Abstract
A soluble form of the CD4 receptor (sCD4) can either enhance or inhibit the infection of cells by simian immunodeficiency virus (SIV) and human immunodeficiency virus. We investigated the basis for these varying effects by studying the entry of three SIV isolates into CD4-positive and CD4-negative cells expressing different chemokine receptors. Infection of CD4-negative cells depended upon the viral envelope glycoproteins and upon the chemokine receptor, with CCR5 and gpr15 being more efficient than STRL33. Likewise, enhancement of infection by sCD4 was observed when CCR5- and gpr15-expressing target cells were used but not when those expressing STRL33 were used. The sCD4-mediated enhancement of virus infection of CD4-negative, CCR5-positive cells was related to the sCD4-induced increase in binding of the viral gp120 envelope glycoprotein to CCR5. Inhibitory effects of sCD4 could largely be explained by competition for virus attachment to cellular CD4 rather than other detrimental effects on virus infectivity (e.g., disruption of the envelope glycoprotein spike). Consistent with this, the sCD4-activated SIV envelope glycoprotein intermediate on the virus was long-lived. Thus, the net effect of sCD4 on SIV infectivity appears to depend upon the degree of enhancement of chemokine receptor binding and upon the efficiency of competition for cellular CD4.
Human immunodeficiency virus type 1 (HIV-1) and HIV-2 are the etiologic agents of AIDS in humans (5, 30). Similarly, simian immunodeficiency virus (SIV) can induce an AIDS-like illness in Old World monkeys (19, 43). AIDS is associated with the depletion of CD4-positive T lymphocytes, which are the major target cells of viral infection in vivo (26). The entry of primate immunodeficiency viruses into target cells is mediated by viral envelope glycoproteins gp120 and gp41, which are organized into trimeric spikes on the virion surface (8, 75). Viral entry usually requires binding of the exterior envelope glycoprotein, gp120, to the primary receptor CD4 (16, 40, 45). The interaction between gp120 and CD4 promotes a series of conformational changes in gp120 that result in the formation or exposure of a binding site for particular members of the chemokine receptor family that serve as coreceptors (73, 76). Binding of gp120 to these seven-transmembrane segment (7-TMS) proteins is thought to induce additional conformational changes that lead to the activation of the transmembrane glycoprotein gp41 and subsequent fusion of the viral and cellular membranes (8, 63, 68, 75).
The CC chemokine receptor CCR5 has been shown to be the major coreceptor for primary HIV-1 isolates (1, 10, 17, 20, 21), while the CXC chemokine receptor CXCR4 is the predominant coreceptor for T-cell-tropic and laboratory-adapted HIV-1 strains (27). Moreover, CCR2b, CCR3, apj, and, to a lesser extent, CCR8 and cytomegalovirus-encoded US28 can function as coreceptors for some HIV-1 isolates (10, 11, 20, 35, 44, 59). HIV-2 and SIV are more distantly related to HIV-1 and form a distinct group of phylogenetically and antigenically related viruses (13, 19, 36, 74). A broad range of coreceptors can be used by HIV-2 (6, 18, 33, 49, 67). Like HIV-1 and HIV-2, SIV can use CCR5 as a coreceptor (9, 46). In addition, SIV strains have been shown to use the orphan 7-TMS receptors STRL33 (Bonzo), gpr15 (BOB), gpr1, apj, and ChemR23 (dez) (2, 11, 18, 25, 61, 62). Thus, with the exception of CCR5 and possibly apj, HIV-1 and SIV use different coreceptors.
Although most HIV-1 isolates depend on CD4 for entry, certain primate immunodeficiency viruses are able to infect cells independently of CD4. Some HIV-2 isolates have been shown to enter CD4-negative cells by using CXCR4 (12, 24, 60). More recently, a CD4-independent HIV-1 isolate that uses CXCR4 has been derived by tissue culture adaptation (22). Moreover, it has been demonstrated that the neurovirulent strain SIV/17E-Fr, as well as other SIV strains, can infect CD4-negative brain capillary endothelial cells by using CCR5 as the primary receptor (23). This potentially provides a direct route across the blood-brain barrier, in contrast to infection of peripheral macrophages and subsequent migration of these cells into the brain. The phenomenon of CD4-independent entry suggests at least partial exposure of the coreceptor binding site on some immunodeficiency viruses, so that CD4 is not necessary to induce the relevant conformational changes in gp120. Indeed, some SIV gp120 glycoproteins have been shown to bind rhesus monkey CCR5 in the absence of CD4 (48).
The role of CD4 binding in HIV-1 and SIV entry has been studied by using a soluble form of the CD4 glycoprotein (sCD4). Both positive and negative effects of sCD4 on virus infection have been observed (3, 4, 12, 16a, 28, 34, 66, 72). The efficacy of inhibition of virus infection by sCD4 is dependent on the affinity with which the sCD4 glycoprotein binds the functional envelope glycoprotein spike (15, 51–53, 57, 64, 71). In addition, sCD4 can induce the shedding of the gp120 envelope glycoprotein from the envelope glycoprotein complex of some virus isolates (7, 29, 31, 39, 50). Conversely, infection by some primary HIV-1, HIV-2, and SIV isolates is enhanced by sCD4 (3, 4, 12, 65, 69, 70).
This study used three SIV variants to analyze the interaction of their envelope glycoproteins with the viral receptors. Pathogenic, molecularly cloned SIVmac239 replicates well in lymphocytes but poorly in macrophages (38, 55). SIVmac316 was isolated from alveolar macrophages of a rhesus monkey infected with SIVmac239 (54). SIVmac316 exhibited an increased ability to infect cultured primary macrophages, a change of cell tropism determined by eight amino acid residues in the viral envelope glycoproteins. SIVmac316BSS was isolated from the brain of a monkey infected with SIVmac316 (41). Compared with the amino acid sequence of SIVmac239, those of the SIVmac316BSS envelope glycoproteins exhibited amino acid changes in nine positions. Two changes (158 T→A and 371 N→S) result in the loss of two glycosylation sites. A particularly surprising change in SIVmac316BSS is 385 D→N in the CD4-binding site that, in HIV-1 and SIVmac239, has been shown to reduce the CD4-binding ability of gp120 (56, 58).
Here, we examined the ability of recombinant viruses carrying the three described SIV envelope glycoproteins to infect CD4-positive and CD4-negative cells and studied the effects of sCD4 on these infections. We analyzed these findings in light of the binding affinities of monomeric gp120 for CD4 and CCR5 and the stability of the activated sCD4-gp120 complex on the virion.
MATERIALS AND METHODS
Cells.
HeLa and Cf2Th cells (obtained from the American Type Culture Collection) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS), penicillin at 100 U/ml, and streptomycin at 100 U/ml. CCR5F-L1.2 (76) cells were cultured in RPMI 1640 medium supplemented with 10% FCS, xanthine at 125 μg/ml, mycophenolic acid at 2.5 μg/ml, HT Supplement (Gibco BRL), and antibiotics. Drosophila Schneider 2 cells were grown at 25°C in MRD4 medium containing 5% FCS, 0.1% pluronic F-68, and hygromycin B (Boehringer, Mannheim, Germany) at 300 μg/ml.
Plasmids.
The pHXBH10ΔenvCAT and pSIVΔgpv plasmids used to produce recombinant virions carrying the envelope glycoproteins of SIVmac239 and SIVmac316 have been previously described (47). The pSIVΔgpv316BSS plasmid expressing the envelope glycoproteins of SIVmac316BSS (41) was constructed by replacing the env gene of pSIVΔgpv316 with that of SIVmac316BSS. For expression of the SIVmac316 and SIVmac316BSS gp120 in drosophila Schneider 2 cells, the DNA sequences were PCR amplified with Pfu polymerase (Stratagene) for 30 cycles. The PCR steps included denaturation at 94°C for 20 s, annealing at 50°C for 1 min, and elongation at 72°C for 4 min. The corresponding envelope-expressing plasmids described above were used as templates with primers RW5SIVwt (5′-TTT TAG ATC TAC TCT ATA TGT CAC AGT CTT TTA TGG; starting within a sequence encoding residue 23 in the signal peptide and containing a flanking BglII site) and RW3SIVwt (5′-TTT TGC TAG CTC ATC TTT TAT TTC TTG AGG TGC CAC C; containing a premature stop codon at the position of the natural gp120/41 proteolytic cleavage site and a flanking NheI site). The BglII-NheI fragments of the PCR products were cloned into the pMt vector (14) by using the corresponding restriction sites in the vector. A similar plasmid expressing SIVmac239 gp120 was provided by R. Wyatt, Dana-Farber Cancer Institute, and was previously described (48). Human CCR5, rhesus CCR5, gpr15, and STRL33 were expressed in the pcDNA3 vector (Invitrogen), which has been described elsewhere (10, 25, 46). The pcDNA3 plasmids expressing full-length human and rhesus CD4 have also been published previously (37).
Env complementation assay.
A single round of virus infection was measured by using a previously described env complementation assay (32). Briefly, HeLa cells were cotransfected by the calcium phosphate method with 18 μg of pHXBH10ΔenvCAT and 3 μg of pSIVΔgpv to produce recombinant virions. The pHXBH10ΔenvCAT plasmid contains an HIV-1 provirus with a deletion in the env gene and a chloramphenicol acetyltransferase (CAT)-encoding gene replacing the nef gene. The functional env gene was provided in trans by the pSIVΔgpv plasmid. The Cf2Th target cells had been transfected 48 h before infection by the calcium phosphate method with either 8 μg of a plasmid encoding human CD4 and 20 μg of a plasmid encoding one of the chemokine receptors or with 8 μg of the pcDNA3.1 plasmid and 20 μg of a plasmid encoding the chemokine receptor. The Cf2Th cells transfected with the latter combination of plasmids expressed the chemokine receptors but not CD4. Cf2Th cells transiently expressing only CD4 were used as controls. Cf2Th cells (2 × 105) were incubated with 20,000 cpm of reverse transcriptase activity of the recombinant viruses at 37°C. Cells were lysed 60 h after infection, and CAT activity was determined. To examine the effect of sCD4 on virus infection, the viral stocks were incubated for 1 h at 37°C with increasing concentrations of four-domain sCD4 (provided by Raymond Sweet at SmithKline Beecham, King of Prussia, Pa.) before they were added to Cf2Th target cells expressing either CD4 and the chemokine receptor or the chemokine receptor alone.
Stability of the activated sCD4-envelope glycoprotein intermediates.
To determine the stability of the activated sCD4-envelope glycoprotein intermediates, the recombinant virions were incubated for 1 h at 37°C with four-domain sCD4 at 10 μg/ml, pelleted twice at 27,000 rpm in an SW28 rotor (Beckman) for 1 h at 19°C, and resuspended in 6 ml of fresh DMEM containing 10% FCS and antibiotics to wash out excess sCD4. The incubation of the viral stocks was continued in this medium at 37°C for the indicated periods before Cf2Th target cells were added.
Production of gp120 in drosophila cells.
The SIVmac239, SIVmac316, and SIVmac316BSS gp120 glycoproteins were produced from drosophila Schneider 2 cells stably transfected with the gp120-expressing pMt vector and the selectable marker pc hygro as described elsewhere (14). Protein expression was induced in expanded, hygromycin B-selected cell lines by culturing the cells in serum-free MRD4 medium containing 0.1% pluronic F-68, hygromycin B at 300 μg/ml, and 750 mM CuSO4 for 7 days at 25°C. Recombinant glycoproteins were purified over a B23 antibody affinity column (B23 antibody was provided by J. Robinson, Tulane University School of Medicine). After extensive washing with phosphate-buffered saline (PBS)–0.5 M NaCl and re-equilibration in PBS, the gp120 glycoproteins were eluted in 100 mM glycine-HCl, pH 2.8, and the fractions were neutralized with 1 M Tris base. The purified proteins were concentrated by using Centriprep spin filters (Amicon). Protein concentrations were determined by measurement of optical density and Coomassie blue staining of sodium dodecyl sulfate-polyacrylamide gels.
Enzyme-linked immunosorbent assay (ELISA) for binding of sCD4 to monomeric gp120.
Ninety-six-well plates were coated at 100 ng per well with four-domain sCD4 in 100 μl of 100 mM sodium carbonate-bicarbonate buffer, pH 9.6. Plates were incubated at 4°C for 24 h, washed five times with PBS containing 0.2% Tween (PBS-Tween), and incubated for another 24 h at 4°C with 300 μl of blocking buffer (PBS containing 2% nonfat dried milk and 5% heat-inactivated FCS). After removal of the blocking buffer, the plates were incubated for 1 h at 25°C with twofold serial dilutions of the gp120 envelope glycoproteins in PBS-Tween, starting at a concentration of 10−5 M in a total volume of 100 μl. Plates were washed 10 times and incubated for 1 h at 25°C with serum (diluted 1/1,000 in blocking buffer) from SIV-infected monkeys. The plates were washed, incubated with anti-monkey immunoglobulin G-horseradish peroxidase (Sigma; diluted 1/2,000 in blocking buffer) overnight at 4°C, and washed again with PBS-Tween. This was followed by incubation with TMB substrate (Bio-Rad) at 100 μl/well. The reaction was stopped with 100 μl of 1 M HCl per well, and the optical density at 450 nm was read. We observed negligible gp120 binding to control plates that had not been incubated with sCD4 (data not shown).
Binding of gp120 to CCR5.
Binding of SIVmac239, SIVmac316, and SIVmac316BSS gp120 to CCR5 was measured by using L1.2 cells stably expressing human CCR5 as previously described (76). Briefly, 106 CCR5F-L1.2 cells were incubated with 0.1 nM 125I-labelled human MIP-1α (DuPont NEN, Boston, Mass.) and increasing concentrations of unlabelled competitor gp120 in binding buffer (50 mM HEPES [pH 7.2], 1 mM CaCl2, 5 mM MgCl2, 0.5% bovine serum albumin) in a total volume of 100 μl. Binding of gp120 to CCR5 was analyzed either in the absence or in the presence of 100 nM sCD4. After 1 h of incubation at 25°C, cells were pelleted and washed twice with binding buffer containing 0.5 M NaCl. The radioactivity associated with the cell pellets was measured. As a positive control, unlabelled MIP-1α was used as a competitor.
RESULTS
CD4-independent entry of SIV isolates.
The CCR5, gpr15, and STRL33 proteins serve as the major coreceptors for CD4-dependent entry of several SIV strains, including SIVmac239, SIVmac316, and SIVmac316BSS (2, 9, 11, 18, 25, 46, 61). To determine whether CCR5, gpr 15, and STRL33 also support the CD4-independent entry of viruses carrying the different envelope glycoproteins, an env complementation assay was utilized (32). Recombinant HIV-1 virions expressing CAT and containing the SIV envelope glycoproteins were harvested 60 h after transfection of HeLa cells, normalized to 20,000 cpm of reverse transcriptase activity, and incubated with Cf2Th target cells transiently expressing CD4 and the coreceptor or the coreceptor alone. Cf2Th cells expressing only CD4 were used as a negative control.
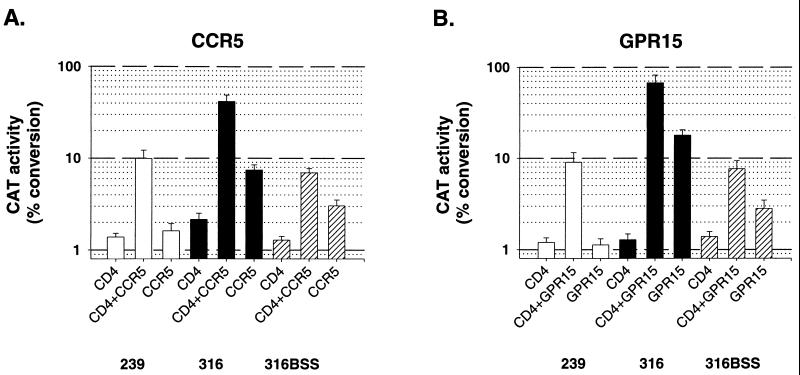
Human CCR5 supported CD4-independent infection of viruses with SIVmac316 and SIVmac316BSS envelope glycoproteins but did not support CD4-independent infection of viruses with SIVmac239 envelope glycoproteins (Fig. 1A). Infection of CD4-negative, CCR5-positive cells by SIVmac316 and SIVmac316BSS was 19 and 43%, respectively, of that of cells expressing both CD4 and CCR5. Similar results were obtained by using CCR5 from rhesus monkeys (data not shown). Cf2Th cells expressing gpr15 supported CD4-independent infection by viruses with SIVmac316 and SIVmac316BSS envelope glycoproteins. By contrast, viruses with the envelope glycoproteins of SIVmac239 could not infect gpr15-expressing cells independently of CD4 (Fig. 1B). None of the SIV envelope glycoproteins tested was able to support entry into Cf2Th cells expressing STRL33 without CD4 (Fig. 1C). These results indicate that the efficiency of CD4-independent infection is influenced by the viral envelope glycoproteins and by the coreceptor on the target cell.
FIG. 1.
Infection of cells expressing CD4 and/or coreceptors. Cf2Th cells transiently expressing either CD4, a 7-TMS receptor, or both proteins were incubated with recombinant CAT-expressing viruses with the envelope glycoprotein of SIVmac239, SIVmac316, or SIVmac316BSS. The coreceptors used were human CCR5 (A), human gpr15 (B), and human STRL33 (C). Viruses with the SIVmac239 envelope glycoprotein are represented by white bars, those with the SIVmac316 envelope glycoprotein are represented by black bars, and those with the SIVmac316BSS envelope glycoprotein are represented by hatched bars. Infection efficiency is reported as the percentage of chloramphenicol acetylated by a standard amount of Cf2Th cell lysate.
Effect of sCD4 on SIV infection.
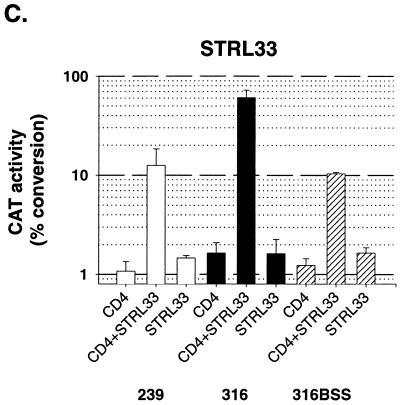
To determine the effect of sCD4 on CD4-dependent and CD4-independent SIV infection, recombinant viruses with the three SIV envelope glycoproteins were incubated with increasing concentrations of sCD4 for 1 h at 37°C prior to infection of the target cells. For infection of cells expressing CD4 and CCR5, sCD4 exhibited only modest inhibitory activity against viruses with the SIVmac239 envelope glycoproteins and no significant inhibitory activity against viruses with SIVmac316 and SIVmac316BSS envelope glycoproteins (Fig. 2). Infection of cells expressing CCR5 but not CD4 was enhanced by incubation of sCD4 with viruses with the SIVmac239 and SIVmac316 envelope glycoproteins. Likewise, sCD4 inhibition of infection of CD4-positive, gpr15-positive cells by the three viruses was minimal, with some inhibition seen for viruses with the SIVmac316BSS envelope glycoproteins. Infection of cells expressing gpr15 but not CD4 by viruses with all three envelope glycoproteins was enhanced by sCD4. The inhibitory effects of sCD4 on infection of CD4-positive cells by all three viruses were more pronounced when STRL33 was used as a coreceptor than when CCR5 or gpr15 was used as a coreceptor. Virus infection of CD4-negative, STRL33-positive cells was not significantly enhanced by the addition of sCD4. These results indicate that the effects of sCD4 on SIV infection depend upon the envelope glycoproteins of the infecting virus, the presence or absence of CD4 on the target cell, and the particular coreceptor used for infection.
FIG. 2.
Effect of sCD4 on infection of CD4-positive and CD4-negative cell lines. Recombinant viruses with the SIVmac239, SIVmac316, or SIVmac316BSS envelope glycoprotein were incubated with various concentrations of sCD4 for 1 h at 37°C. Viruses were then added to Cf2Th target cells transiently expressing human CCR5, gpr15, or STRL33 either with (hatched) or without (black) CD4. Cf2Th cells transiently expressing CD4 alone (white) were included as controls. The results are reported as percentages of chloramphenicol acetylated by a standard amount of Cf2Th cell lysate. The experiment was performed three times with comparable results. A representative experiment is shown.
Receptor-binding abilities of the SIV gp120 envelope glycoproteins.
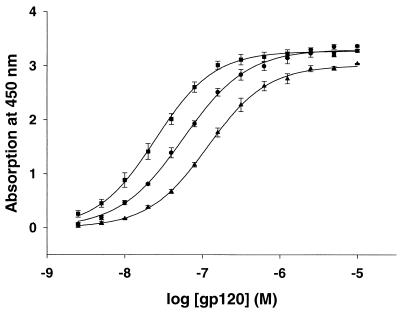
The abilities of the SIVmac239, SIVmac316, and SIVmac316BSS gp120 envelope glycoproteins to bind CD4 and CCR5 were examined. The gp120 glycoproteins of the three SIV isolates were produced in drosophila cells and purified. To measure CD4-binding ability, the SIV gp120 glycoproteins were bound to sCD4 that had been captured on ELISA plates, and bound gp120 was detected by using serum from an SIV-infected monkey. The results indicate that SIVmac316 gp120 binds CD4 with the highest affinity (Kd = 2.8 × 10−8 M) (Fig. 3). The binding affinity of the SIVmac239 gp120 glycoprotein was 5.8 × 10−8 M. The SIVmac316BSS gp120 glycoprotein exhibited the lowest affinity (1.1 × 10−7 M) for CD4. Part of the explanation for the lower CD4-binding ability of the SIVmac316BSS gp120 glycoprotein may be the presence of an asparagine residue at position 385 (41). Most HIV-1 and SIV gp120 glycoproteins have an aspartic acid residue at this position, which corresponds to residue 368 of the prototypic HXBc2 gp120 glycoprotein (42). Alteration of aspartic acid 368 has been shown to reduce the CD4-binding affinity of HIV-1 and SIV gp120 glycoproteins (56, 58), and recent X-ray crystallographic data suggest that aspartic acid 368 forms a critical contact with CD4 (42a).
FIG. 3.
Binding of SIV gp120 glycoproteins to sCD4. ELISA plates were coated with human sCD4 and incubated with various concentrations (molar) of the SIVmac239 (●), SIVmac316 (■), or SIVmac316BSS (▴) gp120 envelope glycoprotein. Plates were washed, incubated with serum from an SIV-infected monkey, and developed by using anti-monkey immunoglobulin G-horseradish peroxidase and TMB substrate. The reaction was stopped with 1 M HCl, and the plates were read at 450 nm.
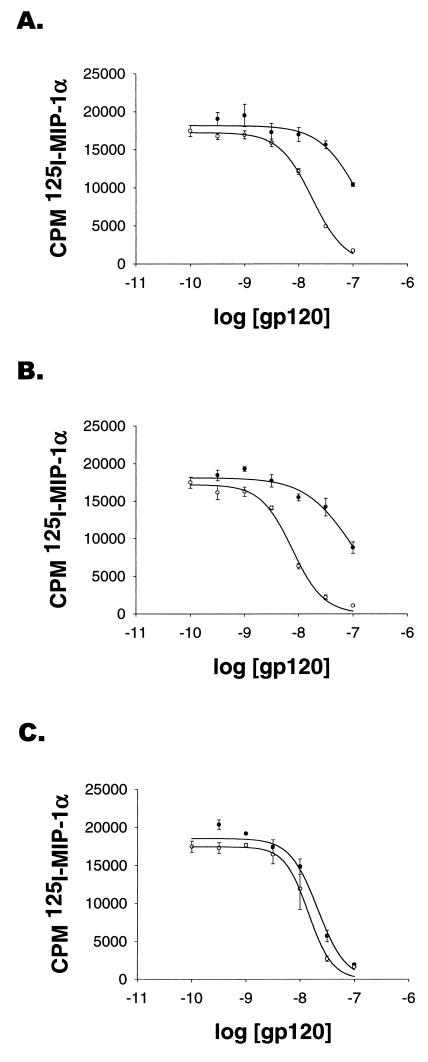
The ability of the three SIV gp120 envelope glycoproteins to bind human CCR5 in the presence or absence of sCD4 was studied (Fig. 4). Increasing concentrations of unlabelled gp120 glycoprotein were used to compete for binding of 125I-labelled MIP-1α to CCR5F-L1.2 cells. In the presence of excess sCD4, the gp120 glycoproteins of all three SIV isolates exhibited similar affinities, with Ki values of approximately 10−8 M. In the absence of sCD4, the SIVmac239 and SIVmac316 gp120 glycoproteins exhibited approximately 10-fold reductions in apparent affinity for CCR5. By contrast, the absence of sCD4 only slightly reduced the apparent affinity of the SIVmac316BSS gp120 envelope glycoprotein for CCR5. These results indicate that some SIV gp120 glycoproteins can bind human CCR5 with high affinity, even in the absence of CD4.
FIG. 4.
Binding of SIV gp120 glycoproteins to CCR5. CCR5-expressing L1.2 cells were incubated for 1 h at 25°C with 0.1 nM 125I-MIP-1α and increasing concentrations of unlabelled gp120 from SIVmac239 (A), SIVmac316 (B), or SIVmac316BSS (C). Incubations were carried out either in the absence (●) or in the presence (○) of 100 nM sCD4. Cells were washed twice with high-salt buffer, and the radioactivity associated with the cell pellets was determined.
Stability of the sCD4-activated envelope glycoprotein intermediate.
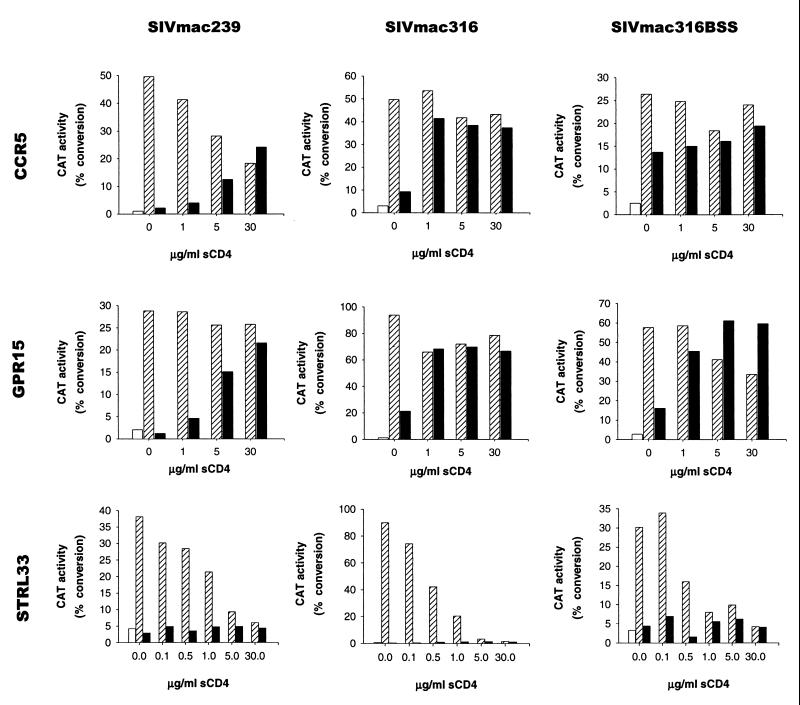
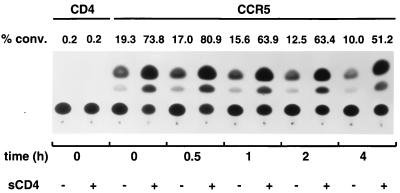
The above-described studies indicate that sCD4 strongly enhanced the infectivity of viruses with the SIVmac316 envelope glycoproteins in target cells expressing CCR5 but not CD4 (Fig. 2). The stability of the sCD4-activated envelope glycoprotein intermediate on these viruses was examined by using the env complementation assay. Recombinant viruses with the SIVmac316 envelope glycoproteins were first incubated in the presence or absence of sCD4. Virions were then pelleted and washed in fresh medium twice, and the incubation was continued for various lengths of time. After this incubation period, the virions were added to Cf2Th cells transiently expressing CCR5. The expression of the CAT-encoding provirus was measured in the Cf2Th cells. The results indicate that the infectivity of virions decreased with time, regardless of whether the virions had been preincubated with sCD4 (Fig. 5 and data not shown). Nonetheless, the virions preincubated with sCD4 exhibited higher infectivity than the virions not exposed to sCD4, even 4 h after the sCD4 incubation. This higher level of infectivity could be discerned even 18 h after sCD4 incubation, although the infectivity of sCD4-treated and untreated virions was very low by this time (data not shown). Thus, the sCD4-induced activation intermediate on the SIV envelope glycoprotein complex is very stable over time.
FIG. 5.
Stability of the sCD4-activated envelope glycoprotein intermediate. Recombinant virions with the envelope glycoprotein of SIVmac316 were incubated for 1 h at 37°C in the presence or absence of sCD4 at 10 μg/ml. Virions were pelleted twice at 27,000 rpm in a Beckman SW28 rotor for 1 h each time at 19°C and resuspended in 5 ml of DMEM. Incubation at 37°C was continued for the indicated periods, after which Cf2Th cells transiently expressing human CCR5 were infected with the virions. Cf2Th cells expressing CD4 without a coreceptor were included as a control. CAT activity was assayed in the target cell lysates 3 days after infection. Percent conversion (conv.) of [14C]chloramphenicol to acetylated forms is indicated above the spots. A representative experiment of two experiments is shown.
DISCUSSION
In this study, we examined the effect of sCD4 on infection of CD4-positive and CD4-negative cells by recombinant viruses with envelope glycoproteins derived from three SIV strains. The net effect of sCD4 on virus infection reflects the degree of enhancement minus any detrimental effects. Enhancing effects might derive from increases in coreceptor binding and from other CD4-induced conformational changes promoting fusogenic conformations of the envelope glycoproteins. The latter effects should be relatively independent of the particular coreceptor used. Thus, our observation that sCD4 enhancement is evident when the target cells express CCR5 or gpr15, but not STRL33, implies that most, if not all, of the enhancing effect of sCD4 on SIV infection is due to positive influences on coreceptor interaction. This is consistent with the observation that the degree of sCD4 enhancement of infection of CD4-negative, CCR5-positive cells by viruses with different SIV envelope glycoproteins could be explained by the receptor-binding data. Thus, viruses with the SIVmac239 and SIVmac316 envelope glycoproteins exhibited sCD4-induced enhancement of infection of CD4-negative target cells, whereas viruses with the SIVmac316BSS envelope glycoproteins did not. In the CCR5-binding assay, the former envelope glycoproteins exhibited increased binding affinity in the presence of sCD4, whereas the latter gp120 glycoprotein did not.
sCD4 can enhance the efficiency of infection of CD4-negative cells by viruses with the SIVmac239 and SIVmac316 envelope glycoproteins to a level close to that seen in CD4-expressing target cells in the absence of sCD4. This implies that the function of CD4 in promoting virus attachment to the target cell can be replaced by a high affinity of the viral envelope glycoprotein-sCD4 complexes for CCR5 or gpr15. The SIVmac316BSS envelope glycoproteins appear to exhibit such a higher affinity for the CCR5 coreceptor spontaneously, in the absence of CD4, thus facilitating attachment to and infection of CD4-negative, CCR5-positive cells.
Hypothetically, the detrimental effects of sCD4 on virus infection might derive from competition for cellular CD4, with a consequent decrease in virus attachment to the target cell surface, and from other disruptive effects on the structure of the virion envelope glycoprotein spike. The latter effects, which should be equivalent for infection of CD4-positive and CD4-negative target cells, include the shedding of the gp120 glycoprotein from the oligomeric envelope glycoprotein complexes (7, 29, 31, 39, 50). We observed no negative effects of sCD4 on infection of cells lacking CD4 by the viruses used in this study. This implies that disruption of the SIV envelope glycoprotein spike, either dramatically as in gp120 shedding or through more subtle effects on the interaction of the envelope glycoprotein components of the spike, does not contribute significantly to inhibitory effects of sCD4 in this system. This is consistent with the observation that the negative effects of sCD4 are observed only with CD4-positive target cells, where competition for cellular CD4 would be operative. It is also consistent with the extremely long-lived activation intermediate induced by sCD4 on the SIV envelope glycoprotein complex. The stability of the SIV envelope glycoproteins complexed to sCD4 contrasts with the shedding of gp120 that results from CD4 binding to some HIV-1 envelope glycoproteins (7, 24, 31, 39, 50). The significance of these differences for the biological function of these envelope glycoproteins remains to be explored fully.
Theoretically, viral envelope glycoprotein complexes activated for subsequent entry events by interaction with CD4 could be transient. However, viruses with long-lived intermediates primed for high-affinity interaction with coreceptors would have a selective advantage because the opportunity for binding of the requisite number of coreceptors would be enhanced. Previous studies have suggested that the conserved coreceptor-binding site on primate immunodeficiency virus gp120 envelope glycoproteins is sterically inaccessible to antibodies after the virus attaches to CD4 on the target cell (70). This would negate any detrimental immunologic consequences for the virus of having a coreceptor-binding site that is exposed for long periods following CD4 binding.
The results suggest that, for at least some primate immunodeficiency viruses, the CD4-bound conformation of the envelope glycoproteins, which is capable of CCR5 binding, is also compatible with maintenance of the gp120-gp41 association in the functional trimer. Further conformational changes in the envelope glycoproteins required for fusion are likely to be triggered by coreceptor binding. The importance of binding to the 7-TMS coreceptors for promotion of these conformational changes is underscored by the observation that although several examples of CD4-independent, 7-TMS coreceptor-dependent viruses have been documented (12, 22–24, 60), coreceptor-independent viruses have not been reported.
Consistent with the results of a previous study (23), we observed fairly efficient infection of target cells lacking CD4 by viruses with certain SIV envelope glycoproteins. The efficiency of CD4-independent infection depended on the SIV strain from which the envelope glycoproteins were derived. Under circumstances in which viruses with the SIVmac316 and SIVmac316BSS envelope glycoproteins exhibited infection efficiencies 18 to 29 and 40 to 43%, respectively, of that seen in CD4-positive target cells, viruses with the SIVmac239 envelope glycoproteins did not efficiently infect CD4-negative cells. The ability of the SIV envelope glycoprotein variants to mediate infection of CD4-negative cells did not always correlate with efficient binding of the monomeric gp120 glycoprotein to CCR5 in the absence of sCD4. Although the SIVmac316BSS envelope glycoproteins exhibited CD4-independent binding to CCR5 and the ability to support CD4-independent infection, CD4-independent infection by viruses with the SIVmac316 envelope glycoproteins was not reflected in the CCR5-binding assay. Likewise, the monomeric SIVmac239 gp120 glycoprotein has been shown to bind rhesus monkey CCR5, but not human CCR5, in a manner that is relatively independent of CD4 (48). Nonetheless, the efficient entry of viruses with the SIVmac239 envelope glycoproteins into target cells expressing either monkey or human CCR5 is dependent upon CD4. These results indicate that some of the interactions between the assembled, trimeric envelope glycoprotein complex and the 7-TMS coreceptors may not be completely mimicked in binding assays using monomeric gp120. The development and use of receptor-binding assays employing trimeric forms of the envelope glycoproteins appears worthwhile.
The efficiency of CD4-independent infection was affected by the particular 7-TMS coreceptor present on the target cell surface. Although human CCR5, rhesus monkey CCR5, and gpr15 supported CD4-independent infection in these assays, STRL33 did not. This observation is not simply explained by the intrinsic coreceptor activity of STRL33, which, in the presence of CD4, is comparable to those of CCR5 and gpr15. Quantitative differences in binding affinity or differences in the degree of exposure of the gp120 binding site in the absence of CD4 may explain the poor ability of STRL33 to support CD4-independent infection.
Further studies are needed to understand the biological relevance of CD4-independent infection. Presumably, viruses that are less dependent upon CD4 would have an advantage when CD4-positive target cells were depleted or in body compartments where CD4-positive cells are less abundant. With respect to the latter, the emergence of SIVmac316BSS in the brain of an infected monkey (41) and the ability of the virus to use two coreceptors, CCR5 and gpr15, which are expressed in the central nervous system, in a CD4-independent manner are intriguing.
ACKNOWLEDGMENTS
We acknowledge Ronald Desrosiers, Raymond Sweet, and Norman Letvin for reagents. We thank Yvette McLaughlin and Sheri Farnum for manuscript preparation.
This work was supported by NIH grants AI24755 and AI41851 and by Center for AIDS Research grant AI28691. We also acknowledge the support of the G. Harold and Leila Mathers Foundation, The Friends 10, Douglas and Judith Krupp, and the late William F. McCarty-Cooper.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC-CKR5: a RANTES, MIP-1a, MIP-1b receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Liao F, Berger E A, Farber J M, Peden K W. A new SIV co-receptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 3.Allan J S. Receptor-mediated activation of immunodeficiency viruses in viral fusion. Science. 1991;252:1322–1323. doi: 10.1126/science.1925547. [DOI] [PubMed] [Google Scholar]
- 4.Allan J S, Strauss J, Buck D W. Enhancement of SIV infection with soluble receptor molecules. Science. 1990;247:1084–1088. doi: 10.1126/science.2309120. [DOI] [PubMed] [Google Scholar]
- 5.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauget C, Axler-Bin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphocyte retrovirus from a patient at risk for acquired immunodeficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 6.Brön R, Klasse P J, Wilkinson D, Clapham P R, Pelchen-Matthews A, Power C, Wells T N C, Kim J, Peiper S, Hoxie J A, Marsh M. Promiscuous use of CC and CXC chemokine receptors in cell-to-cell fusion mediated by a human immunodeficiency virus type 2 ROD envelope protein. J Virol. 1997;71:8405–8415. doi: 10.1128/jvi.71.11.8405-8415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugelski P J, Ellens H, Hart T K, Kirsh R L. Soluble CD4 and dextran sulfate mediate release of gp120 from HIV-1: implications for clinical trials. J Acquired Immune Defic Syndr. 1991;4:923–924. [PubMed] [Google Scholar]
- 8.Chan D C, Fass D, Berger J M, Kim P. Core structure of gp41 from HIV envelope glycoprotein. Cell. 1997;73:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Zhou P, Ho D D, Landau N R, Marx P A. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a cofactor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 11.Choe H, Farzan M, Konkel M, Martin K, Sun Y, Marcon L, Cayabyab M, Berman M, Dorf M E, Gerard N, Gerard C, Sodroski J. The orphan seven-transmembrane receptor Apj supports the entry of primary T-cell-line-tropic and dualtropic human immunodeficiency virus type 1. J Virol. 1998;72:6113–6118. doi: 10.1128/jvi.72.7.6113-6118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clapham P R, McKnight A, Weiss R A. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J Virol. 1992;66:3531–3537. doi: 10.1128/jvi.66.6.3531-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clavel F. HIV-2, the West African AIDS virus. AIDS. 1987;1:135–140. [PubMed] [Google Scholar]
- 14.Culp J S, Johansen H, Hellmig B, Beck J, Matthews T J, Delers A, Rosenberg M. Regulated expression allows high level production and secretion of HIV-1 gp120 envelope glycoprotein in Drosophila Schneider cells. Bio/Technology. 1991;9:173–177. doi: 10.1038/nbt0291-173. [DOI] [PubMed] [Google Scholar]
- 15.Daar E, Li X L, Moudgil T, Ho D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci USA. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalgleish A G, Berverly P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 16a.Deen K, McDougal J S, Inacker R, Folena-Wasserman G, Arthos J, Rosenberg J, Maddon P, Axel R, Sweet R. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature (London) 1988;331:82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- 17.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 18.Deng H K, Unutmaz D, Kewalramani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 19.Desrosiers R C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 20.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Permentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 21.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 22.Dumonceaux J, Nisole S, Chanel C, Quivet L, Amara A, Baleux F, Briand P, Hazan U. Spontaneous mutations in the env gene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent phenotype. J Virol. 1998;72:512–519. doi: 10.1128/jvi.72.1.512-519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edinger A L, Mankowski J L, Doranz B J, Margulies B J, Lee B, Rucker J, Sharron M, Hoffman T L, Berson J F, Zink M C, Hirsch V M, Clements J E, Doms R W. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA. 1997;94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 25.Farzan M, Choe H, Martin K A, Marcon L, Hofmann W, Karlsson G, Sun Y, Barret P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fauci A, Macher A, Longo D, Lane H C, Rook A, Masur H, Gelmann E. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 27.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 28.Fisher R, Bertonis J, Meier W, Johnson V, Costopoulos D, Liu T, Tizard R, Walder B, Hirsch M, Schooley R, Flavell R. HIV infection is blocked in vitro by recombinant soluble CD4. Nature (London) 1988;331:76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y K, Hart T K, Jonak Z L, Bugelski P J. Physicochemical dissociation of CD4-mediated syncytium formation and shedding of human immunodeficiency virus type 1 gp120. J Virol. 1993;67:3818–3825. doi: 10.1128/jvi.67.7.3818-3825.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, White G, Foster P, Markham P D. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 31.Hart T K, Kirsh R, Ellens H, Sweet R W, Lambert D M, Petteway S R, Jr, Leary J, Bugelski P J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc Natl Acad Sci USA. 1991;88:2189–2193. doi: 10.1073/pnas.88.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heredia A, Vallejo A, Soriano V, Epstein J S, Hewlett I K. Chemokine receptors and HIV-2. AIDS. 1997;11:1198–1199. doi: 10.1097/00002030-199709000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Hussey R, Richardson N, Kowalski M, Brown N, Change H, Siliciano R, Dorfman T, Walker B, Sodroski J, Reinherz E. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature (London) 1988;331:78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- 35.Jinno A, Shimizu N, Soda Y, Haraguchi Y, Kitamura T, Hoshino H. Identification of the chemokine receptor TER1/CCR8 expressed in brain-derived cells and T cells as a new coreceptor for HIV-1 infection. Biochem Biophys Res Commun. 1998;243:497–502. doi: 10.1006/bbrc.1998.8130. [DOI] [PubMed] [Google Scholar]
- 36.Kanki P, McLane M, King N, Essex M. Serological identification and characterization of a macaque T-lymphotropic retrovirus closely related to HTLV-III. Science. 1985;228:1199–1201. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard N P, Marcon L, Margolin D, Fanton J, Axthelm M K, Letvin N L, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 39.Kirsh R, Hart T, Ellens H, Miller J, Petteway S, Lambert D, Leary B, Bugelski P. Morphometric analysis of recombinant soluble CD4-mediated release of the envelope glycoprotein gp120 from HIV-1. AIDS Res Hum Retroviruses. 1990;6:1209–1212. doi: 10.1089/aid.1990.6.1209. [DOI] [PubMed] [Google Scholar]
- 40.Klatzmann D, Champagne E, Charmaret S, Gruest J, Guetard D, Hercend T, Gluckman J-C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 41.Kodama T, Mori K, Kawahara T, Ringler D J, Desrosiers R C. Analysis of simian immunodeficiency virus sequence variation in tissues of rhesus macaques with simian AIDS. J Virol. 1993;67:6522–6534. doi: 10.1128/jvi.67.11.6522-6534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korber B, Foley B, Leitner T, McCutchan F, Hahn B, Mellors J, Myers G, Kuiken C, editors. Human retroviruses and AIDS 1997. Los Alamos, N.Mex: Los Alamos National Laboratory; 1997. [Google Scholar]
- 42a.Kwong P D, Wyatt R, Robinson J, Sweet R, Sodroski J, Hendrickson W. Structure of an HIV-1 gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Letvin N L, Daniel M D, Sehgal P K, Desrosiers R C, Hunt R D, Waldron L M, MacKey J J, Schmidt D K, Chalifoux L V, King N W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985;230:71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- 44.Liao F, Alkhatib G, Peden K W, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 46.Marcon L, Choe H, Martin K A, Farzan M, Ponath P D, Wu L, Newman W, Gerard N, Gerard C, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus (SIVmac239) J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcon L, Sodroski J. High degree of sensitivity of the simian immunodeficiency virus (SIVmac) envelope glycoprotein subunit association to amino acid changes in the gp41 ectodomain. AIDS Res Hum Retroviruses. 1997;13:441–447. doi: 10.1089/aid.1997.13.441. [DOI] [PubMed] [Google Scholar]
- 48.Martin K A, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard N P. CD4-independent binding of SIV gp120 to rhesus CCR5. Science. 1997;278:1470–1473. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- 49.McKnight A, Dittmar M, Moniz-Periera J, Ariyoshi K, Reeves J, Hibbits S, Whitby D, Aarons E, Proudfoot A, Whittle H, Clapham P. A broad range of chemokine receptors are used by primary isolates of human immunodeficiency virus type 2 as coreceptors with CD4. J Virol. 1998;72:4065–4071. doi: 10.1128/jvi.72.5.4065-4071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore J, McKeating J, Weiss R, Sattentau Q. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 51.Moore J P, Klasse P J. Thermodynamic and kinetic analysis of sCD4 binding to HIV-1 virions and of gp120 dissociation. AIDS Res Hum Retroviruses. 1992;8:443–450. doi: 10.1089/aid.1992.8.443. [DOI] [PubMed] [Google Scholar]
- 52.Moore J P, McKeating J A, Huang Y X, Ashkenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore J P, McKeating J A, Norton W A, Sattentau Q J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991;65:1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mori K, Ringler D J, Kodama T, Desrosiers R C. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992;66:2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrison G H, Kirchhoff F, Desrosiers R C. Evidence for the cooperation of gp120 amino acids 322 and 448 in SIVmac entry. Virology. 1993;195:167–174. doi: 10.1006/viro.1993.1357. [DOI] [PubMed] [Google Scholar]
- 56.Morrison H, Kirchhoff F, Desrosiers R. Effects of mutations in constant regions 3 and 4 of envelope of simian immunodeficiency virus. Virology. 1995;210:448–455. doi: 10.1006/viro.1995.1361. [DOI] [PubMed] [Google Scholar]
- 57.O’Brien W A, Mao S-H, Cao Y, Moore J P. Macrophage-tropic and T-cell line-adapted chimeric strains of human immunodeficiency virus type 1 differ in their susceptibilities to neutralization by soluble CD4 at different temperatures. J Virol. 1994;68:5264–5269. doi: 10.1128/jvi.68.8.5264-5269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olshevsky U, Helseth E, Furman C, Li J, Haseltine W, Sodroski J. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J Virol. 1990;64:7025–7031. doi: 10.1128/jvi.64.12.5701-5707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 60.Reeves J D, McKnight A, Potempa S, Simmons G, Gray P W, Power C A, Wells T, Weiss R A, Talbot S J. CD4-independent infection by HIV-2 (ROD/B): use of the 7-transmembrane receptors CXCR4, CCR-3, and V28 for entry. Virology. 1997;231:130–134. doi: 10.1006/viro.1997.8508. [DOI] [PubMed] [Google Scholar]
- 61.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samson M, Edinger A L, Stordeur P, Rucker J, Verhasselt V, Sharron M, Govaerts C, Mollereau C, Vassart G, Doms R W, Parmentier M. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 isolates. Eur J Immunol. 1998;28:1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 63.Sattentau Q J, Moore J P, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schutten M, Andeweg A C, Bosch M L, Osterhaus A D. Enhancement of infectivity of a non-syncytium inducing HIV-1 by sCD4 and by human antibodies that neutralize syncytium inducing HIV-1. Scand J Immunol. 1995;41:18–22. doi: 10.1111/j.1365-3083.1995.tb03528.x. [DOI] [PubMed] [Google Scholar]
- 66.Smith D, Byrn R, Marsters S, Gregory T, Groopman J, Capon D. Blocking of HIV-1 infectivity by a soluble secreted form of the CD4 antigen. Science. 1987;238:1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- 67.Sol N, Ferchal F, Braun J, Pleskoff O, Tréboute C, Alizon M. Usage of the coreceptors CCR-5, CCR-3, and CXCR-4 by primary and cell line-adapted human immunodeficiency virus type 2. J Virol. 1997;71:8237–8244. doi: 10.1128/jvi.71.11.8237-8244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stein B S, Gouda S, Lifson J, Penhallow R, Bensch K, Engelman E. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 69.Sullivan N, Sun Y, Li J, Hofmann W, Sodroski J. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J Virol. 1995;69:4413–4422. doi: 10.1128/jvi.69.7.4413-4422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thali M, Olshevsky U, Furman C, Gabuzda D, Li J, Sodroski J. Effects of changes in gp120-CD4 binding affinity on HIV-1 envelope glycoprotein function and soluble CD4 sensitivity. J Virol. 1991;65:5007–5012. doi: 10.1128/jvi.65.9.5007-5012.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Traunecker A, Luke W, Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature (London) 1988;331:84–86. doi: 10.1038/331084a0. [DOI] [PubMed] [Google Scholar]
- 73.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its coreceptor CCR5. Nature. 1996;384:184–186. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 74.Weiss R, Clapham P, Weber J, Dalgleish A, Lasky L, Berman P. Variable and conserved neutralization antigens of human immunodeficiency virus. Nature. 1986;324:572–575. doi: 10.1038/324572a0. [DOI] [PubMed] [Google Scholar]
- 75.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 76.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]