Extraosseous/peripheral ameloblastoma (EPA) is a rare type of benign odontogenic tumor. Although occurring in different anatomical locations, EPA shares both histologic and molecular features with intraosseous ameloblastoma.1,2 Expanding the genetic landscape of ameloblastoma may further the understanding of the molecular basis of its pathogenesis and provide insights into potential therapeutic targets. The aim of this study was to report a novel mutation found by next-generation sequencing in EPA and to briefly review mitogen-activated protein kinase (MAPK) pathway mutations shared between intraosseous and extraosseous ameloblastomas.
Whole-exome sequencing was performed in a case of EPA arising in the mandibular alveolar mucosa of a 65-year-old male (Fig. 1A) by using the SureSelect Human All Exon V8 (Agilent Technologies) as described previously.3 As a result, the NRAS p.G12D (c.35G > A) mutation was detected, and no other MAPK pathway mutations were found. For further analysis, primers for polymerase chain reaction and Sanger sequencing were designed to amplify exon 2 of the NRAS gene (forward: 5′-CAACAGGTTCTTGCTGGTGT-3′, reverse: 5′-CCTCACCTCTATGGTGGGAT-3′), and the NRAS p.G12D (c.35G > A) mutation was identified (Fig. 1B). To confirm the activation of the MAPK pathway, immunohistochemistry for phosphorylated extracellular signal-regulated kinase (p-ERK) was performed as described previously;4 most tumor cells showed strong nuclear expression of p-ERK (Fig. 1C). These findings indicate that previously unidentified NRAS G12D mutations can be found in EPA, mutually exclusive with known genetic alterations, and may lead to activation of the MAPK pathway.
Figure 1.
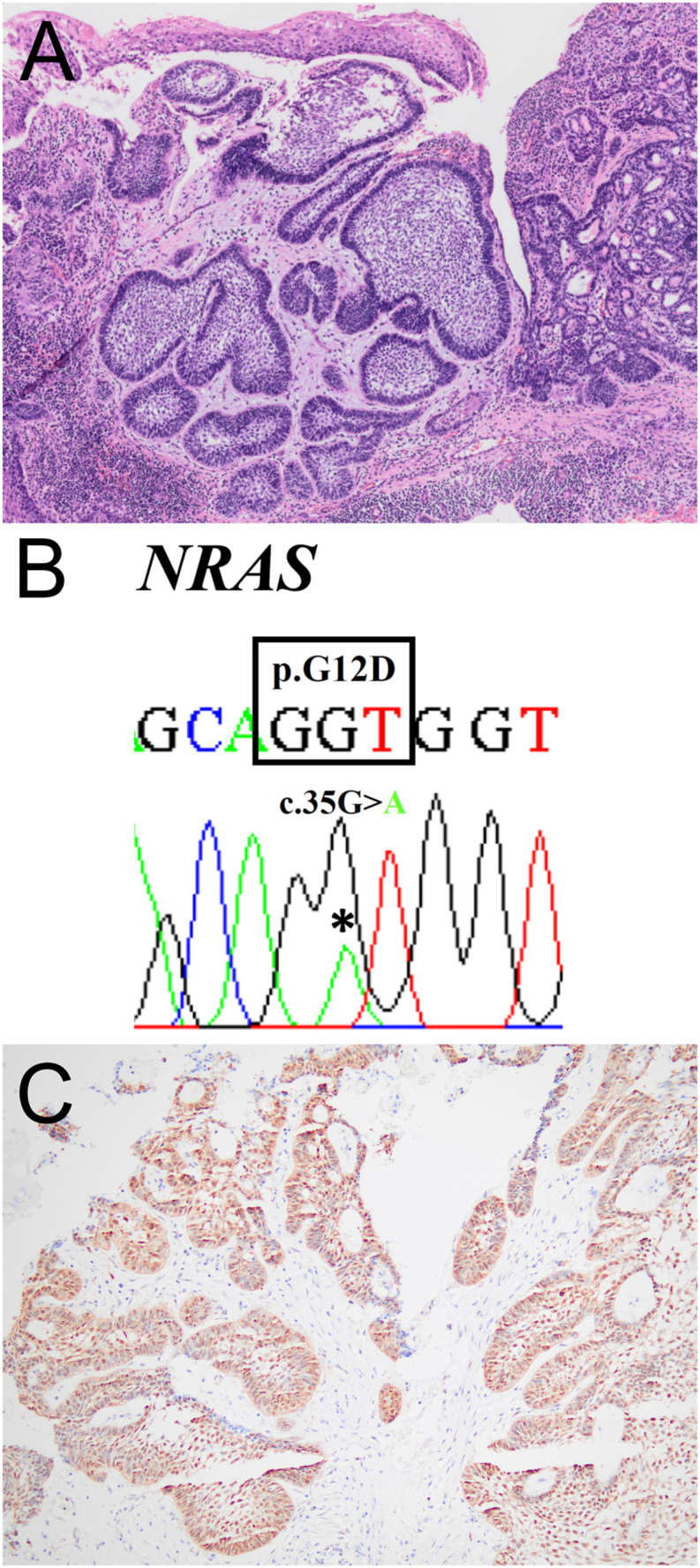
Histologic, immunohistochemical, and molecular findings of a case of extraosseous/peripheral ameloblastoma. (A) Islands of ameloblastic epithelium are found underneath the surface mucosal epithelium (H&E). (B) The NRAS p.G12D (c.35G > A) missense mutation (asterisk) is confirmed by Sanger sequencing. (C) Phosphorylated extracellular signal-regulated kinase (p-ERK) immunohistochemistry shows strong and diffuse staining in the nuclei of tumor cells.
Most of the mutations reported in EPA to date are involved in the MAPK pathway, including BRAF V600E, NRAS Q61R, and FGFR2 C382R mutations.1,2 In addition to these mutations, more diverse MAPK pathway mutations have been identified in intraosseous ameloblastoma compared with EPA, such as KRAS G12C, KRAS G12R, NRAS Q61K, HRAS Q61K, HRAS Q61R, and FGFR2 V395D mutations;1,4 however, there is considerable overlap in molecular features between EPA and intraosseous ameloblastoma: (1) The majority of genetic alterations are found in the MAPK pathway. (2) Among MAPK pathway genes, BRAF is the most frequently mutated gene, with the V600E variant accounting for almost all cases. (3) MAPK pathway mutations are associated with MAPK pathway activation in a tissue context, as demonstrated by p-ERK immunohistochemistry in our previous4 and present studies.
According to the Catalogue of Somatic Mutations in Cancer (COSMIC) database (https://cancer.sanger.ac.uk), NRAS G12D mutations are most commonly reported in leukemia, followed by colorectal adenocarcinoma and malignant melanoma. Although drugs directly targeting NRAS mutations are not currently approved (https://www.fda.gov/drugs), a patient with NRAS G12D-leukemia showed a near-complete response to trametinib, a MEK inhibitor.5 Therefore, the NRAS G12D mutation newly identified in ameloblastoma in this study may represent a new potential therapeutic target for patients with advanced ameloblastoma.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
None.
Contributor Information
Kyu-Young Oh, Email: bigfish@snu.ac.kr.
Seong-Doo Hong, Email: hongsd@snu.ac.kr.
References
- 1.Brown N.A., Rolland D., McHugh J.B., et al. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014;20:5517–5526. doi: 10.1158/1078-0432.CCR-14-1069. [DOI] [PubMed] [Google Scholar]
- 2.Yukimori A., Oikawa Y., Morita K.I., et al. Genetic basis of calcifying cystic odontogenic tumors. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh K.Y., Hong S.D., Yoon H.J. Adenoid ameloblastoma shares clinical, histologic, and molecular features with dentinogenic ghost cell tumor: the histologic spectrum of WNT pathway-altered benign odontogenic tumors. Mod Pathol. 2023;36 doi: 10.1016/j.modpat.2022.100051. [DOI] [PubMed] [Google Scholar]
- 4.Oh K.Y., Kim J.H., Cho S.D., Yoon H.J., Lee J.I., Hong S.D. BRAF V600E and previously unidentified KRAS G12C mutations in odontogenic tumors may affect MAPK activation differently depending on tumor type. Genes Chromosomes Cancer. 2022;61:481–490. doi: 10.1002/gcc.23040. [DOI] [PubMed] [Google Scholar]
- 5.Khanna V., Pierce S.T., Dao K.H., et al. Durable disease control with MEK inhibition in a patient with NRAS-mutated atypical chronic myeloid leukemia. Cureus. 2015;7:e414. doi: 10.7759/cureus.414. [DOI] [PMC free article] [PubMed] [Google Scholar]


