Abstract
Background/purpose
The impact of temporomandibular joint (TMJ) osseous destruction on bone mineral density (BMD) remains unclear due to controversial findings. Besides, no previous study has explored the relationship between idiopathic condylar resorption (ICR) and body composition. This study aimed to investigate the relationship between ICR and BMD or body composition.
Materials and methods
Between July 2018 and August 2022, patients evaluated by an experienced dentist and diagnosed with temporomandibular disorders (TMDs) were referred to our center. They were recruited while they received the magnetic resonance image (MRI) examination, BMD and body composition completely. Patients were further categorized into TMDs with or without ICR groups according to MRI findings. One-way analysis of variance was used to compare the variables of BMD and body composition in the two groups.
Results
In total, 67 patients were included in the analysis, with 42 categorized as TMDs with ICR and 25 as TMDs without ICR. Patients with ICR had a significantly higher lean mass percentage and lower fat mass percentage; lower android/gynoid fat ratio, and visceral adipose tissue area than those without ICR (P < 0.05). Besides, patients above age 30 with ICR had lower Z scores (P = 0.017) compared with subjects without ICR.
Conclusion
TMDs patients with ICR show a relationship with body composition and affect the lean and fat mass distribution, especially android/gynoid fat ratio. The pathophysiological mechanism remains unclear. Further researches to investigate teeth binding, malocclusion and dietary habits are important to understand the association of ICR, BMD and body composition.
Keywords: Body composition, Bone mineral density, Idiopathic condylar resorption, Magnetic resonance imaging, Temporomandibular disorders
Introduction
Temporomandibular disorders (TMDs) are one of many causes of craniofacial pain problems associated with morphological and functional deformities that involve the temporomandibular joints (TMJs), masticatory muscles, and associated head and neck musculoskeletal structures.1,2 Patients with TMDs usually present with orofacial pain, restricted or deviating mandibular motion, TMJ sounds, decreased mouth opening and occlusal changes, etc. The causes of temporomandibular disorders are varied, and among them, idiopathic condylar resorption (ICR) is an aggressive form of dysfunctional remodeling of the TMJ.1,3
Idiopathic condylar resorption, also known as progressive condylar resorption,4,5 idiopathic condylysis,4,5 aggressive condylar resorption,4 or cheerleader syndrome,6 is characterized by progressive resorption of the mandibular condylar heads of unknown etiology. A diagnosis of ICR is based on patient history, clinical and imaging findings, and exclusion of known causes, such as rheumatoid arthritis (RA) or osteoarthritis (OA). ICR is most commonly reported in young to middle-aged women (15–35 years of age).7 Previous studies reported radiographic findings of ICR, including diminished condylar volume,5,8, 9, 10, 11, 12, 13, 14 decreased ramus height,5,10,11,13,15,16 alteration of condylar contour,8, 9, 10, 11,13,17,18 loss of integrity of condylar cortex,5,8,17 increased mandibular plane angle (MPA),17,19, 20, 21 decreased sella–nasion–B-point (SNB) angle,12,17,19, 20, 21 deep bite,10 and anterior open bite,8,10,18 etc.
Condylar resorption not only results in dentofacial deformities including malocclusion, abnormal bite, deformed ramus, increased overjet, facial asymmetry, or retrognathia, but also leads to decreased oropharyngeal airway space.5,14 As for the systemic consequences, the influence of TMJ osseous destruction on bone mineral density (BMD) remains unclear due to conflicting and controversial findings.7,22, 23, 24
The measurement of BMD by dual energy X-ray absorptiometry (DXA) is the gold standard for assessing bone mass status and is an important predictor of fracture risk. The results of DXA are expressed by the T-score and Z-score.25 The number of standard deviations (SD) of the patient's BMD away from the mean BMD of healthy young females aged in the range of 20–29 years yields the T-score. The number of standard deviations away from the mean BMD of healthy age- and sex- and ethnicity-matched reference populations is called the Z-score. As defined by the World Health Organization (WHO), a T-score lower than −2.5 SD is defined as osteoporosis, and a T-score that lies between −1 and −2.5 SD is described as osteopenia or low bone mass. For premenopausal women, men less than 50 years of age, and children, the International Society for Clinical Densitometry (ISCD) recommends using ethnic- or race-adjusted Z-scores: a Z-score lower than −2.0 is defined as “low bone mineral density for chronological age” or “below the expected range for age”.26 Besides, whole-body densitometry using DXA can also measure body composition, which is one of the most widely used methods in clinical practice. This technique enables highly accurate assessment of bone mineral content, lean body mass, and fat mass, making it valuable for evaluating nutritional status and monitoring changes associated with chronic diseases.27 To date, no previous study has explored the relationship between idiopathic condylar resorption (ICR) and whole-body composition. This study aimed to investigate the relationship between ICR and both BMD and body composition.
Materials and methods
Patient selection
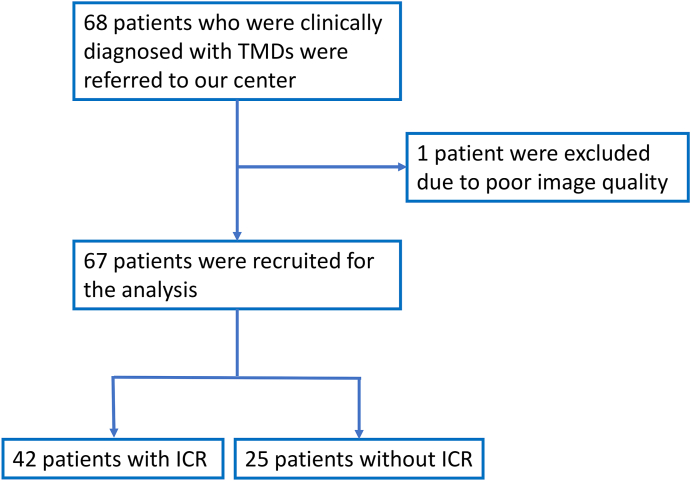
This prospective, single-center study was approved by the Ethics Review Committee of National Taiwan University Hospital (NTUH) and informed consent was obtained from all the study participants. Between July 2018 and August 2022, patients with an established diagnosis of temporomandibular disorders who underwent TMJ MRI examination, BMD, and body composition were eligible. The inclusion criteria were as follows: (1) the patient was clinically diagnosed with TMDs at the outpatient clinics and referred by a specialized dentist (Y. J. Chen), (2) the patient had no history of TMJ surgery, and (3) no systemic diseases that may affect the TMJ, such as inflammatory arthritis and autoimmune/connective tissue diseases. The exclusion criteria included poor image quality. The flow chart of patient selection is represented in Fig. 1. Finally, 67 patients (men: 5, women: 62) were enrolled in the study. The clinical information was collected by reviewing the medical records.
Figure 1.
Study flow diagram. Abbreviations: TMD, temporomandibular disorder; ICR, idiopathic condylar resorption.
Temporomandibular joints magnetic resonance imaging
The images were acquired using a 1.5-T MRI system (Magnetom Sonata, Siemens Healthcare, Erlangen, Germany) with a 16-channel head coil. Images were obtained with the jaws in the closed-mouth neutral position. The MR pulse sequences of TMJ included a gradient echo T2-weighted imaging (repetition time [TR]/echo time [TE] = 433/18 ms, flip angle = 25°, slice thickness = 3 mm) in oblique sagittal view, which was orientated perpendicular to the condylar long axis; a T1-weighted turbo spin echo imaging (repetition time [TR]/echo time [TE] = 542/10 ms, flip angle = 150°, slice thickness = 3 mm) in oblique sagittal view and also in oblique coronal view (repetition time [TR]/echo time [TE] = 451/10 ms, flip angle = 150°, slice thickness = 3 mm). The whole protocol was applied separately to the right and left TMJs during each single examination.
MRI image interpretation
Eligible MRI studies were evaluated by radiologists with 7 and 32 years of experience in musculoskeletal radiology (Y. C. Wang and T.T. F. Shih) according to previous reports about radiographic findings of idiopathic condylar resorption.5,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21
All patients were further categorized into TMDs with or without ICR groups according to MRI findings. If the opinions of the two radiologists differ, they will discuss and reach a consensus decision.
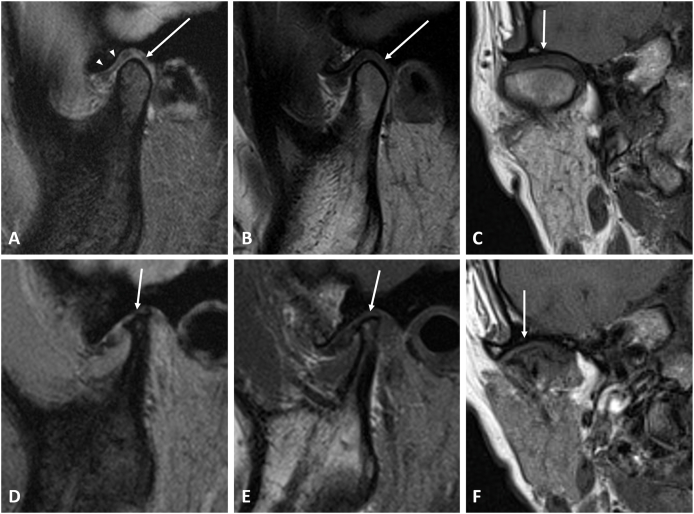
In Fig. 2, we present MR images of TMD patients with normal mandibular condyle and with severe ICR. It is easy to observe the differences in condyle between patients with or without ICR.
Figure 2.
MRI images of the temporomandibular joint. (A–C) Right temporomandibular joint (TMJ) of a 42-year-old woman diagnosed with temporomandibular disorder (TMD) without idiopathic condylar resorption (ICR). (A) T2-weighted gradient-echo image, (B) T1-weighted image, both in the oblique sagittal view and (C) T1-weighted image in the oblique coronal view depict normal morphology of the mandibular condyle (arrows), and normal position of the disc (arrowheads). (D–F) Right TMJ of a 33-year-old woman diagnosed with TMD and also ICR. (D) T2-weighted gradient-echo image and (E) T1-weighted image, both in the oblique sagittal view show severe condylar resorption with reduced antero-posterior diameter, height, and resorbed, irregular superior area of the mandibular condyle (arrows). An irregular, flattened articular eminence is also noted. (F) T1-weighted image in the oblique coronal view demonstrates the deformed superior part of the condyle (arrows).
Measurement of bone mineral density and body composition
Dual energy x-ray absorptiometry (DXA) was used to assess both bone mineral density at the lumber spine (L1–L4) and whole-body composition. The images were acquired using Stratos DR bone densitometer (DMS, Mauguio, France). The results of DXA are presented and analyzed using T-scores and Z-scores. The Z-score is compared with the average bone density of individuals of the same age, gender, and ethnicity. The International Society for Clinical Densitometry (ISCD) recommends using Z-scores for premenopausal women and men less than 50 years of age.26 We further divide the patients into two groups for analysis based on age, categorizing them as older (greater than 30 years old) and younger (less than 30 years old) groups.
Body composition measurements are frequently used in evaluating the nutrition status and monitoring changes associated with chronic diseases.27 We compared the percentage of body fat mass and body lean mass; the fat mass index (FMI); the visceral adipose tissue (VAT) area; as well as the android/gynoid fat ratio within the two groups.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics for Windows, version 23.0 (SPSS Inc, Chicago, IL, USA). Continuous variables are described as means, and categorical variables are as absolute frequencies and percentages. The chi-squared test is employed in the analysis of contingency tables when the sample sizes are large, whereas the Fisher's exact test is valid when the sample sizes are small. Therefore, we used the Fisher's exact test if any of the cells in a contingency table had a count less than 5. Possible differences in the incidence of gender, TMJ symptoms and classification of BMD status within the two groups were analyzed with the chi-squared test or Fisher's exact test based on their sample sizes. One-way analysis of variance (ANOVA) is a statistical test employed to analyze differences among the means of more than two groups. This technique in analysis of variance requires a numeric response dependent variable and a single categorical independent variable. Therefore, we utilized it to assess differences in the means of age, BMI, Z-score, T-score, lean mass percentage, fat mass percentage, fat mass index, android/gynoid fat ratio, and visceral adipose tissue area between the two groups. A P-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics and clinical symptoms
Demographic and clinical characteristics are summarized in Table 1. Sixty-seven patients were reviewed. Forty-two patients were diagnosed with ICR and twenty-five patients were categorized as without ICR group. In the ICR group, there were 39 females and 3 males, with an average age of 32.3 years (range from 20.0 to 60.9 years) and an average body mass index (BMI) of 19.8 kg/m2. In the without ICR group, there were 23 females and 2 males, with an average age of 36.5 years (range from 21.0 to 73.5 years) and an average BMI of 20.6 kg/m2. There was no significant difference in BMI between the two groups.
Table 1.
Characteristics of the study population.
| With ICR | Without ICR | P-value | |
|---|---|---|---|
| Patients, n | 42 | 25 | |
| Age, years; mean (range) | 32.3 (20.0–60.9) | 36.5 (21.0–73.5) | 0.157a |
| Gender, n (%) | 1.000b | ||
| Male | 3 (7.1) | 2 (8) | |
| Female | 39 (92.9) | 23 (92) | |
| Body Mass Index, kg/m2; mean | |||
| Total | 19.8 | 20.6 | 0.333a |
| Male | 20.7 | 26.2 | 0.107a |
| Female | 19.8 | 20.1 | 0.664a |
aP-values based on the one-way analysis of variance (ANOVA).
bP-value based on the Fisher's exact test.
Abbreviations: ICR, idiopathic condylar resorption.
The clinical symptoms are presented in Table 2. Patients with ICR had more TMJ symptoms regarding orofacial pain (83.3% vs 60.0%, P = 0.034) and malocclusion (16.7% vs 12.0%, P = 0.032) compared to patients without ICR. No differences were noted between the two groups in terms of joint sounds, open restriction, muscle strain, and symptom-free.
Table 2.
Clinical symptoms.
| n (%) | With ICR | Without ICR | P-value |
|---|---|---|---|
| Orofacial pain | 35 (83.3) | 15(60.0) | 0.034∗a |
| Joint sounds | 4 (9.5) | 1 (4.0) | 0.643b |
| Open restriction | 2 (4.8) | 3 (12.0) | 0.354b |
| Malocclusion | 7 (16.7) | 3 (12.0) | 0.032∗b |
| Muscle strain | 2 (4.8) | 1 (4.0) | 0.551b |
| Symptom-free | 1 (2.4) | 1 (4.0) | 1.000b |
aP-value based on the chi-squared test.
bP-values based on the Fisher's exact test.
∗ = statistically significant.
Abbreviations: ICR, idiopathic condylar resorption.
Bone mineral density (BMD)
The classification of BMD status in patients with and without ICR groups was presented in Table 3. In total, 53 patients were determined as normal BMD (34 patients with ICR and 19 patients without ICR), 13 patients as osteopenia (7 patients with ICR and 6 patients without ICR), and 1 patient (with ICR) as osteoporosis based on T-scores. After adjusting for age and sex, normal BMD was found in 66 patients (42 patients with ICR and 24 patients without ICR) and low BMD was diagnosed in 1 patient (without ICR). No statistical significance was shown. The sub-group analysis of the bone mineral density of patients with and without ICR was demonstrated in Table 4. Although not statistically significant, ICR patients had lower T-scores and Z-scores than patients without ICR, especially in female patients at chronological age (P = 0.063). We further divided the patients into two groups based on their age, with the younger population defined as less than 30 years old (n = 30, mean age = 24.6 ± 2.9 years) and the older group as 30 years old or older (n = 37, mean age = 41.4 ± 10.7 years). Older patients with ICR had lower Z scores (P = 0.017) compared with older subjects without ICR.
Table 3.
Classification of bone mineral density status in patients with and without ICR groups.
| BMD status | Without ICR, n | With ICR, n | P-valuea | |
|---|---|---|---|---|
| Z-score | 0.373 | |||
| Normal | 24 | 42 | ||
| Low | 1 | 0 | ||
| T-score | 0.583 | |||
| Normal | 19 | 34 | ||
| Osteopenia | 6 | 7 | ||
| Osteoporosis | 0 | 1 |
aP-values based on the Fisher's exact test.
Abbreviations: BMD, bone mineral density; ICR, idiopathic condylar resorption.
Table 4.
Sub-group analysis of bone mineral density in patients with and without ICR groups.
| With ICR |
Without ICR |
P-valuea | |||
|---|---|---|---|---|---|
| mean | range | mean | range | ||
| Z score | |||||
| Total | 0.074 | −1.6∼2.8 | 0.468 | −2.0∼3.1 | 0.135 |
| Male | 0.633 | −0.9∼2.8 | −0.050 | −1.4∼1.3 | 0.723 |
| Female | 0.031 | −1.6∼1.7 | 0.513 | −2.0∼3.1 | 0.063 |
| <30 years old | −0.189 | −1.6∼2.8 | −0.092 | −2.0∼1.5 | 0.819 |
| ≧ 30 years old | 0.271 | −1.3∼1.7 | 0.985 | −0.8∼3.1 | 0.017∗ |
| T score | |||||
| Total | −0.026 | −2.8∼2.8 | 0.160 | −2.0∼2.4 | 0.503 |
| <30 years old | −0.156 | −1.5∼2.8 | −0.050 | −2.0∼1.6 | 0.807 |
| ≧ 30 years old | 0.071 | −2.8∼1.6 | 0.354 | −1.5∼2.4 | 0.442 |
aP-values based on the one-way analysis of variance (ANOVA).
∗ = statistically significant.
Abbreviations: ICR, idiopathic condylar resorption.
Whole body composition
The comparison of body composition in the two groups was presented in Table 5. Patients with ICR had a significantly higher lean mass percentage and lower fat mass percentage (P = 0.036 and 0.035 respectively). The fat mass index and visceral adipose tissue area were lower in patients with ICR than the subjects without ICR (P = 0.049 and 0.008 respectively). The android/gynoid fat ratio was also lower in patients with ICR than the subjects without ICR (P = 0.029).
Table 5.
Body composition in patients with and without ICR groups.
| With ICR |
Without ICR |
P-valuea | |||
|---|---|---|---|---|---|
| mean | range | mean | range | ||
| Lean mass percentage (%) | 61.267 | 45.5–72.1 | 58.460 | 49.3–66.7 | 0.036∗ |
| Fat mass percentage (%) | 34.845 | 23.1–51.9 | 37.875 | 28.7–48.0 | 0.035∗ |
| Fat mass index (kg/m2) | 7.002 | 4.2–14.9 | 8.160 | 4.5–12.7 | 0.049∗ |
| Android/gynoid fat ratio | 0.9026 | 0.74–1.07 | 0.9516 | 0.80–1.25 | 0.029∗ |
| Visceral adipose tissue area (cm2) | 77.669 | 23.5–215.2 | 108.176 | 49.6–233.5 | 0.008∗ |
aP-values based on the one-way analysis of variance (ANOVA).
∗ = statistically significant.
Abbreviations: ICR, idiopathic condylar resorption.
Discussion
Many articles have previously reported on the consequences of idiopathic condylar resorption. This study aims to explore the systemic effects of ICR, such as its impact on bone mineral density and body composition. We found that TMDs patients with ICR have an association with body composition and affect the lean and fat mass distribution, especially android/gynoid fat ratio.
The present study found that patients with ICR exhibit more TMJ symptoms, such as orofacial pain and malocclusion, compared to those without ICR, although no differences were found in age, gender, or BMI in both groups. Among patients with ICR, over 80% reported experiencing pain. This may be explained by previous research that mentioned ICR causing varied dentofacial deformities.5,8,19,20
Yuan et al.7 reported that ICR was associated with an increased probability of low skeletal BMD at chronological age in females but not in males. The average age of the ICR patients with low BMD (23.9 ± 5.7 years) was older than that of the ICR patients with normal BMD (20.7 ± 6.1 years).7 However, osteopenia and osteoporosis based on T-scores were not prevalent in ICR.7 In our study, we also demonstrated similar results. Although not statistically significant, ICR patients had lower T-scores and Z-scores than patients without ICR, especially in female patients at chronological age (P = 0.063). In the sub-group analysis according to patients' age, older patients (above the age of 30) with ICR had lower Z scores (P = 0.017) compared with older subjects without ICR. The T-score is used to diagnose osteopenia and osteoporosis in comparison with the healthy young adult population. The Z-score is beneficial for diagnosing secondary osteoporosis caused by certain illnesses in children, young adults, premenopausal women, and men under the age of 50, and our patients predominantly fall within these demographics. Therefore, for this patients’ population, the Z-score may be more clinically valuable in detecting decreased bone density and facilitating early intervention for treatment.
Although there is no clear consensus, the etiology and risk factors for ICR are usually divided into two main categories: (1) patient factors related to host adaptive capacity, such as the influence of sex hormones (17b-estradiol deficiency), and (2) biomechanical loading, such as post-orthognathic surgery.23 Yang et al.23 reported a significant correlation between the lowest T-score and postoperative relapse after orthognathic surgery in female patients with ICR. Yuan et al.7 suggested that reduced BMD may predispose females to an exacerbated condylar resorption process, in addition to increased joint loading initiated by disc displacement. In our study, we also observed that female patients with ICR had lower Z-scores than subjects without ICR. This finding may suggest that lower bone mineral density could impair host adaptive capacity, which plays a significant role in the pathogenesis of ICR. Furthermore, we found that older patients with ICR had lower Z-scores compared to older subjects without ICR. This observation may suggest that the association between lower BMD and ICR is a long-term effect.
According to our knowledge, this study is the first-known research to investigate the impact of ICR on body composition. While body mass index (BMI) is widely used to assess overweight and obesity, it faces criticism because it does not always reflect true body fatness. Previous studies28,29 have indicated that FMI can provide a more accurate assessment of obesity and is highly correlated with metabolic syndrome compared to BMI. Our research revealed that patients with ICR have a higher lean mass percentage, a lower fat mass percentage, and a lower fat mass index (FMI) although there is no difference in BMI between these two groups. This implies that patients with ICR may have a lower risk of obesity and metabolic syndrome. This may be related to the patients' dietary habits. Faster eating is associated with higher BMI not only in women,30,31 but also in men32 and the total population.33, 34, 35 Lee KS et al.36 and Paz-Graniel I et al.37 also found that fast eating rates are associated with other cardiometabolic risk factors after adjusting for age, alcohol consumption, smoking, exercise, and total energy intake. Patients with ICR experience more TMJ symptoms, which could potentially affect their eating speed. As previous research has shown, eating slowly can help control energy intake, which may further influence the composition of body fat and lean mass. Unfortunately, our study did not collect information on dietary behaviors. Further research to investigate the habit of nutritional intake is important.
Additionally, this study showed that the patients with ICR had a lower android/gynoid fat ratio and visceral adipose tissue area compared to patients without ICR (P = 0.029 and 0.008, respectively). Gynoid fat, located in the pelvis and thighs, is alternatively referred to as peripheral or gluteofemoral fat. It is associated with lower cardiovascular risk compared to android fat, concentrated in the abdominal region, also known as central or truncal fat, which is associated with a higher risk of metabolic complications. Moreover, a visceral adipose tissue (VAT) area equal to or greater than 100 cm2 signifies a heightened cardiovascular risk, while a VAT area equal to or greater than 160 cm2 indicates a very high cardiovascular risk.27 The mean VAT area of patients with or without ICR is 77.669 cm2 (range 23.5–215.2 cm2) and 108.176 cm2 (range 49.6–233.5 cm2), respectively. These results can correspond to the earlier mention that patients with ICR have a lower FMI, suggesting a lower probability of obesity and metabolic syndrome.
This study has some limitations. First, it is a single-center study, so the number of patients is limited. Second, we did not collect information on patients' exercise habits as well as dietary habits, including eating speed, food size and texture, eating frequency, and intervals, all of which could impact patients' body composition. Third, some factors that may affect bone mineral density, such as smoking, alcohol consumption, and menopausal status, etc., were not recorded.
In conclusion, our study suggests that TMD patients with ICR have an association with body composition and affect the lean and fat mass distribution. The TMD patients with ICR had significantly lower android/gynoid fat ratio and visceral adipose tissue area. As for the association between ICR and BMD, there seems to be an increased probability of low BMD at chronological age in both older and female patients with ICR. This study could be quite helpful for the clinical systemic evaluation of ICR patients. The pathophysiological mechanism is still unclear. Further researches to explore the teeth binding, malocclusion, and habit of nutritional intake are crucial for understanding the TMDs, ICR, and whole-body composition.
Ethics approval
Approval was obtained from the ethics committee of National Taiwan University Hospital (Approval number: 201712100RINB). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all the study participants for their anonymized information to be published in this article.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This research was supported by Ministry of Science and Technology, Taiwan (MOST, 107-2314-B-002-103-MY3).
References
- 1.Scrivani S.J., Keith D.A., Kaban L.B. Temporomandibular disorders. N Engl J Med. 2008;359:2693–2705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- 2.Murphy M.K., MacBarb R.F., Wong M.E., Athanasiou K.A. Temporomandibular disorders: a review of etiology, clinical management, and tissue engineering strategies. Int J Oral Maxillofac Implants. 2013;28:e393–e414. doi: 10.11607/jomi.te20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alsabban L., Amarista F.J., Mercuri L.G., Perez D. Idiopathic condylar resorption: a survey and review of the literature. J Oral Maxillofac Surg. 2018;76:2316 e1–e13. doi: 10.1016/j.joms.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Sansare K., Raghav M., Mallya S.M., Karjodkar F. Management-related outcomes and radiographic findings of idiopathic condylar resorption: a systematic review. Int J Oral Maxillofac Surg. 2015;44:209–216. doi: 10.1016/j.ijom.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Wolford L.M., Cardenas L. Idiopathic condylar resorption: diagnosis, treatment protocol, and outcomes. Am J Orthod Dentofacial Orthop. 1999;116:667–677. doi: 10.1016/s0889-5406(99)70203-9. [DOI] [PubMed] [Google Scholar]
- 6.Wolford L.M. Idiopathic condylar resorption of the temporomandibular joint in teenage girls (cheerleaders syndrome) SAVE Proc. 2001;14:246–252. doi: 10.1080/08998280.2001.11927772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan M., Xie Q., Shen P., Yang C. Low skeletal bone mineral density as a potential aetiological factor towards idiopathic condylar resorption. Int J Oral Maxillofac Surg. 2021;50:665–669. doi: 10.1016/j.ijom.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Gunson M.J., Arnett G.W., Formby B., Falzone C., Mathur R., Alexander C. Oral contraceptive pill use and abnormal menstrual cycles in women with severe condylar resorption: a case for low serum 17beta-estradiol as a major factor in progressive condylar resorption. Am J Orthod Dentofacial Orthop. 2009;136:772–779. doi: 10.1016/j.ajodo.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Merkx M.A., Van Damme P.A. Condylar resorption after orthognathic surgery. evaluation of treatment in 8 patients. J Cranio-Maxillo-Fac Surg. 1994;22:53–58. doi: 10.1016/s1010-5182(05)80297-5. [DOI] [PubMed] [Google Scholar]
- 10.Hoppenreijs T.J., Stoelinga P.J., Grace K.L., Robben C.M. Long-term evaluation of patients with progressive condylar resorption following orthognathic surgery. Int J Oral Maxillofac Surg. 1999;28:411–418. [PubMed] [Google Scholar]
- 11.Borstlap W.A., Stoelinga P.J., Hoppenreijs T.J., van't Hof M.A. Stabilisation of sagittal split advancement osteotomies with miniplates: a prospective, multicentre study with two-year follow-up. part III--condylar remodelling and resorption. Int J Oral Maxillofac Surg. 2004;33:649–655. doi: 10.1016/j.ijom.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Wohlwender I., Daake G., Weingart D., Brandstatter A., Kessler P., Lethaus B. Condylar resorption and functional outcome after unilateral sagittal split osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:315–321. doi: 10.1016/j.tripleo.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 13.He Y., Lin H., Lin Q., et al. Morphologic changes in idiopathic condylar resorption with different degrees of bone loss. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128:332–340. doi: 10.1016/j.oooo.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Young A. Idiopathic condylar resorption: the current understanding in diagnosis and treatment. J Indian Prosthodont Soc. 2017;17:128–135. doi: 10.4103/jips.jips_60_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore K.E., Gooris P.J., Stoelinga P.J. The contributing role of condylar resorption to skeletal relapse following mandibular advancement surgery: report of five cases. J Oral Maxillofac Surg. 1991;49:448–460. doi: 10.1016/0278-2391(91)90166-j. [DOI] [PubMed] [Google Scholar]
- 16.Scheerlinck J.P., Stoelinga P.J., Blijdorp P.A., Brouns J.J., Nijs M.L. Sagittal split advancement osteotomies stabilized with miniplates. a 2-5-year follow-up. Int J Oral Maxillofac Surg. 1994;23:127–131. doi: 10.1016/s0901-5027(05)80285-1. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T., Izumi N., Kojima T., Sakagami N., Saito I., Saito C. Progressive condylar resorption after mandibular advancement. Br J Oral Maxillofac Surg. 2012;50:176–180. doi: 10.1016/j.bjoms.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Mercuri L.G. A rationale for total alloplastic temporomandibular joint reconstruction in the management of idiopathic/progressive condylar resorption. J Oral Maxillofac Surg. 2007;65:1600–1609. doi: 10.1016/j.joms.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 19.Hwang S.J., Haers P.E., Seifert B., Sailer H.F. Non-surgical risk factors for condylar resorption after orthognathic surgery. J Cranio-Maxillo-Fac Surg. 2004;32:103–111. doi: 10.1016/j.jcms.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Troulis M.J., Tayebaty F.T., Papadaki M., Williams W.B., Kaban L.B. Condylectomy and costochondral graft reconstruction for treatment of active idiopathic condylar resorption. J Oral Maxillofac Surg. 2008;66:65–72. doi: 10.1016/j.joms.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Hwang S.J., Haers P.E., Sailer H.F. The role of a posteriorly inclined condylar neck in condylar resorption after orthognathic surgery. J Cranio-Maxillo-Fac Surg. 2000;28:85–90. doi: 10.1054/jcms.2000.0129. [DOI] [PubMed] [Google Scholar]
- 22.Jagur O., Kull M., Leibur E., et al. Relationship between radiographic changes in the temporomandibular joint and bone mineral density: a population based study. Stomatol. 2011;13:42–48. [PubMed] [Google Scholar]
- 23.Yang H.J., Hwang S.J. Bone mineral density and mandibular advancement as contributing factors for postoperative relapse after orthognathic surgery in patients with preoperative idiopathic condylar resorption: a prospective study with preliminary 1-year follow-up. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:112–118. doi: 10.1016/j.oooo.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Back K., Ahlqwist M., Hakeberg M., Bjorkelund C., Dahlstrom L. Relation between osteoporosis and radiographic and clinical signs of osteoarthritis/arthrosis in the temporomandibular joint: a population-based, cross-sectional study in an older Swedish population. Gerodontology. 2017;34:187–194. doi: 10.1111/ger.12245. [DOI] [PubMed] [Google Scholar]
- 25.Sozen T., Ozisik L., Basaran N.C. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schousboe J.T., Shepherd J.A., Bilezikian J.P., Baim S. Executive summary of the 2013 international society for clinical densitometry position development conference on bone densitometry. J Clin Densitom. 2013;16:455–466. doi: 10.1016/j.jocd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Chaves L., Goncalves T.J.M., Bitencourt A.G.V., Rstom R.A., Pereira T.R., Velludo S.F. Assessment of body composition by whole-body densitometry: what radiologists should know. Radiol Bras. 2022;55:305–311. doi: 10.1590/0100-3984.2021.0155-en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peltz G., Aguirre M.T., Sanderson M., Fadden M.K. The role of fat mass index in determining obesity. Am J Hum Biol. 2010;22:639–647. doi: 10.1002/ajhb.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P., Ma F., Lou H., Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Publ Health. 2013;13:629. doi: 10.1186/1471-2458-13-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leong S.L., Madden C., Gray A., Waters D., Horwath C. Faster self-reported speed of eating is related to higher body mass index in a nationwide survey of middle-aged women. J Am Diet Assoc. 2011;111:1192–1197. doi: 10.1016/j.jada.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki S., Katagiri A., Tsuji T., Shimoda T., Amano K. Self-reported rate of eating correlates with body mass index in 18-y-old Japanese women. Int J Obes Relat Metab Disord. 2003;27:1405–1410. doi: 10.1038/sj.ijo.0802425. [DOI] [PubMed] [Google Scholar]
- 32.Tanihara S., Imatoh T., Miyazaki M., et al. Retrospective longitudinal study on the relationship between 8-year weight change and current eating speed. Appetite. 2011;57:179–183. doi: 10.1016/j.appet.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Wuren Endoh K., Kuriki K., Shizuoka-Sakuragaoka JMSg. Eating rate as risk for body mass index and waist circumference obesity with appropriate confounding factors: a cross-sectional analysis of the Shizuoka-Sakuragaoka J-MICC study. Asia Pac J Clin Nutr. 2019;28:79–91. doi: 10.6133/apjcn.201903_28(1).0012. [DOI] [PubMed] [Google Scholar]
- 34.Van den Boer J.H.W., Kranendonk J., van de Wiel A., Feskens E.J.M., Geelen A., Mars M. Self-reported eating rate is associated with weight status in a Dutch population: a validation study and a cross-sectional study. Int J Behav Nutr Phys Activ. 2017;14:121. doi: 10.1186/s12966-017-0580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.S., Mishra G., Hayashi K., Watanabe E., Mori K., Kawakubo K. Combined eating behaviors and overweight: eating quickly, late evening meals, and skipping breakfast. Eat Behav. 2016;21:84–88. doi: 10.1016/j.eatbeh.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Lee K.S., Kim D.H., Jang J.S., et al. Eating rate is associated with cardiometabolic risk factors in Korean adults. Nutr Metabol Cardiovasc Dis. 2013;23:635–641. doi: 10.1016/j.numecd.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Paz-Graniel I., Babio N., Mendez I., Salas-Salvado J. Association between eating speed and classical cardiovascular risk factors: a cross-sectional study. Nutrients. 2019;11:83. doi: 10.3390/nu11010083. [DOI] [PMC free article] [PubMed] [Google Scholar]