In affluent societies, coronary artery disease causes severe disability and more death than any other disease, including cancer. It manifests as angina, silent ischaemia, unstable angina, myocardial infarction, arrhythmias, heart failure, and sudden death.
Pathophysiology
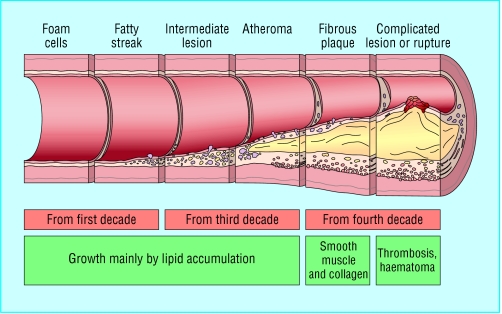
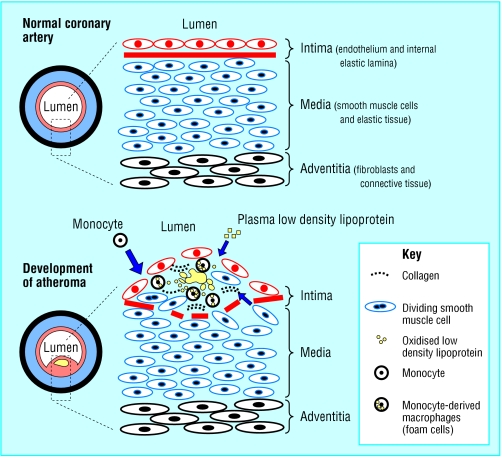
Coronary artery disease is almost always due to atheromatous narrowing and subsequent occlusion of the vessel. Early atheroma (from the Greek athera (porridge) and oma (lump)) is present from young adulthood onwards. A mature plaque is composed of two constituents, each associated with a particular cell population. The lipid core is mainly released from necrotic “foam cells”—monocyte derived macrophages, which migrate into the intima and ingest lipids. The connective tissue matrix is derived from smooth muscle cells, which migrate from the media into the intima, where they proliferate and change their phenotype to form a fibrous capsule around the lipid core.
When a plaque produces a >50% diameter stenosis (or >75% reduction in cross sectional area), reduced blood flow through the coronary artery during exertion may lead to angina. Acute coronary events usually arise when thrombus formation follows disruption of a plaque. Intimal injury causes denudation of the thrombogenic matrix or lipid pool and triggers thrombus formation. In acute myocardial infarction, occlusion is more complete than in unstable angina, where arterial occlusion is usually subtotal. Downstream embolism of thrombus may also produce microinfarcts.
Priorities for cardiology referral
| • Recent onset of symptoms | • Severe symptoms (minimal exertion or nocturnal angina) |
| • Rapidly progressive symptoms | • Angina refractory to medical treatment |
| • Possible aortic stenosis | |
| • Threatened employment | |
Cardiovascular risk factors
Non-modifiable risk factors
| • Positive family history | • Age | • Male sex |
| Modifiable risk factors | • Hypertension | • Smoking |
| • Hypercholesterolaemia | • Sedentary lifestyle | • Diabetes |
| • Left ventricular | • Excessive alcohol | • Uric acid |
| hypertrophy | intake | • Renin |
| • Overweight and obesity | • Lp(a) lipoprotein | |
| Uncertain risk factors | • Fibrinogen | |
| • Hypertriglyceridaemia | • C reactive protein | |
| • Microalbuminuria | ||
| • Hyperhomocysteinaemia | ||
Investigations
Patients presenting with chest pain may be identified as having definite or possible angina from their history alone. In the former group, risk factor assessment should be undertaken, both to guide diagnosis and because modification of some associated risk factors can reduce cardiovascular events and mortality. A blood count, biochemical screen, and thyroid function tests may identify extra factors underlying the onset of angina. Initial drug treatment should include aspirin, a β blocker, and a nitrate. Antihypertensive and lipid lowering drugs may also be given, in conjunction with advice on lifestyle and risk factor modification.
All patients should be referred to a cardiologist to clarify the diagnosis, optimise drug treatment, and assess the need and suitability for revascularisation (which can improve both symptoms and prognosis). Patients should be advised to seek urgent medical help if their symptoms occur at rest or on minimal exertion and if they persist for more than 10 minutes after sublingual nitrate has been taken, as these may herald the onset of an acute coronary syndrome.
Non-invasive investigations
Electrocardiography
An abnormal electrocardiogram increases the suspicion of significant coronary disease, but a normal result does not exclude it.
Exercise stress testing
| Indications | Contraindications |
| • Confirmation of suspected angina | • Cardiac failure |
| • Evaluation of extent of myocardial ischaemia and prognosis | • Any feverish illness |
| • Risk stratification after myocardial infarction | • Left ventricular outflow tract obstruction or hypertrophic cardiomyopathy |
| • Detection of exercise induced symptoms (such as arrhythmias or syncope) | • Severe aortic or mitral stenosis |
| • Evaluation of outcome of interventions (such as percutaneous coronary interventions or coronary artery bypass surgery) | • Uncontrolled hypertension |
| • Assessment of cardiac transplant | • Pulmonary hypertension |
| • Rehabilitation and patient motivation | • Recent myocardial infarction |
| • Severe tachyarrhythmias | |
| • Dissecting aortic aneurysm | |
| • Left main stem stenosis or equivalent | |
| • Complete heart block (in adults) | |
Chest x ray
Patients with angina and no prior history of cardiac disease usually have a normal chest x ray film.
Exercise electrocardiography
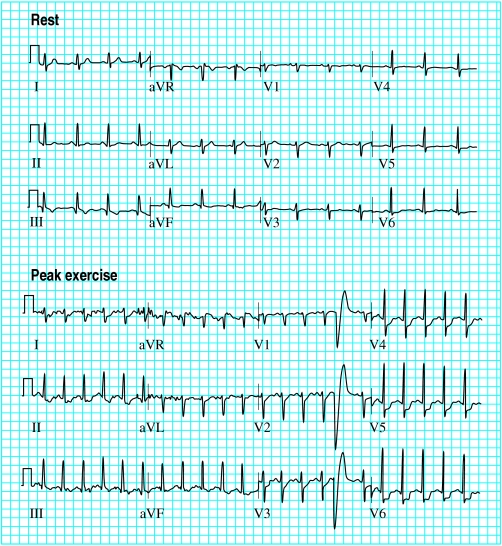
This is the most widely used test in evaluating patients with suspected angina. It is generally safe (risk ratio of major adverse events 1 in 2500, and of mortality 1 in 10 000) and provides diagnostic as well as prognostic information. The average sensitivity and specificity is 75%. The test is interpreted in terms of achieved workload, symptoms, and electrocardiographic response. A 1 mm depression in the horizontal ST segment is the usual cut-off point for significant ischaemia. Poor exercise capacity, an abnormal blood pressure response, and profound ischaemic electrocardiographic changes are associated with a poor prognosis.
Main end points for exercise electrocardiography
Target heart rate achieved (>85% of maximum predicted heart rate)
ST segment depression >1 mm (downsloping or planar depression of greater predictive value than upsloping depression)
Slow ST recovery to normal (>5 minutes)
Decrease in systolic blood pressure >20 mm Hg
Increase in diastolic blood pressure >15 mm Hg
Progressive ST segment elevation or depression
ST segment depression >3 mm without pain
Arrhythmias (atrial fibrillation, ventricular tachycardia)
Features indicative of a strongly positive exercise test
Exercise limited by angina to <6 minutes of Bruce protocol
Failure of systolic blood pressure to increase >10 mm Hg, or fall with evidence of ischaemia
Widespread marked ST segment depression >3 mm
Prolonged recovery time of ST changes (>6 minutes)
Development of ventricular tachycardia
ST elevation in absence of prior myocardial infarction
Stress echocardiography
Stress induced impairment of myocardial contraction is a sensitive marker of ischaemia and precedes electrocardiographic changes and angina. Cross sectional echocardiography can be used to evaluate regional and global left ventricular impairment during ischaemia, which can be induced by exercise or an intravenous infusion of drugs that increase myocardial contraction and heart rate (such as dobutamine) or dilate coronary arterioles (such as dipyridamole or adenosine). The test has a higher sensitivity and specificity than exercise electrocardiography and is useful in patients whose physical condition limits exercise.
Radionuclide myocardial perfusion imaging
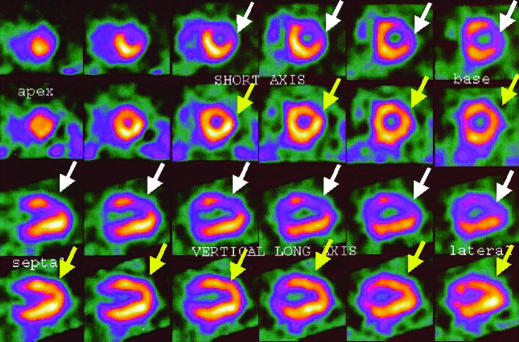
Thallium-201 or technetium-99m (99mTc-sestamibi, 99mTc-tetrofosmin) is injected intravenously at peak stress, and its myocardial distribution relates to coronary flow. Images are acquired with a gamma camera. This test can distinguish between reversible and irreversible ischaemia (the latter signifying infarcted tissue). Although it is expensive and requires specialised equipment, it is useful in patients whose exercise test is non-diagnostic or whose exercise ability is limited.
A multigated acquisition (MUGA) scan assesses left ventricular function and can reveal salvageable myocardium in patients with chronic coronary artery disease. It can be performed with either thallium scintigraphy at rest or metabolic imaging with fluorodeoxyglucose by means of either positron emission tomography (PET) or single photon emission computed tomography (SPECT).
Invasive investigations
Coronary angiography
The only absolute way to evaluate coronary artery disease is by angiography. It is usually performed as part of cardiac catheterisation, which includes left ventricular angiography and haemodynamic measurements, providing a more complete evaluation of an individual's cardiac status. Cardiac catheterisation is safely performed as a day case procedure.
Main indications for coronary angiography
Uncertain diagnosis of angina (coronary artery disease cannot be excluded by non-invasive testing)
Assessment of feasibility and appropriateness of various forms of treatment (percutaneous intervention, bypass surgery, medical)
Class I or II stable angina with positive stress test or class III or IV angina without positive stress test
Unstable angina or non-Q wave myocardial infarction (medium and high risk patients)
Angina not controlled by drug treatment
Acute myocardial infarction—especially cardiogenic shock, ineligibility for thrombolytic treatment, failed thrombolytic reperfusion, re-infarction, or positive stress test
Life threatening ventricular arrhythmia
Angina after bypass surgery or percutaneous intervention
Before valve surgery or corrective heart surgery to assess occult coronary artery disease
Patients must be fully informed of the purpose of the procedure as well as its risks and limitations. Major complications, though rare in experienced hands, include death (risk ratio 1 in 1400), stroke (1 in 1000), coronary artery dissection (1 in 1000), and arterial access complications (1 in 500). Risks depend on the individual patient, and predictors include age, coronary anatomy (such as severe left main stem disease), impaired left ventricular function, valvar heart disease, the clinical setting, and non-cardiac disease. The commonest complications are transient or minor and include arterial access bleeding and haematoma, pseudoaneurysm, arrhythmias, reactions to the contrast medium, and vagal reactions (during sheath insertion or removal).
Before the procedure, patients usually fast and may be given a sedative. Although a local anaesthetic is used, arterial access (femoral, brachial, or radial) may be mildly uncomfortable. Patients do not usually feel the catheters once they are inside the arteries. Transient angina may occur during injection of contrast medium, usually because of a severely diseased artery. Patients should be warned that, during left ventricular angiography, the large volume of contrast medium may cause a transient hot flush and a strange awareness of urinary incontinence (and can be reassured that this does not actually happen). Modern contrast agents rarely cause nausea and vomiting. 
Insertion of an arterial sheath with a haemostatic valve minimises blood loss and allows catheter exchange. Three types of catheter, which come in a variety of shapes and diameters, are commonly used. Two have a single hole at the end and are designed to facilitate controlled engagement of the distal tip within the coronary artery ostium. Contrast medium is injected through the lumen of the catheter, and moving x ray images are obtained and recorded. Other catheters may be used for graft angiography. The “pigtail” catheter has an end hole and several side holes and is passed across the aortic valve into the left ventricle. It allows injection of 30-40 ml of contrast medium over three to five seconds by a motorised pump, providing visualisation of left ventricular contraction over two to four cardiac cycles. Aortic and ventricular pressures are also recorded during the procedure.
Intravascular ultrasound (IVUS)
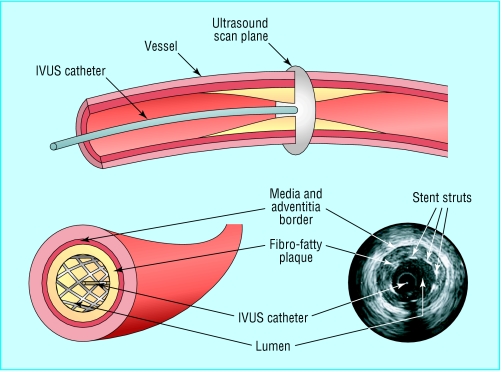
In contrast to angiography, which gives a two dimensional luminal silhouette with little information about the vessel wall, intravascular ultrasound provides a cross sectional, three dimensional image of the full circumference of the artery. It allows precise measurement of plaque length and thickness and minimum lumen diameter, and it may also characterise the plaque's composition.
It is often used to clarify ambiguous angiographic findings and to identify wall dissections or thrombus. It is most useful during percutaneous coronary intervention, when target lesions can be assessed before, during, and after the procedure and at follow up. The procedure can also show that stents which seem to be well deployed on angiography are, in fact, suboptimally expanded. Its main limitations are the need for an operator experienced in its use and its expense; for these reasons it is not routinely used in many centres.
Doppler flow wire and pressure wire
Unlike angiography or intravascular ultrasound, the Doppler flow wire and pressure wire provide information on the physiological importance of a diseased coronary artery. They are usually used when angiography shows a stenosis that is of intermediate severity, or to determine the functional severity of a residual stenosis after percutaneous coronary intervention.
Intracoronary adenosine is used to dilate the distal coronary vessels in order to maximise coronary flow. The Doppler flow wire has a transducer at its tip, which is positioned beyond the stenosis to measure peak flow velocity. The pressure wire has a tip micrometer, which records arterial pressures proximal and distal to the stenosis.
Further reading
Mark DB, Shaw L, Harrell FE Jr, Hlatky MA, Lee KL, Bengtson JR, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med 1991;325: 849-53
Marwick TH, Case C, Sawada S, Rimmerman C, Brenneman P, Kovacs R, et al. Prediction of mortality using dobutamine echocardiography. J Am Coll Cardiol 2001;37:754-60
Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography). J Am Coll Cardiol 1999;33:1756-824
Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). J Am Coll Cardiol 2001;37:1478-92
Figure.
Progression of atheromatous plaque from initial lesion to complex and ruptured plaque
Figure.
Schematic representation of normal coronary artery wall (top) and development of atheroma (bottom)
Figure.
Example of a strongly positive exercise test. After only 2 minutes and 24 seconds of exercise (according to Bruce protocol), the patient developed chest pain and electrocardiography showed marked ischaemic changes (maximum 3 mm ST segment depression in lead V6)
Figure.
99mTc-tetrofosmin perfusion scan showing reversible anterolateral wall ischaemia, induced by intravenous dobutamine infusion (white arrows). Normal rest images are shown by yellow arrows
Figure.
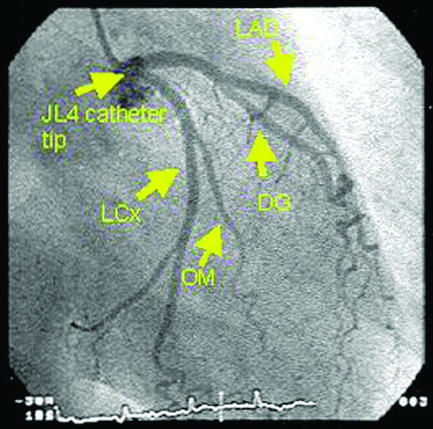
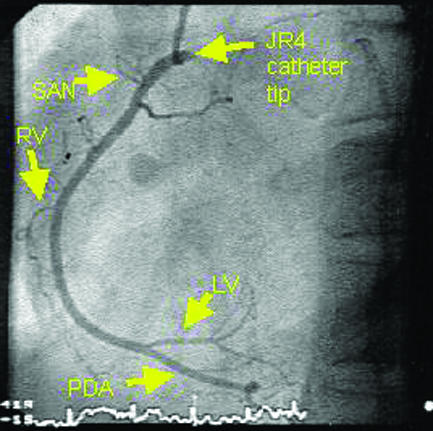
Angiograms of normal coronary arteries (LAD=left anterior descending artery, DG=diagonal artery, LCx=left circumflex artery, OM=obtuse marginal artery, SAN=sino-atrial node artery, RV=right ventricular branch artery, LV=left ventricular branch artery, PDA=posterior descending artery)
Figure.
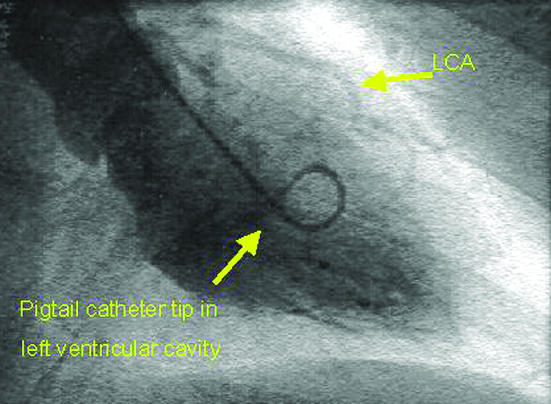
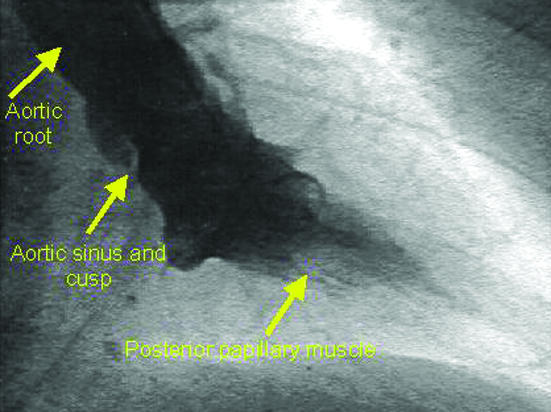
Left ventricular angiogram during diastole (top) and systole (bottom) after injection of contrast medium via a pigtail catheter, showing good contractility (LCA=left coronary artery)
Figure.
Commonly used diagnostic catheters (from left to right): right Judkins, left Judkins, multipurpose, left Amplatz, and pigtail
Figure.
The intravascular ultrasound (IVUS) catheter (above) and images showing a stent within a diseased coronary artery (below)
Figure.
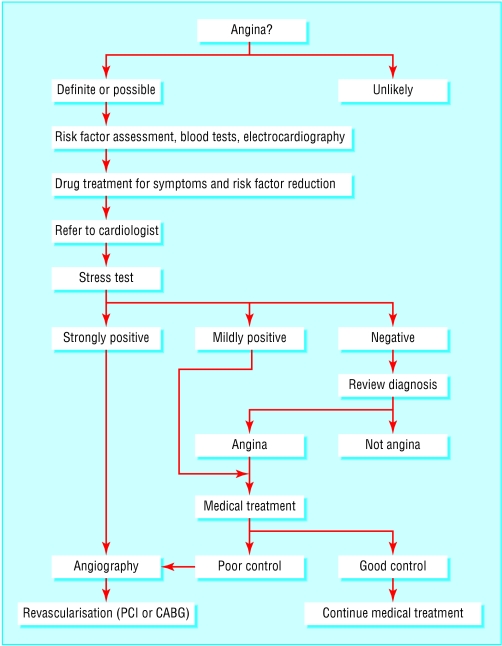
Algorithm for management of suspected angina (PCI=percutaneous coronary intervention, CABG=coronary artery bypass grafting)
Acknowledgments
The figure showing progression of atheromatous plaque from initial lesion is adapted with permission from Pepine CJ, Am J Cardiol 1998;82(suppl 10A):23-7S.
Footnotes
Ever D Grech is consultant cardiologist at the Health Sciences Centre and St Boniface Hospital, Winnipeg, Manitoba, Canada, and assistant professor at the University of Manitoba, Winnipeg.
The ABC of interventional cardiology is edited by Ever D Grech and will be published as a book in summer 2003.
Competing interests: None declared.