Abstract
Background
Protein-based vaccines for coronavirus disease 2019 (COVID-19) provide a traditional vaccine platform with long-lasting protection for non–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogens and may complement messenger RNA vaccines as a booster dose. While NVX-CoV2373 showed substantial early efficacy, the durability of protection has not been delineated.
Methods
The PREVENT-19 vaccine trial used a blinded crossover design; the original placebo arm received NVX-CoV2373 after efficacy was established. Using novel statistical methods that integrate surveillance data of circulating strains with post-crossover cases, we estimated placebo-controlled vaccine efficacy and durability of NVX-CoV2373 against both pre-Delta and Delta strains of SARS-CoV-2.
Results
Vaccine efficacy against pre-Delta strains of COVID-19 was 89% (95% CI, 75–95%) and 87% (72–94%) at 0 and 90 days after 2 doses of NVX-CoV2373, respectively, with no evidence of waning (P = .93). Vaccine efficacy against the Delta strain was 88% (71–95%), 82% (56–92%), and 77% (44–90%) at 40, 120, and 180 days, respectively, with evidence of waning (P < .01). In sensitivity analyses, the estimated Delta vaccine efficacy at 120 days ranged from 66% (15–86%) to 89% (74–95%) per various assumptions of the surveillance data.
Conclusions
NVX-CoV2373 has high initial efficacy against pre-Delta and Delta strains of COVID-19 with little evidence of waning for pre-Delta strains through 90 days and moderate waning against Delta strains over 180 days.
Keywords: COVID-19, vaccine durability, NVX-CoV2373, SARS-CoV-2
In the PREVENT-19 phase 3 trial, NVX-CoV2373 showed high initial efficacy against pre-Delta and Delta strains of COVID-19 with little evidence of waning for pre-Delta strains through 90 days and moderate waning against Delta strains over 180 days.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), continues to be a major threat to public health with the emergence of new variants and waning of immunity following either infection or vaccination [1]. Development of durable and broadly protective COVID-19 vaccines is a major focus of research. Protein-based vaccines have shown long-lasting effects against pathogens other than SARS-CoV-2 [2]. While the durability of messenger RNA (mRNA) and vector-based COVID-19 vaccines for SARS-CoV-2 has been well characterized, less is known about protein-based vaccines [3, 4]. NVX-CoV2373, a recombinant spike protein-based vaccine with Matrix-M adjuvant, was evaluated in 3 efficacy trials. In a phase 2a-b trial of 4337 adults in South Africa during the B.1.351 or Beta wave, vaccine efficacy was 49% (95% confidence interval [CI], 6–73%) with a maximum follow-up of 2 months [5]. In a phase 3 trial of 15 187 adults in the United Kingdom, vaccine efficacy was 89.7% (90.2–94.6%) overall and 86.3% (71.3–93.5%) against the B.1.17 Alpha variant with a median follow-up of 3 months [6]. Prefusion Protein Subunit Vaccine Efficacy Novavax Trial–COVID-19 (PREVENT-19) was a phase 3 trial of 25 452 adults living in the United States and Mexico who were randomized to receive vaccine or placebo between December 2020 and February 2021. Initial vaccine efficacy through April 2021 was 90.4%, with a median follow-up of 3 months [7]. These efficacy estimates for all 3 trials preceded the emergence of the Delta strain and were made after a relatively short follow-up period, precluding assessments of the durability of efficacy against the pre-Delta strain and efficacy against the Delta strain.
In April 2021, PREVENT-19 initiated a blinded crossover period in which the original placebo recipients received NVX-CoV2373 and the original vaccine recipients received placebo [8]. Blinded follow-up of all participants continued through the Delta wave and beyond. In this work, we apply novel statistical methods that integrate surveillance results of circulating strains with post-crossover case detection to estimate the placebo-controlled initial vaccine efficacy and durability against pre-Delta and Delta COVID-19.
METHODS
Study Design and Population
Details of the PREVENT-19 trial have been presented elsewhere [7]. Briefly, 29 949 healthy adults at elevated risk of COVID-19 were randomized 2:1 to receive 2 doses of NVX-CoV2373 or placebo 21 days apart. The primary endpoint was polymerase chain reaction (PCR)–confirmed mild, moderate, or severe COVID-19 via a single central laboratory with onset at least 7 days after the second dose (termed “day 0” for efficacy evaluations). Efficacy was assessed in the per-protocol population of 25 452 SARS-CoV-2–naïve individuals who received 2 doses of NVX-CoV2373 or placebo without major protocol deviations and were event-free through 6 days post–dose 2. Enrollment was between 27 December 2020 and 18 February 2021. Following establishment of efficacy in April 2021, a blinded crossover period was initiated in which volunteers originally randomized to placebo received 2 doses of NVX-CoV2373 and volunteers originally randomized to NVX-CoV2373 received 2 doses of placebo. Our analysis set uses the original per-protocol population augmented with post-crossover follow-up through 1 November 2021, shortly before the Omicron wave occurred. Analogous to the definition of the original per-protocol analysis set, we censored, at the time of the first crossover dose, those who did not receive a crossover dose 2, dropped out, were anti–N antibody positive or SARS-CoV-2 PCR positive at the first crossover visit, or became SARS-CoV-2 positive before 7 days after crossover dose 2. Assessment of efficacy following full immunization began 7 days post–dose 2 of NVX-CoV2373 (ie, day 0 for efficacy evaluations).
GISAID Data
The sequences used for this article are from the corpus of whole-genome sequences submitted to GISAID (Global Initiative on Sharing All Influenza Data) and were downloaded on 26 April 2022 [9, 10]. All sequences in the GISAID database from the United States (N = 2 955 910) and Mexico (N = 51 313) were used. Additionally, each sequence includes the country of origin, the date of submission, and the state or territory of the submitting laboratory. The sequences used in this analysis are available from GISAID with EPI_SET ID “EPI_SET_231018wo”.
Statistical Methods
Cox proportional hazards regression models with calendar time scale were used to estimate vaccine efficacy, relative efficacy, and durability for pre-Delta and Delta strains of COVID-19 for the per-protocol population [11, 12]. Informally, initial efficacy against pre-Delta strains of COVID-19 was established pre-crossover and durability estimated by comparison of the post-crossover incidence of pre-Delta COVID-19 by original arms. Intuitively, a higher incidence in the original vaccine arm post-crossover would indicate waning of vaccine efficacy [8]. Essentially all Delta events occurred post-crossover, which lacked a placebo arm. The incidences for pre-Delta and Delta COVID-19 in a counterfactual placebo group were inferred using the established efficacy against the pre-Delta strain combined with the time-varying community-level incidence of pre-Delta and Delta strains during the same calendar time as reported to GISAID (see Supplementary Figures 1 and 2) [12]. In a complementary analysis for Delta COVID-19, we estimated the hazard ratio curve for time since full immunization versus 40 days since full immunization. The comparison at 40 days since full immunization was chosen to avoid extrapolation and use of a counterfactual placebo group. We also created calibrated vaccine efficacy curves by combining the relative hazard ratio curves with a specified initial vaccine efficacy.
COVID-19 cases were not counted between the first crossover dose through 6 days post–dose 2 for either arm, and all follow-up time was censored on or before 1 November 2021. Piecewise log-linear splines with bend-points at 75 and 140 days post–full immunization were used to model durability for pre-Delta and Delta COVID-19, respectively [13]. Bend-points were selected to be approximately halfway through the follow-up period. Wald tests of the pre-bend slope were used to test for waning of vaccine efficacy. COVID-19 cases with missing strain information used cold-deck imputation where strain was imputed by a Bernoulli draw (coin flip) with the probability of Delta given by the GISAID estimated proportion of Delta cases matched on date and state of the COVID-19 case with missing strain information [14]. Details, assumptions, and sensitivity analyses evaluating different bend-points for the pre-Delta and Delta models are provided in the Supplementary Material. All analyses were conducted using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) and SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Supplementary Figure 3 provides a CONSORT (Consolidated Standards of Reporting Trials) diagram of the PREVENT-19 trial from initiation through 1 November 2021. A total of 25 519 per-protocol participants were evaluated pre-crossover, while 18 495 volunteers received crossover dosing and satisfied per-protocol crossover criteria. The last per-protocol crossover participant received their first dose on 24 July 2021, and 7024 participants were not in the post-crossover per-protocol analysis set, mostly due to withdrawal or censoring at the first crossover dose. A total of 246 participants never initiated crossover dosing and were censored on 24 July 2021. Table 1 displays the characteristics of those who completed crossover by arm demonstrating little difference between the groups, except that approximately 77% of the NVX-CoV2373 arm completed crossover compared to 64% for the placebo arm.
Table 1.
Demographic and Clinical Characteristics by the Original Randomization Group Among Participants Who Received Crossover Doses and Had Follow-up in the Crossover Period
| Characteristic | Overall | NVX-CoV2373a | Placeboa |
|---|---|---|---|
| Did not crossover by 24 July 2021,b n | 7024 | 4033 | 2991 |
| Total N | 18 495 | 13 152 | 5343 |
| Country, n (%) | |||
| United States | 17 362 (93.9) | 12 354 (93.9) | 5008 (93.7) |
| Mexico | 1133 (6.1) | 798 (6.1) | 335 (6.3) |
| Median (range) age, y | 47 (18–95) | 47 (18–95) | 47 (18–90) |
| Age group, n (%) | |||
| 18–64 y | 16 335 (88.3) | 11 627 (88.4) | 4708 (88.1) |
| ≥65 y | 2160 (11.7) | 1525 (11.6) | 635 (11.9) |
| Sex, n (%) | |||
| Male | 9373 (50.7) | 6755 (51.4) | 2618 (49.0) |
| Female | 9122 (49.3) | 6397 (48.6) | 2725 (51.0) |
| Race or ethnic group, n (%) | |||
| White | 13 970 (75.5) | 10 012 (76.1) | 3958 (74.1) |
| Black or African American | 2087 (11.3) | 1406 (10.7) | 681 (12.7) |
| American Indian or Alaska Native, including Mexican Native | 1140 (6.2) | 805 (6.1) | 335 (6.3) |
| Asian | 810 (4.4) | 580 (4.4) | 230 (4.3) |
| Multiple | 338 (1.8) | 237 (1.8) | 101 (1.9) |
| Native Hawaiian or other Pacific Islander | 38 (0.2) | 31 (0.2) | 7 (0.1) |
| Not reported | 112 (0.6) | 81 (0.6) | 31 (0.6) |
| Hispanic or Latino, n (%) | |||
| No | 14 379 (77.7) | 10 242 (77.9) | 4137 (77.4) |
| Yes | 4074 (22.0) | 2878 (21.9) | 1196 (22.4) |
| Not reported | 25 (0.1) | 17 (0.1) | 8 (0.1) |
| Unknown | 17 (0.1) | 15 (0.1) | 2 (0.0) |
| Overall high risk of COVID-19, n (%) | |||
| Yes | 17 635 (95.4) | 12 536 (95.3) | 5099 (95.4) |
| No | 860 (4.6) | 616 (4.7) | 244 (4.6) |
| Coexisting conditions, n (%) | |||
| Any | 8970 (48.5) | 6261 (47.6) | 2709 (50.7) |
| Obesity | 7060 (38.2) | 4908 (37.3) | 2152 (40.3) |
| Chronic lung disease | 2772 (15.0) | 1922 (14.6) | 850 (15.9) |
| Type 2 diabetes | 1531 (8.3) | 1032 (7.8) | 499 (9.3) |
| Cardiovascular disease | 228 (1.2) | 157 (1.2) | 71 (1.3) |
| Chronic kidney disease | 141 (0.8) | 94 (0.7) | 47 (0.9) |
| HIV, n (%) | 141 (0.8) | 110 (0.8) | 31 (0.6) |
| Day of first crossover dose relative to 1 January 2021, median [IQR] | 115.0 [111.0, 118.0] |
115.0 [111.0, 119.0] |
115.0 [111.0, 118.0] |
| Months between second dose of primary series to second dose of crossover series, median [IQR] | 2.8 [2.5, 3.2] | 2.8 [2.5, 3.3] | 2.8 [2.5, 3.2] |
| Months between second dose of NVX-CoV2373 and 24 July 2021, median [IQR] | 4.6 [1.6, 5.2] | 4.9 [4.5, 5.4] | 1.4 [1.3, 1.6] |
Abbreviations: COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; IQR, interquartile range.
aOriginal treatment arm.
b24 July 2021 is the date the last subject received the first crossover dose (includes 76 events in the placebo arm and 18 events in the NVX-CoV2373 arm).
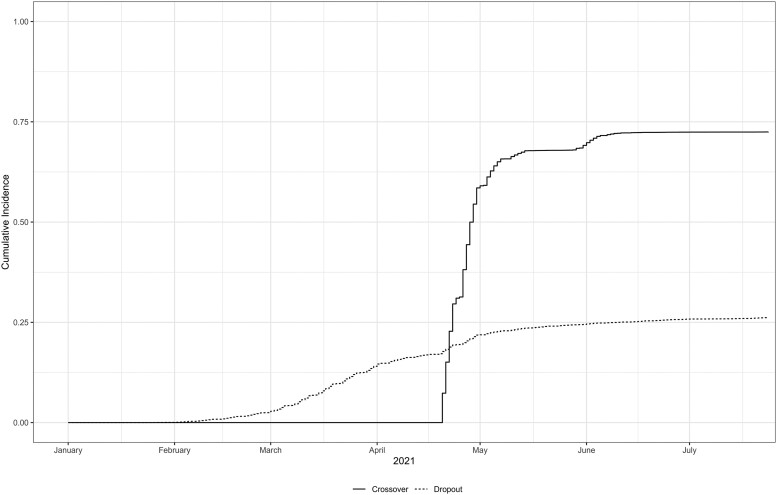
Crossover dosing began on 20 April 2021, with 68% initiating crossover by 21 May 2021 and 72% by 24 July 2021 (see Figure 1). The median time from dose 2 of the primary series to dose 2 of the crossover series was 2.8 months (interquartile range [IQR], 2.5–3.2). The median time from dose 2 of NVX-CoV2373 to 24 July 2021 was 1.4 months (1.3–1.6) for the placebo arm and 4.9 months (4.5–5.4) for the NVX-CoV2373 arm (Table 1), a consequence of randomization where the original vaccine arm received NVX-CoV2373 in December–February and the placebo crossover arm received NVX-CoV2373 in April–May.
Figure 1.
Cumulative incidence of pre-crossover dropout and crossover initiation through the end of the crossover period, 24 July 2021. N = 25 519.
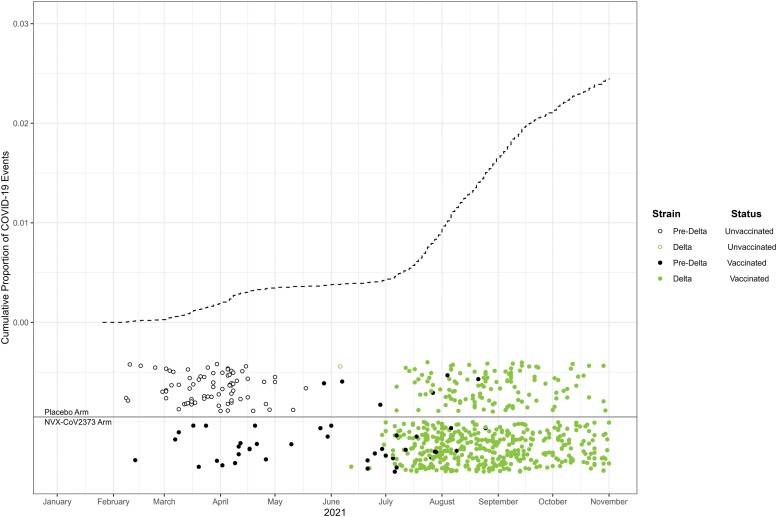
Figure 2 shows the cumulative incidence of COVID-19 from January to November 2021, with triangles denoting COVID-19 cases annotated by randomization arm, vaccination status, and strain. Cases that occurred between the first dose and 6 days post–dose 2, whether for the initial or crossover dosing periods, were excluded. The dearth of events in May–July is due both to the waning of the pandemic and because COVID-19 cases were not counted during each person’s 1-month crossover interlude between crossover dose 1 through 6 days post–crossover dose 2. Of 625 COVID-19 cases, we imputed missing strain information for 176. There were 120 cases of pre-Delta COVID, with 93 occurring before crossover and 505 cases of Delta COVID-19 with 1 occurring before crossover. Supplementary Figure 4 provides a visualization of the imputed and genotyped strains over time.
Figure 2.
Cumulative incidence of the first instance of COVID-19 along with circles denoting the timing of COVID-19 cases annotated by strain and vaccination status (ie, received both doses of NVX-CoV2373). Both sequenced and imputed strains are included. Note the NVX-COV2373:placebo randomization ratio was 2:1. Abbreviation: COVID-19, coronavirus disease 2019.
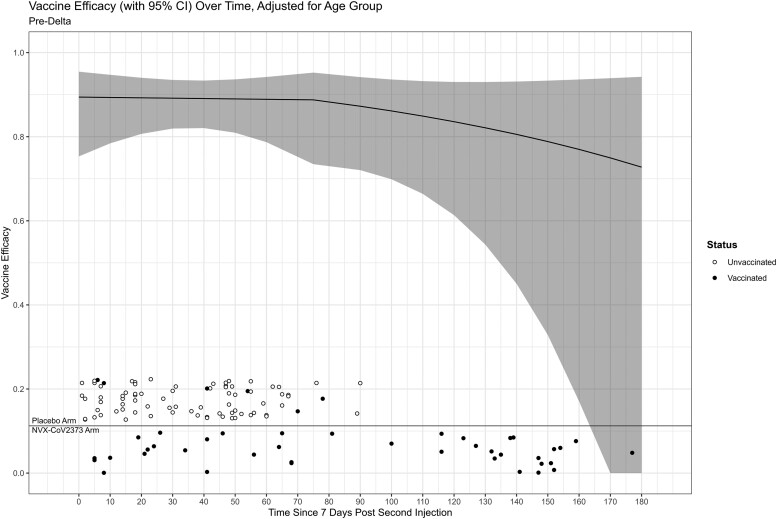
Figure 3 displays the timing of pre-Delta COVID-19 cases 7 days post–dose 2 (ie, day 0) annotated by arm and vaccination status along with vaccine efficacy. The vaccine efficacy was constant through the bend-point of 75 days, with a modest decline after 75 days. A Wald test of a constant vaccine efficacy failed to reject the null hypothesis (P = .93), indicating little evidence of waning. Most cases of pre-Delta COVID-19 occurred within 3 months, with wide CIs at 150–180 days. The vaccine efficacy was 89% (95% CI, 75–95%) and 84% (61–93%) at 0 and 120 days post–full immunization (ie, 7 days post–second dose), respectively.
Figure 3.
Placebo-controlled vaccine efficacy against pre-Delta COVID-19 as a function of days since full immunization. A bend-point is specified at 75 days. Circles denote the time of COVID-19 cases on the x-axis annotated by vaccination status. Time is relative to the second injection of the pre-crossover dosing for placebo unvaccinated cases and NVX-CoV2373 cases and relative to the post-crossover second dose for placebo vaccinated cases. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019.
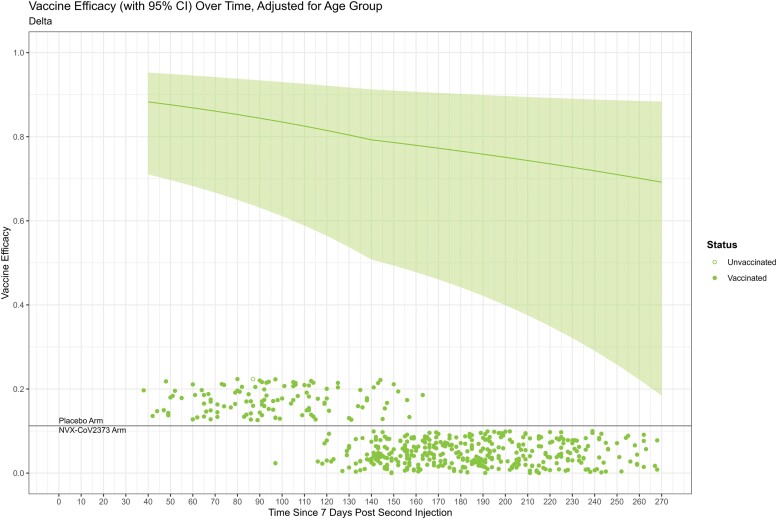
Figure 4 provides an analogous figure for Delta COVID-19. Vaccine efficacy declines moderately through the bend-point of 140 days, with slightly less decline through the rest of follow-up. Cases occurred frequently throughout follow-up, with widening CIs following the bend-point. The vaccine efficacy was 88% (95% CI, 75–95%), 82% (56–92%), and 77% (44–90%) at days 40, 120, and 180 post–full immunization, respectively. A Wald test rejected a constant vaccine efficacy (P = .007), indicating statistically significant waning.
Figure 4.
Counterfactual placebo-controlled vaccine efficacy against Delta COVID-19 as a function of days since full immunization. The vaccine efficacy curve starts at 40 days post–full immunization to avoid extrapolation. A bend-point is specified at 140 days. Circles denote the time of COVID-19 cases on the x-axis annotated by vaccination status. Time is relative to the second injection of the pre-crossover dosing for placebo unvaccinated cases and NVX-CoV2373 cases and relative to the post-crossover second dose for placebo vaccinated cases. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019.
The counterfactual placebo group was constructed using surveillance data and assumes that the reporting lag from infection to testing was approximately the same for the GISAID surveillance data as PREVENT-19. Participants in PREVENT-19 were encouraged to get tested at the start of symptoms. GISAID data include both asymptomatic and symptomatic testing. Lags from infection to testing in the GISAID database might be longer than PREVENT-19 for symptomatic cases as GISAID cases were not encouraged to get tested at the start of symptoms. The lag from infection to testing for asymptomatic cases is likely pseudo-random, which may imply longer lags from infection to testing. Since any difference in lag is unknown, we conducted sensitivity analyses where the lag from infection to testing was 7 days longer or 7 days shorter in GISAID compared to PREVENT-19. At 120 days post–full immunization, the estimates (95% CIs) were 89% (74–95%) and 66% (15–86%), respectively, compared to 82% (56–92%) with equal lag (Supplementary Figure 5).
We also evaluated relative risks for Delta COVID-19 as a function of time since full immunization compared with 40 days post–full immunization. This evaluation did not involve surveillance data. The relative risk (hazard ratio) of COVID-19 was 1.4 (95% CI: 1.1–1.8), 1.8 (1.3–2.7), and 2.2 (1.5–3.3) times greater at 100, 160, or 220 days post–full immunization (Supplementary Figure 6). To translate these curves to vaccine efficacy curves, we need to know the vaccine efficacy just after full immunization. An extension of PREVENT-19 in SARS-CoV-2–naïve adolescents aged 12–17 years had 80% (47–92%) with Delta the only variant sequenced [15]. In Supplementary Figure 7, we specify initial vaccine efficacies of 85% (top panel), 80% (middle panel), and 75% (lower panel) and thus create durability curves under different assumptions. These curves can be viewed as an additional sensitivity analysis. For a specified initial vaccine efficacy of 85%, the efficacy at 120 days was 77% (95% CI, 68–83%); for initial efficacy of 80%, the 120-day efficacy was 69% (57–78%); and for initial efficacy of 75%, the 120-day efficacy was 61% (46–72%).
We also analyzed the data by subgroups defined by age (<50 or ≥50 years), sex (male or female), and pre-existing conditions (yes or no). For pre-Delta, the estimates appear similar across the different subgroups (Supplementary Figure 8). For Delta, there is somewhat more variation, with numerically higher vaccine efficacy for those aged 50 and older, men, and those with pre-existing conditions (Supplementary Figure 9). For both pre-Delta and Delta, the CIs have substantial overlap across the subgroups. Results were similar for the different bend-points in sensitivity analyses (Supplementary Table 1).
DISCUSSION
While the durability of mRNA and vector-based vaccines has been well characterized, durability of protein-based vaccines for SARS-CoV-2 has not been previously described in detail. In this report we evaluated the placebo-controlled vaccine efficacy by time since vaccination with the NVX-CoV2373 vaccine for both pre-Delta and Delta strains of COVID-19, with initial efficacy for both of approximately 90%. There was little evidence of waning against pre-Delta COVID-19 throughout follow-up, although there were few cases past 90 days. The estimated vaccine efficacy against Delta COVID-19 waned moderately to approximately 77% by 6 months in our reference analysis and ranged from 66% to 89% in sensitivity analyses. A natural question is how does this compare to other COVID-19 vaccines.
Three other COVID-19 vaccines have been licensed in the United States. The initial reports of the phase 3 licensure trials conducted during the ancestral era showed that 2 doses of the mRNA-based vaccines, mRNA-1273 and BNT162b2, had overall efficacies of 94% and 95%, respectively, against COVID-19. A single dose of the vector-based AD26.COVS.2 vaccine had an overall efficacy of 67% against moderate or greater COVID-19 during the pre-Delta era [16–18]. NVX-CoV2373 had an overall efficacy of 90% during the pre-Delta era when the Alpha variant predominated and lies between mRNA and vector-based platforms in terms of early efficacy.
Durability was later assessed in the phase 3 trials: mRNA-1273 maintained efficacy of approximately 90% through 6 months of follow-up during the pre-Delta era, with tight CIs [18, 19], whereas AD26.COVS.2 maintained efficacy of approximately 60% through 5 months of follow-up, which was almost entirely pre-Delta. During the July–August 2021 Delta surge, mRNA-1273 demonstrated late waning, with an incidence rate of 77.1 per 1000 person-years for the original vaccine arm (vaccinated July–December 2020) compared with an incidence rate of 49.0 for the crossover placebo arm (vaccinated January–April 2021) [20].
Observational data from North Carolina from December 2020 through September 2021, encompassing both pre-Delta and Delta strain periods, showed efficacies against infection of 96%, 95%, and 71%, at 2 months post–first dose, respectively, for mRNA-1273, BNT162b2, and AD26.COVS.2, which waned to approximately 83%, 76%, and 64% 4 months later. The pattern was similar for hospitalization, although with higher efficacy of 97%, 96%, and 86%, waning to 94%, 91%, and 82%, respectively. Thus, observational data for both mRNA and vector vaccine platforms showed waning over 4 months, with greater waning for the mRNA platforms. While comparison of the durability of the protective efficacy of vaccines is difficult to assess due to differences in study design, endpoints, strains, and duration of follow-up, qualitatively, NVX-CoV2373 aligns with the other vaccines with constant vaccine efficacy against pre-Delta strains through 3 months and waning against Delta over 5 months. Whether NVX-COV2373 has a durability profile similar to mRNA vaccines for later time points is unknown.
Our analysis has a number of strengths and limitations. PREVENT-19 was designed to be inclusive, with a high proportion of historically underserved minorities, was the first trial to use a blinded crossover design, and was launched after the mRNA vaccines were available under Emergency Use Authorization (EUA) in the United States. Knowledge that placebo participants would promptly receive NVX-COV2373 if proven efficacious was critical for successful enrollment. The blinded crossover design should encourage similar behavior between arms throughout follow-up in contrast to an open-label crossover, where knowledge of being in the vaccine arm might influence risk behavior just after unblinding and/or increase dropout. Even so, there were more withdrawals in the placebo arm, likely due to unblinding from side effects and a desire for an EUA vaccine. A similar phenomenon was observed in the evaluation of the mRNA-1273 COVID-19 vaccine [16]. Future studies may consider using a licensed vaccine control to help maintain blinding. Future vaccines will likely not be a perfect match with the circulating strain, as was our evaluation of Delta infecting strains with a Wuhan strain vaccine. This mismatch may help with the extrapolation of our analysis to the current setting. An additional strength is the use of statistical methods that allow recovery of vaccine durability for both pre-Delta and Delta strains, the latter emerging after placebo crossover. A limitation is that these methods have less power than for a placebo-controlled trial. An additional assumption for the Delta vaccine efficacy estimates is that the GISAID data accurately recover the Delta case rate for a counterfactual placebo group. This limitation was addressed with 2 different sensitivity analyses. Assessment of durability using other cohorts could help validate this method. Other limitations include the restriction to SARS-CoV-2–naïve individuals, the restriction to pre-Omicron strains, and the relatively short follow-up for pre-Delta COVID-19, leaving large uncertainty about durability after 90 days.
Nearly all of the world’s population has been vaccinated with at least 1 dose and/or naturally infected with SARS-CoV-2, and COVID-19 vaccines are now used to boost the immune response towards variant strains. Currently, the Centers for Disease Control and Prevention (CDC) and European Medicines Agency recommend COVID-19 booster doses with XBB.1.5 variant vaccines, and both mRNA and protein formulations are available. Because immunity against SARS-CoV-2 wanes, enhancing durability of the boost is a key goal of next-generation vaccines [21]. While our follow-up is shorter and sample size smaller compared with other vaccines, this analysis suggests that, for the period of follow-up studied here, NVX-COV2373 has a durability profile similar to the mRNA and vector-based vaccines for pre-Delta and Delta COVID-19. Heterologous boosting with different vaccine platforms may improve durability [2] and studies to evaluate heterologous boosting would provide useful data to inform vaccine recommendations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Dean Follmann, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Allyson Mateja, Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research, Frederick, Maryland, USA.
Michael P Fay, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Craig A Magaret, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Yunda Huang, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Youyi Fong, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Heather Angier, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Martha Nason, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Cynthia L Gay, School of Medicine, University of North Carolina, Chapel Hill, North Carolina, USA.
Karen Kotloff, School of Medicine, University of Maryland, Baltimore, Maryland, USA.
Wayne Woo, Novavax, Gaithersburg, Maryland, USA.
Iksung Cho, Novavax, Gaithersburg, Maryland, USA.
Lisa M Dunkle, Novavax, Gaithersburg, Maryland, USA.
Notes
Author contributions. D. F. conceived the study, performed statistical analysis, and wrote the first version of manuscript; A. M., M. P. F., and C. A. M.: statistical analysis and manuscript review; Y. H., Y. F., M. N., W. W., and I. C.: manuscript review and trial design; H. A.: editing and manuscript review; C. L. G. and K. K.: manuscript review, trial design, and enrolled patients; L. M. D.: manuscript review, trial design, and PREVENT-19 principal investigator.
Acknowledgments. The authors thank the volunteers who participated in the PREVENT-19 trial. They also acknowledge all the laboratories that submitted sequences to GISAID that were used in this analysis. To view the contributors of each individual sequence with details such as accession number, Virus name, Collection date, Originating Lab and Submitting Lab, and the list of Authors, visit DOI 10.55876/gis8.231018wo.
Data availability. Study information is available at https://clinicaltrials.gov/ct2/show/NCT04611802. The sequences used in this analysis were obtained from GISAID and are available using the EPI_SET ID “EPI_SET_231018wo.” They can also be accessed via DOI “10.55876/gis8.231018wo.”
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by Novavax; the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (contract Operation Warp Speed: Novavax Project Agreement number 1 under Medical CBRN [Chemical, Biological, Radiological, and Nuclear] Defense Consortium base agreement no. 2020-530; Department of Defense no. W911QY20C0077); and the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health. The NIAID provides grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (UM1 AI68614) to Lawrence Corey, the HVTN Statistics and Data Management Center (UM1 AI68635) to Peter B. Gilbert and Yunda Huang, the HVTN Laboratory Center (UM1 AI68618) to M. Julie McElrath, the HIV Prevention Trials Network Leadership and Operations Center (UM1 AI68619) to Myron Cohen, the AIDS Clinical Trials Group Leadership and Operations Center (UM1 AI68636) to Judith Currier, and the Infectious Diseases Clinical Research Consortium leadership group (UM1 AI148684) to David Stephens. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. 75N91019D00024.
References
- 1. Centers for Disease Control and Prevention . Variants of the virus. Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/index.html. Accessed 1 November 2023.
- 2. Couzin-Frankel J. Should you pick Novavax’s COVID-19 shot over mRNA options? Science 2023; 382:141–2. [DOI] [PubMed] [Google Scholar]
- 3. Lin DY, Gu Y, Wheeler B, et al. Effectiveness of COVID-19 vaccines over a 9-month period in North Carolina. N Engl J Med 2022; 386:933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin DY, Xu Y, Gu Y, Zeng D, Sunny SK, Moore Z. Durability of bivalent boosters against omicron subvariants. N Engl J Med 2023; 388:1818–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shinde V, Bhikha S, Hoosain Z, et al. Efficacy of NVX-CoV2373 COVID-19 vaccine against the B.1.351 variant. N Engl J Med 2021; 384:1899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med 2021; 385:1172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunkle LM, Kotloff KL, Gay CL, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med 2022; 386:531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Follmann D, Fintzi J, Fay MP, et al. A deferred-vaccination design to assess durability of COVID-19 vaccine effect after the placebo group is vaccinated. Ann Intern Med 2021; 174:1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khare S, Gurry C, Freitas L, et al. GISAID’s role in pandemic response. China CDC Wkly 2021; 3:1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. GISAID . Home page. Available at: https://gisaid.org/about-us/mission/. Accessed 11 November 2023.
- 11. Fintzi J, Follmann D. Assessing vaccine durability in randomized trials following placebo crossover. Stat Med 2021; 40:5983–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Follmann D, Fay M, Magaret C. Estimation of vaccine efficacy for variants that emerge after the placebo group is vaccinated. Stat Med 2022; 41:3076–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin DY, Zeng D, Gu Y, Krause PR, Fleming TR. Reliably assessing duration of protection for coronavirus disease 2019 vaccines. J Infect Dis 2022; 226:1863–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nordholt ES. Imputation: methods, simulation experiments and practical examples. Int Stat Rev 1998; 66:157–80. [Google Scholar]
- 15. Áñez G, Dunkle LM, Gay CL, et al. Safety, immunogenicity, and efficacy of the NVX-CoV2373 COVID-19 vaccine in adolescents: a randomized clinical trial. JAMA Netw Open 2023; 6:e239135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385:1774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med 2021; 384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin DY, Baden LR, El Sahly HM, et al. Durability of protection against symptomatic COVID-19 among participants of the mRNA-1273 SARS-CoV-2 vaccine trial. JAMA Netw Open 2022; 5:e2215984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baden LR, El Sahly HM, Essink B, et al. Phase 3 trial of mRNA-1273 during the Delta-variant surge. N Engl J Med 2021; 385:2485–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becerra X, Jha A. Project NextGen—defeating SARS-CoV-2 and preparing for the next pandemic. N Engl J Med 2023; 389:773–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.