ABSTRACT
We previously reported that the disruption of cell spreading by v-Crk was dependent on the activation of the MEK/ERK pathway. Here we demonstrate that the activation of that pathway is sufficient to suppress cell spreading. The MEK/ERK pathway regulates the activity of various proteins including AP-1, which is a transcriptional factor composed of heterodimeric proteins. To examine whether AP-1 activity is required for the suppression of cell spreading by the activation of the MEK/ERK pathway, we expressed BATF, which is a negative regulator of AP-1. The expression of BATF clearly restored cell spreading that was suppressed by the activation of MEK/ERK pathway. In addition, a disrupted formation of stress fibers and focal adhesions by such activation was restored by the suppression of AP-1. Our results define an essential role of the MEK/ERK/AP-1 pathway in the disruption of actin cytoskeleton and cell spreading.
Key Words: MEK, ERK, AP-1, BATF, Cell spreading
INTRODUCTION
The transformation of cells results in diverse cellular changes such as adhesion-independent growth, disruption of cell-cell adhesion and changes in cell morphology. Actin cytoskeleton is a major determinant of these cellular changes, and disruptions of actin filaments and focal adhesions are commonly observed features in cells transformed by various oncogenes. The alterations in actin cytoskeleton by oncogenes are often related to the features of transformed cells, such as anchorage-independent growth and tumorigenesis.1,2)
A cell-spreading assay is a simple in vitro experiment to observe changes in actin cytoskeleton. When cells are seeded onto the surface coated with the extracellular matrix (ECM), they first attach to the ECM, and then spread rapidly by extending filopodia-like projections and lamellipodia.3) Cell spreading is often disrupted in transformed cells.4) We previously reported that cell spreading was significantly suppressed in cells transformed by v-Crk5), which is the first member of adaptor-type oncogenes that consisted of a viral Gag sequence fused to the SH2 and SH3 domains.6) v-Crk induces tyrosine phosphorylation of various proteins and activates signal pathways such as C3G/JNK and Ras/MEK/ERK.7) Suppression of the Ras/MEK/ERK pathway restored the ability of v-Crk-transformed cells to spread, whereas the C3G/JNK pathway was not involved in the regulation of cell spreading.5) These results indicated the important role of the Ras/MEK/ERK pathway for the regulation of cell spreading.
In this report, we further investigated the functional role of the MEK/ERK pathway for the regulation of cell spreading, and showed that its activation is sufficient for the disruption of cell spreading. In addition, we showed that AP-1, which is a transcription factor consisting of heterodimeric proteins such as the c-Fos, c-Jun and ATF family of proteins, is essential for the MEK/ERK-mediated disruption of cell spreading.
MATERIALS AND METHODS
Cells, antibodies and chemicals
All cell lines were cultured in DMEM supplemented with 10% fetal bovine serum and antibiotics. Anti-BATF antibody was produced by injecting rabbits with GST-fused full length BATF.8) Antibodies for integrin α5, integrinβ1, fibronectin, vinculin, ERK and talin were obtained from Santa Cruz (Santa Cruz, CA). Antibodies for FAK and paxillin antibodies were purchased from BD Biosciences (San Jose, CA), anti-phospho-ERK was obtained from Cell Signaling Technology (Tokyo, Japan), and dexamethasone from Sigma-Aldrich (Tokyo, Japan).
Establishment of cell lines
To establish cell lines that constitutively express active MEK1 (MEK1EE; substituted Ser218 and Ser222 to Glu), the pBabepuro vector encoding active MEK1 was transfected to 3Y1 cells and then cultured in the presence of 1 μg/ml of puromycin. Colonies were isolated and the expression of active MEK1 was examined for each cell line. To establish cell lines that express BATF in the presence of dexamethasone, BATF was cloned into the pMAM2-BSD vector (Kaken Seiyaku) and transfected to MEK1EE cells. Drug-resistant colonies were isolated, and clones that expressed BATF only in the presence of dexamethasone were selected.
Cell spreading assay
Cells were seeded into a 24-well plate coated with fibronectin at a density of 1 × 105 cells per well and fixed at the indicated time point. Spread and non-spread cells were counted in five representative high-power fields. Non-spread cells were defined as small, round cells with little or no membrane protrusions, whereas spread cells were defined as large cells with extensive visible lamellipodia. Results represent the percentage of spread cells in five high-power fields. The data are presented as the average of the results from three independent experiments.
Luciferase assay
1 × 105 cells were seeded in 6-well plates and treated or untreated with 2 μM dexamethasone for 48 h. Cells were transfected with AP-1 reporter plasmid and PRL-TK control plasmid, and cells were harvested 24 h later for luciferase assay. Luciferase activity was measured and normalized using the dual luciferase kit (Promega, Madison, WI).
RESULTS
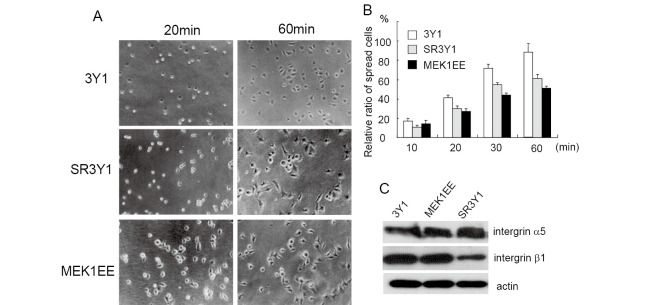
We previously reported that suppression of the Ras/MEK/ERK pathway restored the cell spreading of v-Crk transformed cells;5) therefore, we investigated the effects of MEK/ERK activation for cell spreading. 3Y1 cells, fibroblasts derived from a rat embryo, were transfected with an expression vector encoding active MEK1, and cell lines that constitutively expressed active MEK1 were established (MEK1EE). 3Y1, MEK1EE and SR3Y1 cells were trypsinized and plated onto fibronectin-coated dishes, and cell spreading was observed at different time points. SR3Y1 cells are 3Y1 cells transformed by v-Src. Most 3Y1 cells exhibited a spread morphology after 60 min of plating, whereas 50% of MEK1EE cells still showed round morphology at 60 min, indicating that the activation of MEK inhibits cell spreading (Fig. 1A and 1B). Since integrins are essential for the cell adhesion to the ECM, we examined whether the expression of integrins was reduced in MEK1EE cells. As shown in Fig. 1C, the expression of integrins was not affected by activation of the MEK/ERK pathway.
Fig. 1.
Cell spreading is reduced in MEK1EE cells. (A) 3Y1, SR3Y1 and MEK1EE cells were trypsinized and suspended in serum free media, plated onto fibronectin-coated dishes, after which cell spreading was examined at the indicated time points. Representative images of each experiment are indicated. (B) Spread and non-spread cells were counted and percentages of spread cells are indicated. Spread cells were defined as large cells with extensive visible lamellipodia, whereas non-spread cells were defined as round cells with little or no membrane protrusions. The data are presented as averages of the results from three independent experiments. (C) Expression of indicated proteins in 3Y1 and MEK1EE cells was examined using Western blot.
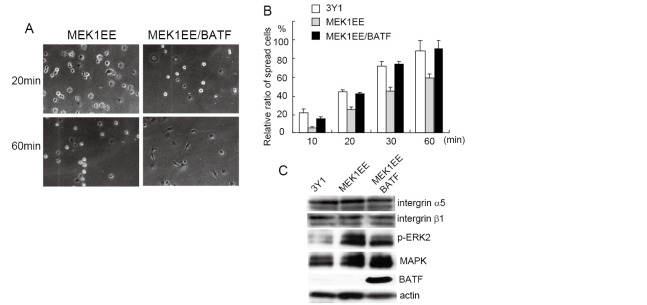
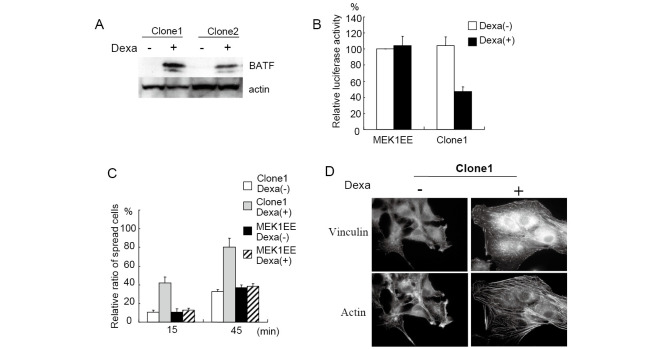
AP-1 is a dimeric transcription factor composed of the Jun and Fos class of proteins.9) Since the MEK/ERK pathway regulates diverse transcriptional factors including AP-1,10) we investigated the role of AP-1 in suppressing cell spreading. To inhibit the activity of AP-1, we used BATF,11) which is a nuclear basic leucine zipper protein consisting of 125 amino acids and is a member of the AP-1 family of transcription factors.8,12,13) BATF forms DNA-binding heterodimers with Jun proteins and negatively regulates AP-1-mediated transcription. We transfected the expression vector encoding BATF to MEK1EE cells and established cell lines that constitutively expressed BATF. MEK1EE and MEK1EE/BATF cells were trypsinized, plated onto fibronectin-coated dishes and observed at indicated time points. As shown in Figs. 2A and 2B, cell spreading of MEK1EE/BATF was clearly recovered to a level similar to that of 3Y1 cells, indicating that the inhibition of cell spreading by active MEK is mediated by the activation of AP-1. Although we examined expression of proteins known to regulate cell adhesion to the extracellular matrix, we did not observe any difference of expression (Fig. 2C). To further confirm the essential role of AP-1 activation for the inhibition of cell spreading, we established cell lines that could express BATF by dexamethasone treatment. Fig. 3A shows that established cell lines expressed BATF only in the presence of dexamethasone. AP-1 activity was clearly reduced by the induction of BATF (Fig. 3B). We then investigated cell spreading with dexamethasone treatment. Cells were treated with dexamethasone for 2 days and were subjected to a cell-spreading assay. As shown in Fig. 3C, cell spreading was recovered by the induction of BATF expression. The dexamethasone treatment had no effect on the cell spreading of MEK1EE cells. We also examined the formation of stress fibers upon the induction of BATF by dexamethasone. Cells were cultured on fibronectin-coated coverslips with or without dexamethasone for 2 days, and were immunostained for vilnculin and stress fibers. As shown in Fig. 3D, the formation of stress fibers and focal adhesions was clearly restored by the expression of BATF.
Fig. 2.
Cell spreading was restored by the expression of BATF. (A) MEK1EE and MEK1EE/BATF cells were trypsinized, suspended in serum free media and then plated onto the fibronectin-coated dishes. Photographs were taken at the indicated time points. (B) Spread and non-spread cells were counted, and percentages of the spread cells were indicated as a graph. Data are presented as averages of the results from three independent experiments. (C) Expression of indicated proteins in MEK1EE and MEK1EE/BATF cells was examined using Western blot.
Fig. 3.
Cell spreading was restored by the induced expression of BATF. (A) Cells were cultured in the presence or absence of 2 μM of dexamethasone for 2 days, and the expression of BATF was examined by Western blot. Dexa is dexamethasone. (B) Cells were cultured in the presence or absence of 2 μM of dexamethasone for 2 days and then subjected to luciferase assay as described in MATERIALS AND METHODS. Relative ratio of luciferase activity is indicated. (C) Cells were cultured with or without 2 μM of dexamethasone for 2 days and subjected to cell spreading assay. Spread and non-spread cells were counted and percentages of spread cells were indicated as a graph. Data are presented as the averages of the results from three independent experiments. (D) Cells were cultured on the fibronectin-coated glass coverslips with or without 2 μM of dexamethasone for 2 days and then immunostained for actin and vinculin.
DISCUSSION
In this report, we demonstrated that activation of the MEK/ERK pathway was sufficient to disrupt cell spreading, and that AP-1 activity was crucial for the MEK/ERK-mediated disruption of actin cytoskeleton and cell spreading, the organization of which is often disrupted in many transformed cells.1) The MEK/ERK pathway is often activated in transformed cells, and has been shown to play critical roles for the disruption of actin cytoskeleton.14) However, exactly how the MEK/ERK pathway regulated the organization of actin cytoskeleton remains uncertain. Activated ERK is known to localize to the site of newly formed focal adhesions, and regulates downstream signals to induce the organization of actin cytoskeleton.15) Activated ERK is also translocated to the nucleus to regulate the transcription of various genes that regulate cell motility and cytoskeletal dynamics. To determine whether AP-1-mediated transcription was required for the disruption of cell spreading by the activation of ERK, we suppressed AP-1 activity by the expression of BATF, a known AP-1 inhibitor.12,13) The expression of BATF clearly restored not only cell spreading but also stress fiber formation and the organization of focal adhesions of active MEK1-expressing cells. These results indicate that disruptions of cell spreading and actin cytoskeleton by the activation of ERK are mediated by AP-1 dependent transcription. It remains unknown which genes that are regulated by AP-1 are essential for the disruption of actin cytoskeleton and cell spreading. Since the expression of a large number of proteins is regulated by AP-1 activity, further studies will be needed to elucidate the AP-1 mediated disruptions of actin cytoskeleton and cell spreading.
ACKNOWLEDGEMENTS
We would like to thank the members of the Division of Cancer Biology for their valuable technical assistance and helpful discussions. This work was funded by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1).Pawlak G, Helfman DM. Cytoskeletal changes in cell transformation and tumorigenesis. Curr Opin Genet Dev, 2001; 11: 41–47. [DOI] [PubMed]
- 2).Button E, Shapland C, Lawson D. Actin, its associated proteins and metastasis. Cell Motil Cytoskeleton, 1995; 30: 247–251. [DOI] [PubMed]
- 3).Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell, 1998; 9: 1863–1871. [DOI] [PMC free article] [PubMed]
- 4).Plantefaber LC, Hynes RO. Changes in integrin receptors on oncogenically transformed cells. Cell, 1989; 56: 281–290. [DOI] [PubMed]
- 5).Liu Y, Hiraiwa Y, Liu E, Kurata H, Thant AA, Matsuda S, Hamaguchi M. Suppression of cell spreading by v-Crk requires Ras-MEK-MAP kinase signaling. Oncogene, 2001; 20: 5908–5912. [DOI] [PubMed]
- 6).Mayer BJ, Hamaguchi M, Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature, 1988; 332: 272–275. [DOI] [PubMed]
- 7).Chodniewicz D, Klemke RL. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta, 2004; 1692: 63–76. [DOI] [PubMed]
- 8).Senga T, Iwamoto T, Humphrey SE, Yokota T, Taparowsky EJ, Hamaguchi M. Stat3-dependent induction of BATF in M1 mouse myeloid leukemia cells. Oncogene, 2002; 21: 8186–8191. [DOI] [PubMed]
- 9).Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer, 2003; 3: 859–868. [DOI] [PubMed]
- 10).Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol, 1997; 9: 180–186. [DOI] [PubMed]
- 11).Hasegawa H, Senga T, Ito S, Iwamoto T, Hamaguchi M. A role for AP-1 in matrix metalloproteinase production and invadopodia formation of v-Crk-transformed cells. Exp Cell Res, 2009; 315: 1384–1392. [DOI] [PubMed]
- 12).Dorsey MJ, Tae HJ, Sollenberger KG, Mascarenhas NT, Johansen LM, Taparowsky EJ. B-ATF: a novel human bZIP protein that associates with members of the AP-1 transcription factor family. Oncogene, 1995; 11: 2255–2265. [PubMed]
- 13).Echlin DR, Tae HJ, Mitin N, Taparowsky EJ. B-ATF functions as a negative regulator of AP-1 mediated transcription and blocks cellular transformation by Ras and Fos. Oncogene, 2000; 19: 1752–1763. [DOI] [PubMed]
- 14).Pullikuth AK, Catling AD. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell Signal, 2007; 19: 1621–1632. [DOI] [PMC free article] [PubMed]
- 15).Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J, 2000; 19: 2911–2923. [DOI] [PMC free article] [PubMed]