Abstract
Human immunodeficiency virus type 1 (HIV-1) gag-encoded proteins play key functions at almost all stages of the viral life cycle. Since these functions may require association with cellular factors, the HIV-1 matrix protein (MA) was used as bait in a yeast two-hybrid screen to identify MA-interacting proteins. MA was found to interact with elongation factor 1-alpha (EF1α), an essential component of the translation machinery that delivers aminoacyl-tRNA to ribosomes. EF1α was then shown to bind the entire HIV-1 Gag polyprotein. This interaction is mediated not only by MA, but also by the nucleocapsid domain, which provides a second, independent EF1α-binding site on the Gag polyprotein. EF1α is incorporated within HIV-1 virion membranes, where it is cleaved by the viral protease and protected from digestion by exogenously added subtilisin. The specificity of the interaction is demonstrated by the fact that EF1α does not bind to nonlentiviral MAs and does not associate with Moloney murine leukemia virus virions. The Gag-EF1α interaction appears to be mediated by RNA, in that basic residues in MA and NC are required for binding to EF1α, RNase disrupts the interaction, and a Gag mutant with undetectable EF1α-binding activity is impaired in its ability to associate with tRNA in cells. Finally, the interaction between MA and EF1α impairs translation in vitro, a result consistent with a previously proposed model in which inhibition of translation by the accumulation of Gag serves to release viral RNA from polysomes, permitting the RNA to be packaged into nascent virions.
Retroviral gag-encoded proteins are synthesized as precursor polyproteins in the cytoplasm of infected cells from unspliced viral mRNA. The human immunodeficiency virus type 1 (HIV-1) Gag polyprotein (Pr55gag) accumulates at the plasma membrane, where it directs formation and release of nascent virions. The Gag polyprotein is sufficient for the assembly of noninfectious particles, and, in addition, it recruits viral genomic RNA and other viral and cellular proteins into virions (reviewed in reference 76). Upon activation of the viral protease during virion assembly, the HIV-1 Gag polyprotein is processed into its mature products, which include the matrix (MA; p17), capsid (CA; p24), nucleocapsid (NC; p7), and p6 domains. As part of the Gag polyprotein, each of these domains exhibits important functions during virion assembly, while the mature proteins play key roles during the early phases of the viral life cycle.
During virion assembly, the MA domain targets the Gag polyprotein to the plasma membrane (82, 85), where it regulates incorporation of env-encoded proteins onto HIV-1 virions, presumably via interaction with the cytoplasmic tail of gp41 (17, 30, 31, 51, 57, 66). Mature MA seems to play several roles as well. Deletion mutations at the C terminus of MA (81), as well as specific MA point mutations (47), cause a defect at an early step in reverse transcription, prior to import of the preintegration complex into the nucleus. MA then associates with the HIV-1 preintegration complex (23, 34, 61) and may play a role in translocation of the complex to the nucleus (10, 23, 34, 40, 61), although the latter point has been questioned by studies from other groups (27, 29).
Many groups have shown that the CA domain of the Gag polyprotein plays an essential function during the assembly process (for a review, see reference 18). As a mature protein, CA is the major constituent of the virion core and has been shown to play a key role in early events of the viral life cycle (6, 21, 32, 58, 71).
As part of the Gag polyprotein, the NC domain is responsible for incorporation of genomic RNA into virions (4) and is the main domain involved in homomeric interactions between Gag polyprotein monomers (3, 11, 59, 84). In its processed form, NC has been shown to promote primer-tRNA annealing on genomic RNA (43, 50) and formation of RNA dimers in vitro (20, 25).
The p6 portion of the Gag polyprotein is required at a late step in the virion budding process (38). Interestingly, the p6 domain is only required for virion release when the viral protease is active (42).
Viruses, as intracellular parasites, are necessarily dependent upon host cell factors for replication. Therefore, cellular factors may be required for any of the many HIV-1 gag-encoded functions. Indeed, interaction between gag-encoded proteins and cellular proteins has already been reported. The association of the HIV-1 Gag polyprotein with the plasma membrane requires cotranslational modification by the cellular myristyl-S-transferase (8, 39). Though of unknown significance, ubiquitin is incorporated into virions, where a subfraction is covalently attached to p6 (64). Via contacts with the Gag polyprotein (53), the cellular protein cyclophilin A is incorporated into HIV-1 virions (28, 78), where it is hypothesized to promote virion core disassembly by blocking contacts between CA monomers (35, 52). HOX3, a cellular protein with homology to histidyl aminoacyl-tRNA synthetase, associates with virions via the MA domain of the Gag polyprotein (48). HIV-1 Gag interacts with filamentous actin (69), and actin is found within HIV-1 virions (65). More recently, a member of the tetratricopeptide repeat family has been shown to associate with HIV-1 Gag and Vpu and may be involved in the Vpu-mediated enhancement of Gag particle release from the plasma membrane (12).
The mature products obtained after processing of the HIV-1 Gag polyprotein by the viral protease have also been shown to interact with cellular proteins. MA is phosphorylated on tyrosine and serine/threonine residues (13, 44), and these modifications are believed to be required for the MA-mediated nuclear translocation of the preintegration complex (9, 33). Phosphorylation on serine/threonine residues has been shown to be mediated by virion-associated mitogen-activated protein kinase ERK2 (44).
Identification of new cellular factors that interact with a specific domain of HIV-1 Gag might help elucidate the function of that domain in virus replication. To this end, HIV-1 MA was used as bait in a yeast two-hybrid screen of a mammalian cDNA library. An interaction between MA and the translation elongation factor 1-alpha (EF1α) was identified. This elongation factor is an essential component of the cellular translational machinery (60). In its GTP-bound form, EF1α delivers aminoacyl-tRNA to ribosomes. Once associated with the ribosome, EF1α hydrolyzes GTP, is released from the tRNA, and leaves the ribosome. EF1α has also been shown to interact with filamentous actin, and it has been hypothesized that this interaction might provide a link between the translation machinery and the cytoskeleton (reviewed in reference 15).
Our studies demonstrate that HIV-1 Gag associates both in vitro and in vivo with EF1α. In addition to the MA domain, the NC domain provides a second, independent binding site for EF1α on the Gag polyprotein. Fine-mapping studies indicate that basic residues in both domains of Gag promote the interaction. EF1α is specifically incorporated into HIV-1 virions, as evidenced by the fact that it is not incorporated into Moloney murine leukemia virus (M-MuLV) virions. We provide evidence that the Gag-EF1α interaction requires tRNA, and EF1α may contribute to tRNA incorporation into HIV-1 virions. Finally, as an indication of a possible functional consequence of the interaction between Gag and EF1α, we show that the portion of MA that interacts with EF1α negatively affects translation in vitro.
MATERIALS AND METHODS
Cloning by PCR.
Many of the expression constructs used here were engineered by using DNA fragments generated by PCR with Pfu DNA polymerase (Stratagene, La Jolla, Calif.). In each case, the identity of the products was confirmed by dideoxy sequencing. The oligonucleotide primers used in the PCRs described below are listed here (5′-to-3′ orientation) and are named sequentially by numbers: 1, GCGCGAATTCATGGGTGCGAGAGCGTC; 2, CGCGCTCGAGTTATTGTGACGAGGGGTCGG; 3, GCGCTCTAGAATGGAAGCCGTCATAAAGG; 4, GCGCGAATTCTTAGCAGGATGTGCCAACGGTT; 5, GCGCTCTAGAATGGGGCAAGAATTAAGCCAG; 6, GCGCGAATTCTTACAGAACTGGGAATCTTTTTGG; 7, GCGCTCTAGAATGGGCCAGACTGTTACCAC; 8, GCGCGAATTCTTAATAAAGGGAGATCGAGGCG; 9, GCGCTCTAGAATGGGCGTGAGAAACTCC; 10, GCGCGAATTCTTAGTAATTTCCTCCTCTGCC; 11, CGCGCCATGGGTGCGAGAGCGTCA; 12, CGCGGGATTCATGATACAGAAAGGCAATTTTAGG; 13, GCGCGTCGACTTAATTAGCCTGTCTCTCAGTAG; 14, GCCCCAGGACTCAGAGACTTTATC; 15, ATGGAAGACGCCAAAAACATAAAG; 16, GCGCCCTAGGGTCGACTTACAATTTGGACTTTCCGCC; 17, GCGCCTGCAGTAATACGACTCACTATAGGTCTCTCTGGTTAGACCAG; 18, CTCTCTCCTTCTAGCCTCCG.
Yeast two-hybrid screen.
The yeast two-hybrid system and the HeLa cDNA library used in this study have already been described (41). This system identifies putative bait-interacting proteins by using selection for leucine prototrophy and for a screen for β-galactosidase (β-Gal) activity in Saccharomyces cerevisiae EGY48 (ura3, his3, trp1, LexAop-leu2). The bait consisted of the entire HIV-1 MA fused at its N terminus to LexA. HIV-1 MA coding sequences were amplified by PCR from the proviral clone NL4-3 (1) by using primers 1 and 2. The product of the PCR was digested with restriction enzymes EcoRI and XhoI and inserted into the same sites of plasmid pEG202 (41). A total of 2 million colonies were screened according to published protocols (41).
Proviral DNAs.
Proviral DNAs were obtained as follows: M-MulV proviral DNA (clone NCA) was obtained from Stephen Goff (Columbia University, New York, N.Y.); Mason-Pfizer monkey virus (M-PMV) was obtained from Eric Hunter (University of Alabama, Birmingham), and wild-type Rous sarcoma virus (RSV; pATV-8 clone) and RSV mutant HB12 (amino acids 12 to 18 of RSV MA substituted for by amino acids 25 to 31 of HIV-1 MA [67]) were obtained from Leslie Parent (Pennsylvania State University College of Medicine, Hershey).
All of the HIV-1 mutations described below were engineered in HXB2-based proviral clones (26) unless otherwise specified and were obtained as follows: MA mutants dB5 (deletion of amino acids 21 to 31 of MA [82]), B5 (amino acids substituted, R21T-K25E-K26E-K27E-K31E [82]), and AAA (amino acids substituted, K25A-K26A-K27A [49]) were obtained from Xiao-Fang Yu (Department of Molecular Microbiology and Immunology, Johns Hopkins University, Baltimore, Md.), HIV-1 MA mutant TT (amino acids substituted, K25T-K26T, in the NL4-3 proviral clone [29]) was obtained from Eric Freed (NIH, Bethesda, Md.), and HIV-1 ΔMA (deletion of 80% of MA [79]) was obtained from Eric Barklis (Oregon Health Sciences University, Portland). The following HIV-1 NC mutants were obtained from Anna Aldovini (Harvard Medical School, Boston, Mass. [68]) and have the indicated amino acids substituted for with alanine: R7, K14, K38-K41-K48, BR (R29-R32-K33-K34), M1-2 (K14-K20-R26-K38-K41-K47), and mutant M1-2/BR (combination of M1-2 and BR). The HIV-1 NC zinc finger mutants (22), C36S, C15S/C36S, and F16A were provided by Heinrich Göttlinger (Division of Human Retrovirology, Dana-Farber Cancer Institute, Boston, Mass.). The HIV-1 proviral clone SVC21BH10, bearing the mutation D25R that inactivates the viral protease, was obtained from Larry Kleiman (Departments of Medicine, Microbiology and Immunology, McGill University, Montreal, Canada). An SphI-EcoRV fragment encompassing this mutation (nucleotides 1443 to 2977, according to reference 62) was subcloned into the NL4-3 proviral clone.
Fragments encompassing the AAA and the M1-2/BR mutations were transferred from the original HXB2-based proviral clones into the proviral clone NL4-3 for detailed analysis in pulse-chase experiments. A BssHII-SphI fragment from the proviral clone AAA (nucleotides 711 to 1443) was subcloned directly into the proviral clone NL4-3 to give plasmid NL4-3 AAA. An SphI-EcoRV fragment from the proviral clone M1-2/BR (nucleotides 1443 to 2977) was first inserted into plasmid pUCNL4-3. This plasmid contains an SphI-EcoRI fragment of NL4-3 (nucleotides 1443 to 5743) inserted into the corresponding sites of pUC19 (New England Biolabs). An SpeI-EcoRI fragment encompassing this mutation (nucleotides 1507 to 5743) was then subcloned from pUCNL4-3 into the unique SpeI-EcoRI sites of the proviral clone NL4-3 AAA in order to obtain the double mutant NL4-3 AAA M1-2/BR.
Construction of glutathione S-transferase (GST) fusion protein expression plasmids.
DNA sequences encoding MA proteins of different retroviruses were PCR amplified from the specific proviral clones listed above with the following primers: RSV, 3 and 4; M-PMV, 5 and 6; M-MuLV, 7 and 8; and simian immunodeficiency virus of macaques 239 (SIVmac239), 9 and 10. The PCR products were cloned as XbaI-EcoRI fragments in the same sites of the vector pGEX2TKPL. pGEXTKPL is a modification of vector pGEXTK (Promega, Madison, Wis.) with an extended polylinker and was obtained from David Shore (Departement de Biologie Moleculaire, Université de Geneve, Geneva, Switzerland). Sequences encoding the different HIV-1 MA mutants were PCR amplified from the specific proviral clones by using primers 11 and 2, cloned in the vector pBSHA (14) as NcoI-XhoI fragments and then subcloned as XbaI-XhoI fragments in the same sites of vector pGEX2TKPL.
Sequences encoding the different HIV-1 NC mutants were PCR amplified with primers 12 and 13 and cloned directly as BamHI-SalI fragments into the same sites of vector pGEX2TKPL. HIV-1 Gag mutants (described below) were cloned as XbaI-XhoI fragments obtained after digestion of the specific plasmid pBS-GagX (see below) into vector pGEX2TKPL. Plasmid SP-p6 was created by ligating the BglII-XhoI fragment obtained after digestion of plasmid pBS-GagX into the vector pGEX2TK linearized with BamHI and XhoI.
Rev-independent Gag mutants.
Coding sequences encompassing gag mutations were introduced into pBS-GagX. In this plasmid, the gag sequence (nucleotides 789 to 2292, according to reference 62) is flanked by an NcoI site at the 5′ end and by an XhoI site at the 3′ end, as described previously (14). The gag sequence in pBS-GagX contains multiple conservative mutations that act at the RNA level to render gag expression Rev independent (70). Mutations in MA (AAA, dB5, or ΔMA) or NC (M1-2/BR) coding sequences were introduced into the gag sequence by replacing an NcoI-NsiI fragment (nucleotides 789 to 1249) or an SphI-XhoI fragment (nucleotides 1443 to 2292) of pBS-GagX with the corresponding fragment obtained after PCR performed on the specific proviral clone with primers 1 and 13. Double mutants in MA (AAA or dB5) and NC (M1-2/BR) were obtained by replacing the NcoI-NsiI fragment containing the specific MA mutation with the corresponding fragment of pBS-GagX M1-2/BR. Mutant GagΔNC-p6 was obtained by digesting pBS-GagX with MfeI (nucleotide 1968), filling the ends with Klenow DNA polymerase, and circularizing the plasmid with T4 DNA ligase. This creates a nonsense codon after the first N-terminal 15 amino acids of NC (pBS-GagX ΔNC-p6).
Eukaryotic gag expression plasmids.
Rev-independent gag coding sequences containing the MA mutation (AAA), NC mutation (M1-2/BR), or a combination of both mutations (AAA M1-2/BR) were released from the specific pBS-GagX plasmids described above by digestion with XbaI and XhoI. The fragments were blunted by treatment with the Klenow fragment of DNA polymerase and ligated into an SRα expression plasmid (7), linearized with EcoRI and XhoI, and blunted by treatment with the Klenow fragment. Expression of gag coding sequences is driven by an SRα promoter (77).
Construction of EF1α bacterial expression plasmids.
All of the EF1α-encoding fragments were cloned into the vector pSE420 (Invitrogen) for bacterial expression. To produce an N-terminal hemagglutinin (HA)-tagged version of EF1α, a JG4-5 two-hybrid clone containing the full-length EF1α cDNA plus 30 nucleotides of the 5′ untranslated region and around 500 nucleotides of the 3′ untranslated region (for a total of 2 kb) was digested with EcoRI and XhoI and subcloned into pBS-HA (14) digested with the same enzymes (pBS-HAEF1α). The cDNA encoding HA-EF1α was then transferred as an NcoI-XhoI fragment from pBS-HAEF1α into the plasmid pSE420.
Restriction sites present in the coding sequence of EF1α were used to create deletion mutants of EF1α. A DNA fragment encoding the N-terminal 74 amino acids of EF1α was excised from pBS-HAEF1α as a NcoI-EcoRV fragment and cloned directly into pSE420. A fragment encoding amino acids 14 to 74 of EF1α was excised from pBSHA-EF1α by using the enzymes AflIII-EcoRV followed by Klenow DNA polymerase treatment and was cloned into plasmid pBS-HA linearized with EcoRV. This fragment was then subcloned as an NcoI-EcoRV fragment into pSE420. As a result of the cloning process, the coding sequences of HAEF1α 1–74 and HAEF1α 14–74 are in frame with the downstream polylinker sequence of pSE420. The resulting fusion proteins have predicted molecular masses of about 20 and 18 kDa, respectively. A fragment encoding the C-terminal portion of EF1α (amino acids 298 to 463) was obtained after digestion of plasmid pBSHA-EF1α with BamHI and HindIII followed by Klenow DNA polymerase treatment and circularization with T4 DNA ligase. This fragment was then subcloned as an NcoI-XhoI fragment into pSE420. Mutant H95L was created with the transformer site-directed mutagenesis kit (Clontech, Palo Alto, Calif.) and mutagenic primer 14. All of the EF1α fragments possess an HA tag at the N terminus.
Translation reporter constructs.
The firefly luciferase cDNA was amplified by PCR from the vector pGL-2 basic (Promega) by using primers 15 and 16 and cloned into the EcoRV site of pBluescript KS− (Stratagene). The orientation of the cDNA was such that it could be transcribed from the T7 promoter (pBS-luciferase). A fragment encompassing the HIV-1 leader region (nucleotides 455 to 788) was amplified by PCR from the proviral clone NL4-3 by using primers 18 and 17 (providing a T7 promoter) and cloned into the SmaI site of the plasmid pGL-2 basic. The resulting plasmid allows transcription from the synthetic T7 promoter of an mRNA that contains the HIV-1 leader sequence upstream of the firefly luciferase cDNA (pGL-leader-luciferase).
Production of proteins.
Cellular lysate obtained from human 293T fibroblasts was used as a source of EF1α. Typically, 107 cells were lysed at 4°C in 1 ml of binding buffer (20 mM HEPES [pH 6.8], 150 mM KOAc, 2 mM MgOAc, 2 mM dithiothreitol, 0.1% Casamino Acids, 1% Tween 20, 1 mM phenylmethylsulfonyl fluoride). The lysate was cleared of insoluble matter by centrifugation at 90,000 rpm in a TL100.1 rotor (Beckman) for 15 min and stored at −80°C with a final glycerol concentration of 20%. Aliquots corresponding to lysate from 5 × 105 cells were used in each binding reaction.
Escherichia coli BL21LysS (Novagen) was used for bacterial protein expression. Bacterial lysates were prepared essentially as described before (53), with binding buffer used as resuspension buffer. The lysate was brought to a final glycerol concentration of 20% and frozen in liquid nitrogen.
GST fusion proteins to be used in the in vitro translation assay were purified from the bacterial lysate on glutathione affinity columns, eluted with 5 mM glutathione, in 50 mM Tris-Cl (pH 8.0), and frozen in liquid nitrogen with a final glycerol concentration of 20%. The purity and quantity of the proteins were checked by Coomassie gel staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by Bradford assay (Bio-Rad).
In vitro binding experiments.
GST fusion proteins were immobilized onto glutathione-agarose beads (Sigma) in binding buffer (20 μl, 50% slurry) for 30 min and washed three times in binding buffer. Beads were then incubated with crude lysates expressing native or recombinant EF1α for 1 h at 4°C in a final volume of 200 μl and then washed three times in binding buffer. Bound proteins were boiled and subjected to SDS-PAGE and Western blotting.
In some in vitro binding experiments, the effects of RNase A on the EF1α–HIV-1 Gag interaction was examined. For these experiments, lysates expressing the two proteins were diluted in buffer containing 50 mM Tris-Cl (pH 8.0), 1 mM EDTA, and RNase A (Sigma), ranging from 0 to 50 μg per 10 mg of total bacterial lysate. The lysates were incubated for 1 h at 37°C and then mixed together and assayed as described above.
Antibodies and Western blot analysis.
Murine monoclonal anti-EF1α antibody was purchased from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Murine monoclonal anti-HIV-1 CA and rabbit polyclonal anti-HIV-1 gp120 antibodies were purchased from Intracel (Cambridge, Mass.). Goat polyclonal anti-M-MuLV CA antibody was a gift from Stephen Goff (Columbia University, New York, N.Y.). Murine monoclonal anti-HA antibody was purchased from Berkeley Antibody Company (Berkeley, Calif.). Western blot analysis was performed essentially as described previously (53).
Immunoprecipitation.
Human 293T fibroblasts were maintained in complete Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. Rev-independent Gag polyproteins were expressed by calcium phosphate transfection (19) of 293T cells by using the SRα expression plasmids described above. Cells were lysed 72 h posttransfection in binding buffer, and the lysate was cleared by centrifugation at 14,000 rpm in an Eppendorf microcentrifuge for 20 min at 4°C. The lysate was preincubated with 100 μl of protein A-Sepharose beads (Sigma; 10% slurry) for 1 h at 4°C. Supernatant was removed from the beads and incubated with rabbit polyclonal anti-HIV-1 CA antibody (obtained from Louis Henderson, Frederick Cancer Research and Development Center, Frederick, Mass.) for 2 h at 4°C. Protein A-Sepharose beads (100 μl) were then added for 1 h at 4°C. Beads were washed three times in binding buffer, and proteins bound to the beads were analyzed by Western blotting.
Virion purification.
293T cells were transfected by calcium phosphate with Gag expression plasmids or with complete proviral DNAs. Seventy-two hours posttransfection, culture supernatant was syringe filtered (0.45-μm-pore-size filter) and purified through a two-step sucrose gradient. Typically 8 to 10 ml of supernatant was layered on 2 ml each of 45 and 25% sucrose. After centrifugation for 2 h at 80,000 × g, the interface between the 45 and the 25% sucrose was collected, diluted in phosphate-buffered saline (PBS), and laid upon 2 ml of 25% sucrose for an additional 2-h centrifugation step at 80,000 × g. Virions were resuspended in loading buffer and analyzed by SDS-PAGE and Western blotting.
Linear sucrose density gradient analysis of virions.
Virions obtained by calcium phosphate transfection of 293T cells with either the HIV-1 proviral clone NL4-3 or the M-MuLV clone NCA were concentrated by centrifugation through 25% sucrose as described above. The pellet was resuspended in 200 μl of DMEM for 4 h on ice and then layered onto a linear sucrose density gradient (20 to 60% [wt/vol]). After 24 h of centrifugation at 80,000 × g, 12 fractions were collected. Each fraction was precipitated with 10% trichloroacetic acid and analyzed by SDS-PAGE and Western blotting.
Subtilisin digestion.
Virions, purified and pelleted through a two-step sucrose gradient as described above, were resuspended in 20 mM Tris-Cl (pH 8.0)–2 mM CaCl2 for 4 h on ice. The solution containing the virions was divided and treated with different concentrations of the protease subtilisin (Boehringer Mannheim) for 18 h at room temperature. Typically, a concentration of 0.1 to 1 mg of protease per ml (stock solution: 100 mg/ml in 20 mM Tris-Cl [pH 8.0] plus 2 mM CaCl2, freshly made) was sufficient to provide efficient digestion of the envelope protein gp120. The reaction was stopped by addition of 5 μM phenylmethylsulfonyl fluoride, and virion-associated proteins were purified by centrifugation through a 25% sucrose cushion prior to analysis by SDS-PAGE and Western blotting.
Metabolic labeling.
HeLa cells maintained in DMEM supplemented with 10% fetal bovine serum were transfected by calcium phosphate with NL4-3 proviral DNAs in 35-mm-diameter plates. Forty-eight hours posttransfection, cells were incubated for 1 h at 37°C with 2 ml of DMEM lacking methionine and cysteine prior to a 45-min pulse with 100 μCi of [35S]Met-Cys, (Translabel; ICN). Cells were washed with PBS and incubated with complete DMEM. Cells were lysed 0, 1, 3, and 6 h later in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris-Cl [pH 8.0]), and immunoprecipitation was carried out as described above. Metabolically labeled virions were purified from the supernatant by ultracentrifugation through 25% sucrose as described above, resuspended in RIPA buffer, and immunoprecipitated with AIDS patient sera (obtained through the AIDS Research and Reference Reagent Program, catalog no. 3957).
tRNA dot blot on immunoprecipitated Gag polyproteins.
The amount of tRNA3Lys or tRNAPhe in Gag immunoprecipitates was determined by dot blot analysis with end-labeled 32P primers and under conditions previously described (54). Immunoprecipitations were carried out as described above, except that the protein A-Sepharose beads were divided into two aliquots after the last washing step. One aliquot was analyzed by Western blotting for the normalization of the Gag polyproteins and the presence of EF1α, and the other aliquot was analyzed by dot blotting. The aliquots were resuspended in the necessary loading buffer according to their final use. The total tRNA fraction was obtained from 293T cell total RNA by a previously described procedure (54). Total yeast tRNA was purchased from Sigma.
In vitro translation assay.
Increasing amounts of purified GST proteins were first incubated with 2 mg of rabbit reticulocyte lysate (RRL; Promega) for 1 h at 4°C. After this incubation, luciferase-encoding mRNA (250 ng) was added to the reaction mixture and translated according to the manufacturer’s instructions (Promega). Capped mRNA was made according to the manufacturer (mMESSAGE-mMACHINE; Ambion) by using either pBS-luciferase or pGL-HIV-1 leader-luciferase as a template for T7 in vitro transcription. Uncapped mRNA encoding the firefly luciferase gene was purchased from Promega. Luciferase activity was measured according to standard protocols. The peptide encompassing the HIV-1 MA basic region was purchased from Intracel (catalog no. 279010; amino acid sequence, LRPGGKKKYKLKHIV), and the peptide encompassing the T-antigen NLS was purchased from Sigma (catalog no. C4547; amino acid sequence, CGYGPKKKRKVGG).
RESULTS
Identification of the interaction between HIV-1 MA and EF1α.
HIV-1 MA was used as bait in a yeast two-hybrid screen (41) to identify interacting proteins encoded by a cDNA library derived from HeLa cells. The cDNAs were expressed as C-terminal fusions with the B42 acid patch activation domain. MA was expressed as a LexA fusion protein in a yeast strain (EGY48) bearing Leu2 and lacZ genes downstream of multimerized LexA-binding sites. Two million transformants with the cDNA expression library were screened, and 16 clones were identified in which both the ability to grow on leucine-deficient media and high-level β-Gal activity were dependent upon MA. cDNA expression plasmids were isolated from the seven clones displaying the strongest β-Gal activity. The cDNA inserts from these clones were sequenced, and all were found to encode the full-length EF1α protein. Of the seven EF1α cDNA clones, two were identical. The other clones differed from each other in the amount of 5′ untranslated sequence present in the fusion with the B42 activation domain.
EF1α binds in vitro to MA proteins encoded by primate lentiviruses.
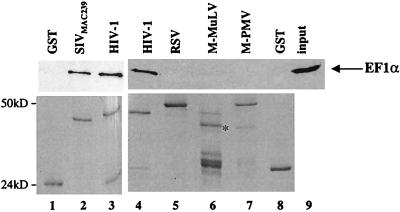
To confirm and extend our observations on the interaction between EF1α and MA in the two-hybrid system, in vitro binding assays were performed with recombinant proteins expressed in bacteria. In addition, the specificity of the interaction was tested by determining if MA proteins encoded by retroviruses other than HIV-1 also associate with EF1α. MA proteins from HIV-1NL4-3, SIVmac239, M-PMV, M-MuLV, and RSV were expressed in bacteria as GST fusion proteins. The GST fusion proteins were immobilized on glutathione-agarose beads, washed, and assayed for the ability to bind EF1α obtained from the soluble lysate of 293T cells. The beads were washed again, and any protein still associated with the beads was boiled in SDS and processed by SDS-PAGE. The ability of EF1α to remain associated with each of the fusion proteins was assayed by Western blotting with a mouse monoclonal anti-EF1α antibody (Fig. 1, top panels). EF1α bound to HIV-1NL4-3 (Fig. 1, lanes 3 and 4) and SIVmac239 (lane 2) GST-MA fusion proteins. In lane 9, 10% of the 293T cell lysate input into each binding reaction is shown. From the signal intensity, the amount of EF1α that remained associated with HIV-1 GST-MA was estimated to be 4 to 8% of the total input under the binding conditions shown here (compare lanes 4 and 9 in Fig. 1). In contrast, EF1α binding to GST (Fig. 1, lanes 1 and 8) or GST-MA fusion proteins from RSV, M-MuLV or M-PMV (lanes 5, 6 and 7, respectively) was undetectable. The inability to detect interaction between EF1α and the nonlentiviral MA proteins was not explained by a failure to express them as GST fusion proteins, since these proteins were expressed at the same level as HIV-1 and SIV-derived proteins (Fig. 1, bottom panels). These results demonstrate that the interaction between HIV-1 GST-MA and EF1α occurs in vitro and appears to be specific for lentivirus MA.
FIG. 1.
EF1α binds in vitro to MA proteins encoded by primate lentiviruses. The MA proteins of the indicated retroviruses were produced as GST fusion proteins, bound to glutathione-agarose beads, and assayed for the ability to interact with EF1α provided by a lysate of 293T cells. After washing, the proteins that remained associated with the beads were analyzed by Western blotting with an anti-EF1α antibody (top panels). The position of EF1α is indicated by the arrow on the right. The amount of GST fusion protein present in each binding reaction mixture was determined by Coomassie staining (lower panels). Lane 9 shows 10% of the total 293T cellular lysate input used in the binding reactions. The positions of migration of molecular mass markers (in kilodaltons) are indicated on the left. The expected position of the M-MuLV GST-MA fusion protein is shown by an asterisk in lane 6.
To exclude the possibility that binding between EF1α and HIV-1 MA was mediated by a third protein present in the eukaryotic cell lysate, similar binding experiments were performed with recombinant EF1α expressed in bacteria. EF1α was expressed with an HA-epitope tag at its N terminus and used in binding experiments with GST-HIV-1 MA. As shown in Fig. 2B, recombinant EF1α bound to MA (lane 3), but not to GST (lane 5). Therefore, it is unlikely that a third protein bridges the association between MA and EF1α.
FIG. 2.
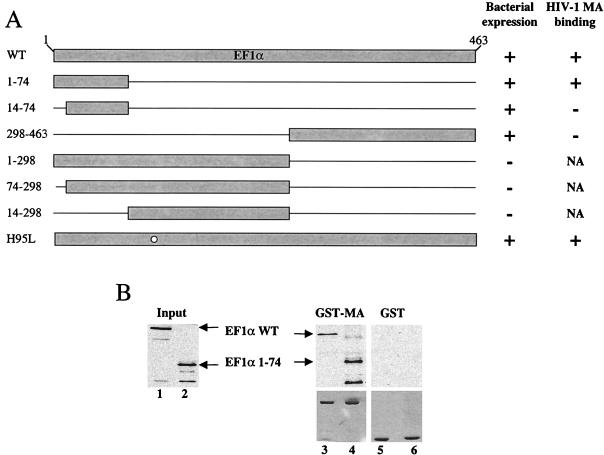
Determination of the region of EF1α involved in binding to HIV-1 MA. A schematic diagram of EF1α mutants expressed in bacteria as HA-tagged fusion proteins is presented in panel A. The names of the mutants are shown to the left of the figure; numbers refer to the amino acid residues retained by the deletion mutants. The bacterial expression status and the in vitro binding results obtained for these mutants are reported qualitatively as + or − to the right of the figure. NA, not applicable; WT, wild type. (B) EF1α mutants were assayed for their ability to bind either GST–HIV-1 MA (lanes 3 and 4) or GST (lanes 5 and 6) as described in the legend to Fig. 1. Samples were analyzed by Western blotting with an anti-HA antibody (top panels) or were Coomassie stained after SDS-PAGE (bottom panels). The positions of the wild type (WT) and the EF1α 1–74 mutant are indicated by arrows. Lanes 1 and 2 represent 10% of total input of HA-tagged wild type and the EF1α 1–74 mutant, respectively.
Determination of the region of EF1α involved in binding to HIV-1 MA.
To determine which portion of EF1α is responsible for association with HIV-1 MA, deletion mutants were engineered within the cDNA encoding EF1α. Each mutant protein was expressed in bacteria with an HA epitope fused at its N terminus. Lysates from bacteria expressing the HA-tagged EF1α mutants were used in binding reactions with GST–HIV-1 MA. The deletion mutants analyzed are shown in the schematic diagram of Fig. 2A. In addition to the deletion mutants, the H95L mutant was tested; the analogous mutation has been shown to dramatically reduce the GTPase activity of EF-Tu, the EF1α homologue in bacteria (83).
Three of the deletion mutants were not stably expressed under the conditions used here (Fig. 2A, bacterial expression). Of the mutants that were expressed, only EF1α 1–74 and H95L were capable of binding MA (Fig. 2A, + HIV-1 MA binding). Figure 2B shows a binding experiment with bacterially expressed wild-type EF1α and the EF1α 1–74 mutant. Both the wild type and the EF1α 1–74 mutant bound to GST–HIV-1 MA (Fig. 2B, lanes 3 and 4, respectively), but not to GST (lanes 5 and 6, respectively). Identical results were obtained with the H95L mutant (data not shown). In lanes 1 and 2, 10% of the EF1α protein input in the binding reaction is shown for both the wild type and the EF1α 1–74 mutant, respectively. These results demonstrate that the EF1α fragment encoding the N-terminal 74 amino acids is sufficient for binding to HIV-1 MA. This portion of EF1α is part of domain I, as deduced from the crystal structure of the complex of GTP–EF-Tu from Thermus aquaticus with aminoacyl-tRNA (63). It contains one of the three GTP-binding sites of EF1α, as well as contact points for aminoacyl-tRNA. In addition, the ability of the H95L mutant to bind indicates that GTPase activity is not required for interaction with MA.
Determination of the region of HIV-1 MA involved in binding to EF1α.
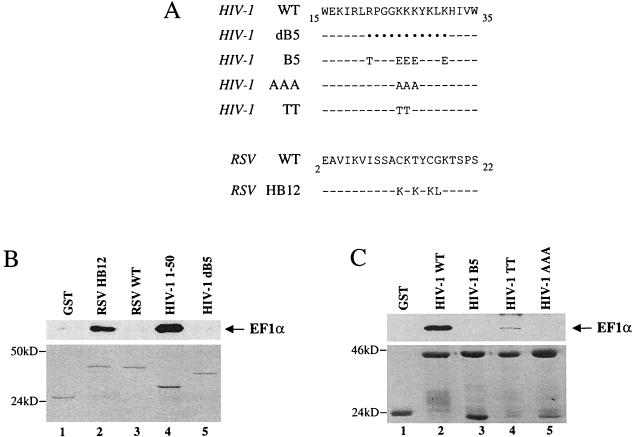
To define the region of HIV-1 MA responsible for binding to EF1α, different portions of MA were produced as GST fusion proteins and assayed for the ability to associate in vitro with EF1α. The N-terminal 50 amino acids of MA were found to be sufficient for interaction with EF1α (Fig. 3B, lane 4). This region contains a cluster of basic residues that have been shown to be important for targeting and binding of the Gag polyprotein to the plasma membrane (82, 85). A role in nuclear translocation of the preintegration complex has also been proposed for this basic region (10, 23, 34, 40, 61), although others failed to detect this activity (27, 29).
FIG. 3.
Determination of the region of HIV-1 MA involved in binding to EF1α. (A) Amino acid sequences of the mutants used here. In the top portion, HIV-1 mutants are compared with the wild-type (WT) HIV-1 MA sequence. In the lower portion, an RSV–HIV-1 chimera is compared to the wild-type RSV MA sequence. HIV-1 dB5 has a deletion from amino acids 21 to 31 that encompasses the MA basic region. HIV-1 mutants of the MA basic region include B5, AAA, and TT, which have five, three, or two basic residues substituted as indicated, respectively. RSV HB12 has four residues substituted that recreate the sequence KKKYKL of HIV-1 MA (amino acids 25 to 30 of HIV-1 [compare with the top sequence]). In the amino acid alignment, black dots indicate that a residue was deleted, and dashes indicate that the residue is identical to that in the wild type. The MA mutants shown in panel A, as well as one additional mutant that includes the first 50 amino acids of MA (HIV-1 1–50), were expressed as GST fusion proteins and assayed for their ability to associate in vitro with EF1α as described in the legend of Fig. 1. Samples were analyzed by Western blotting probed with an anti-EF1α antibody (B and C, top panels) or by Coomassie staining (B and C, bottom panels) after SDS-PAGE. The position of EF1α is indicated by the arrows on the right, and the positions of molecular mass markers (in kilodaltons) are indicated to the left of each panel.
The importance of the HIV-1 MA basic region for association with EF1α was assessed next. Not only did deletion of the basic region abrogate binding of HIV-1 MA to EF1α (Fig. 3B, lane 5), but transfer of the HIV-1 basic region into RSV MA (RSV HB12 [Fig. 3A]), a chimeric protein previously described by Parent et al. (67), converted the nonbinding RSV MA (Fig. 3B, lane 3) into an EF1α binder (Fig. 3B, lane 2). Finally, replacement of two, three, or five basic residues with nonbasic amino acids disrupted MA’s interaction with EF1α (Fig. 3C, lanes 4, 5, and 3, respectively). These results demonstrate that the basic region of HIV-1 MA is required for binding to EF1α.
The NC domain provides a second binding site on HIV-1 Gag for EF1α.
MA is translated as part of the Gag polyprotein. Experiments were therefore performed to determine if EF1α binds the HIV-1 Gag polyprotein. Using the yeast two-hybrid system, it was determined that full-length Gag polyproteins encoded by HIV-1 and SIVmac239 interact with EF1α; no EF1α interaction was detected with the Gag polyproteins of M-MuLV or MPMV (data not shown). These results concur with the previous finding (Fig. 1) that EF1α interacts with MA proteins encoded by lentiviruses but not by other retroviruses.
The ability of the HIV-1 Gag polyprotein to interact with EF1α was next examined by using the in vitro binding assay. As expected, EF1α bound to wild-type GST-Gag (Fig. 4A, lane 2) but not to GST (lane 1). Surprisingly, a GST-Gag mutant in which the MA basic region had been deleted retained the ability to interact with EF1α (dB5, lane 5). Furthermore, a GST-Gag mutant in which the majority of MA had been deleted interacted with EF1α (ΔMA, lane 3). These results suggested that a second binding site for EF1α must be present within the Gag polyprotein.
FIG. 4.
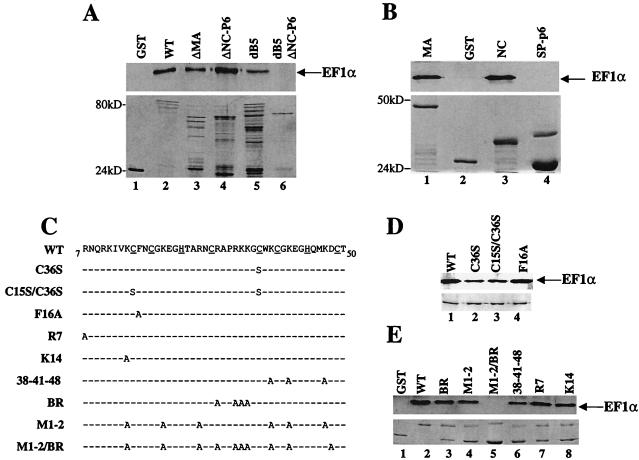
NC provides a second binding site for EF1α on the HIV-1 Gag polyprotein. (A) Deletion mutants of the HIV-1 Gag polyprotein were expressed as GST fusion proteins and assayed for their ability to associate with EF1α in vitro as described in the legend to Fig. 1. Samples were analyzed by Western blotting and probed with an anti-EF1α antibody (top panels) or by Coomassie staining (bottom panels). The position of EF1α is indicated by an arrow on the right. Binding of EF1α to GST or wild-type (WT) GST-Gag is shown in lanes 1 and 2, respectively. Binding of EF1α to GST-Gag deletion mutants is as indicated: ΔMA is GST-Gag with the majority of MA deleted (lane 3); ΔNC-p6 is GST-Gag with p6 and most of NC deleted (lane 4); dB5 is GST-Gag with an 11-amino-acid deletion encompassing the MA basic region (lane 5); dB5ΔNC-p6 is GST-Gag with deletion of both the basic region of MA and the NC/p6 regions (lane 6). (B) GST-NC (lane 3) and GST-SP-p6 (lane 4) fusion proteins were used to better define the second EF1α-binding site in Gag. Binding to GST and GST–HIV-1 MA is shown in lanes 2 and 1, respectively. (C) Amino acid sequence alignment showing wild-type HIV-1 NC and the NC mutants used in (D and E). Underlined sequences indicate residues involved in the two Cys-His boxes; dashes indicate amino acid residues identical to those of the wild type. Mutants were produced as GST fusion proteins and assayed for the ability to interact with EF1α as described above. (D) Mutants in the zinc fingers of NC. (E) Mutants in the basic amino acid residues of NC.
To map the putative second EF1α-binding site, Gag polyprotein deletion mutants were expressed as GST fusions. A GST-Gag polyprotein with a deletion of the NC and p6 regions (Fig. 4A, ΔNC-p6, lane 4) bound to EF1α. In combination with a deletion of the MA basic region, ΔNC-p6 completely abolished binding to EF1α (Fig. 4A, ΔNC-p6 dB5, lane 6). These results suggest that the second EF1α binding site is located at the C terminus of the Gag polyprotein. More specifically, GST-NC (Fig. 4B, lane 3), but not a fusion containing a portion of the spacer peptide plus p6 (Fig. 4B, SP-p6, lane 4), was able to associate in vitro with EF1α. Thus, the HIV-1 Gag polyprotein possesses two independent binding sites for EF1α, one located in MA, the second located in NC.
To determine if the portion of EF1α that binds NC is the same portion that binds MA, EF1α mutants shown in Fig. 2 were tested for their ability to associate with NC. The mutant EF1α 1–74 bound NC (data not shown), demonstrating that the same portion of EF1α is involved in the interaction with MA and NC.
Basic amino acid residues in NC are necessary for binding to EF1α.
HIV-1 NC is rich in basic residues and contains two zinc fingers. Both of these features have been demonstrated to be important for packaging the viral RNA genome into virions (22, 68). To determine if either of these features is necessary for NC binding to EF1α, a panel of NC mutants (Fig. 4C) was produced as GST fusion proteins and assayed for the ability to bind EF1α in vitro (Fig. 4D). Mutants predicted to significantly disrupt the function of the first zinc finger (F16A, lane 4), the second zinc finger (C36S, lane 2), or both zinc fingers (C15S/C36S, lane 3) were tested for EF1α-binding activity. None of these mutants had a significant effect on NC’s interaction with EF1α, indicating that the zinc fingers are dispensable for this activity.
A second group of NC mutants were tested in which basic amino acid residues were replaced with alanine. As shown in Fig. 4E, EF1α bound NC proteins with a single basic amino acid substitution (R7 and K14, lanes 7 and 8, respectively), as well as mutants with three (38-41-48, lane 6), four (BR, lane 3), or six (M1-2, lane 4) basic residues substituted. The only NC mutant unable to bind EF1α was M1-2/BR, in which a total of 10 basic residues were substituted with alanine (lane 5). These results indicate that a critical number of basic residues are required for binding of NC to EF1α.
HIV-1 Gag associates with EF1α in vivo.
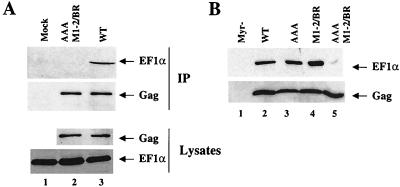
To confirm that the interaction between Gag and EF1α occurs in vivo, attempts were made to immunoprecipitate a Gag-EF1α complex. To express the HIV-1 Gag polyprotein in the absence of other viral proteins, 293T cells were transfected with a plasmid in which gag coding sequences contain multiple, conservative mutations that act at the RNA level to render gag expression Rev independent (70). Seventy-two hours posttransfection, the cellular lysate was immunoprecipitated with a rabbit polyclonal anti-CA antibody. The proteins in the precipitate were then analyzed by Western blotting for the presence of bound Gag and EF1α. As shown in the top panel of Fig. 5A, EF1α coimmunoprecipitated with wild-type Gag polyprotein (lane 3), but not with the double MA and NC mutant AAA M1-2/BR Gag (lane 2) nor with a mock-transfected control (lane 1). The inability to detect EF1α associated with the AAA M1-2/BR Gag mutant was not explained by a failure of the rabbit polyclonal anti-CA antibody to immunoprecipitate the mutant Gag polyprotein (Fig. 5A, second panel from the top, compare lanes 2 and 3). Similar amounts of EF1α and Gag proteins were also present in the cellular lysates prior to the immunoprecipitation (lower panels, as indicated). The anti-EF1α antibody did not function in immunoprecipitation, so it was not possible to perform the reciprocal coimmunoprecipitation experiment. These results nonetheless indicate that the interaction between Gag and EF1α occurs in vivo and that it requires the MA and NC domains as defined in our in vitro binding assays.
FIG. 5.
Effect of MA and NC mutations on HIV-1 Gag’s ability to associate with EF1α in vivo. (A) Anti-CA antibody was used to immunoprecipitate (IP) wild-type (WT) (lane 3) or AAA M1-2/BR double mutant (lane 2) Gag polyproteins from transfected 293T cell lysates. Immunoprecipitated proteins were analyzed by Western blotting with anti-EF1α and anti-CA antibodies for associated EF1α and for immunoprecipitated Gag (upper panels, as indicated). The same lysates used in the immunoprecipitation were analyzed directly in Western blots with an anti-CA and with an anti-EF1α antibody (lower panels, as indicated). Lane 1 shows results from mock-transfected cells. (B) Gag virions were produced by transfection of 293T cells with plasmids expressing myristylation-deficient (Myc−) Gag (lane 1), wild-type Gag (lane 2), MA mutant Gag AAA (lane 3), NC mutant Gag M1-2/BR (lane 4) or MA and NC double mutant Gag AAA M1-2/BR (lane 5). See Fig. 2 and 6 for the amino acid sequences of the AAA and M1-2/BR mutations. Virions were purified by centrifugation onto a sucrose step gradient (25 to 45%). The interface was harvested and then layered onto a 25% sucrose cushion for a final centrifugation step. Samples were analyzed by Western blotting for the presence of EF1α (top panel) and for the presence of Gag (lower panel). The positions of the EF1α and Gag proteins are indicated by arrows to the right of the figure.
As a complement to the coimmunoprecipitation studies, experiments were performed to determine if EF1α associates with HIV-1 Gag virions. 293T cells were transfected with the Rev-independent gag expression plasmid. Gag virions were purified from the cell culture supernatant by centrifugation onto a sucrose step gradient (25 to 45%). The interface was harvested and layered onto a 25% sucrose cushion for a final centrifugation step. Samples were then analyzed by Western blotting for the presence of EF1α (Fig. 5B, top panel) and Gag (Fig. 5B, lower panel). EF1α associated with Gag virions purified by this method (Fig. 5B, lane 2). EF1α was not detected when cells were transfected with Gag harboring a mutation that precludes myristylation and blocks virion release (Fig. 5B, lane 1); this control demonstrates that the detection of EF1α in association with particulate material in the supernatant requires the extracellular release of Gag protein.
As expected from our previous data, mutations that disrupted the interaction between EF1α and MA (AAA, lane 3) or NC (M1-2/BR, Fig. 5B, lane 4) had no obvious effect on the association of EF1α with Gag virions when either of these mutants was introduced in Gag (compare mutants with the wild type in lane 2). However, the combination of both mutants in the same Gag polyprotein severely impaired association of EF1α with Gag virions (AAA M1-2/BR, lane 5). These results demonstrate that two regions in the Gag polyprotein are independently sufficient for the association of EF1α with Gag virions.
EF1α associates with virions produced by a replication-competent HIV-1 provirus.
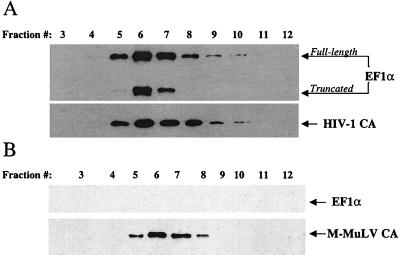
Experiments were performed to determine if EF1α associates with complete infectious HIV-1 virions. Virions produced by transfection of the infectious proviral clone NL4-3 into 293T cells were concentrated by centrifugation through a 25% sucrose cushion, resuspended, and layered onto a linear sucrose density gradient (20 to 60%). Following centrifugation, the gradient was harvested in 12 fractions. The proteins in each fraction were precipitated with trichloroacetic acid and analyzed by Western blotting for the presence of EF1α and CA proteins. EF1α comigrated in the gradient with the CA protein of HIV-1 (compare the top and bottom panels of Fig. 6A). A faster-mobility band reacting with the anti-EF1α antibody was also observed to migrate with the CA protein in the sucrose gradient (Fig. 6A). The identity of this band was further characterized in subsequent experiments.
FIG. 6.
EF1α associates specifically with HIV-1 virions. Virions were produced by transfection of 293T cells with HIV-1 (A) or M-MuLV (B) proviral DNAs. Virions were purified and concentrated from the supernatant by being pelleted through 25% sucrose and then were layered onto a linear sucrose gradient (20 by 60%). After centrifugation, fractions were collected from the top of the gradient and numbered from 1 to 12. Samples 3 to 12 are shown as indicated. Samples were analyzed by Western blotting and probed with an anti-EF1α antibody (A and B, top panels), with an anti-HIV-1 CA antibody (A, bottom panel) or with an anti-M-MLV CA antibody (B, bottom panel). The position and identity of the proteins are indicated by arrows on the right. The Western blot of the HIV-1 virion-associated proteins (A, top panel) shows the presence of a truncated form in addition to the full-length EF1α.
To determine if the association of EF1α with HIV-1 virions is specific for HIV-1, M-MuLV virions were similarly produced by transfection of 293T cells and purified on a linear sucrose density gradient. Fractions were collected and analyzed by Western blotting for the presence of EF1α and M-MuLV CA proteins. As shown in Fig. 6B (top panel), EF1α was not detected in association with M-MuLV CA protein.
HIV-1 and M-MuLV virions were also produced by transfection of 293T cells in the presence of [35S]Met-Cys. Virions were purified from the cell culture supernatant by centrifugation onto a sucrose step gradient as described above, resuspended, and normalized, as determined by quantitation of the signals obtained from the CA protein present in the two viruses. EF1α was not detected in M-MuLV viral preparations, even when fivefold more M-MuLV virions than HIV-1 virions were examined (data not shown). EF1α was also not detected in association with M-MuLV virions produced from an NIH 3T3 M-MuLV producer cell line (24), demonstrating that the failure to detect EF1α was not attributable to species-specific differences in the producer cell line (data not shown). These experiments demonstrate that EF1α associates with HIV-1 virions and not with M-MuLV virions, a result consistent with our in vitro binding results (Fig. 1).
Virion-associated EF1α is protected from subtilisin digestion and is processed by the viral protease.
To determine if EF1α is incorporated within the HIV-1 virion membrane, the resistance of virion-associated EF1α to exogenously added protease was examined. Protease protection assays have been used for many years by cell biologists to determine the orientation of membrane proteins (2) and, more recently, to show that HIV-1-associated proteins are contained within the viral membrane (65).
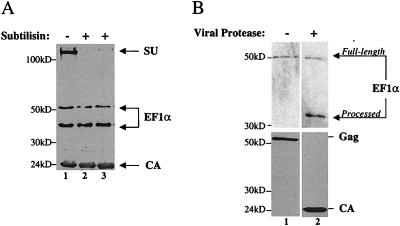
HIV-1 virions were obtained by transfection of 293T cells and purified by centrifugation through a 25% sucrose cushion. Virions were resuspended and either mock treated (Fig. 7A, lane 1) or treated with 1 or 0.1 mg of subtilisin per ml (Fig. 7A, lanes 2 and 3, respectively). After protease treatment, samples were again purified by centrifugation through 25% sucrose and analyzed by Western blotting for the presence of the envelope protein SU (gp120), EF1α, and CA proteins. As expected, SU was degraded by subtilisin, consistent with the location of this protein on the outside of the virion membrane (Fig. 7A, compare lane 1 with lanes 2 or 3). In contrast to SU, the virion core protein CA was resistant to digestion (Fig. 7A). Virion-associated EF1α was also resistant to digestion, indicating that EF1α is located within the virion membrane.
FIG. 7.
EF1α is incorporated within the HIV-1 virion membrane and is processed by the viral protease. (A) HIV-1 virions were purified by centrifugation onto a sucrose step gradient (25 to 45%). The interface was harvested and then layered onto a 25% sucrose cushion for a final centrifugation step. The virions thus obtained were mock treated (lane 1) or treated with 1 or 0.1 mg of subtilisin per ml (lanes 2 and 3, respectively). Samples were analyzed by Western blotting with antibodies against EF1α, gp120 (SU), and CA. The position of these proteins is indicated on the right; the positions of molecular mass markers (in kilodaltons) are indicated on the left. Note again that the anti-EF1α antibody detects two bands. (B) HIV-1 virions produced by a provirus with a functional protease (lane 2) or by a provirus bearing the protease-inactivating mutation D25R (lane 1) were purified as described for panel A and analyzed by Western blotting with anti-EF1α (top panels) or anti-CA (bottom panels) antibodies. The positions of migration of full-length and viral protease-processed forms of EF1α, as well as the positions of unprocessed Gag polyprotein (p55) and CA protein (p24), are indicated by arrows on the right. The positions of molecular mass markers (in kilodaltons) are indicated on the left.
A truncated form of EF1α with an apparent size of 34 to 36 kDa was observed in association with virions, but not in cell lysates (Fig. 6A and 7A). To determine if the truncated EF1α resulted from proteolytic processing by the active viral protease inside virions, EF1α present in wild-type virions was compared to EF1α present in virions in which the active site of the viral protease had been inactivated by a point mutation (D25R) (73). HIV-1 virions were produced and purified by two-step sucrose gradient. Samples were analyzed by Western blotting and probed with anti-CA (Fig. 7B, lower panels) and anti-EF1α (Fig. 7B, top panels) antibodies. Detection of the truncated form of EF1α was dependent upon the presence of a functional viral protease, providing further evidence that EF1α is a bona fide HIV-1 virion protein.
The interaction between HIV-1 Gag and EF1α is disrupted by RNase.
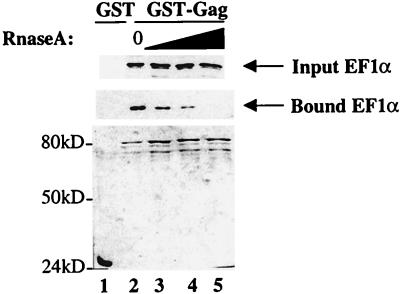
Both EF1α and Gag bind RNA. EF1α binds aminoacyl-tRNA, which it delivers to the ribosome during translation elongation (60). Gag binds and packages HIV-1 genomic mRNA into virions (4). Since basic amino acid residues are found in RNA-binding motifs, and basic amino acid residues in Gag are required for binding to EF1α, experiments were performed to determine if RNA is required for the association between these two proteins. Bacterial lysates expressing GST-Gag and EF1α were treated with RNase A before mixing them together in a typical binding reaction. Samples were then analyzed by Western blotting for the presence of EF1α in association with GST-Gag. With increasing concentrations of RNase A, decreasing amounts of EF1α remained associated with GST-Gag (Fig. 8, middle panel, bound EF1α; compare lane 2 with lanes 3 to 5). Treatment of the lysates with RNase A didn’t affect the stability of EF1α (Fig. 8, top panel, input EF1α) nor that of GST-Gag (bottom panel). These results suggest that the in vitro interaction between Gag and EF1α requires RNA.
FIG. 8.
The interaction between HIV-1 Gag and EF1α is disrupted by RNase A. Bacterial lysate expressing GST-Gag and 293T lysate used as a source of EF1α were each treated separately with increasing amounts of RNase A (lanes 3 to 5). Samples were then used in a typical binding reaction with glutathione-agarose beads as in Fig. 1. Proteins that remained associated with the beads at the conclusion of the binding experiment were analyzed by Western blotting for the presence of EF1α (bound EF1α, middle panel) and by Coomassie blue staining after SDS-PAGE (lower panel). Ten percent of the total EF1α input for each sample was loaded on a Western blot (input EF1α, top panel). Lane 1 shows binding to GST (RNase A untreated). Lanes 2 through 5 show binding to GST-Gag treated with 0, 1, 10, and 50 μg of RNase A, respectively.
Effect on virion assembly of a Gag mutant that doesn’t bind EF1α.
To obtain evidence that the interaction between Gag and EF1α is functionally relevant, several experimental approaches were tried. In the first approach, EF1α mutants EF1α 1–74 and H95L (Fig. 2A) were expressed in cells along with HIV-1 proviral DNA. Both of these EF1α mutants associate with Gag and therefore might compete with wild-type EF1α for binding to Gag. Since the first mutant is severely truncated and the second disrupts EF1α’s GTPase activity, either mutant might inhibit an EF1α-dependent Gag function in trans. No effect on virion yield or on virion assembly kinetics (pulse-chase experiments) was observed with either mutant (data not shown). In addition, no effect was seen on virion infectivity, as assessed by semiquantitative PCR of reverse transcription products within 8 h postinfection or by MAGI (multinuclear activation of a galactosidase indicator) assay (data not shown). The mutant proteins were expressed with two different high-level promoters (SRα and EF1α), but the interpretation of these experiments must be tempered by the fact that EF1α is one of the most abundant proteins in the cell (72), and it may not be possible to express the mutant proteins at levels sufficient for inhibition in trans.
A second experimental approach to address the function of EF1α in HIV-1 replication is to examine the infectivity of Gag mutants that don’t bind EF1α. Only one such mutant was identified (AAA M1-2/BR in Fig. 5) and it was subcloned into pNL4-3 for further studies. The previously characterized M1-2/BR mutant was reported to abolish viral infectivity but to have no effect on virion assembly (68); the AAA mutant was reported to show only a slight replication defect (49). Virions with the AAA M1-2/BR mutation were not infectious (data not shown), and transfection of an expression construct bearing this mutation demonstrated a defect in virion assembly. For example, to obtain sufficient AAA M1-2/BR mutant Gag virions for the analysis in Fig. 5B, at least fivefold more starting material was required than for the wild-type Gag.
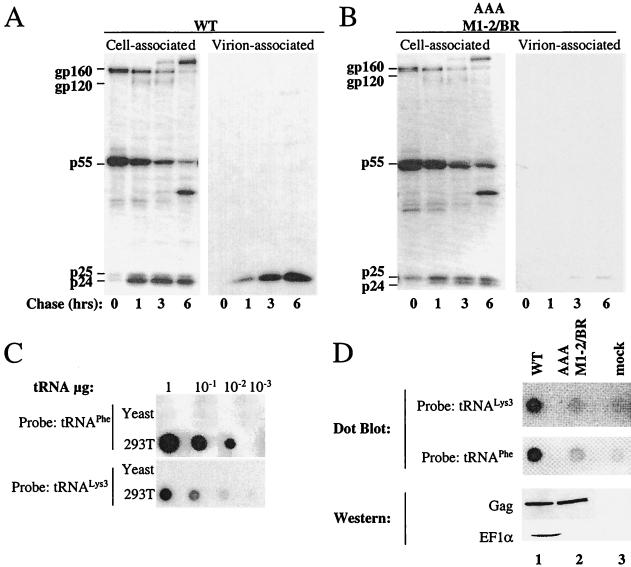
The effect of the AAA M1-2/BR mutation on the HIV-1 assembly process was examined in further detail. HeLa cells were transfected with wild-type and AAA M1-2/BR mutant proviral DNAs. After 48 h, cells were pulsed for 45 min with [35S]Met-Cys and chased for 1, 3, and 6 h as indicated (Fig. 9A and 9B). Virions were purified from culture supernatant by ultracentrifugation through 25% sucrose, and cells were lysed in RIPA buffer. Virion and cell-associated proteins were then immunoprecipitated with serum from an HIV-1-infected person. Compared to the wild-type NL4-3 (Fig. 9A), the AAA M1-2/BR mutant (Fig. 9B) showed at least a 10-fold reduction in virion yield over 6 h. This effect did not appear to be due to decreased stability of the mutant protein, since the amount of Gag polyprotein retained in the cell was greater for the mutant than for the wild type. Furthermore, the mutant showed an increased proportion of incompletely processed p25 in the cell-associated fraction, consistent with failure to assemble virions and to complete processing by the viral protease.
FIG. 9.
A Gag mutant that disrupts interaction with EF1α impairs virion assembly and Gag’s ability to bind tRNA. HeLa cells transfected with wild-type (WT) (A) and AAA M1-2/BR (B) NL4-3 were labeled with [35S]Met-Cys for 45 min and chased for 0, 1, 3, or 6 h, as indicated. MW, molecular mass markers. Virion-associated proteins were purified by ultracentrifugation through 25% sucrose. Virion and cell-associated viral proteins were immunoprecipitated with an HIV-1 antiserum and analyzed by SDS-PAGE. (C) Controls for quantitative dot blot assays specific for either human tRNAPhe or human tRNA3Lys. Increasing amounts of purified total human and yeast tRNAs (as indicated on the left of the panels) were immobilized on a nylon membrane and probed with a tRNAPhe probe (upper panel) or with a tRNA3Lys probe (lower panel). The amount of total tRNA loaded onto each spot is indicated on the top of the figure. (D) Wild-type and AAA M1-2/BR Gag polyproteins were expressed by transfection of 293T cells (lanes 1 and 2) and immunoprecipitated with an anti-CA antibody. A fraction of the samples were analyzed by Western blotting and probed with anti-CA or anti-EF1α antibodies (lower two panels, as indicated). A second aliquot of the samples was analyzed by dot blotting and probed with either tRNAPhe or tRNA3Lys-specific probes (upper two panels, as indicated). Lane 3, mock-transfected cells.
A Gag mutant that doesn’t interact with EF1α is impaired in its ability to bind tRNA.
Virions with the AAA M1-2/BR mutation are impaired in assembly (Fig. 9B). Therefore, effects that this mutation might have on other steps of the HIV-1 life cycle could not be studied. To better characterize the interaction between Gag and EF1α, experiments were performed to determine if there is a correlation between Gag’s ability to bind EF1α and its ability to bind RNA. These experiments were suggested by the fact that RNase A disrupts the interaction between Gag and EF1α in vitro (Fig. 8) and that EF1α is a major tRNA binding protein (60). NC has also been shown to bind tRNA (4, 46).
A dot blot assay was established for the quantification of tRNA associated with Gag polyproteins that had been immunoprecipitated from transfected cells. Two 32P-labeled oligonucleotide probes were used, one specific for human tRNA3Lys, the other specific for human tRNAPhe (55). tRNA obtained from 293T cells was blotted onto nylon membranes (Fig. 9C). Densitometric analysis of the signal obtained after hybridization with either probe demonstrated a linear increase in signal with increasing quantity of tRNA, as previously reported (55). Probe specificity was confirmed by showing that neither probe hybridized with total yeast tRNA (Fig. 9C).
Wild-type and AAA M1-2/BR mutant Gag were expressed by transfection of 293T cells. Seventy-two hours posttransfection, the Gag polyproteins in the cell lysates were immunoprecipitated with an anti-CA antibody (Fig. 9D). The presence of tRNA associated with CA immunoprecipitates from cells expressing wild-type Gag (Fig. 9D, lane 1), the AAA M1-2/BR Gag mutant (lane 2), or mock-transfected cells (lane 3), was determined by dot blotting. A specific signal was obtained with either the tRNA3Lys or the tRNAPhe probe (top panels, as indicated), indicating that tRNA associates with wild-type HIV-1 Gag polyprotein in the cytoplasm of transfected cells. Quantification of the signal obtained in dot blotting showed that the amount of tRNA associated with the AAA M1-2/BR mutant Gag polyprotein was reduced 10-fold compared to the wild type. This difference was reproducibly seen regardless of the probe used in the dot blot. This difference was not due to the decreased amount of mutant Gag polyprotein present in the immunoprecipitate (Fig. 9D, lower panels, as indicated). As expected, EF1α was detected only in association with wild-type but not mutant Gag (lower panels, as indicated).
HIV-1 MA inhibits in vitro translation.
EF1α is an essential translation factor (60). Since Gag binds EF1α, the effect of Gag on translation efficiency in an RRL system was examined. For technical reasons related to protein purification and quantification, recombinant MA was used in these experiments rather than the complete Gag polyprotein. GST-MA was purified from bacterial lysate by affinity chromatography. As a control, GST was prepared in the same way, as well as GST-MA dB5, which contains a deletion encompassing the basic region of MA (Fig. 3A). The purity of the three proteins was assayed by SDS-PAGE with Coomassie staining (Fig. 10A), and concentrations were determined by Bradford assay. Increasing amounts of each purified GST fusion protein were added to a constant amount of RRL. Firefly luciferase encoded by an in vitro-synthesized, capped mRNA was used as a translation reporter. After incubation of the RRL with the GST fusion proteins at 4°C, reporter mRNA and amino acids were added, and translation was allowed to proceed by shifting the temperature to 30°C. In pilot experiments, the amount of luciferase activity recovered in the lysate correlated perfectly with the amount of luciferase protein produced in the RRL, as determined by metabolic labeling (data not shown). Therefore, in all subsequent experiments, the luciferase activity produced in the samples was used as a measure of translation efficiency.
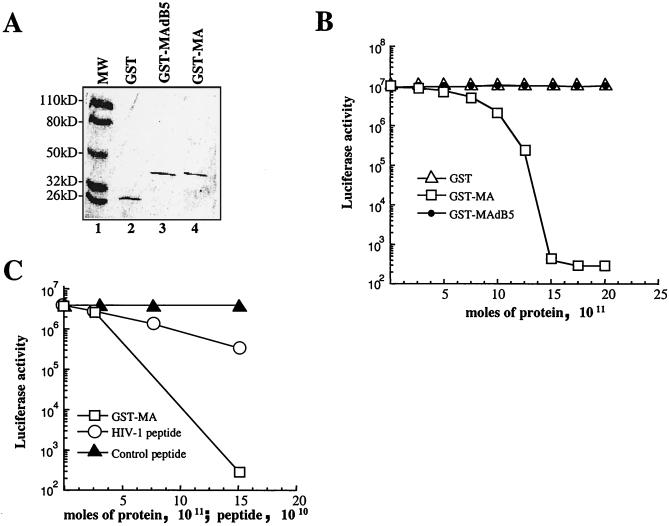
FIG. 10.
HIV-1 MA inhibits in vitro translation. (A) GST, wild-type GST-MA, and GST-MA dB5 (with the basic region deleted and unable to bind to EF1α) were expressed in bacteria, purified, and analyzed by Coomassie staining after SDS-PAGE, as indicated. (B) Increasing amounts of purified GST proteins (abscissa) were added to a constant amount of RRL. Luciferase-encoding mRNA was added to the reaction mixture, and the activity of the luciferase produced was used as a measure of translation efficiency (ordinate). Similar results were obtained in three separate experiments. (C) Increasing amounts of peptide encompassing the HIV-1 MA basic region or the simian virus 40 T-antigen NLS were added to a constant amount of RRL and analyzed as described above.
In Fig. 10B, the luciferase activity produced in each sample is plotted on the ordinate against the quantity of GST fusion protein added to the RRL on the abscissa. Addition of purified GST-MA decreased luciferase activity almost 5 orders of magnitude to levels of activity observed with control reactions in which amino acids or luciferase mRNA were omitted. Equimolar amounts of GST or GST-MA dB5 had no effect on luciferase activity. Inhibition of translation by MA was not dependent on the presence of a cap on the mRNA, since similar inhibition was observed with an uncapped mRNA (data not shown). To determine if HIV-1 RNA possesses properties that allow it to be efficiently translated in the presence of MA, an mRNA reporter was synthesized in which the HIV-1 leader sequence was placed upstream of luciferase coding sequences. Inhibition of translation by MA with this construct was identical to that obtained with the conventional reporter (data not shown).
As a complement to the experiments described above, a peptide encompassing the MA basic region was assayed for its ability to inhibit translation in the same RRL system (Fig. 10C). A peptide encompassing the simian virus 40 T-antigen nuclear localization signal, with a comparable number of basic residues, was used as control peptide. Inhibition of translation was observed with the peptide encompassing the HIV-1 MA basic region, but not with the control peptide. The magnitude of the inhibition by the peptide was less than that observed with the entire MA protein.
DISCUSSION
In the present study, we demonstrated that EF1α binds to two independent domains in the HIV-1 Gag polyprotein, one in MA and the second in NC. As a consequence of its interaction with HIV-1 Gag, EF1α is incorporated into HIV-1 virions, where it is cleaved by the viral protease and protected within the virion membrane from digestion with exogenously added subtilisin. The exact role of the interaction between Gag and EF1α in viral replication could not be pinpointed. We were unable to disrupt the interaction in trans by expressing mutant EF1α molecules, most probably due to the abundance of native EF1α. Despite the extensive mutagenesis presented here, we were only able to identify a single Gag mutant that abolished binding to EF1α (Fig. 5). This mutant severely impairs virion assembly, but due to the drastic nature of this mutant (alanine is substituted for 3 basic residues in MA and 10 basic residues in NC), we are not comfortable concluding that this phenotype is related solely to disruption of the interaction with EF1α. As a consequence of the Gag mutant assembly defect, we have been unable to produce amounts of EF1α-deficient virions sufficient to accurately determine if EF1α is necessary for virion infectivity.
Previous reports showed that the cellular proteins actin and moesin are incorporated into HIV-1 virions, where they are cleaved by the viral protease (65). The significance of protease cleavage of cellular proteins in virions is not known, but the presence of actin and EF1α in HIV-1 virions is interesting. EF1α binds actin and bundles actin microfilaments (15), and the HIV-1 Gag polyprotein has been shown to associate with filamentous actin (69). To test if incorporation of EF1α into HIV-1 virions requires actin microfilaments or interaction with actin, we produced HIV-1 virions in the presence of cytochalasin D. The drug had no effect on EF1α incorporation into HIV-1 virions (data not shown), suggesting that EF1α incorporation doesn’t require intact actin microfilaments. Furthermore, the association between Gag and EF1α appears to be direct, since bacterially expressed HIV-1 Gag and EF1α interact in vitro (Fig. 2). Therefore, we infer that actin is not required for EF1α incorporation into HIV-1 virions.
The interaction between Gag and EF1α requires RNA. This is suggested by the requirement for basic residues for the association of Gag with EF1α (Fig. 3 and 4) and by the finding that interaction between Gag and EF1α is disrupted in vitro by RNase (Fig. 8). What is the identity of this RNA? EF1α binds to the RNA genomes of viruses that possess tRNA-like structures (5, 36, 37) and might therefore bind to secondary structures present in the HIV-1 genomic RNA. Our experiments demonstrated that the complete HIV-1 genomic RNA is not required for interaction between Gag and EF1α (Fig. 1, 3, and 4), and we hypothesized that tRNA is the most likely candidate to mediate this interaction. EF1α binds aminoacyl-tRNA during translation (60). NC has been shown to bind tRNA (46) and to promote primer tRNA annealing onto viral genomic RNA (56). Consistent with this hypothesis, a Gag mutant unable to bind EF1α is impaired in tRNA binding. Therefore, our current view is that the EF1α-tRNA complex is recognized by the MA and NC domains of the Gag polyprotein in the cytoplasm of infected cells and is incorporated into HIV-1 virions.
What determines the specificity of the interaction between HIV-1 Gag and EF1α? It is clear that the presence of basic residues is not sufficient for binding to EF1α: EF1α binds in vitro to HIV-1 and SIVmac239 MAs but doesn’t bind to MAs obtained from M-PMV and M-MuLV, although the latter proteins display clusters of basic residues (16, 74). In addition, EF1α is incorporated into HIV-1 virions, but not into M-MuLV virions. At present, despite the extensive mutagenesis presented here, the molecular basis for the specificity of the interaction between HIV-1 Gag and EF1α remains unclear. As mentioned above, we believe that EF1α is incorporated into virions as a complex with tRNA. However, the EF1α-tRNA complex cannot be responsible for the bulk incorporation of tRNA into virions, since M-MuLV virions also contain tRNA, but they don’t incorporate EF1α. Therefore, the tRNA-EF1α complex must serve some specific function that is unique for the HIV-1 life cycle.
Intriguingly, addition of wild-type HIV-1 MA to an RRL inhibits translation of a reporter gene. The same assay was recently used to show that the herpes simplex virus protein ICP0 interacts with EF1δ and inhibits translation (45). The amount of recombinant MA protein that must be added to obtain complete inhibition of translation equals the estimated concentration of EF1α present in RRL (72). Evidence that MA does not inhibit translation by binding directly to RNA comes from experiments in which we varied the concentration of RNA present in the assay by 100-fold and observed no change in the magnitude of the effect of MA on translation (data not shown). If MA were inhibiting translation by binding to RNA, we would have expected that larger quantities of MA would have been required under these conditions to obtain complete inhibition. Also, our peptide inhibition experiments comparing the HIV-1 basic region with the simian virus 40 NLS show that inhibition of translation is not simply due to the presence of basic amino acids.
In vivo translation inhibition has not been conclusively demonstrated for HIV-1, although it has been suggested that the Tat protein specifically inhibits cellular translation, without inhibiting viral translation, by binding to EF1δ (80). In our system, however, inhibition of translation by MA occurred when the reporter gene was placed upstream of the HIV-1 leader, indicating no differential effect of MA on translation of HIV-1 mRNAs. Similar results were also recently shown in vivo for RSV (75).
Gag-mediated inhibition of translation might be used to rout viral genomic RNA between translation and packaging. Upon leaving the nucleus, the unspliced HIV-1 transcript has two functions. It serves as mRNA encoding gag and pol proteins and as genomic RNA that is packaged into virions. The factors that determine the path taken by a given RNA molecule are not known. The observation by others that the accumulation of RSV Gag inhibits translation of viral RNA, prompted these investigators to propose that this inhibition of translation might free genomic RNA from the translation machinery, making it available for packaging (75). Similarly, as the local concentration of HIV-1 Gag increases in a cell, Gag association with the EF1α-tRNA complex might inhibit translation and, in effect, free viral RNA from the translation machinery. Once this initial step occurs, nucleation of the Gag polyproteins on genomic RNA might lead to further translation inhibition and subsequently to packaging of viral RNA into the nascent virions. Inhibition of translation by Gag might only occur locally in the cell at the site of Gag translation. We were unable to demonstrate inhibition of translation of a luciferase reporter cotransfected with a gag expression plasmid. Gag polyproteins of other retroviruses might have evolved a specific interaction with components of the translation machinery other than EF1α in order to attain the same aim.
ACKNOWLEDGMENTS
We thank Anna Aldovini, Eric Barklis, Roger Brent, Ariberto Fassati, Eric Freed, Heinrich Göttlinger, Stephen Goff, Eric Hunter, Larry Kleiman, Leslie Parent, George Pavlakis, David Shore, John Wills, and Xiao-Fang Yu for generously providing plasmid DNAs and other reagents. We thank Douglas Braaten and Cagan Gurer for critical reading of the manuscript.
This work was supported by grant AI 41857 (J.L.) and by shared core facilities of the Columbia-Rockefeller Center for AIDS Research (P30 AI42848), both from the National Institutes of Health. For part of this project period, A.C. was a recipient of a fellowship from the Istituto Superiore di Sanita′ (Rome, Italy).
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D J, Walter P, Blobel G. Signal recognition protein is required for the integration of acetylcholine receptor delta subunit, a transmembrane glycoprotein, into the endoplasmic reticulum membrane. J Cell Biol. 1982;93:501–506. doi: 10.1083/jcb.93.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett R P, Nelle T D, Wills J W. Functional chimeras of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J Virol. 1993;67:6487–6498. doi: 10.1128/jvi.67.11.6487-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkowitz R, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell J L, Brinton M A. Translation elongation factor-1 alpha interacts with the 3′ stem-loop region of West Nile virus genomic RNA. J Virol. 1997;71:6433–6444. doi: 10.1128/jvi.71.9.6433-6444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braaten D, Franke E K, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bram R J, Hung D T, Martin P K, Schreiber S L, Crabtree G R. Identification of the immunophilins capable of mediating inhibition of signal transduction by cyclosporin A and FK506: roles of calcineurin binding and cellular location. Mol Cell Biol. 1993;13:4760–4769. doi: 10.1128/mcb.13.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burniston, M., A. Cimarelli, J. Colgan, S. Curtis, and J. Luban. Submitted for publication.
- 12.Callahan M A, Handley M A, Lee Y-H, Talbot K J, Harper J W, Panganiban A T. Functional interaction of human immunodeficiency virus type 1 Vpu and Gag with a novel member of the tetratricopeptide repeat protein family. J Virol. 1998;72:5189–5197. doi: 10.1128/jvi.72.6.5189-5197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camaur D, Gallay P, Swingler S, Trono D. Human immunodeficiency virus matrix tyrosine phosphorylation: characterization of the kinase and its substrate requirements. J Virol. 1997;71:6834–6841. doi: 10.1128/jvi.71.9.6834-6841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colgan J, Yuan H E H, Franke E K, Luban J. Binding of the human immunodeficiency virus type 1 Gag polyprotein to cyclophilin A is mediated by the central region of capsid and requires Gag dimerization. J Virol. 1996;70:4299–4310. doi: 10.1128/jvi.70.7.4299-4310.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Condeelis J. Elongation factor 1 alpha, translation and the cytoskeleton. Trends Biochem Sci. 1995;20:169–170. doi: 10.1016/s0968-0004(00)88998-7. [DOI] [PubMed] [Google Scholar]
- 16.Conte M R, Klikova M, Hunter E, Ruml T, Matthews S. The three-dimensional solution structure of the matrix protein from the type D retrovirus, the Mason-Pfizer monkey virus, and implications for the morphology of retroviral assembly. EMBO J. 1997;16:5819–5826. doi: 10.1093/emboj/16.19.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosson P. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 1996;15:5783–5788. [PMC free article] [PubMed] [Google Scholar]
- 18.Craven R C, Parent L J. Dynamic interactions of the Gag polyprotein. Curr Top Microbiol Immunol. 1996;214:65–94. doi: 10.1007/978-3-642-80145-7_3. [DOI] [PubMed] [Google Scholar]
- 19.Cullen B R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- 20.Darlix J L, Gabus C, Nugeyre M T, Clavel F, Barre-Sinoussi F. Cis elements and trans-acting factors involved in the RNA dimerization of the human immunodeficiency virus HIV-1. J Mol Biol. 1990;216:689–699. doi: 10.1016/0022-2836(90)90392-Y. [DOI] [PubMed] [Google Scholar]
- 21.Dorfman T, Bukovsky A, Öhagen Å, Höglund S, Göttlinger H G. Functional domains of the capsid protein of human immunodeficiency virus type 1. J Virol. 1994;68:8180–8187. doi: 10.1128/jvi.68.12.8180-8187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorfman T, Luban J, Goff S P, Haseltine W A, Göttlinger H G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993;67:6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farnet C M, Haseltine W A. Integration of human immunodeficiency virus type 1 DNA in vitro. Proc Natl Acad Sci USA. 1990;87:4164–4168. doi: 10.1073/pnas.87.11.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fassati A, Wells D J, Walsh F S, Dickson G. Transplantation of retroviral producer cells for in vivo gene transfer into mouse skeletal muscle. Hum Gene Ther. 1996;7:595–602. doi: 10.1089/hum.1996.7.5-595. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y X, Copeland T D, Henderson L E, Gorelick R J, Bosche W J, Levin J G, Rein A. HIV-1 nucleocapsid protein induces “maturation” of dimeric retroviral RNA in vitro. Proc Natl Acad Sci USA. 1996;93:7577–7581. doi: 10.1073/pnas.93.15.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher A G, Collalti E, Ratner L, Gallo R C, Wong-Staal F. A molecular clone of HTLV-III with biological activity. Nature. 1985;316:262–265. doi: 10.1038/316262a0. [DOI] [PubMed] [Google Scholar]
- 27.Fouchier R A, Meyer B E, Simon J H, Fischer U, Malim M H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franke E K, Yuan H E H, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 29.Freed E O, Englund G, Martin M A. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J Virol. 1995;69:3949–3954. doi: 10.1128/jvi.69.6.3949-3954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freed E O, Martin M A. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J Virol. 1995;69:1984–1989. doi: 10.1128/jvi.69.3.1984-1989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freed E O, Martin M A. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J Virol. 1996;70:341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuta R A, Shimano R, Ogasawara T, Inubushi R, Amano K, Akari H, Hatanaka M, Kawamura M, Adachi A. HIV-1 capsid mutants inhibit the replication of wild-type virus at both early and late infection phases. FEBS Lett. 1997;415:231–234. doi: 10.1016/s0014-5793(97)01132-0. [DOI] [PubMed] [Google Scholar]
- 33.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 34.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 35.Gamble T R, Vajdos F F, Yoo S, Worthylake D K, Houseweart M, Sundquist W I, Hill C P. Crystal structure of human cyclophilin A bound to the amino-terminal domain of HIV-1 capsid. Cell. 1996;87:1285–1294. doi: 10.1016/s0092-8674(00)81823-1. [DOI] [PubMed] [Google Scholar]
- 36.Giege R. Interplay of tRNA-like structures from plant viral RNAs with partners of the translation and replication machineries. Proc Natl Acad Sci USA. 1996;93:12078–12081. doi: 10.1073/pnas.93.22.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodwin J B, Dreher T W. Transfer RNA mimicry in a new group of positive-strand RNA plant viruses, the furoviruses: differential aminoacylation between the RNA components of one genome. Virology. 1998;246:170–178. doi: 10.1006/viro.1998.9193. [DOI] [PubMed] [Google Scholar]
- 38.Göttlinger H G, Dorfman T, Sodroski J G, Haseltine W A. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc Natl Acad Sci USA. 1991;88:3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Göttlinger H G, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gulizia J, Dempsey M P, Sharova N, Bukrinsky M I, Spitz L, Goldfarb D, Stevenson M. Reduced nuclear import of human immunodeficiency virus type 1 preintegration complexes in the presence of a prototypic nuclear targeting signal. J Virol. 1994;68:2021–2025. doi: 10.1128/jvi.68.3.2021-2025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 42.Huang M, Orenstein J M, Martin M A, Freed E O. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J Virol. 1995;69:6810–6818. doi: 10.1128/jvi.69.11.6810-6818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Khorchid A, Gabor J, Wang J, Li X, Darlix J-L, Wainberg M A, Kleiman L. The role of nucleocapsid and U5 stem/A-rich loop sequences in tRNA3Lys genomic placement and initiation of reverse transcription in human immunodeficiency virus type 1. J Virol. 1998;72:3907–3915. doi: 10.1128/jvi.72.5.3907-3915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacque J M, Mann A, Enslen H, Sharova N, Brichacek B, Davis R J, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawaguchi Y, Bruni R, Roizman B. Interaction of herpes simplex virus 1 α regulatory protein ICP0 with elongation factor 1 δ: ICP0 affects translational machinery. J Virol. 1997;71:1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan R, Giedroc D P. Recombinant human immunodeficiency virus type 1 nucleocapsid (NCp7) protein unwinds tRNA. J Biol Chem. 1992;267:6689–6695. [PubMed] [Google Scholar]
- 47.Kiernan R E, Ono A, Englund G, Freed E O. Role of matrix in an early postentry step in the human immunodeficiency virus type 1 life cycle. J Virol. 1998;72:4116–4126. doi: 10.1128/jvi.72.5.4116-4126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lama J, Trono D. Human immunodeficiency virus type 1 matrix protein interacts with cellular protein HO3. J Virol. 1998;72:1671–1676. doi: 10.1128/jvi.72.2.1671-1676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee Y-M, Tang X-B, Cimakasky L M, Hildreth J E K, Yu X-F. Mutations in the matrix protein of human immunodeficiency virus type 1 inhibit surface expression and virion incorporation of viral envelope glycoproteins in CD4+ T lymphocytes. J Virol. 1997;71:1443–1452. doi: 10.1128/jvi.71.2.1443-1452.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Quan Y, Arts E J, Li Z, Preston B D, de Rocquigny H, Roques B P, Darlix J-L, Kleiman L, Parniak M A, Wainberg M A. Human immunodeficiency virus type 1 nucleocapsid protein (NCp7) directs specific initiation of minus-strand DNA synthesis primed by human tRNA3Lys in vitro: studies of viral RNA molecules mutated in regions that flank the primer binding site. J Virol. 1996;70:4996–5004. doi: 10.1128/jvi.70.8.4996-5004.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lodge R, Göttlinger H, Gabuzda D, Cohen É, Lemay G. The intracytoplasmic domain of gp41 mediates polarized budding of human immunodeficiency virus type 1 in MDCK cells. J Virol. 1994;68:4857–4861. doi: 10.1128/jvi.68.8.4857-4861.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luban J. Absconding with the chaperone: essential cyclophilin-Gag interaction in HIV-1 virions. Cell. 1996;87:1157–1159. doi: 10.1016/s0092-8674(00)81811-5. [DOI] [PubMed] [Google Scholar]
- 53.Luban J, Bossolt K A, Franke E K, Kalpana G V, Goff S P. Human immunodeficiency virus type 1 gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 54.Mak J, Jiang M, Wainberg M A, Hammarskjöld M-L, Rekosh D, Kleiman L. Role of Pr160gag-pol in mediating the selective incorporation of tRNALys into human immunodeficiency virus type 1 particles. J Virol. 1994;68:2065–2072. doi: 10.1128/jvi.68.4.2065-2072.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mak J, Khorchid A, Cao Q, Huang Y, Lowy I, Parniak M A, Prasad V R, Wainberg M A, Kleiman L. Effects of mutations in Pr160gag-pol upon tRNA(Lys3) and Pr160gag-pol incorporation into HIV-1. J Mol Biol. 1997;265:419–431. doi: 10.1006/jmbi.1996.0742. [DOI] [PubMed] [Google Scholar]
- 56.Mak J, Kleiman L. Primer tRNAs for reverse transcription. J Virol. 1997;71:8087–8095. doi: 10.1128/jvi.71.11.8087-8095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mammano F, Kondo E, Sodroski J, Bukovsky A, Göttlinger H G. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J Virol. 1995;69:3824–3830. doi: 10.1128/jvi.69.6.3824-3830.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mammano F, Öhagen Å, Höglund S, Göttlinger H G. Role of the major homology region of human immunodeficiency virus type 1 in virion morphogenesis. J Virol. 1994;68:4927–4936. doi: 10.1128/jvi.68.8.4927-4936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDermott J, Farrell L, Ross R, Barklis E. Structural analysis of human immunodeficiency virus type 1 Gag protein interactions, using cysteine-specific reagents. J Virol. 1996;70:5106–5114. doi: 10.1128/jvi.70.8.5106-5114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merrick W C, Hershey J W B. The pathway and mechanism of eukaryotic protein synthesis. In: Hershey J W B, editor. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 31–69. [Google Scholar]
- 61.Miller M D, Farnet C M, Bushman F D. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Myers G, Wain-Hobson S, Korber B, Smith R F, Pavlakis G N. Human retroviruses and AIDS. A compilation of nucleic acid and amino acid sequences. Los alamos, N.Mex: Theoretical Biology and Biophysics, Los Alamos National Laboratory; 1994. [Google Scholar]
- 63.Nissen P, Kjeldgaard M, Thirup S, Polekhina G, Reshetnikova L, Clark B F, Nyborg J. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science. 1995;270:1464–1472. doi: 10.1126/science.270.5241.1464. [DOI] [PubMed] [Google Scholar]
- 64.Ott D E, Coren L V, Copeland T D, Kane B P, Johnson D G, Sowder II R C, Yoshinaka Y, Oroszlan S, Arthur L O, Henderson L E. Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type 1 and simian immunodeficiency virus and to the p12Gag protein of Moloney murine leukemia virus. J Virol. 1998;72:2962–2968. doi: 10.1128/jvi.72.4.2962-2968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ott D E, Coren L V, Kane B P, Busch L K, Johnson D G, Sowder II R C, Chertova E N, Arthur L O, Henderson L E. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J Virol. 1996;70:7734–7743. doi: 10.1128/jvi.70.11.7734-7743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Owens R J, Dubay J W, Hunter E, Compans R W. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc Natl Acad Sci USA. 1991;88:3987–3991. doi: 10.1073/pnas.88.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parent L J, Wilson C B, Resh M D, Wills J W. Evidence for a second function of the MA sequence in the Rous sarcoma virus Gag protein. J Virol. 1996;70:1016–1026. doi: 10.1128/jvi.70.2.1016-1026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poon D T K, Wu J, Aldovini A. Charged amino acid residues of human immunodeficiency virus type 1 nucleocapsid p7 protein involved in RNA packaging and infectivity. J Virol. 1996;70:6607–6616. doi: 10.1128/jvi.70.10.6607-6616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rey O, Canon J, Krogstad P. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology. 1996;220:530–534. doi: 10.1006/viro.1996.0343. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber B K, Pavlakis G N. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol. 1992;66:7176–7182. doi: 10.1128/jvi.66.12.7176-7182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimano R, Iida S, Fukumori T, Yamamoto Y, Kawamura M, Furuta R A, Adachi A. Inhibition of HIV replication by capsid mutant C6b. Biochem Biophys Res Commun. 1998;242:313–316. doi: 10.1006/bbrc.1997.7963. [DOI] [PubMed] [Google Scholar]
- 72.Slobin L I. The role of eucaryotic factor Tu in protein synthesis. The measurement of the elongation factor Tu content of rabbit reticulocytes and other mammalian cells by a sensitive radioimmunoassay. Eur J Biochem. 1980;110:555–563. doi: 10.1111/j.1432-1033.1980.tb04898.x. [DOI] [PubMed] [Google Scholar]
- 73.Smith A J, Srinivasakumar N, Hammarskjold M-L, Rekosh D. Requirements for incorporation of Pr160gag-pol from human immunodeficiency virus type 1 into virus-like particles. J Virol. 1993;67:2266–2275. doi: 10.1128/jvi.67.4.2266-2275.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soneoka Y, Kingsman S M, Kingsman A J. Mutagenesis analysis of the murine leukemia virus matrix protein: identification of regions important for membrane localization and intracellular transport. J Virol. 1997;71:5549–5559. doi: 10.1128/jvi.71.7.5549-5559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonstegard T S, Hackett P B. Autogenous regulation of RNA translation and packaging by Rous sarcoma virus Pr76gag. J Virol. 1996;70:6642–6652. [PMC free article] [PubMed] [Google Scholar]
- 76.Swanstrom R, Wills J W. Synthesis, assembly and processing of viral proteins. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 263–334. [PubMed] [Google Scholar]
- 77.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K-I, Yoshida M, Arai N. SRα promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh C T, Sodroski J, Gottlinger H G. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 79.Wang C-T, Zhang Y, McDermott J, Barklis E. Conditional infectivity of a human immunodeficiency virus matrix domain deletion mutant. J Virol. 1993;67:7067–7076. doi: 10.1128/jvi.67.12.7067-7076.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiao H, Neuveut C, Benkirane M, Jeang K T. Interaction of the second coding exon of Tat with human EF-1 delta delineates a mechanism for HIV-1-mediated shut-off of host mRNA translation. Biochem Biophys Res Commun. 1998;244:384–389. doi: 10.1006/bbrc.1998.8274. [DOI] [PubMed] [Google Scholar]
- 81.Yu X, Yu Q-C, Lee T-H, Essex M. The C terminus of human immunodeficiency virus type 1 matrix protein is involved in early steps of the virus life cycle. J Virol. 1992;66:5667–5670. doi: 10.1128/jvi.66.9.5667-5670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan X, Yu X, Lee T-H, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeidler W, Egle C, Ribeiro S, Wagner A, Katunin V, Kreutzer R, Rodnina M, Wintermeyer W, Sprinzl M. Site-directed mutagenesis of Thermus thermophilus elongation factor Tu. Replacement of His85, Asp81 and Arg300. Eur J Biochem. 1995;229:596–604. doi: 10.1111/j.1432-1033.1995.tb20503.x. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Barklis E. Effects of nucleocapsid mutations on human immunodeficiency virus assembly and RNA encapsidation. J Virol. 1997;71:6765–6776. doi: 10.1128/jvi.71.9.6765-6776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou W, Parent L J, Wills J W, Resh M D. Identification of a membrane-binding domain within the amino-terminal region of the human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]