Abstract
Background:
Lower extremity trauma can have a significant impact on a patient’s quality of life. The LIMB-Q is a recently developed and validated patient-reported outcome measure that assesses patient-specific outcomes and experience of health care. The aim of this study was to translate and linguistically validate the LIMB-Q from English to German.
Methods:
The translation was performed by combining World Health Organization and Professional Society for Health Economics and Outcomes Research guidelines. The process consisted of forward translations, a backward translation, expert panel meetings, cognitive debriefing interviews with patients, and several rounds of discussion and reconciliation with the creators of LIMB-Q. The goal was to obtain a culturally and conceptually accurate translation of LIMB-Q into German for use in Switzerland.
Results:
From the two forward translations, there was one primary discrepancy between the two translators that was discussed to determine the most conceptually accurate translation. From the backward translations, there were 63 items that required discussion and re-translation. Nine patients participated in the cognitive debriefing interviews, which led to three items being modified. The translation process led to a linguistically validated and conceptually equivalent German version of the LIMB-Q.
Conclusions:
The German (Switzerland) version of LIMB-Q is now available. This will offer a valuable tool for lower extremity trauma research and clinical care in German-speaking populations.
Takeaways
Question: We aimed to translate the LIMB-Q (a lower extremity trauma patient-reported outcome measure) into German, using International Society for Pharmacoeconomics and Outcomes Research (ISPOR) best-practice guidelines.
Findings: The LIMB-Q was successfully translated into German.
Meaning: The German version of LIMB-Q is now available as a tool for lower extremity trauma research and clinical care.
INTRODUCTION
Lower extremity traumatic injuries can have a significant impact on patients. These injuries are complex and can impact patients’ quality of life and well-being in various ways.1,2 Treatment is dependent on the injury, but it often includes tissue debridement; fracture reduction and fixation; and, in severe injuries, soft tissue reconstruction with a flap or amputation of the limb.3,4 Given the significant impact of lower extremity traumatic injuries on patients’ lives, it becomes imperative to fully understand and integrate the patient perspective into care. This need is particularly acute in German-speaking regions because there is a growing emphasis on integrating patient-reported outcome measures (PROMs) into their healthcare systems.5–11
PROMs are becoming an essential component of patient-centered care. They ensure that the patient’s perspective is not only captured but also valued and considered integral to the treatment process. Generic PROMs (eg, SF-36 or EQ-5D) are designed to capture the general quality of life and satisfaction outcomes that can be compared across disease groups, such as comparing the quality of life between patients with diabetes and lung disease.11–14 Alternatively, condition- or disease-specific PROMs capture outcomes that are determined to be relevant to a specific population of patients. Disease-specific PROMs have content validity; that is, they have been developed specifically to ensure that they measure outcomes relevant to a specific patient population in a comprehensive manner.15,16 The use of specific PROMs ensures that the data collected is reflective of issues and concepts that are important to patients with a given disease or injury pattern.
To meet this need for lower extremity trauma patients, the LIMB-Q was developed.8,9,17,18 Conceived and validated following international standards for PROM development, the LIMB-Q is available for adult patients presenting with injuries distal to the mid-femur requiring fracture surgery, soft tissue reconstruction with a flap, and/or amputation.2,19,20 Details of the LIMB-Q development and validation are published elsewhere but briefly summarized here.21 Qualitative interviews were performed with 33 lower extremity trauma patients, which informed the development of the preliminary scales. Cognitive debriefing interviews were then performed with 12 patients, and expert opinion was obtained from 43 experts, which allowed for further refinement of the LIMB-Q. Finally, an international field test recruited 713 patients, leading to the finalized LIMB-Q with 164 items across 16 independently functioning scales. To date, the LIMB-Q is the only PROM that has been thoroughly developed to address the entire spectrum of care for patients with lower extremity trauma, including those who have undergone amputation or soft tissue reconstruction.4
The aim of this study was to translate and linguistically validate the LIMB-Q into German, adhering to international standards for PROM translations. Having reliable and valid instrument for assessing the outcomes and experiences of German-speaking patients with traumatic lower limb injuries is vital for enhancing care quality and guiding future treatment strategies.
METHODS
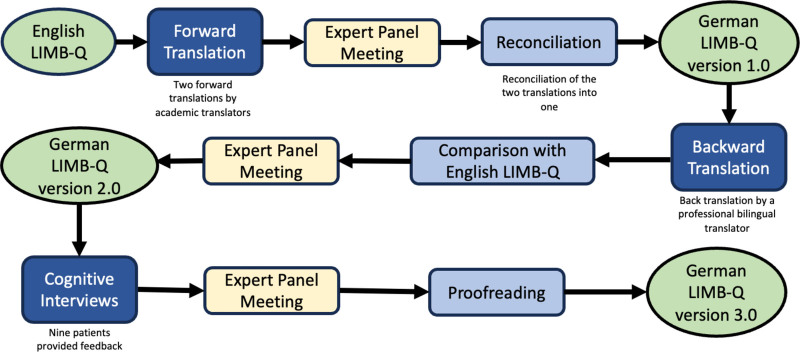
We used the World Health Organization (WHO) and the Professional Society for Health Economics and Outcomes Research (ISPOR) guidelines to conduct this translation.20,22 This study was approved by the Kantonal Ethics Committee of Zurich, Switzerland (BASEC No. 2022-00232). Figure 1 depicts our translation process.
Fig. 1.
A figure that shows our translation process.
Forward Translation
Two academic translators performed independent forward translations of the field-test version of the LIMB-Q scales. Both translators were native German speakers and fluent in English. At an expert panel meeting, the two translations were compared, and discrepancies were discussed and resolved. The two translations were reconciled into one translation. The final forward translation produced was the German LIMB-Q version 1.0.
Backward Translations
A translator bilingual in German and English performed the backward translation of German LIMB-Q version 1.0. This backward translation was compared with the English version of LIMB-Q. At an expert panel meeting, all discrepancies were noted and discussed with the LIMB-Q developers. Following the meeting, items were re-translated and discussed until a consensus was reached, leading to the German LIMB-Q version 2.0.
Cognitive Debriefing Interviews
Cognitive debriefing interviews with patients were performed to assess the relevancy and clarity of the translated LIMB-Q. Nine patients with various lower extremity injuries were recruited to participate. Each patient completed all appropriate scales of the LIMB-Q. This process involved face-to-face interviews. The patients were methodically guided to answer all LIMB-Q items, and to identify any items that were challenging to understand. For any items deemed difficult, patients were encouraged to propose alternative wordings for the question or make improvement suggestions. Any difficulties encountered and solutions proposed were recorded in an Excel spreadsheet. Consistency was maintained throughout the process, with all interviews conducted in the same manner and by the same individual. Findings were discussed with LIMB-Q developers at an expert panel meeting, and discussion continued until satisfactory results were achieved, leading to the German LIMB-Q version 3.0.
RESULTS
Forward and backward translations were conducted from January 1, 2021 to March 17, 2021, followed by cognitive debriefing interviews and several rounds of discussion for reconciliation. Subsequently, a final version of the German (Switzerland) LIMB-Q was produced.
Forward Translation
The primary discrepancy was the terminology for the term “lower limb.” The first translator used the German phrase for “lower limb” (Gliedmaße), whereas the second used the German phrase for “lower extremity” (Extremität). After discussions, the term “Gliedmaße” was selected because it was determined to be more colloquially used than the term “Extremität” to describe the lower limb.
Backward Translations
A comparison of the back translation of the German version and the original English version highlighted 63 items that differed, required discussion, and resulted in alterations. These differences could be categorized into three types: rewording from a yes/no item to work with the response options (n = 30), rewording for meaning and cultural accuracy (n = 30), rewording to include parts that were missing after the back-translation (n = 6). (See table, Supplemental Digital Content 1, which displays a table that shows the variations in the forward translation, backward translation, and the cognitive debriefing interviews. http://links.lww.com/PRSGO/D361.)
There were several instances of items needing to be reworded from a yes/no question into statements. Most of these items occurred in the “Sexual Function” and “Work Function” scales. The structure of the scale was an overarching stem that asked how strongly the respondents agreed with the following statements, four response options (“definitely disagree,” “somewhat disagree,” “somewhat agree,” “definitely agree”), and a list of statements. The German LIMB-Q 1.0 had translated a statement into a yes/no question. For example, “Had to reduce the amount of work you do in a day” was translated into “Did you have to reduce the amount of work you do in one day?” All items that were yes/no questions were reworded into statements that allowed respondents to agree or disagree on a scale, consistent with the original LIMB-Q.
All the rewording discrepancies were resolved through discussion to ensure cultural accuracy. For example, changes included re-translations of “insecure” to “self-conscious,” “bothering” to “interfering,” and “treatment” to “care.” Instances of the German LIMB-Q 1.0 missing parts of the backtranslation were resolved by adding the missing adjective or adverb. There was one instance of a cultural difference in translation. “Exercise” was back-translated into “sport activities.” Discussion centered on the fact that there did not seem to be a German word for “exercise” and the German word “sport” should be avoided to ensure the right conceptual translation. Ultimately, the statement was rephrased to “physical activity” after discussion during the expert panel meeting. Following this, the German LIMB-Q version 2.0 was produced.
Cognitive Debriefing Interviews
A varied sample of nine patients participated in the cognitive debriefing interviews (Table 1). There were three translations that were revised to enhance clarity. For example, in a response option, patients found it difficult to distinguish between “ein bisschen,” which was used as a translation for “a little,” and “massig,” which was used as a translation for “moderate.” “Ein bisschen” was changed to “eher” to better reflect the gradient of choices used to describe difficulty with quality-of-life task. Following the cognitive interviews and reconciliation, the German LIMB-Q version 3.0 was produced. Minor grammatical mistakes, spelling, and punctuation were corrected.
Table 1.
Patient Demographics
| n | |
|---|---|
| Gender | |
| Male | 5 |
| Female | 4 |
| Age, y, mean (range) | 49.1 (34–71) |
| Type of injury | |
| Fracture | 3 |
| Infection | 2 |
| Burn | 2 |
| Chronic ulcer | 1 |
| Other | 1 |
| Location of injury | |
| Foot | 4 |
| Leg | 3 |
| Foot and leg | 1 |
| Not specified | 1 |
| Laterality | |
| Unilateral | 7 |
| Bilateral | 2 |
| Treatment outcomes | |
| Free flap | 6 |
| Amputation | 2 |
| Not specified | 2 |
DISCUSSION
In this study, we translated the LIMB-Q, a new PROM, from English to German according to WHO and ISPOR guidelines.22–24 Our process involved several rounds of translation, patient interviews, and expert panel meetings. Feedback from the LIMB-Q development team and native German speakers ensured a culturally sensitive and accurate adaptation of the LIMB-Q. The final German (Switzerland) LIMB-Q consists of 16 scales with 164 items, consistent with the English (United States) version.
Conceptual and cultural accuracy was ensured through adherence to WHO and ISPOR guidelines. WHO guidelines encourage conceptual translation over literal translation and emphasize the utility of expert panels to resolve discrepancies and of cognitive debriefing interviews to include the patients’ perspectives. For example, during the backward translation process, there were 30 items that required discussion and rewording. Although many of these changes were a one-word change, this fine-tuning was critical to ensure cultural accuracy and a conceptual translation. In addition, ISPOR guidelines outlined specific recommendations for the translation process, including having two independent forward translations and having five to eight patients participate in the cognitive debriefing interviews. These guidelines have been successfully used in the Danish translation of LIMB-Q and in translations of other PROMs.4,20,25
The Danish version of the LIMB-Q was successfully translated through a similar rigorous process.22 The Danish LIMB-Q was validated through a forward translation, backward translation, an expert panel meeting, two rounds of cognitive interviews, and harmonization. Conceptual and cultural accuracy were heavily emphasized throughout the whole process. For example, during the forward translation, performed by a professional translator and a clinician, the primary discrepancy was the translation of the term “lower limb.” As the term “lower limb” does not exist in the Danish language, the clinician used “lower extremity,” whereas the professional translator used “lower leg.” The developers decided to add examples [eg, “leg (eg, foot, ankle, lower leg, knee, thigh)”] to clarify the term. In addition, following the cognitive debriefing interviews, several items were removed for problematic translations. Ultimately, Danish patients found the final version of the Danish LIMB-Q to be relevant to them. In addition to German and Danish, the LIMB-Q has been translated into Dutch, and additional language translations are ongoing.
Like the Danish LIMB-Q, the German LIMB-Q will serve as an important adjunct of care for German speakers. There are around 130 million people who speak German as their native language, and it is the official language of Germany, Austria, Belgium, Luxembourg, Switzerland, and Liechtenstein.26 Across these countries, there is a growing trend toward integrating PROMs into clinical assessments to improve the quality and efficiency of their healthcare systems.10,11 The relevance of a lower extremity PROM is even more pronounced due to the rising incidence of lower extremity fractures in Germany.10 Certain types of fractures, such as distal femur fractures, have seen increases as much as 30% over a 10-year period.10 Furthermore, Switzerland, which has one of the longest life expectancies in the world and the highest health expenditure per capita, stands uniquely positioned to benefit from a lower extremity PROM.27 This tool will offer a deeper understanding of the quality of lives of patients who have experienced lower extremity injuries, contributing to healthcare advancements in several European countries.
There are limitations to this study. The panel discussions and cognitive debriefing sessions were not recorded, limiting our ability to precisely analyze the decision-making process behind changes in the translations. However, notetakers were used in each step of the process. Findings were recorded in a spreadsheet during these sessions and were easy to reference. We conducted only one round of cognitive debriefing interviews, potentially limiting the representation of patients who may be affected by lower extremity trauma. However, we interviewed nine patients, which exceeds the ISPOR recommendation of interviewing a minimum of five patients.22 The nine patients interviewed represented a diverse range of injury causes and locations, and we reached a point of content saturation where no additional translation concerns were detected.
Finally, the purpose of this study was to ensure a culturally relevant and accurate PROM for German speakers, which meant we could only edit or remove existing scales. The LIMB-Q was field-tested in an international cohort of patients from over 20 countries, optimizing its relevance to German-speaking patients.1 However, because the scales were developed in the United States and Canada, there could be additional metrics that would be more relevant or culturally specific to German speakers that are not included in the current set of scales.
CONCLUSIONS
The LIMB-Q is now available in German for use in research and clinical care (QPortfolio.org). It is available upon request, subject to completion of a licensing agreement. The German (Switzerland) LIMB-Q was created through adherence to WHO and ISPOR guidelines to ensure it was conceptually and culturally accurate. The LIMB-Q may be used to capture outcomes related to quality of life and satisfaction in German-speaking patients with lower extremity injuries below the mid-femur.
DISCLOSURES
Drs. Klassen and Mundy are co-developers of the LIMB-Q and could potentially receive a share of any license revenue on the inventor sharing policies from the institutions that own the LIMB-Q. Nicole Lindenblatt acts as consultant and scientific advisor for Medical Microinstruments. All the other authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 19 July 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Drs. Besmens and Lindenblatt contributed equally to this work.
REFERENCES
- 1.Mundy LR, Grier AJ, Weissler EH, et al. Patient-reported outcome instruments in lower extremity trauma: a systematic review of the literature. Plast Reconstr Surg Glob Open. 2019;7:e2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mundy LR, Klassen AF, Grier J, et al. Development of a patient-reported outcome instrument for patients with severe lower extremity trauma (LIMB-Q): protocol for a multiphase mixed methods study. JMIR Res Protoc. 2019;8:e14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devendra A, Nishith PG, Dilip Chand Raja S, et al. Current updates in management of extremity injuries in polytrauma. J Clin Orthop Trauma. 2021;12:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonsen NV, Rölfing JD, Mundy LR, et al. Danish translation and linguistic validation of the LIMB-Q, a PROM for traumatic lower limb injuries and amputations. Eur J Plast Surg. 2023;46:1255–1264. [Google Scholar]
- 5.Hovd MH, Mariussen E, Uggerud HT, et al. Population pharmacokinetic modeling of CSF to blood clearance: prospective tracer study of 161 patients under work-up for CSF disorders. Fluids Barriers CNS. 2022;19:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulsen L, Klassen AF, Jhanwar SM, et al. Patient expectations of bariatric and body contouring surgery. Plast Reconstr Surg Glob Open. 2016;4:e694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reavey PL, Klassen AF, Cano S, et al. Measuring quality of life and patient satisfaction after body contouring: a systematic review of patient-reported outcome measures. Aesthet Surg J. 2011;31:807–813. [DOI] [PubMed] [Google Scholar]
- 8.Song AY, Rubin JP, Thomas V, et al. Body image and quality of life in post massive weight loss body contouring patients. Obesity. 2006;14:1626–1636. [DOI] [PubMed] [Google Scholar]
- 9.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46:1417–1432. [DOI] [PubMed] [Google Scholar]
- 10.Rupp M, Walter N, Pfeifer C, et al. The incidence of fractures among the adult population of Germany. Dtsch Arztebl Int. 2021;118:665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerry MJ, Volken T, Biller-Andorno N, et al. A Swiss digital Delphi study on patient-reported outcomes. Swiss Med Wkly. 2023;153. Available at https://smw.ch/index.php/smw/article/view/3489. Accessed June 16, 2024. [DOI] [PubMed] [Google Scholar]
- 12.Sousa VD, Rojjanasrirat W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. J Eval Clin Pract. 2011;17:268–274. [DOI] [PubMed] [Google Scholar]
- 13.Stolz M, Albus C, Beutel ME, et al. Assessment of health-related quality of life in individuals with depressive symptoms: validity and responsiveness of the EQ-5D-3L and the SF-6D. Eur J Health Econ. 2023;24:1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters N, Bello-Haas VD, Packham T, et al. Do generic preference-based measures accurately capture areas of health-related quality of life important to individuals with amyotrophic lateral sclerosis: a content validation study. Pat Relat Outcome Meas. 2021;12:191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terwee CB, Elders PJM, Langendoen-Gort M, et al. Content validity of patient-reported outcome measures developed for assessing health-related quality of life in people with type 2 diabetes mellitus: a systematic review. Curr Diab Rep. 2022;22:405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebecca W-H, Pamela B, Nicola W, et al. Generation of evidence supporting the content validity of SF-36, FACIT-F, and LupusQoL, and novel patient-reported symptom items for use in patients with systemic lupus erythematosus (SLE) and SLE with lupus nephritis (LN). Lupus Sci Med. 2022;9:e000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogler A, Janssens J, Nyffeler T, et al. German translation and validation of the “freezing of gait questionnaire” in patients with Parkinson’s disease. Parkinsons Dis. 2015;2015:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rathore FA, Ayaz SB, Mansoor SN, et al. Demographics of lower limb amputations in the pakistan military: a single center, three-year prospective survey. Cureus. 2016;8:e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mundy LR, Klassen AF, Sergesketter AR, et al. Content validity of the LIMB-Q: a patient-reported outcome instrument for lower extremity trauma patients. J Reconstr Microsurg. 2020;36:625–633. [DOI] [PubMed] [Google Scholar]
- 20.Wild D, Grove A, Martin M, et al. ; ISPOR Task Force for Translation and Cultural Adaptation. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8:94–104. [DOI] [PubMed] [Google Scholar]
- 21.Al Sayah F, Jin X, Johnson JA. Selection of patient-reported outcome measures (PROMs) for use in health systems. J Patient Rep Outcomes. 2021;5:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Translation and Linguistic Evaluation Protocol and Supporting Material. Available at https://terrance.who.int/mediacentre/data/WHODAS/Guidelines/WHODAS%202.0%20Translation%20guidelines.pdf. Published 2009. Accessed November 8, 2023. [Google Scholar]
- 23.Poulsen L, Rose MR, Klassen AF, et al. Danish translation and linguistic validation of the BODY-Q: a description of the process. Eur J Plast Surg. 2016;40:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogt B, Fresen J, Gosheger G, et al. LIMB-Q kids—German translation and cultural adaptation. Children (Basel). 2022;9:1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marlene T. German around the world. Deutschland.de. Available at https://www.deutschland.de/en/topic/knowledge/mother-tongue-german-in-42-countries-around-the-world. Accessed January 22, 2024. [Google Scholar]
- 26.Borchert K, Altevers J, Braun S, et al. The value of patient reported outcomes in German Amnog Dossiers. Value Health. 2016;19:A483. [Google Scholar]
- 27.Heim C, Bosisio F, Roth A, et al. Is trauma in Switzerland any different? Epidemiology and patterns of injury in major trauma - a 5-year review from a Swiss trauma centre. Swiss Med Wkly. 2014;144:w13958. (In eng). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.