Abstract
Background
Meniscus tears are a common cause of knee pain encountered in orthopedics and sports medicine. There are numerous management strategies, from physical therapy and oral medications to surgery. Recent evidence is more favorable for conservative management, as operative treatment has limited clinical benefits and is associated with an accelerated progression toward osteoarthritis. Injections with orthobiologic therapies, such as platelet-rich plasma (PRP), are emerging as an alternative therapeutic tool for degenerative tears. This study aims to evaluate the latest evidence regarding the efficacy of PRP injections for the nonoperative management of degenerative meniscal pathology.
Data sources
Articles were obtained from Embase, PubMed, World of Science, Cochrane, and Galileo databases after searching “Platelet-rich plasma” AND “Meniscus.” Inclusion criteria consisted of original, human studies evaluating the use of platelet-rich plasma for nonoperative management of meniscus tears.
Main results
A total of 384 articles were screened, with ten studies selected for final inclusion. The pooled study population comprised 686 patients, with an average age ranging from 33 to 53 years, and a 38% female population. Three different injection approaches were utilized, categorized as intra-articular alone (IA), intra-meniscal alone (IM), or a combination of both. Most studies demonstrated improved pain and functionality by 3 months that persisted for at least one year. Within the IA and IM groups, the majority of patients were either radiographically stable (30–70%) or demonstrated interval healing (40–60%). Several studies within IM and combined treatment groups evaluated rates and time to arthroscopy, and found lower failure rates and greater arthroscopy-free survival time than control comparison groups.
Conclusion
PRP appears to be a safe and efficacious treatment strategy for degenerative meniscal pathology. However, due to diverse periprocedural techniques, PRP injectate characteristics, and a lack of high-quality studies, additional trials are needed to provide greater a degree of confidence in PRP's clinical impact on patients with meniscus tears.
Level of evidence
Systematic Review.
Keywords: Meniscus tears, Meniscus degeneration, Platelet-rich plasma, PRP, Non-operative, Regenerative medicine, Orthobiologics
1. Introduction
Meniscal tears are a common cause of knee pain and dysfunction, affecting roughly 60 per 100,0000 people in the United States, with a prevalence of 12–14%.1,2 The meniscus provides various biomechanical roles, including axial load redistribution, shock absorption, joint lubrication, and joint stabilization; thus, tears result in symptoms of clicking, locking, or feelings of instability.3 Management varies based on injury type and severity, ranging from conservative approaches like physical therapy (PT) and oral non-steroidal anti-inflammatories (NSAIDs) to more invasive options like meniscectomy or repair. Surgery is preferred in cases of significant mechanical symptoms, injuries unresponsive to conservative management, and those with concomitant ligamentous injury.4 Routine meniscectomies for degenerative tears have become less favorable due to limited clinical benefit, compared to placebo,5,6 and an increased risk of early-onset osteoarthritis (OA).7 This shift in clinical practice has prompted clinicians and researchers to explore alternative management strategies.
Platelet-rich plasma (PRP), an autologous blood product with supraphysiologic concentrations of platelets and growth factors, has gained significant attention over the past two decades within orthopedics and sports medicine. PRP is obtained from centrifugation and filtration of whole blood to isolate plasma from the cellular components and increase the platelet concentration. This PRP is then injected into the target, commonly under image guidance. Clinical applications include tendinopathy, osteoarthritis, and ligament sprains, as well as meniscal tears. However, the literature evaluating the efficacy of PRP for meniscal pathology is limited and largely focuses on augmenting arthroscopic meniscus repairs. Although multiple meta-analyses have demonstrated that augmentation provides additional pain relief, it does not improve functional outcomes or healing.8,9 Available studies evaluating PRP for the non-operative management of degenerative meniscus tears suggest clinical promise, however, the existing literature is complicated by a heterogeneous body of evidence with scant randomized control trials and a lack of standardized PRP preparations. As such, we aim to comprehensively review and evaluate the available evidence to provide insights into the efficacy of PRP for degenerative meniscus tears.
2. Methods
Database search within Pubmed, Embase, Galileo, and Web of Science was performed in July 2023. Articles were considered if “platelet-rich plasma” AND “meniscus” were mentioned within the title or article.
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed for article appraisal and inclusion (Fig. 1). Before article appraisal, duplicates between databases were removed. Literature was excluded during title review if any of the following were identified: 1) Systematic or narrative reviews; 2) Editorials or expert opinion pieces; 3) Conference abstracts; 4) Individual case reports; 5) In vitro or nonhuman animal studies; 6) Studies involving surgically managed meniscal tears; 7) PRP for non-meniscal pathology. Articles included for final review required the following criteria: 1) Human clinical study; 2) The focal pathology needed to include meniscal tears or degeneration; 3) Patients had to receive PRP injection(s) in a nonoperative context; 4) The studies must measure and evaluate patient-reported outcome measures (PROMs); 5) The text must be written in English. Article search and appraisals were performed by the lead authors (J.E., E.A.); identified articles were independently reviewed for inclusion. If article discrepancies occurred, a tiebreaker was performed by the senior author (B.C.).
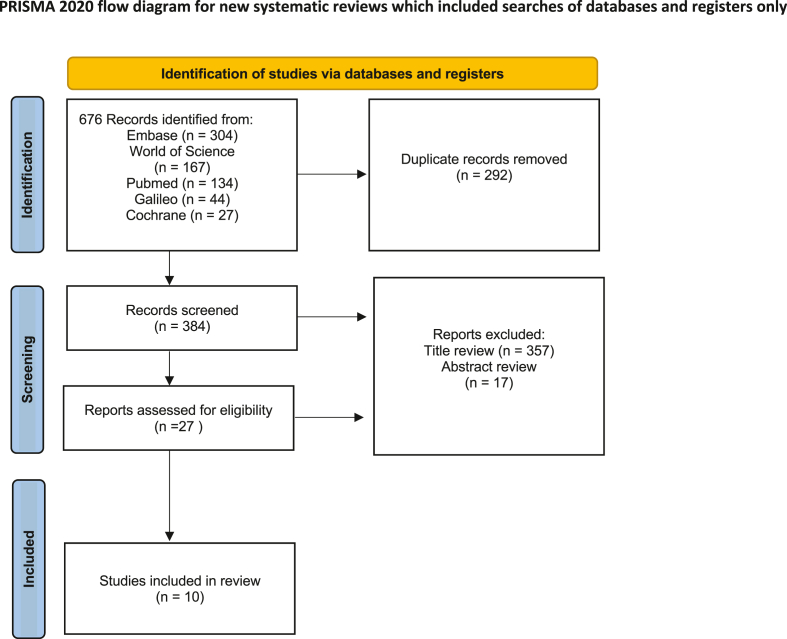
Fig. 1.
PRISMA flow diagram of Article Review Process.
From each article, study size, patient demographics, PRP characteristics, method of injection, as well as PROMs were organized into Table 1, Table 2.
Table 1.
Study characteristics.
| Author | Level of Evidence | Number of Participants | Age (Years) | Sex | OA Grade |
Pathology | Laterality of injury | Intervention technique and approach | Time periods assessed | PROMs |
|---|---|---|---|---|---|---|---|---|---|---|
| Alessio-Mazzola et al. (2021) | Level IV Prospective case series |
69 | 52.1 ± 7.8 | 21 M (30.4%) 48 F (69.6%) |
KL 0 47(68%) KL 1 22(32%) |
Crues Grade 3 | Medial 69/69 (100%) Lateral 0 |
IA (Fluoroscopic) | 1 Months | Lysholm |
| 3 Months | Tegner | |||||||||
| 6 Months | VAS | |||||||||
| 12 Months | WOMAC | |||||||||
| Blanke et al. (2015) | Level IV Prospective case series |
10 | 53.3 ± 13.9 | 6 M (60%) 4 F (40%) |
KL 0-2 | Reicher Grade 2 |
NA | IA (Fluoroscopic) | 6 Months | Knee MRI |
| Level of sport activity | ||||||||||
| NRS | ||||||||||
| Bonodariev et al. (2022) | Level II Non-Blinded Prospective RCT |
53 | 42 ± 16 | 33 M (62.2%) 20 F (37.8%) | Less than Grade 2 | Stoller 2nd Stage 41 (77.3%) 3rd Stage 12 (22.7%) |
Medial 126/154 (81.8%) Lateral 9/154 (5.8%) |
IA (Method not described) | 3 Months | KOOS |
| 6 Months | VAS | |||||||||
| 12 Months | WOMAC | |||||||||
| 24 Months | ||||||||||
| 36 Months | ||||||||||
| Di Matteo et al. (2021) | Level IV Prospective Case Series |
12 | 51.7 ± 19.1; | 10 M (83%) 2 F (17%) |
KL 0-1 | Medial Degenerative Meniscopathy | Medial 12/12 (100%) | IA + IM (US-Guided | 6 Months | IKDC |
| 12 Months | VAS | |||||||||
| 18 Months | ||||||||||
| Guenoun et al. (2020) | Level IV Retrospective Case Series |
10 | 40.4 ± 13.6 | 7 M (70%) 3 F (30%) |
Excluded if any evidence of OA | Stoller Grade 1 (n = 2) Grade 2 (n = 4) Grade 3 (n = 4) |
NA | IM + IA (US-Guided) | 3 Months | KOOS |
| 6 Months | MRI (6 Months) | |||||||||
| Return to competition | ||||||||||
| VAS | ||||||||||
| Kaminski et al. (2019) | Level II Prospective, Double Blinded RCT |
72 30 control 42 PRP |
Control 46 (27–68); PRP 44 (18–67) |
Control 19 M (63%) 11 F (37%) PRP 22 M (52%) 20 F (48%) |
Grade 0-1 Control: KL 0: 23 (77%) KL 1: 7 (23%) PRP: KL 0 30 (71%) KL 1: 12 (29%) |
Chronic Horizontal Lesions | Medial Control 30/30 (100%) PRP 41/42 (97.6%) Lateral Control 0 PRP 1/42 (2.4%) |
meniscal trephination ± PRP augmentation IM (US-Guided) |
3 Months | Arthroscopy-Free Survival |
| 6 Months | IKDC | |||||||||
| 12 Months | KOOS | |||||||||
| 24 Months | MRI | |||||||||
| VAS | ||||||||||
| WOMAC | ||||||||||
| Medina-Porqueres et al. (2022) | Level IV Retrospective Case Series |
38 | 50.68 ± 9.65 | 30 M (79%)/8 F (21%) | NA | Reicher Grade I-III |
Medial 32/38 (84.2%) Lateral 6/38 (15.8%) |
IA Infrapatellar Approach (Blind) |
76 ± 32 days (range 39–190) | KOOS |
| Patient Satisfaction | ||||||||||
| Quality of life | ||||||||||
| Tegner | ||||||||||
| VAS | ||||||||||
| Mitev et al. (2019) | Level IV Prospective Cohort |
15 | 49.3 ± 6.3 | 5 M (33.3%) 10 F (66.7%) | NA | Degenerative meniscus lesions | NA | IA (Blind) | 3 Months | Tegner Lysholm Knee Scoring Scale |
| 6 Months | ||||||||||
| Özyalvaç et al. (2019) | Level IV Retrospective Case series |
15 | 33.2 ± 8.2 | 6 M (40%) 9 F (60%) |
Ahlbäck grade 2 or milder | Grade 2 Degenerative lesion | Medial 15/15 (100%) Lateral 0 |
IM (US-Guided) | Average of 32 Months | Lysholm |
| MRI | ||||||||||
| Sanchez et al. (2023) | Level IV Prospective Survival Study |
392 | 52.0 ± 2.0 | 264 M (67.4%) 128 F (32.6%) |
NA | “Meniscal injury identified on MRI” | Medial −323/392 (82.4%) Lateral −69/392 (17.6%) |
Injection 1/3: IA + IM 2/3: IA alone 3/3: IA + IM (US-Guided) |
6 months | Arthroscopy-Free Survival |
| 18 months | KOOS |
F: Female; IA: Intra-articular; IM: Intra-meniscal; IKDC: International Knee Documentation Committee; KL: Kellgren-Lawrence, KOOS: Knee Injury and Osteoarthritis Outcome Score; M: Male; MRI: Magnetic Resonance Imaging; NA: Not Available; NRS: Numerical Rating Scale; OA: Osteoarthritis; PROM: Patient Reported Outcome Measure; RCT, Randomized Control Trial; US: Ultrasound; VAS: Visual Analog Scale, WOMAC: Western Ontario and McMaster Universities Arthritis Index.
Table 2.
Platelet rich plasma characteristics.
| Author | Initial Platelet Count (x103/μl) | Final Platelet Count (x103/μl) | Platelet increase Factor | Leukocyte Status | RBC Status | Activating Factor | Injectate Volume (ml) | Number of Injections | Platelet Dose Per Injection (x109) | Total Platelet Dose (x109) | Filtering system |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alessio-Mazzola et al. (2021) | 250 | 600 | 2.4x | Leukocyte Poor | RBC poor | None | 5.0 | 4 | 3.0 | 12 | NA |
| Blanke et al. (2015) | NA | NA | >5x | Leukocyte Poor | NA | None | 2.0–5.0 | 3 | NA | NA | Arthrex ACP Double Syringe System™ |
| Bonodariev et al. (2022) | NA | 1000 | NA | Leukocyte Rich | NA | None | 3.0 + 15 mg/ml LMWHA |
3–6 | 3.0 | 9–18 | NA |
| Di Matteo et al. (2021) | NA | NA | NA | Leukocyte Poor | NA | NA | 2.0 | 3 | NA | NA | Arthrex ACP Double Syringe System™ |
| Guenoun et al. (2020) | 265 ± 106 | 497 ± 154 | 1.9x | Leukocyte poor | RBC rich | No | 4.0 | 1 | 2.0 ± 0.6 | 2.0 ± 0.6 | Hy Tissue 20 device (Fidia, Abano) |
| Kaminski et al. (2019) | NA | 900–1700 | NA | Leukocyte rich | RBC rich | CaCl2 + Thrombin | 6.0–8.0 | 1 | 5.4 ± 0.5 | 5.4 ± 0.5 | Cell-saver/separator PRP devices |
| Medina-Porqueres et al. (2022) | NA | NA | NA | NA | NA | CaCl2 | 1.5–2.0 | 3 | NA | NA | Auxilab, Nahita Blue, FugelabGB10, Navarra, Spain) |
| Mitev et al. (2019) | NA | NA | NA | NA | NA | NA | 5.0–6.0 | 3 | NA | NA | Arthrex ACP Double Syringe System™ |
| Özyalvaç et al. (2019) | NA | NA | 4.8x | Leukocyte rich | NA | Activated, but method not reported | 4.0 | 1 | NA | NA | T-LAB PRP kit (T-Biotechnology Laboratory, Istanbul, Turkey) |
| Sanchez et al. (2023) | NA | 322 ± 114 | 1.5–2.5x | Leukocyte poor | RBC poor | CaCl2 |
IA: 8.0 IA + IM 10.0–12.0 |
3 |
IA: 1.7–3.5 IA + IM: 2.1–5.2 |
5.9–13.9 | NA |
IA: Intra-articular; IM: Intra-meniscal; LMWHA: Low molecular weight hyaluronic Acid; NA: not available; RBC: Red Blood Cell.
3. Results
3.1. Study characteristics
On initial review, 676 articles were extracted from five separate databases. Of these articles, 292 duplicates were removed. Following the title review, 357 articles were excluded, leaving 27 for abstract and full-length review. Ten articles were selected for final review and data extraction (Fig. 1).
Seven articles were prospective studies,10, 11, 12, 13, 14, 15, 16 with three case series,15,17,18 one cohort,13 and one survival study.14 Two studies utilized a control group,12,16 with only one being blinded.16 The remaining three studies were retrospective.17, 18, 19 The Level of evidence for each article ranged from II to IV (Table I).
3.2. Patient demographics
On average, study participants were at least 40 years of age (average 40.4–53.1 years), except for Özyalvaç,19 where patients averaged 33.2 years. The pooled studies consisted of 686 patients, 38% of whom were female. Most groups aimed to exclude patients with knee OA, as it has been established that PRP is less efficacious in those with moderate-to-severe OA.20 Six papers excluded patients with Kellgren-Lawrence (KL) severity of greater than one10,12,15, 16, 17,19 or two.11,12
Although meniscus laterality was predominantly medial (86%), and pathology was degenerative, the injury classification nomenclature was inconsistent.10, 11, 12, 13,15,19 Two groups included patients with both acute and chronic injuries.14,18 Even degenerative tear classification systems were assorted, with Reicher,11,18, Stoller,16,17 and Crues.10
3.3. Platelet-rich plasma
The PRP qualities were also diverse. PRP characteristics reported in Table II were organized based on the constituents of Kon's classification.21 Two studies explicitly referred to Mishra's classification system, which describes fold differences in platelets–rather than precise concentrations– and the presence or absence of leukocytes and activators.11,19,22 Six provided enough information to calculate the final platelet concentration.10, 11, 12,14,16,17 Two reported using RBC-rich PRP.12,17 Only one study listed using leukocyte-rich PRP,16 while five reported leukocyte-poor formulation.10,11,14,15,17 Three studies utilized external activators, with Kaminski providing both CaCl2 and autologous thrombin,12 while Sanchez and Medina-Porqueres added only CaCl2.14,18
Platelet concentrations for each injection ranged from 2.0 to 5.4 × 109 platelets, with total platelet treatment doses of 2.0–18 × 109. The number of injections ranged from one to six, with four groups performing a single injection,12,17,19, five performing three injections,11,13, 14, 15,18 one with 4 injections,10 and one with up to 6 injections.16 Of these studies, only Di Matteo15 and Alessio-Mazzola10 incorporated a protocol based on prior literature to justify three23 and four injections,24 respectively.
PRP was administered via one of three methods: Intra-articular alone (IA),10,11,13,16,18, intrameniscal alone (IM),12,19 or a combination of IA and IM.14,15,17 Injections were performed under fluoroscopy,10,11 ultrasound-guidance,12,14,15,17,19 or blind.13,18 Two articles did not describe the technique.16,
Multiple time points were utilized, from one to 36 months. Most studies compared baseline characteristics to 3 months post-injection,10,12,13,16,17, as well as six months10, 11, 12, 13, 14, 15, 16; however, assessments were also collected at one,10 12,10,12,15,16 18,14,15 24,16 and 36 months.16 Two of the retrospective studies did not have structured time points, but rather averages of 76 days18 and 32 months.19
The studies also varied in pre and post-injection protocols. Only two studies advised patients to avoid NSAIDs between two to 10 days before the first injection.16,18 Following injections, five groups advised no NSAID use for up to six months,10,11,14,18, four had no formal physical therapy,10,11,14,19 and six recommended a more gradual, self-guided return to activity.10,11,14,16,18, However, one study did utilize a hinged knee brace with a structured 12-week PT program12
Five articles evaluated post-injection complications.12,15, 16, 17, 18 Outside of injection site pain, the groups reported no adverse reactions or complications.
3.4. Patient Reported Outcome Measures (PROMs)
Table III synthesizes PROMs, grouped by the injection methods.
Table 3.
Patient reported outcome measures.
| Method of Delivery | Author | VAS/NRS | Lysholm | Tegner Activity | KOOS Total | WOMAC | Imaging | Other | |
|---|---|---|---|---|---|---|---|---|---|
| Intra-Articular (IA) | Alessio-Mazzola et al. (2021) | Baseline | 5.3 ± 2.0 | 72.9 ± 7.3 | 4.3 ± 1.1 | NA | 77.7 ± 11.3 | NA | ROM (Flexion + Extension) |
| 1 Month | 4.1 ± 2.0 | 78.2 ± 8.0 | NA | 81.5 ± 10.2 | 131 ± 7.7 | ||||
| 3 Months | 2.8 ± 1.5 | 85.7 ± 7.1 | NA | 89.4 ± 6.5 | 131 ± 13.5 | ||||
| 6 Months | 2.6 ± 1.2 | 87.8 ± 5.9 | NA | 91.9 ± 4.9 | 133 ± 5.2 | ||||
| 12 Months | 2.6 ± 1.3 | 85.6 ± 5.6 | NA | 90.7 ± 4.7 | 133 ± 5.2 | ||||
| P | <0.001 | <0.001 | >0.05 | <0.001 | 133 ± 5.2 | ||||
| <0.001 | |||||||||
| Blanke et al. (2015) | Baseline 6 Months P |
6.9 ± 1.0 4.5 ± 3.0 0.027 |
NA | NA | NA | NA | 9 IIb, 1 IIa | 6/10 had increased sporting activity | |
| 4 improved | |||||||||
| 2 worsened | |||||||||
| 4 unchanged | |||||||||
| 0.889 | |||||||||
| Mitev et al. (2019) | Baseline | NA | 61.9 ± 7.4 | NA | NA | NA | NA | NA | |
| 3 Months | 83.4 ± 4.6 | ||||||||
| 6 Months | 83.4 ± 4.3 | ||||||||
| P | <0.001 | ||||||||
| Medina-Porqueres et al. (2022) | Baseline Range of 1–6 Months (39–190 days) P |
5.9 ± 1.9 | NA | 3.7 ± 1.7 | 41.9 ± 23.0 | NA | NA | Feeling thermometer: | |
| 1.6 ± 1.3 | 4.7 ± 1.7 | 85.9 ± 13.5 | 67.9 ± 16.4 | ||||||
| <0.001 | 0.001 | <0.001 | 86.3 ± 6.7 | ||||||
| <0.001 | |||||||||
| Bonodariev et al. (2022) | Baseline | 7.4 ± 1.3 | NA | NA | 69.3 ± 5.3 | 50.6 ± 8.7 | NA | NA | |
| 3 Months | 3.8 ± 1.7 | 78.3 ± 4.7 | 20.8 ± 9.4 | ||||||
| 6 Months | 2.0 ± 1.1 | 85.7 ± 5.9 | 10.6 ± 6.7 | ||||||
| 12 Months | 2.2 ± 1.4 | 91.5 ± 4.9 | 8.9 ± 3.7 | ||||||
| 24 Months | 1.2 ± 0.3 | 94.4 ± 7.1 | 3.5 ± 1.7 | ||||||
| 36 Months | 1.2 ± 0.5 | 94.5 ± 4.8 | 4.0 ± 1.9 | ||||||
| P | <0.05 | <0.01 | <0.01 | ||||||
| Intra-Meniscal (IM) | Özyalvaç et al. (2019) | Baseline | NA | 71.1 ± 6.9 | NA | NA | NA | 15 grade 2 | NA |
| 31.9 ± 5.6 Months | 91.9 ± 6.6 | 4 Healed | |||||||
| P | <0.001 | 6 Improved | |||||||
| 4 Unchanged | |||||||||
| 1 Horizontal | |||||||||
| Kaminski et al. (2019) | Baseline (vs control) | 5.4 ± 0.1 vs | NA | NA |
Subscales discussed intext >0.05 |
34.4 ± 0.4 vs |
Healed: 10/27 vs 5/27 Partially Healed: 4/27 vs 3/27 Failed: 13/27 vs 19/27 0.41 |
IKDC | |
| 92 weeks (54–157) | 4.4 ± 0.1 | 28.9 ± 0.6 | 52.0 ± 0.3 vs | ||||||
| P | 2.0 ± 0.1 vs | 9.7 ± 0.3 vs | 54.9 ± 0.5 | ||||||
| 2.1 ± 0.1 | 7.5 ± 0.6 | 86.0 ± 0.5 vs | |||||||
| 0.39 | 0.36 | 88.1 ± 0.9 | |||||||
| 0.36 | |||||||||
| Combined IA and IM | Di Matteo et al. (2021) | Baseline | 6.5 ± 2.0 | NA | NA | NA | NA | NA | IKDC |
| 6 Months | 4.3 ± 1.7 | 50.1 ± 18.0 | |||||||
| 12 Months | 2.9 ± 2.2 | 71.5 ± 14.2 | |||||||
| 18 Months | 2.5 ± 2.5 | 78.6 ± 11.9 | |||||||
| P | <0.001 | 81.6 ± 13.9 | |||||||
| <0.001 | |||||||||
| Guenoun et al. (2020) | Baseline | 5.7 ± 1.2 | NA | NA | 56.6 ± 15.7 70.2 ± 16.8 72.7 ± 18.5 0.007 |
NA | Stoller Grade: | ||
| 3 Months | NA | 1: 2/10 | |||||||
| 6 Months | 3.6 ± 3.1 | 2: 4/10 | |||||||
| P | 0.18 | 3: 4/10 | |||||||
| 7/10 had no radiographic change | |||||||||
| Sanchez et al. (2023) | Baseline | NA | NA | NA | Subscales discussed intext | NA | NA | Survival Analysis: 90.3% (354/392) did not undergo surgery; estimated mean survival of 54 months |
|
| 6 Months | <0.001 | ||||||||
| 18 Months | |||||||||
| P |
IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; NA, Not Available; NRS, Numerical Rating Scale; ROM, Range of Motion; VAS, Visual Analog Scale.
3.4.1. Intra-articular (IA)
Five studies evaluated pain with either a Visual Analog Scale (VAS) or Numeric Rating System (NRS).10,11,16,18, All studies evaluating pain demonstrated statistically significant improvements from baseline. Benefits in pain were noted within the first one to three months after injection,10,16, and relief was sustained for at least 24–36 months.16 Medina-Porqueres also utilized the feeling thermometer, which is a visual scale from 0 to 100 to assess overall feelings and satisfaction. The group demonstrated a significant rise, from 67.9 to 86.3 after 10 weeks.
Two articles evaluated symptoms and function with Lysholm scores.10,13, Findings were consistent between studies, with significant improvements by 3 months that persisted through six10,13 to 12 months.10 Reported score classifications, as described by the original publication by Tegner, progressed from “unsatisfactory” or “fair” to “good” after treatment.10,13 Two groups utilized the Knee injury and Osteoarthritis Outcome Score (KOOS), which is another tool assessing symptoms, and functionality, through five questionnaire subscales (Symptoms, Pain, Activities of Daily Living, Quality of Life (QOL), and Sport/Recreation). Similar to Lysholm scores, KOOS total scores rose within the first three months, with stable improvements at 36 months.16,18 However, when delving into subscales, Medina only reported a significant rise in Pain and ADL scores within the 76 days of follow-up. This trend was also observed with Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores.10 In the Blanke cohort, sporting activity levels were also assessed and notably improved to previous levels of sporting performance in 60% (6/10) of patients.11 However the level of sporting proficiency was not described.
Interestingly, Bonodariev compared PRP injections to both control (NSAIDs) and surgical management and reported significantly better KOOS scores at 12 months for the treatment group relative to surgery and control. Although the surgical group did have better pain control in the first six months, this advantage dissipated soon after, whereas the treatment group's improvements did not falter.16
Blanke's group did evaluate pre- and post-treatment changes on MRI.11 The cohort originally consisted of nine Reicher grade IIb and one grade IIa lesions. By six months, four (40%) improved by at least one grade, four (40%) were radiographically unchanged, and two (20%) worsened by at least one grade.
3.4.2. Intra-meniscal (IM)
One of the two IM studies evaluated pain,12 and did not demonstrate statistically significant improvements. However, short and midterm pain relief cannot be commented on since the average responder follow-up assessment was nearly two years after treatment (92 weeks). Despite this, Kaminski did attain a greater percentage of symptom improvement past the Minimally Clinically Important Difference (MCID) for VAS (2-point improvement; 65% vs 39%, p = 0.027) than the control group (meniscus trephination). Özyalvaç demonstrated significant symptomatic improvements, via the Lysholm scale, from ‘fair’ to ‘excellent,’ within the 32-month assessment period.19 Kaminski revealed significantly greater function through improvement in all KOOS subsections, but there were no meaningful differences in average scores between treatment and control groups at any time point. However, more patients achieved the MCID (16.7) in the treatment group for the KOOS Symptoms subsection (76% vs 48%, p = 0.016). Similar to KOOS results, there were significant improvements in WOMAC and IKDC scores with PRP at an average of 92 weeks, but this was similar to the control group.12
Both groups performed pre and post-treatment MRI to evaluate for meniscus healing. Özyalvaç started with 15 grade 2 tears. After 32 months, six (40%) demonstrated partial healing, four (27%) were completely healed, and five (33%) either did not change or converted to a horizontal tear.19 Kaminski revealed a relatively greater healing rate after PRP treatment than trephination alone, although it was not deemed to be statistically significant (60% vs 42%, p = 0.41).12 However, the group did reveal a significantly lower failure rate (48% vs 70%; p = 0.04) and greater arthroscopy-free survival (92% vs 72%, p = 0.032) in the PRP group.
3.4.3. Combined IA and IM
Three articles utilized a combination of intra-articular/perimeniscal (IA) and intra-meniscal (IM) injections.14,15,17 However, their injection techniques and therapeutic approaches differed. Sánchez and colleagues performed three injections (once weekly), with the first and third combining IA and IM (10–12 ml) and the second being IA alone (8 ml). The combined injection consisted of 8 ml in the IA space, then 1–2 ml in the meniscal wall, and 1–2 ml in the meniscal capsule.14 Guenon used considerably less volume (4 ml) for a single injection, providing 0.5 ml within the meniscus tear, 1.5 ml in the meniscal wall, and 2 ml in the perimeniscal space.17 Di Matteo injected even less, with 2 ml total, divided into three to four depots within the meniscus and menisco-capsular junction.15
Of these studies, Di Matteo and Guenoun evaluated pain.15,17 Di Matteo reported a significant reduction in pain at the initial six-month follow-up (p = 0.003), with additional improvements in pain at 12 months post-treatment (p = 0.004).15 This is in contrast to Guenon, who found no pain reduction at three or six months (p = 0.18). Guenon and Sanchez utilized KOOS subscales to evaluate symptoms and function following treatment. Although Guenoun had significant improvements in KOOS Total, Symptoms, and QOL at three and six months, they did not achieve meaningful improvement in the Pain and Sport and Recreation subscales.17 Sanchez reported an excellent rise in all KOOS subscales at six and 18 months (p < 0.001) following intra-articular and intra-meniscal injections.14 Similarly, Di Matteo demonstrated significant long-term IKDC score improvement at both the 6-month and 12-month follow-up.15 The 18-month reassessment was also higher but did not reach statistical significance. Within Guenoun's study population, 60% (6 out of 10) of the patients returned to preinjury competition or training activities.
Imaging was evaluated in one of the combined injection studies.17 Although Guenon did not demonstrate radiographic healing, MR imaging was stable at six months in seven of the 10 patients.17 Sanchez evaluated rates of arthroscopic meniscus surgery after PRP treatment and noted a 90% survival rate, with an estimated survival time of 54.3 months.14 The group found that radial or complex meniscal lesions, as well as medial femorotibial compartment chondropathy, were risk factors for failing nonoperative management with PRP.
4. Discussion
The results of this review demonstrate that patients receiving PRP injections for degenerative meniscus tears may experience significant improvements in pain and function. However, wide variations in the injection technique, PRP characteristics, and injection frequency are noted. Improvements in pain and function for those receiving exclusively intra-articular injections were most prominent within the first three months of treatment and persisted for at least one year in many cases. Radiographic healing was evident in roughly 40% of patients within IA and IM groups. Although data are limited amongst these studies, the reported clinical outcomes are promising.
Multiple in vitro studies provide a molecular basis for PRP's meniscus-repairing potential, with meniscocyte and fibroblast proliferation, migration, and extracellular matrix (ECM) deposition being the main mechanisms.25, 26, 27, 28 Another theoretical influence is angiogenic growth factors such as EGF, VEGF, and PDGF, which are thought to promote healing by stimulating blood vessel formation within diseased tissue and enhancing nutrient delivery. Additionally, work by Wang indicates that PRP may provide a less catabolic milieu in the face of inflammatory stimuli, such as fragmented fibronectin, following meniscus and hyaline cartilage damage. The group demonstrated that PRP attenuated meniscocyte and chondrocyte proinflammatory interleukin and catabolic matrix metalloproteinase expression elicited by fragmented fibronectin.29
Within the orthobiologics and regenerative medicine community, discussions are growing regarding the importance of platelet doses, but a consensus has not yet been reached. Literature suggests that larger doses, approaching 10 billion total platelets, are ideal for osteoarthritis30,31 but there are a lack of comparative trials evaluating the efficacy of various platelet concentrations. When comparing PROMs between those studies that reported platelet dosing, we note that patients receiving more than 5.5 billion platelets (range 5.9–18 billion)10,14,16 had significant improvements compared to no statistical difference with those receiving 2.0–5.4 billion.12,17 Moreso, a recent meta-analysis suggests that three injections provide better pain relief than one for knee osteoarthritis, with a single dose being equivalent to double, after one year of follow-up.32 However, the optimal number of injections remains unclear. In the current review, seven of the 10 studies utilized three or more injections,10,11,13, 14, 15,18 which more consistently provided statistically significant symptomatic and functional improvement compared to the three studies utilizing a single injection.10,14,16
Multiple studies included in this review performed additional treatments that may have influenced outcomes. Kaminski utilized US-guided meniscus trephination, a technique used to stimulate meniscus healing during arthroscopic repair, with PRP.12 Although there were impressive improvements in pain and function, the group demonstrated no significant difference in PROMs between treatment and control. However, the addition of PRP was associated with greater meniscus healing on repeat MRI. Di Matteo performed a similar treatment with percutaneous meniscus needling with ACP injection and reported promising improvements in pain and function.15 However, since the study was a case series without a comparator group, the impact of the orthobiologic therapy is not completely appreciated.
Multiple injection methods were described, including blind, fluoroscopy, and US-guided. Although injections were tolerated well and no serious complications were reported, the accuracy of each injection method is not equivalent. A recent meta-analysis by Fang compared US-guided and blind knee injections, including 1315 knee injections. The group demonstrated that US-guided injections were more reliable than blind in each of the 12 studies.33 They also noted that the superolateral approach was the most precise. Works comparing fluoroscopic injections to the other methods are limited, as fluoroscopy is typically reserved for shoulders and axial joints. Amber and colleagues compared US and fluoroscopy-guided shoulder injections. Much like with knees, US was generally more accurate (93% vs 80%), but the group did not find a statistically meaningful difference.34
Bondariev complicated our comparison by incorporating hyaluronic acid (HA) with PRP treatments.16 The group demonstrated significantly greater pain and functional improvements in the treatment arm compared to controls with conservative and surgical (partial meniscectomy) interventions. However, HA's clinical contribution is dubious since recent literature does not suggest any added benefit of HA in conjunction with PRP for OA. A meta-analysis by Zhang in 2022, evaluated 13 studies with a pool of 1118 patients, compared PRP alone to a combination of PRP and HA, and demonstrated no statistical difference in pain or functional outcomes between treatment modalities after 12 months.35
Alessio-Mazzola utilized four PRP treatments, with the first injection being a fresh sample, while the remaining three were stored at −80C and thawed for weekly injections. To our knowledge, the clinical impact of PRP cryopreservation has not been evaluated in human subjects. Based on basic science research, however, freezing PRP at −80C has mixed effects on growth factor concentrations, but these do not seem to be substantial, even after 4 weeks.36,37 This work does pose questions regarding the potential of banking autologous or allogeneic PRP for an off-the-shelf product, as some reports suggest that PRP is minimally immunogenic.38,39
Although PRP appears to be a promising treatment, it is not the only orthobiologic to be evaluated for degenerative meniscus tears. Malanga evaluated intra-articular and intra-meniscal micro-fragmented adipose tissue (MFAT) injection in patients with meniscal tears and accompanying osteoarthritis.40 Similar to PRP findings, the group noted significant improvement in pain and KOOS by three months that persisted into a one-year follow-up. However, the group tended to include more severe pathology, such as large complex tears (74%), that usually are managed operatively. The future appears bright for various orthobiologics for meniscus pathologies, however, at this time significantly more work is needed to make confident claims of their safety and efficacy. For now, PRP is the most accessible orthobiologic with the most evidence to support its use.
5. Limitations
This systematic review is not without limitations. Most prominently are the limited high-quality randomized controlled trials, which limit confidence in clinical conclusions. Although meniscus tears are common, isolated degenerative pathology without accompanying OA is less prevalent, which makes identifying appropriate patients for these studies difficult. The aforementioned studies attempted to exclude OA, but in clinical practice, degenerative meniscus tears tend to accompany 60% of patients with KL grade of 2 or greater OA.41 However, given the frequency of meniscal tears in arthritic knees, the meniscus may be an addressable pain source in patients with OA and a direction for future research. Moreover, the heterogeneity of injection approaches, the number of injections, and PRP qualities tack additional complexity to study interpretation and comparison and do not permit for meta-analysis. Overall it does appear that patients tolerate the treatments well and experience clinical improvement. However, it is tricky to separate therapeutic impact from natural disease course, as degenerative meniscal tears tend to symptomatically improve spontaneously within two years.42
6. Conclusion
Overall, PRP appears to be a safe and effective treatment for degenerative meniscal pathology, significantly improving pain and functionality. Clinical improvements were most prominent within the first three months and may persist for over one year. Additionally, the most consistent improvements were observed in those receiving at least three treatments of PRP or a platelet dose greater than 5.5 billion. However, the confidence of these assertions is limited by the availability of quality of evidence and heterogeneity of methodologies. Further randomized controlled trials are needed to more convincingly report optimal dosing and PRP delivery methods for meniscus tears.
Ethical statement
This is not human-subject research. The authors agree that this systematic review represents honest and original research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Guardian patients consent
This is a systematic review and therefore parent/guardian consent is not applicable.
CRediT authorship contribution statement
Joseph W. Elphingstone: Conceptualization, Investigation, Literature Review, Methodology, Writing – original draft, Visualization, Writing – review & editing, Project administration. Elijah T. Alston: Literature Review, Methodology, Writing – original draft, Visualization. Berdale S. Colorado: Writing – review & editing, Project administration, Supervision.
Declaration of competing interest
The authors have no relevant conflicts of interest to report.
Acknowledgements
None.
References
- 1.Bhan K. Meniscal tears: current understanding, diagnosis, and management. Cureus. 2020;12(6) doi: 10.7759/cureus.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Logerstedt D.S., Scalzitti D.A., Bennell K.L., et al. Knee pain and mobility impairments: meniscal and articular cartilage lesions revision 2018. J Orthop Sports Phys Ther. 2018;48(2):A1–A50. doi: 10.2519/jospt.2018.0301. [DOI] [PubMed] [Google Scholar]
- 3.Makris E.A., Hadidi P., Athanasiou K.A. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–7431. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laible C., Stein D.A., Kiridly D.N. Meniscal repair. J Am Acad Orthop Surg. 2013;21(4):204–213. doi: 10.5435/JAAOS-21-04-204. [DOI] [PubMed] [Google Scholar]
- 5.Thorlund J.B., Juhl C.B., Roos E.M., Lohmander L.S. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350(jun16 3):h2747. doi: 10.1136/bmj.h2747. h2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Graaff S.J.A., Eijgenraam S.M., Meuffels D.E., et al. Arthroscopic partial meniscectomy versus physical therapy for traumatic meniscal tears in a young study population: a randomised controlled trial. Br J Sports Med. 2022;56(15):870–876. doi: 10.1136/bjsports-2021-105059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S.B., Shetty G.M., Lee D.H., et al. Unfavorable results of partial meniscectomy for complete posterior medial meniscus root tear with early osteoarthritis: a 5- to 8-year follow-up study. Arthrosc J Arthrosc Relat Surg. 2010;26(10):1326–1332. doi: 10.1016/j.arthro.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Li Z., Weng X. Platelet-rich plasma use in meniscus repair treatment: a systematic review and meta-analysis of clinical studies. J Orthop Surg Res. 2022;17(1):446. doi: 10.1186/s13018-022-03293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y lei, Jiang H., Wang S., et al. Effect of platelet-rich plasma on meniscus repair surgery: a meta-analysis of randomized controlled trials. Medicine. 2022;101(33) doi: 10.1097/MD.0000000000030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alessio-Mazzola M., Felli L., Trentini R., et al. Efficacy of autologous platelet-rich plasma injections for grade 3 symptomatic degenerative meniscal lesions: a 1-year follow-up prospective study. Sport Health. 2022;14(2):227–236. doi: 10.1177/19417381211011074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanke F., Vavken P., Haenle M., von Wehren L., Pagenster G., Majewski M. Percutaneous injections of Platelet rich plasma for treatment of intrasubstance meniscal lesions. Muscle Ligaments and Tendons J. 2015;5(3):162. doi: 10.32098/mltj.03.2015.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaminski R., Maksymowicz-Wleklik M., Kulinski K., Kozar-Kaminska K., Dabrowska-Thing A., Pomianowski S. Short-term outcomes of percutaneous trephination with a platelet rich plasma intrameniscal injection for the repair of degenerative meniscal lesions. A prospective, randomized, double-blind, parallel-group, placebo-controlled study. IJMS. 2019;20(4):856. doi: 10.3390/ijms20040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitev K., Longurov A. Intra-articular platelet-rich plasma injections for treating knee pain associated with articular cartilage and degenerative meniscal lesions. Open Access Maced J Med Sci. 2019;7(15):2484–2487. doi: 10.3889/oamjms.2019.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez M., Jorquera C., Bilbao A.M., et al. High survival rate after the combination of intrameniscal and intraarticular infiltrations of platelet-rich plasma as conservative treatment for meniscal lesions. Knee Surg Sports Traumatol Arthrosc. 2023;12 doi: 10.1007/s00167-023-07470-4. Published online June. [DOI] [PubMed] [Google Scholar]
- 15.Di Matteo B., Altomare D., Garibaldi R., La Porta A., Manca A., Kon E. Ultrasound-guided meniscal injection of autologous growth factors: a brief report. Cartilage. 2021;13(1_suppl):387S–391S. doi: 10.1177/19476035211037390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bondariev G, Strafun S, Holiuk Y, Saulenko K, Darovskyi O, Syvak A. Comparative analysis of the use of L-PRP/L-PCP injections, arthroscopic partial resection and nonsteroidal anti-inflammatory drugs in the treatment of the meniscus tears. Accessed December 18, 2023. https://transplantology.org/2022-10-1/article-01/.
- 17.Guenoun D., Magalon J., de Torquemada I., et al. Treatment of degenerative meniscal tear with intrameniscal injection of platelets rich plasma. Diagnostic and Interventional Imaging. 2020;101(3):169–176. doi: 10.1016/j.diii.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Medina-Porqueres I., Martin-Garcia P., Sanz-De-Diego S., et al. Clinical and functional outcome of meniscal injuries treated with platelet-rich plasma: a single-center case series. IJERPH. 2022;19(12):7118. doi: 10.3390/ijerph19127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Özyalvaç O.N., Tüzüner T., Gürpinar T., Obut A., Acar B., Akman Y.E. Radiological and functional outcomes of ultrasound-guided PRP injections in intrasubstance meniscal degenerations. J Orthop Surg. 2019;27(2) doi: 10.1177/2309499019852779. [DOI] [PubMed] [Google Scholar]
- 20.Saita Y., Kobayashi Y., Nishio H., et al. Predictors of effectiveness of platelet-rich plasma therapy for knee osteoarthritis: a retrospective cohort study. J Clin Med. 2021;10(19):4514. doi: 10.3390/jcm10194514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kon E., Di Matteo B., Delgado D., et al. Platelet-rich plasma for the treatment of knee osteoarthritis: an expert opinion and proposal for a novel classification and coding system. Expet Opin Biol Ther. 2020;20(12):1447–1460. doi: 10.1080/14712598.2020.1798925. [DOI] [PubMed] [Google Scholar]
- 22.Mishra A., Harmon K., Woodall J., Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharmaceut Biotechnol. 2012;13(7):1185–1195. doi: 10.2174/138920112800624283. [DOI] [PubMed] [Google Scholar]
- 23.Smith P.A. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884–891. doi: 10.1177/0363546515624678. [DOI] [PubMed] [Google Scholar]
- 24.Repetto I., Biti B., Cerruti P., Trentini R., Felli L. Conservative treatment of ankle osteoarthritis: can platelet-rich plasma effectively postpone surgery? J Foot Ankle Surg. 2017;56(2):362–365. doi: 10.1053/j.jfas.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Freymann U., Degrassi L., Krüger J.P., Metzlaff S., Endres M., Petersen W. Effect of serum and platelet-rich plasma on human early or advanced degenerative meniscus cells. Connect Tissue Res. 2017;58(6):509–519. doi: 10.1080/03008207.2016.1260563. [DOI] [PubMed] [Google Scholar]
- 26.Wong C.C., Kuo T.F., Yang T.L., et al. Platelet-rich fibrin facilitates rabbit meniscal repair by promoting meniscocytes proliferation, migration, and extracellular matrix synthesis. Int J Mol Sci. 2017;18(8):1722. doi: 10.3390/ijms18081722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tumia N.S., Johnstone A.J. Platelet derived growth factor-AB enhances knee meniscal cell activity in vitro. Knee. 2009;16(1):73–76. doi: 10.1016/j.knee.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Xiao W.F., Yang Y.T., Xie W.Q., et al. Effects of platelet-rich plasma and bone marrow mesenchymal stem cells on meniscal repair in the white-white zone of the meniscus. Orthop Surg. 2021;13(8):2423–2432. doi: 10.1111/os.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C.C., Lee C.H., Peng Y.J., Salter D.M., Lee H.S. Platelet-rich plasma attenuates 30-kDa fibronectin fragment-induced chemokine and matrix metalloproteinase expression by meniscocytes and articular chondrocytes. Am J Sports Med. 2015;43(10):2481–2489. doi: 10.1177/0363546515597489. [DOI] [PubMed] [Google Scholar]
- 30.Bansal H., Leon J., Pont J.L., et al. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci Rep. 2021;11(1):3971. doi: 10.1038/s41598-021-83025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nouri F., Babaee M., Peydayesh P., Esmaily H., Raeissadat S.A. Comparison between the effects of ultrasound guided intra-articular injections of platelet-rich plasma (PRP), high molecular weight hyaluronic acid, and their combination in hip osteoarthritis: a randomized clinical trial. BMC Muscoskel Disord. 2022;23(1):856. doi: 10.1186/s12891-022-05787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao X., Aw A.A.L., Leeu J.J., Bin Abd Razak H.R. Three doses of platelet-rich plasma therapy are more effective than one dose of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthroscopy. 2023;39(12):2568–2576.e2. doi: 10.1016/j.arthro.2023.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Fang W.H., Chen X.T., Vangsness C.T. Ultrasound-guided knee injections are more accurate than blind injections: a systematic review of randomized controlled trials. Arthrosc Sports Med Rehabil. 2021;3(4):e1177–e1187. doi: 10.1016/j.asmr.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amber K.T., Landy D.C., Amber I., Knopf D., Guerra J. Comparing the accuracy of ultrasound versus fluoroscopy in glenohumeral injections: a systematic review and meta-analysis. J Clin Ultrasound. 2014;42(7):411–416. doi: 10.1002/jcu.22154. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q., Liu T., Gu Y., Gao Y., Ni J. Efficacy and safety of platelet-rich plasma combined with hyaluronic acid versus platelet-rich plasma alone for knee osteoarthritis: a systematic review and meta-analysis. J Orthop Surg Res. 2022;17(1):499. doi: 10.1186/s13018-022-03398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiga Y., Kubota G., Orita S., et al. Freeze-dried human platelet-rich plasma retains activation and growth factor expression after an eight-week preservation period. Asian Spine J. 2017;11(3):329–336. doi: 10.4184/asj.2017.11.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen Y.H., Lin W.Y., Lin C.J., et al. Sustained or higher levels of growth factors in platelet-rich plasma during 7-day storage. Clin Chim Acta. 2018;483:89–93. doi: 10.1016/j.cca.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z.Y., Huang A.W., Fan J.J., et al. The potential use of allogeneic platelet-rich plasma for large bone defect treatment: immunogenicity and defect healing efficacy. Cell Transplant. 2013;22(1):175–187. doi: 10.3727/096368912X653183. [DOI] [PubMed] [Google Scholar]
- 39.He M., Guo X., Li T., et al. Comparison of allogeneic platelet-rich plasma with autologous platelet-rich plasma for the treatment of diabetic lower extremity ulcers. Cell Transplant. 2020;29 doi: 10.1177/0963689720931428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malanga G.A., Chirichella P.S., Hogaboom N.S., Capella T. Clinical evaluation of micro-fragmented adipose tissue as a treatment option for patients with meniscus tears with osteoarthritis: a prospective pilot study. Int Orthop. 2021;45(2):473–480. doi: 10.1007/s00264-020-04835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Englund M., Guermazi A., Gale D., et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359(11):1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg B., Roos E.M., Kise N.J., Engebretsen L., Holm I., Risberg M.A. On a trajectory for success-9 in every 10 people with a degenerative meniscus tear have improved knee function within 2 Years after treatment: a secondary exploratory analysis of a randomized controlled trial. J Orthop Sports Phys Ther. 2021;51(6):289–297. doi: 10.2519/jospt.2021.10025. [DOI] [PubMed] [Google Scholar]