Abstract
Background
Epidemiologic studies have shown decreasing vancomycin susceptibility among clinical Clostridioides difficile isolates, but the impact on patient outcomes is unknown. We hypothesized that reduced vancomycin susceptibility would be associated with decreased rates of sustained clinical response (SCR).
Methods
This multicenter cohort study included adults with C. difficile infection (CDI) treated with oral vancomycin between 2016 and 2021. Clostridioides difficile isolates underwent agar dilution vancomycin susceptibility testing, ribotyping, and Sanger sequencing of the vancomycin resistance vanR gene. Reduced susceptibility was defined as vancomycin minimum inhibitory concentration (MIC) >2 μg/mL. The primary outcome was 30-day SCR; secondary outcomes were 14-day initial cure, 30-day recurrence, and 30-day mortality. Exploratory analysis assessed the association between the VanR Thr115Ala polymorphism, susceptibility, and outcomes.
Results
A high proportion (34% [102/300]) of C. difficile isolates exhibited reduced vancomycin susceptibility (range, 0.5–16 μg/mL; MIC50/90 = 2/4 μg/mL). Ribotype 027 accounted for the highest proportion (77.4% [41/53]) of isolates with reduced vancomycin susceptibility. Overall, 83% (249) of patients achieved 30-day SCR. Reduced vancomycin susceptibility was associated with lower rates of 30-day SCR (76% [78/102]) than vancomycin-susceptible strains (86% [171/198]; P = .031). A significantly lower rate of 14-day initial cure was also observed among individuals infected with strains with reduced vancomycin susceptibility (89% vs 96%; P = .04). Reduced susceptibility remained an independent predictor of 30-day SCR in multivariable modeling (odds ratio, 0.52 [95% confidence interval, .28–.97]; P = .04).
Conclusions
Reduced vancomycin susceptibility in C. difficile was associated with decreased odds of 30-day SCR and lower 14-day initial cure rates in the studied patient cohort.
Keywords: vancomycin, Clostridioides difficile, antimicrobial resistance, anaerobes, susceptibility testing
A vancomycin minimum inhibitory concentration >2 μg/mL in Clostridioides difficile was associated with decreased odds of 30-day sustained clinical response and lower 14-day initial cure rates in a cohort of hospitalized patients with C. difficile infection.
Clostridioides difficile is the most common healthcare-acquired pathogen in the United States (US) and causes nearly 500 000 annual cases, leading the Centers for Disease Control and Prevention to list it as 1 of 5 urgent antimicrobial resistance threats [1–3]. Clostridioides difficile infection (CDI) is the leading cause of death due to gastroenteritis in the US and causes gastrointestinal symptoms ranging from diarrhea and abdominal pain to toxic megacolon, sepsis, and death [4]. Although metronidazole has long served as a mainstay of treatment, oral vancomycin and fidaxomicin recently became the only 2 antibiotics recommended as first-line therapy to treat CDI by clinical practice guidelines [5, 6]. Oral vancomycin is more commonly used than fidaxomicin due to its greater accessibility, with usage ranging between half and three-quarters of all cases of CDI [7–10]. The use of oral vancomycin has also recently increased by 54% following updated treatment recommendations published in 2018 [7]. However, vancomycin clinical cure rates have decreased from nearly 100% in the early 2000s to approximately 70% in contemporary clinical trials for reasons that are largely unknown; furthermore, even lower rates have been observed for infections caused by “hypervirulent” ribotype (RT) 027 [11–14]. In light of these findings, we hypothesize that increased vancomycin use may be contributing to reduced susceptibility to vancomycin in C. difficile, in addition to decreased clinical cure rates.
As CDI diagnosis is based on molecular testing, C. difficile antibiotic susceptibility testing is not part of the diagnostic workup in the clinical setting. However, several surveillance studies have noted an emergence of C. difficile with reduced susceptibility to vancomycin, defined as a minimum inhibitory concentration (MIC) >2 μg/mL [15–21]. This phenomenon has been most common in RT 027, including 18%–39% of isolates in early surveillance studies between 2011 and 2013 in the US [16, 18]. Several underlying resistance mechanisms have been proposed, the most well-described of which involves constitutive expression of the C. difficile vanG operon due to point mutations in its VanSR 2-component regulatory system [22, 23]. The expression of the vanG operon changes the vancomycin binding site from lipid-II molecules bearing D-alanine-D-alanine to D-alanine-D-serine, which is associated with a 7-fold decrease in vancomycin affinity [22]. A mutation involving Thr115Ala in the VanR regulator has been associated with increased vancomycin MICs and it is a common variant in RT 027, confounding investigations into its clinical implications [22, 24, 25]. Of RT 027 isolates, almost half (47.4%) carry the Ala-115 variation and have higher MIC50 and MIC90 values of 2 and 4 μg/mL, respectively, compared to RT 027 carrying the wild-type Thr-115 (MIC50 and MIC90 of 0.5 and 2 μg/mL, respectively) [24]. Whether the Thr115Ala polymorphism affects vancomycin susceptibility and clinical outcomes independent from other virulence properties of RT 027 strains is not known [14, 26, 27].
Oral vancomycin stool concentrations range from 500 to 2000 μg/mL and the clinical paradigm has therefore been that any moderate increase in vancomycin MIC should not appreciably impact patient outcomes [12, 28, 29]. However, several factors should challenge this paradigm, including that the luminal concentrations of vancomycin have not been measured, the likelihood these concentrations may vary along the length of the colon, and previous demonstrations that clinical isolates and laboratory-generated mutants exhibiting moderate MICs of 4–16 μg/mL, exhibit profoundly elevated minimum bactericidal concentrations (64 to >1000 μg/mL) [22, 27, 28, 30–33]. As vancomycin treatment results in CDI recurrence in up to one-fourth of cases, and more than 80% of recurrences following vancomycin treatment are caused by the same C. difficile strain, it seems plausible to consider whether reduced susceptibility to vancomycin plays a role in clinical outcomes [34–36].
The purpose of this study was to evaluate whether reduced vancomycin susceptibility in C. difficile could be associated with decreased rates of CDI sustained clinical response (SCR); thus, we focused our research on a geographical area where vancomycin resistance has been described [15]. An exploratory goal was to further assess whether clinically relevant resistance was associated with the Thr115Ala substitution in VanR.
METHODS
Study Population and Sample Collection
This was a secondary database analysis conducted on samples prospectively collected as a part of a multicenter, 14-hospital cohort study. The cohort in this analysis included adult patients admitted to 1 of 2 university-affiliated quaternary care hospitals or their respective associated community hospitals in the greater Houston area between 2016 and 2021. Leftover diagnostic stool samples were collected and brought to a centralized research laboratory at the University of Houston College of Pharmacy for additional microbiologic analysis. Patients were included in the study if they tested positive for CDI as part of routine clinical care, using either nucleic acid amplification testing or toxin testing, as dictated by local protocols, and were treated with vancomycin monotherapy, as defined below. Additionally, local protocols required liquid stool sample consistency for testing and no administration of laxatives within 48 hours of testing. Vancomycin monotherapy was defined as oral vancomycin therapy initiated within 48 hours of positive diagnostic test and continued through the end of treatment. Concomitant metronidazole was only allowed within the initial 48 hours following diagnosis. This study was approved by the Committee for the Protection of Research Subjects at the University of Houston (CPHS 000128) and associated hospitals.
Vancomycin Susceptibility Testing
Stool samples were enriched using brain-heart infusion broth (Criterion Media), then plated onto selective cefoxitin-cycloserine-fructose agar plates (Anaerobe Systems). Specimens were incubated under strict anaerobic conditions for 48–72 hours and then subcultured to obtain pure isolates. It is worth noting that vancomycin was not included in agars used to isolate C. difficile. Fluorescent ribotyping was performed as previously described [37].
Agar dilution antibiotic susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) methods for susceptibility testing of anaerobic bacteria [38]. In brief, a C. difficile inoculum of approximately 105 colony-forming units/mL was spotted onto Brucella agar plates (Criterion Media) that were supplemented with 5 µg/mL hemin (Sigma), 10 µg/mL vitamin K (Sigma), and 5% v/v defibrinated sheep blood (Northeast Laboratory Services). Agar contained doubling dilutions of vancomycin ranging from 0.25 to 16 μg/mL. Vancomycin hydrochloride powder (Sigma) was utilized in all experiments.
Susceptibility testing was repeated in duplicate and included the reference strain R20291 as a vancomycin-susceptible control strain (MIC = 1 μg/mL) [22]. MICs between laboratories must have been within 1 dilution of each other, and the higher of the 2 dilutions was recorded as the final MIC used for analysis. Reduced susceptibility to vancomycin was defined as an MIC >2 μg/mL based the CLSI epidemiological cutoff value (ECV) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) tentative epidemiological cutoff value ([T]ECOFF) [39, 40].
Sanger Sequencing of vanSR
Following lysozyme treatment, genomic DNA was isolated using the QIAamp DNA mini kit (Qiagen) and vanSR was amplified using primers P-forward-1: GCTTGTAATTATTTGATATG and P-reverse-1: ACAAGAGTGCTCTTTATTTATTTATC in a polymerase chain reaction (PCR) containing 25 ng of genomic DNA in a 50-μL reaction volume using Phusion high-fidelity PCR master mix (Thermo Fisher Scientific). PCR amplicons were sequenced, by Azenta Life Sciences, using P-forward-2: CGAAATTTATAAAAACTGTATGG, and P-forward-3: CAAATATATCACTTGAATACAG. The Thr115Ala polymorphism was identified in VanR following comparison with the wild-type sequence from R20291.
Clinical Outcomes
The primary study outcome, SCR, was a composite 30-day endpoint including (i) 14-day initial cure, (ii) absence of disease recurrence by day 30, and (iii) alive at day 30. All endpoints were assessed using day of positive diagnostic test as day 0. Initial cure was defined by the absence of abdominal pain and diarrhea by day 14. CDI recurrence was defined as presence of an additional positive stool test for C. difficile accompanied by compatible CDI symptoms and the restart of CDI-directed antibiotic therapy following 14-day initial cure. Patients must have discontinued vancomycin therapy for at least 48 hours prior to a second diagnostic test to be classified as CDI recurrence. Secondary endpoints included 14-day initial cure, 30-day CDI recurrence, and 30-day all-cause mortality. Planned post hoc analyses also included the association between Thr115Ala polymorphism with reduced vancomycin susceptibility and clinical outcomes.
Outcomes were determined by an infectious diseases–trained clinician blinded to vancomycin MIC based on review of electronic medical records (EMRs). To minimize potential bias, a subcohort of patients underwent independent assessment by a second clinician to validate outcomes determination. If the investigating clinicians were unable to determine initial cure or SCR following a thorough review of the EMR, the patient was assumed to have experienced a successful treatment outcome.
Statistical Analysis
Binary and categorical variables were compared using χ2 or Fisher exact tests and continuous variables were compared using Student t tests or Wilcoxon rank-sum tests. Multivariable regression modeling was performed for the primary, composite endpoint including variables yielding a P < .2 in univariate analyses. All variables with a P < .05 were included in the final model and defined as statistically significant. For subanalysis of the VanR Thr115Ala mutation, MIC values were assessed using Fisher exact tests in isolates with and without Thr115Ala and stratified according to strain type (RT 027 vs non-027). All statistical analyses were performed using SPSS version 29.0.0.0 (IBM Corporation, Armonk, New York) or R Studio version 4.1.0 (R: A Language and Environment for Statistical Computing, Vienna, Austria) software.
RESULTS
The study cohort consisted of 300 patients, with the majority being non-Hispanic White (56%) and female (56%) (Table 1). CDI diagnosis dates spanned across 6 years: 2016 (n = 6), 2017 (n = 58), 2018 (n = 95), 2019 (n = 86), 2020 (n = 48), and 2021 (n = 7). Almost half (145 [48%]) of patients had severe (n = 130) or fulminant (n = 15) CDI based on the Infectious Diseases Society of America/Society for Healthcare Epidemiology of America criteria [41]. Most patients exclusively received oral vancomycin therapy, except for 66 (22%) patients who received concomitant metronidazole within the first 48 hours of diagnosis. Patient and disease characteristics were similar between those who achieved 30-day SCR and those who did not. However, those individuals who failed to achieve a 30-day SCR had higher Charlson Comorbidity Index (CCI) scores (P = .02) and a higher incidence of RT 027 infection (P < .001).
Table 1.
Characteristics of Study Cohort Stratified by 30-Day Sustained Clinical Response
| Characteristic | 30-Day SCR | P Value | |
|---|---|---|---|
| Yes (n = 249) |
No (n = 51) |
||
| Demographics | |||
| Age >65 y | 141 (57) | 33 (65) | .29 |
| Female sex | 140 (56) | 29 (57) | .93 |
| Race/Ethnicity | |||
| White, non-Hispanic | 138 (55) | 29 (57) | .83 |
| Black, non-Hispanic | 49 (20) | 12 (24) | |
| Hispanic | 34 (14) | 6 (12) | |
| Others/not reported | 28 (11) | 4 (8) | |
| CCI score, mean (SD) | 6.3 (3.5) | 7.6 (3.4) | .02 |
| CDI characteristics | |||
| PCR diagnosis | 232 (93) | 44 (86) | .10 |
| CDI classification, HO-CDIa | 111 (45) | 27 (53) | .28 |
| CDI disease severitya | |||
| Nonsevere | 132 (53) | 23 (45) | .30 |
| Severe/fulminant | 117 (47) | 28 (55) | |
| Initial episode | 180 (72) | 37 (73) | .97 |
| Prior treatment with oral vancomycinb | 27 (11) | 6 (12) | .85 |
| Clostridioides difficile strain typec | |||
| RT 027 | 37 (16) | 16 (34) | <.01 |
| RT 014-020 | 39 (16) | 6 (13) | .48 |
| RT 106 | 22 (9) | 9 (19) | .06 |
| RT 002 | 21 (9) | 3 (6) | .54 |
| CDI treatment | |||
| Concomitant initial metronidazole (<48 h) | 53 (21) | 13 (26) | .51 |
| Days of oral vancomycin therapy, mean (SD) | 16.6 (14.4) | 15.0 (12.2) | .46 |
| Vancomycin standard dosingd | 185 (74) | 37 (73) | .80 |
Data are presented as No. (%) unless otherwise indicated. Bold values indicate a P value <.05.
Abbreviations: CCI, Charlson Comorbidity Index; CDI, Clostridioides difficile infection; HO-CDI, healthcare facility–onset Clostridioides difficile infection; PCR, polymerase chain reaction; RT, ribotype; SCR, sustained clinical response; SD, standard deviation.
aAs defined per the 2017 Infectious Diseases Society of America/Society for Healthcare Epidemiology of America clinical guideline.
bAny receipt in the 90 days prior to current CDI diagnosis.
cPresented as percentage of 238 (30-day SCR) or 47 (no 30-day SCR) isolates with ribotyping complete.
d125 mg administered 4 times daily.
Isolates included demonstrated vancomycin MICs ranging from 0.5 to 16 μg/mL, with MIC50 and MIC90 values of 2 μg/mL and 4 μg/mL, respectively. Overall, 34% (102/300) of the cohort was infected with strains showing reduced vancomycin susceptibility. None of the 102 isolates with reduced susceptibility displayed high-level resistance of MICs ≥32 μg/mL, according to the CLSI ECV and EUCAST (T)ECOFF [39, 40]. The distribution of MICs did not exhibit appreciable changes across the years of CDI diagnosis. Ribotype results were attained for 285 (95%) isolates, of which 53 (19%) were RT 027, 45 (16%) were RT 014-020, and 31 (11%) were RT 106. RT 027 demonstrated the highest proportion of reduced vancomycin susceptibility (77% [41/53]) and comprised 40% (41/102) of all isolates with an MIC of >2 μg/mL. Severe/fulminant disease was also significantly more common among patients with RT 027 infection (62% [33/53]) compared to non-RT 027 infection (45% [112/247]; P = .03).
Clinical Outcomes
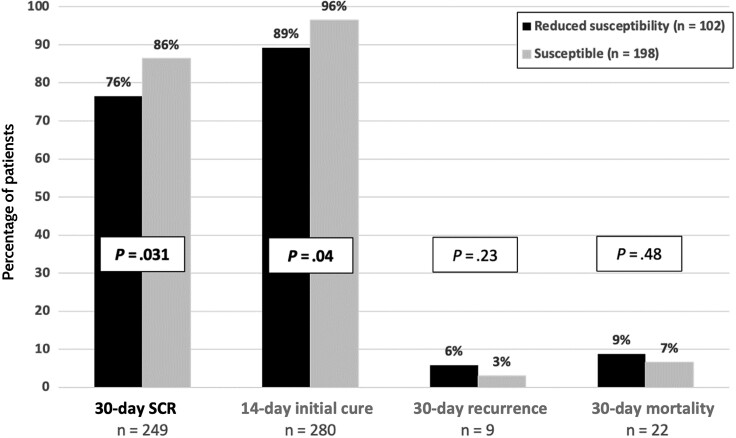
Within the entire cohort, 249 (83%) patients met the primary endpoint of 30-day SCR (Figure 1). Of the 51 patients who did not achieve SCR, 20 (39%) had continued diarrhea on day 14, 9 (18%) had disease recurrence within 30 days, and 22 (43%) experienced mortality within 30 days of CDI diagnosis; 17 of 22 deaths occurred within 12 days of CDI diagnosis. Overall, patients infected with C. difficile exhibiting reduced susceptibility to vancomycin had lower rates of 30-day SCR (76% [78/102]) than those infected with vancomycin-susceptible strains (86% [171/198]; P = .031). The proportion of patients experiencing 14-day initial cure was also significantly lower among those infected with strains with reduced susceptibility (P = .04). Notably, infection with RT 027 also demonstrated lower 30-day SCR rates (70% [37/53]) compared to infection with non-027 ribotypes (86% [212/247]; P = .005).
Figure 1.
Clinical outcomes stratified by vancomycin susceptibility. Reduced susceptibility defined as vancomycin minimum inhibitory concentration >2 μg/mL. Abbreviation: SCR, sustained clinical response.
Eight variables were tested in univariate analyses as outcome predictors (Table 2). CCI, RT 027, Thr115Ala polymorphism, and vancomycin MIC >2 μg/mL were associated with decreased odds of 30-day SCR (P < .2), while only vancomycin MIC >2 μg/mL was associated with decreased 14-day initial cure and RT 027 was associated with increased 30-day recurrence. CCI score was associated with increased odds of 30-day mortality; notably, neither concomitant initial metronidazole use nor severe/fulminant disease demonstrated a correlation with mortality. Two variables (CCI score and vancomycin >2 μg/mL) were included in multivariable modeling for our composite 30-day SCR endpoint. Both CCI score (odds ratio [OR], 0.90 [95% confidence interval {CI}: .82–.98]; P = .021) and MIC >2 μg/mL (OR, 0.52 [95% CI: .28–.97]; P = .04) remained independent predictors of 30-day SCR.
Table 2.
Univariate Analyses for Predictors of Composite and Secondary Outcomes
| Covariate | 30-Day SCR | 14-Day Initial Cure | 30-Day Recurrence | 30-Day Mortality | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age >65 y | 0.71 (.38–1.33) | .29 | 0.73 (.28–1.89) | .51 | 3.78 (.81–17.56) | .09 | 0.71 (.30–1.68) | .43 |
| CCI score | 0.90 (.83–.98) | .02 | 0.97 (.85–1.11) | .66 | 1.04 (.88–1.23) | .59 | 1.20 (1.06–1.35) | .005 |
| Concomitant initial metronidazole (<48 h) | 0.79 (.39–1.59) | .51 | 0.64 (.23–1.73) | .38 | 1.19 (.31–4.53) | .80 | 0.77 (.25–2.37) | .65 |
| HO-CDI | 0.72 (.39–1.31) | .28 | 0.68 (.27–1.69) | .41 | 1.68 (.52–5.41) | .39 | 1.45 (.61–3.46) | .41 |
| RT 027 | 0.38 (.19–.76) | .006 | 0.62 (.22–1.79) | .38 | 3.57 (1.09–11.7) | .04 | 1.84 (.69–4.96) | .23 |
| Severe/fulminant disease | 0.73 (.40–1.33) | .30 | 0.93 (.38–2.31) | .88 | 1.07 (.34–3.40) | .91 | 1.96 (.79–4.83) | .14 |
| Initial episode | 0.99 (.50–1.94) | .97 | 1.13 (.42–3.05) | .81 | 0.76 (.22–2.58) | .66 | 1.33 (.47–3.72) | .59 |
| Vancomycin MIC >2 μg/mL | 0.51 (.28–.95) | .03 | 0.39 (.16–.98) | .046 | 2.0 (.63–6.37) | .24 | 1.38 (.57–3.34) | .48 |
| Thr115Ala mutation | 0.36 (.18–.75) | .006 | 0.49 (.17– 1.41) | .18 | 3.1 (.89–10.78) | .08 | 2.84 (1.09–7.36) | .03 |
Bolded values represent P < .05.
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; HO-CDI, healthcare facility–onset Clostridioides difficile infection; OR, odds ratio; RT, ribotype; SCR, sustained clinical response. Bolded values represent P < .05.
Analysis of Role of Thr115Ala Substitution
An exploratory goal was to assess the association between the Thr115Ala polymorphism and vancomycin susceptibility. The Thr115Ala substitution was present in 14.7% (44/300) of strains overall, including 42% (43/102) of isolates with MIC >2 μg/mL compared to 0.5% (1/198) of isolates with MIC ≤2 μg/mL (P ≤ .001). Thr115Ala was also more prevalent in RT 027 strains (70% [37/53] of RT 027 vs 3% [7/247] of non-027 strains; P < .001), which also more often exhibited higher vancomycin MICs. However, the Thr115Ala polymorphism was notably more common among isolates with MIC >2 μg/mL, even within RT 027 strains (Supplementary Table 1).
Overall, we observed a significant difference in 30-day SCR based on presence (68% [30/44]) or absence (85% [219/256]) of SNP Thr115Ala mutation (P = .005). Given its high co-occurrence with RT 027, we conducted an additional analysis to explore a potential independent impact of Thr115Ala. However, after stratifying outcomes by RT 027, Thr115Ala was not predictive of 30-day SCR in either RT 027 or non-RT 027 subgroups (RT 027: 68% [25/37] with Thr115Ala vs 75% [12/16] in those without [P = .59]; non-RT 027: 71% [5/7] with Thr115Ala vs 86% [207/240] in those without [P = .27]).
DISCUSSION
Despite the increasing prevalence of strains showing reduced susceptibility to vancomycin, there is a significant lack of understanding regarding whether these strains affect the clinical response to vancomycin therapy. As these strains exhibit MICs of 4–16 μg/mL, the prevailing view is that they are unlikely to impact clinical outcomes, given the high concentrations of vancomycin in stools. To investigate the clinical relevance of these strains, we initially hypothesized that they may potentially survive better in vancomycin and may influence recurrence. A similar hypothesis has been proposed following observations that C. difficile strains with reduced susceptibility were associated with poorer vancomycin treatment outcomes in a mouse model [27]. Therefore, we used CLSI-recommended susceptibility testing methods to analyze the susceptibility of isolates in a cohort of 300 patients with CDI who were treated with vancomycin monotherapy. We used a composite, 30-day SCR endpoint to assess multiple potential impacts of elevated vancomycin MICs. This revealed that 30-day SCR and 14-day clinical cure were both negatively impacted by reduced susceptibility to vancomycin. However, reduced vancomycin susceptibility did not correlate with decreased rates of 30-day recurrence. The decrease in 30-day SCR observed was driven by a decrease in the 14-day initial cure, of which a vancomycin MIC >2 μg/mL was the only predictor, implicating that true antibiotic failure was the major consequence of reduced vancomycin susceptibility.
In addition to correlating phenotypic reduced susceptibility with outcomes, we also sought to explore whether the VanR Thr115Ala polymorphism influences outcomes. The occurrence of Thr115Ala, especially in the RT027 population, tends to be associated with higher vancomycin MICs, although the mutation is also found in vancomycin-susceptible strains [24]. Thr115Ala requires further research to explore its use as an indicator of reduced susceptibility to vancomycin, at least in RT 027 [22, 25]. In our study, Thr115Ala was an independent predictor of 30-day SCR overall; however, we were underpowered to detect a difference once outcomes were stratified by RT027 due to the high occurrence of Thr115Ala in this ribotype [22, 25]. This suggests that Thr115Ala did not appear to impact outcomes independent of RT 027. Additionally, 42% of strains with reduced vancomycin susceptibility carried this variation, indicating that it is not the main or sole mechanism responsible for elevated vancomycin MICs. However, a limitation of our analysis is that sequence analysis of vanR is not a direct surrogate for vanG expression. Thus, it is possible that Thr115Ala may affect vanG expression in a strain-specific manner or that alternate mechanisms of vanG regulation occur across strains. Therefore, the role of Thr115Ala may be multifactorial and co-dependent on there being sufficient expression of vanG and the synthesis of the lipid-II D-Ala-D-Ser motif; the latter factor is also critical in vancomycin-resistant enterococci that produce a lipid-II D-Ala-D-Lac motif [22, 42]. Further genetic studies will be required to determine correlation between mutations in VanSR, their impact on both vanG expression and D-Ala-D-Ser production, and their potential clinical significance.
Our study design and findings are strengthened by several factors. First, we rigorously determined vancomycin MICs in 2 independent research laboratories using validated and CLSI-recommended agar dilution methods. Additionally, our patient cohort represented a diverse array of patients and characteristics included from 14 different hospitals, including academic and community settings. All patients received vancomycin therapy and there was no difference in the mean days of therapy between those achieving and not achieving 30-day SCR, minimizing potential treatment confounding and/or selection bias. The majority of our cohort (72%) was also experiencing an initial episode at study inclusion. As recurrent CDI episodes often have lower cure rates than initial infection, regardless of antibiotic used, due to more severe or continued microbiome disruptions, host immunocompromise, and/or other nonantibiotic factors, our cohort likely allowed for a more direct analysis of vancomycin efficacy [43, 44]. Indeed, CDI episode categorization as initial versus first or more recurrence was not associated with 30-day SCR in our cohort, indicating this was a minimally confounding characteristic. Last, a clinician blinded to vancomycin MIC collected clinical outcomes, which were standardized and defined according to those used in contemporary clinical trials [35, 36, 45, 46].
Our study has certain limitations. First, we conducted our study in a region with a high prevalence of vancomycin-resistant C. difficile that has not yet been identified elsewhere [15, 47]. Therefore, our findings might be representative of a local resistance mechanism, which requires genetic elucidation, and may not extrapolate to other regions. Nonetheless, our MIC50 and MIC90 values were identical to those reported in national surveillance of US isolates between 2011 and 2012 [16]. Clearly, more studies will be needed to validate and expand upon our findings. Similarly, as C. difficile susceptibilities are not conducted in the clinical setting and there are no clinical breakpoints representing vancomycin resistance, we used an ECV/(T)ECOFF to define reduced susceptibility [39, 40]. Although we found an association between this ECV/(T)ECOFF and outcomes, it is plausible there may be an MIC threshold that is a better predictor. Continued outcomes studies are warranted to define this threshold as our cohort only contained isolates with a relatively low upper range of MICs (16 μg/mL) compared to those reported particularly in other regions outside the US (MICs 32–256 μg/mL) (Supplementary Figure 1) [15, 20]. Additionally, the vast majority (92%) of our cohort was diagnosed using a toxin PCR assay alone. Although we used a stringent CDI definition for study entry, use of the toxin PCR is associated with detection of C. difficile colonization and it is possible some of patients may not have had true CDI [48]. However, PCR diagnosis has been and continues to serve as the most common diagnostic method in the US for CDI; thus, our cohort may be an accurate representation of patients being treated for CDI in many institutions [3, 10]. Indeed our 14-day initial cure rate was high (93%) compared to clinical trials, which may be due in part to inclusion of colonized patients, but also likely reflects the challenge and limitation of determining CDI outcomes through retrospective chart review [49].
To our knowledge, this is the first study demonstrating an impact of elevated vancomycin MICs on clinical outcomes with respect to CDI. These findings, paired with reports of clinical failure in strains with metronidazole MICs ≥1 μg/mL and the recent emergence of fidaxomicin resistance, argue for increased C. difficile susceptibility testing in the clinical setting, which would represent a paradigm shift [50, 51]. Although the underlying resistance mechanism(s) remain unclear, surveillance efforts and outcomes studies should be paramount, given the paucity of anti–C. difficile antibiotics available to treat this persistent and challenging infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Taryn A Eubank, Department of Pharmacy Practice and Translational Research, University of Houston College of Pharmacy.
Chetna Dureja, Center of Infectious and Inflammatory Diseases, Institute of Biosciences and Technology, Texas A&M Health Science Center, Houston, Texas.
Kevin W Garey, Department of Pharmacy Practice and Translational Research, University of Houston College of Pharmacy.
Julian G Hurdle, Center of Infectious and Inflammatory Diseases, Institute of Biosciences and Technology, Texas A&M Health Science Center, Houston, Texas.
Anne J Gonzales-Luna, Department of Pharmacy Practice and Translational Research, University of Houston College of Pharmacy.
Notes
Author Contributions. T. A. E., A. J. G.-L., K. W. G., C. D., and J. G. H. conceptualized and designed the study. T. A. E. and C. D. performed microbiological and molecular data analyses. T. A. E. collected clinical data. T. A. E. and K. W. G. performed statistical analyses. T. A. E. and C. D. interpreted data and wrote the manuscript. T. A. E. and K. W. G. have full access to all the data in the study and verified the underlying data. All authors had final responsibility for the decision to submit for publication. All authors read and approved the final version of the manuscript.
Patient consent. This study was approved by the Committee for the Protection of Research Subjects at the University of Houston (CPHS 000128) and does not include factors necessitating patient consent. This study conforms to standards currently applied in the United States.
Disclaimer. The funders had no role in the study design, data collection, interpretation of the findings, or writing and submission of the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant numbers 2R01AI139261 and T32 A1141349); the American College of Clinical Pharmacy Foundation Junior Investigator Research Award (G0507743); and the Society of Infectious Diseases Pharmacists Early Career Research Award (G0507861).
References
- 1. Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 2018; 379:1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC) . Antibiotic resistance threats in the United States, 2019. Atlanta, GA: CDC, 2019.
- 3. Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382:1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 2012; 55:216–23. [DOI] [PubMed] [Google Scholar]
- 5. Johnson S, Lavergne V, Skinner AM, et al. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73:755–7. [DOI] [PubMed] [Google Scholar]
- 6. Kelly CR, Fischer M, Allegretti JR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol 2021; 116:1124–47. [DOI] [PubMed] [Google Scholar]
- 7. Clancy CJ, Buehrle D, Vu M, Wagener MM, Nguyen MH. Impact of revised Infectious Diseases Society of America and Society for Healthcare Epidemiology of America clinical practice guidelines on the treatment of Clostridium difficile infections in the United States. Clin Infect Dis 2021; 72:1944–9. [DOI] [PubMed] [Google Scholar]
- 8. Crowell KT, Julian KG, Katzman M, et al. Compliance with Clostridium difficile treatment guidelines: effect on patient outcomes. Epidemiol Infect 2017; 145:2185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feuerstadt P, Boules M, Stong L, et al. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: a real-world data analysis. SAGE Open Med 2021; 9:2050312120986733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention (CDC) . 2021 annual report for the Emerging Infections Program for Clostridioides difficile infection. Atlanta, GA: CDC, 2021.
- 11. Zar FA, Bakkanagari SR, Moorthi KMLST, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–7. [DOI] [PubMed] [Google Scholar]
- 12. Louie T, Nord CE, Talbot GH, et al. Multicenter, double-blind, randomized, phase 2 study evaluating the novel antibiotic cadazolid in patients with Clostridium difficile infection. Antimicrob Agents Chemother 2015; 59:6266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vickers RJ, Tillotson GS, Nathan R, et al. Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: a phase 2, randomised, double-blind, active-controlled, non-inferiority study. Lancet Infect Dis 2017; 17:735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petrella LA, Sambol SP, Cheknis A, et al. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin Infect Dis 2012; 55:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Darkoh C, Keita K, Odo C, et al. Emergence of clinical Clostridioides difficile isolates with decreased susceptibility to vancomycin. Clin Infect Dis 2022; 74:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Snydman DR, McDermott LA, Jacobus NV, et al. U.S.-based national sentinel surveillance study for the epidemiology of Clostridium difficile–associated diarrheal isolates and their susceptibility to fidaxomicin. Antimicrob Agents Chemother 2015; 59:6437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Snydman DR, McDermott LA, Thorpe CM, et al. Antimicrobial susceptibility and ribotypes of Clostridium difficile isolates from a phase 2 clinical trial of ridinilazole (SMT19969) and vancomycin. J Antimicrob Chemother 2018; 73:2078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tickler IA, Goering RV, Whitmore JD, et al. Strain types and antimicrobial resistance patterns of Clostridium difficile isolates from the United States, 2011 to 2013. Antimicrob Agents Chemother 2014; 58:4214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Putsathit P, Hong S, George N, et al. Antimicrobial resistance surveillance of Clostridioides difficile in Australia, 2015–18. J Antimicrob Chemother 2021; 76:1815–21. [DOI] [PubMed] [Google Scholar]
- 20. Baghani A, Mesdaghinia A, Kuijper EJ, Aliramezani A, Talebi M, Douraghi M. High prevalence of Clostridioides difficile PCR ribotypes 001 and 126 in Iran. Sci Rep 2020; 10:4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adler A, Miller-Roll T, Bradenstein R, et al. A national survey of the molecular epidemiology of Clostridium difficile in Israel: the dissemination of the ribotype 027 strain with reduced susceptibility to vancomycin and metronidazole. Diagn Microbiol Infect Dis 2015; 83:21–4. [DOI] [PubMed] [Google Scholar]
- 22. Shen WJ, Deshpande A, Hevener KE, et al. Constitutive expression of the cryptic vanGCd operon promotes vancomycin resistance in Clostridioides difficile clinical isolates. J Antimicrob Chemother 2020; 75:859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eubank TA, Gonzales-Luna AJ, Hurdle JG, Garey KW. Genetic mechanisms of vancomycin resistance in Clostridioides difficile: a systematic review. Antibiotics (Basel) 2022; 11:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gargis AS, Karlsson M, Paulick AL, et al. Reference susceptibility testing and genomic surveillance of Clostridioides difficile, United States, 2012–17. Clin Infect Dis 2022; 75:1677–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wickramage I, Peng Z, Chakraborty S, et al. The vanR Cd mutation 343A > G, resulting in a Thr115Ala substitution, is associated with an elevated minimum inhibitory concentration (MIC) of vancomycin in Clostridioides difficile clinical isolates from Florida. Microbiol Spectr 2023; 11:e0377722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldstein EJ, Citron DM, Sears P, Babakhani F, Sambol SP, Gerding DN. Comparative susceptibilities to fidaxomicin (OPT-80) of isolates collected at baseline, recurrence, and failure from patients in two phase III trials of fidaxomicin against Clostridium difficile infection. Antimicrob Agents Chemother 2011; 55:5194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pu M, Cho JM, Cunningham SA, et al. Plasmid acquisition alters vancomycin susceptibility in Clostridioides difficile. Gastroenterology 2021; 160:941–5 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonzales M, Pepin J, Frost EH, et al. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect Dis 2010; 10:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garey KW, Begum K, Lancaster C, et al. A randomized, double-blind, placebo-controlled, single and multiple ascending dose phase 1 study to determine the safety, pharmacokinetics and food and faecal microbiome effects of ibezapolstat administered orally to healthy subjects. J Antimicrob Chemother 2020; 75:3635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jarrad AM, Blaskovich MAT, Prasetyoputri A, Karoli T, Hansford KA, Cooper MA. Detection and investigation of eagle effect resistance to vancomycin in Clostridium difficile with an ATP-bioluminescence assay. Front Microbiol 2018; 9:1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corbett D, Wise A, Birchall S, et al. In vitro susceptibility of Clostridium difficile to SMT19969 and comparators, as well as the killing kinetics and post-antibiotic effects of SMT19969 and comparators against C. difficile. J Antimicrob Chemother 2015; 70:1751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Odenholt I, Walder M, Wullt M. Pharmacodynamic studies of vancomycin, metronidazole and fusidic acid against Clostridium difficile. Chemotherapy 2007; 53:267–74. [DOI] [PubMed] [Google Scholar]
- 33. Dureja C, Olaitan AO, Hurdle JG. Mechanisms and impact of antimicrobial resistance in Clostridioides difficile. Curr Opin Microbiol 2022; 66:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Figueroa I, Johnson S, Sambol SP, Goldstein EJ, Citron DM, Gerding DN. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Infect Dis 2012; 55(Suppl 2):S104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. [DOI] [PubMed] [Google Scholar]
- 36. Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12:281–9. [DOI] [PubMed] [Google Scholar]
- 37. Alam MJ, Anu A, Walk ST, Garey KW. Investigation of potentially pathogenic Clostridium difficile contamination in household environs. Anaerobe 2014; 27:31–3. [DOI] [PubMed] [Google Scholar]
- 38. Clinical and Laboratory Standards Institute (CLSI) . Methods for antimicrobial susceptibility testing of anaerobic bacteria. 9th ed. CLSI M11. Wayne, PA: CLSI, 2018. [Google Scholar]
- 39. European Committee on Antimicrobial Susceptibility Testing . Data from the EUCAST MIC distribution website. Available at: http://www.eucast.org. Accessed 30 March 2023.
- 40. Clinical and Laboratory Standards Institute (CLSI) . Performance standards for antimicrobial susceptibility testing. 33rd ed. CLSI supplement M100. Wayne, PA: CLSI, 2023. [Google Scholar]
- 41. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Arthur M, Depardieu F, Reynolds P, Courvalin P. Quantitative analysis of the metabolism of soluble cytoplasmic peptidoglycan precursors of glycopeptide-resistant enterococci. Mol Microbiol 1996; 21:33–44. [DOI] [PubMed] [Google Scholar]
- 43. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002; 97:1769–75. [DOI] [PubMed] [Google Scholar]
- 44. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect 2012; 18(Suppl 6):21–7. [DOI] [PubMed] [Google Scholar]
- 45. Mikamo H, Tateda K, Yanagihara K, et al. Efficacy and safety of fidaxomicin for the treatment of Clostridioides (Clostridium) difficile infection in a randomized, double-blind, comparative phase III study in Japan. J Infect Chemother 2018; 24:744–52. [DOI] [PubMed] [Google Scholar]
- 46. Guery B, Menichetti F, Anttila VJ, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis 2018; 18:296–307. [DOI] [PubMed] [Google Scholar]
- 47. Greentree DH, Rice LB, Donskey CJ. Houston, we have a problem: reports of Clostridioides difficile isolates with reduced vancomycin susceptibility. Clin Infect Dis 2022; 75:1661–4. [DOI] [PubMed] [Google Scholar]
- 48. Koo HL, Van JN, Zhao M, et al. Real-time polymerase chain reaction detection of asymptomatic Clostridium difficile colonization and rising C. difficile–associated disease rates. Infect Control Hosp Epidemiol 2014; 35:667–73. [DOI] [PubMed] [Google Scholar]
- 49. Carlson TJ, Gonzales-Luna AJ. Utilizing antibiotics to prevent Clostridioides difficile infection: does exposure to a risk factor decrease risk? A systematic review. J Antimicrob Chemother 2020; 75:2735–42. [DOI] [PubMed] [Google Scholar]
- 50. Gonzales-Luna AJ, Olaitan AO, Shen WJ, et al. Reduced susceptibility to metronidazole is associated with initial clinical failure in Clostridioides difficile infection. Open Forum Infect Dis 2021; 8:ofab365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marchandin H, Anjou C, Poulen G, et al. In vivo emergence of a still uncommon resistance to fidaxomicin in the urgent antimicrobial resistance threat Clostridioides difficile. J Antimicrob Chemother 2023; 78:1992–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.