Abstract
Background
Stratification to categorize patients with Staphylococcus aureus bacteremia (SAB) as low or high risk for metastatic infection may direct diagnostic evaluation and enable personalized management. We investigated the frequency of metastatic infections in low-risk SAB patients, their clinical relevance, and whether omission of routine imaging is associated with worse outcomes.
Methods
We performed a retrospective cohort study at 7 Dutch hospitals among adult patients with low-risk SAB, defined as hospital-acquired infection without treatment delay, absence of prosthetic material, short duration of bacteremia, and rapid defervescence. Primary outcome was the proportion of patients whose treatment plan changed due to detected metastatic infections, as evaluated by both actual therapy administered and by linking a adjudicated diagnosis to guideline-recommended treatment. Secondary outcomes were 90-day relapse-free survival and factors associated with the performance of diagnostic imaging.
Results
Of 377 patients included, 298 (79%) underwent diagnostic imaging. In 15 of these 298 patients (5.0%), imaging findings during patient admission had been interpreted as metastatic infections that should extend treatment. Using the final adjudicated diagnosis, 4 patients (1.3%) had clinically relevant metastatic infection. In a multilevel multivariable logistic regression analysis, 90-day relapse-free survival was similar between patients without imaging and those who underwent imaging (81.0% versus 83.6%; adjusted odds ratio, 0.749; 95% confidence interval, .373–1.504).
Conclusions
Our study advocates risk stratification for the management of SAB patients. Prerequisites are follow-up blood cultures, bedside infectious diseases consultation, and a critical review of disease evolution. Using this approach, routine imaging could be omitted in low-risk patients.
Keywords: Staphylococcus aureus, bacteremia, low risk, diagnostics, risk stratification
This retrospective multicenter cohort study supports a risk-informed approach for management of Staphylococcus aureus bacteremia (SAB) patients. In low-risk SAB patients, routine imaging could be omitted as its yield is very low and not associated with lower 90-day relapse-free survival.
Traditionally, Staphylococcus aureus bacteremia (SAB) is divided into uncomplicated and complicated disease [1, 2]. This dichotomous classification insufficiently reflects the heterogeneity of SAB and thus prevents a more precise clinical diagnosis that, in certain patients, would allow for more personalized treatment [3, 4]. Moreover, risk factors for metastatic infection and confirmed metastatic infection have traditionally been considered equivalent in this classification [5, 6]. The disadvantage of this classification is that it is not conducive to the detection of metastatic infections, whereas adequate control of these infectious foci is associated with lower mortality [7].
Recently, a risk stratification model for categorizing SAB patients as “high risk” or “low risk” for metastatic infection has been proposed to allow for a more personalized diagnostic workup and avoid unnecessary diagnostic imaging [8]. Risk factors relate to host factors, features of bacteremia, and clinical course [8]. In this 2-step system, high-risk SAB patients should undergo a more extensive diagnostic workup to identify or exclude infective endocarditis (IE) and other metastatic infections; in low-risk patients, diagnostic imaging can remain limited.
Although distinct patient groups are at increased risk of metastatic infections and risk-based imaging is used in daily practice [3, 4], studies to determine which patients require specific investigations are limited. Most of these studies focused on the need for transesophageal echocardiography (TEE) in IE, usually as a follow-up examination after transthoracic echocardiography (TTE). Two studies examined clinical risk factors for a complicated course, including metastatic infection, and found that 10%–20% of low-risk patients still had a complicated course [1, 9]. However, neither study had the primary objective of examining the safety of omitting diagnostic imaging in low-risk patients. Moreover, the usefulness of these studies in guiding imaging studies is complicated by the fact that definitions of complicated disease included both risk factors, metastatic disease, and poor clinical outcome. Therefore, in a large multicenter study, we investigated how often metastatic infections are found in low-risk SAB patients, what their clinical relevance is, and whether omitting routine imaging is associated with worse outcomes.
METHODS
Study Design
We performed a retrospective multicenter cohort study at 7 hospitals in the Netherlands. We obtained approval from the research ethics boards of all hospital sites.
All adults with low-risk SAB [8] (see definition below) between January 2013 and December 2022 were eligible for inclusion. Supplementary Table A provides exact time periods per hospital.
Exclusion criteria were aged <18 years, death or deemed suitable for palliative care ≤72 hours after the index blood culture, contaminated or polymicrobial blood cultures, or immediate transfer to another hospital. If follow-up blood cultures were missing, we considered them to be negative. Blood cultures were considered contaminated when S. aureus grew in only 1 blood culture bottle, the cultures were documented as contaminated by the treating physician or clinical microbiologist, no antibiotic treatment was initiated, and no relapse of S. aureus bacteremia occurred. Follow-up was 90 days after discontinuation of antibiotic treatment to determine infection-related mortality [10], all-cause mortality, and relapse. Infection-related mortality was assessed as previously described [10].
Low-Risk SAB
Low-risk SAB was defined as 1 or more blood cultures positive for S. aureus in the absence of all of the following risk factors: community acquisition [11], implanted prosthetic material (vascular and intracardiac prostheses [except for coronary artery stents], prosthetic joints, and osteosynthesis material), failure to remove central venous catheter (CVC), positive blood cultures more than 48 hours after initiation of antibiotics with in vitro susceptibility to the isolated S. aureus (appropriate treatment), fever (≥38°C) more than 72 hours after initiation of appropriate antibiotic treatment, treatment delay (signs of infection for more than 48 hours before initiation of appropriate antibiotic treatment), and clinical signs of metastatic infection (Supplementary Table B) [8].
SAB Management
The Dutch national guideline provides recommendations on diagnosis and treatment of SAB patients. Clinical microbiologists are required to report all S. aureus blood culture results to the treating physician promptly, ideally within 4 hours. Follow-up blood cultures are recommended every 48 hours until negative. Bedside consultation by an infectious diseases (ID) physician should be standard care for all patients with SAB. The guideline recommends a TTE in all patients. Patients with uncomplicated SAB should be treated with intravenous (IV) antibiotics for 2 weeks after initial negative blood cultures. We considered 14 ± 4 days of antibiotics appropriate, given the variation in prescription practices between hospitals. In patients with metastatic infection, antibiotic treatment for 4–6 weeks, depending on focus of infection, is required.
Data Collection
We manually retrieved data on comorbidity [12], laboratory results, microbiology, imaging, and antibiotic treatment from electronic medical records. All diagnostic imaging modalities except chest X ray were included from 1 day prior to the first positive blood culture until discontinuation of antibiotics or hospital discharge, whichever occurred first. We included diagnostic modalities performed to search for metastatic infection as well as for other clinical indications.
Outcome Measures
The primary outcome was the proportion of low-risk SAB patients whose treatment plan changed due to detection of clinically relevant metastatic infections by additional imaging. All metastatic infections were considered clinically relevant, except right-sided IE or pulmonary septic emboli because these can be treated with short-term (2 weeks) treatment [13, 14].
This outcome was determined twice: first, by including how the clinician had acted based on imaging studies, if performed (ie, actual therapy given), and second, by linking a final adjudicated diagnosis to the treatment duration recommended in the guideline in order to correct for under- and overtreatment. This final adjudicated diagnosis was established retrospectively by 2 ID physicians (I. K., J. t O.) and an ID physician-in-training (M. H.) based on all available clinical information, including the results of additional imaging and disease course (Supplementary Table C). Discrepancies were discussed until consensus was reached. Secondary outcomes were differences in 90-day relapse-free survival between patients who underwent additional imaging and those who did not and factors associated with the performance of diagnostic imaging.
Statistics
Clinical features of SAB and the primary outcome were reported using the appropriate descriptive statistics for normally and nonnormally distributed variables. Secondary outcomes were determined using multilevel binary logistic regression models to correct for possible clustering because of the hierarchical structure of the multicenter data. Patient characteristics with a univariate P value <.1 were entered into the multivariable model to assess factors associated with the performance of diagnostic imaging. In the regression model with 90-day relapse-free survival as the dependent variable, we included additional imaging (yes/no) as the fixed independent variable. We corrected for known confounders for 90-day relapse-free survival (age, Charlson comorbidity index, predisposing factors for endocarditis, immunocompromised status, and intensive care unit [ICU] admission). We used available case analysis because few data were missing. A sensitivity analysis was conducted by excluding patients with missing follow-up blood cultures. Statistical significance was tested at a 2-sided P value of .05, and 95% confidence intervals (CI) were reported for all inferential statistics. All statistical analyses were performed using SPSS (version 29.0, SPSS, Inc).
RESULTS
Patient Characteristics
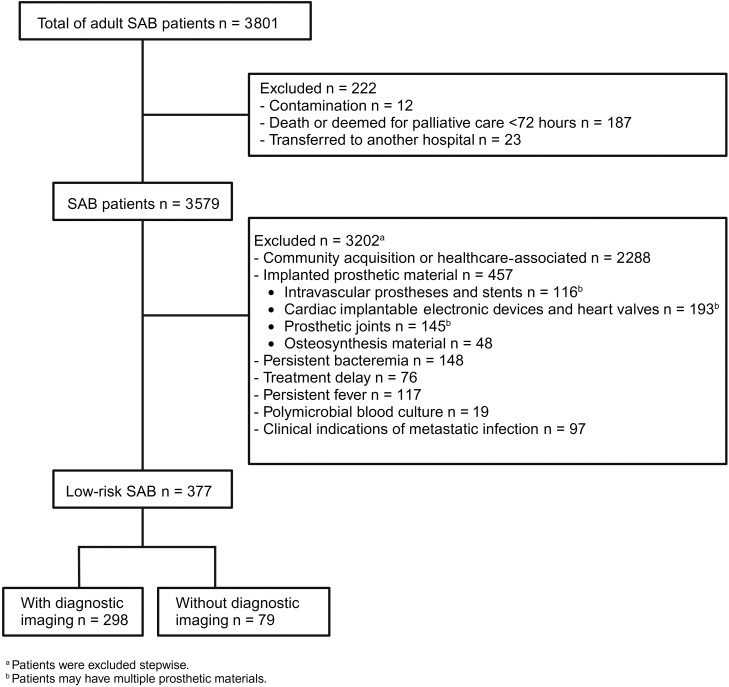
Between January 2013 and December 2022, 3801 patients were diagnosed with SAB, of whom 377 (9.9%) fulfilled inclusion criteria (Figure 1, Table 1). All S. aureus isolates were methicillin-susceptible. Patients who underwent diagnostic imaging were consulted by an ID physician more frequently compared to those who did not undergo diagnostic imaging (81.2% versus 60.8%, P < .001).
Figure 1.
Flowchart of patient inclusion. Abbreviation: SAB, Staphylococcus aureus bacteremia.
Table 1.
Baseline Characteristics
| Characteristic | All Patients N = 377 |
No Diagnostic Imaging n = 79 (21%) |
Diagnostic Imaging n = 298 (79%) |
|---|---|---|---|
| Male sex | 219 (58.1) | 46 (58.2) | 173 (58.1) |
| Age (median + IQR), y | 68 (20.0) | 67 (27.0) | 68.5 (18.0) |
| Infectious diseases specialist bedside consultation | 290 (76.9) | 48 (60.8) | 242 (81.2) |
| Predisposing factors for infective endocarditis | 36 (9.5) | 5 (6.3) | 31 (10.4) |
| Complex cyanotic congenital heart disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| History of endocarditis | 1 (0.3) | 0 (0.0) | 1 (0.3) |
| Congenital cardiac malformations | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Acquired valvar dysfunction | 33 (8.8) | 5 (6.3) | 28 (9.4) |
| Hypertrophic cardiomyopathy | 3 (0.8) | 1 (1.3) | 2 (0.7) |
| Mitral valve prolapse and valve regurgitation or thickened leaflets | 1 (0.3) | 0 (0.0) | 1 (0.3) |
| Intravenous drug use | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Portal of entry | |||
| Unknown | 34 (9.0) | 7 (8.9) | 27 (9.1) |
| Phlebitis | 232 (61.5) | 50 (63.3) | 182 (61.1) |
| Central line | 61 (16.2) | 14 (17.7) | 47 (15.8) |
| Skin | 26 (6.9) | 4 (5.1) | 22 (7.4) |
| Lungs | 11 (2.9) | 0 (0.0) | 11 (3.7) |
| Urinary tract | 8 (2.1) | 3 (3.8) | 5 (1.7) |
| Other | 5 (1.3) | 1 (1.3) | 4 (1.3) |
| CVC | 84 (22.3) | 17 (21.5) | 67 (22.5) |
| Visual signs of infected CVC | 34 (9.0) | 9 (52.9) | 25 (38.5) |
| Positive tip culture | 42 (11.1) | 9 (52.9) | 33 (50.8) |
| C-reactive protein on day of index blood culture (median + IQR)a | 70 (108.5) | 74 (108.0) | 70 (108.5) |
| Immunocompromised | 62 (16.4) | 12 (15.2) | 50 (16.8) |
| Charlson comorbidity index (median + IQR) | 5 (4.0) | 4.0 (5.0) | 5.0 (4.0) |
| Methicillin-resistant Staphylococcus aureus | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Positive follow-up blood culture <48 hb | 90 (23.9) | 12 (17.1) | 78 (27.4) |
| Intensive care unit admission | 66 (17.5) | 8 (10.1) | 58 (19.5) |
All data are absolute numbers (%) unless shown otherwise.
Abbreviations: CVC, central venous catheter; IQR, interquartile range.
aC-reactive protein on day of blood culture was missing for 12 patients: 9 with diagnostic imaging and 3 without diagnostic imaging.
bRepeat blood cultures were missing for 22 patients: 13 with diagnostic imaging and 9 without diagnostic imaging.
Diagnostic Imaging
Diagnostic imaging was performed in 298 (79.0%) patients (529 investigations), including TTE in 222 (58.9%), TEE in 68 (12.9%), 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography with combined computed tomography ([18F]FDG-PET/CT) in 68 (12.9%), CT in 80 (15.1%), magnetic resonance imaging (MRI) in 24 (4.5%), and ultrasound in 67 (12.7%), as shown in Supplementary Table D. A total of 241 patients (63.9%) underwent at least 1 imaging study because of SAB. Of the 529 radiological investigations, 383 (72.4%) were requested to identify SAB-related infectious foci. Echocardiography (91.7%) and [18F]FDG-PET/CT (92.6%) were predominantly requested because of SAB, whereas CT (6.3%) and MRI (25.0%) were mostly performed for suspected stroke, malignancies, or coronavirus disease 2019. Median time interval until any of the diagnostic imaging modalities was 3 days (interquartile range, 1–5). Multilevel logistic regression analysis showed that bedside consultation (81.2% versus 60.8%; odds ratio [OR], 2.876; 95% CI, 1.512–5.470; P < .001) and ICU admission (19.5% versus 10.1%; OR, 2.753; 95% CI, 1.139–6.654; P .025) were independently associated with the use of imaging (Table 2). The intraclass correlation coefficient among hospitals was low (0.35%), indicating that only 0.35% of the probability of diagnostic imaging usage is explained by between-hospital variability [15]. Focusing on SAB-specific diagnostic imaging showed that bedside consultation (64.7% versus 83.8%; OR, 2.562; 95% CI, 1.437–4.568; P < .001) and follow-up blood culture positivity <48 hours (18.9% versus 28.8%; OR, 1.862; 95% CI, 1.063–3.262; P .03) were independently associated with imaging.
Table 2.
Univariate and Multivariable Multilevel Logistic Regression Analysis to Identify Predictors for Use of Diagnostic Imaging
| Variable | Univariate OR for Performing Diagnostic Imaging (95% CI) | Multivariable OR for Performing Diagnostic Imaging (95% CI) | Multivariable OR for Performing Staphylococcus aureus Bacteremia–Specific Diagnostic Imaging (95% CI) |
|---|---|---|---|
| Male | .993 (.600–1.642) | … | … |
| Age | 1.007 (.993–1.022) | … | … |
| Infectious diseases specialist bedside consultation | 2.791 (1.631–4.775)**** | 2.876 (1.512–5.470)**** | 2.562 (1.43–4.568)**** |
| Predisposing heart condition for infective endocarditis | 1.718 (.646–4.574) | … | … |
| Complex cyanotic congenital heart disease | … | … | … |
| History of endocarditis | … | … | … |
| Congenital cardiac malformations | … | … | … |
| Acquired valvar dysfunction | 1.535 (.573–4.113) | … | … |
| Hypertrophic cardiomyopathy | .527 (.047–5.888) | … | … |
| Mitral valve prolapse and valve regurgitation or thickened leaflets | … | … | … |
| Portal of entry | … | … | … |
| Unknown | ref. | … | … |
| Skin | 1.426 (.369–5.508) | … | … |
| Central line | .870 (.313–2.422) | … | … |
| Phlebitis | .440 (.880–2.294) | … | … |
| Lungs | … | … | … |
| Urinary tract | .432 (.083–2.262) | … | … |
| Other | 1.037 (.100–10.806) | … | … |
| Central venous catheter | 1.058 (.580–1.930) | … | … |
| C-reactive protein on day of index blood culturea | 1.001 (.998–1.003) | … | … |
| Immunocompromised | 1.126 (.567–2.234) | … | … |
| Charlson comorbidity index | 1.027 (.939–1.125) | … | … |
| Positive follow-up blood culture <48 hb | 1.821 (.928–3.573)* | 1.842 (.918–3.696) | 1.862 (1.063–3.262)** |
| Intensive care unit admission | 2.145 (.978–4.703)* | 2.753 (1.139–6.654)** | 1.483 (1.063–3.262) |
Abbreviations: CI, confidence interval; OR, odds ratio.
aC-reactive protein on day of blood culture was missing for 12 patients: 9 with diagnostic imaging and 3 without diagnostic imaging.
bRepeat blood cultures were missing for 22 patients: 13 with diagnostic imaging and 9 without diagnostic imaging.
* P < .1, ** P < .05, **** P < .001.
Metastatic Infections and Clinical Outcome
In 15 of the 298 patients (5.0%), imaging findings during patients' admission had been interpreted as metastatic infections that should extend treatment duration (Table 3). No source control procedures were performed based on these imaging results. By linking the final adjudicated diagnosis to the guideline-recommended treatment duration, only 4 patients (1.3%) would have required a change in treatment. One patient was diagnosed with possible muscle and soft tissue abscesses on [18F]FDG-PET/CT. Three other patients had deep arm vein thrombosis that originated from superficial phlebitis and were therefore considered infected thrombi. In the case of 1 patient, the treatment team deliberately opted not to extend antibiotic therapy despite deep arm vein thrombosis. All deep arm vein thromboses were diagnosed by ultrasound. In 1 of these patients, [18F]FDG-PET/CT was performed and showed local FDG-uptake in the thrombus. The diagnostic yield for clinically relevant metastatic infection that required extended treatment was 4.5% for ultrasound, 2.9% for [18F]FDG-PET/CT, and 0% for the other modalities.
Table 3.
Imaging Findings That Led to or Should Have Led to Prolonged Antibiotic Treatment
| Patient | Diagnostic Modality | Diagnosis Before Imaging | Diagnosis After Imaging | Final Adjudicated Diagnosis | Length of Actual Treatment Given, Days |
|---|---|---|---|---|---|
| Clinically relevant metastatic infection | |||||
| 1 | Ultrasound [18F]FDG-PET/CTa | Superficial phlebitis | Infected deep vein thrombosis | Infected deep vein thrombosis | 38 |
| 2 | [18F]FDG-PET/CTa | Superficial phlebitis | Muscle and soft tissue abscesses | Muscle and soft tissue abscesses | 43 |
| 3 | Ultrasound | Superficial phlebitis | Infected deep vein thrombosis (ultrasound) | Infected deep vein thrombosis | 39 |
| 4 | Ultrasound | Superficial phlebitis | Deep vein thrombosis | Infected deep vein thrombosis | 14 |
| Not clinically relevant metastatic infection | |||||
| 5 | Ultrasound [18F]FDG-PET/CTa,b | Superficial phlebitis | Superficial phlebitis (ultrasound) and pulmonary metastatic infection ([18F]FDG-PET/CT) | Superficial phlebitis; pulmonary metastatic infection |
43 |
| 6 | TEE [18F]FDG-PET/CTa | Superficial phlebitis | Infected superficial vein thrombosis (ultrasound) and possible NVIE (TEE) | Superficial phlebitis; NVIE rejected in multidisciplinary team based on TEE findings |
43 |
| 7 | [18F]FDG-PET/CTa,b | Pneumonia | Pneumonia | Pneumonia | 43 |
| 8 | [18F]FDG-PET/CT CT MRIa,b |
CLABSI | Possible osseous metastatic foci | CLABSI; suspected osseous metastatic infections turned out to be cancer metastases (MRI) |
42 |
| 9 | TEEa | CLABSI | NVIE tricuspid valve | NVIE tricuspid valve | 64 |
| 10 | Ultrasound TEE [18F]FDG-PET/CTa |
Superficial phlebitis | Superficial phlebitis (ultrasound) and possible NVIE (TEE); vegetations could not be excluded | Superficial phlebitis TEE after 6 weeks of antibiotic therapy showed calcified spots; diagnosis of NVIE rejected |
42 |
| 11 | TEEa | Wound infection | NVIE because of new chordae tendinae rupture, despite absence of vegetations | Wound infection; new mitral valve insufficiency on TEE due to traumatic chordae tendinae rupture; no vegetations; NVIE rejected |
44 |
| 12 | Ultrasound [18F]FDG-PET/CTa | Superficial phlebitis | Infected superficial vein thrombosis | Superficial phlebitis | 44 |
| 13 | Ultrasound [18F]FDG-PET/CTa,b | Superficial phlebitis | Infected superficial vein thrombosis | Superficial phlebitis | 42 |
| 14 | Ultrasound [18F]FDG-PET/CTa,a | Superficial phlebitis | Infected superficial vein thrombosis | Superficial phlebitis | 41 |
| 15 | Ultrasound [18F]FDG-PET/CTa | Superficial phlebitis | Infected superficial vein thrombosis | Superficial phlebitis | 49 |
Abbreviations: [18F]FDG-PET/CT, 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography with combined computed tomography; CLABSI, central line–associated blood stream infection; CT, computed tomography; MRI, magnetic resonance imaging; NVIE, native valve infective endocarditis; TEE, transesophageal echocardiography.
aTransthoracic echocardiography (TTE) was also performed but did not contribute to final diagnosis.
bTEE was also performed but did not contribute to final diagnosis.
Median treatment duration was 15 days for both groups. Fourteen patients received extended antibiotic therapy due to imaging findings (Table 3). Twenty-three other patients with a working diagnosis of uncomplicated SAB received treatment for longer than 18 days, mainly due to coinfections, logistical reasons, or local practice to give flucloxacillin for at least 2 weeks (Supplementary Figure E).
Overall, 90-day relapse-free survival was 83.0% (313 of 377); 81.0% (64 of 79) in patients without diagnostic imaging versus 83.6% (249 of 298) in patients with diagnostic imaging (P = .592). At 90 days, 63 patients had died and 1 patient had a relapse. Death was adjudicated as infection-related in 12 (19.0%), as possibly infection-related in 23 patients (36.5%), and as not infection-related in 28 (44.4%).
Multivariable multilevel logistic regression analysis with correction for confounders showed an adjusted OR (aOR) for 90-day relapse-free survival of 0.749 (95% CI .373–1.504; P = .416) in patients without diagnostic imaging (Table 4). A sensitivity analysis by excluding 22 patients without follow-up blood cultures showed a similar aOR for 90-day relapse-free survival in patients without diagnostic imaging (aOR, 0.823; 95% CI, .394–1.718; P = .541; Supplementary Table F). A subgroup analysis in patients with echocardiography showed no differences in 90-day relapse-free survival in patients who did not undergo echocardiography (N = 143) compared with those who did (N = 234) when corrected for confounders (80.4% versus 84.6%; aOR, 0.695; 95% CI, .379–1.272; P = .237).
Table 4.
Univariate and Multivariable Multilevel Logistic Regression Analysis to Assess the Association Between the Omission of Diagnostic Imaging and 90-Day Relapse-Free Survival
| Variable | Relapse-Free Survival n = 313 |
Relapse or Mortality n = 64 |
Univariate OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|---|---|
| Male sex | 171 (54.6) | 48 (75.0) | .401 (.219–.737)*** | … |
| Age (per year) (median + IQR) | 67 (21.0) | 73 (17.0) | .969 (.950–.988)**** | .995 (.970–1.021) |
| Infectious diseases specialist bedside consultation | 242 (77.3) | 48 (75.0) | 1.136 (.608–2.122) | … |
| Predisposing heart condition for infective endocarditis | 25 (8.0) | 11 (17.2) | .418 (.194–.901)** | .436 (.189–1.006)* |
| Portal of entry | … | … | … | … |
| Unknown | 26 (8.3) | 8 (12.5) | ref. | … |
| Skin | 21 (6.7) | 5 (7.5) | 1.292 (.368–4.541) | … |
| Central line | 52 (16.6) | 9 (14.1) | 1.778 (.614–5.144) | … |
| Phlebitis | 196 (62.6) | 36 (56.3) | 1.675 (.703–3.992) | … |
| Lungs | 8 (2.6) | 3 (4.7) | .821 (.175–3.849) | … |
| Urinary tract | 6 (1.9) | 2 (3.1) | .923 (.155–5.505) | … |
| Other | 4 (1.3) | 1 (1.6) | 1.231 (.120–12.652) | … |
| Central venous catheter | 74 (23.6) | 10 (15.6) | 1.672 (.811–3.447) | … |
| C-reactive protein on day of index blood culturea (median + IQR) | 67 (107.3) | 88 (115) | 1.000 (.997–1.003) | … |
| Immunocompromised | 47 (15.0) | 15 (23.4) | .577 (.29–1.113) | .657 (.310–1.394) |
| Charlson comorbidity index (median + IQR) | 4 (4.0) | 6 (4.0) | .734 (.658–.819)**** | .740 (.651–.842)*** |
| Positive follow-up blood culture <48 hb | 73 (23.3) | 17 (29.8) | .809 (.434–1.507) | … |
| Intensive care unit admission | 55 (17.6) | 11 (17.2) | 1.027 (.504–2.093) | .719 (.322–1.606) |
| No diagnostic imaging | 64 (20.4) | 15 (23.4) | .840 (.443–1.593) | .749 (.373–1.504) |
All data are absolute numbers (%) unless shown otherwise.
Abbreviations: CI, confidence interval; IQR, interquartile range; OR, odds ratio.
aC-reactive protein on day of blood culture was missing for 12 patients: 9 with diagnostic imaging and 3 without diagnostic imaging.
bRepeat blood cultures were missing for 22 patients: 13 with diagnostic imaging and 9 without diagnostic imaging.
* P < .1 ** P < .05, *** P < .01, **** P < .001.
DISCUSSION
In this observational, multicenter study that focused on patients with low-risk SAB, we found that 79% of patients underwent imaging studies, three-quarters of which were specifically requested to detect metastatic infections. However, clinically relevant metastatic infections were rare, as imaging results led to a change in therapy in only 4.7% of the patients, and a change in therapy was indicated in only 1.3%. There was no difference in 90-day relapse-free survival between patients who underwent radiographic imaging and those who did not.
Two previous studies concluded that clinical risk scores in SAB patients lack sufficient negative predictive value to exclude complicated bacteremia [1, 9]. The probability of developing complicated disease in the absence of all risk factors defined in these studies was 16%–17%. These percentages are relatively high, given the impact of complicated SAB. In contrast to these findings, our study demonstrated that clinical risk stratification is useful. Such an approach is valuable for identifying patients at negligible risk of metastatic infections. A significant reason for this inconsistency is rooted in the varying definitions used. Fowler et al and Lambregts et al [1, 9] considered both factors associated with metastatic infection, that is, persistent bacteremia and poor clinical outcomes, as their outcome of interest, whereas we used a more actionable definition. By focusing only on factors associated with metastatic infections, our risk stratification guides the diagnostic workup or, in this case, its omission, to search for infectious foci. Our study is therefore a call to reserve complicated disease for metastatic infection and to use patient characteristics, features of bacteremia, and clinical course to guide imaging [8].
In addition to the importance of distinguishing the risk of metastatic infection from the outcome itself, we emphasize prerequisites for implementing a risk stratification model that eliminates the need for further diagnostics. Baseline patient and disease characteristics alone, as used in the studies mentioned [1, 9], fall short in this regard. It is crucial to collect follow-up blood cultures because approximately 25% of patients with SAB have persistent bacteremia [16, 17], which significantly increases the risk of metastatic infection and mortality [17]. It is also essential to have a bedside ID consultation performed, as numerous studies have shown that compliance with treatment guidelines improves with such consultations [18–22]. In our study, patients who underwent diagnostic imaging were more likely to have received a bedside ID consultation. Better adherence to recommendations to perform echocardiography and the experience of ID physicians in managing complicated SAB for which additional imaging is important may have played a role in the increased frequency of imaging in low-risk patients. Because clinical manifestations of metastatic infection were an exclusion criterion, patient factors are unlikely to explain this difference. However, this is exactly the reason why bedside ID consultations should take place for patients at low risk of complications: to look for signs of a complicated course. The foregoing implies that it is not possible to identify patients at a negligible risk of metastatic infection until day 3 or 4. This requires negative follow-up blood cultures, a comprehensive evaluation by an ID physician, thorough clinical follow-up, and, if applicable, removal of a CVC.
A nonrandomized study on the added value of [18F]FDG-PET/CT in patients with SAB suggested a survival benefit in low-risk patients [23]. Sixty-one percent of low-risk patients had infectious foci on [18F]FDG-PET/CT. However, the precise localization of these foci in this patient group and the part that affected treatment were not described. Furthermore, patients with prosthetic material and treatment delay were not considered to be at high risk, and patients with existing clinical signs of metastatic infection were also included.
Most clinical decision rules for imaging in SAB focus on IE and aim to determine the necessity of TEE [24, 25]. While current guidelines recommend performing TTE in all SAB patients, emerging evidence suggests that TTE may not be necessary for well-defined low-risk SAB patients [26, 27]. A cost-effectiveness analysis has also shown that in patients with a low probability of IE (<2%), treating bacteremia without echocardiography can be cost-effective [28]. Moreover, scenario-based research also indicated that clinicians feel confident in using risk stratification when deciding on echocardiography strategies [3]. Consistent with these findings, our study revealed that echocardiography was not performed in all patients. TTE was performed in 58.9% and TEE was performed in 18.0% of the patients; 1 showed valvular vegetations (tricuspid valve; Table 4, Supplementary Table E). Importantly, we observed similar 90-day relapse-free survival in patients who did not undergo echocardiography compared with those who did. This suggests that risk stratification can be a valuable tool in deciding whether to perform TTE [3, 26, 27, 29]. Additionally, incorporating variables such as time to blood culture positivity, which clinicians can readily access at the time of SAB diagnosis, can enhance the precision of risk stratification models and improve accuracy when identifying patients who may not require invasive imaging [29].
There are several strengths of our study. One is the study’s multicenter design, which reduced the potential referral bias and enhanced generalizability. Additionally, the use of well-established and easy-to-obtain risk factors to identify a homogeneous group of low-risk SAB patients further contributes to the study's generalizability. Our study has some limitations as well. First, there is the risk that due to the retrospective, nonrandomized design, the outcome will be biased by confounding by indication. However, we tried to reduce this as much as possible by including a relatively homogeneous low-risk group in which patients with significant predictors of metastatic infection were excluded. Additionally, multilevel logistic regression was performed to further adjust for any remaining known confounders. Second, the observational design of the study may have introduced immortal time bias, which frequently occurs in diagnostic studies, as has also been recently demonstrated for studies that investigate the added value of [18F]FDG-PET/CT in SAB [30]. We attempted to minimize this impact by excluding all patients who died or were deemed suitable for palliative care only within 72 hours from the index blood culture. Third, as the prevalence of metastatic infections was low, the study lacked power for a formal noninferiority analysis on the safety of omitting imaging. It should be noted that our study included more patients than the recently completed Staphylococcus aureus bacteremia antibiotic treatment options (SABATO) trial of early oral switch in low-risk SAB with a similar 90-day relapse-free survival [31]. Fourth, we assigned equal weight to all diagnostic modalities in the analysis, even though they vary in sensitivity for detecting metastatic foci and the extent to which the body is examined. Fifth, the absence of methicillin-resistant Staphylococcus aureus bacteremia and IV drug users may limit generalizability to these patients.
In conclusion, this study advocates for a structured approach along with risk stratification for the management of SAB patients. Several days after bacteremia onset, patients with low risk of clinically significant metastatic infections can be identified using easily obtainable clinical parameters. This strategy necessitates the performance of (repeated) bedside ID consultation, the collection of follow-up blood cultures, and a critical review of disease evolution. Our study found that metastatic infections were rare using this approach and that the omission of radiological imaging was not associated with lower 90-day relapse-free survival, suggesting that routine imaging may be unnecessary for low-risk SAB patients. To enhance the effectiveness of risk stratification beyond 10% of the overall SAB population, further investigations should concentrate on identifying additional factors that ascertain patients at low risk for metastatic infections.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Marianne M C Hendriks, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Nijmegen, The Netherlands.
Kris S A Schweren, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Nijmegen, The Netherlands.
Ayden Kleij, Department of Internal Medicine, Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands.
Marvin A H Berrevoets, Department of Internal Medicine, Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands.
Emma de Jong, Department of Internal Medicine, Amphia Hospital, Breda, The Netherlands.
Peter van Wijngaarden, Department of Internal Medicine, Amphia Hospital, Breda, The Netherlands.
Heidi S M Ammerlaan, Department of Internal Medicine, Catharina Hospital, Eindhoven, The Netherlands.
Anja Vos, Department of Internal Medicine, Treant, Emmen, The Netherlands.
Sander van Assen, Department of Internal Medicine, Treant, Emmen, The Netherlands.
Kitty Slieker, Department of Internal Medicine, Bernhoven Hospital, Uden, The Netherlands.
Jet H Gisolf, Department of Intenal Medicine, Rijnstate Hospital, Arnhem, The Netherlands.
Mihai G Netea, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Nijmegen, The Netherlands; Department of Immunology and Metabolism, Life and Medical Sciences Institute, University of Bonn, Bonn, Germany.
Jaap ten Oever, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Nijmegen, The Netherlands.
Ilse J E Kouijzer, Department of Internal Medicine and Radboud Center for Infectious Diseases, Radboud University Medical Center, Nijmegen, The Netherlands.
Notes
Author contributions. M. M. C. H., J. t. O., and I. J. E. K. conceptualized the study and wrote the analysis plan. M. M. C. H. and K. S. A. S. performed the data analysis. All authors contributed to data acquisition. M. M. C. H. and K. S. A. S. wrote the first draft of the manuscript. All authors critically reviewed the manuscript.
Acknowledgments. The authors thank Scott Maurits for his help with the statistical analysis and Marike Koekkoek for additional data acquisition.
References
- 1. Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 2. Hagel S, Bahrs C, Schumann R, Pletz M, Weis S. Complicated and uncomplicated S. aureus bacteraemia: an international Delphi survey among infectious diseases experts on definitions and treatment. Clin Microbiol Infect 2022; 28:1026.e7–e11. [DOI] [PubMed] [Google Scholar]
- 3. Heriot GS, Tong SYC, Cheng AC, Liew D. A scenario-based survey of expert echocardiography recommendations for patients with Staphylococcus aureus bacteremia at varying risk for endocarditis. JAMA Netw Open 2020; 3:e202401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vos FJ, Bleeker-Rovers CP, Sturm PD, et al. 18F-FDG PET/CT for detection of metastatic infection in gram-positive bacteremia. J Nucl Med 2010; 51:1234–40. [DOI] [PubMed] [Google Scholar]
- 5. Naber CK, Baddour LM, Giamarellos-Bourboulis EJ, et al. Clinical consensus conference: survey on gram-positive bloodstream infections with a focus on Staphylococcus aureus. Clin Infect Dis 2009; 48(Suppl 4):S260–70. [DOI] [PubMed] [Google Scholar]
- 6. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52:285–92. [DOI] [PubMed] [Google Scholar]
- 7. Escrihuela-Vidal F, Kaasch AJ, Von Cube M, et al. Impact of adherence to individual quality-of-care indicators on the prognosis of bloodstream infection due to Staphylococcus aureus: a prospective observational multicentre cohort. Clin Microbiol Infect 2023; 29:498–505. [DOI] [PubMed] [Google Scholar]
- 8. Kouijzer IJE, Fowler VG Jr, Ten Oever J. Redefining Staphylococcus aureus bacteremia: a structured approach guiding diagnostic and therapeutic management. J Infect 2023; 86:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lambregts MMC, Molendijk EBD, Meziyerh S, et al. Early differentiation between uncomplicated and complicated Staphylococcus aureus bacteraemia: potential value and limitations of a clinical risk score. Int J Clin Pract 2020; 74:e13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Vaart TW, Prins JM, Soetekouw R, et al. All-cause and infection-related mortality in Staphylococcus aureus bacteremia, a multicenter prospective cohort study. Open Forum Infect Dis 2022; 9:ofac653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mun SJ, Kim SH, Huh K, et al. Role of echocardiography in uncomplicated Staphylococcus aureus catheter-related bloodstream infections. Medicine (Baltimore) 2021; 100:e25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 13. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–128. [DOI] [PubMed] [Google Scholar]
- 14. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 15. Wu S, Crespi CM, Wong WK. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemp Clin Trials 2012; 33:869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holland TL, Raad I, Boucher HW, et al. Effect of algorithm-based therapy vs usual care on clinical success and serious adverse events in patients with staphylococcal bacteremia: a randomized clinical trial. JAMA 2018; 320:1249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuehl R, Morata L, Boeing C, et al. Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis 2020; 20:1409–17. [DOI] [PubMed] [Google Scholar]
- 18. Cobussen M, van Tiel FH, Oude Lashof AML. Management of S. aureus bacteraemia in the Netherlands; infectious diseases consultation improves outcome. Neth J Med 2018; 76:322–9. [PubMed] [Google Scholar]
- 19. Ariaans M, Roovers EA, Claassen MAA, Hassing RJ, Swanink CMA, Gisolf EH. Increased overall survival after introduction of structured bedside consultation in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 2018; 37:1187–93. [DOI] [PubMed] [Google Scholar]
- 20. Rieg S, Peyerl-Hoffmann G, de With K, et al. Mortality of S. aureus bacteremia and infectious diseases specialist consultation—a study of 521 patients in Germany. J Infect 2009; 59:232–9. [DOI] [PubMed] [Google Scholar]
- 21. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore) 2009; 88:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forsblom E, Ruotsalainen E, Ollgren J, Järvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2013; 56:527–35. [DOI] [PubMed] [Google Scholar]
- 23. Ghanem-Zoubi N, Kagna O, Abu-Elhija J, et al. Integration of FDG-PET/CT in the diagnostic workup for Staphylococcus aureus bacteremia: a prospective interventional matched-cohort study. Clin Infect Dis 2021; 73:e3859–66. [DOI] [PubMed] [Google Scholar]
- 24. van der Vaart TW, Prins JM, Soetekouw R, et al. Prediction rules for ruling out endocarditis in patients with Staphylococcus aureus bacteremia. Clin Infect Dis 2022; 74:1442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bai AD, Agarwal A, Steinberg M, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23:900–6. [DOI] [PubMed] [Google Scholar]
- 26. Barton T, Moir S, Rehmani H, Woolley I, Korman TM, Stuart RL. Low rates of endocarditis in healthcare-associated Staphylococcus aureus bacteremia suggest that echocardiography might not always be required. Eur J Clin Microbiol Infect Dis 2016; 35:49–55. [DOI] [PubMed] [Google Scholar]
- 27. Khatib R, Sharma M. Echocardiography is dispensable in uncomplicated Staphylococcus aureus bacteremia. Medicine (Baltimore) 2013; 92:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heidenreich PA, Masoudi FA, Maini B, et al. Echocardiography in patients with suspected endocarditis: a cost-effectiveness analysis. Am J Med 1999; 107:198–208. [DOI] [PubMed] [Google Scholar]
- 29. Simos PA, Holland DJ, Stewart A, et al. Clinical prediction scores and the utility of time to blood culture positivity in stratifying the risk of infective endocarditis in Staphylococcus aureus bacteraemia. J Antimicrob Chemother 2022; 77:2003–10. [DOI] [PubMed] [Google Scholar]
- 30. van der Vaart TW, Prins JM, van Werkhoven CH, et al. Positive impact of [18F]FDG-PET/CT on mortality in patients with Staphylococcus aureus bacteremia explained by immortal time bias. Clin Infect Dis 2023; 77:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaasch AJ, López-Cortés LE, Rodríguez-Baño J, et al. Efficacy and safety of an early oral switch in low-risk Staphylococcus aureus bloodstream infection (SABATO): an international, open-label, parallel-group, randomised, controlled, non-inferiority trial. Lancet Infect Dis 2024; doi: 10.1016/S1473-3099(23)00756-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.