ABSTRACT
Tissue engineering of bone is an interesting field of research. Many approaches to bone tissue engineering such as from bone marrow stromal cells in vitro have been reported. Furthermore, a model of vascularized tissue-engineered bone flap has been reported. However, there has been no report of bone tissue engineering using omentum. We present a study of tissue engineering of bone from omentum in a rabbit model. The omentum, which was elevated based on the right gastroepiploic vessels, was wrapped by the periosteum from cranial bone in the abdomen of rabbits. We harvested the omentum thus wrapped 1, 2, 4, 6, 8, 12, or 24 weeks after surgery. Within 1 week after surgery, woven bone was formed and clusters of osteoblasts were observed. At 8 weeks, medullization, including the presence of granulocytes, was confirmed. This technique might prove useful for creating tissue-engineered bone flaps for reconstructive surgery.
Key Words: Omentum, Bone, Tissue-engineering, Reconstruction
INTRODUCTION
Vascularized bone transfers using microsurgical techniques have become a common reconstruction technique for correcting osseous defects. However, such a procedure is limited by donor site availability and the required shaping of the bone. Tissue engineering of bone, which would be useful in such cases is mainly used in two different ways. One procedure is to regenerate bone by injecting bone marrow-derived stem cells into the defective site.1-5) The other procedure is to transplant bone to that site using a bone graft previously prepared for it as a so-called prefabricated flap.6-7) In reconstructive surgery for cancer, since the periosteum does not usually remain at the bone defect site, it is difficult to regenerate bone using the former procedure. In addition, defects of bone alone rarely occur, and most cases of bone defects also require the filling of soft tissues.8-12)
Higher flexibility of the greater omentum is useful for reconstructive surgery as it facilitates not only filling of the site of infections such as myelitis, but also filling of complicated defects of soft tissues.13-15) If a bone graft can be regenerated from the highly flexible greater omentum, it would allow reconstructing the bone defect site and filling soft tissues. We present a study establishing a unique in vivo model in rabbits for examining the tissue engineering of bone using omentum.
MATERIALS AND METHODS
Twenty-one Japanese white rabbits were used in our study. First, the periosteum of the cranial bone was removed under general anesthesia. The size of the periosteum was 1.5 × 1.5 cm. The omentum was then elevated based on the right gastroepiploic vessels in an open laparotomy. The end of the omentum was wrapped by the periosteum of the cranial bone in the abdomen of each rabbit (Fig. 1). Three omentums wrapped by periosteum were harvested at 1, 2, 4, 6, 8, 12, and 24 weeks after surgery. The harvested specimens were evaluated by radiography and a histologic examination. The density of the regenerated bone and tail bone was examined by radiography. One sample was prepared from each animal by excision of the greater omentum covered with the periosteum at the center. To examine osteoblasts and osteoclasts in fat tissue of the greater omentum covered with the periosteum, a histological examination was performed using hematoxylin-eosin and Azan staining. The bone marrow, which is important to the bone quality, was examined in the samples. Findings obtained from all 3 animals 1, 2, 4, 6, 8, 12, and 24 weeks after surgery were evaluated.
Fig. 1.
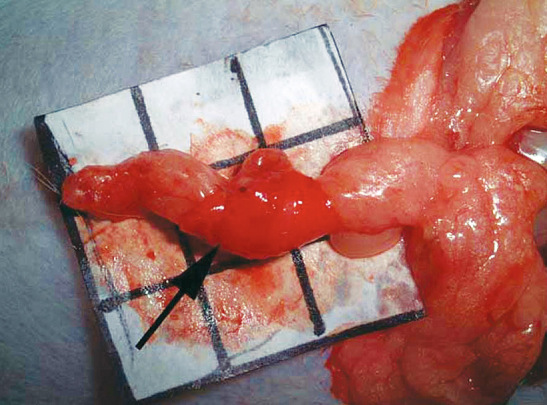
The omentum was wrapped by the periosteum from cranial bone in the abdomen. Arrow indicates the periosteum from cranial bone.
RESULTS
Macroscopic and radiographic findings
The omentum wrapped by periosteum for up to 2 weeks was soft as a fat, and no bone was formed macroscopically, as shown by radiography. Four weeks after surgery, the harvested omentum was as soft as cartilage, though radiography revealed areas with a density similar to that of bone (Fig. 2). Six weeks after surgery, the harvested omentum was slightly hard (Fig. 3), and radiography revealed areas with a density similar to that of bone (Fig. 4). At 8, 12 and 24 weeks, the omentum wrapped by periosteum was as hard as bone, while radiography revealed a density identical to that of bone.
Fig. 2.
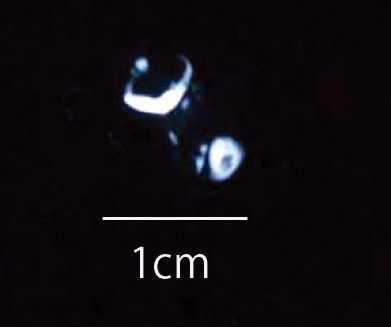
Four weeks after surgery, radiography showed areas with a density similar to that of bone.
Fig. 3.
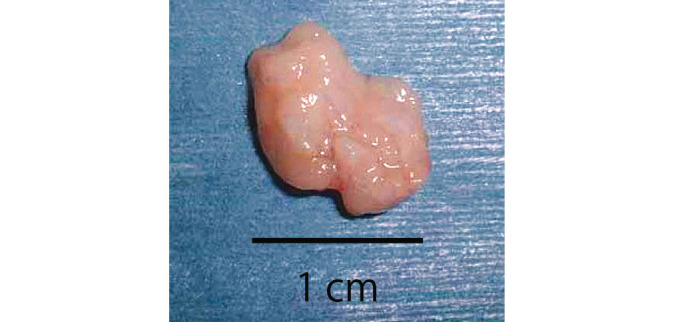
Six weeks after surgery, the harvested omentum was slightly hard.
Fig. 4.

Radiography revealed areas with a density similar to that of bone 6 weeks after surgery.
Histologic findings
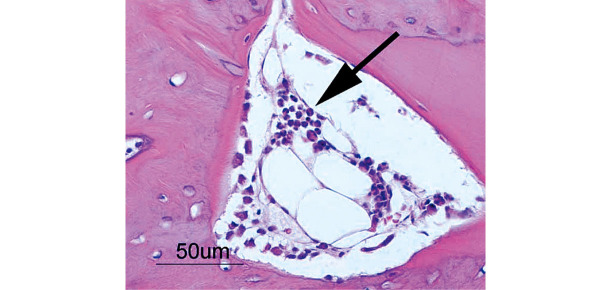
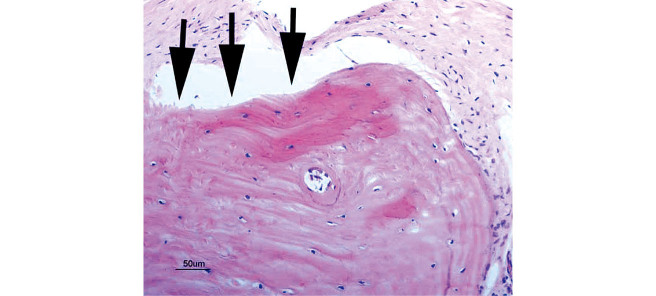
At 1 week, 2–3 pieces of woven bone were formed in all samples, and many clusters of osteoblasts were observed (Fig. 5). At 2 weeks, 3–5 pieces of woven bone had clearly formed in all samples, including the formation of cortical bone and haversian canals (Fig. 6). At 4 and 6 weeks, complete cortical bone was observed. There was proliferation of lipoblasts in 2–3 regions of all samples, which differed from omental fat. Canalization, which looked just like medullization, was observed (Fig. 7). At 8 weeks, a decrease in the number of osteoblasts was observed in all samples. The hematopoiesis of granulocytes in the canals was observed, and the medullization was complete (Fig. 8). At 12 weeks, a disorder of the bone lamination was observed, and the number of osteocytes was reduced in all samples. At 24 weeks, bone resorption occurred and bone marrow regressed showing no function (Fig. 9).
Fig. 5.
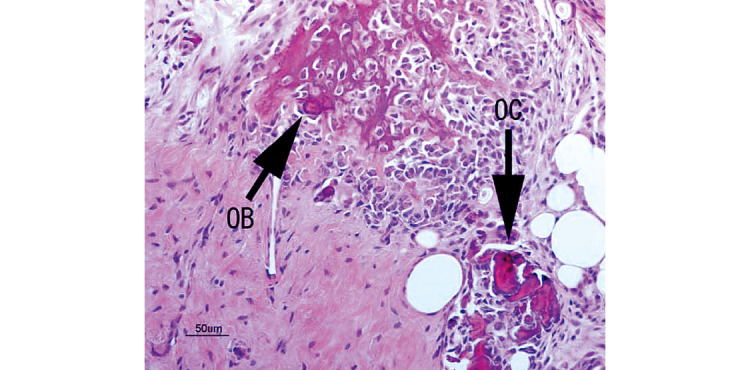
Woven bone was formed and clusters of osteoblasts were observed at 1 week. (OB: osteoblast; OC: osteoclast)
Fig. 6.
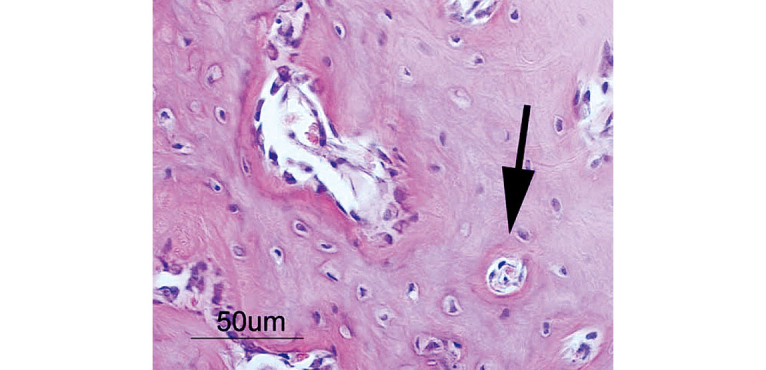
Woven bone has clearly formed, including cortical bone and haversian canals (arrow) at 2 weeks.
Fig. 7.
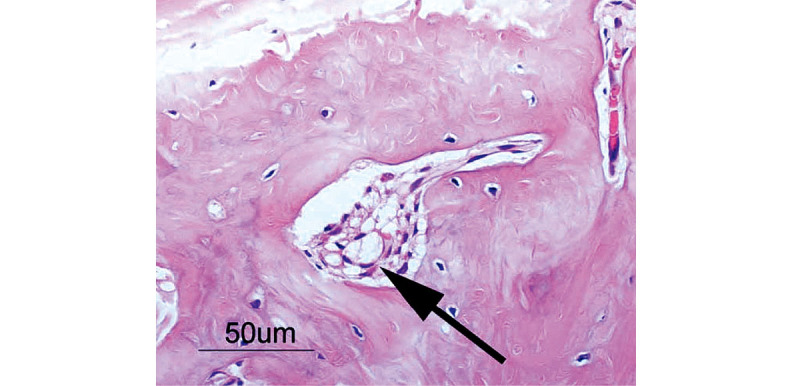
Complete cortical bone is observed. Note proliferation of lipoblasts, which are different from omental fat. Arrow indicates lipoblasts.
Fig. 8.
Hematopoiesis of granulocytes in the canals is observed and medullization is complete.
Fig. 9.
Bone lamination disorder is observed and the number of osteocytes is reduced. Pimelosis of the bone is occurring (arrows); bone marrow has decreased and has no function.
DISCUSSION
Many studies of bone regeneration from bone marrow-derived stem cells have been reported in recent years.1-5),16-19) Furthermore, a model of a vascularized tissue-engineered bone flap has been reported.6,7) However, there has been no report of bone tissue engineering using omentum. From rabbit omentum we successfully regenerated bone that had both osteoblasts and osteoclasts. Furthermore, at 6 weeks there was a proliferation of lipoblasts, which were different from omental fat. This was consistent with the early stages of bone marrow formation and medullization, which included differentiated granulocytes, was confirmed at 8 weeks. There may be three origins of this regenerated bone. One is direct regeneration from the omentum, the second is regeneration from the periosteum due to stimulation by the omentum, and the third is regeneration by transmission from the bone marrow through signaling. Although our experiment did not determine the exact mechanism, the regeneration of bone marrow was confirmed. Such regenerated bone marrow may have a hematopoietic function, since it contained blood cell components. To demonstrate this, it is necessary to confirm the presence of hematopoietic stem cells. Since the purpose of this study was to examine whether bone regeneration from the greater omentum was possible, statistical analysis and analysis by immunostaining will be required in the future. However, with regard to the establishment of an experimental model of bone regeneration from the greater omentum, this method is useful because of the simplicity of its model production and the absence of tissues involved in bone regeneration in the abdominal cavity. The regeneration of bone marrow started 4–6 weeks after surgery, and both the formation of bone marrow and the completion of bone regeneration were observed 8 weeks after surgery. Bone regeneration has a major influence on vascularization,20-22) while the omentum has abundant vessels and a high potential for angiogenesis.23-25) Omentum, therefore, has characteristics suitable for bone regeneration.
In our study, the formation of periosteum around regenerated bone was observed at 8 weeks, and bone resorption occurred after 12 weeks, because such regenerated bone was not subjected to a pressure load. The stimulation of bone remodeling is defined as a continuous stress or tension on bone, such as signals inducing bone tissue absorption, regeneration, maintenance, and mechanical loading. Although it has yet to be confirmed what biological stimulation is useful for bone remodeling, speculations include mechanical stimulation such as tension,26) stress,27) tension energy, tension change rate,28) and microdamage.29) We consider that such bone regenerated from the greater omentum requires a pressure load for remodeling, though it is not useful for reconstructive surgery at the present time. However, this bone can be regenerated without any growth factors or medications, and the method used to regenerate it is very easy and uncomplicated. We consider that bone regeneration using omentum may prove an attractive option in regenerative medicine for the reconstruction of bone defects.
REFERENCES
- 1).Urist MR. Bone formation by autoinduction. Science, 1965; 150: 893–899. [DOI] [PubMed]
- 2).Hanada K, Dennis JE, Caplan AI. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res, 1997; 12: 1606–1614. [DOI] [PubMed]
- 3).Kuznetsov SA, Krebsbach PH, Satomura K., Kerr J, Riminucci M, Benayahu D, Robey PG. Single colony derived strains of human marrow stromal fibroblasts from bone after transplantation in vivo. J Bone Miner Res, 1997; 12: 1335–1347. [DOI] [PubMed]
- 4).Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest, 2002; 110: 751–759. [DOI] [PMC free article] [PubMed]
- 5).Isogai N, Landis W, Kim TH, Gerstenfeld LC, Upton J, Vacanti JP. Formation of phalanges and small joints by tissue-engineering. J Bone Joint Surg Am, 1999; 81: 306–316. [DOI] [PubMed]
- 6).Vogelin E, Jones NF, Lieberman JR, Baker JM, Tsingotjidou AS, Brekke JH. Prefabrication of bone by use of a vascularized periosteal flap and bone morphgenetic protein. Plast Reconstr Surg, 2002; 109: 190–198. [DOI] [PubMed]
- 7).Miller MJ, Goldberg DP, Yasko AW, Lemon JC, Satterfield WC, Wake MC, Mikos AG. Guided bone growth in sheep: A model for tissue-engineered bone flaps. Tissue Eng, 1996; 2: 51–59. [DOI] [PubMed]
- 8).Taylor GI, Townsend P, Corlett R. Superiority of deep circumflex iliac vessels as the supply for free groin flaps, clinical work. Plast Reconstr Surg, 1979; 64: 745–759. [DOI] [PubMed]
- 9).Serafin D, Villarreal-Rios A, Georgiade NG. A rib-containing free flap to reconstruct mandibular defects. Br J Plast Surg, 1977; 30: 263–266. [DOI] [PubMed]
- 10).Swartz WM, Banis JC, Newton ED, Ramasastry SS, Jones NF, Acland R. The osteocutaneous scapular flap for mandibular and maxillary reconstruction. Plast Reconstr Surg, 1986; 77: 530–545. [DOI] [PubMed]
- 11).Soutar DS, McGregor IA. The radial forearm flap in intraoral reconstruction: the experience of 60 consecutive cases. Plast Reconstr Surg, 1986; 78: 1–8. [DOI] [PubMed]
- 12).Hidalgo DA. Fibula free flap mandibula reconstruction. Clin Plast Surg, 1994; 21: 25–35. [PubMed]
- 13).Harii K, Ohmori K. Use of the gastroepiploic vessels as recipient or donor vessels in the free transfer of composite flaps by microvascular anastomosis. Plast Reconstr Surg, 1973; 52: 541–548. [DOI] [PubMed]
- 14).McLean DH, Buncke HJ Jr. Autotransplant of omentum to large scalp defect with microsurgical revascularization. Plast Reconstr Surg, 1972; 49: 268–274. [DOI] [PubMed]
- 15).Kamei Y, Torii S. A new composite gastric seromuscular and omental pedicle flap. Ann Surg, 1994; 220: 97–101. [DOI] [PMC free article] [PubMed]
- 16).Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol, 1976; 47: 327–359. [DOI] [PubMed]
- 17).Owen M. Lineage of osteogenic cells and their relationship to the stromal system. In: Bone and Mineral, edited by Peck WA. pp. 1–25, 1985, Elsevier Inc,, Amsterdam.
- 18).Dennis JE, Caplan AI. Differentiation potential of conditionally immortalized mesenchymal progenitor cells from adult marrow of a H-2Kb-tsA58 transgenic mouse. J Cell Physiol, 1996; 167: 523–538. [DOI] [PubMed]
- 19).Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science, 1999; 284: 143–147. [DOI] [PubMed]
- 20).Drushel RF, Pechak DG, Caplan AI. The anatomy, ultrastructure and fluid dynamics of the developing vasculature of the embryonic chick wing bud. Cell Differ, 1985; 16: 13–28. [DOI] [PubMed]
- 21).Caplan AI, Pechak D. The cellular and molecular biology of bone formation. In: Bone and Mineral Research, edited by Peck WA. pp. 117–183, 1987, Elsevier Inc., New York.
- 22).Collin-Osdoby P. Role of vascular endothelial cells in bone biology. J Cell Biochem, 1994; 55: 304–309. [DOI] [PubMed]
- 23).Silverman KJ, Lund DP, Zetter BR, Lainey LL, Freiman DG, Folkman J, Barger AC. Angiogenic activity of adipose tissue. Biochem Biophys Res Commun, 1988; 153: 347–352. [DOI] [PubMed]
- 24).Suzuki S, Issiki N, Ogawa Y, Nishimura R, Kurokawa M. Effect of intravenous prostaglandin E1 on experimental flaps. Ann Plast Surg, 1987; 19: 49–53. [DOI] [PubMed]
- 25).Bikfalvi A, Alterio J, Inyang AL, Dupuy E, Laurent M, Hartmann MP, Vigny L, Raulais D, Courtois Y, Tobelem G. Basic fibroblast growth factor expression in human omental microvascular endothelial cells and the effect of phorbol ester. J Cell Physiol, 1990; 144: 151–158. [DOI] [PubMed]
- 26).Cowin SC, Hegedus DM. Bone remodeling I: A theory of adaptive elasticity. J Elasticity 1976; 6: 313–325.
- 27).Fyhrie DP, Carter DR. A unifying principle relating stress to trabecular bone morphology. J Orthop Res, 1986; 4: 304–317. [DOI] [PubMed]
- 28).Hert J, Pribylova E, Liskova M. Reaction of bone to mechanical stimuli. Part 3. Microstructure of compact bone of rabbit tibia after intermittent loading. Acta Anat, 1972; 82: 218–230. [PubMed]
- 29).Carter DR, Hayes WC. Bone compressive strength: The influence of density and strain rate. Science, 1976; 194: 1174–1176. [DOI] [PubMed]