Abstract
Background/purpose
There are reports on the relationship between periodontal treatment and the whole body. The purpose of the present study was to investigate the effect of periodontal initial treatment on brain function activity by improving periodontal tissue and the occlusal status of subjects with periodontitis.
Materials and methods
The subjects were 13 patients with periodontitis. Following the patient's informed written consent, the periodontal initial treatment provided to the patient included tooth brushing instruction, scaling and root planning, however, occlusal adjustment was not performed at this stage. Periodontal examination, occlusal force examination and fMRI results were also evaluated at the initial and the reevaluation examinations.
Results
After the periodontal initial treatment had been performed, periodontal tissue had significantly improved. In addition, cerebral blood flow in the insula and primary motor cortex was also improved, as confirmed by fMRI.
Conclusion
This result suggests that the periodontal ligament has recovered and the periodontal ligament neuron have been further subjected to clenching in the insula.
Keywords: Brain activation, Clenching task, Functional magnetic resonance imaging, Occlusion, Periodontal initial treatment
Introduction
We have demonstrated that tooth movement and displacement in periodontal diseases are caused by the destruction of periodontal tissue.1 It was found that the improvement of periodontal tissue resulting from periodontal initial treatment significantly increased the occlusal force.2 Thus, periodontal treatment not only improved the periodontal tissue, but also increased the occlusion.
Periodontal disease is considered to be not only a local oral infection, but also a chronic inflammatory disease in which recent and inflammatory substances in periodontal tissues affect various systemic diseases. In addition, periodontal medicine in relation to periodontal and systemic diseases has previously been reported.3 Nobel et al.4 noted that periodontal disease caused functional changes in both memory and cognition. In recent years, the relationship between periodontal disease and Alzheimer's disease has been described in studies involving laboratory mice and it was noted that periodontal disease did impact upon brain function.5 The purpose of periodontal therapy is to improve the function of the tissue as well as to improve occlusion function. Therefore, we hypothesized that the improvement of periodontal tissue and the occlusal state should also impact upon brain function. Functional magnetic resonance imaging (fMRI) is one method for evaluating brain activity and the analysis of the task dominant region of the brain at the central level using fMRI, is both a noninvasive and simple procedure.
It has been reported that there is a relationship between chewing function and brain function.6 However, there is no report that examines the change in brain function resulting from periodontal treatment. We hypothesized that periodontal treatment would recover brain function, as periodontal disease has been reported to affect the brain.4,5 The purpose of this study was to investigate the effect of periodontal treatment on brain function activity by improving periodontal tissue and the occlusal status of subjects with periodontitis.
Materials and methods
Study design
This study was approved by the human subjects ethics board of the Kyushu Dental University Ethics Committee (No. 11–39) and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Our subjects were thirteen patients (4 males and 9 females), who were diagnosed as having periodontitis via clinical and radiographic examination at the Kyushu Dental University Hospital. The subjects average age was 62.5 ± 8.5 (range: 45–74 years old), including 1 subject in 40s, 4 in 50s, 5 in 60s, and 3 in 70s. The subjects average number of teeth were 22.9 ± 4.6 (range: 15–29), including 3 between 10 and 20 and 10 with more than 20. The occlusal conditions of the upper and lower teeth of the subjects were selected according to the Eichner's index.7 The numbers of subjects belonging to Eichner's A1, A2, A3, B1, B2, B3 and B4 categories were 1, 2, 5, 1, 1, 1 and 2, respectively.
The 6 subjects with systemic diseases included 1 with hyperlipidemia, 3 with hypertension, and 2 with type 2 diabetes mellitus. All subjects were controlled within the standard values of oral administration.
Examination
The following examinations were carried out at the first visit and at the subsequent reevaluation.
At the first visit and reevaluation, periodontal clinical parameters such as probing pocket depth (PPD), clinical attachment level (CAL) and bleeding on probing as a percentage of the measured area (%BOP(+)) were examined. PPD and CAL measurements of the mesial, central and distal of the buccal and lingual side of each tooth were achieved using a William's probe. The %BOP(+) was considered positive when bleeding occurred within 30 s after probing.
Additionally, the periodontal inflamed surface area (PISA) and the periodontal epithelial surface area (PESA) were methods used by Nasse et al.8 measured in all participants and were calculated using an automatically computable EXCEL form known as PISA and PESA as an alternative to the parsprototo. info website.
In order to measure occlusal force, the subjects sat in the seated position and a pressure-sensitive measurement sheet (Dental Prescale® 50H–R type, GC Co, Tokyo, Japan) was inserted into the mouth. At this time, the subject chewed at the occlusal engagement position with the maximum occlusal force for 3 s and the occlusion state was recorded. This procedure was then repeated three times.
WBC (white blood cell), RBC (red blood cell) and Hb (hemoglobin) of complete blood count, AST, ALT, γ-GTP (γ-glutamyl transpeptidase), HbA1c and leptin of blood chemistry test, High-sensitivity CRP of the immunological test were measured. The subjects didn't eat or drink after dinner on the day before their medical check-up. Blood samples were taken in the morning and blood testing was then outsourced to the clinical laboratory (SRL Inc, Tokyo, Japan).
The MRI apparatus used (1.5-T full-body MR system, EXCELART Vantage™ Powered by Atlas, Toshiba, Tokyo, Japan) was provided by the Dental Radiology Department of the Kyushu Dental University Hospital. Observance of the brain activity site using the fMRI measurement was a method used by Oda et al.9 The subjects entered the MRI room and were provided with MRI room headphones instructions and ear protection from noise during the MRI examination. They were then photographed in a supine position with the eye ear plane set so as to be perpendicular to the floor surface. In addition, to prevent the patient's head from moving, the forehead was held in position with a pad and belt. The subjects were then given instructions from the control room and were asked to carry out a chewing motion on both sides of their mouth. The task of double-sided bite occlusion for 30 s and non-occlusion for 30 s was repeated three times.
Periodontal treatment
The nature of periodontal disease was explained to the subjects at their initial visits and each treatment was set as tooth brushing instruction by Bass method, scaling, root planning and the use of an ultrasonic scaler for each visit and no occlusal adjustment was undertaken for any of the subjects. Adhered plaque was evaluated based on O'Leary's PCR method.10 Plaque remains on buccal, lingual and palatal surfaces of each tooth were calculated. In addition, the plaque control records using the O'Leary method for all subjects were instructed to become 20% or less throughout this period. The duration of the periodontal initial treatment period was 163.1 ± 62.1 days (periodontal initial treatment range 93–288 days).
Statistical analysis
Comparisons of occlusal force, ALT, HbA1c, leptin, and high-sensitivity CRP between the initial visit and reevaluation were analyzed by using the Wilcoxon test. Other parameters were analyzed by t-test. JMP8.0.2® (SAS Ins, Cary, NC, USA) was used for statistical analysis.
fMRI's data analysis was performed using SPM 8 (Minitab, State College, PA, USA) and executed by Matlab 7.11 (Mathworks, Sherborn, MA, USA). The images from the functional scans were normalized to the standard Montreal Neurological Institute template brain. The t-test was used to determine the significance of the voxels and activation areas were characterized by their peak heights (P < 0.01). The pre- and post-treatment image data and numerical data were integrated for all subjects.
Results
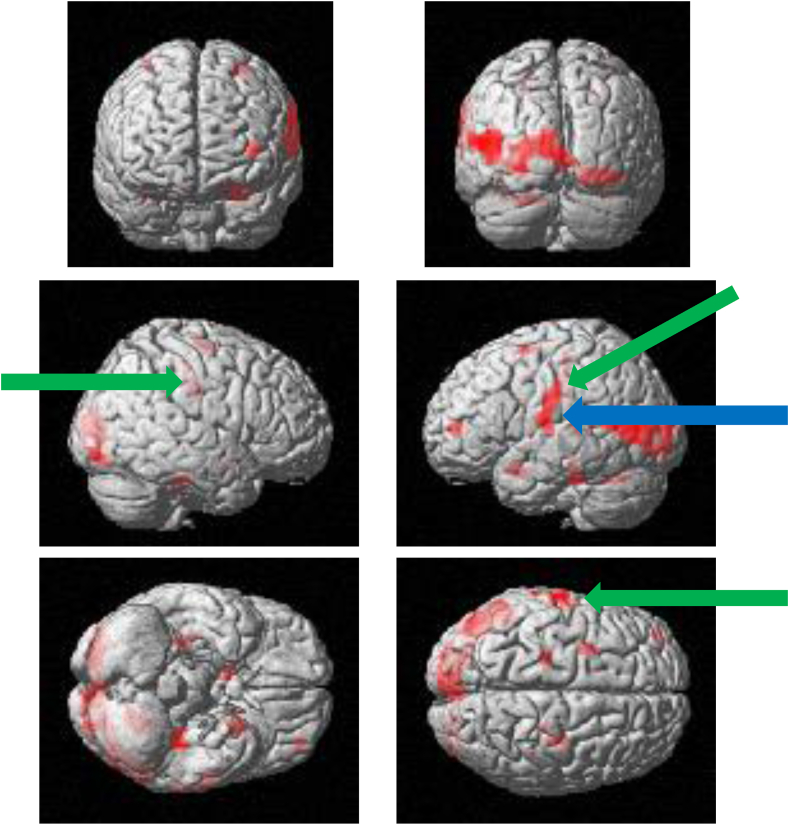
Figure 1, Figure 2, Figure 3, Figure 4 show the brain activation sites using fMRI. Brain activity from the initial visit occurred in the motor area of the insula, the primary motor cortex, the primary somatosensory cortex and so on (Fig. 1). The T-value in the left insula was recorded as 3.63 as can be seen in Fig. 2. Increased brain activity from the reevaluation occurred in the same area as that of the first visit (Fig. 3). However, the T-value in the left insula was significantly higher than that of the initial visit. The T-value was recorded as 6.58 as can be seen in Fig. 4.
Figure 1.
Surface projection of statistical parametric maps superimposed onto a standard Montreal Neurological Institute template brain (P < 10−3) during the clenching task at the initial visit. The primary motor cortex (green arrows) and the insula (blue arrow) were activated.
Figure 2.
T-value of brain activity of clenching task at the initial visit. Arrows indicate insula, the T-value calculation region of the brain. The T-value was recored as 3.63.
Figure 3.
Surface projection of statistical parametric maps superimposed onto a standard Montreal Neurological Institute template brain (P < 10−3) during the clenching task at the reevaluation. The primary motor cortex (green arrows) and the insula (blue arrows) were activated.
Figure 4.
T-value of brain activity of clenching task at the reevaluation. The arrows indicated T-value of brain. Arrows indicate insula, the T-value calculation region of the brain. The T-value was recorded as 6.58.
Table 1 shows comparison of periodontal tissue and occlusal force at the initial visit and the reevaluation. Periodontal treatment showed significant improvement of periodontal tissues in the average PPD (the initial visit: 3.2 ± 0.9 mm, the reevaluation: 2.4 ± 0.6 mm), the average CAL (the initial visit: 3.5 ± 1.0 mm, the reevaluation: 2.9 ± 1.0 mm) and the average %BOP (+) (the initial visit: 39.2 ± 25.5%, the reevaluation: 17.4 ± 10.5%). And ave PISA and ave PESA also improved significantly with periodontal treatment (Ave PISA: the initial visit: 636.6 ± 535.7 mm2, the reevaluation: 251.2 ± 187.4 mm2), (Ave PESA: the initial visit: 1381.3 ± 503.0 mm2, the reevaluation: 1039.6 ± 354.8 mm2).
Table 1.
Comparison of periodontal tissue and occlusal force at the initial visit and the reevaluation (n = 13).
| Initial | Reevaluation | P | |
|---|---|---|---|
| Ave PPD (mm) | 3.1 ± 0.8 | 2.4 ± 0.6 | 0.0050 |
| Ave CAL (mm) | 3.5 ± 1.0 | 2.9 ± 1.0 | 0.0039 |
| %BOP (+) | 37.7 ± 25.5 | 16.5 ± 11.1 | 0.0022 |
| Ave PISA (mm2) | 636.6 ± 535.7 | 251.2 ± 187.4 | 0.0075 |
| Ave PESA (mm2) | 1381.3 ± 503.0 | 1039.6 ± 354.8 | 0.0107 |
| Ave occlusal force (N) | 210.4 ± 134.9 | 233.7 ± 96.6 | 0.3051 |
Ave PPD: Probing pocket depth.
Ave CAL: Clinical attachment level.
%BOP(+): Bleeding on probing as a percentage of the measured area.
Ave PISA: The periodontal inflamed surface area.
Ave PESA: The periodontal epithelial surface area.
Ave occlusal force: Occlusal forces were measured using a Dental Prescale®.
Values are presented as mean ± standard deviation.
The difference in average occlusal forces were not statistically significant between the initial visit and the reevaluation. However, the average occlusal forces tended to increase at the reevaluation as compared with the first visit.
All blood test parameter differences were not statistically significant between the initial visit and the reevaluation. However, the leptin in particular tended to decrease at the reevaluation when compared to the first visit.
Discussion
Periodontal treatment is aimed at eliminating the cause of periodontal disease, stopping the progress of lesions and to eliminate factors such as plaque and calculus from occurring. Makino et al.2 concluded that occlusal force increases with periodontal treatment and that the amount of change was seen to be significantly larger in the molars than in the anterior teeth, and that inflammatory changes in periodontal tissue and occlusal force are closely related. PISA and PESA have recently been used as indicators of the inflammatory states of periodontal tissue. The results of this study demonstrated that periodontal initial treatment significantly improved periodontal tissue. The differences in occlusal force were not statistically significant between the initial visit and the reevaluation. However, the occlusal force tended to increase at the reevaluation as compared to the first visit. No statistically significant differences in occlusal force due to milder periodontal tissue inflammation were found in this study as opposed to that reported by Makino et al.2
WBC blood testing has been reported to cause diurnal variation.11 Even with diurnal variation, the WBC count increases in the evening and at night.12 To eliminate these variations, blood samples were taken in the morning and on an empty stomach. The differences in the blood tests were not statistically significant between the initial visit and the reevaluation. However, some of the blood tests tended to decrease at the reevaluation when compared to the first visit. Leptin has been reported to act as an appetite suppressant hormone, inhibiting neural activity in the brain and acting on the hippocampus.13 It has also been reported that the serum leptin level significantly decreased after periodontal treatment in systemically healthy patients with periodontitis.14 However, the differences in level of serum leptin in this study was not statistically significant as compared with that reported by Kardeşler et al.14 which was more severe than in this study. The inflammation of the periodontal tissue in the present study was mild (WBC: the initial visit: 6580.8 ± 2218.7/μl, the reevaluation: 6087.7 ± 1789.7/μl, P = 0.0826), therefore, the differences in leptin level was not statistically significant between the initial visit and the reevaluation. Although, the leptin level tended to decrease at the reevaluation visit.
We assumed that the occlusion state was controlled at the central level of local movement and perception and that by using fMRI, we could see changes in the central nerve level control due to changes in periodontal tissue before and after periodontal treatment.
Bllineau et al.15 were the first to perform fMRI. fMRI is a technique that captures the slight signal enhancement of blood oxygenation in blood vessels caused by increased neural activity. The fMRI technique uses the blood oxygenation level-dependent (BOLD) effect16 to detect changes in the balance between regional cerebral blood flow and oxygen metabolism, and then reconstructs them in functional images. Because the fMRI is non-invasive, has excellent temporal and spatial resolution, and does not involve radiation exposure, it has been used in a variety of settings. To date, there have been few reports conducted on the relationship between periodontal treatment and the brain using fMRI, so in this study our objective was to investigate this particular relationship.
Results from fMRI at the initial visit showed that in the state of biting, cerebral activity was evident in the insula. In the reevaluation, after periodontal initial treatment and in the state of biting, increased brain activity was observed in the motor area of the insula, when compared to the initial examination. In addition, new activity was found in the ventral part, including the primary motor cortex.
The insular cortex is involved in tongue and jaw movements, and the ventral part of the insular cortex, including the primary motor cortex, is involved in the expression of voluntary movements. It was reported that electrical stimulation of the insular in rats induced rhythmic jaw movements similar to masticatory movements and active salivation.17 In addition, Sörös et al.18 reported that the insular was activated during water swallowing in humans. When swallowing is accompanied by jaw movement, we assumed that the insular is activated as reported by Maeda et al.17
The T-value is a t-map illustrating the test statistic calculated as a result of the t-test performed for each voxel on the brain image template. This means that the spatial specificity is higher and the brain activation can be localized. In this case study, we could identify the brain activity site from the T-value at the initial examination (Fig. 2) and the T-value at the reevaluation (Fig. 4).
There were clear findings and alterations in the insula and the primary motor cortex on fMRI, even at levels that were not significantly different in the occlusal force examination and blood examinations in this study.
Kiuchi et al.19 reported that the development of subjective cognitive complaints (SCC) was associated with dysphagia, dry mouth, tooth loss, and reduced occlusal function. Poor oral health may increase the risk of SCC. Since tooth loss and loss of masticatory function are mostly associated with periodontal disease, it is necessary to maintain a good oral environment.
We conclude that the periodontal initial treatment may recover the periodontal ligament and the periodontal ligament neuron is further subjected to clenching in the insula.
These results show that periodontal initial treatment is a necessary item for improving the brain function. However, this study was short term as only periodontal initial treatment was targeted. In the future, we will plan to investigate the brain function over a longer period of time in subjects with SPT. The number of samples is also small and will be increased to evaluate the brain function in more detail.
Periodontal disease may interfere with the higher brain functions of working memory. Periodontal treatment maintains and improves not only oral functions, but also higher brain functions such as cognitive functions and behavioral choices. The results of this study suggest that periodontal treatment improves not only periodontal tissue but also activity in brain.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgement
The article was funded by grant-in-aid for Scientific Research(C) (Number 26463140).
References
- 1.Muraoka K., Kubota K., Tashiro Y., Yokota M. Effect of root planing on pathologic tooth migration in experimental periodontitis in beagles. J J Soc Periodontol. 2002;44:148–158. [Google Scholar]
- 2.Makino M., Muraoka K., Yokota M. A study of alterations of the occlusal force following nonsurgical treatment. J J Soc Periodontol. 2007;49:37–46. [Google Scholar]
- 3.Offenbacher S. Periodontal disease: pathogenesis. Ann Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 4.Noble J.M., Borrell L.N., Papapanou P.N., Elkind M.S.V., Scarmeas N., Wright C.B. Periodontitis is associated with congnitive impairment among older adults: analysis of nhanes-Ⅲ. J Neurol Neurosurg Psychiatry. 2009;80:1206–1211. doi: 10.1136/jnnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishida N., Ishihara Y., Ishida K., et al. Periodontitis induced by bacterial infection exacerbates features of Alzheimer's disease in transgenic mice. NPJ Aging Mech Dis. 2017;3:15. doi: 10.1038/s41514-017-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onozuka M., Fujita M., Watanabe K., et al. Age-related changes in brain regional activity during chewing: a functional magnetic resonance imaging study. J Dent Res. 2003;82:657–660. doi: 10.1177/154405910308200817. [DOI] [PubMed] [Google Scholar]
- 7.Eichner K. A group classification of missing teeth for prosthodontics. Dtsch Zahnärztl Z. 1955;10:1831–1834. [Google Scholar]
- 8.Nesse W., Abbas F., van der Ploeg I., Spijkervet F.K.L., Dijkstra P.U., Vissink A. Periodontal inflamed surface area: quantifying unflammatory burden. J Clin Periodontol. 2008;35:668–673. doi: 10.1111/j.1600-051X.2008.01249.x. [DOI] [PubMed] [Google Scholar]
- 9.Oda M., Yoshino K., Tanaka T., et al. Identification and adjustment of experimental occlusal interference using function magnetic resonance imaging. BMC Oral Health. 2014;14:124. doi: 10.1186/1472-6831-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Leary T.J., Drake R.B., Naylor J.E. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 11.Pigatto P.D., Radaelli A., Tadini G., Brambilla L., Altomare G., Carandente F. Circadian rhythm of the in vivo migration of neutrophils in psoriatic patients. Arch Dermatol Res. 1985;277:185–189. doi: 10.1007/BF00404314. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki T., Taga K., Nagaoki T., Seki H., Suzuki Y., Taniguchi N. Circadian changes of t lymphocyte subsets in human peripheral blood. Clin Exp Immunol. 1984;55:618–622. [PMC free article] [PubMed] [Google Scholar]
- 13.Lieb W., Beiser A.S., Vasan R.S., et al. Association of plasma leptin levels with incident alzheimer's disease and mri measures of brain aging: the framingham study. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kardeşler L., Buduneli N., Cetinkalp S., Kinane D.F. Adipokines and inflammatory mediators after initial periodontal treatment in patients with type2 diabetes and chronic periodontitis. J Periodontol. 2010;81:24–33. doi: 10.1902/jop.2009.090267. [DOI] [PubMed] [Google Scholar]
- 15.Belliveau J.W., Kennedy D.N., Mckinstry R.C., et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa S., Lee T.M., Kay A.R., Tank D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda N., Kobayashi M., Mitoh Y., Fujita M., Minagi S., Matsuo R. Differential involvement of two cortical masticatory areas in submandibular salivary secretion in rats. Brain Res. 2014;1543:200–208. doi: 10.1016/j.brainres.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Sörös P., Inamoto Y., Martin R. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2009;30:2426–2439. doi: 10.1002/hbm.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiuchi S., Kusama T., Sugiyama K., et al. Longitudinal association between oral status and cognitive decline using fixed-effects analysis. J Epidemiol. 2022;32:330–336. doi: 10.2188/jea.JE20200476. [DOI] [PMC free article] [PubMed] [Google Scholar]