Abstract
Background
This is an update of a Cochrane review first published in The Cochrane Library in Issue 4, 2007 and previously updated in 2011.
Unilateral peripheral vestibular dysfunction (UPVD) can occur as a result of disease, trauma or postoperatively. The dysfunction is characterised by complaints of dizziness, visual or gaze disturbances and balance impairment. Current management includes medication, physical manoeuvres and exercise regimes, the latter known collectively as vestibular rehabilitation.
Objectives
To assess the effectiveness of vestibular rehabilitation in the adult, community‐dwelling population of people with symptomatic unilateral peripheral vestibular dysfunction.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; ISRCTN and additional sources for published and unpublished trials. The most recent search was 18 January 2014.
Selection criteria
Randomised controlled trials of adults living in the community, diagnosed with symptomatic unilateral peripheral vestibular dysfunction. We sought comparisons of vestibular rehabilitation versus control (e.g. placebo), other treatment (non‐vestibular rehabilitation, e.g. pharmacological) or another form of vestibular rehabilitation. Our primary outcome measure was change in the specified symptomatology (for example, proportion with dizziness resolved, frequency or severity of dizziness). Secondary outcomes were measures of function, quality of life and/or measure(s) of physiological status, where reproducibility has been confirmed and shown to be relevant or related to health status (for example, posturography), and adverse effects
Data collection and analysis
We used the standard methodological procedures expected by The Cochrane Collaboration.
Main results
We included 39 studies involving 2441 participants with unilateral peripheral vestibular disorders in the review. Trials addressed the effectiveness of vestibular rehabilitation against control/sham interventions, medical interventions or other forms of vestibular rehabilitation. Non‐blinding of outcome assessors and selective reporting were threats that may have biased the results in 25% of studies, but otherwise there was a low risk of selection or attrition bias.
Individual and pooled analyses of the primary outcome, frequency of dizziness, showed a statistically significant effect in favour of vestibular rehabilitation over control or no intervention (odds ratio (OR) 2.67, 95% confidence interval (CI) 1.85 to 3.86; four studies, 565 participants). Secondary outcomes measures related to levels of activity or participation measured, for example, with the Dizziness Handicap Inventory, which also showed a strong trend towards significant differences between the groups (standardised mean difference (SMD) ‐0.83, 95% CI ‐1.02 to ‐0.64). The exception to this was when movement‐based vestibular rehabilitation was compared to physical manoeuvres for benign paroxysmal positional vertigo (BPPV), where the latter was shown to be superior in cure rate in the short term (OR 0.19, 95% CI 0.07 to 0.49). There were no reported adverse effects.
Authors' conclusions
There is moderate to strong evidence that vestibular rehabilitation is a safe, effective management for unilateral peripheral vestibular dysfunction, based on a number of high‐quality randomised controlled trials. There is moderate evidence that vestibular rehabilitation resolves symptoms and improves functioning in the medium term. However, there is evidence that for the specific diagnostic group of BPPV, physical (repositioning) manoeuvres are more effective in the short term than exercise‐based vestibular rehabilitation; although a combination of the two is effective for longer‐term functional recovery. There is insufficient evidence to discriminate between differing forms of vestibular rehabilitation.
Keywords: Adult; Humans; Vestibule, Labyrinth; Vestibule, Labyrinth/physiopathology; Dizziness; Dizziness/rehabilitation; Exercise Movement Techniques; Postural Balance; Randomized Controlled Trials as Topic; Sensation Disorders; Sensation Disorders/rehabilitation; Vertigo; Vertigo/rehabilitation; Vestibular Diseases; Vestibular Diseases/physiopathology; Vestibular Diseases/rehabilitation
Plain language summary
Vestibular rehabilitation to improve dizziness, balance and mobility in patients with unilateral peripheral vestibular dysfunction
Background
People with vestibular problems often experience dizziness and trouble with vision, balance or mobility. The vestibular disorders that are called unilateral and peripheral (UPVD) are those that affect one side of the vestibular system (unilateral) and only the portion of the system that is outside of the brain (peripheral ‐ part of the inner ear). Examples of these disorders include benign paroxysmal positional vertigo (BPPV), vestibular neuritis, labyrinthitis, one‐sided Ménière's disease or vestibular problems following surgical procedures such as labyrinthectomy or removal of an acoustic neuroma. Vestibular rehabilitation for these disorders is becoming increasingly used and involves various movement‐based regimes. Components of vestibular rehabilitation may involve learning to bring on the symptoms to 'desensitise' the vestibular system, learning to co‐ordinate eye and head movements, improving balance and walking skills, and learning about the condition and how to cope or become more active.
Study characteristics
We found 39 randomised controlled trials (involving 2441 participants) that investigated the use of vestibular rehabilitation in this group of disorders. All studies used a form of vestibular rehabilitation and involved adults who lived in the community with symptomatic, confirmed UPVD. The studies were varied in that they compared vestibular rehabilitation with other forms of management (for example, medication, usual care or passive manoeuvres), with control or placebo interventions or with other forms of vestibular rehabilitation. Another source of variation between studies was the use of different outcome measures (for example, reports of dizziness, improvements in balance, vision or walking, or ability to participate in daily life).
Key results
Due to the variation between studies, only limited pooling (combining) of data was possible. The results of four studies could be combined, which demonstrated that vestibular rehabilitation was more effective than control or sham interventions in improving subjective reports of dizziness, and in improving participation in life roles. Two studies gave a combined result in favour of vestibular rehabilitation for improving walking. Other single studies all found in favour of vestibular rehabilitation for improvements in areas such as balance, vision and activities of daily living. The exception to these findings was for the specific group of people with BPPV, where comparisons of vestibular rehabilitation with specific physical repositioning manoeuvres showed that these manoeuvres were more effective in dizziness symptom reduction, particularly in the short term. However, other studies demonstrated that combining the manoeuvres with vestibular rehabilitation was effective in improving functional recovery in the longer term. There were no reports of adverse effects following any vestibular rehabilitation. In the studies with a follow‐up assessment (3 to 12 months) positive effects were maintained. There was no evidence that one form of vestibular rehabilitation is superior to another. There is a growing and consistent body of evidence to support the use of vestibular rehabilitation for people with dizziness and functional loss as a result of UPVD.
Quality of the evidence
The studies were generally of moderate to high quality but were varied in their methods. This evidence is up to date to 18 January 2014.
Background
This is an update of a Cochrane review first published in The Cochrane Library in Issue 4, 2007 and previously updated in 2011.
Description of the condition
People with dysfunction within the vestibular system (vestibulopathy) often complain of dizziness, visual or gaze disturbances, and balance disorders. Dizziness alone accounts for nearly seven million doctor visits per annum in the US (Gans 2002). These impairments lead to significant activity and participation restrictions for the person affected (Perez 2001). The cause of the dysfunction can be a disease‐related pathology or trauma and can be sited in the central (brain) or peripheral (inner ear) portions of the vestibular system. More specifically, because the vestibular system is replicated symmetrically in the periphery, many commonly presenting vestibulopathies involve unilateral (asymmetrical) peripheral vestibular dysfunction (UPVD). Examples of these disorders include benign paroxysmal positional vertigo (BPPV), vestibular neuritis, Ménière's disease (and endolymphatic hydrops) and perilymphatic fistula. Unilateral peripheral dysfunction can also occur after surgical interventions such as unilateral labyrinthectomy or neurectomy (acoustic or vestibular) (Curthoys 2000; Fetter 2000). This review will only address the management of these unilateral peripheral diagnoses.
Table 1 contrasts the incidence, aetiology, symptomatology, diagnosis and specific management of the most prevalent unilateral peripheral vestibulopathies. Whilst there are many aspects specific to each group, there are commonalities in terms of presentation of symptoms that have been reported to be amenable to interventions such as vestibular rehabilitation.
1. Unilateral peripheral vestibulopathies.
| Vestibulopathy | Incidence | Aetiology | Symptoms | Diagnosis | Treatment |
| Benign paroxysmal positional vertigo (BPPV) (idiopathic) (Cabrera Kang 2013; Hilton 2014) | All age groups Peak 40 to 60 years 11 to 64 per 100,000 pa Females > males |
Various: Canalithiasis (free‐floating debris in semicircular canals) Cupulolithiasis (debris attached to cupula) |
Episodic vertigo after rapid head motion, lasting seconds to 1 minute; +/‐ nausea; some balance deficits; nystagmus (latency, fatigue, rotatory and beating) | Dix‐Hallpike test (post) (Dix 1952) Lateral head‐trunk tilt (Brandt 1999) etc. Use of ENG to record nystagmus |
1. Repositioning manoeuvre/s relative to semicircular canal (Cabrera Kang 2013; Epley 1992; Semont 1988) 2. VR 3. Vestibular suppressant medication for symptom relief 4. Vestibular neurectomy or post‐semicircular canal obliteration |
| Vestibular neuritis (Gans 2002)/neuronitis and labyrinthitis (Strupp 1998) | Unknown | Unclear Viral, autoimmune or vascular mechanisms Viral or bacterial infection of labyrinthine fluids (labyrinthitis) or CN VIII (neuritis) |
Acute onset Distressing tonal imbalance producing: rotatory vertigo; spontaneous nystagmus (horizontal); falls to the affected side; nausea |
From history and presentation ENG and caloric irrigation show reduced or no response in horizontal semicircular canal; ocular tilt reaction |
Symptomatic medication (vestibular suppressants) Bacterial/viral management VR |
| Ménière's disease (Scott 1994; Strupp 2013) | Unknown Equal males and females Greatest in 3rd and 4th decades |
Unclear Endolymphatic hydrops |
Acute: unpredictable and episodic hearing loss, tinnitus and vertigo, +/‐ nausea, vomiting, visual disturbance, anxiety, motion sensitivity Chronic: UPVD or bilateral PVD |
History and presentation Audiogram ENG with calorics Imaging the inner ear with high‐resolution MRI after tympanic gadolinium injection |
Acute: medication (transtympanic glucocorticoids, antihistamines, suppressants)
diet; low salt; diuretics Chronic: VR, psychological support, surgery (see next row) |
| Postoperative:
Labyrinthectomy Neurectomy Intra‐tympanic injection of gentamycin |
Unknown | For management of intractable UPVD, tumour removal, Ménière's | UPVD, i.e. spontaneous nystagmus, vertigo, disequilibrium, VOR gain, postural instability | — | VR Symptomatic medication (Dowdal‐Osborn 2002) |
| Perilymphatic fistula (Baloh 2003) | Unknown | History of head trauma, barotraumas or sudden strain; may be associated with chronic otitis or cholesteatoma; perforation of tympanic membrane | Unilateral hearing loss, vertigo, nystagmus | Induce symptoms by pressure in external ear canal Positive head thrust ENG Audiography |
Symptomatic medication Surgical packing |
ENG: electronystagmography MRI: magnetic resonance imaging pa: per year UPVD: unilateral peripheral vestibular disorder VOR: vestibular ocular reflex VR: vestibular rehabilitation
General treatment and management options
It has been reported that in many cases of chronic vestibular dysfunction, pharmacological and surgical interventions offer limited improvement (Smith‐Wheelock 1991). Medication is often directed at vestibular suppression and/or control of symptoms, such as nausea, or for specific disease processes, such as control of infection. Surgery has a limited role in the management of patients with vestibular dysfunction. It can be used as a 'last resort' in patients whose symptoms are attributable to episodic fluctuation in peripheral function. In such patients, a procedure may be undertaken to remove function from a peripheral vestibular structure (by, for example, labyrinthectomy) or to interrupt the central input of vestibular signals (by vestibular nerve section). Fluctuating vestibular function is thereby replaced with a fixed vestibular deficit. Surgery may also have a role in certain specific conditions, such as the repair of a perilymphatic fistula or removal of an acoustic neuroma.
Description of the intervention
There has been increasing interest in the use of vestibular rehabilitation for the treatment or management of patients with vestibular dysfunction (Chang 2008; Giray 2009; Hoffer 2011). Vestibular rehabilitation is an exercise‐based group of approaches that began with the aim of maximising central nervous system compensation for vestibular pathology (Hoffer 2011). The original protocols by Cooksey and Cawthorne used group activities in a hierarchy of difficulty to challenge the central nervous system (Cooksey 1946). More recently, specific components have been further defined in the vestibular rehabilitation armamentarium (Herdman 2000), each having differing physiological or behavioural rationales as summarised below:
Compensatory responses (for positional or motion‐provoked symptoms), based on the inherent plasticity of the central nervous system and using motion to habituate or reduce responsiveness to repetitive stimuli and to re‐balance tonic activity within the vestibular nuclei (Gans 2002). Whilst this process is often termed habituation it is more likely to be a compensatory or neuroplastic process (Hain 2011), rather than a physiological synaptic habituation response.
Adaptation for visual‐vestibular interaction (gaze stabilisation) and possibly eye/hand co‐ordination, using repetitive and provocative movements of the head and/or eyes to reduce error and restore vestibulo‐ocular reflex (VOR) gain (Balaban 2012; Cullen 2009).
Substitution promotes the use of individual or combinations of sensory inputs (such as visual or somatosensory) to bias use away from the dysfunctional vestibular input or conversely to strengthen use and drive compensation.
Postural control exercises, falls prevention, relaxation training, (re)conditioning activities and functional/occupational retraining are based on motor learning principles to change movement behaviour and/or to promote movement fitness.
In addition, there are specific repositioning manoeuvres that may be incorporated into the overall vestibular rehabilitation package for particular diagnostic groups of vestibular dysfunction (for example, benign paroxysmal positional vertigo) (Hilton 2014; Hunt 2012). These manoeuvres (e.g. canalith repositioning manoeuvres or Epley, Semont and Liberatory) are performed on the patient (rather than the patient performing exercises) and are based on a mechanical rationale to shift vestibular debris. Such techniques are not the focus of this review.
Why it is important to do this review
The symptoms and signs of vestibular dysfunction of varying aetiologies are frequent, and often chronic and disabling. Differential diagnosis between possible pathologies is often difficult, with many patients receiving a label of 'unilateral vestibulopathy of unknown cause' (Baloh 2003). Vestibular rehabilitation is a growing method used to reduce resultant impairments and drive adaptation, and is predominantly management‐based (in that it is not 'curative'). Furthermore, vestibular rehabilitation tends to be delivered, and investigated, as a package and prescription is based on the presence of symptoms rather than a specific diagnosis. This review updates the previous Cochrane reviews of 2011 and 2007 for vestibular rehabilitation and a second general review also published in 2007 for a broader range of vestibular disorders conducted by Hansson (Hansson 2007).
Objectives
To assess the effectiveness of vestibular rehabilitation in the adult, community‐dwelling population of people with symptomatic unilateral peripheral vestibular dysfunction.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Community‐dwelling adults with vestibular dysfunction of unilateral peripheral origin, experiencing a combination of symptoms that may include one or all of the following: dizziness, vertigo, balance deficits (dysequilibrium), visual or gaze disturbances.
Participants with a diagnosis of a symptomatic unilateral, peripheral vestibular dysfunction, named as: peripheral vestibular hypofunction, vestibular neuritis, acoustic neuroma/schwannoma, perilymphatic fistula, Ménière's disease, benign paroxysmal positional vertigo or a combination of these. In the case of a diagnosis of Ménière's disease the participants are in the late stage with a fixed (non‐fluctuating) vestibular deficit. In some instances the authors reported including individuals with central or bilateral vestibular disorders. We contacted authors to obtain results separately for those with UPVD, and if this was not possible we included studies provided those with central and/or peripheral disorders numbered less than 10% of the sample size.
Types of interventions
Interventions described as 'vestibular rehabilitation' that are predominantly exercise and movement‐based, excluding specific (passive) repositioning manoeuvres.
Vestibular rehabilitation does not include medical, electrophysiological or pharmacological management.
Possible comparison interventions from the literature included:
vestibular rehabilitation versus control (placebo, sham or usual care);
vestibular rehabilitation versus other treatment (e.g. pharmacological or surgical); and
vestibular rehabilitation of one type versus another form of vestibular rehabilitation.
Types of outcome measures
Primary outcomes
Measure(s) of change in the specified symptomatology (for example, proportion with dizziness resolved, frequency or severity of dizziness). Symptomatic ratings must be reported and recorded pre‐ and post‐trial.
Secondary outcomes
Measure of function, quality of life and/or measure(s) of physiological status, where reproducibility has been confirmed and shown to be relevant or related to health status (for example, posturography). We also included adverse effects a secondary outcome.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 18 January 2014, following previous searches in July 2010 and March 2007.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL 2013, Issue 12); PubMed; EMBASE; AMED; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; ISRCTN; ClinicalTrials.gov; ICTRP; Google Scholar and Google. In searches prior to 2013, we also searched BIOSIS Previews 1926 to 2012 and CNKI.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Handbook 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Selection of studies
One of the authors retrieved papers from the identified lists on the basis of the title and abstract. The two authors then reviewed these in full against the established criteria and confirmed them as eligible for consideration. Where there was disagreement between the authors about the inclusion/exclusion criteria, we consulted a third expert and reached a consensus decision.
Data extraction and management
The two authors extracted data from the included studies independently using standardised data forms. Data included participant characteristics (number, age, gender), eligibility and exclusion criteria, setting, description of intervention/s and outcomes. Both authors independently extracted data and we resolved any differences in opinion by discussion and consensus, or by consulting a third expert if needed. In the event of unpublished studies, particularly those with published protocols and where data were incomplete in the published papers, we contacted the trial authors to obtain further details. We did not transform data for reproduction in figures or graphs.
Assessment of risk of bias in included studies
The two authors undertook assessment of the risk of bias of the included trials independently, with the following taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We used the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involves describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias.
We also reported risk of bias as part of the analysis of findings.
Data synthesis
We extracted and analysed data to calculate odds ratios (OR) (fixed‐effect), 95% confidence intervals (CI) and individual and total effect sizes. This required the identification of the number of participants in each group in each trial and total number (for dichotomous data) and number of participants plus mean and standard deviations for each group (for continuous outcome data). We used the standardised mean difference (SMD) for continuous data, and the mean difference (MD) for outcomes from single studies.
There was considerable variation between trials with respect to clinical presentation, the types of exercises included in vestibular rehabilitation and the settings in which the trial was conducted (e.g. community with a booklet‐guided approach compared to a laboratory setting). We assessed heterogeneity between trials with the I2 statistic. Where significant heterogeneity was present, we attempted to explain the differences based on the patient clinical characteristics and interventions of the included studies. We performed neither sensitivity analysis nor subgroup analyses due to the small number of trials that could be pooled for the analysis of the primary outcome.
Results
Description of studies
Results of the search
The current search in January 2014 yielded 1184 titles: 1077 were removed in first‐level screening (i.e. removal of duplicates and clearly irrelevant references), leaving 107 studies which we retrieved in full, where possible. After excluding protocols and trials in progress, we reviewed 96 studies and 12 of these met the inclusion criteria (Basta 2011; Cakrt 2010; Foster 2012; Garcia 2013; Karanjai 2010; Marioni 2013; Morozetti 2011; Pavlou 2012; Rossi‐Izquierdo 2011; Rossi‐Izquierdo 2013; Winkler 2011; Yardley 2012). Five studies are currently in progress and we contacted all authors but results were not available for meta‐analysis. We excluded a further 13 studies (Amor‐Dorado 2012; Bielinska 2012; Cronin 2011; Gurkov 2012; Ipek 2011; Krueger 2010; Lauenroth 2012; Maciaszek J, Osinski 2012; Miranda 2010; Rossi‐Izquierdo 2013a; Sparrer 2013; Steenerson 1996; Wrisley 2011). The current review therefore includes a total of 39 studies (2441 participants). Figure 1 provides a summary of the search process.
1.
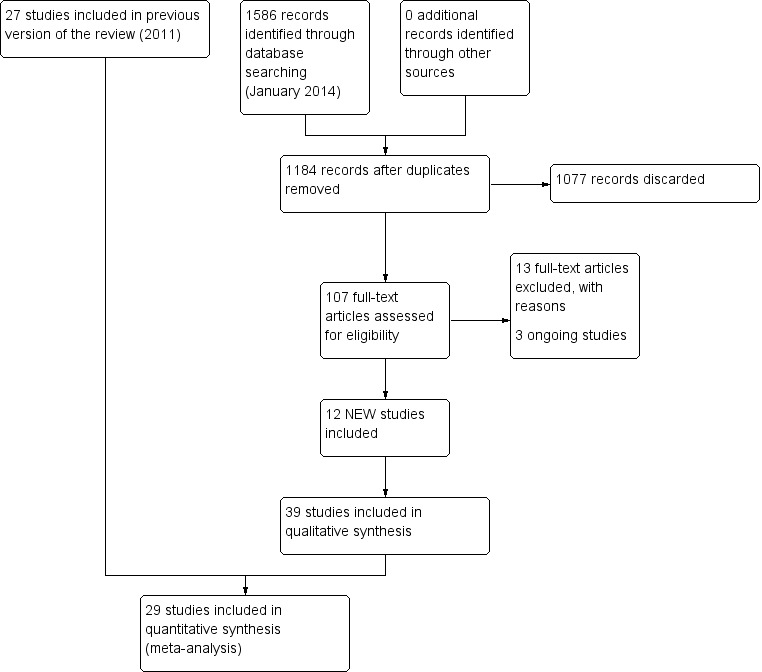
Study flow diagram for 2014 update.
From the 2011 update searches we retrieved a total of 802 references: we removed 652 of these after screening, leaving 150 references for further consideration. Of the 15 retrieved from this list, we ultimately included six studies and added these to the original 21 studies. We excluded a further 10 because they did not meet the review inclusion criteria (see Characteristics of excluded studies). A further four citations reported trial protocols, however the authors did not respond to our request for clarification of completion. The 2011 review therefore included a total of 27 studies (1668 participants) and we excluded a total of 21 studies.
In searches for the 2007 review, we retrieved a total of 232 papers and reviewed them against the inclusion criteria, with 32 being accepted for initial inclusion and quality assessment. After quality appraisal and full consideration we excluded a further 11 for reasons such as subject inclusion of mixed aetiology (e.g. unilateral and bilateral vestibular dysfunction, inclusion of vestibulopathy of central origin or of unknown aetiology), lack of clear intervention or lack of randomisation (see Characteristics of excluded studies table). We included several studies investigating patients with dizziness from a variety of aetiologies (unilateral and bilateral vestibular dysfunction) because they differentiated between the two groups in the analyses (Krebs 2003; Pavlou 2004; Scott 1994; Szturm 1994). This enabled the UPVD patients to be analysed separately. Yardley 1998 and Yardley 2004 also included subjects with dizziness of vestibular origin with mixed aetiology but stipulated that central pathology was excluded. We also decided that because these authors confirmed dizziness as the primary symptom that this would effectively confirm an asymmetrical pathology. We also noted that several papers reported the same trial but with differing outcome measures in each of the papers, notably Cohen 2003 and McGibbon 2004, although the two reports of the latter study were later excluded due to mixed aetiology.
Included studies
See Characteristics of included studies table.
Design
All studies were of parallel design and while they all reported randomisation the majority were unclear in their description of the method of allocation or generation (see Risk of bias in included studies).
The comparisons varied, with 16 investigating vestibular rehabilitation versus placebo or sham interventions. Seven studies compared vestibular rehabilitation with a non‐vestibular rehabilitation intervention. Eighteen studies compared a form of vestibular rehabilitation with one or more other forms of vestibular rehabilitation. Some studies involved multiple comparisons, for example vestibular rehabilitation versus control (sham) versus non‐vestibular rehabilitation (medication).
Sample sizes
A total of 2441 participants participated in the 39 studies, with a mean sample size of 64.7 and a range of 14 to 360. Sample size calculations were rarely reported and this omission (with probable poor statistical power) in the smaller studies was a frequent methodological flaw.
Settings
Five studies investigated vestibular rehabilitation in an acute hospital setting, with the remainder being conducted in community or outpatient environments. Some studies required the vestibular rehabilitation intervention to be performed in the outpatient clinic, others established programmes to be performed in the home or more frequently a combination of the two was administered.
Participants
Participants were all adults, living in the community under normal circumstances. The five studies investigating vestibular rehabilitation in the hospital setting recruited participants who were community dwellers pre‐ and postoperatively. Whilst the acute hospital inpatients were ultimately community dwellers, we separated these out in the final discussion. Age range varied, with most studies reporting a higher recruitment of people in the 65 plus range, reflecting the increasing incidence of dizziness with increasing age.
Eight studies investigated benign paroxysmal positional vertigo, six investigated acute unilateral vestibular loss, five investigated postoperative patients (either acoustic neuroma resection, removal of vestibular schwannoma or ablative vestibular surgery), three specifically investigated Ménière's (non‐acute phase) and the rest reported their sample variously as having chronic unilateral vestibular weakness, hypofunction, dysfunction or dizziness of vestibular origin (including labyrinthitis, neuronitis and other mixed or idiopathic unilateral peripheral vestibular dysfunction pathologies).
Interventions
As expected most studies included a mixture of the various components of vestibular rehabilitation, the most common combination being habituation (movement‐provoking) with gaze stabilising (adaptation), balance and gait/activity training (27). Other additions to this type of package included education (three), booklet‐based (three), sensory substitution (three) and relaxation (two). Five studies described single component vestibular rehabilitation: these included Varela 2001 that investigated Brandt‐Daroff exercises (a form of habituation), Cohen 2003 that investigated rapid versus slow head movements (habituation) and Scott 1994 that investigated relaxation. Two studies compared individualised vestibular rehabilitation with a generic vestibular rehabilitation programme (Szturm 1994; Zimbelman 1999).
Control or placebo interventions involved either usual care or some form of sham exercise that did not target compensatory or adaptation processes (e.g. sham manoeuvres, range of motion, general conditioning, general instructions or strength training).
Studies that compared vestibular rehabilitation with non‐vestibular rehabilitation interventions were also varied. Chang 2008, Cohen 2005, Toledo 2000 and Varela 2001 compared exercise‐based vestibular rehabilitation with repositioning manoeuvres; Kulcu 2008 and Horak 1992 compared vestibular rehabilitation with medication; Scott 1994 compared vestibular rehabilitation (relaxation) with electrical stimulation; and Barozzi 2006 compared oculomotor exercises (adaptation vestibular rehabilitation) with electrical stimulation.
Outcomes
There was considerable variation in the outcome measures used. We considered those that related to symptomatology (dizziness, dysequilibrium or visual disturbance) or functional status (gait, activities of daily living ‐ ADL). Secondary outcome measures that have previously been shown to relate to function, such as visual acuity or posturography (also described as computerised dynamic posturography or Equi‐test), were also considered (Balaguer Garcia 2012). Other reported physiological measures, such as electronystagmography (ENG) and tests for vestibular ocular reflex (VOR) and ocular torsion, subjective visual vertical or biomechanical tests of kinematic and kinetic parameters, were not considered because they have not been directly related to health or functional status.
The outcome measures included in the analyses were as follows.
Primary outcomes
Subjective measures of change in symptoms (impairments):
Dizziness cure rate ‐ 'cure' defined as the disappearance of the sensation of dizziness (Karanjai 2010; Varela 2001): dichotomous data of proportion cured.
Subjective improvement in dizziness ‐ subjects asked to nominate improvement (better) or no change/worsening in subjective experience of dizziness (dichotomous) (Foster 2012; Horak 1992; Karanjai 2010; Morozetti 2011; Yardley 1998; Yardley 2004; Yardley 2006; Zimbelman 1999).
Vertigo Symptom Scale (VSS) ‐ shortened version (14‐item), measuring frequency of dizziness/vertigo, imbalance and related autonomic symptoms during the past month, with a higher score indicating greater symptoms (score range 0 to 60) (Basta 2011; Pavlou 2004; Yardley 1998; Yardley 2004; Yardley 2006; Yardley 2012). (Component related to vertigo reported (VSS‐V), second component related to autonomic/somatic anxiety (VSS‐A)).
Vertigo visual analogue scale (VAS) ‐ subjective rating of vertigo on a closed VAS ranging from 0 mm (no symptoms) to 100 mm (worst possible symptoms) (Kammerlind 2005).
Vertigo intensity ‐ subjective rating of intensity of vertigo on a five‐point qualitative scale from 1 (no vertigo) to 5 (severe) (Chang 2008; Cohen 2002; Cohen 2003; Garcia 2013; Morozetti 2011).
Vertigo frequency ‐ subjective rating of frequency of vertigo experiences on a four‐point scale from 0 (no episodes per day) to 3 (more than 10 episodes per day or constantly) (Cohen 2003).
Secondary outcomes
Objective measures of change in impairment, activity or participation:
Repetitive head movement task ‐ measure of standard head movements and resultant provocation (or not) of symptoms, scored as time to perform and intensity of elicited vertigo. Reduction in time and intensity scores indicates improvement (intensity scores not analysed) (Cohen 2003).
Dynamic visual acuity ‐ tests for visual acuity during head movements either under predictable conditions (patient moved own head) or unpredictable (head moved by tester), related to oscillopsia and scored as number of errors during tests (Herdman 2003).
Romberg test ‐ a measure of standing balance, as dichotomous data, scored as number of pass or fail scores (Herdman 1995). Also (sharpened) Romberg test (scores) ‐ static standing balance tests, timed in seconds where a higher score indicates better (longer) balance (Kammerlind 2005; Yardley 1998).
Sway path ‐ measure of standing balance, recording the length of the path of the centre of force (in two planes) during a given time and potentially under differing stance conditions, giving a total sway path measured in metres per minute where the smaller path indicates greater balance proficiency (Strupp 1998).
Posturography ‐ (computerised dynamic posturography) a battery of standing balance tests under prescribed variable conditions (sensory organisation test), which can be scored as composite scores and sensory ratios (compared to normative data, other variables available) (Basta 2011; Cakrt 2010; Cohen 2002; Cohen 2003; Marioni 2013; Pavlou 2004; Rossi‐Izquierdo 2011; Rossi‐Izquierdo 2013).
Dynamic Gait Index (DGI) ‐ scores eight mobility tasks (ranging from straight walking through to stair ascent/descent) to give a total score of 24 points (Chang 2008; Pavlou 2012; Teggi 2009; Vereeck 2008; Winkler 2011).
Gait ataxia ‐ dichotomous data, scored as the presence or absence of abnormal co‐ordination during walking (Herdman 1995), or as continuous data from deviations along a lined walking task (Cohen 2003).
Tandem walk ‐ test of dynamic balance and gait proficiency where the patient walks 15 steps forward then backward along a line, scored as the number of correct steps (performed heel to toe and on line), with a higher score indicating greater proficiency (Kammerlind 2005).
Vestibular dysfunction in activities of daily living (VD‐ADL) ‐ questionnaire to rate the impact of dizziness or vestibular dysfunction on primary activities of daily life, with a higher score indicating greater functional loss (Cohen 2003; Yardley 1998).
Vertigo Handicap Questionnaire (VHQ) ‐ shortened version (14‐item), which measures restriction of activity caused by dizziness and the social effects of this activity restriction (score range 0 to 56) (Cohen 2003; Yardley 1998).
Dizziness Handicap Inventory (DHI) ‐ measures patient perception of handicap related to dizziness (an indication of the effect of the symptom on participation or quality of life), where a higher score indicates greater dysfunction (Barozzi 2006; Basta 2011; Garcia 2013; Giray 2009; Morozetti 2011; Rossi‐Izquierdo 2011; Rossi‐Izquierdo 2013; Teggi 2009; Winkler 2011; Yardley 2004; Yardley 2006; Zimbelman 1999).
Beck Anxiety Inventory ‐ a self report measure of anxiety state (Pavlou 2012).
Situational Vertigo Questionnaire ‐ a self report measure of visually induced vertigo (Pavlou 2012).
Subjective health ‐ self report of current health status with respect to dizziness (Yardley 2012).
Follow‐up assessment
Follow‐up was variable, from none (12 studies) to between two, three, six and 12 months for the remaining studies.
Excluded studies
We excluded a total of 34 studies from the review (see Characteristics of excluded studies table). We excluded the majority of these because the participants included mixed aetiologies without separate analysis for those with UPVD (19) or because the study was not randomised (seven).
Ongoing studies
Our search identified two published protocols and a further three trials, which were identified from clinical trial registries. We contacted the primary investigators to determine whether results were available for inclusion in this review. Results are not yet available (see Characteristics of ongoing studies table).
Risk of bias in included studies
The risk of bias for each of the six domains is reported for each trial in the individual 'Risk of bias' tables (see Characteristics of included studies). A summary is also illustrated in Figure 2 and Figure 3. These figures most significantly demonstrate a marked deficiency in the reporting of the methods used to generate and conceal the randomisation process across the majority of studies. The other domains were more clearly reported and we generally evaluated them as low risk of bias.
2.
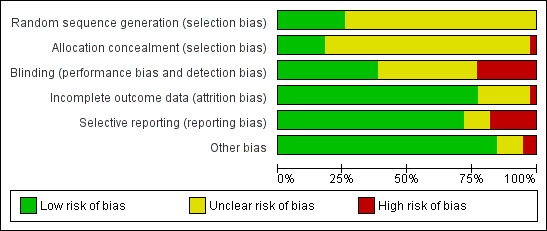
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
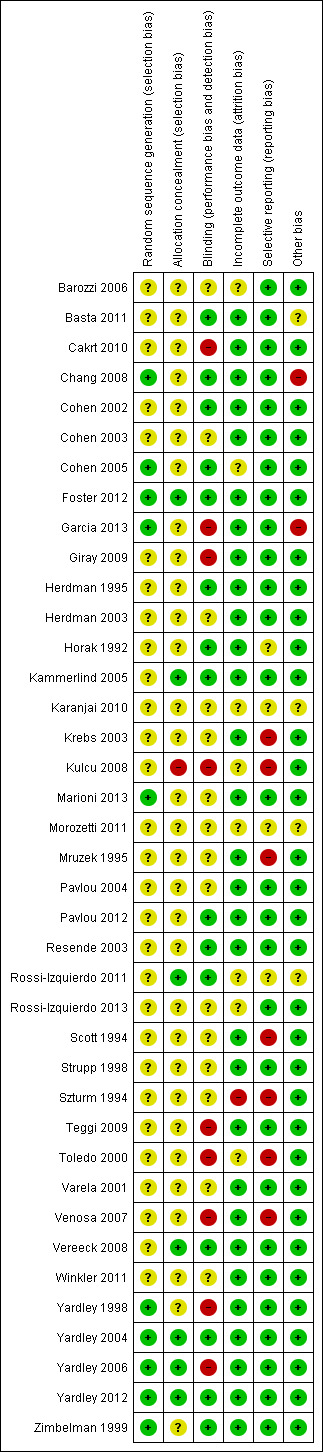
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
The majority of studies measured more than one aspect (symptomatology and/or function), therefore some participants appear in more than one section. Ten studies did not provide the necessary data to enable further analyses and therefore appear among the included studies but not in the meta‐analyses. The majority of all analyses contain data from only one study each, due to the heterogeneity of outcome measures within each comparison. Three studies potentially appear in more than one comparison as they had three‐way (or more) group comparisons (Cohen 2005; Horak 1992; Yardley 2006). Data from Vereeck 2008 appear twice in one analysis but this is reporting separate subgroups based on age (under 50 and over 50 years old).
A summary of individual study results can be found in Table 2.
2. Study results.
| Study ID | Inclusion criteria | Intervention/comparator | Result |
| Barozzi 2006 | Unilateral peripheral vestibular deficit, 1 to 6 months after the acute phase, diagnosed by clinical examination, CDP, videonystagmography, rotatory chair and caloric tests demonstrating a canal paresis of at least 25% | Intervention groups (n not stated): oculomotor rehabilitation (adaptation) Comparator group (n not stated): vestibular electrical stimulation |
No significant differences between groups |
| Basta 2011 | Experienced balance disorder for more than 12 months due to the following conditions: canal paresis, otolith disorder, removal of an acoustic neuroma, microvascular compression syndrome, Parkinson's disease, presbyvertigo | Intervention group (n = 59): vibrotactile neurofeedback training and vestibular rehabilitation exercises performed daily (15 minutes) over 2 weeks with the Vertiguard system Comparator group (n = 9): sham Vertiguard device and vestibular rehabilitation exercises |
Significant reduction in trunk and ankle sway and improved VSS scores on the Vertiguard group. No changes observed in the sham Vertiguard group |
| Cakrt 2010 | Participants undergoing retrosigmoid microsurgical removal of vestibular schwannoma | Intervention group (n = 9): received visual feedback while performing VR using the BalanceMaster Comparator group (n = 8): control group received VR without feedback |
2‐week intervention post acoustic neuroma removal, significant improvement in 5 out of 7 centre of pressure parameters in quiet stance on foam in the visual feedback group only |
| Chang 2008 | First ever attack of unilateral posterior canal BPPV, diagnosed by neurologist and clinical examination | Intervention group (n = 13): canalith repositioning technique (CRT) and vestibular exercises Comparator group (n = 13): CRT only |
Intervention group demonstrated a significant improvement in single leg stance with eyes closed at the 2‐week assessment, and static balance and DGI at the 4‐week assessment |
| Cohen 2002 | Acoustic neuroma resection ‐ postoperative (1 week ‐ acute) diagnosed by history, audiometry, MRI | Intervention group (n = 16): VR (head exercises) Comparator group (n = 15): control (attention only) |
No significant difference between groups |
| Cohen 2003 | Chronic vestibulopathy (labyrinthitis or neuronitis of more than 2 months) diagnosed by physician using posturography, calorics and oculomotor test battery | Intervention group (n = 13): VR (slow head exercises ‐ habituation) Comparator group 1 (n = 22): VR (rapid head exercises) Comparator group 2 (n = 18): VR (rapid plus attention) |
All groups significantly improved for VI, VF, DHI, VSS VHQ no change |
| Cohen 2005 | Unilateral BPPV (post SC) diagnosed by physician (D‐H test), with dizziness for at least 1 week | Intervention group (n = 25): B‐D exercises Comparator group 1 (n = 25): habituation exercises Comparator group 2 (n = 24): CRM Comparator group 3 (n = 25): LM Comparator group 4 (n = 25): sham manoeuvre |
Manoeuvres (CRM and LM) better results than exercises (B‐D, habituation), both better than sham |
| Foster 2012 | Adults with a history suggestive of BPPV and Dix‐Hallpike manoeuvre consistent with unilateral posterior canal BPPV | Intervention group: (n = 33) half‐somersault manoeuvre was performed twice in the clinic and also given as a home exercise Comparator group: (n = 35) Epley manoeuvre was performed twice in the clinic and also given as a home exercise |
Significantly less nystagmus observed after the initial half‐somersault manoeuvre, but no difference in recurrence over the 6‐month follow‐up period |
| Garcia 2013 | Participants were included if they had Ménière's disease diagnosed by an ENT specialist, and had complaints of dizziness between exacerbations of their disease | Intervention group (n = 23): 12 rehabilitation sessions (twice weekly for 45 minutes) with virtual reality stimuli in a Balance Rehabilitation Unit, plus diet and lifestyle advice and betahistine Intervention group (n = 21): 12 stimulus enriched exercise sessions (twice weekly) on the Balance Rehabilitation Unit, plus diet and lifestyle advice and betahistine |
Intervention participants improved significantly on the DHI, dizziness analogue scale and had greater stability on posturography compared to control participants |
| Giray 2009 | Participants were diagnosed by a neuro‐otologist or neurologist with chronic decompensated unilateral peripheral vestibular deficit, secondary to peripheral vestibular dysfunction. Diagnosed by ENG, bithermal caloric test, ocular motor testing and positional testing | Intervention group (n = 20): VR incorporating adaptation, substitution, visual desensitisation and balance exercises Comparator group (n = 21): control, no input |
Significant improvements were seen in all parameters for the intervention group while there were no changes in the control group |
| Herdman 1995 | Participants post removal of acoustic neuroma. Diagnosed by MRI and surgically resected ‐ study performed in acute post period | Intervention group (n = 11): VR (adaptation to increase gain) plus ambulation exercises Comparator group (n = 8): smooth pursuit exercises (no head movement) plus ambulation exercises |
Intervention group significant improvements for dysequilibrium VAS, VOR to slow head movements, gait and posturography on day 6 compared to control group |
| Herdman 2003 | Unilateral vestibular hypofunction with abnormal DVA, diagnosed by caloric, rotary chair, positive head thrust | Intervention group (n = 13): VR (adaptation to enhance VOR) Comparator group (n = 8): placebo exercises designed to be "vestibular neutral" |
12/13 improved DVA in intervention group
1/8 improved DVA in comparator group Both improved VAS |
| Horak 1992 | Peripheral vestibular dysfunction diagnosed by neuro‐otologist for BPPV, inner ear concussion syndrome, reduced unilateral vestibular function, 18 to 60 years of age | Intervention group (n = 14): VR Comparator group 1 (n = 4): general conditioning exercises Comparator group 2 (n = 8): medication (meclizine or Valium) |
VR ‐ superior reduction in sway and increased SOOL DI decreased for both VR and medication 92% improvement rate with VR (75% with comparator group 1, 75% with comparator group 2) |
| Kammerlind 2005 | Acute unilateral vestibular loss confirmed by ENG with calorics | Intervention group (n = 28): VR (home exercises plus extra PT (habituation, adaptation, balance and gait) (extra PT included individualised instruction and further exercises) Comparator group (n = 26): VR (home exercises only) |
No significant difference between groups ‐ intensity not supported |
| Karanjai 2010 | Diagnosed with posterior canal BPPV through history and clinical examination (Dix‐Hallpike manoeuvre) | Intervention group: Brandt‐Daroff exercises 3 times a day for 2 weeks, n = 16 Comparator group 1: single Epley manoeuvre followed by post‐treatment instructions, n = 16 Comparator group 2: single Semont manoeuvre followed by post‐treatment instructions (sleep upright for 2 nights, then on the unaffected side for the next 5 nights), n = 16 |
Statistical analysis of the differences between groups not performed; 73% of participants overall reported resolution of symptoms with no recurrence at 3 months follow‐up |
| Krebs 2003 | Mixed diagnoses ‐ unilateral and bilateral peripheral vestibular dysfunction. Diagnosed by VOR gain, calorics etc. | Intervention group (n = 42): VR (adaptation, balance) Comparator group (n = 44): control (strength exercises) |
VR group significantly improved for gait speed and base of support measures UPVD and BVD groups improved equally though BVD were less functional at baseline |
| Kulcu 2008 | Diagnosed with BPPV and has undergone repositioning techniques by their otorhinolaryngologists but were still complaining of vertigo and dysequilibrium | Intervention group (n = 19): VR (Cawthorne‐Cooksey exercises) Comparator group (n = 19): medication (betahistine) |
The intervention group demonstrated significant improvements in the VSS and VDI at the end of the study (8 weeks) |
| Marioni 2013 | Adults aged 18 to 65 with acute unilateral peripheral vestibular disorder occurring within 2 weeks of entry into the study, with at least 50% weakness on videonystagmography with caloric testing | Intervention group (n = 15): posturography‐assisted VR Comparator group 1 (n = 15): group awaiting spontaneous compensation, no VR Comparator group 2 (controls, n = 10): healthy adults without a vestibular disorder |
Both groups of participants with vestibular dysfunction improved over the 6‐week intervention but only the posturography‐assisted VR improved postural control, which approximated the healthy controls |
| Morozetti 2011 | Adults with a chronic vestibular disorder diagnosed by otorhinolaryngologists | Intervention group (n = 10): home exercises based on vertical and horizontal vestibulo‐ocular reflex stimulation (VRS) Comparator group (n = 10): personalised VR home exercise programme |
Both groups improved over time but the personalised VR group reported less dizziness on VAS and greater gains on the DHI |
| Mruzek 1995 | Participants had been reviewed by a physician for acoustic neuroma or Ménière's disease and were referred for ablative surgery | Intervention group (n = 8): VR plus social reinforcement, 15 minutes, 2 x day plus a daily walk Comparator group 1 (n = 8): VR no social reinforcement Comparator group 2 (n = 8): general range of motion exercises plus social reinforcement |
All the same at 4 weeks Intervention group and comparator group 1 significant improvement for MSQ at 7 weeks Intervention group significant improvement for DHI at 8 weeks CDP no difference between groups |
| Pavlou 2004 | Peripheral vestibular disorder diagnosed by full vestibular examination | Intervention group (n = 20): VR (customised exercises, including gaze control and stability, balance training) Comparator group (n = 20): simulator (optokinetic disc to produce visual‐vestibular conflict plus above) |
Both groups improved significantly on posturography: intervention group more than comparator group Subjective symptom reports reduced for both (? any difference) Visual‐vertigo symptoms improved for intervention comparator group Depression reduced significantly for both groups: intervention group more than comparator group Anxiety reduced for both BBS not sensitive |
| Pavlou 2012 | Participants with a history of acute onset of vertigo and had a confirmed peripheral vestibular deficit on the basis of the caloric tests and/or rotational tests on ENG | Intervention group (n = 5): dynamic virtual reality, performed for 45 minutes twice weekly for 4 weeks plus home exercises and general conditioning programme (walking) Comparator group 1 (n = 11): static virtual reality image rehabilitation, performed for 45 minutes twice weekly for 4 weeks plus home exercises and general conditioning programme (walking) Comparator group 1 (n = 5): cross‐over of 5 group 1 participants who then received dynamic virtual reality (not included in our analysis) |
After 4 weeks the dynamic groups reported significantly less visual vertigo, but depression improved in the static virtual reality VR group only |
| Resende 2003 | Participants with BPPV diagnosed by ENT using history, ENT examination, ENG | Intervention group: VR (compensation, adaptation, sensory substitution, balance: C‐C) Comparator group: control (nil) |
Intervention group significantly improved Comparator group no change |
| Rossi‐Izquierdo 2011 | Participants with instability due to chronic unilateral peripheral vestibular disorders, which had not spontaneously resolved after a month. Hypofunction was defined with caloric tests, at least 25% labyrinthic preponderance according to defined criteria | Intervention group (n = 12): computerised dynamic posturography (CDP), 5 sessions of approximately 15 to 20 minutes on consecutive days Comparator group (n = 12): optokinetic stimulation (OKN), 5 sessions lasting 5 to 15 minutes on consecutive days |
Outcomes assessed 3 weeks after treatment. Both groups improved, with the CDP group showing greater gains in the visual and vestibular input and limits of stability, while the OKN group showed greater improvement in visual preference |
| Rossi‐Izquierdo 2013 | Participants with instability due to chronic unilateral peripheral vestibular disorders, which had not spontaneously resolved after a month | Intervention group (n = 13): 5 sessions of posturography‐assisted VR over a 2‐week period Comparator group (n = 13): 10 sessions of posturography‐assisted VR over a 2‐week period |
Outcomes assessed 3 weeks after the intervention and both groups improved over time, with the 5‐session group reporting greater gains on the DHI, but some items of posturography improved to a greater extent in the 10‐session group |
| Scott 1994 | Ménière's disease diagnosed by medical and audiological examination (5 were bilateral but had one "worse" ear) | Intervention group (n = 10): applied relaxation Comparator group (n = 10): transcutaneous nerve stimulation to the hand |
No change in either group for relevant measures (dizziness etc.) Intervention group improved on hearing ability more than comparator group Comparator group improved on psychoacoustic tests more than intervention group |
| Strupp 1998 | Vestibular neuritis (acute/sub‐acute). Diagnosed by history, examination ‐ nystagmus, postural imbalance, ENG, calorics, ocular tilt reaction | Intervention group (n = 19): VR (home exercises, based on Cooksey‐Cawthorne, Norre ‐ habituation, gaze exercises, sensory substitution, functional retraining) Comparator group (n = 20): control (nil exercise but encouragement to move) |
For OT and SVV tests, intervention group equal to comparator group For SP, intervention group improved significantly more than comparator group, i.e. balance improved |
| Szturm 1994 | Clinical diagnosis of peripheral vestibular dysfunction, persistent dizziness, disorientation or imbalance for at least 1 year, and abnormal balance performance during CDP at baseline | Intervention group (n = 11): VR Comparator group (n = 12): VR (home, C‐C) |
Intervention group had reduced falls, improved CDP values and reduced VOR asymmetry compared with comparator group |
| Teggi 2009 | Participants were recently hospitalised for an acute episode of rotational vertigo which lasted several days and were diagnosed with vestibular neuritis | Intervention group (n = 20): VR Comparator group (n = 20): control ("perform usual daily activities") |
Significant improvement in DHI between groups and reduction in anxiety. For both groups, there was a significant correlation between change in anxiety and change in DHI/DGI |
| Toledo 2000 | BPPV diagnosed with clinical assessment and electronystagmography | Intervention group (n = 10): VR (PC, head‐eye and habituation) Comparator group 1 (n = 10): Semont manoeuvre Comparator group 2 (n = 20): Semont + VR |
Intervention group 80% cure rate at day 15 versus comparator group 1 45% Intervention group 66% cure rate at 3 months versus comparator group 2 100% |
| Varela 2001 | BPPV, diagnosed by history and D‐H test (nystagmus) | Intervention group (n = 29): VR (B‐D habituation exercises) Comparator group 1 (n = 35): Semont manoeuvre Comparator group 2 (n = 42): Epley manoeuvre |
Comparator groups 1 and 2 had a similar cure rate at 1 week; by 3 months comparator group 2 were superior but comparator group 1 more stable CRM superior to habituation (B‐D) for BPPV |
| Venosa 2007 | Acute episode of rotational vertigo within the last 5 days | Intervention group (n = 45): VOR adaptation exercises (X1 and X2 viewing exercises) Comparator group (n = 42): placebo exercises (sham visual fixation task) |
Intervention group recovered more quickly in all parameters measured and required significantly less medication by the end of the follow‐up period (21 days) |
| Vereeck 2008 | Consecutive patients post removal of an acoustic neuroma | Intervention group (n = 31): customised VR (exercises for balance, head motion, mobility, gaze and treadmill walking) Comparator group (n = 22): general instructions |
Participants were stratified according to age (above and below 50 years). Older participants performed significantly better than the control group for balance, TUG and tandem gait compared to the control group. There was no group effect for the younger participants |
| Winkler 2011 | Participants with chronic dizziness (greater than 6 months duration) who had completed a VR programme, functional range of motion and strength in the lower limbs and trunk, intact sensation in the lower limbs, ability to stand unassisted for 1 minute | Intervention group (n = 10): platform tilt perturbations only Comparator group 1 (n = 7): platform tilt perturbations and VR exercise programme Comparator group 2 (n = 12): VR only |
Outcomes were assessed after the 3‐week intervention and a follow‐up at 2 months later. The VR group only demonstrated significant improvement on the DHI but the platform tilt groups improved activity and participation domain outcomes |
| Yardley 1998 | Dizziness of vestibular origin. Mixed aetiology ‐ diagnosed where possible by medical records (1/3) Possibility of central pathology |
Intervention group (n = 67): VR (education, head and body movements, relaxation, breathing, encouragement to function) Comparator group (n = 76): control |
Intervention group improved significantly on all measures more than comparator group, except VHQ (no difference) Overall intervention group 4 times more likely to report subjective improvement than comparator group |
| Yardley 2004 | Dizziness of vestibular origin diagnosed by case history and MPD | Intervention group (n = 83): VR (primary care: demonstration, booklet and follow‐up) Comparator group (n = 87): control, usual medical care |
All measures improved significantly in VR group compared with control group Clinical improvement 67% VR; 38% control |
| Yardley 2006 | Participants with Ménière's disease (non‐acute phase) who had experienced dizziness of imbalance in the last 12 months, had consulted their GP regarding involvement in the study | Intervention group (n = 120): VR (booklet of exercises) Comparator group 1 (n = 120): SC (booklet for self management) Comparator group 2 (n = 120): waiting list control |
At 3 months intervention group had greater improvement on 5 measures compared with comparator group 1 (2 measures) compared with comparator group 2 (0 measures) At 6 months intervention group and comparator group 1 both reported significant improvement, more than comparator group 2 Correlation between adherence and outcome |
| Yardley 2012 | Chronic dizziness, as diagnosed by their GP | Intervention group (n = 112): VR (self management booklet with phone support from a vestibular therapist) Comparator group 1 (n = 113): SC (self management booklet only) Comparator group 2 (n = 112): routine medical care | At 12 weeks all groups showed some improvement in the VSS, and at 1 year both intervention groups improved significantly compared to usual care |
| Zimbelman 1999 | Unilateral peripheral vestibular dysfunction diagnosed by neuro‐otological tests | Intervention group (n = 6): VR (individual with adaptation and postural control) Comparator group (n = 8): VR (general C‐C) |
Intervention group improved dizziness over time, comparator group did not No change for either on the BBS (insensitive) No between‐group differences ‐ but 100% of intervention group reported improvement compared with 62.5% of comparator group Intervention group had more Ménière's disease |
BBS: Berg Balance Scale B‐D: Brandt‐Daroff BPPV: benign paroxysmal positional vertigo BVD: bilateral vestibular dysfunction C‐C: Cooksey‐Cawthorne CDP: computerised dynamic posturography CRM: canalith repositioning manoeuvre CRT: canalith repositioning technique DGI: Dynamic Gait Index D‐H test: Dix‐Hallpike test DHI: Dizziness Handicap Inventory DI: dizziness intensity DVA: dynamic visual acuity ENG: electronystagmography GP: general practitioner LM: liberatory manoeuvre MPD: motion‐provoked dizziness MRI: magnetic resonance imaging MSQ: motion sensitivity quotient OKN: optokinetic reflex OT: ocular tilt PC: postural control PT: physical therapy SC: symptom control SOOL: standing on one leg SP: sway path SVV: subjective visual vertical TUG: Timed Up and Go VAS: visual analogue scale VDI: Vertigo Dizziness Imbalance questionnaire VF: vertigo frequency VHQ: Vestibular Handicap Questionnaire VI: vertigo intensity VOR: vestibular ocular reflex VSS: Vertigo Symptom Scale VR: vestibular rehabilitation
Comparison 1: Vestibular rehabilitation versus control (placebo, sham, usual care or no intervention)
We analysed 13 trials in this comparison (Cohen 2002; Cohen 2005; Giray 2009; Herdman 1995; Herdman 2003; Horak 1992; Resende 2003; Strupp 1998; Teggi 2009; Vereeck 2008; Yardley 1998; Yardley 2004; Yardley 2006). Three other studies performed this comparison (Krebs 2003; Marioni 2013; Venosa 2007), however they could not supply data to enable meta‐analysis.
We found statistically significant differences between vestibular rehabilitation and control/placebo interventions in favour of vestibular rehabilitation for the following outcomes.
Primary outcome
Subjective improvement in dizziness (odds ratio (OR) fixed‐effect 2.67, 95% confidence interval (CI) 1.85 to 3.86, P value < 0.0001; four studies, 565 participants) (Analysis 1.1).
Vertigo Symptom Scale (VSS) (standardised mean difference (SMD) fixed‐effect ‐0.68, 95% CI ‐0.87 to ‐0.49, P value < 0.00001; three studies, 553 participants) (Analysis 1.2).
1.1. Analysis.
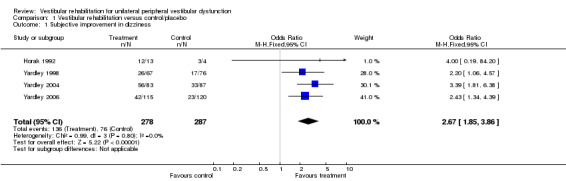
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 1 Subjective improvement in dizziness.
1.2. Analysis.
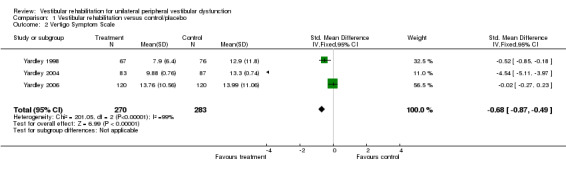
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 2 Vertigo Symptom Scale.
Secondary outcomes
Gait ataxia (OR fixed‐effect 0.04, 95% CI 0.00 to 0.77, P value = 0.03; one study, 19 participants) (Analysis 1.3).
Vestibular disorders activities of daily living (VD‐ADL) (mean difference (MD) fixed‐effect ‐10.50, 95% CI ‐14.09 to ‐6.91, P value < 0.0001; one study, 16 participants) (Analysis 1.4).
Sway path (posturography data) (MD fixed‐effect ‐13.70, 95% CI ‐16.51 to ‐10.89, P value < 0.00001; one study, 39 participants) (Analysis 1.5).
Dynamic visual acuity (OR fixed 84.00, 95% CI 4.51 to 1564.26, P value = 0.003; one study, 21 participants) (Analysis 1.6).
Vertigo Handicap Questionnaire (VHQ) (MD fixed‐effect ‐3.40, 95% CI ‐6.76 to ‐0.04, P value = 0.05; one study, 143 participants) (Analysis 1.7).
Sharpened Romberg test scores (balance) (MD fixed‐effect 9.90, 95% CI 0.80 to 19.00, P value = 0.03; one study, 143 participants) (Analysis 1.8).
Dizziness Handicap Inventory (DHI) (SMD fixed‐effect ‐0.83, 95% CI ‐1.02 to ‐0.64, P value < 0.00001; five studies, 535 participants) (Analysis 1.9).
Dynamic Gait Index (DGI) (SMD fixed‐effect ‐0.92, 95% CI ‐1.38 to ‐0.46, P value < 0.0001; two studies, 93 participants) (Analysis 1.10) (Teggi 2009; Vereeck 2008, under 50 and over 50 years old).
1.3. Analysis.
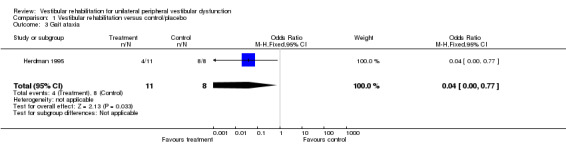
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 3 Gait ataxia.
1.4. Analysis.
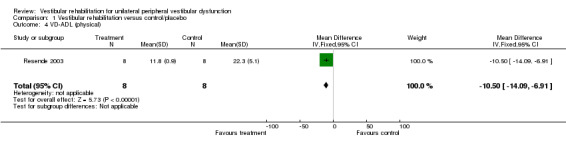
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 4 VD‐ADL (physical).
1.5. Analysis.
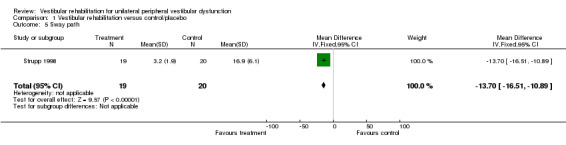
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 5 Sway path.
1.6. Analysis.
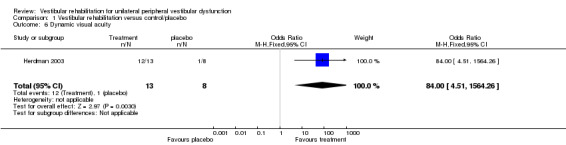
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 6 Dynamic visual acuity.
1.7. Analysis.
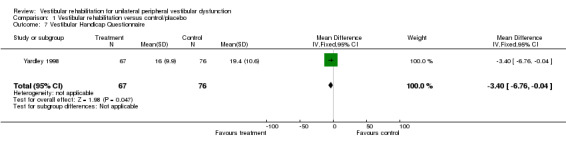
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 7 Vestibular Handicap Questionnaire.
1.8. Analysis.
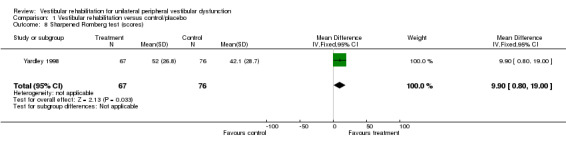
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 8 Sharpened Romberg test (scores).
1.9. Analysis.
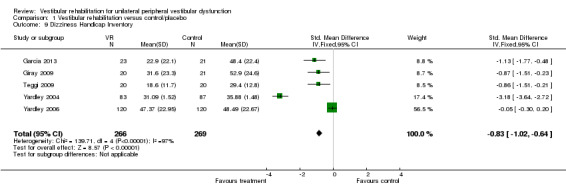
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 9 Dizziness Handicap Inventory.
1.10. Analysis.
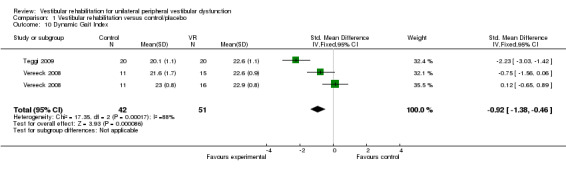
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 10 Dynamic Gait Index.
Differences were non‐significant for the other four measures: Romberg test, vertigo intensity (two separate comparisons) and posturography.
The three studies that could not be included in the meta‐analysis, due to inadequate reporting of data, supported the positive findings of vestibular rehabilitation improving gait and reducing the duration of dizziness symptoms compared to a control group (Krebs 2003; Marioni 2013; Venosa 2007).
We calculated heterogeneity as being high in three analyses in this comparison. On visual inspection of Analysis 1.2 (Vertigo Symptom Scale) and Analysis 1.9 (Dizziness Handicap Inventory), we noted the same study to have markedly larger effects than the other pooled studies (Yardley 2004). Comparison of methods and clinical parameters did not reveal any clear reasons for the difference. Furthermore, removal of the study from each analysis still retained the statistically significant effects. In the third analysis (Analysis 1.10, Dynamic Gait Index) the Teggi 2009 study provided a higher effect size than the other pooled study results; again there were no obvious clinical or methodological differences to explain this, as all studies had acceptably low risk of bias and usual care control groups. However, in this instance removal of the study also removed the significant effect.
Comparison 2: Vestibular rehabilitation versus other treatment (non‐vestibular rehabilitation)
There were seven studies in this comparison (Barozzi 2006; Chang 2008; Cohen 2002; Cohen 2005; Horak 1992; Karanjai 2010; Varela 2001), with a further three studies with inadequate data (Kulcu 2008; Scott 1994; Toledo 2000).
Primary outcome
Statistically significant differences between vestibular rehabilitation and other interventions (manoeuvres) in favour of 'other' (where 'other' were physical manoeuvres for benign paroxysmal positional vertigo (BPPV)) were found for the following.
Dizziness cure rate (OR fixed 0.19, 95% CI 0.07 to 0.49, P value = 0.006; two studies, 119 participants) (Analysis 2.1).
2.1. Analysis.
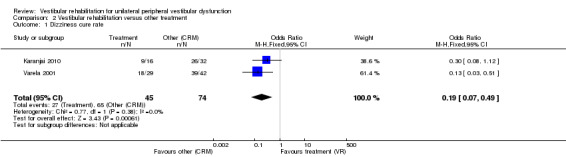
Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 1 Dizziness cure rate.
Secondary outcomes
Statistically significant differences between vestibular rehabilitation plus canalith repositioning manoeuvres (physical manoeuvres for BPPV) and canalith repositioning manoeuvres (CRM) only, in favour of vestibular rehabilitation plus CRM were found for the following.
Dynamic Gait Index (MD fixed‐effect ‐1.00, 95% CI ‐1.85 to ‐0.15, P value = 0.02; one study, 26 participants) (Analysis 2.2).
2.2. Analysis.
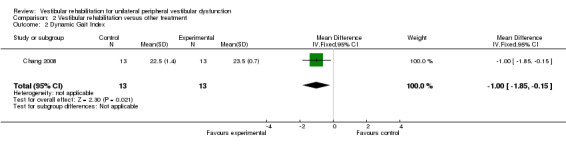
Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 2 Dynamic Gait Index.
Differences were non‐significant for all other measures (four): subjective improvement in dizziness, vertigo intensity (two) and Dizziness Handicap Inventory.
One study not included in the meta‐analysis compared a home‐based exercise programme with betahistine medication and found that the exercise programme improved dizziness symptoms and health‐related quality of life to a greater extent (Kulcu 2008). The second study compared relaxation with electrical stimulation and found no significant differences (Scott 1994). The third study not included in the meta‐analysis compared only the Semont manoeuvre with combined manoeuvre and vestibular rehabilitation for people with BPPV (Toledo 2000). The manoeuvre was found to be superior in cure rate in the short term (15 days), but the combination approach was superior in the longer term (three months). Details of the results of these studies are in the table Characteristics of included studies.
Comparison 3: Vestibular rehabilitation versus other form of vestibular rehabilitation
We included 12 studies in these analyses (Basta 2011; Cohen 2003; Kammerlind 2005; Morozetti 2011; Pavlou 2004; Pavlou 2012; Rossi‐Izquierdo 2011; Rossi‐Izquierdo 2013; Winkler 2011; Yardley 2006; Yardley 2012; Zimbelman 1999). Another four studies also performed this comparison but did not provide appropriate data (Cakrt 2010; Foster 2012; Mruzek 1995; Szturm 1994).
We found statistically significant differences between one form of vestibular rehabilitation and another form of vestibular rehabilitation for the following.
Primary outcome
Vertigo Symptom Scale ‐ vertigo component (VSS‐V) (SMD fixed‐effect ‐1.12, 95% CI ‐1.80 to ‐0.45, P value = 0.001; one study, 40 participants) (Analysis 3.1 section 2), in favour of the inclusion of simulator activities, however the overall vertigo symptom score was non‐significant (P value = 0.18).
3.1. Analysis.
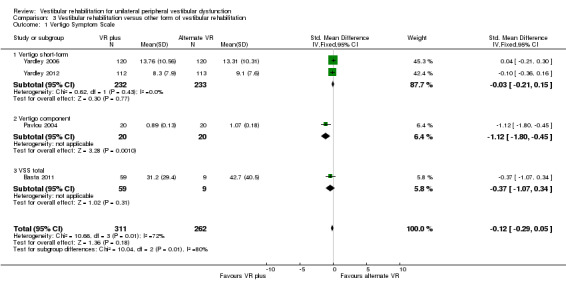
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 1 Vertigo Symptom Scale.
Secondary outcomes
Dizziness Handicap Inventory (SMD fixed‐effect ‐0.96, 95% CI ‐1.78 to ‐0.14, P value = 0.02; one study, 26 participants) (Analysis 3.2 section 4), in favour of five sessions of balance training compared to 10.
3.2. Analysis.
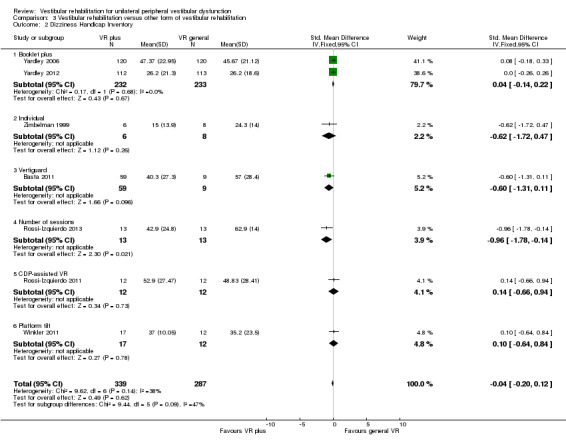
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 2 Dizziness Handicap Inventory.
Differences were non‐significant for all other measures (18) in these comparisons between different forms of vestibular rehabilitation: repetitive head movement task, vertigo visual analogue scale (VAS), tandem walk, posturography (five), VSS (four), DHI (seven), subjective improvement in dizziness, vertigo intensity, vertigo frequency, VHQ, ataxia, VD‐ADL and subjective health.
Four studies were not included in the meta‐analysis. One reported that after surgical removal of a schwannoma patients' recovered balance (as measured by posturography) was greater with visual feedback on training than without feedback (Cakrt 2010). Another found varying results when comparing a half‐somersault versus the Epley manoeuvre for BPPV, with the former superior in improving exercise‐induced dizziness (Foster 2012). One study reported similar results whether vestibular rehabilitation was performed with or without social support (Mruzek 1995). A final single study reported that a formal vestibular rehabilitation programme was more effective in improving balance/reducing falls than a home‐based Cooksey‐Cawthorne programme (Szturm 1994).
We evaluated heterogeneity as high, as indicated by the I2 statistic for two analyses. Visual inspection of the forest plot for Analysis 3.1 (Vertigo Symptom Scale) revealed that Pavlou 2004 had reported a larger effect size using the Vertigo Symptom Scale vertigo component ‐ this is to be expected clinically given that vertigo reduction is the primary goal and outcome of vestibular rehabilitation. The second analysis (Analysis 3.7) revealed that a larger effect size was produced by the Rossi‐Izquierdo 2011 study than other studies in the meta‐analysis. The overall effect was not significant and there was no obvious clinical or methodological explanation for the effect, other than that computerised dynamic posturography or posturography measures have multiple interpretations and parameters, which may not be appropriate for pooling.
3.7. Analysis.
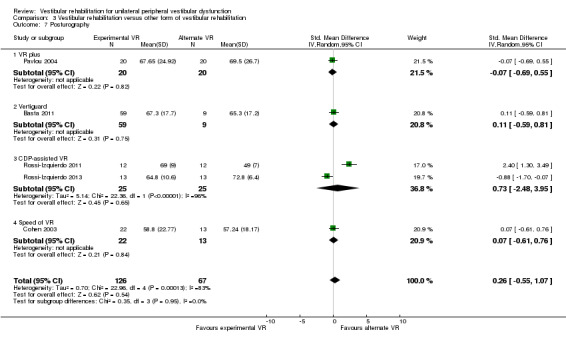
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 7 Posturography.
Discussion
Summary of main results
If consideration is directed solely at the clinical question, 'Is vestibular rehabilitation effective in improving the symptoms of unilateral peripheral vestibular dysfunction?', then the evidence from this review is sufficient to answer yes, given the number of moderate to high quality studies reporting outcomes in favour of the vestibular rehabilitation intervention. This 2014 update has served to strengthen the original findings. The heterogeneity of the 39 studies still acts as a qualifier to this strong conclusion. The study variability lies in three domains: the varied comparators and the nature of the vestibular rehabilitation intervention, the sample characteristics (for example sub‐categories of unilateral peripheral vestibular dysfunction (UPVD), or acute versus chronic) and the outcome measures. In the following section we discuss the studies by grouping them in these three domains in turn, to answer the following subsidiary questions:
Is vestibular rehabilitation better than no or other interventions?
What form of vestibular rehabilitation is most effective?
Do different categories of unilateral peripheral vestibular dysfunction respond differently and what signs/symptoms are affected?
Unless otherwise indicated, we will only discuss the studies where data could be extracted.
Comparisons
Taken at the strictest level of evidence provided by meta‐analysis, the low risk of bias studies Giray 2009, Horak 1992, Teggi 2009, Vereeck 2008, Yardley 1998, Yardley 2004 and Yardley 2004 offer support for the use of vestibular rehabilitation to improve subjective measures of dizziness (including the Vertigo Symptom Scale (VSS)), level of participation (DHI) and gait performance (DGI) in people with chronic peripheral vestibulopathy, as compared to sham exercises or no vestibular rehabilitation/usual care. Individually the studies of Herdman 1995, Herdman 2003, Resende 2003 and Strupp 1998 also offer evidence of effectiveness in terms of improvement in measures of balance, activities of daily living and vision compared to no or sham interventions. These studies, as a body of evidence, therefore offer strong support for the effectiveness of vestibular rehabilitation across a broad range of outcomes in unilateral peripheral vestibular dysfunction as compared to placebo, sham or no intervention. It should be noted that a large degree of heterogeneity was found for the comparisons using the VSS and the DHI. We examined the studies that contributed to this finding, Yardley 2004 and Yardley 2006, and found that the only clinical source of heterogeneity was in the population, where one was general UPVD and the other Ménière's disease. However, these populations are both versions of chronic UPVD.
Studies that compared vestibular rehabilitation to other forms of unilateral peripheral vestibular dysfunction management (non‐vestibular rehabilitation) include Barozzi 2006 (electrical stimulation), Horak 1992 and Kulcu 2008 (medication), Chang 2008 (physical manoeuvres for benign paroxysmal positional vertigo (BPPV) (canalith repositioning manoeuvres (CRM)) plus vestibular rehabilitation versus CRM alone), Toledo 2000 (Semont manoeuvre), and Varela 2001 and Karanjai 2010 (Semont and Epley manoeuvres). Horak 1992 and Kulcu 2008 found that vestibular rehabilitation was superior to medication in improving subjective reports of dizziness in people with unilateral peripheral vestibular dysfunction. In contrast, Toledo 2000, Varela 2001 and Karanjai 2010 found in favour of manoeuvres over vestibular rehabilitation as defined for this review. The difference in findings can be explained by considering the different subject groups ‐ Horak recruited a pool of people with general peripheral vestibular dysfunction, whereas Varela and Karanjai investigated confirmed BPPV diagnoses only. This specific issue of BPPV will be discussed later. The studies by Cohen 2002 and Cohen 2005 failed to reach a sufficient effect size despite statistical significance in the original 2005 paper. Barozzi 2006 reported no difference in effect size between the vestibular rehabilitation and electrical stimulation groups.
Considering the comparative or relative effectiveness of different forms of vestibular rehabilitation, three studies reached statistical significance in our review. Pavlou 2004 compared customised home‐based vestibular rehabilitation exercises with the same programme plus simulator‐based visual and self motion stimulation, finding in favour of the supplemented programme. Therefore there is some evidence to support the addition of simulator‐based activities in a vestibular rehabilitation approach. A later study by Pavlou 2012 found that dynamic versus static virtual reality vestibular rehabilitation was superior in reducing visually induced dizziness. Rossi‐Izquierdo 2013 found that only five sessions of balance training (versus 10) were needed to improve dizziness experiences on the DHI, but that 10 were superior to five in improving balance. The lack of homogeneity means that it is not possible to draw strong conclusions about the other studies that compared different versions of vestibular rehabilitation. Further studies with a larger sample size are needed to clarify the questions of which exercises should be used, in what environment, administered by whom and for how long or how intensively (dosage).
Sub‐diagnoses of unilateral peripheral vestibular dysfunction
Acute UPVD
Five studies considered vestibular rehabilitation in the acute stage immediately post‐surgery for acoustic neuroma resection, removal of schwannoma or vestibular ablation. Vereeck 2008 reported that older participants in particular (over 50 years old) regained postural control more quickly with vestibular rehabilitation compared to general instructions, and that the greater benefits for postural control were maintained 12 months postoperatively. Herdman 1995 found a variable picture comparing vestibular rehabilitation that targeted vestibular gain versus eye movements that did not influence gain, reporting that balance and gait tests were superior in the vestibular rehabilitation group at day six postoperatively. Cohen 2002 found no difference between vestibular rehabilitation and sham interventions at day six; Cakrt 2010 found that patients post schwannoma removal, who received visual feedback as part of their vestibular rehabilitation, had greater improvement in balance parameters than those who did not receive feedback; and finally Mruzek 1995 found that vestibular rehabilitation (with or without social reinforcement) had better effects than a sham exercise for several dizziness and sensitivity quotients in the longer term (seven weeks post operation). Neither of the two latter studies could be included in a meta‐analysis.
Kammerlind 2005 investigated acute unilateral vestibular loss, comparing two forms of vestibular rehabilitation and finding them equally effective. Teggi 2009 (vestibular rehabilitation versus control) and Venosa 2007 (adaptation vestibular rehabilitation versus placebo) both reported greater benefits for people with acute vestibular presentations receiving vestibular rehabilitation, in terms of reduced symptom duration and medication use. Marioni 2013 found that posturography‐assisted vestibular rehabilitation compared to no vestibular rehabilitation had similar results but only the vestibular rehabilitation group improved to a level similar to healthy controls.
Benign paroxysmal positional vertigo
Eight studies investigated BPPV specifically. Resende 2003 investigated elderly patients with BPPV and compared vestibular rehabilitation (Cooksey‐Cawthorne type exercises) with no intervention ‐ both groups had received prior Ginkgo biloba. The vestibular rehabilitation group performed significantly better on measures of activities of daily living post‐intervention. In contrast, the study Varela 2001 also investigated participants with confirmed BPPV and found that manoeuvres (either Epley or Semont) were more effective in producing resolution than habituation exercises (Brandt 1999). They concluded that a hierarchy of interventions should be offered to people with BPPV, starting with a canalith repositioning manoeuvre. This suggestion has found favour in current clinical practice and is supported by the similar study of Cohen 2005 (though not in the meta‐analysis), who also found in favour of manoeuvres (canalith repositioning manoeuvre and modified Liberatory) compared to two versions of vestibular rehabilitation habituation exercise, noting that the exercises were also superior to a sham manoeuvre. Further, more recent, support is provided by Foster 2012 and Karanjai 2010, who both found in favour of the Epley manoeuvre compared to the Semont or Brandt‐Daroff manoeuvres. Similarly, Toledo 2000 found the Semont manoeuvre to be superior to vestibular rehabilitation alone at 15 days, however by three months a combination of Semont and vestibular rehabilitation was superior to either of the sole interventions. The Semont only group had a > 30% recurrence rate by this time leading these authors to suggest that vestibular rehabilitation has a preventative role. This result was confirmed more recently by Chang 2008, who compared canalith repositioning manoeuvres (CRM) with vestibular rehabilitation versus CRM alone. They reported that the combination promoted greater mobility skills (improved DGI) than the CRM alone. This body of evidence suggests that for people with BPPV the primary intervention should include manoeuvres to actually treat the condition and that this should be supported by vestibular rehabilitation to aid in longer‐term functional recovery. The evidence for the effectiveness of manoeuvres for BPPV is the subject of other Cochrane reviews (Hilton 2014; Hunt 2012).
Chronic and mixed forms of UPVD
The majority of studies investigated chronic dizziness of broad unilateral peripheral vestibular dysfunction origin and hence attract the general recommendations of this review.
More specifically vestibular neuritis was investigated firstly by Strupp 1998, who found postural control measures improved more in a group of patients with vestibular neuritis who performed vestibular rehabilitation (physical therapy and home‐based) compared to no specific intervention (other than encouragement to move). More recently Teggi 2009 also reported that vestibular rehabilitation significantly reduced anxiety in people with acute neuritis compared to the control group.
Scott 1994 investigated people with Ménière's disease but found no difference between applied relaxation training versus transcutaneous nerve stimulation on dizziness scores (could not be included in meta‐analysis). Yardley 2006 also investigated people in a non‐acute phase of Ménière's disease using booklet‐based forms of vestibular rehabilitation or symptom management and reported significant effects for subjective improvement in dizziness compared to control.
Outcome measures
Nineteen different measures were included in the results of this review, as summarised in the Results section. They covered impairments (dizziness and visual disturbances), activity restrictions (balance and gait parameters, activities of daily living) and participation restrictions (quality of life and social roles). As reported, the four common outcome measures available to pool were dizziness reduction scores and the vertigo symptom scale (measures of impairment), the Dizziness Handicap Inventory (measure of participation) and the Dynamic Gait Index (measure of activity). Future studies should consider evaluation at these three levels and should wherever possible use the vestibular‐specific scales.
Overall completeness and applicability of evidence
Clinical applicability of the evidence is impacted by the previously discussed areas of variance or heterogeneity. Clinicians are advised to read specifically for pertinent comparisons, outcomes and specific diagnostic groups. Another key aspect of applicability is the benefits over a longer time period and the existence of any mitigating adverse effects. Follow‐up was performed in the majority of studies and confirmed that any positive effects gained lasted for the three, six or 12‐month period. This lends further support to the conclusions in favour of the use of vestibular rehabilitation for unilateral peripheral vestibular dysfunction, as does the lack of reported adverse events. Studies also reported nil or low to moderate drop‐out rates and loss to follow‐up, although there was some suggestion that compliance may be an issue in some groups. Yardley 2006 reported a strong correlation between adherence and positive outcomes using booklet‐based vestibular rehabilitation, and again in 2012 confirmed superior outcomes for this intervention along with findings in favour of cost‐effectiveness (Yardley 2012). These issues warrant further investigation both within future randomised controlled trials and with qualitative methodology to establish individual experiences regarding patient acceptability of vestibular rehabilitation interventions.
Quality of the evidence
The overall quality of the evidence was acceptable. As can be seen in the 'Risk of bias' tables, there are few areas where there is a known high risk of bias that would cause readers to reconsider the strength of the evidence. However, there is a tendency for poor reporting particularly in the area of how the randomisation sequence was generated and to a lesser extent how randomisation was allocated. Although, it would be nice to see this as an historic problem and largely resolved in more recent studies with a higher awareness of trial conduct and reporting standards, this does not seem to be the case and therefore we make a strong plea for improved diligence by clinical researchers to improve both attention to trial conduct and to trial reporting.
There were isolated cases of high heterogeneity as assessed by the I2 statistic. Given the overall high level of clinical heterogeneity this was not unexpected but nevertheless again highlights the need for larger, standardised trials using consistent methods and outcomes.
Potential biases in the review process
We have applied a rigorous process of review and therefore expect minimal biases in extracting and reporting of data (both review authors selected studies for inclusion, and both independently extracted data and checked analyses with assistance from the editorial team). We have conducted extensive literature searches at each update of this review. The possibility of some publication bias cannot be ruled out, as our attempts to retrieve unpublished studies only included review of trial registries and contacting authors. Studies that were not registered nor published may therefore still exist.
Agreements and disagreements with other studies or reviews
There are still no alternate comprehensive systematic reviews covering the question of the effectiveness of vestibular rehabilitation for UPVD. There are many non‐systematic reviews and we have used these for their reference lists to ensure that we have found all known studies.
Authors' conclusions
Implications for practice.
There is moderate to strong evidence that vestibular rehabilitation (movement, exercise‐based) is a safe and effective approach for unilateral peripheral vestibular disorders. This is based on (at least) 13 moderate to high quality studies comparing vestibular rehabilitation to placebo, sham or non‐vestibular rehabilitation interventions. Improvements are reported across a range of outcomes including symptom reduction (dizziness), gait, activities of daily living, visual impairments, balance and quality of life domains, although the number of studies supporting these latter individual outcome measures is small.
There is also moderate evidence that there is maintenance of improvements over the following months post‐intervention.
The evidence for the dosage (frequency, intensity, timing) and specifics of vestibular rehabilitation (e.g. compensatory, adaptation, substitution, task‐specific) is still limited due to the largely heterogeneous studies. It appears that even a minimalist approach of education, demonstration and home exercises may be effective.
For the specific diagnosis of benign paroxysmal positional vertigo (BPPV), on balance there is more evidence for the use of repositioning manoeuvres in the first instance, with evidence that vestibular rehabilitation should be incorporated in the long term as a preventative measure or to promote functional recovery, or both.
There is moderate evidence that vestibular rehabilitation is effective in improving function in post‐surgical patients, patients with vestibular neuritis or patients with acute unilateral peripheral vestibular dysfunction.
There is some evidence for the use of vestibular rehabilitation in patients with Ménière's disease in reducing dizziness.
Implications for research.
Further research in this field should consider:
Patient diagnosis: in general researchers follow clinical practice and group all unilateral peripheral vestibular dysfunction patients together. It may also be useful to consider sub‐diagnoses, however it is very difficult to diagnose differentially for the majority of unilateral peripheral vestibular dysfunction presentations. We rejected several studies because they included bilateral peripheral vestibular dysfunction.
Power: small patient numbers reduce the strength of evidence. This is an issue for vestibular research where patient numbers in specific diagnostic categories may be small. Strong recommendations are made for multicentre trials to boost power and to allow for stratification of sub‐diagnoses.
Generally study methods were strong (given the inability to blind participants in these clinical trials), however poor reporting of randomisation methods introduced uncertainty about risk of bias and poor reporting of basic means and standard deviations prevented more comprehensive data pooling.
Consistent use of valid and reliable, vestibular‐specific outcome measures that cover the levels of impairment (subjective and objective), activity and participation restrictions is needed. International consensus could confirm a more consistent adoption of such scales.
Further quantitative and qualitative examination of patient compliance, cost‐effectiveness and adverse events is also required.
Comparisons of different vestibular rehabilitation components would be useful to clarify questions of process, dosage and delivery. Whilst these studies are being performed, they require more appropriate methods, as noted above, to enable meta‐analysis.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2015 | New citation required but conclusions have not changed | We included 12 new studies and adjusted the text accordingly (Basta 2011; Cakrt 2010; Foster 2012; Garcia 2013; Karanjai 2010; Marioni 2013; Morozetti 2011; Pavlou 2012; Rossi‐Izquierdo 2011; Rossi‐Izquierdo 2013; Winkler 2011; Yardley 2012). We excluded 13 further studies and identified three further ongoing studies. There are no changes to the conclusions of the review. |
| 18 January 2014 | New search has been performed | New searches run. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 4, 2007
| Date | Event | Description |
|---|---|---|
| 13 January 2011 | New citation required but conclusions have not changed | The review authorship has changed. |
| 1 July 2010 | New search has been performed | We ran new searches on 1 July 2010. Six new studies were included in the review. The review conclusions have been strengthened. |
| 30 October 2008 | Amended | Converted to new review format. |
Notes
This review will be updated again in 2015.
Acknowledgements
Staff at the International Centre for Allied Health Evidence, Sansom Institute for Health Research, University of South Australia. Staff at the Cochrane ENT Group ‐ Jenny Bellorini, Gemma Sandberg and Carolyn Doree for their patient and efficient assistance with searching, editing and translating. Malcolm Hilton for his assistance with the initial proposal.
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | EMBASE (Ovid) | CINAHL (EBSCO) |
| #1 MeSH descriptor Vestibular Diseases explode all trees with qualifiers: NU,RH #2 MeSH descriptor Vertigo explode all trees with qualifiers: NU,RH #3 MeSH descriptor Dizziness explode all trees with qualifiers: NU,RH #4 MeSH descriptor Labyrinth Diseases explode all trees #5 MeSH descriptor Vestibulocochlear Nerve Diseases explode all trees #6 (VERTIGO OR VESTIBULOPATH* OR DIZZINESS):ti #7 ((VESTIBULAR NEAR DISORDER*) OR (VESTIBULAR NEAR HYPOFUNCTION*) OR (VESTIBULAR NEAR DYSFUNCTION*) OR (VESTIBULAR NEAR IMPAIR*) OR (VESTIBULAR NEAR DISABILIT*) OR (VESTIBULAR NEAR PATHOLOG*) OR (VESTIBULAR NEAR DISTURBANCE*)):ti #8 ((BALANCE NEAR DISORDER*) OR (BALANCE NEAR HYPOFUNCTION*) OR (BALANCE NEAR DYSFUNCTION*) OR (BALANCE NEAR IMPAIR*) OR (BALANCE NEAR DISABILIT*) OR (BALANCE NEAR PATHOLOG*) OR (BALANCE NEAR DISTURBANCE*)):ti #9 (NEUROLABYRINTHITIDES OR NEUROLABYRINTHITIS OR VESTIBULAR NEAR NEURITIS OR VESTIBULAR NEAR NEURONITIS OR VESTIBULAR NEAR NEURITIDES):ti #10 (VESTIBULAR NERVE NEAR INFLAMMATION OR VESTIBULAR NERVE NEAR COMPRESSION):ti #11 (ACOUSTIC NEUROMA* OR ACOUSTIC NEURINOMA* OR ACOUSTIC NEURILEMOMA* OR ACOUSTIC NEURILEMMOMA*):ti #12 (VESTIBULAR SCHWANNOMA* OR ACOUSTIC SCHWANNOMA*):ti #13 (MOTION SENSITIVITY OR VESTIBULAR NEAR PERIPHERAL OR PERILYMPHATIC NEAR FISTULA*):ti #14 (MENIERE* OR ENDOLYMPHATIC NEXT HYDROPS):ti #15 ((LABYRINTH* NEAR HYDROPS) OR (LABYRINTH* NEAR SYNDROME)):ti #16 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) #17 MeSH descriptor Occupational Therapy explode all trees #18 MeSH descriptor Physical Therapy Modalities explode all trees #19 MeSH descriptor Exercise Therapy explode all trees #20 MeSH descriptor Exercise explode all trees #21 MeSH descriptor Head Movements explode all trees #22 MeSH descriptor Vestibular Function Tests explode all trees #23 REHABILITAT* OR PHYSIOTHERAP* OR (PHYSICAL NEAR THERAP*) OR EXERCIS* OR HABITUAT* #24 EPLEY OR CANALITH OR SEMONT OR MANOEUVRE* OR MANEUVER* OR (RECONDITIONING ADJ ACTIVIT*) #25 POSTUROGRAPHY OR POSTURAL ADJ CONTROL OR PFPP #26 (SENSORY NEAR RELEARN*) OR (SENSORY NEAR RETRAIN*) OR (POSTURAL NEAR RELEARN*) OR (POSTURAL NEAR RETRAIN*) #27 (POSITION* NEAR PROCEDURE*) OR (REPOSITION* NEAR PROCEDURE*) OR (REPOSITION* NEAR PARTICLE*) #28 (VISUAL NEAR VESTIBULAR) OR (FUNCTIONAL NEAR RETRAIN*) OR (OCCUPATIONAL NEAR RETRAIN*) OR (OCCUPATIONAL ADJ ADAPTATION) #29 COOKSEY AND CAWTHORNE #30 (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29) #31 (#16 AND #30) #32 (#1 OR #2 OR #3 OR #31) | #1 "Vestibular Diseases/nursing"[Mesh] OR "Vestibular Diseases/rehabilitation"[Mesh] OR "Vertigo/nursing"[Mesh] OR "Vertigo /rehabilitation"[Mesh] OR "Dizziness /nursing"[Mesh] OR "Dizziness /rehabilitation"[Mesh] #2 (VESTIBULAR [tiab] AND (REHABILITATION [tiab] OR ADAPTATION [tiab] OR HABITUATION [tiab])) #3 “LABYRINTH DISEASES” [Mesh] OR “VESTIBULOCOCHLEAR NERVE DISEASES” OR (“PERILYMPH” [MeSH] AND “FISTULA” [Mesh]) #4 Vertigo [tiab] OR vestibulopath* [tiab] OR dizziness [tiab] OR ((vestibular [ti] OR balance* [ti]) AND (disorder [ti] OR hypofunction* [ti] OR dysfunction* [ti] OR impair* [ti] OR disability* [ti] OR pathology* [ti] OR disturbance* [ti])) #5 NEUROLABYRINTHITIDES [tiab] OR NEUROLABYRINTHITIS [tiab] OR (VESTIBULAR [tiab] AND (NEURITIS [tiab] OR NEURONITIS [tiab] OR NEURITIDES[tiab])) #6 “VESTIBULAR NERVE” [tiab] AND (INFLAMMATION [tiab] OR COMPRESSION [tiab])) #7 “ACOUSTIC NEUROMA” [tiab] OR “ACOUSTIC NEURINOMA” [tiab] OR “ACOUSTIC NEURILEMOMA” [tiab] OR “ACOUSTIC NEURILEMMOMA” [tiab] OR “VESTIBULAR SCHWANNOMA” [tiab] OR “ACOUSTIC SCHWANNOMA” [tiab] OR “MOTION SENSITIVITY” [tiab] OR (VESTIBULAR [tiab] AND PERIPHERAL [tiab]) OR (PERILYMPHATIC [tiab] AND FISTULA [tiab]) OR MENIERE* [tiab] OR “ENDOLYMPHATIC HYDROPS” [tiab] OR (LABYRINTH* [tiab] AND HYDROPS [tiab]) OR (LABYRINTH* [tiab] AND SYNDROME [tiab]) OR BPV [tiab] OR BPPV [tiab] OR ANTBPPV [tiab] #8 #3 OR #4 OR #5 OR #6 OR #7 #9 “OCCUPATIONAL THERAPY” [Mesh] OR “PHYSICAL THERAPY MODALITIES” [Mesh] OR “EXERCISE THERAPY” [Mesh] OR “EXERCISE” [Mesh] OR “HEAD MOVEMENTS” [Mesh] OR “VESTIBULAR FUNCTION TESTS” [Mesh] #10 REHABILITATION [tiab] OR PHYSIOTHERAP* [tiab] OR (PHYSICAL [tiab] AND THERAP* [tiab]) OR EXERCIS* [tiab] OR HABITUAT* [tiab] OR EPLEY [tiab] OR CANALITH [tiab] OR SEMONT [tiab] OR MANOEUVRE* [tiab] OR MANEUVER* [tiab] OR “RECONDITIONING ACTIVIT*” [tiab] OR POSTUROGRAPHY [tiab] OR “POSTURAL CONTROL” [tiab] OR PFPP [tiab] OR (SENSORY [tiab] AND RELEARN* [tiab]) OR (SENSORY [tiab] AND RETRAIN* [tiab]) OR (POSTURAL [tiab] AND RELEARN* [tiab]) OR (POSTURAL [tiab] AND RETRAIN* [tiab]) #11 (POSITION* [tiab] AND PROCEDURE* [tiab]) OR (REPOSITION* [tiab] AND PROCEDURE* [tiab]) OR (REPOSITION* [tiab] AND PARTICLE* [tiab]) OR (VISUAL [tiab] AND VESTIBULAR [tiab]) OR (FUNCTIONAL [tiab] AND RETRAIN* [tiab]) OR (OCCUPATIONAL [tiab] AND RETRAIN* [tiab]) OR (OCCUPATIONAL [tiab] AND ADAPTATION [tiab]) OR (COOKSEY [tiab] AND CAWTHORNE [tiab]) #12 #9 OR #10 OR #11 #13 #8 AND #12 #14 #1 OR #2 OR #13 | 1 exp vestibular disorder/rh [Rehabilitation] 2 exp vertigo/rh [Rehabilitation] 3 dizziness/rh [Rehabilitation] 4 (VESTIBULAR and (REHABILITATION or ADAPTATION or HABITUATION)).tw. 5 exp *inner ear disease/ 6 perilymph/ and fistula/ 7 (Vertigo or vestibulopath* or dizziness or ((vestibular or balance*) and (disorder or hypofunction* or dysfunction* or impair* or disability* or pathology* or disturbance*))).ti. 8 (NEUROLABYRINTHITIDES or NEUROLABYRINTHITIS or (VESTIBULAR and (NEURITIS or NEURONITIS or NEURITIDES))).ti. 9 ((ACOUSTIC adj NEUROMA) or (ACOUSTIC adj NEURINOMA) or (ACOUSTIC adj NEURILEMOMA) or (ACOUSTIC adj NEURILEMMOMA) or (VESTIBULAR adj SCHWANNOMA) or (ACOUSTIC adj SCHWANNOMA) or (MOTION adj SENSITIVITY) or (VESTIBULAR and PERIPHERAL) or (PERILYMPHATIC and FISTULA) or MENIERE* or (ENDOLYMPHATIC and HYDROPS) or (LABYRINTH* and HYDROPS) or (LABYRINTH* and SYNDROME) or BPV or BPPV or ANTBPPV).ti. 10 5 or 6 or 7 or 8 or 9 11 VOCATIONAL REHABILITATION/ or exp KINESIOTHERAPY/ or exp EXERCISE/ or exp HEAD MOVEMENT/ 12 (REHABILITATION or PHYSIOTHERAP* or (PHYSICAL and THERAP*) or EXERCIS* or HABITUAT* or EPLEY or CANALITH or SEMONT or MANOEUVRE* or MANEUVER* or (RECONDITIONING adj ACTIVIT*) or POSTUROGRAPHY or (POSTURAL adj CONTROL) or PFPP or (SENSORY and RELEARN) or (SENSORY and RETRAIN*) or (POSTURAL and RELEARN*) or (POSTURAL and RETRAIN*)).tw. 13 ((POSITION* and PROCEDURE*) or (REPOSITION* and PROCEDURE*) or (REPOSITION* and PARTICLE*) or (VISUAL and VESTIBULAR) or (FUNCTIONAL and RETRAIN*) or (OCCUPATIONAL and RETRAIN*) or (OCCUPATIONAL and ADAPTATION) or (COOKSEY and CAWTHORNE)).tw. 14 11 or 12 or 13 15 10 and 14 16 1 or 2 or 3 or 4 or 15 | S1 (MH "Vestibular Diseases+/NU/RH") S2 (MH "Vertigo+/NU/RH") S3 (MH "Dizziness/NU/RH") S4 TX vestibular and TX (REHABILITATION or ADAPTATION or HABITUATION) S5 (MH "Labyrinth Diseases+") S6 (MH "VESTIBULOCOCHLEAR NERVE DISEASES+") S7 TX Vertigo or vestibulopath* or dizziness or ((vestibular or balance*) and (disorder or hypofunction* or dysfunction* or impair* or disability* or pathology* or disturbance*)) S8 TI NEUROLABYRINTHITIDES or NEUROLABYRINTHITIS or (VESTIBULAR and (NEURITIS or NEURONITIS or NEURITIDES)) S9 TX (ACOUSTIC adj NEUROMA) or (ACOUSTIC adj NEURINOMA) or (ACOUSTIC adj NEURILEMOMA) or (ACOUSTIC adj NEURILEMMOMA) or (VESTIBULAR adj SCHWANNOMA) or (ACOUSTIC adj SCHWANNOMA) or (MOTION adj SENSITIVITY) or (VESTIBULAR and PERIPHERAL) or (PERILYMPHATIC and FISTULA) or MENIERE* or (ENDOLYMPHATIC and HYDROPS) or (LABYRINTH* and HYDROPS) or (LABYRINTH* and SYNDROME) or BPV or BPPV or ANTBPPV S10 S5 or S6 or S7 or S8 or S9 S11 (MH "Occupational Therapy+") S12 (MH "Physical Therapy+") S13 (MH "Exercise+") S14 (MH "Vestibular Function Tests+") S15 TX REHABILITATION or PHYSIOTHERAP* or (PHYSICAL and THERAP*) or EXERCIS* or HABITUAT* or EPLEY or CANALITH or SEMONT or MANOEUVRE* or MANEUVER* or (RECONDITIONING adj ACTIVIT*) or POSTUROGRAPHY or (POSTURAL adj CONTROL) or PFPP or (SENSORY and RELEARN) or (SENSORY and RETRAIN*) or (POSTURAL and RELEARN*) or (POSTURAL and RETRAIN*) S16 TX (POSITION* and PROCEDURE*) or (REPOSITION* and PROCEDURE*) or (REPOSITION* and PARTICLE*) or (VISUAL and VESTIBULAR) or (FUNCTIONAL and RETRAIN*) or (OCCUPATIONAL and RETRAIN*) or (OCCUPATIONAL and ADAPTATION) or (COOKSEY and CAWTHORNE) S17 S11 or S12 or S13 or S14 or S15 or S16 S18 S10 and S17 S19 S1 or S2 or S3 or S4 or S18 |
| Web of Science | BIOSIS Previews (Ovid) | CAB Abstracts (Ovid) | ISRCTN (mRCT) |
| #1 TS=(VESTIBULAR and (REHABILITATION or ADAPTATION or HABITUATION)) #2 TI=(Vertigo or vestibulopath* or dizziness or ((vestibular or balance*) and (disorder or hypofunction* or dysfunction* or impair* or disability* or pathology* or disturbance*))) #3 TI=(NEUROLABYRINTHITIDES or NEUROLABYRINTHITIS or (VESTIBULAR and (NEURITIS or NEURONITIS or NEURITIDES))) #4 TI=((ACOUSTIC adj NEUROMA) or (ACOUSTIC adj NEURINOMA) or (ACOUSTIC adj NEURILEMOMA) or (ACOUSTIC adj NEURILEMMOMA) or (VESTIBULAR adj SCHWANNOMA) or (ACOUSTIC adj SCHWANNOMA) or (MOTION adj SENSITIVITY) or (VESTIBULAR and PERIPHERAL) or (PERILYMPHATIC and FISTULA) or MENIERE* or (ENDOLYMPHATIC and HYDROPS) or (LABYRINTH* and HYDROPS) or (LABYRINTH* and SYNDROME) or BPV or BPPV or ANTBPPV) #5 #4 OR #3 OR #2 #6 TS=(REHABILITATION or PHYSIOTHERAP* or (PHYSICAL and THERAP*) or EXERCIS* or HABITUAT* or EPLEY or CANALITH or SEMONT or MANOEUVRE* or MANEUVER* or (RECONDITIONING adj ACTIVIT*) or POSTUROGRAPHY or (POSTURAL adj CONTROL) or PFPP or (SENSORY and RELEARN) or (SENSORY and RETRAIN*) or (POSTURAL and RELEARN*) or (POSTURAL and RETRAIN*)) #7 TS=((POSITION* and PROCEDURE*) or (REPOSITION* and PROCEDURE*) or (REPOSITION* and PARTICLE*) or (VISUAL and VESTIBULAR) or (FUNCTIONAL and RETRAIN*) or (OCCUPATIONAL and RETRAIN*) or (OCCUPATIONAL and ADAPTATION) or (COOKSEY and CAWTHORNE)) #8 #7 OR #6 #9 #8 AND #5 #10 #9 OR #1 | #1 TS=(VESTIBULAR and (REHABILITATION or ADAPTATION or HABITUATION)) #2 TI=(Vertigo or vestibulopath* or dizziness or ((vestibular or balance*) and (disorder or hypofunction* or dysfunction* or impair* or disability* or pathology* or disturbance*))) #3 TI=(NEUROLABYRINTHITIDES or NEUROLABYRINTHITIS or (VESTIBULAR and (NEURITIS or NEURONITIS or NEURITIDES))) #4 TI=((ACOUSTIC adj NEUROMA) or (ACOUSTIC adj NEURINOMA) or (ACOUSTIC adj NEURILEMOMA) or (ACOUSTIC adj NEURILEMMOMA) or (VESTIBULAR adj SCHWANNOMA) or (ACOUSTIC adj SCHWANNOMA) or (MOTION adj SENSITIVITY) or (VESTIBULAR and PERIPHERAL) or (PERILYMPHATIC and FISTULA) or MENIERE* or (ENDOLYMPHATIC and HYDROPS) or (LABYRINTH* and HYDROPS) or (LABYRINTH* and SYNDROME) or BPV or BPPV or ANTBPPV) #5 #4 OR #3 OR #2 #6 TS=(REHABILITATION or PHYSIOTHERAP* or (PHYSICAL and THERAP*) or EXERCIS* or HABITUAT* or EPLEY or CANALITH or SEMONT or MANOEUVRE* or MANEUVER* or (RECONDITIONING adj ACTIVIT*) or POSTUROGRAPHY or (POSTURAL adj CONTROL) or PFPP or (SENSORY and RELEARN) or (SENSORY and RETRAIN*) or (POSTURAL and RELEARN*) or (POSTURAL and RETRAIN*)) #7 TS=((POSITION* and PROCEDURE*) or (REPOSITION* and PROCEDURE*) or (REPOSITION* and PARTICLE*) or (VISUAL and VESTIBULAR) or (FUNCTIONAL and RETRAIN*) or (OCCUPATIONAL and RETRAIN*) or (OCCUPATIONAL and ADAPTATION) or (COOKSEY and CAWTHORNE)) #8 #7 OR #6 #9 #8 AND #5 #10 #9 OR #1 | 1 (VESTIBULAR and (REHABILITATION or ADAPTATION or HABITUATION)).tw. 2 (Vertigo or vestibulopath* or dizziness or ((vestibular or balance*) and (disorder or hypofunction* or dysfunction* or impair* or disability* or pathology* or disturbance*))).ti. 3 (NEUROLABYRINTHITIDES or NEUROLABYRINTHITIS or (VESTIBULAR and (NEURITIS or NEURONITIS or NEURITIDES))).ti. 4 ((ACOUSTIC adj NEUROMA) or (ACOUSTIC adj NEURINOMA) or (ACOUSTIC adj NEURILEMOMA) or (ACOUSTIC adj NEURILEMMOMA) or (VESTIBULAR adj SCHWANNOMA) or (ACOUSTIC adj SCHWANNOMA) or (MOTION adj SENSITIVITY) or (VESTIBULAR and PERIPHERAL) or (PERILYMPHATIC and FISTULA) or MENIERE* or (ENDOLYMPHATIC and HYDROPS) or (LABYRINTH* and HYDROPS) or (LABYRINTH* and SYNDROME) or BPV or BPPV or ANTBPPV).ti. 5 2 OR 3 OR 4 6 VOCATIONAL REHABILITATION/ or exp KINESIOTHERAPY/ or exp EXERCISE/ or exp HEAD MOVEMENT/ 7 (REHABILITATION or PHYSIOTHERAP* or (PHYSICAL and THERAP*) or EXERCIS* or HABITUAT* or EPLEY or CANALITH or SEMONT or MANOEUVRE* or MANEUVER* or (RECONDITIONING adj ACTIVIT*) or POSTUROGRAPHY or (POSTURAL adj CONTROL) or PFPP or (SENSORY and RELEARN) or (SENSORY and RETRAIN*) or (POSTURAL and RELEARN*) or (POSTURAL and RETRAIN*)).tw. 8 ((POSITION* and PROCEDURE*) or (REPOSITION* and PROCEDURE*) or (REPOSITION* and PARTICLE*) or (VISUAL and VESTIBULAR) or (FUNCTIONAL and RETRAIN*) or (OCCUPATIONAL and RETRAIN*) or (OCCUPATIONAL and ADAPTATION) or (COOKSEY and CAWTHORNE)).tw. 9 6 OR 7 OR 8 10 5 AND 9 11 1 OR 10 | (vestibular OR vertigo OR dizziness) AND (rehab% OR adaptation OR habituation OR exercis%) |
Data and analyses
Comparison 1. Vestibular rehabilitation versus control/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Subjective improvement in dizziness | 4 | 565 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.85, 3.86] |
| 2 Vertigo Symptom Scale | 3 | 553 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.87, ‐0.49] |
| 3 Gait ataxia | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.77] |
| 4 VD‐ADL (physical) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐10.5 [‐14.09, ‐6.91] |
| 5 Sway path | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐13.7 [‐16.51, ‐10.89] |
| 6 Dynamic visual acuity | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 84.0 [4.51, 1564.26] |
| 7 Vestibular Handicap Questionnaire | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐6.76, ‐0.04] |
| 8 Sharpened Romberg test (scores) | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | 9.90 [0.80, 19.00] |
| 9 Dizziness Handicap Inventory | 5 | 535 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐1.02, ‐0.64] |
| 10 Dynamic Gait Index | 2 | 93 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐1.38, ‐0.46] |
| 11 Romberg test | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.33, 21.98] |
| 12 Vertigo intensity | 2 | 75 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.14, 0.26] |
| 13 Posturography | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐7.09, 9.29] |
| 14 Vertigo intensity (BD versus sham) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.04, 0.24] |
1.11. Analysis.
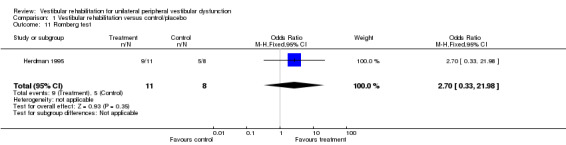
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 11 Romberg test.
1.12. Analysis.
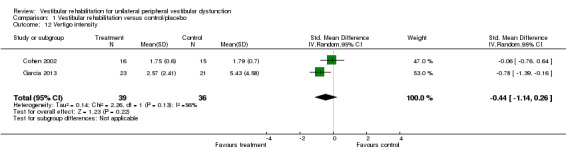
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 12 Vertigo intensity.
1.13. Analysis.
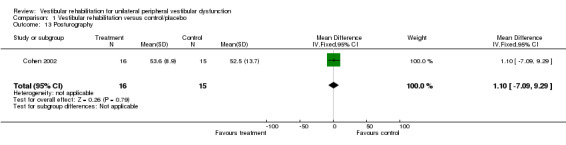
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 13 Posturography.
1.14. Analysis.
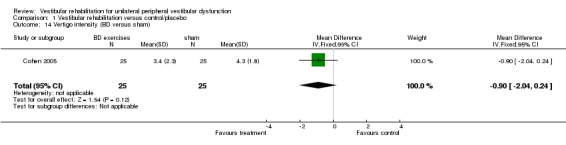
Comparison 1 Vestibular rehabilitation versus control/placebo, Outcome 14 Vertigo intensity (BD versus sham).
Comparison 2. Vestibular rehabilitation versus other treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dizziness cure rate | 2 | 119 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.49] |
| 2 Dynamic Gait Index | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.85, ‐0.15] |
| 3 Subjective improvement in dizziness | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.30, 53.47] |
| 4 Vertigo intensity (BD versus CRM) | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.35, 0.95] |
| 5 Vertigo intensity (XS versus CRM) | 2 | 75 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.61, 0.30] |
| 6 Dizziness Handicap Inventory | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.85, 1.85] |
2.3. Analysis.
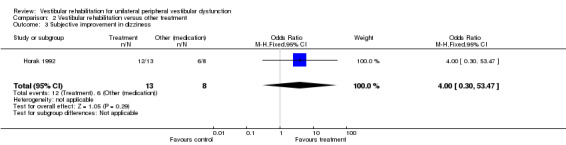
Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 3 Subjective improvement in dizziness.
2.4. Analysis.
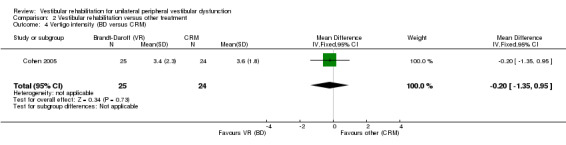
Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 4 Vertigo intensity (BD versus CRM).
2.5. Analysis.
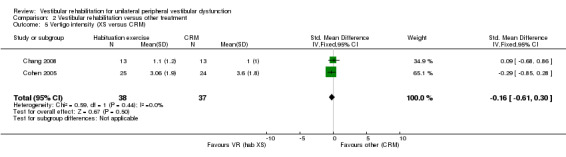
Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 5 Vertigo intensity (XS versus CRM).
2.6. Analysis.
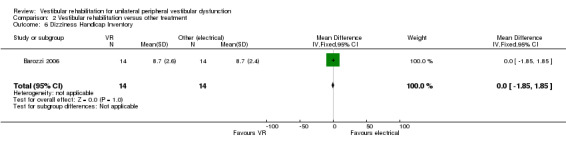
Comparison 2 Vestibular rehabilitation versus other treatment, Outcome 6 Dizziness Handicap Inventory.
Comparison 3. Vestibular rehabilitation versus other form of vestibular rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vertigo Symptom Scale | 4 | 573 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.29, 0.05] |
| 1.1 Vertigo short‐form | 2 | 465 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.21, 0.15] |
| 1.2 Vertigo component | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.12 [‐1.80, ‐0.45] |
| 1.3 VSS total | 1 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐1.07, 0.34] |
| 2 Dizziness Handicap Inventory | 7 | 626 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.20, 0.12] |
| 2.1 Booklet plus | 2 | 465 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.14, 0.22] |
| 2.2 Individual | 1 | 14 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐1.72, 0.47] |
| 2.3 Vertiguard | 1 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.31, 0.11] |
| 2.4 Number of sessions | 1 | 26 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.78, ‐0.14] |
| 2.5 CDP‐assisted VR | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.66, 0.94] |
| 2.6 Platform tilt | 1 | 29 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.64, 0.84] |
| 3 Repetitive head movement task | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 9.10 [0.12, 18.08] |
| 4 Vertigo VAS | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 4.5 [‐6.44, 15.44] |
| 5 Romberg test (eyes closed) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐7.18, 0.58] |
| 6 Tandem walk | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.58, 1.58] |
| 7 Posturography | 5 | 193 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.55, 1.07] |
| 7.1 VR plus | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.69, 0.55] |
| 7.2 Vertiguard | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.59, 0.81] |
| 7.3 CDP‐assisted VR | 2 | 50 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [‐2.48, 3.95] |
| 7.4 Speed of VR | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.61, 0.76] |
| 8 Subjective improvement in dizziness | 1 | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.27 [0.35, 197.61] |
| 9 Vertigo intensity | 2 | 55 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.03, 0.35] |
| 10 Vertigo frequency | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.05, 1.65] |
| 11 Vertigo Handicap Questionnaire | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 7.35 [‐4.94, 19.64] |
| 12 Ataxia | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.65, 0.19] |
| 13 Vestibular disorders ‐ activities of daily living scale | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.28, 0.68] |
| 14 Dynamic Gait Index | 2 | 45 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.41, 0.81] |
| 15 Beck Depression Inventory | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐8.01, 6.91] |
| 16 Subjective health | 2 | 435 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.65, 1.41] |
| 16.1 Booklet | 1 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.58, 1.71] |
| 16.2 Booklet plus | 1 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.60] |
| 17 Beck Anxiety Inventory | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐4.18 [‐10.50, 2.14] |
| 18 Situational vertigo questionnaire | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐1.21, ‐0.05] |
3.3. Analysis.
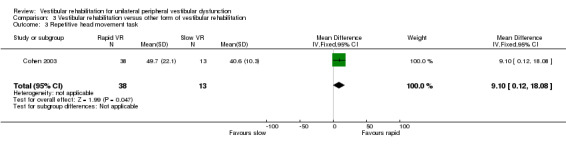
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 3 Repetitive head movement task.
3.4. Analysis.
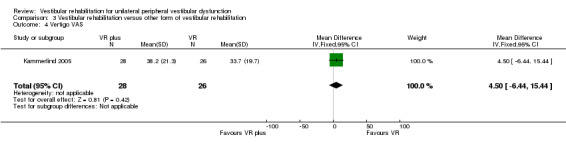
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 4 Vertigo VAS.
3.5. Analysis.
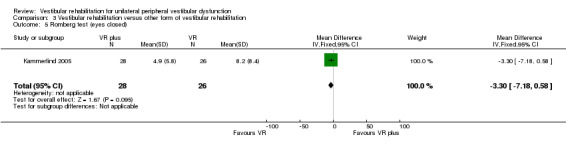
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 5 Romberg test (eyes closed).
3.6. Analysis.
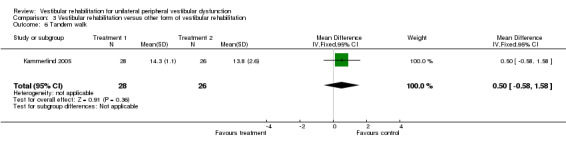
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 6 Tandem walk.
3.8. Analysis.
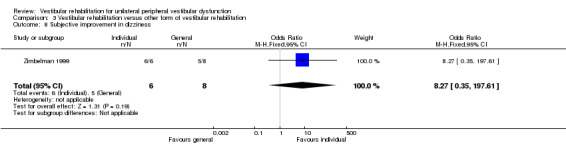
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 8 Subjective improvement in dizziness.
3.9. Analysis.
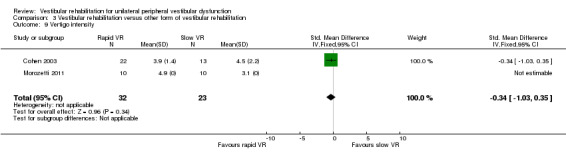
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 9 Vertigo intensity.
3.10. Analysis.
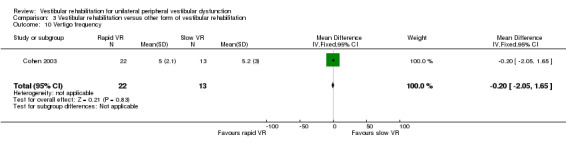
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 10 Vertigo frequency.
3.11. Analysis.
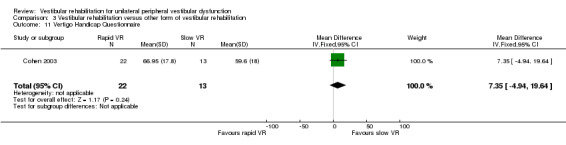
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 11 Vertigo Handicap Questionnaire.
3.12. Analysis.
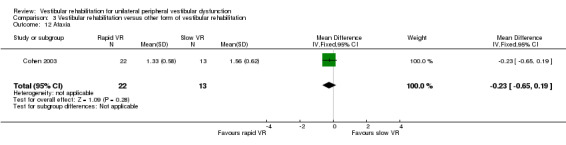
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 12 Ataxia.
3.13. Analysis.
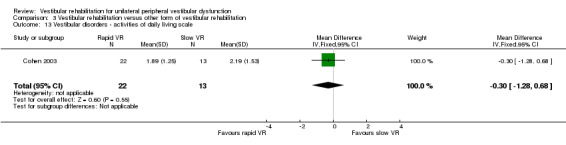
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 13 Vestibular disorders ‐ activities of daily living scale.
3.14. Analysis.
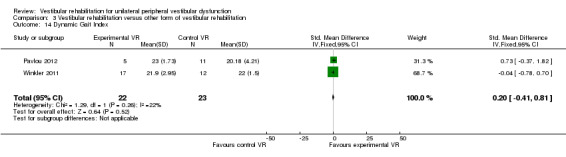
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 14 Dynamic Gait Index.
3.15. Analysis.
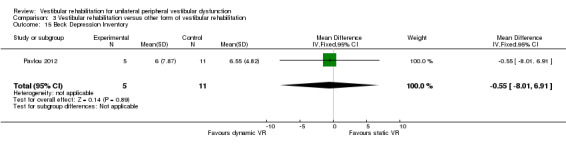
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 15 Beck Depression Inventory.
3.16. Analysis.
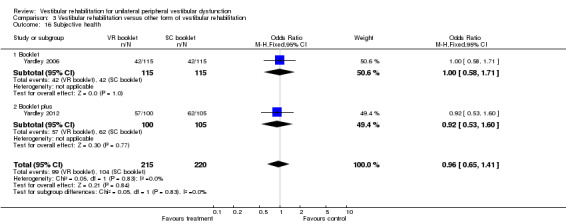
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 16 Subjective health.
3.17. Analysis.
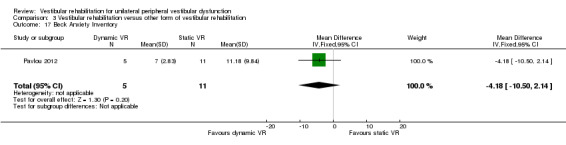
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 17 Beck Anxiety Inventory.
3.18. Analysis.
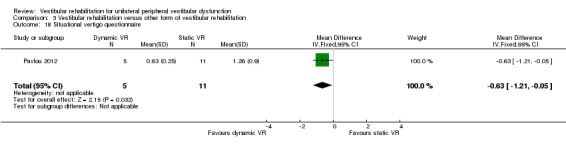
Comparison 3 Vestibular rehabilitation versus other form of vestibular rehabilitation, Outcome 18 Situational vertigo questionnaire.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barozzi 2006.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 28 Age: mean age 59 (SD 6) years Gender: 8 men Setting: not reported Eligibility criteria: unilateral peripheral vestibular deficit, 1 to 6 months after the acute phase, diagnosed by clinical examination, CDP, videonystagmography, rotatory chair and caloric tests demonstrating a canal paresis of at least 25% Baseline characteristics: not reported |
|
| Interventions |
Intervention group: oculomotor rehabilitation (adaptation) (n not stated) Comparator group: vestibular electrical stimulation (n not stated) VR versus non‐VR |
|
| Outcomes | Primary outcomes: DHI Secondary outcome: posturography | |
| Notes | No details given regarding participants lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | There is insufficient information regarding the blinding of participants and assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The total numbers of participants in each group at follow‐up was not reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Basta 2011.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 155; only data from 68 participants were included in meta‐analysis Age: intervention group mean age 60.6 (SD 13.3), comparator group mean age 61.3 (SD 9.2) Gender: intervention group 57.2% male, comparator group 57.1% male Setting: participants were recruited from neuro‐otologic or neurologic clinics Eligibility criteria: experienced balance disorder for more than 12 months due to the following conditions: canal paresis, otolith disorder, removal of an acoustic neuroma, microvascular compression syndrome, Parkinson's disease, presbyvertigo Exclusion criteria: use of drugs which actively influence the vestibular system, sensory deficits exceeding age‐related values, combination of different types of vestibular disorder in the one patient, an acute vestibular disorder, or receiving other treatment for their balance disorder Baseline characteristics: there were no significant differences between the age and sex of the groups at baseline |
|
| Interventions | Data included in this review were obtained from the authors and only included participants with UPVD Intervention group: vibrotactile neurofeedback training and vestibular rehabilitation exercises performed daily (15 minutes) over 2 weeks with the Vertiguard system (n = 59) Comparator group: sham Vertiguard device and vestibular rehabilitation exercises (n = 9) VR versus VR |
|
| Outcomes |
Primary outcome: DHI Secondary outcomes: VSS, posturography (BalanceMaster) |
|
| Notes | No participants were lost to follow‐up. Only data from those with UPVD were included in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The patients as well as the supervisor did not know the group classification" (double‐blinded study design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers of participants contributing to outcome measures were reported and the authors propose that the 40% of participants who did not attend follow‐up sessions were likely to have no remaining vestibular symptoms |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Unclear risk | There was no disclosure regarding authors' potential financial interests in the Vertiguard device |
Cakrt 2010.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 17 Age: intervention group: mean age 37 (range 19 to 56), comparator group: mean age 44 (range 26 to 62) Gender: intervention group: 8 males, comparator group: 5 males Setting: Department of Otolaryngology, University Hospital Eligibility criteria: participants undergoing retrosigmoid microsurgical removal of vestibular schwannoma Exclusion criteria: proven pre‐operative vestibular loss, central nervous system or other musculoskeletal system deficits Baseline characteristics: no significant differences in mean age, posturography measures or tumour size, although there were more males in the intervention group |
|
| Interventions |
Intervention group: received visual feedback while performing VR using the BalanceMaster (n = 9) Comparator group: control group received VR without feedback (n = 8) Both commenced on the 5th postoperative day and progressively increased amount of exercise until discharge at approximately day 15 VR versus VR |
|
| Outcomes | Primary outcome: posturography | |
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants, investigators nor outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed up after 2 weeks |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Chang 2008.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 26 Age: mean age 56.4 (SD 11.4) years Gender: 11 males Setting: medical centre Eligibility criteria: first ever attack of unilateral posterior canal BPPV, diagnosed by neurologist and clinical examination Exclusion criteria: peripheral vestibular hypofunction and central vestibular lesions Baseline characteristics: no differences between groups |
|
| Interventions |
Intervention group: canal repositioning technique (CRT) and vestibular exercises (n = 13) Comparator group: CRT only (n = 13) VR versus other (CRT) |
|
| Outcomes |
Primary outcome: DGI Secondary outcomes: posturography (BalanceMaster), vertigo intensity (VAS), tandem walk |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were then randomly assigned to either group by an independent person who picked one of the sealed envelopes before the start of the intervention" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants was possible but was not described. Outcomes were assessed by the same evaluator who was blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | High risk | Authors acknowledge that the intensity and duration of treatment were greater in the experimental group, which received 6.6 hours of treatment compared with 0.3 hours in the control group (pg 345) |
Cohen 2002.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 31 Age: mean age 51 years (range 35 to 77) Gender: 17 males Setting: Department of Otolaryngology, university Eligibility criteria: acoustic neuroma resection ‐ postoperative (1 week ‐ acute) diagnosed by history, audiometry, MRI Exclusion criteria: nil stated Baseline characteristics: no significant difference between the groups, participants did not complain of vertigo |
|
| Interventions |
Intervention group: VR (head exercises) (n = 16) Comparator group: control (attention only) (n = 15) VR versus control (nil) |
|
| Outcomes | Primary outcome: VOR Secondary outcomes: posturography, VI and VF, WOL | |
| Notes | All 31 participants were available for follow‐up on postoperative day 5 or 6. 9 participants were lost to follow‐up at later assessments, but their group allocation was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors and treating physiotherapists were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 29% of participants were lost to follow‐up but the authors attempted to correct for this in the statistical analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Cohen 2003.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 53 Age: mean 51.1 years (range 25 to 84) Gender: 15 men Setting: tertiary care centre Eligibility criteria: chronic vestibulopathy (labyrinthitis or neuronitis of more than 2 months) diagnosed by physician using posturography, calorics and oculomotor test battery Exclusion criteria: Ménière's disease, BPPV, acute vestibular neuronitis or labyrinthitis, significant orthopaedic limitations, a history of head trauma or neurologic disease, prior otologic disease or taking vestibular suppressants Baseline characteristics: no differences reported |
|
| Interventions |
Intervention group: VR (slow head exercises ‐ habituation) (n = 13) Comparator group 1: VR (rapid head exercises) (n = 22) Comparator group 2: VR (rapid plus attention) (n = 18) VR versus VR versus VR |
|
| Outcomes | Primary outcome: VSS Secondary outcomes: VD‐ADL, VHQ, DHI, VI, VF | |
| Notes | 71 participants were recruited originally but this analysis only included those who completed all sessions and follow‐up assessments | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Outcomes were questionnaires and not likely to be affected by bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for drop‐outs following initial assessment were reported although final numbers in each group were not |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Cohen 2005.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 124 Age: 58.3 years (SD 12.8) Gender: 48 males Setting: hospitals Eligibility criteria: unilateral BPPV (post SC) diagnosed by physician (D‐H test), with dizziness for at least 1 week Exclusion criteria: those with whiplash, head trauma, significant orthopaedic, neurological and other otologic disorders Baseline characteristics: not reported |
|
| Interventions |
Intervention group: B‐D exercises (n = 25) Comparator group 1: habituation exercises (n = 25) Comparator group 2: CRM (n = 24) Comparator group 3: LM (n = 25) Comparator group 4: sham manoeuvre (n = 25) VR versus other (CRMs) versus placebo |
|
| Outcomes | Primary outcome: VI Secondary outcomes: VF, posturography | |
| Notes | 24 participants dropped out of the study for a variety of reasons and their data were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated by the senior investigator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16% of participants dropped out of the study with reasons. Further drop‐outs after the first post‐test assessment were not adequately described (at 3 and 6 months) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Foster 2012.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 68 Age: not reported Gender: 19 males Setting: university outpatient clinic Eligibility criteria: adults with a history suggestive of BPPV and Dix‐Hallpike manoeuvre consistent with unilateral posterior canal BPPV Exclusion criteria: those with cupulolithiasis, horizontal canal BPPV, bilateral BPPV, nystagmus due to central or other peripheral vestibular disorders, those without nystagmus on the D‐H, those unable to bend the neck or turn the head safely, or sit up, lie down, roll over or kneel on hands and knees, or those who could not tolerate the D‐H, the CRM or assume the half‐somersault position Baseline characteristics: not reported |
|
| Interventions |
Intervention group: half‐somersault manoeuvre was performed twice in the clinic and also given as a home exercise (n = 33) Comparator group: Epley manoeuvre was performed twice in the clinic and also given as a home exercise (n = 35) VR versus VR |
|
| Outcomes |
Primary outcome: nystagmus intensity score Secondary outcome: BPPV recurrence |
|
| Notes | All participants completed the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Researcher... assigned them via a randomised list prepared by a statistician" |
| Allocation concealment (selection bias) | Low risk | Participants were removed to another training room prior to randomisation to treatment group |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6‐month follow‐up 5 participants dropped out from the Epley group and 6 from the half‐somersault group |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Garcia 2013.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 44 Age: intervention group age = 48 (range 20 to 60), control group age = 48 (range 19 to 60) Gender: intervention group 9 males, control group 7 males Setting: university medical school Eligibility criteria: Participants were included if they had Ménière's disease diagnosed by an ENT specialist and had complaints of dizziness between exacerbations of their disease Exclusion criteria: Participants were excluded if they had suffered a bout immediately before the study, if they had rheumatic disease, uncontrolled hypertension, heart disease, severe visual involvement or decompensated involvement despite corrective lenses, orthopaedic disorders or joint replacements affecting the lower limbs, psychiatric disorders, were unable to communicate or stand independently, those who had been involved in balance rehabilitation programmes in the past 6 months, those in the intervention group who did not attend 3 consecutive intervention sessions, and those who failed to follow the diet and other advice to cease alcohol, refined sugar, coffee and smoking and take betahistine Baseline characteristics: At baseline participants reported their frequency and duration of dizzy spells, with no differences between the groups |
|
| Interventions |
Intervention group: 12 rehabilitation sessions (twice weekly for 45 minutes) with virtual reality stimuli in a Balance Rehabilitation Unit, plus diet and lifestyle advice and betahistine (n = 23) Control group: 12 stimulus enriched exercise sessions (twice weekly) in the Balance Rehabilitation Unit, plus diet and lifestyle advice and betahistine (n = 21) VR versus control (usual care) |
|
| Outcomes |
Primary outcome: dizziness analogue scale scores Secondary outcomes: DHI, posturography Intervention participants were assessed 6 weeks after completion of the 12 sessions, while comparator group participants were assessed immediately after the 12 sessions (6 weeks) |
|
| Notes | Participants lost to follow‐up: nil, but intervention group participants who missed more than 3 consecutive sessions were excluded from the study (number not reported) Intervention participants improved significantly on the DHI, dizziness analogue scale and had greater stability on posturography compared to control participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using "a table with uniformly distributed random numbers produced by a computer program" pg 368 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The evaluations and the rehabilitation program were carried out by the head researcher" pg 369 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were available for follow‐up assessments |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | High risk | Excluding participants who missed intervention sessions does not allow for evaluation of participant compliance. The different time periods for assessing outcomes post intervention allows for the potential bias that the intervention group may have simply recovered over time due to the lifestyle changes |
Giray 2009.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 41 Age: intervention group: mean age 50 (range 26 to 78), comparator group: mean age 55.5 (range 18 to 73) Gender: intervention group: 6 males, comparator group: 8 males Setting: university hospital outpatient department Eligibility criteria: participants were diagnosed with chronic decompensated unilateral peripheral vestibular deficit, secondary to peripheral vestibular dysfunction by a neuro‐otologist or neurologist. Diagnosed by ENG, bithermal caloric test, ocular motor testing and positional testing Exclusion criteria: any problem that compromised rehabilitation, visual or somato‐sensorial disorders, fluctuating and intermittent vertigo, BPPV, less than 2 months duration of symptoms Baseline characteristics: the only difference between groups was superior performance standing on foam with eyes closed in the intervention group |
|
| Interventions |
Intervention group: VR incorporating adaptation, substitution, visual desensitisation and balance exercises (n = 20) Comparator group: control, no input (n = 21) VR versus control (no input) |
|
| Outcomes |
Primary outcome: unsteadiness (VAS) Secondary outcomes: DHI, BBS, posturography (BalanceMaster) |
|
| Notes | 1 participant from the control group was lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants, investigators nor outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient in the control group dropped out because of difficulty commuting to the hospital |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Herdman 1995.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 19 Age: intervention group: mean age = 59.3 (SD 10.9 years), comparator group: mean age = 47.9 (SD 10.4 years) Gender: intervention group: 3 males, comparator group: 3 males Setting: university Eligibility criteria: participants post removal of acoustic neuroma. Diagnosed by MRI and surgically resected ‐ study performed in acute post period Exclusion criteria: other CNS involvement or other musculoskeletal disorders Baseline characteristics: the experimental group was significantly older than the comparator group and they were more likely to have had a translabyrinthine approach. There were no differences in clinical assessments before surgery |
|
| Interventions |
Intervention group: VR (adaptation to increase gain) plus ambulation exercises (n = 11) Comparator group: smooth pursuit exercises (no head movement) plus ambulation exercises (n = 8) VR versus control (placebo) |
|
| Outcomes |
Primary outcomes: vertigo intensity (VAS)
Dysequilibrium (VAS) Secondary outcomes: Romberg ‐ normal and sharpened, Fukuda, gait analysis, oculomotor tests, posturography |
|
| Notes | All participants were available for follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants were excluded from analysis and numbers are provided for each group |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Herdman 2003.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 21 Age: intervention group: mean age 65.2 (SD 16.5), comparator group: mean age 64.9 (SD 16.2) Gender: not reported Setting: university Eligibility criteria: unilateral vestibular hypofunction with abnormal DVA, diagnosed by caloric, rotary chair, positive head thrust Exclusion criteria: nil specified Baseline characteristics: there were no differences between the groups |
|
| Interventions |
Intervention group: VR (adaptation to enhance VOR) (n = 13) Comparator group: placebo exercises designed to be "vestibular neutral" (n = 8) VR versus control (placebo) |
|
| Outcomes | Primary outcome: DVA during head movements (predictable and unpredictable) Secondary outcome: oscillopsia intensity (VAS) | |
| Notes | 2 participants dropped out of the study from the comparator group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs were explained (9%) |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Horak 1992.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 25 Age: not reported Gender: not reported Setting: not reported Eligibility criteria: peripheral vestibular dysfunction diagnosed by neuro‐otologist for BPPV, inner ear concussion syndrome, reduced unilateral vestibular function, 18 to 60 years of age Exclusion criteria: CNS involvement, spontaneous fluctuating vestibular symptoms, significant orthopaedic or cardiac problems, or non‐compliance with the treatment programme Baseline characteristics: no differences reported |
|
| Interventions |
Intervention group: VR (n = 14) Comparator group 1: general conditioning exercises (n = 4) Comparator group 2: medication (meclizine or Valium) (n = 8) VR versus control (sham) versus other non‐VR (medication) |
|
| Outcomes | Primary outcome: DI Secondary outcomes: posturography, SOOL, questionnaire, positional vertigo ‐ number of positions, DI and duration | |
| Notes | Number of participants available for post intervention assessments not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, physicians and outcome assessors were all blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were available for follow‐up assessments |
| Selective reporting (reporting bias) | Unclear risk | Some outcome data not reported for meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kammerlind 2005.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 54 Age: intervention group: mean age 52 (SD 12) years, comparator group: mean age 52 (SD 15) years Gender: intervention group: 11 male, comparator group: 18 male Setting: ENT departments of 3 hospitals Eligibility criteria: acute unilateral vestibular loss confirmed by ENG with calorics Exclusion criteria: central neurologic or auditory symptoms or other vertigo disease Baseline characteristics: the groups were similar for most measures except gender, as there were more males in the home training group |
|
| Interventions |
Intervention group: VR (home exercises plus extra PT (habituation, adaptation, balance and gait) (extra PT included individualised instruction and further exercises) (n = 28) Comparator group: VR (home exercises only) (n = 26) VR versus VR |
|
| Outcomes | Primary outcome: balance tests (clinical) Secondary outcomes: ENG, vertigo (VAS), balance (VAS) | |
| Notes | 2 participants were lost to follow‐up at the 6‐month assessments in the intervention group and 1 in the comparator group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used to inform participants of group allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs and missed sessions were reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Karanjai 2010.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 48 Age: average 48, range 32 to 52 Gender: 20 male Setting: outpatient department, medical college Eligibility criteria: diagnosed with posterior canal BPPV through history and clinical examination (Dix‐Hallpike manoeuvre) Exclusion criteria: lateral canal BPPV, bilateral disease, history of middle or inner ear problems, ototoxic drug use, previous neurological disorder Baseline characteristics: not reported |
|
| Interventions |
Intervention group: Brandt‐Daroff exercises 3 times a day for 2 weeks (n = 16) Comparator group 1: single Epley manoeuvre followed by post‐treatment instructions (n = 16) Comparator group 2: single Semont manoeuvre followed by post‐treatment instructions (sleep upright for 2 nights, then on the unaffected side for the next 5 nights) (n = 16) VR (BD) versus other (CRM ‐ Epley) versus other (CRM ‐ Semont) |
|
| Outcomes |
Primary outcome: BPPV cure rate Secondary outcomes: no secondary outcomes were reported Statistical analysis of the differences between groups not performed |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were no participants lost to follow‐up at 3 months |
| Selective reporting (reporting bias) | Unclear risk | All study data appear to be reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
Krebs 2003.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 33 (UPVD), n = 51 (bilateral VD) Age: intervention group: mean age 51.8 (SD 19.3) years, comparator group: mean age 67.8 (SD 16.1) years Gender: not reported Setting: tertiary care hospital Eligibility criteria: mixed diagnoses ‐ unilateral and bilateral peripheral vestibular dysfunction. Diagnosed by VOR gain, calorics etc Exclusion criteria: BPPV, Ménière's disease, unstable vestibulopathies Baseline characteristics: not reported |
|
| Interventions |
Intervention group: VR (adaptation, balance) (n = 42) Comparator group: control (strength exercises) (n = 44) VR versus control (sham) |
|
| Outcomes |
Primary outcome: gait speed Secondary outcomes: locomotor stability, base of support |
|
| Notes | Only 27 of the 86 who completed the exercise intervention returned for the 1‐year follow‐up assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing data explained for both groups and analysis done only on participants who completed the study |
| Selective reporting (reporting bias) | High risk | Data not reported adequately to enable meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kulcu 2008.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 38 Age: intervention group: 47.1 (SD 12.2) years, comparator group: 45.6 (SD 13.1 years) Gender: intervention group: 5 males, comparator group: 5 males Setting: university hospital outpatient department Eligibility criteria: patients diagnosed with BPPV who had undergone repositioning techniques by their otorhinolaryngologists but were still complaining of vertigo and dysequilibrium. Participants were included in the study if they had no intervention for at least the last 3 months Exclusion criteria: simultaneous occurrence of central or peripheral neurological disease, other causes of vertigo affecting balance Baseline characteristics: no differences between age and sex |
|
| Interventions |
Intervention group: VR (Cawthorne‐Cooksey exercises) (n = 19) Comparator group: medication (betahistine) (n = 19) VR versus medication |
|
| Outcomes |
Primary outcome: Vertigo, Dizziness, Imbalance questionnaire (VDI) incorporating the symptom subscale and health‐related quality of life subscale Secondary outcome: Vertigo Symptom Scale (VSS) |
|
| Notes | 1 participant dropped out of the exercise group due to increased severity of symptoms and was not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was done using a sequence of random numbers before the baseline assessments were recorded" |
| Allocation concealment (selection bias) | High risk | Randomisation was performed using an open random allocation schedule |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants, investigators nor outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 patient in the exercise group dropped out because of increased severity of symptoms |
| Selective reporting (reporting bias) | High risk | Appropriate data not reported for meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Marioni 2013.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 30 Age: intervention group: mean age 45 (SD 7) years, comparator group 1: mean age 42 (SD 9) years, comparator group 2 (controls): mean age 48 (SD 4) years Gender: intervention group: 10 males, comparator group 1: 8 males, comparator group 2 (controls): 5 males Setting: Department of Otolaryngology, university hospital Eligibility criteria: adults aged 18 to 65 with acute unilateral peripheral vestibular disorder occurring within 2 weeks of entry into the study, with at least 50% weakness on videonystagmography with caloric testing. Exclusion criteria: abnormal visual acuity, other neurological or musculoskeletal disorders Baseline characteristics: no differences between sides for the UPVD groups, but marked differences in posturography between both UPVD and control groups |
|
| Interventions |
Intervention group: posturography‐assisted VR (n = 15) Comparator group 1: group awaiting spontaneous compensation, no VR (n = 15) Comparator group 2: healthy adults without a vestibular disorder (controls, n = 10) VR versus no vestibular control versus healthy controls |
|
| Outcomes | Primary outcome: posturography | |
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedule was computer generated using the SAS 6.12" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether participants, physical therapist/otolaryngologist or outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Low risk | All study data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Morozetti 2011.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 20 Age: mean age 55 years Gender: 8 male Setting: university Eligibility criteria: adults with a chronic vestibular disorder diagnosed by otorhinolaryngologists Exclusion criteria: those with any central vestibulopathy, BPPV, unstable Meniere's disease Baseline characteristics: not reported |
|
| Interventions |
Intervention group: home exercises based on vertical and horizontal vestibulo‐ocular reflex stimulation (VRS) (n = 10) Comparator group: personalised VR home exercise programme (n = 10) VR versus VR |
|
| Outcomes |
Primary outcome: DHI Secondary outcome: VAS |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether participants, physical therapists or outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants who completed the study was not reported |
| Selective reporting (reporting bias) | Unclear risk | Appropriate data not reported |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
Mruzek 1995.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 24 Age: intervention group: mean age 52, range 40 to 77, comparator group 1: mean age 50, range 37 to 79, comparator group 2: mean age 50, range 27 to 65 Gender: intervention group: 2 males, comparator group 1: 2 males, comparator group 2: 7 males Setting: balance disorders clinic Eligibility criteria: participants had been reviewed by a physician for acoustic neuroma or Ménière's disease and were referred for ablative surgery Exclusion criteria: not reported Baseline characteristics: no differences in baseline measures between groups |
|
| Interventions |
Intervention group: VR plus social reinforcement, 15 minutes, 2 x day plus a daily walk (n = 8) Comparator group 1: VR no social reinforcement (n = 8) Comparator group 2: general range of motion exercises plus social reinforcement (n = 8) VR versus other VR versus control (no VR) |
|
| Outcomes |
Primary outcome: DHI Secondary outcomes: CDP, MSQ |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data were reported |
| Selective reporting (reporting bias) | High risk | Data not reported adequately to enable meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pavlou 2004.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 40 Age: intervention group: mean age 43.8, range 23 to 77; comparator group: mean age 43.0, range 22 to 64 Gender: intervention group: 5 males; comparator group: 7 males Setting: clinic Eligibility criteria: clinical diagnosis of a peripheral vestibular disorder; stable symptoms; 18 to 80 years of age; previous completion of a vestibular rehabilitation programme with partial or no improvement Exclusion criteria: CNS involvement, fluctuating symptoms, e.g. Ménière's disease or active BPPV, inability to attend sessions or other medical conditions in the acute phase, e.g. orthopaedic injury Baseline characteristics: no significant difference in characteristics between groups |
|
| Interventions | Intervention group: VR (customised exercises, including gaze control and stability, balance training) (n = 20) Comparator group: simulator (optokinetic disc to produce visual‐vestibular conflict plus above) (n = 20) VR versus VR |
|
| Outcomes | Primary outcome: posturography Secondary outcomes: VSS‐V and VSS‐A, HADS, BBS, SCQ, STAI, CMSSQ | |
| Notes | BBS not sensitive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data were reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pavlou 2012.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 16 Age: intervention group: mean age 42.0, range 25 to 51, comparator group: mean age 42.1, range 28 to 54 Gender: intervention group: 2 males, comparator group: 7 males Setting: university Eligibility criteria: participants with a history of acute onset of vertigo and with a confirmed peripheral vestibular deficit on the basis of the caloric tests and/or rotational tests on ENG Exclusion criteria: those with migrainous vertigo, Ménière's disease, BPPV, central vestibular disorders, other neurological disorders, significant systemic illness or psychiatric disorders Baseline characteristics: symptom duration was significantly longer in the intervention group |
|
| Interventions |
Intervention group: dynamic virtual reality, performed for 45 minutes twice weekly for 4 weeks plus home exercises and general conditioning programme (walking) (n = 5) Comparator group 1: static virtual reality image rehabilitation, performed for 45 minutes twice weekly for 4 weeks plus home exercises and general conditioning programme (walking) (n = 11) Comparator group 2: cross‐over of 5 group 1 participants who then received dynamic virtual reality (not included in our analysis) (n = 5) VR versus VR versus VR |
|
| Outcomes |
Primary outcome: Dynamic Gait Index Secondary outcomes: Beck Depression Inventory, Beck Anxiety Inventory, Fear Questionnaire, Situational Vertigo Questionnaire, virtual reality exercise symptom scores |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It is not clear whether participants were blinded to the purpose of the experiment or whether they were aware that there were 2 types of virtual reality training groups. An independent observer was used to collect the Dynamic Gait Index outcome data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant did not complete the study in the static virtual reality group and their data were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Resende 2003.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 16 Age: intervention group: mean age 70.5 years, range 61 to 82, comparator group: mean age 69.3 years, range 60 to 78 Gender: intervention group: no males, comparator group: no males Setting: hospital Eligibility criteria: participants with BPPV diagnosed by ENT using history, ENT examination, ENG Exclusion criteria: visual disorders, severe auditory disorders, systemic diseases such as diabetes, significant neurological disorders, musculoskeletal disorders, psycho‐emotional abnormalities Baseline characteristics: no differences in any parameters between the 2 groups |
|
| Interventions |
Intervention group: VR (compensation, adaptation, sensory substitution, balance: C‐C) (n = 8) Comparator group: control (n = 8) VR versus control (nil) Both groups had Ginkgo biloba prior to exercises |
|
| Outcomes | Primary outcome: VD‐ADL | |
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It is not clear whether outcome assessors were blinded to group allocation; questionnaire results unlikely to be affected by bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data were reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Rossi‐Izquierdo 2011.
| Methods | Design: randomised controlled trial with balanced, block randomisation | |
| Participants |
Number: 24 Age: intervention group: mean age 54.5, range 30 to 82, comparator group: mean age 48.8 years, range 28 to 75 Gender: intervention group: 5 males, comparator group: 3 males Setting: Department of Otolaryngology, university hospital Eligibility criteria: participants with instability due to chronic unilateral peripheral vestibular disorders, which had not spontaneously resolved after a month. Hypofunction was defined with caloric tests, at least 25% labyrinthic preponderance according to defined criteria Exclusion criteria: inner ear and pontocerebellar lesions, post‐traumatic conditions, locomotor disturbance preventing standing, previous instrumental VR or the lack of a complete evaluation Baseline characteristics: mixed aetiology reported but no differences in age, gender or duration of symptoms |
|
| Interventions |
Intervention group: computerised dynamic posturography (CDP), 5 sessions of approximately 15 to 20 minutes on consecutive days (n = 12) Comparator group: optokinetic stimulation (OKN), 5 sessions lasting 5 to 15 minutes on consecutive days (n = 12) VR versus VR |
|
| Outcomes |
Primary outcome: DHI Secondary outcome: posturography |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We used block randomisation" |
| Allocation concealment (selection bias) | Low risk | An independent researcher assigned participants to groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The person in charge of the VR was neither of the two who assigned patients to groups and evaluated the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not clear from the results or the figures whether the data from all participants are included |
| Selective reporting (reporting bias) | Unclear risk | Data not reported adequately to enable meta‐analysis |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
Rossi‐Izquierdo 2013.
| Methods | Design: randomised controlled trial, with balanced block randomisation | |
| Participants |
Number: 26 Age: intervention group: mean age 59.3 (SD 13.5), comparator group: mean age 63.3 (SD 16.1) Gender: intervention group: 7 males, comparator group: 3 males Setting: Department of Otolaryngology, university hospital Eligibility criteria: participants with instability due to chronic unilateral peripheral vestibular disorders which had not spontaneously resolved after a month. Hypofunction was defined with caloric tests, at least 25% labyrinthic preponderance according to defined criteria Exclusion criteria: inner ear and pontocerebellar lesions, post‐traumatic conditions, locomotor disturbance preventing standing, previous instrumental VR or the lack of a complete evaluation Baseline characteristics: there were no differences in age, gender or duration of symptoms, but 2 of the baseline posturography measures were significantly different between the groups at baseline |
|
| Interventions |
Intervention group: 5 sessions of posturography‐assisted VR over a 2‐week period (n = 13) Comparator group: 10 sessions of posturography‐assisted VR over a 2‐week period (n = 13) VR versus VR |
|
| Outcomes |
Primary outcome: DHI Secondary outcome: posturography |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We used balanced block randomisation" |
| Allocation concealment (selection bias) | Unclear risk | An independent researcher assigned participants to groups |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The person who performs the VR in each hospital was neither of the other people who assigned the patients to groups and evaluated the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It appears as though all participants completed the study but numbers of participants are not provided in the results |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Scott 1994.
| Methods | Design: randomised controlled trial (cross‐over ‐ analysed first phase as experimental phase) | |
| Participants |
Number: 20 Age: mean age 54 years, range 29 to 77 Gender: 14 males Setting: Department of Audiology, university hospital Eligibility criteria: Ménière's disease diagnosed by medical and audiological examination (5 were bilateral but had one "worse" ear) Exclusion criteria: diagnosed coronary artery problems Baseline characteristics: no differences reported |
|
| Interventions |
Intervention group: applied relaxation (n = 10) Comparator group: transcutaneous nerve stimulation to the hand (n = 10) VR (relaxation) versus other non‐VR (TNS) |
|
| Outcomes |
Primary outcome: dizziness Secondary outcomes: ENG, interview/questionnaire, psychoacoustic measures (not relevant), hearing ability (not relevant), tinnitus discomfort (not relevant) |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data were reported |
| Selective reporting (reporting bias) | High risk | Data not reported adequately to enable meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Strupp 1998.
| Methods |
Design: randomised controlled trial Did not justify sample size; did not report validity and reliability of measures |
|
| Participants |
Number: 39 (43 spontaneous resolution participants were removed from the study) Age: intervention group: mean age 51.7 (SD 11.1) years, comparator group: mean age 52.4 (SD 9.9) years Gender: not reported Setting: neurology department of hospital Eligibility criteria: vestibular neuritis (acute/sub‐acute) diagnosed by history, examination ‐ nystagmus, postural imbalance, ENG, calorics, ocular tilt reaction Exclusion criteria: history of other vestibular dysfunction, central vestibular disorder, polyneuropathy, marked decreased visual acuity, other diseases that might impair mobilisation Baseline characteristics: reported to be similar between the groups |
|
| Interventions |
Intervention group: VR (home exercises, based on Cooksey‐Cawthorne, Norre ‐ habituation, gaze exercises, sensory substitution, functional retraining) (n = 19) Comparator group: control (nil exercise but encouragement to move) (n = 20) VR versus control |
|
| Outcomes |
Primary outcome: sway path values (vestibulo‐spinal system) Secondary outcomes: ocular tilt (vestibular‐ocular system), subjective visual vertical (perception) |
|
| Notes | On initial assessment 82 patients were included but 43 were later excluded due to partial or complete recovery of labyrinth function | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data were reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Szturm 1994.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 23 (3 participants with bilateral vestibulopathy) Age: intervention group: mean age 50.3 (SD 7.0), comparator group: mean age 48.1 (SD 10.9) Gender: intervention group: 6 males, comparator group: 6 males Setting: Department of Otolaryngology, university Eligibility criteria: clinical diagnosis of peripheral vestibular dysfunction, persistent dizziness, disorientation or imbalance for at least 1 year, and abnormal balance performance during CDP at baseline Exclusion criteria: other neurological disorders, taking medication for their vestibular condition Baseline characteristics: no differences were reported |
|
| Interventions |
Intervention group: VR (n = 11) Comparator group: VR (home, C‐C) (n = 12) VR versus VR |
|
| Outcomes | Primary outcome: CDP Secondary outcomes: VOR, OKN (step chair rotations) | |
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It appears that data are missing from Group B participants but this is not adequately explained in the results |
| Selective reporting (reporting bias) | High risk | Data not reported adequately to enable meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Teggi 2009.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 40 Age: intervention group: mean age 53.5 (SD 9.8) years, 8 males, comparator group: mean age 51.4 (SD 9.1) years, 9 males Gender: intervention group: 8 males, comparator group: 9 males Setting: hospital department Eligibility criteria: participants were recently hospitalised for an acute episode of rotational vertigo that lasted several days and were diagnosed with vestibular neuritis Exclusion criteria: previous vertiginous episodes, other neurological disorders such as migraine, previous psychiatric disorders, visual deficits, acute orthopaedic disorders Baseline characteristics: not reported |
|
| Interventions |
Intervention group: VR (n = 20) Comparator group: control ("perform usual daily activities") (n = 20) VR versus control (nil) |
|
| Outcomes |
Primary outcome: DHI Secondary outcomes: posturography, DGI, anxiety (VAS) |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants, investigators nor outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Toledo 2000.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 40 Age: intervention group: mean age 53.7, comparator group 1: mean age 55.4, comparator group 2: mean age 58.9 Gender: intervention group: 3 males, comparator group 1: 2 males, comparator group 2: 5 males Setting: not reported Eligibility criteria: BPPV diagnosed with clinical assessment and electronystagmography Exclusion criteria: CNS disturbances Baseline characteristics: described as similar between the groups but not reported |
|
| Interventions |
Intervention group: VR (PC, head‐eye and habituation) (n = 10) Comparator group 1: Semont manoeuvre (n = 10) Comparator group 2: Semont + VR (n = 20) VR versus other versus VR + other |
|
| Outcomes | Primary outcome: Dix‐Hallpike cure rate | |
| Notes | Number of participants at follow‐up assessments was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding of assessors or participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not clear from the results or the figures whether the data from all participants are included |
| Selective reporting (reporting bias) | High risk | Numbers of participants in each group not provided in figures of results; data not reported adequately to enable meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Varela 2001.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 106 Age: 55 years (SD 12.9), range 18 to 77 Gender: 31.1% male Setting: university clinic Eligibility criteria: BPPV, diagnosed by history and D‐H test (nystagmus) Exclusion criteria: other associated causes of vertigo Baseline characteristics: no difference between sex, affected sides, age or time since onset of symptoms |
|
| Interventions |
Intervention group: VR (B‐D habituation exercises) (n = 29) Comparator group 1: Semont manoeuvre (n = 35) Comparator group 2: Epley manoeuvre (n = 42) VR versus others (CRM) |
|
| Outcomes |
Primary outcome: cure rate with Dix‐Hallpike Secondary outcomes: number of sessions required for resolution (Group 2 and 3), relapse frequency, subjective rating of outcome |
|
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not clear whether outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data were reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Venosa 2007.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 87 Age: intervention group: mean age 46 years, comparator group: mean age 42 years Gender: intervention group: 18 males, comparator group: 19 males Setting: hospital Eligibility criteria: acute episode of rotational vertigo within the last 5 days Exclusion criteria: BPPV, central nervous system disorders and perilymphatic fistula were excluded Baseline characteristics: no differences between groups |
|
| Interventions |
Intervention group: VOR adaptation exercises (X1 and X2 viewing exercises) (n = 45) Comparator group: placebo exercises (sham visual fixation task) (n = 42) VR versus control (sham) |
|
| Outcomes |
Primary outcome: dizziness intensity (VAS) Secondary outcomes: use of medication (dimenhydrinate), spontaneous nystagmus incidence, Romberg test, Fukuda test, post head‐shaking nystagmus (PHSN) |
|
| Notes | 13 participants were lost to follow‐up, 6 and 7 in the intervention and comparator groups respectively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Outcomes were assessed by the principal investigator who was not blinded to group allocation; participants were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The drop‐outs were similar between the study (13%) and control (16%) groups |
| Selective reporting (reporting bias) | High risk | Data not reported adequately to enable meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Vereeck 2008.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 53 Age: participants were stratified according to age (above and below 50 years) Intervention group: young: mean age 41.6 (SD 5.9), older mean age 58.5 (SD 6.2) Comparator group: young: mean age 40.8 (SD 7.4), older mean age 60.6 (SD 6.6) Gender: not reported Setting: recruited following hospital admission Eligibility criteria: consecutive patients post removal of an acoustic neuroma Exclusion criteria: central neurological disorders affecting postural control prior to surgery Baseline characteristics: the younger participants performed more favourably on the balance tests |
|
| Interventions |
Intervention group: customised VR (exercises for balance, head motion, mobility, gaze and treadmill walking) (n = 31) Comparator group: general instructions (n = 22) VR versus control (nil) |
|
| Outcomes |
Primary outcome: balance assessment (Standing Balance Sum of 7 timed tests) Secondary outcomes: ENG (pre‐operative only), DHI, Timed Up and Go (TUG), tandem gait, DGI |
|
| Notes | No participants were lost to follow‐up but some did not attend all of the assessments | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | Closed envelopes were used to conceal allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although no participants withdrew from the study there were multiple occasions of missing data but the authors attempted to deal with this in the analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Winkler 2011.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 29 Age: intervention group: mean age 55.7, comparator group 1: mean age 54.0, comparator group 2: mean age 52.9 Gender: intervention group: 2 males, comparator group 1: 4 males, comparator group 2: 6 males Setting: university Eligibility criteria: participants with chronic dizziness (greater than 6 months duration) who had completed a VR programme, functional range of motion and strength in the lower limbs and trunk, intact sensation in the lower limbs, ability to stand unassisted for 1 minute Exclusion criteria: acute episodes of vertigo in the past 6 months for those with hydrops, BPPV, bilateral involvement or other neurological, postural or orthopaedic deficits that could affect posture and balance Baseline characteristics: the only significant difference at baseline was better performance on the DGI for those in the exercise group |
|
| Interventions |
Intervention group: platform tilt perturbations only (n = 10) Comparator group 1: platform tilt perturbations and VR exercise programme (n = 7) Comparator group 2: VR only (n = 12) VR versus VR versus VR |
|
| Outcomes |
Primary outcome: DGI Secondary outcomes: temporospatial gait measures, DHI, Patient Specific Functional Scale (PSFS), Perceived Outcome Scale (POS) |
|
| Notes | A total of 5 additional participants were randomised but were either lost to follow‐up (n = 1), did not receive the allocated intervention (n = 3) or were not compliant with the exercise intervention (n = 1) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Individuals were randomly assigned by drawing to 1 of 2 experimental groups or a group receiving traditional VR exercises" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was stated to be a single‐blind design, although it was not explicitly stated that the outcome assessor was blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant was excluded from analysis due to non‐compliance in the exercise only group |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Yardley 1998.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 143 Age: intervention group: mean age 60.1 (SD 15.2), comparator group: mean age 59.6 (SD 15.9) Gender: intervention group: 15 males, comparator group: 13 males Setting: conducted in 10 general practices, delivered by primary care nurse Eligibility criteria: dizziness of vestibular origin. Mixed aetiology ‐ diagnosed where possible by medical records (1/3). Possibility of central pathology Exclusion criteria: vigorous head or body movement contraindicated, non‐vestibular cause for dizziness, multiple, life‐threatening or progressive CNS disorders Baseline characteristics: nil reported |
|
| Interventions |
Intervention group: VR (education, head and body movements, relaxation, breathing, encouragement to function) (n = 67) Comparator group: control (n = 76) VR versus control (usual medical care) |
|
| Outcomes | Primary outcome: VSS Secondary outcomes: VHQ, HADS, sharpened Romberg, provocative movements | |
| Notes | 16 participants dropped out of the study before follow‐up and were excluded from the analysis; those who dropped out were more likely to report a higher number of movements that provoked their dizziness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used in the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither the therapists, outcome assessors nor participants were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were missing for various measures across many time points but this is adequately explained |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Yardley 2004.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 170 Age: intervention group: mean age 62.9 (SD 15.2), comparator group: mean age 61.0 (SD 14.4) Gender: intervention group: 24 males, comparator group: 25 males Setting: conducted in 20 general practices, delivered by primary care nurse Eligibility criteria: dizziness of vestibular origin diagnosed by case history and MPD Exclusion criteria: non‐vestibular cause for dizziness, duration of dizziness less than 2 months in the past 2 years, vigorous head or body movement contraindicated, serious comorbid conditions Baseline characteristics: no differences between groups |
|
| Interventions |
Intervention group: VR (primary care: demonstration, booklet and follow‐up) (n = 83) Comparator group: control, usual medical care (n = 87) VR versus control (usual medical care) |
|
| Outcomes |
Primary outcome: VSS (short form) Secondary outcomes: CDP, DHI, MPD |
|
| Notes | 25 participants were lost to follow‐up: 5 from each group at the end of the 3‐month intervention, then a further 7 and 8 respectively from the intervention and comparator groups at the 6‐month follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation was performed by an independent researcher |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were missing at several time points but this was accounted for in the intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Yardley 2006.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 360 Age: mean age 59.2 (SD 12.3) Gender: 113 males, 31.4% Setting: participants received the intervention in the community after being recruited from a Ménière's society Eligibility criteria: participants with Ménière's disease (non‐acute phase) who had experienced dizziness of imbalance in the last 12 months, had consulted their GP regarding involvement in the study Exclusion criteria: other vestibular disorder Baseline characteristics: no differences between groups on any of the participant characteristics |
|
| Interventions |
Intervention group: VR (booklet of exercises) (n = 120) Comparator group 1: SC (booklet for self management) (n = 120) Comparator group 2: waiting list control (n = 120) VR versus other VR versus control |
|
| Outcomes | Primary outcomes: questionnaire (better versus same/worse), VSS, PEI Secondary outcomes: DHI, HADS, DBS, adherence | |
| Notes | Only 17 participants of the sample of 360 failed to complete the final follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent research administrator allocated participants to the intervention arms using a computer randomisation program" |
| Allocation concealment (selection bias) | Low risk | Participants were sent a letter directly by the independent research administrator informing them of group allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants nor assessors were blinded to group allocation. Outcomes were assessed by the use of questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The drop‐out rate was reported to be 5% |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Yardley 2012.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 276 Age: 59.4 (SD 15.3) Gender: 98 male Setting: participants received the intervention in the community after being recruited from their local general practice Eligibility criteria: chronic dizziness, as diagnosed by their GP Exclusion criteria: dizziness attributed to a non‐vestibular cause, any contraindications to VR Baseline characteristics: significant differences were observed between groups for sex, age when leaving school, duration of dizziness and number of patients exceeding the threshold for anxiety and depression according to the HADS. The sensitivity analysis was adjusted for these baseline differences |
|
| Interventions |
Intervention group: VR (self management booklet with phone support from a vestibular therapist) (n = 112)
Comparator group 1: SC (self management booklet only) (n = 113)
Comparator group 2: routine medical care (n = 112) VR versus other VR versus control |
|
| Outcomes |
Primary outcome: VSS and total healthcare costs related to dizziness per quality life year (QALY) Secondary outcomes: questionnaire (better versus same/worse), DHI, HADS, EuroQol EQ‐5D, adherence |
|
| Notes | Only 82% of participants completed all clinical measures at the primary endpoint, 12 weeks and 78% at 12 months follow‐up. At 12 weeks, 27 had dropped out of the intervention group, 21 from comparator group 1 and 14 from comparator group 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent randomisation service was used, stratified for symptom severity |
| Allocation concealment (selection bias) | Low risk | The trial administrator informed participants of group allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Participants, therapists, and the trial administrator could not be blinded to treatment allocation but the researchers who assessed and analysed outcomes remained blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All drop‐outs were similar across the groups and multiple imputation was used for missing data |
| Selective reporting (reporting bias) | Low risk | The trial protocol was published and all outcomes are reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Zimbelman 1999.
| Methods | Design: randomised controlled trial | |
| Participants |
Number: 14 Age: intervention group: mean age 53.5, range 35 to 69, comparator group: mean age 58.3, range 40 to 79 Gender: intervention group: 2 males, comparator group: 3 males Setting: neuro‐otology department, hospital Eligibility criteria: unilateral peripheral vestibular dysfunction diagnosed by neuro‐otological tests Exclusion criteria: central vestibular deficits, cognitive deficits, joint replacements, arthritic joint problems, significant cardiovascular disease or previous stroke, multiple sclerosis, cervical vertigo, peripheral neuropathy or uncorrected visual deficits Baseline characteristics: no significant differences in age, gender or duration of symptoms |
|
| Interventions |
Intervention group: VR (individual with adaptation and postural control) (n = 6) Comparator group: VR (general C‐C) (n = 8) VR versus VR |
|
| Outcomes | Primary outcome: DHI Secondary outcome: BBS | |
| Notes | No participants were lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing random numbers was used to generate the random sequence |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors for balance tests were blinded to group allocation (not for DHI) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data were reported |
| Selective reporting (reporting bias) | Low risk | Study protocol not available but all data appear to be reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
BBS: Berg Balance Scale B‐D: Brandt‐Daroff BPPV: benign paroxysmal positional vertigo C‐C: Cooksey‐Cawthorne CDP: computerised dynamic posturography CMSSQ: Childhood Motion Sickness Short‐form Questionnaire CNS: central nervous system CRM: canalith repositioning manoeuvre DBS: Dizziness Belief Scale D‐H: Dix‐Hallpike test DGI: Dynamic Gait Index DHI: Dizziness Handicap Inventory DI: dizziness intensity DVA: dynamic visual acuity ENG: electronystagmography GP: general practitioner HADS: Hospital Anxiety and Depression Scale LM: liberatory manoeuvre MPD: motion‐provoked dizziness MRI: magnetic resonance imaging MSQ: motion sensitivity quotient OKN: optokinetic reflex OT: ocular tilt PC: postural control PEI: patient enablement instrument PT: physical therapy SC: symptom control (e.g. stress reduction techniques aspects of cognitive behavioural therapy approach) SCQ: situational characteristics questionnaire SD: standard deviation SOLEC: stand on one leg, eyes closed SOOL: standing on one leg SP: sway path SR: social reinforcement STAI: Spielberger State Trait Anxiety Inventory SVV: subjective visual vertical TNS: transcutaneous nerve stimulation TUG: Timed Up and Go test UPVD: unilateral peripheral vestibular disorder VAS: visual analogue scale VD: vestibular disorder VD‐ADL: vestibular disorders activities of daily living scale VDI: Vertigo Dizziness Imbalance questionnaire VF: vertigo frequency VHQ: Vestibular Handicap Questionnaire VI: vertigo intensity VOR: vestibular ocular reflex VR: vestibular rehabilitation VSS: Vertigo Symptom Scale VSS‐A: Vertigo Symptom Scale anxiety component VSS‐V: Vertigo Symptom Scale vestibular component WOL: walk on line
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amor‐Dorado 2012 | OUTCOME Participants were assessed for cure rate of nystagmus on Dix‐Hallpike manoeuvre |
| Andersson 2006 | PARTICIPANTS Mixed aetiology, no separate analyses |
| Angeli 2003 | ALLOCATION Study 2 (with VR intervention) not randomised |
| Bielinska 2012 | PARTICIPANTS Mixed aetiology of dizziness (included central) |
| Cronin 2011 | PARTICIPANTS Dizziness due to aging, not unilateral peripheral vestibular dysfunction |
| Ellialtioglu 2003 | ALLOCATION
Randomised but unclear INTERVENTION Comparison predominantly one for manoeuvres |
| Enticott 2008 | PARTICIPANTS Mixed aetiology of dizziness (included bilateral) |
| Gurkov 2012 | INTERVENTION Not routine vestibular rehabilitation |
| Hall 2010 | PARTICIPANTS Dizziness was not due to a vestibular disorder |
| Hansson 2004 | PARTICIPANTS Dizziness of central or age‐related origin |
| Hansson 2006 | PARTICIPANTS Dizziness due to whiplash‐associated disorders |
| Ipek 2011 | ABSTRACT ONLY |
| Jauregui‐Renaud 2007 | PARTICIPANTS Mixed aetiology of dizziness (included bilateral) |
| Johansson 2001 | PARTICIPANTS Mixed aetiology of dizziness |
| Krueger 2010 | PARTICIPANTS Mixed aetiology ‐ over half reported motion sickness only and were not assessed for unilateral peripheral vestibular dysfunction |
| Lauenroth 2008 | INTERVENTION Not routine vestibular rehabilitation |
| Lauenroth 2012 | ALLOCATION Non‐randomised |
| Lillet‐Leclercq 1989 | ALLOCATION Not adequately randomised (year of birth) |
| Loader 2007 | INTERVENTION Computerised optokinetic therapy not routine vestibular rehabilitation |
| Maciaszek J, Osinski 2012 | PARTICIPANTS Mixed aetiology ‐ reported dizziness but not assessed for unilateral peripheral vestibular dysfunction |
| McGibbon 2004 | PARTICIPANTS Mixed unilateral and bilateral vestibular dysfunction ‐ no separate analysis |
| Meli 2006 | ALLOCATION Non‐randomised |
| Miranda 2010 | PARTICIPANTS Unclear aetiology |
| Oh 2009 | INTERVENTION Comparison predominantly one for manoeuvres |
| Orendorz 2002 | ALLOCATION
Unclear randomisation PARTICIPANTS Unclear aetiology INTERVENTION Investigating use of adjunct pharmacology with VR |
| Prasansuk 2004 | PARTICIPANTS Unclear aetiology; elderly people with a history of balance disorders |
| Rossi‐Izquierdo 2013a | PARTICIPANTS Parkinson's disease only |
| Rzewnicki 2008 | ALLOCATION Unclear randomisation |
| Simoceli 2008 | ALLOCATION
Unclear randomisation PARTICIPANTS Elderly people with body balance disorder |
| Sparrer 2013 | INTERVENTION Not routine vestibular rehabilitation, focus on balance only using the Nintendo Wii® Balance Board |
| Steenerson 1996 | ALLOCATION Alternate allocation, not randomised |
| Viirre 2002 | ALLOCATION Control group selected, not randomised |
| Wrisley 2011 | PARTICIPANTS Mixed unilateral, bilateral and central vestibular dysfunction ‐ no separate analysis |
| Yardley 2001 | PARTICIPANTS
Symptomatic dizziness INTERVENTIONS No intervention analysed |
VR: vestibular rehabilitation
Characteristics of ongoing studies [ordered by study ID]
ACTRN12609000284268.
| Trial name or title | Does adding otolith specific exercises to a standard vestibular rehabilitation program improve outcomes for adults with inner ear dizziness? |
| Methods | RCT |
| Participants | 48 with unilateral peripheral vestibular dysfunction |
| Interventions | Group 1 ‐ VR (home exercise programme) plus otolith‐specific exercises Group 2 ‐ VR (home exercise programme) |
| Outcomes | Primary outcome: degree of perceived impairment associated with dizziness via the Dizziness Handicap Inventory Secondary outcomes: computerised dynamic posturography ‐ composite score and condition eyes closed + sway reference |
| Starting date | April 2008 |
| Contact information | Arimbi Winoto, 32 Gisborne Street East Melbourne Victoria 3002, Australia; awinotosuatmadji@students.latrobe.edu.au |
| Notes | Recruitment complete, publication pending |
Aquaroni Ricci 2012.
| Trial name or title | Effects of conventional versus multimodal vestibular rehabilitation on functional capacity and balance control in older people with chronic dizziness from vestibular disorders: design of a randomized clinical trial |
| Methods | RCT |
| Participants | Older individuals with a clinical diagnosis of chronic dizziness resulting from vestibular disorders |
| Interventions | Group 1: multimodal Cawthorne‐Cooksey protocols Group 2: conventional protocol The protocols will be performed during individual 50‐minute sessions, twice a week, for 2 months (a total of 16 sessions) |
| Outcomes | Primary outcomes will be determined in accordance with the Dizziness Handicap Inventory (functional capacity) and the Dynamic Gait Index (body balance) Secondary outcomes include dizziness features, functional records, body balance control tests and psychological information |
| Starting date | April 2010 |
| Contact information | Natalia Aquaroni Ricci, Universidade Federal de Sao Paulo |
| Notes | Recruitment completed but publication still in preparation |
ISRCTN86912968.
| Trial name or title | Online dizziness intervention for older adults: a randomised controlled trial |
| Methods | RCT |
| Participants | Adults aged over 50 who have reported symptoms of dizziness over the past 2 years, who have access to the internet and an email account |
| Interventions | Intervention group: standalone, web‐based information about dizziness and the balance system, instructions, advice, video demonstrations and tailored feedback about VR exercises, and advice and instructions about psychological techniques to assist with stress management and relaxation |
| Outcomes | Primary: Vertigo Symptom Scale at baseline, 3 and 6 months Secondary: subjective improvement in health, DHI, HADS, EQ5D |
| Starting date | 19 August 2013 |
| Contact information | Miss Rosie Essery, School of Psychology, University of Southampton |
| Notes | Anticipated end date 25 July 2014 |
Meldrum 2012.
| Trial name or title | Effectiveness of conventional versus virtual reality based vestibular rehabilitation in the treatment of dizziness, gait and balance impairment in adults with unilateral peripheral vestibular loss: a randomised controlled trial |
| Methods | RCT |
| Participants | 80 patients with unilateral peripheral vestibular loss |
| Interventions | Group 1: virtual reality‐based VR for 6 weeks Group 2: conventional VR for 6 weeks |
| Outcomes | Primary outcome: gait speed measured with 3‐dimensional gait analysis Secondary outcomes: computerised posturography, dynamic visual acuity, validated questionnaires on dizziness, confidence and anxiety/depression Assessed post‐treatment (8 weeks) and at 6 months |
| Starting date | February 2011 |
| Contact information | Dara Meldrum, MSc. Royal College of Surgeons in Ireland |
| Notes | This study is ongoing, but not currently recruiting participants |
NCT00702832.
| Trial name or title | Effects of vestibular rehabilitation in the treatment of patients with acute vestibular loss ‐ a randomised controlled trial |
| Methods | RCT |
| Participants | Patients aged 18 to 70 years with acute symptoms of dizziness (vestibular injury) diagnosed by videonystagmography; inclusion within 1 week after symptom onset Exclusion criteria: chronic dizziness; psychiatric diagnosis that might interfere with participation |
| Interventions | Group 1: vestibular rehabilitation (daily home training (4 to 6 specific exercises) 2 to 3 times per day; group training led by a physiotherapist twice per week during the first 10 weeks and once per week from 10 weeks to 12 months or until symptoms are cured) Group 2: no intervention |
| Outcomes | Primary outcome measure: Vertigo Symptom Scale Secondary outcome measures: Dizziness Handicap Inventory; UCLA‐DQ; HADS; VAS on dizziness; registration of provoked dizziness; accelerometer; sick leave; adverse effects |
| Starting date | January 2008 |
| Contact information | Dr Siv Mørkved, Norwegian University of Science and Technology |
| Notes | Recruitment complete and publication in preparation |
HADS: Hospital Anxiety and Depression Scale RCT: randomised controlled trial UCLA‐DQ: University of California Los Angeles Dizziness Questionnaire VAS: visual analogue scale VR: vestibular rehabilitation
Contributions of authors
Michelle McDonnell: search and retrieval, quality assessment, data extraction and analysis. Susan Hillier: protocol development, design of search strategy, quality assessment, data extraction and analysis.
Sources of support
Internal sources
International Centre for Allied Health Evidence, Australia.
External sources
No sources of support supplied
Declarations of interest
Michelle McDonnell: none known. Susan Hillier: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barozzi 2006 {published data only}
- Barozzi S, Berardino F, Arisi E, Cesarini A. A comparison between oculomotor rehabilitation and vestibular electrical stimulation in unilateral peripheral vestibular deficit. International Tinnitus Journal 2006;12:45‐9. [PubMed] [Google Scholar]
Basta 2011 {published and unpublished data}
- Basta D, Rossi‐Izquierdo M, Soto‐Varela A, Greters ME, Bittar RS, Steinhagen‐Thiessen E, et al. Efficacy of a vibrotactile neurofeedback training in stance and gait conditions for the treatment of balance deficits: a double‐blind, placebo‐controlled multicenter study. Otology & Neurology 2011;32:1492‐9. [DOI] [PubMed] [Google Scholar]
Cakrt 2010 {published data only}
- Cakrt O, Chovanec M, Funda T, Kalitova P, Betka J, Zverina E, et al. Exercise with visual feedback improves postural stability after vestibular schwannoma surgery. European Archives of Oto‐Rhino‐Laryngology 2010;236:1355‐60. [DOI] [PubMed] [Google Scholar]
Chang 2008 {published data only}
- Chang W, Yang Y, Hsu L, Chern C, Wang R. Balance improvement in patients with benign paroxysmal positional vertigo. Clinical Rehabilitation 2008;22(4):338‐47. [DOI] [PubMed] [Google Scholar]
Cohen 2002 {published data only}
- Cohen HS, Kimball KT, Jenkins HA. Factors affecting recovery after acoustic neuroma resection. Acta Otolaryngologica 2002;122:841‐50. [PubMed] [Google Scholar]
Cohen 2003 {published data only}
- Cohen HS, Kimball KT. Changes in a repetitive head movement task after vestibular rehabilitation. Clinical Rehabilitation 2004;18:125‐31. [DOI] [PubMed] [Google Scholar]
- Cohen HS, Kimball KT. Decreased ataxia and improved balance after vestibular rehabilitation. Otolaryngology ‐ Head and Neck Surgery 2004;130:418‐25. [DOI] [PubMed] [Google Scholar]
- Cohen HS, Kimball KT. Increased independence and decreased vertigo after vestibular rehabilitation. Otolaryngology ‐ Head and Neck Surgery 2003;128:60‐70. [DOI] [PubMed] [Google Scholar]
Cohen 2005 {published data only}
- Cohen HS, Kimball KT. Effectiveness of treatments for benign paroxysmal positional vertigo of the posterior canal. Otology and Neurology 2005;26:1034‐40. [DOI] [PubMed] [Google Scholar]
Foster 2012 {published data only}
- Foster CA, Ponnapan A, Zaccaro K, Strong D. A comparison of two home exercises for benign positional vertigo: half somersault versus Epley maneuver. Audiology and Neurotology. Extra 2012;2:16‐23. [Google Scholar]
Garcia 2013 {published data only}
- Garcia AP, Gananca MM, Cusin FS, Tomaz A, Gananca FF, Caovilla HH. Vestibular rehabilitation with virtual reality in Meniere's disease. Brazilian Journal of Otorhinolaryngology 2013;79(3):366‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giray 2009 {published data only}
- Giray M, Kirazli Y, Karapolat H, Celebisoy N, Bilgen C, Kirazli T. Short‐term effects of vestibular rehabilitation in patients with chronic unilateral vestibular dysfunction: a randomised controlled study. Archives of Physical Medicine and Rehabilitation 2009;90(8):1325‐31. [DOI] [PubMed] [Google Scholar]
Herdman 1995 {published data only}
- Herdman SJ, Clendaniel RA, Mattox DE, Holliday MJ, Niparko JK. Vestibular adaptation exercises and recovery: acute stage after acoustic neuroma resection. Otolaryngology ‐ Head and Neck Surgery 1995;113:77‐87. [DOI] [PubMed] [Google Scholar]
Herdman 2003 {published data only}
- Herdman SJ, Schubert MC, Das VE, Tusa RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Archives of Otolaryngology ‐ Head and Neck Surgery 2003;129:819‐24. [DOI] [PubMed] [Google Scholar]
Horak 1992 {published data only}
- Horak FB, Jones‐Rycewicz C, Black FO, Shumway‐Cook A. Effects of vestibular rehabilitation on dizziness and imbalance. Otolaryngology ‐ Head and Neck Surgery 1992;106(2):175‐80. [PubMed] [Google Scholar]
Kammerlind 2005 {published data only}
- Kammerlind AC, Ledin TEA, Odkvist LM, Skargren EIB. Effects of home training and additional physical therapy on recovery after acute unilateral vestibular loss ‐ a randomized study. Clinical Rehabilitation 2005;19:54‐62. [DOI] [PubMed] [Google Scholar]
Karanjai 2010 {published data only}
- Karanjai S, Saha AK. Evaluation of vestibular exercises in the management of benign paroxysmal positional vertigo. Indian Journal of Otolaryngology and Head and Neck Surgery 2010;62:202‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krebs 2003 {published data only}
- Krebs DE, Gill‐Body KM, Parker SW, Ramirez JV, Wernick‐Robinson M. Vestibular rehabilitation: useful but not universally so. Otolaryngology ‐ Head and Neck Surgery 2003;128:240‐50. [DOI] [PubMed] [Google Scholar]
Kulcu 2008 {published data only}
- Kulcu D, Yanik B, Boynukalin S, Kurtais Y. Efficacy of a home‐based exercise program on benign paroxysmal positional vertigo compared with betahistine. Journal of Otolaryngology, Head and Neck Surgery 2008;37(3):373‐9. [PubMed] [Google Scholar]
Marioni 2013 {published data only}
- Marioni G, Fermo S, Zanon D, Bri N, Staffieri A. Early rehabilitation for unilateral peripheral vestibular disorders: a prospective, randomized investigation using computerized posturography. European Archives of Oto‐Rhino‐Laryngology 2013;270:425‐35. [DOI] [PubMed] [Google Scholar]
Morozetti 2011 {published data only}
- Morozetti PG, Gananca CF, Chiari BM. Comparison of different protocols for vestibular rehabilitation in patients with peripheral vestibular disorders. Jornal da Sociedade Brasileira de Fonoaudiologia 2011;23:44‐50. [DOI] [PubMed] [Google Scholar]
Mruzek 1995 {published data only}
- Mruzek M, Barin K, Nichols DS, Burnett CN, Welling DB. Effects of vestibular rehabilitation and social reinforcement on recovery following ablative vestibular surgery. Laryngoscope 1995;105:686‐92. [DOI] [PubMed] [Google Scholar]
Pavlou 2004 {published data only}
- Pavlou M, Lingeswaran A, Davies RA, Gresty MA, Bronstein AM. Simulator based rehabilitation in refractory dizziness. Journal of Neurology 2004;251:983‐95. [DOI] [PubMed] [Google Scholar]
Pavlou 2012 {published data only}
- Pavlou M, Kanegaonkar RG, Swapp D, Bamiou DE, Slater M, Luxon LM. The effect of virtual reality on visual vertigo symptoms in patients with peripheral vestibular dysfunction: a pilot study. Journal of Vestibular Research 2012;22:273‐81. [DOI] [PubMed] [Google Scholar]
Resende 2003 {published data only}
- Resende CR, Taguchi CK, Almeida JG, Fujita RR. Vestibular rehabilitation in elderly patients with benign paroxysmal positional vertigo. Brazilian Journal of Otorhinolaryngology 2003;69(4):535‐40. [Google Scholar]
Rossi‐Izquierdo 2011 {published data only}
- Rossi‐Izquierdo M, Santos‐Perez S, Soto‐Varela A. What is the most effective vestibular rehabilitation technique in patients with unilateral peripheral vestibular disorders?. European Archives of Oto‐Rhino‐Laryngology 2011;268:1569‐74. [DOI] [PubMed] [Google Scholar]
Rossi‐Izquierdo 2013 {published data only}
Scott 1994 {published data only}
- Scott B, Larsen H‐C, Lyttkens L, Melin L. An experimental evaluation of the effects of transcutaneous nerve stimulation and applied relaxation on hearing ability, tinnitus and dizziness in patients with Menieres disease. British Journal of Audiology 1994;28:131‐40. [DOI] [PubMed] [Google Scholar]
Strupp 1998 {published data only}
- Strupp M, Arbusow V, Maag KP, Gall C, Brandt T. Vestibular exercises improve central vestibulospinal compensation after vestibular neuritis. Neurology 1998;51:838‐44. [DOI] [PubMed] [Google Scholar]
Szturm 1994 {published data only}
- Szturm T, Ireland DJ, Lessing‐Turner M. Comparison of different exercise programs in the rehabilitation of patients with chronic peripheral vestibular dysfunction. Journal of Vestibular Research 1994;4:461‐79. [PubMed] [Google Scholar]
Teggi 2009 {published data only}
- Teggi R, Caldirola D, Fabiano B, Pecanati P, Bussi M. Rehabilitation after acute vestibular disorders. Journal of Laryngology and Otology 2009;123:397‐402. [DOI] [PubMed] [Google Scholar]
Toledo 2000 {published data only}
- Toledo H, Cortés ML, Pane C, Trujillo V. Semont maneuver and vestibular rehabilitation exercises in the treatment of benign paroxysmal postural vertigo. A comparative study. Neurologia 2000;15(4):152‐7. [PubMed] [Google Scholar]
Varela 2001 {published data only}
- Soto Varela A, Bartual Magro J, Santos Perez S, Velez Regueiro M, Lechuga Garcia R, Perez‐Carro Rios A, et al. Benign paroxysmal vertigo: a comparative prospective study of the efficacy of Brandt and Daroff exercises, Semont and Epley manoeuvre. Revue de Laryngologie Otologie Rhinologie 2001;122:179‐83. [PubMed] [Google Scholar]
Venosa 2007 {published data only}
- Venosa A, Bittar R. Vestibular rehabilitation exercises in acute vertigo. Laryngoscope 2007;117:1482‐7. [DOI] [PubMed] [Google Scholar]
Vereeck 2008 {published data only}
- Vereeck L, Wuyts F, Truijen S, Valck C, Heyning P. The effect of early customised vestibular rehabilitation on balance after acoustic neuroma resection. Clinical Rehabilitation 2008;22:698‐713. [DOI] [PubMed] [Google Scholar]
Winkler 2011 {published data only}
- Winkler PA, Esses B. Platform tilt perturbation as an intervention for people with chronic vestibular dysfunction. Journal of Neurologic Physical Therapy 2011;35:105‐15. [DOI] [PubMed] [Google Scholar]
Yardley 1998 {published data only}
- Yardley L, Beech S, Zander L, Evans T, Weinman J. A randomised controlled trial of exercise therapy for dizziness and vertigo in primary care. British Journal of General Practice 1998;48:1136‐40. [PMC free article] [PubMed] [Google Scholar]
Yardley 2004 {published data only}
- Yardley L, Donovan‐Hall M, Smith HE, Walsh BM, Mullee M, Bronstein AM. Effectiveness of primary care‐based vestibular rehabilitation for chronic dizziness. Annals of Internal Medicine 2004;141:598‐605. [DOI] [PubMed] [Google Scholar]
Yardley 2006 {published data only}
- Yardley L, Kirby S. Evaluation of booklet‐based self management of symptoms in Meniere Disease: a randomised controlled trial. Psychosomatic Medicine 2006;68:762‐9. [DOI] [PubMed] [Google Scholar]
Yardley 2012 {published data only}
- Yardley L, Barker F, Muller I, Turner D, Kirby S, Mullee M, et al. Clinical and cost effectiveness of booklet based vestibular rehabilitation for chronic dizziness in primary care: single blind, parallel group, pragmatic, randomised controlled trial. BMJ 2012;355:e2237. [DOI: 10.1136/bmj.e2237] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimbelman 1999 {published data only}
- Zimbelman JE, Stoecker J, Haberkamp TJ. Outcomes in vestibular rehabilitation. Physical Therapy Case Reports 1999;2:232‐40. [Google Scholar]
References to studies excluded from this review
Amor‐Dorado 2012 {published data only}
- Amor‐Dorado JC, Barreira‐Fernandez MP, Aran‐Gonzalez I, Casariego‐Vales E, Llorca J, Gonzalez‐Gay MA. Particle repositioning maneuver versus Brandt‐Daroff exercise for treatment of unilateral idiopathic BPPV of the posterior semicircular canal: a randomized prospective clinical trial with short‐ and long‐term outcome. Otology & Neurotology 2012;33(8):1401‐7. [DOI] [PubMed] [Google Scholar]
Andersson 2006 {published data only}
- Andersson G, Asmundson G, Denev J, Nilsson J, Larsen HC. A controlled trial of cognitive‐behaviour therapy combined with vestibular rehabilitation in the treatment of dizziness. Behaviour Research and Therapy 2006;44:1265‐73. [DOI] [PubMed] [Google Scholar]
Angeli 2003 {published data only}
- Angeli S, Hawley R, Gomez O. Systematic approach to benign paroxysmal positional vertigo in the elderly. Otolaryngology ‐ Head and Neck Surgery 2003;128(5):719‐25. [DOI] [PubMed] [Google Scholar]
Bielinska 2012 {published data only}
- Bielinska MH, Zielinska‐Blizniewska H, Pietkiewicz P, Olszewski J. The evaluation of the efficiency of kinesitherapy in the patients with mixed‐type vertigo. Otolaryngologia Polska 2012;66(5):337‐41. [DOI] [PubMed] [Google Scholar]
Cronin 2011 {published data only}
- Cronin GW, Steenerson RL. Disequilibrium of aging: response to a 3‐month program of vestibular therapy. Physical & Occupational Therapy in Geriatrics 2011;29(2):148‐55. [Google Scholar]
Ellialtioglu 2003 {published data only}
- Ellialtioglu A, Karan A, Erdamar B, Aksoy C. The beneficial effect of habituation exercises added on particle repositioning manoeuvre on life quality of patients with BPPV. Turkiye Fiziksel Tip Ve Rehabilitasyon Dergisi 2003;49:36‐41. [Google Scholar]
Enticott 2008 {published data only}
- Enticott J, Vitkovic J, Reid B, O'Neill P, Paine M. Vestibular rehabilitation in individuals with inner‐ear dysfunction: a pilot study. Audiology and Neuro‐otology 2008;13(1):19‐28. [DOI] [PubMed] [Google Scholar]
Gurkov 2012 {published data only}
- Gurkov R, Filipe Mingas LB, Rader T, Louza J, Olzowy B, Krause E. Effect of transtympanic low‐pressure therapy in patients with unilateral Meniere's disease unresponsive to betahistine: a randomised, placebo‐controlled, double‐blinded, clinical trial. Journal of Laryngology and Otology 2012;126(4):356‐62. [DOI] [PubMed] [Google Scholar]
Hall 2010 {published data only}
- Hall CD, Heusel‐Gillig L, Tusa RJ, Herdman SJ. Efficacy of gaze stability exercises in older adults with dizziness. Journal of Neurological Physical Therapy 2010;34:64‐9. [DOI] [PubMed] [Google Scholar]
Hansson 2004 {published data only}
- Hansson EE, Mansson N‐O, Hakansson A. Effects of specific rehabilitation for dizziness among patients in primary health care: a randomised controlled trial. Clinical Rehabilitation 2004;18:558‐65. [DOI] [PubMed] [Google Scholar]
Hansson 2006 {published data only}
- Hansson EE, Mansson N‐O, Ringsberg KAM, Hakansson A. Dizziness among patients with whiplash‐associated disorder: a randomized controlled trial. Journal of Rehabilitation Medicine 2006;38:387‐90. [DOI] [PubMed] [Google Scholar]
Ipek 2011 {published data only}
- Ipek D, Tekat A, Imamoglu O. The effect of head neck motion on vertigo in vestibular rehabilitation. Fizyoterapi Rehabilitasyon 2011;22(2):122. [Google Scholar]
Jauregui‐Renaud 2007 {published data only}
- Jauregui‐Renaud K, Villanueva‐Padron L, Cruz G. The effect of vestibular rehabilitation supplemented by training of the breathing rhythm or proprioception exercises in patients with chronic peripheral vestibular disease. Journal of Vestibular Research 2007;17(1):63‐72. [PubMed] [Google Scholar]
Johansson 2001 {published data only}
- Johansson M, Akerlund D, Larsen HC, Andersson G. Randomized controlled trial of vestibular rehabilitation combined with cognitive‐behavioral therapy for dizziness in older people. Otolaryngology ‐ Head and Neck Surgery 2001;125(3):151‐6. [DOI] [PubMed] [Google Scholar]
Krueger 2010 {published data only}
- Krueger WW. Controlling motion sickness and spatial disorientation and enhancing vestibular rehabilitation with a user‐worn see‐through display. Laryngoscope 2011;121(Suppl 2):S17‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauenroth 2008 {published data only}
- Lauenroth A, Pudszuhn A, Bloching M, Esperer MD, Hottenrott K, Becker S, et al. Three‐dimensional training therapy in vestibular neuropathy. Manuelle Medizin 2008;46:219‐27. [Google Scholar]
Lauenroth 2012 {published data only}
- Lauenroth A, Knipping S, Schwesig R. Vestibular disorders. Effects of sensorimotor training on postural regulation and on recovery process. HNO 2012;60(8):692‐9. [DOI] [PubMed] [Google Scholar]
Lillet‐Leclercq 1989 {published data only}
- Lillet‐Leclercq C, Lillet M, Demanez JP. Benign paroxysmal vertigo: comparison of 2 rehabilitation methods. Acta Oto‐Laryngologica Belgica 1989;43:351‐60. [PubMed] [Google Scholar]
Loader 2007 {published data only}
- Loader B, Grunther W, Mueller CA, Neuwirth G, Thurner S, Ehrenberger K, et al. Improved postural control after computerized optokinetic therapy based on stochastic visual stimulation in patients with vestibular dysfunction. Journal of Vestibular Research 2007;17:131‐6. [PubMed] [Google Scholar]
Maciaszek J, Osinski 2012 {published data only}
- Maciaszek J, Osinski W. Effect of Tai Chi on body balance: randomized controlled trial in elderly men with dizziness. American Journal of Chinese Medicine 2012;40(2):245‐53. [DOI] [PubMed] [Google Scholar]
McGibbon 2004 {published data only}
- McGibbon CA, Krebs DE, Parker SW, Scarborough DM, Wayne PM, Wolf SL. Tai Chi and vestibular rehabilitation improve vestibulopathic gait via different neuromuscular mechanisms: preliminary report. BMC Neurology 2005;5(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGibbon CA, Krebs DE, Wolf SL, Wayne PM, Scarborough DM, Parker SW. Tai chi and vestibular rehabilitation effects on gaze and whole‐body stability. Journal of Vestibular Research 2004;14:467‐78. [PubMed] [Google Scholar]
Meli 2006 {published data only}
- Meli A, Zimatore G, Badaracco C, Angelis E, Tufarelli D. Vestibular rehabilitation and 6‐month follow‐up using objective and subjective measures. Acta Oto‐Laryngologica 2006;126:259‐66. [DOI] [PubMed] [Google Scholar]
Miranda 2010 {published data only}
- Miranda MÁ, Santana Álvarez J, Fernandez Alvarez AC. Peripheral vertiginous syndrome: individualized program of physical exercises for its rehabilitation [Síndrome vertiginoso periférico: programa individualizado de ejercicios físicos para su rehabilitación]. Revista Archivo Médico de Camagüey 2010;14(3):0‐0. [Google Scholar]
Oh 2009 {published data only}
- Oh S‐Y, Kim J‐S, Jeong S‐H, Oh Y‐M, Choi K‐D, Kim B‐K, et al. Treatment of apogeotropic benign positional vertigo: comparison of therapeutic head‐shaking and modified Semont maneuver. Journal of Neurology 2009;256:1330‐6. [DOI] [PubMed] [Google Scholar]
Orendorz 2002 {published data only}
- Orendorz‐Fraczkowska K, Pospiech L, Gawron W. Associated treatment of vestibular receptor impairment by means of physical therapy and Gingko biloba extract. Otolaryngologia Polska 2002;1:83‐9. [PubMed] [Google Scholar]
Prasansuk 2004 {published data only}
- Prasansuk S, Siriyananda C, Nakorn AN, Atipas S, Chongvisal S. Balance disorders in the elderly and the benefit of balance exercise. Journal of the Medical Association of Thailand 2004;87:1225‐33. [PubMed] [Google Scholar]
Rossi‐Izquierdo 2013a {published data only}
- Rossi‐Izquierdo M, Ernst A, Soto‐Varela A, Santos‐Perez S, Faraldo‐Garcia A, Sesar‐Ignacio A, et al. Vibrotactile neurofeedback balance training in patients with Parkinson's disease: reducing the number of falls. Gait and Posture 2013;37:195‐200. [DOI] [PubMed] [Google Scholar]
Rzewnicki 2008 {published data only}
- Rzewnickin I, Rogowski M. Vestibular rehabilitation of vertigo and dizziness. Polski Merkuriusz Lekarski 2008;24:244‐6. [PubMed] [Google Scholar]
Simoceli 2008 {published data only}
- Simoceli L, Bittar RSM, Sznifer J. Adaptation exercises of vestibulo‐ocular reflex on balance in the elderly. International Archives of Otorhinolaryngology 2008;12:183‐8. [Google Scholar]
Sparrer 2013 {published data only}
- Sparrer I, Duong Dinh TA, Ilgner J, Westhofen M. Vestibular rehabilitation using the Nintendo(R) Wii Balance Board ‐ a user‐friendly alternative for central nervous compensation. Acta Oto‐Laryngologica 2013;133(3):239‐45. [DOI] [PubMed] [Google Scholar]
Steenerson 1996 {published data only}
- Steenerson RL, Cronin GW. Comparison of the canalith repositioning procedure and vestibular habituation training in forty patients with benign paroxysmal positional vertigo. Otolaryngology ‐ Head and Neck Surgery 1996;114(1):61‐4. [DOI] [PubMed] [Google Scholar]
Viirre 2002 {published data only}
- Viirre E, Sitarz R. Vestibular rehabilitation using visual displays: preliminary study. Laryngoscope 2002;112:500‐3. [DOI] [PubMed] [Google Scholar]
Wrisley 2011 {published data only}
- Wrisley D, Stephens MJ. The effects of rotational platform training on balance and ADLs. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2011;2011:3529‐32. [DOI] [PubMed] [Google Scholar]
Yardley 2001 {published data only}
- Yardley L, Beech S, Weinman J. Influence of beliefs about the consequences of dizziness on handicap in people with dizziness, and the effect of therapy on beliefs. Journal of Psychosomatic Research 2001;50:1‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12609000284268 {unpublished data only}
- Winoto A. Does adding otolith specific exercises to a standard vestibular rehabilitation programme improve outcomes for adults with inner ear dizziness?. http://apps.who.int/trialsearch/trial.aspx?trialid=ACTRN12609000284268 (accessed 25 August 2010). [ACTRN12609000284268]
Aquaroni Ricci 2012 {published data only}
- Aquaroni Ricci N, Aratani MC, Caovilla HH, Gananca FF. Effects of conventional versus multimodal vestibular rehabilitation on functional capacity and balance control in older people with chronic dizziness from vestibular disorders: design of a randomized clinical trial. Trials 2012;13:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN86912968 {unpublished data only}
- Essery R. Online dizziness intervention for older adults: a randomised controlled trial. http://www.isrctn.com/ISRCTN86912968 2013. [ISRCTN86912968]
Meldrum 2012 {published data only}
- Meldrum D, Herdman S, Moloney R, Murray D, Duffy D, Malone K, et al. Effectiveness of conventional versus virtual reality based vestibular rehabilitation in the treatment of dizziness, gait, balance impairment in adults with unilateral peripheral vestibular loss: a randomised controlled trial. BMC Ear, Nose & Throat Disorders 2012;12:3‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00702832 {unpublished data only}
- Mørkved S. Effects of vestibular rehabilitation in the treatment of patients with acute vestibular loss ‐ a randomized controlled trial. http://clinicaltrials.gov/ct2/show/NCT00702832 (accessed 25 August 2010). [NCT00702832]
Additional references
Balaban 2012
- Balaban C, Hoffer M, Gottshall K. Top‐down approach to vestibular compensation: translational lessons from vestibular rehabilitation. Brain Research 2012;1482:101‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balaguer Garcia 2012
- Balaguer Garcia R, Corresa S, Bertomeu J, Suarez‐Varela M. Static posturography with dynamic tests. Usefulness of biomechanical parameters in assessing vestibular patients. Acta Otorrinolaringologica Espanola 2012;63(5):332‐8. [DOI] [PubMed] [Google Scholar]
Baloh 2003
- Baloh RW. Vestibular neuritis. New England Journal of Medicine 2003;348:1027‐32. [DOI] [PubMed] [Google Scholar]
Brandt 1999
- Brandt T. Benign paroxysmal positional vertigo. Vestibular Dysfunction and its Therapy. Basel: Karger, 1999:169‐94. [Google Scholar]
Cabrera Kang 2013
- Cabrera Kang CM, Tusa RJ. Vestibular rehabilitation: rationale and indications. Seminars in Neurology 2013;33:276‐85. [DOI] [PubMed] [Google Scholar]
Cooksey 1946
- Cooksey FS. Rehabilitation and vestibular injuries. Proceedings of the Royal Society of Medicine. 1946; Vol. 39:273. [PMC free article] [PubMed]
Cullen 2009
- Cullen K, Minor L, Beraneck M, Sadeghi S. Neural substrates underlying vestibular compensation: contribution of peripheral versus central processing. Journal of Vestibular Research 2009;19:171‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curthoys 2000
- Curthoys IS, Halmagyi GM. Clinical changes in vestibular function with time after unilateral vestibular loss. In: Herdman SJ editor(s). Vestibular Rehabilitation. Second Edition. Philadelphia: FA Davis Company, 2000. [Google Scholar]
Dix 1952
- Dix R, Hallpike CS. The pathology, symptomatology and diagnosis of certain common disorders of the vestibular system. Annals of Otology, Rhinology, and Laryngology 1952;6:987‐1016. [DOI] [PubMed] [Google Scholar]
Dowdal‐Osborn 2002
- Dowdal‐Osborn M. Early vestibular rehabilitation in patients with Meniere's disease. Otolaryngologic Clinics of North America 2002;35:683‐90. [DOI] [PubMed] [Google Scholar]
Epley 1992
- Epley JM. The canalith repositioning procedure: for treatment of BPPV. Otolaryngology ‐ Head and Neck Surgery 1992;107:399‐404. [DOI] [PubMed] [Google Scholar]
Fetter 2000
- Fetter M. Vestibular system disorders. In: Herdman SJ editor(s). Vestibular Rehabilitation. 2nd Edition. Philadelphia: FA Davis Company, 2000. [Google Scholar]
Gans 2002
- Gans RE. Vestibular rehabilitation: critical decision analysis. Seminars in Hearing 2002;23:149‐59. [Google Scholar]
Hain 2011
- Hain T. Neurophysiology of vestibular rehabilitation. NeuroRehabilitation 2011;29:127‐41. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hansson 2007
- Hansson EE. Vestibular rehabilitation ‐ for whom and how? A systematic review. Advances in Physiotherapy 2007;9:106‐16. [Google Scholar]
Herdman 2000
- Herdman S. Vestibular Rehabilitation. 2nd Edition. Philadelphia: FA Davis, 2000. [Google Scholar]
Hilton 2014
- Hilton M, Pinder D. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD003162.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffer 2011
- Hoffer M, Balaban C. Vestibular rehabilitation: ready for the mainstream. NeuroRehabilitation 2011;29:125. [DOI] [PubMed] [Google Scholar]
Hunt 2012
- Hunt W, Zimmerman E, Hilton M. Modifications of the Epley (canalith repositioning) manoeuvre for posterior canal benign paroxysmal positional vertigo (BPPV). Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008675.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez 2001
- Perez N, Garmendia I, Garcia‐Granero M, Martin E, Garcia‐Tapia R. Factor analysis and correlation between Dizziness Handicap Inventory and dizziness characteristics and impact on quality of life scales. Acta Otolaryngologica 2001;545:145‐54. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Semont 1988
- Semont A, Freyss G, Vitte E. Curing the BPPV with the liberatory maneuver. Advances in Oto‐rhino‐laryngology 1988;42:290‐3. [DOI] [PubMed] [Google Scholar]
Smith‐Wheelock 1991
- Smith‐Wheelock M, Shepard NT, Telian SA. Physical therapy program for vestibular rehabilitation. American Journal of Otology 1991;12:218‐25. [PubMed] [Google Scholar]
Strupp 2013
- Strupp M, Brandt T. Peripheral vestibular disorders. Current Opinion in Neurology 2013;26:81‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hillier 2005
- Hillier SL, Hollohan V, Hilton MP. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005397] [DOI] [PubMed] [Google Scholar]
Hillier 2007
- Hillier SL, McDonnell M. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005397.pub2] [DOI] [PubMed] [Google Scholar]
Hillier 2011
- Hillier SL, McDonnell M. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD005397.pub3] [DOI] [PubMed] [Google Scholar]


