CD47 antibodies stimulate the phagocytosis of tumor cells by macrophages and anti-GD2 antibodies have been recently shown to upregulate calreticulin expression and interrupts the interaction of GD2 with its newly identified ligand, the inhibitory immunoreceptor Siglec-7 [58]. As a result, a potent synergy for a combination of anti-GD2 and anti-CD47 in syngeneic and xenograft mouse models of neuroblastoma was recently demonstrated. These pre-clinical data supported the initiation of a phase I clinical trial combining Dinutuximab with Magrolimab, a humanized anti-CD47 monoclonal antibody, in children and young adults with recurrent or refractory neuroblastoma by the United States National Cancer Institute. The study is however presently on hold because of its toxicity (NCT04751383).
3.4. Approaches targeting macrophages and MSCs/CAFs
The contribution of M2 macrophages to a pro-tumorigenic TME in several cancers has been well demonstrated. Macrophages may be polarized into pro-tumorigenic M2 macrophages by monocyte colony stimulating factor-1 (M-CSF). Recently, M2 macrophages infiltration has been shown to be more abundant in relapse/refractory neuroblastoma tumors [59]. Blocking macrophages has thus been tested and considered in clinical trials for many years [60]. Therapies targeting M-CSF and CSF-1R have been explored in many cancers in numerous clinical trials however with mixed results. In neuroblastoma, pre-clinical studies in immunodeficient mice demonstrated that depletion of macrophages with anti-CSF-1R and anti-CSF-1 was associated with increased chemotherapeutic efficacy without requiring a contribution from T-lymphocytes [59,61]. However, clinical trials have not been initiated.
Approaches to block the recruitment of macrophages to tumors by targeting chemokines attracting myeloid cells to the TME like monocyte chemoattractant protein (MCP)-1 or macrophage migration inhibitory factor (MIF) have also been tested in pre-clinical models. Inhibition of MIF by 4-Iodo-6-phenylpyrimidine (4-IPP) in xenograft models improved drug response while delaying neuroblastoma tumor growth, improving survival and decreasing the risk of bone marrow metastasis [62]. The inhibition of CXCR4 by Plerixafor, a MIF receptor, has been clinically tested for tolerability and toxicity in patients (NCT01288573). The combination of Plerixafor with GM-CSF and chemotherapy was well tolerated and efficacious when used to mobilize CD34+ cells [63,64]. In another patient-derived xenograft (PDX) model, treatment of immunodeficient mice with anti-MCP-1 antibody combined with Etoposide significantly increased survival after resection of primary tumors, and limited macrophage recruitment into tumors [65].
Targeting CAFs and MSCs is more complex due to the heterogeneity of these populations, as recently shown by single-cell transcriptomics [27,28] and an absence of specific targetable surface markers. TRC105 (TRACON Pharmaceuticals), an anti-endoglin (CD105) monoclonal antibody expressed by MSCs and by ECs, when tested in xenotransplanted models of neuroblastoma in combination with Dinutuximab, inhibited tumor growth and infiltration with NK cells and prolonged mice survival [38,66]. However, no clinical trial in neuroblastoma could be initiated as the company terminated all clinical trials in 2019 based on lack of meaningful clinical efficacy in adult patients.
3.5. Approaches normalizing the tumor vasculature
Neuroblastoma cells and in particular MYCN-amplified cells, produce VEGF which is transcriptionally upregulated by MYCN. Excess VEGF results in the formation of an abnormal tumor vasculature characterized by an increase in ECs lacking coverage with pericytes, increased leakiness and impaired recruitment and extravasation of native and adaptive immune cells [67]. Inhibition of VEGF with agents like Avastin, an anti-VEGF antibody or Anlotinib, a multiple kinase inhibitor targeting VEGF, PDGF and FGF receptors, were initially tested for their anti-angiogenic effect. However, these agents also renormalize the tumor vasculature promoting drug delivery to tumor tissue and egression of immune cells, providing the rationale for combinations of BET inhibitors and ICB agents discussed above. It was reported that Anlotinib facilitated tumor vessel normalization at least partially through CD4+ T cells, reprogrammed the immunosuppressive TME into an immunostimulatory TME, and inhibited tumor growth in a syngeneic TH-MYCN mouse model [68,69]. Phase I and II clinical trials combining anti-VEGF with chemotherapy (Temozolomide, Irinotecan, Cyclophosphamide) have been initiated in refractory and relapsed neuroblastoma (NCT04842526, NCT02308527, NCT01114555).
3.6. Approaches reprogramming the TME
Spleen tyrosine kinase (Syk) is a marker of neuroblastoma-associated macrophages and plays a crucial role in promoting immunosuppression in the TME by stabilizing HIF-1α. Indeed, blocking Syk in TH-MYCN neuroblastoma tumor-bearing mice with R788 (Fostamatinib) impaired tumor growth and induced a reprogramming of pro-tumor M2 macrophages into immunostimulatory M1 macrophages [70]. Furthermore, combining Fostamatinib with anti-PD-L1 mAb provided a synergistic effect leading to complete tumor regression and durable anti-tumor immunity in mice bearing small tumors from the murine 9464D cell line but not larger tumors [70]. While Fostamatinib is not presently tested in clinical trials for neuroblastoma, it is tested in phase III and I clinical trials in autoimmune diseases and ovarian cancers, respectively.
3.7. Approaches targeting immune-suppressive cytokines and extracellular vesicles (EVs)
TME cells are an important source of immune-suppressive cytokines. TGF-β1 is one of the main cytokines secreted by CAFs and macrophages that stimulate the production of other cytokines like IL-6, IL-8 or MCP-1 by neuroblastoma cells. TGF-β directly promotes proliferation and resistance to chemotherapy in neuroblastoma cells and inhibits the cytotoxicity of NK and T cells. In vitro, the inhibition of TGF-β1 by Galunisertib, a TGFβ-RI inhibitor, decreases the production of cytokines and restores the cytotoxicity of NK cells and drug sensitivity in tumor cells [71]. In TH-MYCN tumor-bearing immunocompetent mice and patient-derived tumor spheroids in immunodeficient mice, the combination of low-dose of Mitoxantrone and anti-TGF-β treatment with PD-1 blockade improved antitumor immunity by promoting the infiltration and the activation of DCs, IFNγ- and granzyme B-expressing CD8+ T cells and NK cells and overcoming the immunosuppressive microenvironment [72].
In addition to cytokines, cells from the TME and neuroblastoma tumor cells produce EVs that contain proteins, nucleic acids, and lipids which may have an immunosuppressive function in tumors. In an immunocompetent in vivo model using the neuroblastoma cell line 9464D derived from a TH-MYCN tumor, small EVs produced by neuroblastoma cells were shown to create an immunosuppressive TME that contained an increased number of macrophages and fewer tumor-infiltrating NK cells [73]. The combination of anti-GD2 and Tipifarnib, a farnesyltransferase inhibitor that blocks the release of EVs by neuroblastoma cells, inhibited tumor growth and prevented the immunosuppressive effects of neuroblastoma-derived EVs.
Considering the diversity of soluble factors contributing to the interaction between neuroblastoma cells and the TME, it is uncertain that targeting a single factor may have a significant effect.
3.8. Approaches targeting pathways activated in neuroblastoma by TME cells
Through the production of cytokines and chemokines, macrophages and CAFs activate several signaling pathways in neuroblastoma cells promoting growth and resistance to apoptosis. Among these are the JAK2/STAT3 or TAK1/NF-κB pathways activated by IL-6 or TGF-β1, respectively. Neuroblastoma cells do not endogenously express IL-6 but it is upregulated by macrophages and CAFs [71]. Other signaling pathways often activated by cytokines and tumor growth factors are PI3K/mTOR and RAF/MEK, that induce proliferation and apoptosis resistance. These represent more promising avenues targeting both the tumor cells and an effect of the TME. Phase I clinical trials targeting PI3K/BRD4 with SF1126 or more recently RAF/MEK with Avutometinib in neuroblastoma patients have been conducted (NCT02337309, NCT06104488) [74].
3.9. Approaches targeting cells with chimeric antigen receptor (CAR)-T cells
The use of CAR-T cells in neuroblastoma has been the subject of only recent studies in vitro and clinical trials [75]. Early studies used GD2 as a tumor specific target. The safety and efficacy of GD2-CAR-T cells in ten patients with progressive disease completed at the Zhujiang Hospital in Guangzhou, China (NCT02765243) indicated that six patients had stable disease six months and four at one year (and being alive with stable disease after four years). Toxicities (cytokine release syndrome and neuropathic pain) were manageable [76]. However, in a recent phase I study, among twelve patients with neuroblastoma who were treated with GD2 autologous CAR-T cells, none had the predetermined objective clinical response but three of six patients who received the highest doses demonstrated other positive outcomes —regression of soft tissue and bone marrow disease— and two had evidence of immunological activity similar to that seen in CAR-T therapy-treated lymphomas and leukemias [77]. More recently, Del Bufalo et al. reported encouraging results of a clinical trial in 27 children with refractory or relapsed high-risk neuroblastoma with GD2 CAR-T cells engineered with a caspase-9 suicide gene [78]. Seventeen children (63%) had a response with nine achieving a complete response and eight a partial response. Among those who received the recommended dose, the 3-year OS and EFS were 60% and 38%, respectively. Straathof et al. also recently reported the results of a clinical study in twelve children with relapsed/refractory neuroblastoma treated with escalating doses of second-generation GD2-directed CAR-T cells and increasing intensity of preparative lymphodepletion. Overall, no patients had objective clinical response at +28 days after CAR-T cell infusion but of the six patients receiving ≥108/meter2 CAR-T cells after Fludarabine/Cyclophosphamide conditioning, three demonstrated regression of soft tissue and bone marrow disease [77]. As mentioned above, reproducible expansion of TILs from neuroblastoma samples obtained from surgical resection or biopsies is now feasible and such expanded TILs have been successfully transduced with second-generation GD2 specific CAR constructs [32]. These CAR TILs exhibited a higher killing capacity against cell lines expressing GD2 compared to their non-transduced counterparts. These results indicate a specific reactivity of CAR cells against neuroblastoma and provide the first rationale for using adoptive cell therapy with CAR TILs as a new immunotherapy approach [32].
Because of concerns of off target effects with CAR-T cells targeting GD2, especially neurotoxicity, as deciphered in a mouse model of neuroblastoma using GD2-specific CAR-T [79], other target antigens such as B7H3, ALK antigen and dual antigen GD2 and B7H3 with a gating mechanism have been developed and tested in pre-clinical models providing proof of concept for future clinical trials [80–82]. Another group has developed CAR-T cells expressing a pluripotent pro-inflammatory neutrophil-activating protein (NAP) from Helicobacter pylori, to induce the formation of an immunologically “hot” microenvironment supporting DCs maturation and infiltration by cytotoxic CD8+ T cells [83]. Glypican-2/GPC2 is another surface molecule expressed by neuroblastoma cells considered as a target for engineered CAR-T cells [84,85]. A better understanding of the TME in neuroblastoma will allow to develop approaches to circumvent mechanisms of immune escape and improve the efficacy of CAR-T cells.
4. Conclusions
It is now evident that the TME in neuroblastoma provides multiple mechanisms allowing tumor cells to escape the broad spectrum of therapies currently used and those under development. With a better understanding of the TME landscape, its heterogeneity and the cells involved and mechanisms of action, we will be able to design and test combination of optimal approaches that – for example – in addition to stimulating the infiltration of lymphoid cells in “cold” tumors, block the immunosuppression of the TME or suppress the activation of pathways leading toward resistance. Recent characterization of various immune cell populations by single-cell transcriptomics, and subsequent identification of interactions between different cell types now provide new resources for the development of biologically stratified immunotherapies in children with neuroblastoma. The development of spatial transcriptomic and proteomic methods will provide additional information to define the spatial distribution of tumor cells and their relation to the TME as well as specific signaling patterns between different cell populations. Efforts are also ongoing to integrate several datasets obtained at the single-cell level into a harmonized cell atlas, offering an increased power in the characterization of tumor and non-tumor cells and facilitating the comparison of distinct cohorts of tumors or distinct classes of tumors defined according to specific clinical or biological features. Investigating the features of the TME at sites of metastasis will be also crucial to understand the tumor microenvironment interactions. Adding information on the TME landscape to the genomic landscape at the time of diagnosis or relapse, becomes essential in the design of future umbrella and biomarker-informed clinical trials and in our efforts to continue to improve the lives of children with high-risk neuroblastoma.
Funding
This work was supported by the National Cancer Institute under Grant P01 CA217959–06 (PI J. Maris) to Y.A.D., by The Saban Research (Children’s Hospital Los Angeles) under a Research Career Development Fellowship (RCDF) Grant (PI K. Louault); and by Institut Curie, Inserm, the Institut National du Cancer (PEDIAC consortium, INCA_15670), the ERC Synergy program “Kill-or-differentiate” (PI O. Delattre), and by the following associations: Association Hubert Gouin-Enfance et Cancer, Les Bagouz à Manon and les amis de Claire.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Isabelle Janoueix-Lerosey: Writing – original draft, Conceptualization. Kevin Louault: Writing – original draft. Yves A De Clerck: Writing – original draft, Conceptualization.
References
- [1].Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, et al. , Neuroblastoma, Nat. Rev. Dis. Prim. 2 (2016) 1–21, 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- [2].Blavier L, Yang R-M, DeClerck YA, The tumor microenvironment in neuroblastoma: new players, new mechanisms of interaction and new perspectives, Cancers (Basel) 12 (2020) 2912, 10.3390/cancers12102912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wienke J, Dierselhuis MP, Tytgat GAM, Künkele A, Nierkens S, Molenaar JJ, The immune landscape of neuroblastoma: challenges and opportunities for novel therapeutic strategies in pediatric oncology, Eur. J. Cancer 144 (2021) 123–150, 10.1016/j.ejca.2020.11.014. [DOI] [PubMed] [Google Scholar]
- [4].Joshi S, Targeting the tumor microenvironment in neuroblastoma: recent advances and future directions, Cancers (Basel) 12 (2020), 10.3390/cancers12082057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA, Profiling tumor infiltrating immune cells with CIBERSORT, Methods Mol. Biol. 1711 (2018) 243–259, 10.1007/978-1-4939-7493-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Batchu S, Immunological landscape of Neuroblastoma and its clinical significance, Cancer Treat. Res Commun. 26 (2021) 100274, 10.1016/j.ctarc.2020.100274. [DOI] [PubMed] [Google Scholar]
- [7].Schaafsma E, Jiang C, Cheng C, B cell infiltration is highly associated with prognosis and an immune-infiltrated tumor microenvironment in neuroblastoma, J. Cancer Metastas-.-. Treat. 7 (2021), 10.20517/2394-4722.2021.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cheng C, Yan X, Sun F, Li LM, Inferring activity changes of transcription factors by binding association with sorted expression profiles, BMC Bioinforma. 8 (2007) 452, 10.1186/1471-2105-8-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Varn FS, Andrews EH, Mullins DW, Cheng C, Integrative analysis of breast cancer reveals prognostic haematopoietic activity and patient-specific immune response profiles, Nat. Commun. 7 (2016) 10248, 10.1038/ncomms10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Varn FS, Wang Y, Mullins DW, Fiering S, Cheng C, Systematic pan-cancer analysis reveals immune cell interactions in the tumor microenvironment, Cancer Res 77 (2017) 1271–1282, 10.1158/0008-5472.CAN-16-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feng C, Li T, Xiao J, Wang J, Meng X, Niu H, et al. , Tumor microenvironment profiling identifies prognostic signatures and suggests immunotherapeutic benefits in neuroblastoma, Front Cell Dev. Biol. 10 (2022) 814836, 10.3389/fcell.2022.814836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, et al. , Inferring tumour purity and stromal and immune cell admixture from expression data, Nat. Commun. 4 (2013) 2612, 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].He E, Shi B, Liu Z, Chang K, Zhao H, Zhao W, et al. , Identification of the molecular subtypes and construction of risk models in neuroblastoma, 11790, Sci. Rep. 13 (2023), 10.1038/s41598-023-35401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jin W, Zhang Y, Liu Z, Che Z, Gao M, Peng H, Exploration of the molecular characteristics of the tumor-immune interaction and the development of an individualized immune prognostic signature for neuroblastoma, J. Cell Physio 236 (2021) 294–308, 10.1002/jcp.29842. [DOI] [PubMed] [Google Scholar]
- [15].Melaiu O, Chierici M, Lucarini V, Jurman G, Conti LA, De Vito R, et al. , Cellular and gene signatures of tumor-infiltrating dendritic cells and natural-killer cells predict prognosis of neuroblastoma, Nat. Commun. 11 (2020) 5992, 10.1038/s41467-020-19781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang W, Yu Y, Hertwig F, Thierry-Mieg J, Zhang W, Thierry-Mieg D, et al. , Comparison of RNA-seq and microarray-based models for clinical endpoint prediction, Genome Biol. 16 (2015) 133, 10.1186/s13059-015-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. , Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity, Cancer Cell 26 (2014) 638–652, 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Melaiu O, Lucarini V, Giovannoni R, Fruci D, Gemignani F, News on immune checkpoint inhibitors as immunotherapy strategies in adult and pediatric solid tumors, Semin. Cancer Biol. 79 (2022) 18–43, 10.1016/j.semcancer.2020.07.001. [DOI] [PubMed] [Google Scholar]
- [19].Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G, Reciprocal activating interaction between natural killer cells and dendritic cells, J. Exp. Med 195 (2002) 327–333, 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bao R, Spranger S, Hernandez K, Zha Y, Pytel P, Luke JJ, et al. , Immunogenomic determinants of tumor microenvironment correlate with superior survival in high-risk neuroblastoma, J. Immunother. Cancer 9 (2021) e002417, 10.1136/jitc-2021-002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mina M, Boldrini R, Citti A, Romania P, D’Alicandro V, De Ioris M, et al. , Tumor-infiltrating T lymphocytes improve clinical outcome of therapy-resistant neuroblastoma, Oncoimmunology 4 (2015) e1019981, 10.1080/2162402X.2015.1019981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bao R, Stapor D, Luke JJ, Molecular correlates and therapeutic targets in T cell-inflamed versus non-T cell-inflamed tumors across cancer types, Genome Med 12 (2020) 90, 10.1186/s13073-020-00787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yarchoan M, Johnson BA, Lutz ER, Laheru DA, Jaffee EM, Targeting neoantigens to augment antitumour immunity, Nat. Rev. Cancer 17 (2017) 569, 10.1038/nrc.2017.74. [DOI] [PubMed] [Google Scholar]
- [24].Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, et al. , Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma, Proc. Natl. Acad. Sci. USA 113 (2016) E7759–E7768, 10.1073/pnas.1609376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dong R, Yang R, Zhan Y, Lai H-D, Ye C-J, Yao X-Y, et al. , Single-cell characterization of malignant phenotypes and developmental trajectories of adrenal neuroblastoma, Cancer Cell 38 (2020) 716–733.e6, 10.1016/j.ccell.2020.08.014. [DOI] [PubMed] [Google Scholar]
- [26].Verhoeven BM, Mei S, Olsen TK, Gustafsson K, Valind A, Lindström A, et al. , The immune cell atlas of human neuroblastoma, Cell Rep. Med 3 (2022) 100657, 10.1016/j.xcrm.2022.100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Costa A, Thirant C, Kramdi A, Pierre-Eugène C, Louis-Brennetot C, Blanchard O, et al. , Single-cell transcriptomics reveals shared immunosuppressive landscapes of mouse and human neuroblastoma, J. Immunother. Cancer 10 (2022) e004807, 10.1136/jitc-2022-004807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu Q, Wang Z, Jiang Y, Shao F, Ma Y, Zhu M, et al. , Single-cell landscape analysis reveals distinct regression trajectories and novel prognostic biomarkers in primary neuroblastoma, Genes Dis. 9 (2022) 1624–1638, 10.1016/j.gendis.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wienke J, Visser LL, Kholosy WM, Keller KM, Barisa M, Poon E, et al. , Integrative analysis of neuroblastoma by single-cell RNA sequencing identifies the NECTIN2-TIGIT axis as a target for immunotherapy, Cancer Cell 42 (2024) 283, 10.1016/j.ccell.2023.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Slyper M, Porter CBM, Ashenberg O, Waldman J, Drokhlyansky E, Wakiro I, et al. , A single-cell and single-nucleus RNA-Seq toolbox for fresh and frozen human tumors, Nat. Med 26 (2020) 792–802, 10.1038/s41591-020-0844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Davoodzadeh Gholami M, Kardar GA, Saeedi Y, Heydari S, Garssen J, Falak R, Exhaustion of T lymphocytes in the tumor microenvironment: Significance and effective mechanisms, Cell Immunol. 322 (2017) 1–14, 10.1016/j.cellimm.2017.10.002. [DOI] [PubMed] [Google Scholar]
- [32].Ollé Hurtado M, Wolbert J, Fisher J, Flutter B, Stafford S, Barton J, et al. , Tumor infiltrating lymphocytes expanded from pediatric neuroblastoma display heterogeneity of phenotype and function, PLoS One 14 (2019) e0216373, 10.1371/journal.pone.0216373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. , CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy, Nature 572 (2019) 392–396, 10.1038/s41586-019-1456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grover A, Sanseviero E, Timosenko E, Gabrilovich DI, Myeloid-derived suppressor cells: a propitious road to clinic, Cancer Discov. 11 (2021) 2693–2706, 10.1158/2159-8290.CD-21-0764. [DOI] [PubMed] [Google Scholar]
- [35].Pelon F, Bourachot B, Kieffer Y, Magagna I, Mermet-Meillon F, Bonnet I, et al. , Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms, Nat. Commun. 11 (2020) 404, 10.1038/s41467-019-14134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mhaidly R, Mechta-Grigoriou F, Fibroblast heterogeneity in tumor microenvironment: role in immunosuppression and new therapies, Semin Immunol. 48 (2020) 101417, 10.1016/j.smim.2020.101417. [DOI] [PubMed] [Google Scholar]
- [37].Borriello L, Nakata R, Sheard MA, Fernandez GE, Sposto R, Malvar J, et al. , Cancer-associated fibroblasts share characteristics and protumorigenic activity with mesenchymal stromal cells, Cancer Res 77 (2017) 5142–5157, 10.1158/0008-5472.CAN-16-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Di Matteo S, Avanzini MA, Pelizzo G, Calcaterra V, Croce S, Spaggiari GM, et al. , Neuroblastoma tumor-associated mesenchymal stromal cells regulate the cytolytic functions of NK cells, Cancers (Basel) 15 (2022) 19, 10.3390/cancers15010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hochheuser C, van Zogchel LMJ, Kleijer M, Kuijk C, Tol S, van der Schoot CE, et al. , The metastatic bone marrow niche in neuroblastoma: altered phenotype and function of mesenchymal stromal cells, Cancers (Basel) 12 (2020) 3231, 10.3390/cancers12113231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fetahu IS, Esser-Skala W, Dnyansagar R, Sindelar S, Rifatbegovic F, Bileck A, et al. , Single-cell transcriptomics and epigenomics unravel the role of monocytes in neuroblastoma bone marrow metastasis, Nat. Commun. 14 (2023) 3620, 10.1038/s41467-023-39210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yu AL, Gilman AL, Ozkaynak MF, Naranjo A, Diccianni MB, Gan J, et al. , Long-term follow-up of a phase iii study of ch14.18 (Dinutuximab) + cytokine immunotherapy in children with high-risk neuroblastoma: COG study ANBL0032, Clin. Cancer Res 27 (2021) 2179–2189, 10.1158/1078-0432.CCR-20-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hara J, Nitani C, Kawamoto H, Taguchi T, Kimura T, Yoshimura K, et al. , A phase I/IIa study of antidisialoganglioside antibody dinutuximab in japanese patients with neuroblastoma, J. Pedia Hematol. Oncol. 43 (2021) e358–e364, 10.1097/MPH.0000000000001684. [DOI] [PubMed] [Google Scholar]
- [43].Furman WL, McCarville B, Shulkin BL, Davidoff A, Krasin M, Hsu C-W, et al. , Improved outcome in children with newly diagnosed high-risk neuroblastoma treated with chemoimmunotherapy: updated results of a phase ii study using hu14.18K322A, J. Clin. Oncol. 40 (2022) 335–344, 10.1200/JCO.21.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Flaadt T, Ladenstein RL, Ebinger M, Lode HN, Arnardóttir HB, Poetschger U, et al. , Anti-GD2 antibody dinutuximab beta and low-dose interleukin 2 after haploidentical stem-cell transplantation in patients with relapsed neuroblastoma: a multicenter, phase i/ii trial, J. Clin. Oncol. 41 (2023) 3135–3148, 10.1200/JCO.22.01630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ladenstein R, Pötschger U, Valteau-Couanet D, Luksch R, Castel V, Ash S, et al. , Investigation of the role of dinutuximab beta-based immunotherapy in the SIOPEN high-risk neuroblastoma 1 trial (HR-NBL1), E309, Cancers (Basel) 12 (2020), 10.3390/cancers12020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nguyen R, Zhang X, Sun M, Abbas S, Seibert C, Kelly MC, et al. , Anti-GD2 antibodies conjugated to IL15 and IL21 mediate potent antitumor cytotoxicity against neuroblastoma, Clin. Cancer Res 28 (2022) 3785–3796, 10.1158/1078-0432.CCR-22-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lerman BJ, Li Y, Carlowicz C, Granger M, Cash T, Sadanand A, et al. , Progression-free survival and patterns of response in patients with relapsed high-risk neuroblastoma treated with irinotecan/temozolomide/dinutuximab/granulocyte-macrophage colony-stimulating factor, J. Clin. Oncol. 41 (2023) 508–516, 10.1200/JCO.22.01273. [DOI] [PubMed] [Google Scholar]
- [48].Pathania AS, Prathipati P, Murakonda SP, Murakonda AB, Srivastava A, null Avadhesh, et al. , Immune checkpoint molecules in neuroblastoma: a clinical perspective, Semin Cancer Biol. 86 (2022) 247–258, 10.1016/j.semcancer.2022.06.013. [DOI] [PubMed] [Google Scholar]
- [49].Shirinbak S, Chan RY, Shahani S, Muthugounder S, Kennedy R, Hung LT, et al. , Combined immune checkpoint blockade increases CD8+CD28+PD-1+ effector T cells and provides a therapeutic strategy for patients with neuroblastoma, Oncoimmunology 10 (2021) 1838140, 10.1080/2162402X.2020.1838140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Berckmans Y, Ceusters J, Vankerckhoven A, Wouters R, Riva M, Coosemans A, Preclinical studies performed in appropriate models could help identify optimal timing of combined chemotherapy and immunotherapy, Front Immunol. 14 (2023) 1236965, 10.3389/fimmu.2023.1236965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Webb ER, Moreno-Vincente J, Easton A, Lanati S, Taylor M, James S, et al. , Cyclophosphamide depletes tumor infiltrating T regulatory cells and combined with anti-PD-1 therapy improves survival in murine neuroblastoma, iScience 25 (2022) 104995, 10.1016/j.isci.2022.104995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Boboila S, Okochi S, Banerjee D, Barton S, Street C, Zenilman AL, et al. , Combining immunotherapy with high-dose radiation therapy (HDRT) significantly inhibits tumor growth in a syngeneic mouse model of high-risk neuroblastoma, Heliyon 9 (2023) e17399, 10.1016/j.heliyon.2023.e17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wu X, Nelson M, Basu M, Srinivasan P, Lazarski C, Zhang P, et al. , MYC oncogene is associated with suppression of tumor immunity and targeting Myc induces tumor cell immunogenicity for therapeutic whole cell vaccination, J. Immunother. Cancer 9 (2021) e001388, 10.1136/jitc-2020-001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sauvage D, Bosseler M, Viry E, Kanli G, Oudin A, Berchem G, et al. , The BET protein inhibitor JQ1 decreases hypoxia and improves the therapeutic benefit of anti-PD-1 in a high-risk neuroblastoma mouse model, Cells 11 (2022) 2783, 10.3390/cells11182783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pasqualini C, Rubino J, Brard C, Cassard L, André N, Rondof W, et al. , Phase II and biomarker study of programmed cell death protein 1 inhibitor nivolumab and metronomic cyclophosphamide in paediatric relapsed/refractory solid tumours: arm G of AcSé-ESMART, a trial of the European innovative therapies for children with cancer consortium, Eur. J. Cancer 150 (2021) 53–62, 10.1016/j.ejca.2021.03.032. [DOI] [PubMed] [Google Scholar]
- [56].Ehlert K, Hansjuergens I, Zinke A, Otto S, Siebert N, Henze G, et al. , Nivolumab and dinutuximab beta in two patients with refractory neuroblastoma, J. Immunother. Cancer 8 (2020) e000540, 10.1136/jitc-2020-000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Davis KL, Fox E, Isikwei E, Reid JM, Liu X, Minard CG, et al. , A Phase I/II trial of nivolumab plus ipilimumab in children and young adults with relapsed/refractory solid tumors: a children’s oncology group study ADVL1412, Clin. Cancer Res 28 (2022) 5088–5097, 10.1158/1078-0432.CCR-22-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Theruvath J, Menard M, Smith BAH, Linde MH, Coles GL, Dalton GN, et al. , Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication, Nat. Med 28 (2022) 333–344, 10.1038/s41591-021-01625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Valind A, Verhoeven BM, Enoksson J, Karlsson J, Christensson G, Mañas A, et al. , Macrophage infiltration promotes regrowth in MYCN-amplified neuroblastoma after chemotherapy, Oncoimmunology 12 (2023) 2184130, 10.1080/2162402X.2023.2184130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Vitale C, Bottino C, Castriconi R, Monocyte and macrophage in neuroblastoma: blocking their pro-tumoral functions and strengthening their crosstalk with natural killer cells, Cells 12 (2023) 885, 10.3390/cells12060885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Webb MW, Sun J, Sheard MA, Liu W-Y, Wu H-W, Jackson JR, et al. , Colony stimulating factor 1 receptor blockade improves the efficacy of chemotherapy against human neuroblastoma in the absence of T lymphocytes, Int J. Cancer 143 (2018) 1483–1493, 10.1002/ijc.31532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Garcia-Gerique L, García M, Garrido-Garcia A, Gómez-González S, Torrebadell M, Prada E, et al. , MIF/CXCR4 signaling axis contributes to survival, invasion, and drug resistance of metastatic neuroblastoma cells in the bone marrow microenvironment, BMC Cancer 22 (2022) 669, 10.1186/s12885-022-09725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Malinowska I, Romiszewski M, Smalisz K, Stelmaszczyk-Emmel A, Nasilowska-Adamska B, Krol M, et al. , Plerixafor combined with G-CSF for stem cell mobilization in children qualified for autologous transplantation-single center experience, Transfus. Apher. Sci. 60 (2021) 103077, 10.1016/j.transci.2021.103077. [DOI] [PubMed] [Google Scholar]
- [64].Morland B, Kepak T, Dallorso S, Sevilla J, Murphy D, Luksch R, et al. , Plerixafor combined with standard regimens for hematopoietic stem cell mobilization in pediatric patients with solid tumors eligible for autologous transplants: two-arm phase I/II study (MOZAIC), Bone Marrow Transpl. 55 (2020) 1744–1753, 10.1038/s41409-020-0836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lascano D, Zobel MJ, Lee WG, Chen SY, Zamora A, Asuelime GE, et al. , Anti-CCL2 antibody combined with etoposide prolongs survival in a minimal residual disease mouse model of neuroblastoma, Sci. Rep. 13 (2023) 19915, 10.1038/s41598-023-46968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wu H-W, Sheard MA, Malvar J, Fernandez GE, DeClerck YA, Blavier L, et al. , Anti-CD105 antibody eliminates tumor microenvironment cells and enhances anti-GD2 antibody immunotherapy of neuroblastoma with activated natural killer cells, Clin. Cancer Res 25 (2019) 4761–4774, 10.1158/1078-0432.CCR-18-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Roy Choudhury S, Karmakar S, Banik NL, Ray SK, Targeting angiogenesis for controlling neuroblastoma, J. Oncol. 2012 (2012) 782020, 10.1155/2012/782020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Su Y, Luo B, Lu Y, Wang D, Yan J, Zheng J, et al. , Anlotinib Induces a T cell-inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD-1 checkpoint blockade in neuroblastoma, Clin. Cancer Res 28 (2022) 793–809, 10.1158/1078-0432.CCR-21-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Park JA, Espinosa-Cotton M, Guo H-F, Monette S, Cheung N-KV, Targeting tumor vasculature to improve antitumor activity of T cells armed ex vivo with T cell engaging bispecific antibody, J. Immunother. Cancer 11 (2023) e006680, 10.1136/jitc-2023-006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rohila D, Park IH, Pham TV, Jones R, Tapia E, Liu KX, et al. , Targeting macrophage Syk enhances responses to immune checkpoint blockade and radiotherapy in high-risk neuroblastoma, Front Immunol. 14 (2023) 1148317, 10.3389/fimmu.2023.1148317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Louault K, Porras T, Lee M-H, Muthugounder S, Kennedy RJ, Blavier L, et al. , Fibroblasts and macrophages cooperate to create a pro-tumorigenic and immune resistant environment via activation of TGF-β/IL-6 pathway in neuroblastoma, Oncoimmunology 11 (2022) 2146860, 10.1080/2162402X.2022.2146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lucarini V, Melaiu O, D’Amico S, Pastorino F, Tempora P, Scarsella M, et al. , Combined mitoxantrone and anti-TGFβ treatment with PD-1 blockade enhances antitumor immunity by remodelling the tumor immune landscape in neuroblastoma, J. Exp. Clin. Cancer Res 41 (2022) 326, 10.1186/s13046-022-02525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liu X, Wills CA, Chen L, Zhang J, Zhao Y, Zhou M, et al. , Small extracellular vesicles induce resistance to anti-GD2 immunotherapy unveiling tipifarnib as an adjunct to neuroblastoma immunotherapy, J. Immunother. Cancer 10 (2022) e004399, 10.1136/jitc-2021-004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lukoseviciute M, Holzhauser S, Pappa E, Mandal T, Dalianis T, Kostopoulou ON, Efficacy of combined targeted therapy with PI3K and CDK4/6 or PARP and WEE1 inhibitors in neuroblastoma cell lines, Oncol. Rep. 50 (2023) 166, 10.3892/or.2023.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].DuBois SG, Macy ME, Henderson TO, High-risk and relapsed neuroblastoma: toward more cures and better outcomes, Am. Soc. Clin. Oncol. Educ. Book (42) (2022) 1–13, 10.1200/EDBK_349783. [DOI] [PubMed] [Google Scholar]
- [76].Yu L, Huang L, Lin D, Lai X, Wu L, Liao X, et al. , GD2-specific chimeric antigen receptor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients, J. Cancer Res Clin. Oncol. 148 (2022) 2643–2652, 10.1007/s00432-021-03839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Straathof K, Flutter B, Wallace R, Jain N, Loka T, Depani S, et al. , Antitumor activity without on-target off-tumor toxicity of GD2-chimeric antigen receptor T cells in patients with neuroblastoma, Sci. Transl. Med 12 (2020), 10.1126/scitranslmed.abd6169. [DOI] [PubMed] [Google Scholar]
- [78].Del Bufalo F, De Angelis B, Caruana I, Del Baldo G, De Ioris MA, Serra A, et al. , GD2-CART01 for relapsed or refractory high-risk neuroblastoma, N. Engl. J. Med 388 (2023) 1284–1295, 10.1056/NEJMoa2210859. [DOI] [PubMed] [Google Scholar]
- [79].Richman SA, Nunez-Cruz S, Moghimi B, Li LZ, Gershenson ZT, Mourelatos Z, et al. , High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model, Cancer Immunol. Res 6 (2018) 36–46, 10.1158/2326-6066.CIR-17-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bergaggio E, Tai W-T, Aroldi A, Mecca C, Landoni E, Nüesch M, et al. , ALK inhibitors increase ALK expression and sensitize neuroblastoma cells to ALK.CAR-T cells, Cancer Cell 41 (2023) 2100–2116.e10, 10.1016/j.ccell.2023.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Halliwell E, Vitali A, Muller H, Alonso-Ferrero M, Barisa M, Gavriil A, et al. , Targeting of low ALK antigen density neuroblastoma using AND logic-gate engineered CAR-T cells, Cytotherapy 25 (2023) 46–58, 10.1016/j.jcyt.2022.10.007. [DOI] [PubMed] [Google Scholar]
- [82].Moghimi B, Muthugounder S, Jambon S, Tibbetts R, Hung L, Bassiri H, et al. , Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma, Nat. Commun. 12 (2021) 511, 10.1038/s41467-020-20785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jin C, Ma J, Ramachandran M, Yu D, Essand M, CAR T cells expressing a bacterial virulence factor trigger potent bystander antitumour responses in solid cancers, Nat. Biomed. Eng. 6 (2022) 830–841, 10.1038/s41551-022-00875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Heitzeneder S, Bosse KR, Zhu Z, Zhelev D, Majzner RG, Radosevich MT, et al. , GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity, Cancer Cell 40 (2022) 53–69.e9, 10.1016/j.ccell.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sun M, Cao Y, Okada R, Reyes-González JM, Stack HG, Qin H, et al. , Preclinical optimization of a GPC2-targeting CAR T-cell therapy for neuroblastoma, J. Immunother. Cancer 11 (2023) e005881, 10.1136/jitc-2022-005881. [DOI] [PMC free article] [PubMed] [Google Scholar]


