Abstract
Background
Ambient air pollution, including traffic-related air pollution (TRAP), increases cardiovascular disease risk, possibly through vascular alterations. Limited information exists regarding in-vehicle TRAP exposure and vascular changes.
Objective
To determine via particle filtration the effect of on-roadway TRAP exposure on blood pressure and retinal vasculature.
Design
Randomized cross-over trial.
Setting
In-vehicle scripted commutes driven through Seattle, WA traffic over 2014–2016.
Participants
Normotensive individuals aged 22–45 (n=16).
Intervention
On two days, on-road air was entrained into the vehicle. On another day, the vehicle was equipped with HEPA filtration. Participants were blinded to exposure and randomized to sequence.
Measurements
Fourteen three-minute periods of blood pressure were recorded before, during, and up to 24 hours after drive. Image-based central retinal arteriolar equivalents (CRAE) were measured pre/post. Brachial artery diameter and gene expression were measured and will be reported separately.
Results
Mean age was 29.7 years, pre-drive diastolic blood pressure (DBP) was 122.7 mmHg, systolic blood pressure (SBP) was 70.8, and drive duration was 122.3 minutes (IQR: 4). Filtration reduced particle count by 86%. Among individuals with complete data (n=13), at 1 hour, mean DBP, adjusted for pre-drive levels, order, and carryover, was 4.7 mmHg higher (95% CI: 0.9, 8.4) for unfiltered drives compared to filtered drives, and mean adjusted SBP was 4.5 mmHg higher (−1.2, 10.2). At 24 hours, adjusted mean DBP (unfiltered) was 3.8 mmHg higher (0.02, 7.5) and adjusted mean SBP was 1.1 higher (−4.6, 6.8). Adjusted mean CRAE (unfiltered) was 2.7 μm wider (−1.5, 6.8).
Limitations
Imprecise estimates due to small sample size; seasonal imbalance by exposure order.
Conclusion
Filtration of TRAP may mitigate its adverse effects on blood pressure rapidly and at 24 hours. Validation is required in larger samples and different settings.
Primary Funding source:
US Environmental Protection Agency and US National Institutes of Health
Background
Traffic related air pollution (TRAP) exposure is recognized as a risk factor for cardiovascular disease and other health effects. It has been proposed that the relationship between air pollution exposure and cardiovascular disease is mediated through inflammation and autonomic dysregulation (1). In vivo studies have shown atherosclerotic changes in response to controlled exposure (2–4). Experimental research in humans primarily relies on controlled laboratory exposures with limited generalizability.
In our prior controlled exposure research, using diesel exhaust as a proxy for TRAP exposure, we observed alteration in blood pressure during and up to 24 hours after exposure (5–7). These and other experimental results suggest acute effects of TRAP-like exposures, but the exposure concentrations typically used in these studies better reflect occupational exposures and are high compared to typical ambient on-roadway air. Additionally, the chemical components and particulate size of the mixtures used do not exactly replicate on-roadway ambient air pollution.
TRAP is a complex air pollution mixture generated by roadway sources—i.e., vehicle exhaust and brake and tire wear. Pollutants commonly measured to characterize TRAP have higher concentrations near roadways and include ultrafine particles, black carbon, oxides of nitrogen, carbon monoxide, carbon dioxide, and to a lesser extent PM2.5 (8). TRAP is the major source of air pollution contrasts within U.S. metropolitan areas.
In the United States, average travel time to work for commuters was over 27 minutes in 2019 (9). Time in traffic is associated with higher pollution exposures and has been observationally associated with increased cardiovascular risk. One study found increased odds of myocardial infraction shortly after exposure to traffic (10), but the observational design was unable to conclusively implicate TRAP in this effect due to the co-occurring traffic exposures of psychological stress and noise.
Scripted commute studies, in which volunteers are driven through traffic, are a novel approach to assess the health effects of air pollution in a realistic setting (11). To determine real-world acute changes in both blood pressure and retinal arteriolar diameter in response to on-roadway TRAP compared to filtered air, we conducted a double-blind crossover trial of passenger compartment filtration in rush-hour motor vehicle traffic.
Methods
Design Overview
The trial was a randomized-crossover trial of in-vehicle filtration during a scripted commute. Participants were randomized to order of exposure. Participants and study coordinator were blinded to exposure status. Drives occurred over November 2014 to July 2016.
The protocol was finalized prior to study initiation and not modified except as described in this paper. While this environmental intervention study was not a typical clinical trial as usually contemplated by trial registration programs at the time, we did initiate registration of this protocol with clincaltrials.gov concurrent with study onset (study number NCT05454930). Due to challenges in adapting a non-pharmaceutical intervention to the clinicaltrials.gov system, the publication of the protocol was initially made in July 2015 but was subsequently reset by the Protocol Registration and Results system, leading to the appearance of an unregistered trial. Ultimately these issues were resolved and the trial registration completed, though not prior to completion of data collection.
Settings and Participants
Participants were recruited in Seattle, WA through flyers posted on the University of Washington campus as well as through the University of Washington Institute of Translational Health Sciences research recruitment site. Inclusion criteria required that participants be aged 18–49 years. Participants additionally were screened to be free of hypertension, asthma, diabetes, hypercholesterolemia, cardiovascular disease, and any chronic medical conditions based on self-reported medical history, spirometry, fasting glucose, lipids, and electrocardiogram. Participants were excluded if they were taking antihypertensive medications. Participants were also screened to be normotensive at recruitment via blood pressure reading. To determine blood pressure at screening, we used three seated measurements of blood pressure with an arm cuff (Omron HEM-907XL; Bannockburn, IL) measured 1 minute apart after participants sat for 5 minutes. The average of the last two measurements was used to determine eligibility (inclusion criteria: >=90/60 and <=131/86 mmHg).
Additionally, at screening, potential volunteers were tested for a common genotypic single nucleotide polymorphism (SNP) in the irritant receptor gene TRPV1 (rs8065080). We selected 16 participants for the commute trial such that there were approximately equal numbers of participants in each allele group of the SNP. We had previously identified this SNP as a modifier of the effect of diesel exhaust exposure on blood pressure (6).
Our initial protocol for the scripted commute trial was for a trial with 24 participants, each with two drive sessions (one filtered and one unfiltered). Following an additional consultation with our External Scientific Advisory Committee, but prior to recruitment, the protocol was modified (IRB modification filed in October 2014) to have three drive sessions (one filtered and two unfiltered) per subject, resulting in a reduction in sample size from 24 (two drives each) to 16 (three drives each). This was the protocol ultimately followed.
Sixteen subjects initiated the drive protocol, although complete outcome data for the blood pressure endpoints is only available in 13 subjects due to failure to collect adequate outcome data (n =1 Raynaud’s syndrome, which made taking readings using the fingertip blood pressure sensor impossible), and data loss on the measurement device (n = 2). Retinal images were collected on all drives in all participants.
The Human Subjects Division of the University of Washington approved subject consent forms and the study protocol.
Randomization and Intervention
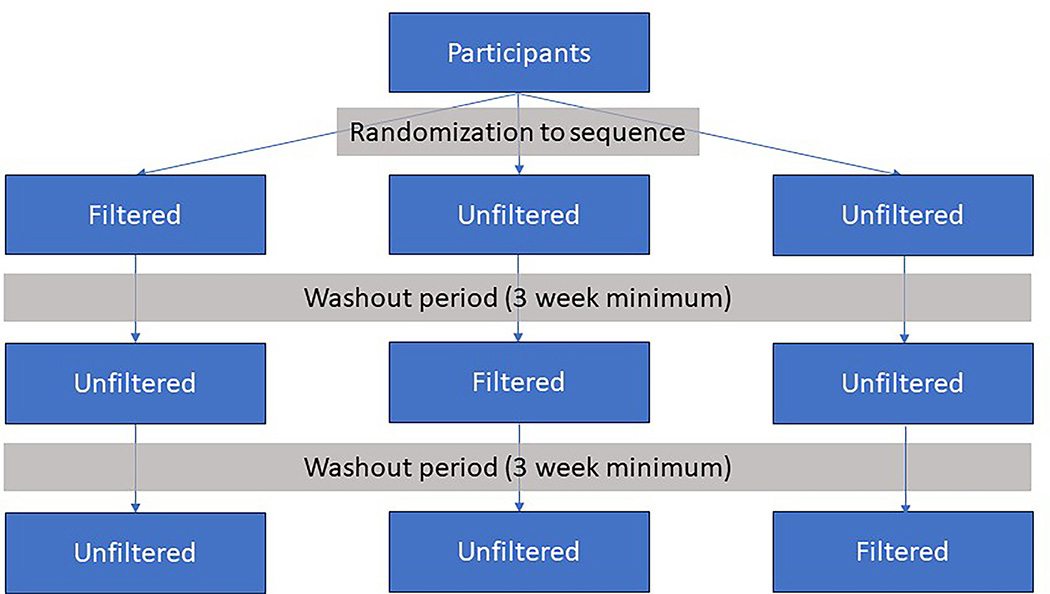
On each study day, a participant was driven through rush-hour Seattle traffic for two hours following a pre-planned route (Supplementary figure S1). These drives occurred three times for each participant and the drives were each separated by at least three weeks. Pairs of adjacent drives for the same subject took place between 21 and 218 days apart. On one drive day, the vehicle was equipped with two filtration devices: a commercially available car cabin air filter (FRAM FreshBreeze model CF10743) and a home HEPA + active carbon air purifier (Whirlpool Whispure 510) which was placed in the front seat with air directed towards the participant using a purpose-built vent and diffusor. On two days, on-road air was entrained into the cabin through the vehicle’s factory air vents with filter elements removed from both units. An overview of the randomization, sequences, and washout is visualized in Figure 1.
Figure 1: Overview of randomization.
Participants were randomized to sequence with a minimum 3 week washout period between drives. Every participant drive occurred separately.
The order of the filtered and unfiltered days was randomized prior to initiation of recruitment by generating values from a discrete uniform distribution corresponding to one of the three possible sequences (filtered-unfiltered-unfiltered, unfiltered-filtered-unfiltered, unfiltered-unfiltered-filtered) for each participant. Randomization was not designed to guarantee an equal number of sequences and was not blocked with respect to genotype or other factors.
All drives occurred on weekdays, Mondays through Thursdays, with a planned start time of 9:30 am and a duration of 2 hours. As detailed above, each participant had multiple drive days. These days were not constrained to be on the same day of the week, but no drives were scheduled on holidays. Drives occurred during all seasons of the year.
The vehicle was the same for each drive (Dodge Grand Caravan) and the driver was the same for all drives except one. The vehicle’s windows were always closed, and the car’s HVAC system was set so that recirculation was off. The air conditioning was always on, and heat was adjusted to maintain a comfortable temperature.
Participants received instructions to fast beginning at 10 pm the night prior to each study drive and again beginning at 10 pm after the drive (because additional measurements were collected at 24 hours post-drive). Participants received a pre-determined lunch shortly after each drive. This lunch was designed by the research kitchen and consisted of a sandwich consisting of turkey and Swiss cheese, chips, milk, and an apple. Dietary restrictions were accommodated such that if a participant received a substitution, that substitution was the same for every one of that participant’s drives. Specifically, vegetarians received a chickpea sandwich instead of a turkey sandwich. Individuals who did not consume dairy did not receive cheese or milk.
Blinding
Participants and study coordinator were blinded to exposure status. Research technicians set exposure status ahead of time via removing or installing filters and were responsible for monitoring in-vehicle exposure levels during the drive. These research technicians did not interact with the study participant. In order to assess the effectiveness of blinding, each day participants were asked whether they thought filtration was used, no filtration was used, or whether they did not know.
Adherence
Because the intervention was controlled by the technician, non-adherence is not strictly relevant in this design. However, we were interested in assessing the effectiveness of filtration to determine the extent to which filtration altered in-vehicle exposure concentrations. In order to assess the effectiveness of filtration, we performed continuous in-vehicle exposure measurement during drives. The exposure instruments were situated on a platform at the rear of the drive vehicle, and the sample inlet for these instruments was connected to a manifold, the inlet to which was located near the breathing zone of the study participant. This was the case for all drives.
We measured in-vehicle particle count via a TSI P-Trak 8525, black carbon (BC) via Aethlabs Micro-Aethelometer AE51, PM2.5 via a Radiance Research Nephelometer M903, and NO2 via an Aerodyne Research Cavity Attenuated Phase Shift Monitor. Nephelometry-based measurements of the particle scattering coefficient of light (bscat) were converted to PM2.5 mass based on a conversion factor specific to the Seattle-Duwamish monitoring site (an area traversed in the drive studied here) and adjusted for the wavelength used by the M903 nephelometer. The equation used was micrograms per cubic meter of PM2.5 equals , where bscat is in units of reciprocal meters.
Outcomes and Follow-up
Primary pre-specified outcomes in this protocol were blood pressure, brachial artery diameter, retinal arteriolar diameter, and gene expression. Of these endpoints, we focus here on blood pressure as it is the only one of these outcomes which has direct clinical relevance. This is also the only one of the outcomes with a long time-series of measurements, including repeated measurements during the drive and the following day. Central retinal arteriolar diameter (CRAE) is also reported in this manuscript. Other outcomes will be reported separately.
Blood pressure measurements were taken at the finger using a pulse waveform device (Finometer Pro Model-1, Finapres Medical Systems, The Netherlands) for three-minute periods. Pulse waveform measurement of blood pressure allows for beat to beat pressure assessment and has been validated for physiological research (12). We selected a finger pulse waveform device because the continuous beat-to-beat approach used permits continuous physiological assessment throughout the drive and is more robust to vibrations and noise from driving compared to brachial artery devices using discrete auscultatory/oscillometric measurement. The protocol for measurement of blood pressure was designed to increase consistency across all measurement periods. Specifically, the seat in the vehicle and the seat used for post-drive measurements were always set to the same angle. This angle was checked every day using a goniometer. Measurements were always taken on the right hand, and during the measurement periods, participants were directed to place their feet flat on the floor.
We recorded these three-minute measurement periods of continuous blood pressure at fourteen pre-specified times occurring throughout the day. One measurement period was taken immediately prior to the drive at approximately 9:30 am, nine measurement periods were taken during the two-hour drive, three measurement periods were after the drive on the same day, and a single measurement period was taken 24 hours after drive initiation. These measurement times were initiated manually by the study coordinator according to a measurement schedule defined in relation to minutes since drive start (5, 15, 30, 45, 60, 75, 90, 105, 120, 150, 300, 420, and 1440 minutes). Beat-to-beat blood pressure measurements were averaged separately over each three-minute measurement period (supplementary table S1).
Retinal photographs were taken 30 minutes before and 30 minutes after the two hour drive period. Retinal photographs were taken using a digital nonmydriatic camera (Canon CR6–45nm non-mydriatic retinal camera) and values of CRAE were calculated using the IVAN (Interactive Vessel Analyzer) software (University of Wisconsin) (13).
Statistical Analysis
The originally proposed controlled exposure design included a sample size assessment for different endpoints. Fundamentally, sample sizes were chosen empirically, based on previously conducted results from controlled exposure studies. Specifically, we had observed alterations in brachial artery diameter, finding a 0.11 mm (95% CI: 0.02, 0.18) decrease in response to diesel exhaust exposure compared to filtered air in a sample size of 22 (14). Based on these results, we chose a sample size of 24 participants and 48 sessions. Subsequently, the design of this experiment changed based on revised requirements from the EPA (see Role of the Funding Source). We did not update the sample size assessment because the number of sessions was fixed based on allocated resources. On the advice of our external science advisory committee, we modified the protocol to include two unfiltered drives (and one filtered drive) per subject, to capitalize on expected variation in ambient exposure on unfiltered days while keeping the total number of drive sessions constant at 48. Again, the sample size assessment was not updated because the number of drives was fixed based on resources available.
We estimated the effect of roadway air pollutants (unfiltered air) on blood pressure at each time interval using a single mixed effects model (per outcome). The model was specified with an interaction between exposure (filtered vs unfiltered) and categorical measurement time, with a main effect for categorical measurement time. We additionally adjusted for pre-drive measurement, drive order (categorical), participant (as a fixed effect, categorical), and carryover. Carryover was defined as the preceding exposure value (15). We included a term for drive day as a random intercept (since there were two unfiltered days) nested within participant. The entire time-course of blood pressure response was of interest, so we report all measured time points from a fully saturated model. Additional details on model formulation can be found in the statistical appendix.
The effect of unfiltered days on CRAE was estimated using a slightly altered model. Since CRAE was collected exactly twice on each day (pre-drive and post-drive), we fit a model without interaction by time and without a random effect for day within participant.
We performed a chi squared test on perception of filtration against actual exposure status. We also performed a sensitivity analysis on the blood pressure results in which we additionally adjusted for season.
Role of the Funding Source
This study was part of the University of Washington Center for Clean Air Research, which was overseen by an External Scientific Advisory Committee (ESAC). The primary funder of the Center, the US Environmental Protection Agency, had ex-officio representation on the ESAC. The funder had no role in data collection, analysis, interpretation, or writing of the study reports. The study was modified from its original design as a controlled exposure study at the request of the EPA, and the study as conducted was proposed as an alternate approach by the Center investigators and, after modifications to the protocol, was approved by the ESAC. All authors were provided complete access to the data and all authors share responsibility for the decision to submit the manuscript.
Results
Study sample and randomization
Mean age was 29.7 years (range: 22–45). Mean pre-drive blood pressure was 122.7 (IQR: 22.4) mmHg for systolic and 70.8 (IQR: 14.6) for diastolic. Mean drive duration was 122.3 minutes (IQR: 4). Sample sizes were approximately equal by gender (male, n = 9; female, n =7) and by TRPV1 (rs8065080) allele groups (C/T alleles, n = 4; C/C alleles, n = 6; T/T alleles, n = 6). Three participants were excluded from the blood pressure analysis. For one participant, blood pressure could not be measured via finger cuffs due to Raynaud’s syndrome. Two participants were excluded due to data loss when blood pressure data was inadvertently overwritten on the Finapres device.
There were differing numbers of participants receiving each drive sequence, and we observed modest imbalance by age and blood pressure with respect to drive sequence (Table 1). Mean systolic blood pressures by sequence were 115.6, 117.7, and 128.2 mmHg, with some imbalance also occurring in diastolic blood pressure. Drive characteristics including season, day of week, drive duration, and drive start time are described by filtration status in Table 2. We observed imbalance in filtration status by season; winter drives, for example, accounted for 1 of 13 filtered and 8 of 26 unfiltered drives.
Table 1:
Characteristics of participants by drive sequence. Participants were randomized to one of three drive schedules. FUU, etc. denotes the sequence of drives where FUU stands for Filtered Unfiltered Unfiltered. A total of 16 participants initiated the trial, but blood pressure data was unavailable for some or all drives for three participants, resulting in an analytic sample of 13 for the blood pressure analysis (denoted as “complete data available”). sd is the standard deviation and IQR is the interquartile range.
| FUU | UFU | UUF | ||||
|---|---|---|---|---|---|---|
| Initiated trial (n = 6) | Complete data available (n = 4) | Initiated trial (n = 2) | Complete data available (n = 2) | Initiated trial (n = 8) | Complete data available (n = 7) | |
| Age in years | ||||||
| Mean (sd) | 30.2 (8.0) | 29.3 (7.8) | 25.0 (2.8) | 25.0 (2.8) | 30.5 (9.1) | 30.7 (9.9) |
| Median (IQR) | 29.5 (13.3) | 29.0 (12.8) | 25.0 (2.0) | 25.0 (2.0) | 27.5 (10.5) | 26.0 (14.0) |
| Gender percentages | ||||||
| Male (count) | 50.0 % (3) | 50.0 % (2) | 50.0 % (1) | 50.0 % (1) | 62.5 % (5) | 57.1 % (4) |
| Female (count) | 50.0 % (3) | 50.0 % (2) | 50.0 % (1) | 50.0 % (1) | 37.5 % (3) | 42.9 % (3) |
| Average days between drives | ||||||
| Mean (sd) | 31.8 (8.0) | 33.8 (7.8) | 30.0 (2.8) | 30.0 (2.82) | 53.0 (9.1) | 47.8 (9.9) |
| Median (IQR) | 29.3 (3.3) | 31.0 (7.25) | 30.0 (5.0) | 30.0 (5.0) | 40.3 (31.8) | 38.5 (18) |
| Pre-drive systolic blood pressure in mmHg | ||||||
| Mean (sd) | 115.6 (9.7) | 117.7 (7.8) | 128.2 (11.0) | |||
| Median (IQR) | 116.3 (9.0) | 117.7 (5.5) | 122.5 (19.3) | |||
| Pre-drive diastolic blood pressure in mmHg | ||||||
| Mean (sd) | 70.3 (11.4) | 67.1 (4.1) | 72.1 (6.3) | |||
| Median (IQR) | 70.8 (18.9) | 67.1 (2.9) | 73.7 (7.3) | |||
| CRAE in microns | ||||||
| Mean (sd) | 169.1 (9.7) | 171.9 (9.7) | 159.8 (31.1) | 159.8 (31.1) | 162.2 (10.7) | 161.9 (11.5) |
| Median (IQR) | 170.1 (11.0) | 172.0 (9.4) | 159.8 (22.0) | 159.8 (22.0) | 163.8 (12.9) | 163.4 (15.0) |
Table 2:
Characteristics of drives by filtration status. Drive characteristics are listed for the 13 participants in the blood pressure analysis
| Filtered (n drives = 13) | Unfiltered (n drives = 26) | |
|---|---|---|
| Season percentages (count) | ||
| Winter | 7.7 % (1) | 30.1 % (8) |
| Spring | 46.2 % (6) | 23.1 % (6) |
| Summer | 30.1 % (4) | 15.4 % (4) |
| Fall | 15.4 % (2) | 30.8 % (8) |
| Day percentages (count) | ||
| Monday | 23.0 % (3) | 19.2 % (5) |
| Tuesday | 38.4 % (5) | 46.2 % (12) |
| Wednesday | 15.4 % (2) | 3.8 % (1) |
| Thursday | 23.1% (3) | 30.8 (8) |
| Drive duration in minutes | ||
| Mean (sd) | 121.9 (3.7) | 122.5 (4.01) |
| Median (IQR) | 120.0 (2.0) | 120.0 (4.75) |
| Drive start time percentages (9:30 am target) | ||
| Within 10 minutes of target (<= 10) | 84.6 % (11) | 84.6 % (22) |
| > 10 minutes, <= 30 minutes | 7.7 % (1) | 15.4% (4) |
| > 30 minutes < 60 minutes | 7.7 % (1) | 0 % (0) |
Filtration and in-cabin measurements
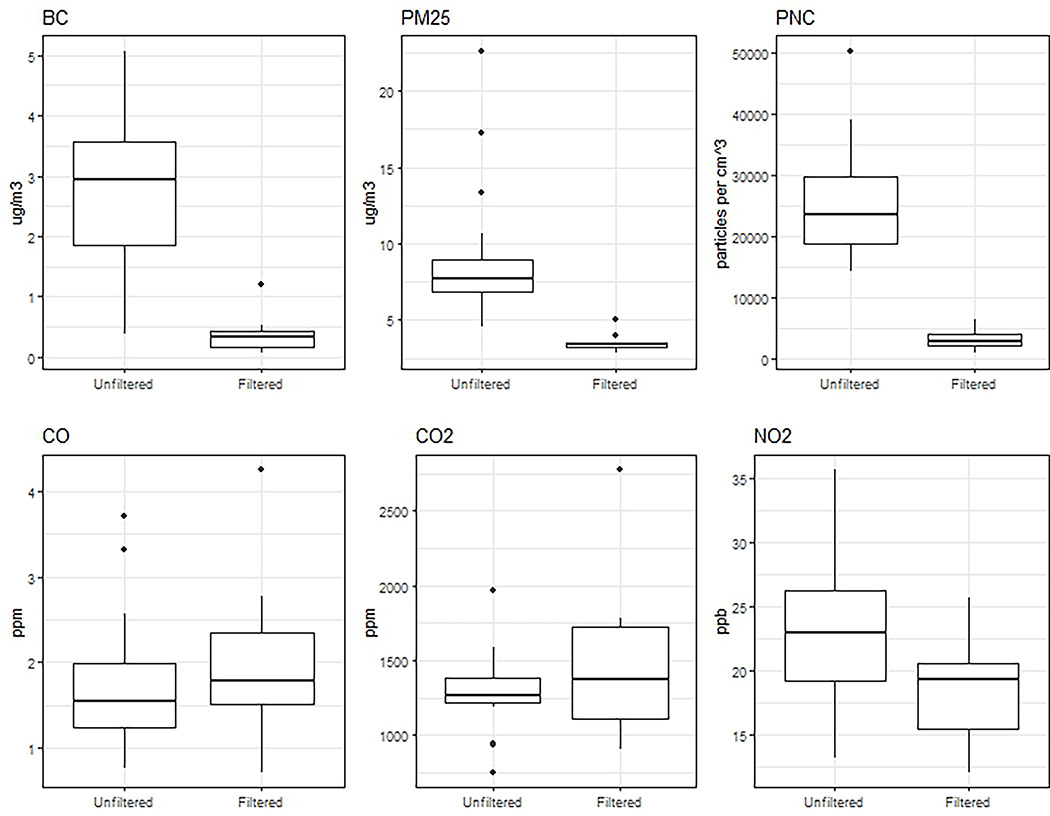
Filtration was highly effective for particles (Figure 2, Table 3). Drive-average particle number count (PNC) was reduced by 86%. Average PM2.5 concentrations was reduced by 60%, and black carbon was reduced by 86%. Filtration was ineffective for gases. NO2 concentrations were reduced by 19%. CO and CO2 were effectively unaltered. Correlations between pollutants are presented in supplementary figure S2.
Figure 2: Effectiveness of Filtration.
Distribution of drive-average (2 hour duration in traffic) pollutant measurements, comparing filtered and unfiltered days, Seattle, WA.
Table 3:
Distribution of drive-average (2 hour duration) pollutant measurements. Drives had on average 815 measurements (at 10 second frequency) each resulting in a single ~2-hour average. Pollution data was available for 37 of the 39 drives in the analytic sample. Note that the standard deviations correspond to the distribution of 2 hour averages, and not for measurements at the raw sampling frequency.
| Unfiltered | Filtered | |||
|---|---|---|---|---|
| Mean (sd) | Median (IQR) | Mean (sd) | Median (IQR) | |
| PNC (per cm3) | 25204.6 (8740.8) | 23648.3 (18790.6, 29758.5) | 3405.9 (1824.9) | 3023.5 (2289.4, 4086.9) |
| PM2.5 (μg/m3) | 8.7 (4) | 7.7 (6.9, 8.9) | 3.5 (0.6) | 3.4 (3.2, 3.5) |
| BC (μg/m3) | 2.8 (1.3) | 2.9 (1.9, 3.6) | 0.4 (0.3) | 0.3 (0.2, 0.4) |
| NO2 (ppb) | 22.9 (5.1) | 22.9 (19.2, 26.2) | 18.6 (4.4) | 19.4 (15.5, 20.6) |
| CO (ppm) | 1.7 (0.8) | 1.5 (1.2, 2) | 2 (1) | 1.8 (1.5, 2.3) |
| CO2 (ppm) | 1314.4 (282.8) | 1269 (1215.1, 1387.1) | 1491.2 (534.3) | 1374.9 (1115.9, 1724.3) |
Effect of filtration on blood pressure and CRAE
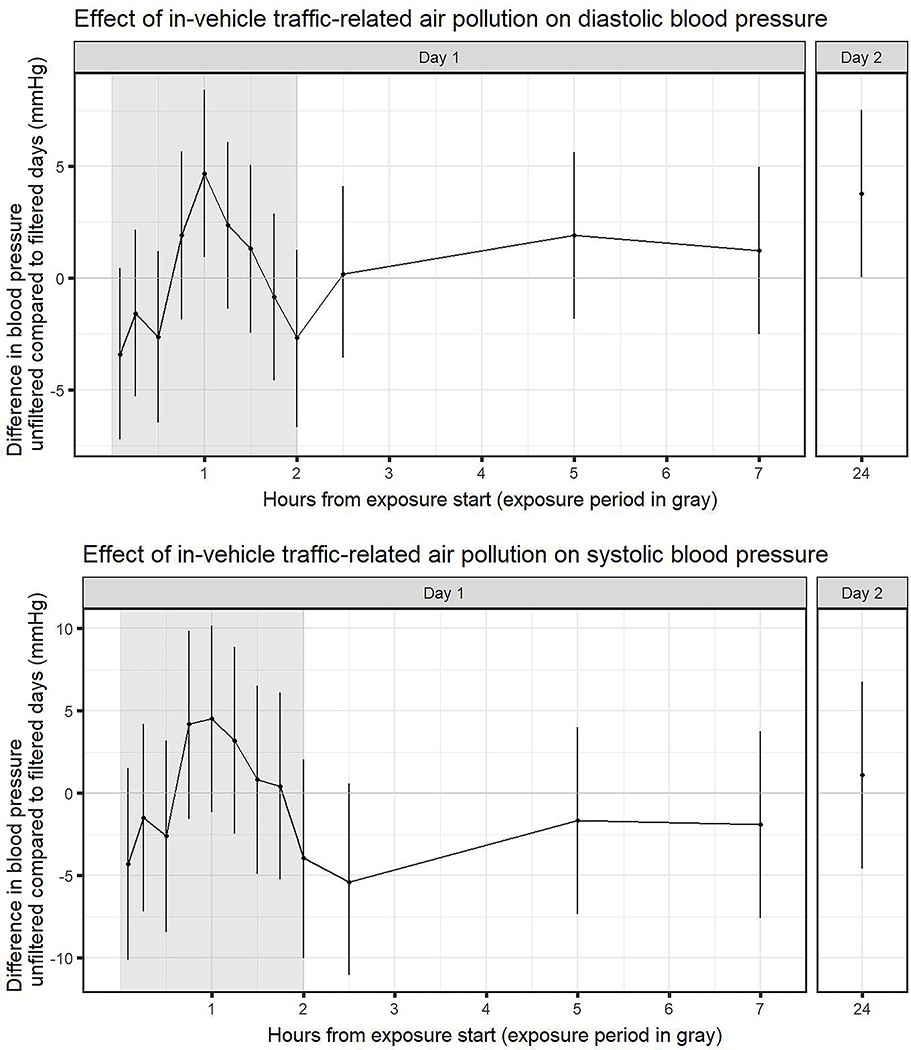
Raw mean blood pressure values generally show decreases from the pre-drive measures in both groups, with larger decreases in the filtered group (Table 4; supplementary figures S3, S4). The peak effect from the statistical analysis was observed at 1 hour after drive start for both systolic and diastolic blood pressure (Figure 3). Diastolic blood pressure at 1 hour, adjusted for pre-drive levels, drive order, participant, and carryover, was on average 4.67 mmHg higher (95% CI: 0.93, 8.42) in unfiltered drives compared to filtered drives. Adjusted systolic blood pressure was on average 4.50 mmHg higher (95% CI: −1.2, 10.18) in unfiltered drives compared to filtered drives. At approximately 24 hours, adjusted blood pressure was on average 3.77 mmHg higher (95% CI: 0.022, 7.51) for diastolic and 1.09 (95% CI: −4.58, 6.76) higher for systolic. Adjusted mean CRAE was 2.66 μm wider (CI: −1.45, 6.78) on unfiltered days compared to filtered days.
Table 4:
Mean blood pressure values and mean changes from predrive values values with 95% confidence intervals.
| Systolic (mmHg) | Diastolic (mmHg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Raw measure | Change from pre-drive | Raw measure | Change from pre-drive | |||||
| Time (minutes) | Filtered | Unfiltered | Filtered | Unfiltered | Filtered | Unfiltered | Filtered | Unfiltered |
| Pre-drive | 124.7 (116.7,132.6) | 121.7 (115.2,128.3) | 72.8 ( 67.8, 77.8) | 69.8 ( 65.9, 73.6) | ||||
| 5 | 119.3 (111.9,126.6) | 115.0 (109.7,120.4) | −4.0 ( −7.4, −0.5) | −6.7 (−11.7, −1.7) | 72.3 ( 67.6, 76.9) | 68.1 ( 64.5, 71.8) | 0.0 ( −3.8, 3.9) | −1.6 ( −3.8, 0.5) |
| 15 | 119.2 (111.0,127.3) | 117.1 (112.6,121.5) | −5.5 (−10.7, −0.3) | −4.7 ( −9.9, 0.6) | 71.7 ( 67.4, 76.1) | 69.2 ( 65.9, 72.5) | −1.1 ( −5.0, 2.8) | −0.6 ( −3.3, 2.1) |
| 30 | 122.0 (114.5,129.4) | 118.3 (114.7,122.0) | −3.1 ( −8.8, 2.6) | −3.4 ( −8.8, 2.0) | 73.9 ( 68.9, 78.9) | 70.1 ( 67.4, 72.9) | 0.6 ( −4.0, 5.3) | 0.4 ( −2.7, 3.4) |
| 45 | 116.1 (109.8,122.4) | 119.5 (114.6,124.4) | −8.5 (−14.7, −2.4) | −2.4 ( −8.3, 3.5) | 70.1 ( 66.4, 73.7) | 71.2 ( 67.8, 74.7) | −2.7 ( −6.7, 1.2) | 1.0 ( −2.3, 4.3) |
| 60 | 112.6 (105.3,120.0) | 116.5 (112.0,121.0) | −12.1 (−19.6, −4.5) | −5.2 (−12.2, 1.8) | 66.6 ( 62.5, 70.8) | 70.4 ( 67.1, 73.6) | −6.2 (−12.2, −0.1) | 0.6 ( −3.0, 4.2) |
| 75 | 116.0 (109.8,122.1) | 118.6 (114.2,122.9) | −8.7 (−13.6, −3.8) | −3.2 ( −9.9, 3.6) | 70.4 ( 66.1, 74.7) | 71.8 ( 68.6, 75.1) | −2.4 ( −7.9, 3.2) | 2.1 ( −1.6, 5.8) |
| 90 | 116.6 (111.0,122.1) | 117.0 (113.0,121.0) | −8.1 (−14.9, −1.3) | −5.3 (−11.7, 1.2) | 70.2 ( 66.3, 74.1) | 70.7 ( 68.2, 73.2) | −2.6 ( −8.0, 2.8) | 0.8 ( −2.6, 4.2) |
| 105 | 118.1 (111.2,125.1) | 118.0 (113.7,122.2) | −6.6 (−12.4, −0.8) | −3.8 (−10.4, 2.8) | 72.6 ( 68.1, 77.0) | 70.8 ( 67.9, 73.6) | −0.2 ( −5.0, 4.6) | 1.0 ( −2.5, 4.5) |
| 120 | 120.7 (112.0,129.3) | 114.0 (109.2,118.9) | −6.1 (−14.2, 2.1) | −6.8 (−13.9, 0.2) | 73.7 ( 67.2, 80.1) | 69.1 ( 66.1, 72.2) | −0.5 ( −6.4, 5.4) | −0.6 ( −4.9, 3.7) |
| 150 | 121.3 (114.9,127.8) | 116.2 (111.6,120.7) | −3.5 (−10.4, 3.4) | −5.6 (−11.2, 0.0) | 69.3 ( 64.3, 74.4) | 69.4 ( 65.8, 72.9) | −2.8 ( −7.6, 2.1) | −0.4 ( −4.3, 3.4) |
| 300 | 119.6 (110.4,128.7) | 117.3 (112.9,121.6) | −5.1 (−14.3, 4.0) | −4.5 (−10.4, 1.5) | 67.0 ( 61.8, 72.2) | 68.0 ( 64.4, 71.5) | −5.8 ( −8.7, −2.8) | −1.8 ( −5.7, 2.1) |
| 420 | 121.1 (113.4,128.7) | 118.6 (113.6,123.5) | −3.6 (−11.9, 4.7) | −3.2 ( −8.7, 2.3) | 70.9 ( 65.5, 76.4) | 71.2 ( 67.6, 74.8) | −1.9 ( −6.5, 2.8) | 1.4 ( −2.1, 5.0) |
| 1440 | 112.0 (104.8,119.3) | 112.5 (106.6,118.5) | −12.6 (−23.6, −1.6) | −9.2 (−16.8, −1.6) | 65.7 ( 60.7, 70.7) | 68.5 ( 65.0, 72.0) | −7.1 (−11.4, −2.9) | −1.3 ( −5.3, 2.7) |
Direct comparison of treatment-specific confidence intervals from this table does not reflect the within-individual study design and statistical adjustments accounted for in the main results.
Figure 3: Effect of unfiltered TRAP on blood pressure.
Modeled estimates and 95% confidence intervals of blood pressure comparing unfiltered days to filtered days, adjusted for pre-drive blood pressure. Models were estimated using data from 39 drives by 13 individuals.
We found no carryover effect (t values: diastolic, 0.24; diastolic, −0.93; CRAE, 0.30). For the perception of filtration survey, participants responded “do not know” for 26 out of 39 drives, and were ineffective at correctly guessing exposure (days where participants did guess: χ2= 0.17, ν = 1). Results were robust to seasonal adjustment (supplementary table S2).
Discussion:
In our small crossover trial of in-vehicle filtration of traffic-related air pollution (TRAP), we found that drives in vehicles with unfiltered TRAP resulted in net increases of blood pressure of more than 4.5 mmHg when compared to drives with in-vehicle filtration although estimates were imprecise and the confidence intervals for the increases included zero at various times points. Changes in blood pressure occurred rapidly—peaked within 60 minutes--and persisted over 24 hours. In-vehicle filtration of TRAP did not alter CRAE.
There has been increasing interest in filtration research designs (16). Most filtration studies are set in-home, where ambient air pollution levels are relatively low (17,18), with fewer studies set near roadways. A cross-over trial of filtration of TRAP set in rooms near a roadway found a 2.8 mmHg change in blood pressure at 20 minutes for a PNC of 30,000 particles/cm3 (n = 77) (17), motivating an upcoming trial of residential filtration on blood pressure (19). Randomized cross-over studies found facemasks decreased on-roadway blood pressure, although the unblinded designs limits interpretability (n = 15, n = 24) (20,21). A randomized cross-over trial of TRAP, also found blood pressure effects, but relied on unblinded location for exposure (n = 28) (22). A randomized crossover on-roadway study of facemasks was blinded via sham-control; this study found differences in exhaled nitric oxide and in one arterial stiffness indicator (n = 15) (23).
Our study is unique in studying the effect of in-vehicle filtration on blood pressure. A prior study using a randomized sham-controlled crossover design assessed the acute effects of in-vehicle filtration on heart rate, finding increases in high-frequency power HRV, but did not report post-exposure blood pressure (n = 48) (11). Our current study extends to a real-world setting our previous controlled exposure laboratory findings. For a controlled exposure of freshly generated, diluted, aged diesel exhaust, we found similar magnitude blood pressure changes as the current study (4.4 mmHg increase combining 30-minute and 90-minute readings, 95% CI: 1.1–7.7) (n = 45) (5). This study had considerably higher PM2.5 mass concentrations (200 μg/m3), but PNC was on the same order of magnitude (53,000 particles/cm3), suggesting the importance of PNC (generally reflecting the smallest particles--i.e., ultrafine particles). The time-response of blood pressure observed in the present study is consistent with prior studies where we identified changes at 60 minutes (5–7) and 24 hours after exposure start (7).
Air pollution-induced changes in blood pressure are thought to occur partly due to autonomic alterations (1). Our prior diesel exhaust study found that blood pressure response is modified by an Alpha 1 Adrenergic Blockade (n = 20) (7). Additionally, the irritant receptor TRPV1 may in part mediate air pollution-induced autonomic alterations (6,24). We examined CRAE to assess microvasculature effects, since arterioles are key resistance vessels. However, retinal vessels are atypical--lacking autonomic innervation and being regulated through local factors (25)--which may explain the divergent results.
The ineffectiveness of the studied filtration system for gaseous pollutants implicates the particle component of TRAP in observed effects. We are unable to distinguish specific causal pollutants, since filtration reduced all measures of particulates--though PM2.5 was least reduced. Ultrafine particles (measured as particulate count) and black carbon are not regulated under EPA standards nor WHO air quality guidelines. PM2.5 concentrations were consistently below current United States regulatory limits. These results suggest the potential for particle filtration to mitigate the acute effects of air pollution exposure, and justifies further research into the effects of filtration in other acute and long-term exposure settings.
Several limitations should be considered when interpreting our results. Blood pressure measured using finger pulse waveforms may more strongly reflect specific hemodynamic changes than conventional brachial artery measurement, although good estimates of brachial blood pressure can be reconstructed from measurements taken using Finapres devices. The equipment in this study dates from 2014–2016, though reflects current filtration and measurement technology and on-road pollution characteristics. Our study was small, included healthy participants, and had genotype-stratified recruitment--all of which limits generalizability. The small sample size and long periods between drives meant that randomization did not balance all time-varying variables such as season, leading to potential confounding. A sensitivity analysis adjusting for season did not appreciably alter the results, but further research is needed to confirm our findings in different settings and larger populations. Finally, the clinical implications of transient changes in blood pressure are not well understood, and further research is also needed to determine whether these changes might contribute to acute risk of cardiovascular disease events or long-term vascular alterations.
Conclusion
In a realistic on-road exposure trial, we found that unfiltered traffic-related air pollutant exposure may result in net increases in blood pressure that occurred and peaked within 60 minutes and are sustained at 24 hours when compared to filtered exposure suggesting that the effects of air pollution on blood pressure may be reduced with effective cabin air filtration. Our study reinforces existing literature suggesting that traffic-derived particulate matter may be implicated in hypertension.
Supplementary Material
Funding
Research reported in this (publication/press release) was supported by the University of Washington EDGE Center of the National Institutes of Health under award number: P30ES007033. This publication was made possible by USEPA grant (RD-83479601–0). The content is solely the responsibility of the grantee and do not necessarily represent the official views of the funding agencies. Further, the funding agencies do not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Protocol details and the analytical dataset can be made available through written agreements with the authors.
Statistical code is available to interested readers by contacting M. Young at myoung3@uw.edu.
Contributor Information
Michael T Young, Department of Environmental and Occupational Sciences, University of Washington, Seattle, WA.
Karen Jansen, Department of Environmental and Occupational Sciences, University of Washington, Seattle, WA.
Kristen E Cosselman, Department of Environmental and Occupational Sciences, University of Washington, Seattle, WA.
Timothy R Gould, Department of Civil and Environmental Engineering, University of Washington, Seattle, WA, USA.
James A Stewart, Department of Environmental and Occupational Health Sciences, University of Washington, Seattle, WA.
Timothy Larson, Department of Civil and Environmental Engineering, University of Washington, Seattle, WA, USA; Department of Environmental and Occupational Sciences, University of Washington, Seattle, WA.
Coralynn Sack, Department of Medicine, University of Washington, Seattle, WA; Department of Environmental and Occupational Sciences, University of Washington, Seattle, WA.
Sverre Vedal, Department of Environmental and Occupational Sciences, University of Washington, Seattle, WA.
Adam A Szpiro, Department of Biostatistics, University of Washington, Seattle, WA.
Joel D Kaufman, Department of Environmental and Occupational Sciences, University of Washington, Seattle, WA; Department of Medicine, University of Washington, Seattle, WA; Department of Epidemiology, University of Washington, Seattle, WA.
References
- 1.Brook RD, Rajagopalan S, Pope CA, et al. Particulate Matter Air Pollution and Cardiovascular Disease An Update to the Scientific Statement From the American Heart Association. Circulation. 2010;121(21):2331–2378. doi:Hoe [DOI] [PubMed] [Google Scholar]
- 2.Araujo Jesus A, Berenice Barajas, Michael Kleinman, et al. Ambient Particulate Pollutants in the Ultrafine Range Promote Early Atherosclerosis and Systemic Oxidative Stress. Circ Res. 2008;102(5):589–596. doi: 10.1161/CIRCRESAHA.107.164970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai N, Kido T, Suzuki H, et al. Changes in atherosclerotic plaques induced by inhalation of diesel exhaust. Atherosclerosis. 2011;216(2):299–306. doi: 10.1016/j.atherosclerosis.2011.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T, Jia G, Wei Y, Li J. Beijing ambient particle exposure accelerates atherosclerosis in ApoE knockout mice. Toxicol Lett. 2013;223(2):146–153. doi: 10.1016/j.toxlet.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 5.Cosselman KE, Krishnan R, Oron AP, et al. BLOOD PRESSURE RESPONSE TO CONTROLLED DIESEL EXHAUST EXPOSURE IN HUMAN SUBJECTS. Hypertension. 2012;59(5). doi: 10.1161/HYPERTENSIONAHA.111.186593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosselman K, Krishnan RM, Oron AP, et al. Abstract 17098: Blood Pressure Response to Controlled Diesel Exhaust Inhalation in Human Subjects is Modified by Functional Variation in TRPV1. Circulation. 2011;124(suppl_21):A17098–A17098. doi: 10.1161/circ.124.suppl_21.A17098 [DOI] [Google Scholar]
- 7.Cosselman KE, Jansen K, Sack C, Larson TV, Kaufman JD. Abstract 20747: Systolic Blood Pressure Response is Eliminated by Alpha 1 Adrenergic Blockade in Human Subjects. Circulation. 2016;134(suppl_1):A20747–A20747. doi: 10.1161/circ.134.suppl_1.20747 [DOI] [Google Scholar]
- 8.Near-Roadway Air Quality: Synthesizing the Findings from Real-World Data | Environmental Science & Technology. Accessed August 30, 2023. https://pubs.acs.org/doi/10.1021/es100008x [DOI] [PubMed]
- 9.Burd C, Burrows M, McKenzie B. Travel Time to Work in the United States: 2019,” American Community Survey Reports. US Censu Bur Wash DC ACS-47. Published online 2021. [Google Scholar]
- 10.Peters A, von Klot S, Heier M, et al. Exposure to Traffic and the Onset of Myocardial Infarction. N Engl J Med. 2004;351(17):1721–1730. doi: 10.1056/NEJMoa040203 [DOI] [PubMed] [Google Scholar]
- 11.Mallach G, Shutt R, Thomson EM, Valcin F, Kulka R, Weichenthal S. Randomized Cross-Over Study of In-Vehicle Cabin Air Filtration, Air Pollution Exposure, and Acute Changes to Heart Rate Variability, Saliva Cortisol, and Cognitive Function. Environ Sci Technol. 2023;57(8):3238–3247. doi: 10.1021/acs.est.2c06556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guelen I, Westerhof BE, van der Sar GL, et al. Validation of brachial artery pressure reconstruction from finger arterial pressure. J Hypertens. 2008;26(7):1321–1327. doi: 10.1097/HJH.0b013e3282fe1d28 [DOI] [PubMed] [Google Scholar]
- 13.Yip W, Tham YC, Hsu W, et al. Comparison of Common Retinal Vessel Caliber Measurement Software and a Conversion Algorithm. Transl Vis Sci Technol. 2016;5(5):11. doi: 10.1167/tvst.5.5.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peretz A, Sullivan JH, Leotta DF, et al. Diesel Exhaust Inhalation Elicits Acute Vasoconstriction in Vivo. Environ Health Perspect. 2008;116(7):937–942. doi: 10.1289/ehp.11027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones B, Kenward MG. Design and Analysis of Cross-Over Trials. 3rd edition. Chapman and Hall/CRC; 2014. [Google Scholar]
- 16.Newman JD, Bhatt DL, Rajagopalan S, et al. Cardiopulmonary Impact of Particulate Air Pollution in High-Risk Populations. J Am Coll Cardiol. 2020;76(24):2878–2894. doi: 10.1016/j.jacc.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudda N, Eliasziw M, Hersey SO, et al. Effect of Reducing Ambient Traffic-Related Air Pollution on Blood Pressure: A Randomized Crossover Trial. Hypertens Dallas Tex 1979. 2021;77(3):823–832. doi: 10.1161/HYPERTENSIONAHA.120.15580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen R, Zhao A, Chen H, et al. Cardiopulmonary Benefits of Reducing Indoor Particles of Outdoor Origin. J Am Coll Cardiol. 2015;65(21):2279–2287. doi: 10.1016/j.jacc.2015.03.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugge D, Lerman Ginzburg S, Hudda N, et al. A randomized crossover trial of HEPA air filtration to reduce cardiovascular risk for near highway residents: Methods and approach. Contemp Clin Trials. 2021;108:106520. doi: 10.1016/j.cct.2021.106520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langrish JP, Mills NL, Chan JK, et al. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Part Fibre Toxicol. 2009;6(1):8. doi: 10.1186/1743-8977-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J, Lin Z, Chen R, et al. Cardiovascular Benefits of Wearing Particulate-Filtering Respirators: A Randomized Crossover Trial. Environ Health Perspect. 2017;125(2):175–180. doi: 10.1289/EHP73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubesch N, De Nazelle A, Guerra S, et al. Arterial blood pressure responses to short-term exposure to low and high traffic-related air pollution with and without moderate physical activity. Eur J Prev Cardiol. 2015;22(5):548–557. doi: 10.1177/2047487314555602 [DOI] [PubMed] [Google Scholar]
- 23.Guan T, Hu S, Han Y, et al. The effects of facemasks on airway inflammation and endothelial dysfunction in healthy young adults: a double-blind, randomized, controlled crossover study. Part Fibre Toxicol. 2018;15(1):30. doi: 10.1186/s12989-018-0266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, Gonzalez-Flecha B. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol Sci Off J Soc Toxicol. 2008;102(2):328–336. doi: 10.1093/toxsci/kfn005 [DOI] [PubMed] [Google Scholar]
- 25.Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32(6):249–256. doi:55622 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.