Abstract
Background/purpose
Periodontitis is associated with various systemic diseases, potentially facilitated by the passage of Porphyromonas gingivalis outer membrane vesicles (Pg-OMVs). Several recent studies have suggested a connection between Pg-OMVs and neuroinflammation and neurodegeneration, but the precise causal relationship remains unclear. This study aimed to investigate the mechanisms underlying these associations using in vitro models.
Materials and methods
Isolated Pg-OMVs were characterized by morphology, size, and gingipain activity. We exposed SH-SY5Y neuroblastoma cells and BV-2 microglial cells to various concentrations of Pg-OMVs. Cell morphology, a 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, an enzyme-linked immunosorbent assay, and Western blot analysis were used to evaluate the cellular mechanism underlying Pg-OMV-induced neurotoxicity in neuronal cells and inflammatory responses in microglial cells.
Results
Exposure to Pg-OMVs induced neurotoxicity in SH-SY5Y cells, as evidenced by cellular shrinkage, reduced viability, activation of apoptotic pathways, and diminished neuronal differentiation markers. Gingipain inhibition mitigated these effects, suggesting that gingipain mediates Pg-OMVs-induced neurotoxicity in SH-SY5Y cells. Our research on neuroinflammation suggests that upon endocytosis of Pg-OMVs by BV-2 cells, lipopolysaccharide (LPS) can modulate the production of inducible nitric oxide synthase and tumor necrosis factor-alpha by activating pathways that involve phosphorylated AKT and the phosphorylated JNK pathway.
Conclusion
Our study demonstrated that following the endocytosis of Pg-OMVs, gingipain can induce neurotoxicity in SH-SY5Y cells. Furthermore, the Pg-OMVs-associated LPS can trigger neuroinflammation via AKT and JNK signaling pathways in BV-2 cells.
Keywords: Porphyromonas gingivalis, Outer membrane vesicles, Neuron, Microglial cell, Gingipain, Lipopolysaccharide
Introduction
Periodontitis ranks as the sixth most prevalent human disease, affecting approximately 11.2% of adults worldwide.1 Previous studies have shown an association between periodontal disease and systemic diseases such as diabetes, cardiovascular disease, rheumatoid arthritis, and non-alcoholic fatty liver disease.2,3 Moreover, recent research has indicated an association between periodontitis and neurodegenerative diseases.4,5 Central to this association between periodontitis and systemic disease is the presence of Porphyromonas gingivalis (P. gingivalis), a pivotal bacterium within the oral microbiome recognized for its substantial role in the development of periodontal diseases.6,7 P. gingivalis produces multiple virulence factors including fimbriae, hemolysin, hemagglutinins, outer membrane vesicles (OMVs), lipopolysaccharides (LPS), and gingipains.8,9 Among these virulence factors, P. gingivalis OMVs (Pg-OMVs) carry LPS on their membrane surface and encapsulate a range of proteins, including fimbrial proteins and gingipains, particularly arginine-specific gingipains (RgpA and RgpB) and lysine-specific gingipain (Kgp).10, 11, 12, 13 The importance of Pg-OMVs lies in their capacity to traverse the bloodstream, serving as carriers of multiple virulence factors to tissues outside the oral cavity, thereby linking periodontal disease with systemic health issues.12,14, 15, 16
Several recent studies have illuminated the intricate relationships among P. gingivalis-related factors, neuroinflammation, and potential contributions to the progression of neurodegenerative disease.4,17, 18, 19 The persistent expression of gingipains by P. gingivalis could harm neurons, resulting in the formation of autophagic vacuoles in neurites, tau degradation, and the loss of synapses.20 Additionally, an association has emerged between a diagnosis of Alzheimer's disease and the load of gingipains in the brain.21 Animal studies have revealed that P. gingivalis infection in mice increases the expression of neurotoxic mediators such as nitric oxide (NO), inducible NO synthase (iNOS), and a series of pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and IL-1β in brain tissues, consequently leading to brain inflammation and neurodegeneration,12,22,23 and amyloid-beta production.23 Additionally, a review study highlighted the involvement of LPS in the pathology of Alzheimer's disease, including neuroinflammation via microglial activation and neuronal cell death induction.24 Moreover, recent studies have found that introducing Pg-OMVs into mouse brains triggers microglial activation and tau hyperphosphorylation,15,16 subsequently contributing to cognitive impairment.15
Although these studies have provided compelling evidence linking P. gingivalis and neurodegenerative pathways, some research gaps remain. The specific mechanisms identified also vary across studies,12,25,26 indicating potential complexities in the pathways involved. This study aimed to identify the pathways involved in the progression of these neuroinflammation and neurodegenerative conditions.
Materials and methods
Preparation and characterization of P. gingivalis-derived OMVs (Pg-OMVs)
Wild-type P. gingivalis strain ATCC 33277 was maintained on anaerobic blood agar plates under anaerobic conditions at 37 °C. In a liquid culture, bacteria were grown in brain-heart infusion broth supplemented with 5 mg/mL yeast extract and 0.5 mg/mL l-cysteine hydrochloride. The isolation of Pg-OMVs followed the methodology outlined in a prior study.14 The isolated Pg-OMVs were visualized by transmission electron microscopy (TEM; JEM-1400, JEOL, Tokyo, Japan). The concentration and size of Pg-OMVs were analyzed through nanoparticle tracking analysis, using NanoSight NS300 instrument (Malven, Worcestershire, UK). For the gingipain activity assay, Pg-OMVs were incubated with the specific synthetic substrates Z-His-Glu-Lys-MCA for Kgp and Z-Phe-Arg-MCA for Rgp for 10 min at 40 °C. The gingipain activity was measured using a Synergy microplate reader (BioTek, Winooski, VT, USA) at 460 nm.
Cell culture
Mouse microglial BV-2 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C with 5% CO2.27 Human neuroblastoma SH-SY5Y cells were maintained in DMEM/F-12 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. For neuronal differentiation, SH-SY5Y cells were treated with 10 μM all-trans retinoic acid for 6 days.28
Cell viability assay
Cells were seeded on 96-well plates (2 × 104 cells/well) and exposed to different concentrations of Pg-OMVs for 24 h. The cells were then treated with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/mL) for 1 h, and then 100 μL of dimethyl sulfoxide was added to dissolve the formazan crystals. The optical density at 570 nm was measured using a Multiskan FC microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Western blot analysis
Cells were homogenized in RIPA buffer supplemented with protease inhibitor and 1 mM PMSF. The samples were separated using SDS PAGE and transferred onto PVDF membranes. After blocking, the membranes were incubated overnight at 4 °C with primary antibodies for poly (ADP-ribose) polymerase (PARP), growth associated protein (GAP43), synaptophysin (Syn), iNOS, p-AKT, p-JNK, GAPDH, and β-actin. After incubation with secondary antibodies, the immune complexes were detected with enhanced chemiluminescence reagents (Millipore, Billerica, MA, USA) and images were obtained using an LAS-4000 imaging system (Fujifilm, Tokyo, Japan). The relative intensity of the blot band was quantified using densitometry with Image J software.
Enzyme-linked immunosorbent assay
After Pg-OMVs treatment, the cell culture supernatant was collected and the levels of TNF-α were measured using a mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Thermo Fisher Scientific). The total amount of TNF-α in the media were normalized to the levels of total protein.
Endocytosis assay of Pg-OMVs
Pg-OMVs were labeled with membrane fluorescent dye DiO3 according to the manufacturer's instructions (Biotium, Bay Area, CA, USA). SH-SY5Y cells and BV-2 cells were incubated with 10 μg/mL labeled Pg-OMVs for 24 h at 37 °C. To inhibit endocytosis, cells were pretreated with 2 μM Pitstop-2 for 30 min. After incubation, cells were fixed with 4% paraformaldehyde for 30 min at room temperature. The cells were washed and added to a diamidino-2-phenylindole (DAPI) solution for 10 min at room temperature. Cellular internalization of Pg-OMVs was imaged with an Olympus IX71 fluorescence microscope (Olympus Corp., Tokyo, Japan) using a 488 nm fluorescence filter.
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM) and analyzed using a one-way analysis of variance (ANOVA). Statistical significance between groups was determined using a one-way ANOVA, followed by Fisher's least significant difference test, using IBM SPSS Statistics software. A P-value less than 0.05 was considered statistically significant and was marked with an asterisk in the figures.
Results
Characterization of Pg-OMVs
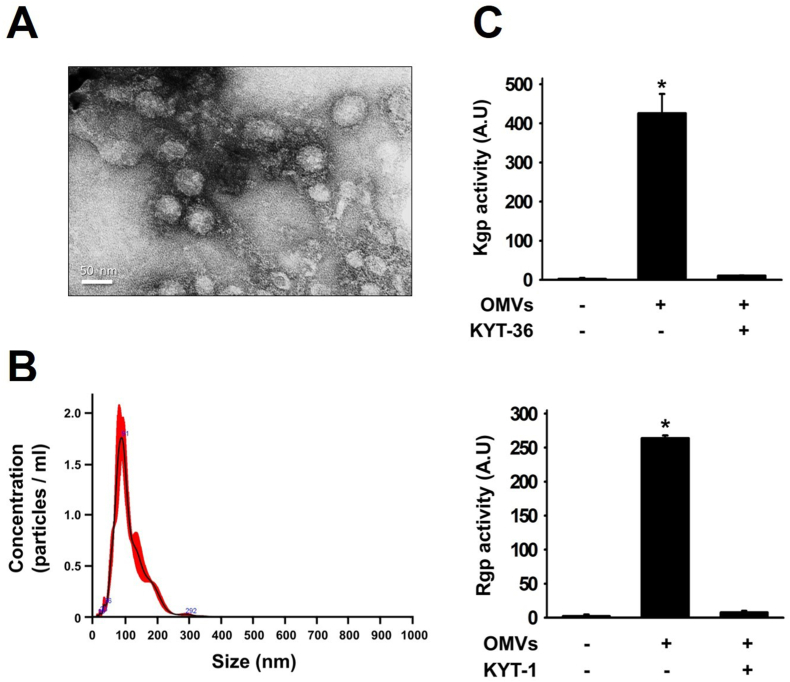
TEM images revealed successful isolation of spherical Pg-OMVs from the P. gingivalis culture supernatant (Fig. 1A), with particle diameters predominantly ranging between 50 and 150 nm (Fig. 1B). The results from gingipain activity assays indicated that the Pg-OMVs isolated from the P. gingivalis bacterial culture exhibited activity for both Rgp and Kgp (Fig. 1C). This confirmed the presence of active forms of Rgp and Kgp within the isolated Pg-OMVs.
Figure 1.
Characterization of Pg-OMVs. (A) The morphology of Pg-OMVs was evaluated using transmission electron microscope (TEM). (B) Analysis of Pg-OMVs particle diameters and concentration using nanoparticle tracking analysis. (C) Analysis of Pg-OMVs gingipain activity and its inhibition with KYT-1 and KYT-36. The bar represents the mean ± SEM of three different experiments (∗P < 0.05).
Pg-OMVs-induced neurotoxicity in SH-SY5Y cells
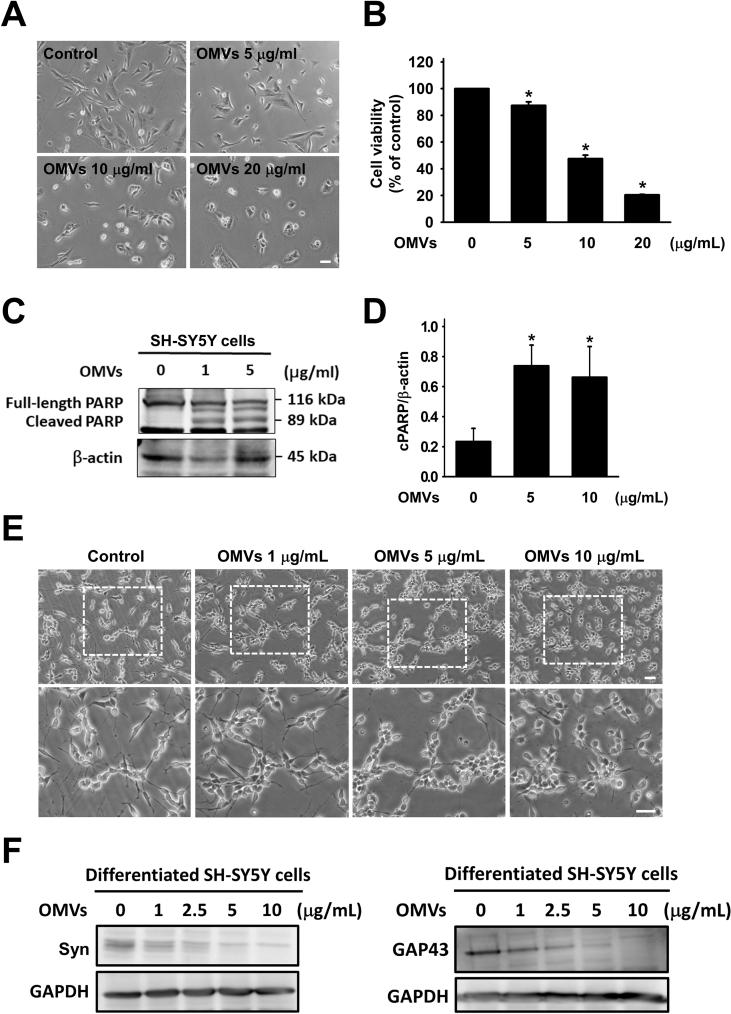
With the increasing concentration of Pg-OMVs, SH-SY5Y cells became more centralized and exhibited signs of shrinkage (Fig. 2A). Furthermore, increasing concentrations of Pg-OMVs also led to a dose-dependent decrease in cell viability (Fig. 2B). Western blot analysis revealed an increased induction of PARP cleavage in SH-SY5Y cells upon exposure to Pg-OMVs, indicating the activation of apoptotic pathways (Fig. 2C). Further quantification revealed significant differences in cleaved PARP (cPARP) levels between the control group and the group exposed to Pg-OMVs (Fig. 2D). Neurites exhibited signs of damage, including reduction in neurite length, which was exacerbated with increasing concentrations of Pg-OMVs (Fig. 2E). Further investigation into neurite markers GAP43 and Syn revealed a decrease in their expression levels, correlating with increased Pg-OMVs concentrations (Fig. 2F).
Figure 2.
Pg-OMVs-induced neurotoxicity in SH-SY5Y cells. (A) Bright-field morphology of undifferentiated SH-SY5Y treated with different concentrations of Pg-OMVs for 24 h. Scale bar, 100 μm. (B) MTT assay evaluation of the effect of Pg-OMVs on neuronal cell viability. For dose-dependence, Pg-OMVs were given at concentrations of 5, 10, and 20 μg/mL for 24 h. (C) Western blot analysis of cleaved PARP (cPARP) in SH-SY5Y cells upon exposure to different concentrations of Pg-OMVs for 24 h. (D) The relative quantification of cPARP expression normalized to β-actin. The bar represents the mean ± SEM of three different experiments (∗P < 0.05). (E) Bright-field morphology of differentiated SH-SY5Y cells treated with different concentrations of Pg-OMVs for 24 h. Scale bar, 100 μm. (F) Western blot analysis of Syn and GAP43 in differentiated SH-SY5Y cells upon exposure to different concentrations of Pg-OMVs for 24 h.
Gingipain mediates Pg-OMVs-induced neurotoxicity in SH-SY5Y cells
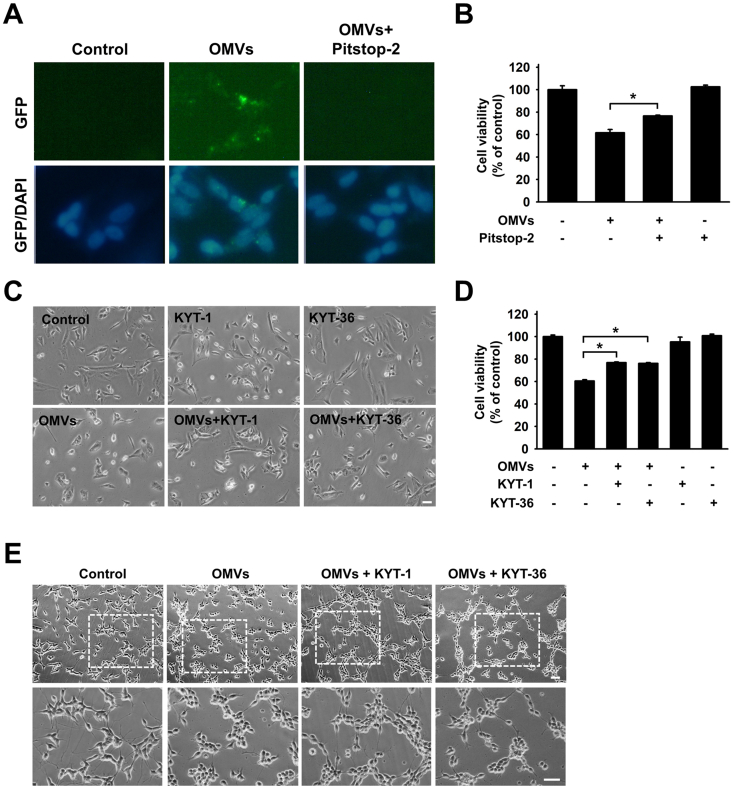
The clathrin inhibitor Pitstop-2 significantly inhibited Pg-OMVs uptake in SH-SY5Y cells and effectively reversed the neurotoxic effects induced by Pg-OMVs (Fig. 3A and B). In addition, following the introduction of KYT-1 and KYT-36, morphological changes and reduced cell viability were markedly alleviated (Fig. 3C and D). Moreover, neurites exhibited a prominent decrease in length after exposure to Pg-OMVs, but the introduction of KYT-1 and KYT-36 significantly protected against neurite shortening (Fig. 3E).
Figure 3.
Pg-OMVs-induced neurotoxicity via clathrin-dependent endocytosis and gingipain. (A) The undifferentiated SH-SY5Y cells were pretreated with Pitstop (2 μM) for 30 min and then incubated with DiO3-labeled Pg-OMVs (green) for another 24 h. Nuclei were stained with DAPI (blue). (B) MTT assay evaluation of the effect of Pitstop on Pg-OMVs-induced neurotoxicity. The bar represents the mean ± SEM of three different experiments (∗P < 0.05). (C) The undifferentiated SH-SY5Y cells were pretreated with KYT-1 (1 μM) or KYT-36 (1 μM) for 30 min followed by treatment with Pg-OMVs (10 μg/mL) for 24 h. The morphology was observed using bright-field microscopy. Scale bar, 100 μm. (D) MTT assay evaluation of the effect of KYT-1 and KYT-36 on Pg-OMVs-induced neurotoxicity. (E) Bright-field morphology of differentiated SH-SY5Y cells pretreated with KYT-1 and KYT-36 followed by treatment with Pg-OMVs for 24 h. Scale bar, 100 μm.
These findings strongly suggest that gingipains may impact neurotoxicity by clathrin-dependent endocytosis in response to Pg-OMVs. Additionally, they highlight the significant influence of gingipains on alterations in neurite morphology.
Pg-OMVs activate microglial cells via LPS
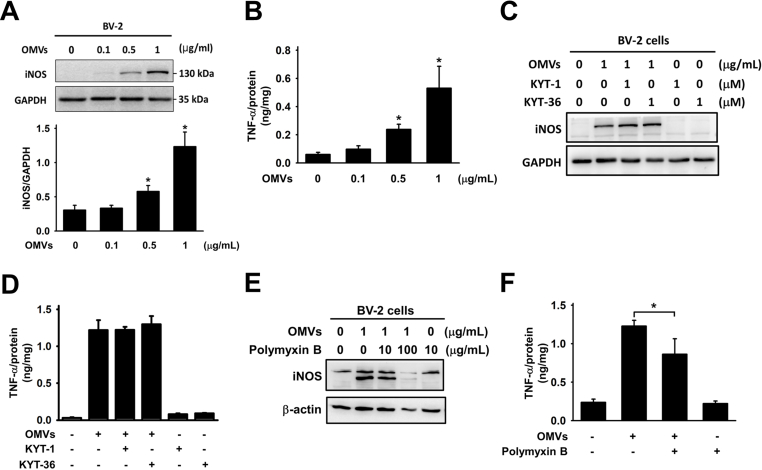
A significant increase of iNOS production was observed at a Pg-OMVs concentration of 0.5 μg/mL (Fig. 4A). Furthermore, Pg-OMVs induced production of TNF-α in microglial cells and exerted dose-dependent effects (Fig. 4B). However, the introduction of KYT-1 and KYT-36 did not reduce iNOS levels or the TNF-α production induced by Pg-OMVs (Fig. 4C and D). In contrast, polymyxin B significantly reduced iNOS production (Fig. 4E). Similarly, the production of TNF-α in microglial cells induced by Pg-OMVs notably diminished (Fig. 4F). These observations suggested that gingipain might not directly influence these specific inflammatory responses initiated by Pg-OMVs in microglial cells. Instead, they suggest that LPS probably has a crucial role in facilitating microglial activation triggered by Pg-OMVs, thereby influencing iNOS and TNF-α production in these cells.
Figure 4.
Pg-OMVs activate microglial cells via LPS. (A) Western blot analysis of iNOS expression in BV-2 cells exposed to different concentrations of Pg-OMVs for 24 h. The relative quantification of iNOS expression normalized to GAPDH. The bar represents the mean ±SEM of three different experiments (∗P < 0.05). (B) ELISA analysis of the levels of TNF-α in BV-2 cells exposed to different concentrations of Pg-OMVs for 24 h. The levels of TNF-α were normalized to total protein. The bar represents the mean ± SEM of three different experiments (∗P < 0.05). (C) Western blot analysis of the levels of iNOS in BV-2 cells pretreated with KYT-1 (1 μM) or KYT-36 (1 μM) for 30 min and then incubated with Pg-OMVs (1 μg/mL) for 24 h. (D) ELISA analysis of the levels of TNF-α in BV-2 cells pretreated with KYT-1 (1 μM) or KYT-36 (1 μM) for 30 min and then incubated with Pg-OMVs (1 μg/mL) for 24 h. The levels of TNF-α were normalized to total protein. The bar represents the mean ± SEM of three different experiments (∗P < 0.05). (E) Western blot analysis of iNOS production in BV-2 cells exposed to Pg-OMVs and LPS inhibitor polymyxin B. (F) ELISA analysis of TNF-α production in BV-2 cells exposed to Pg-OMVs and LPS inhibitor polymyxin B. The levels of TNF-α were normalized to total protein. The bar represents the mean ± SEM of three different experiments (∗P < 0.05).
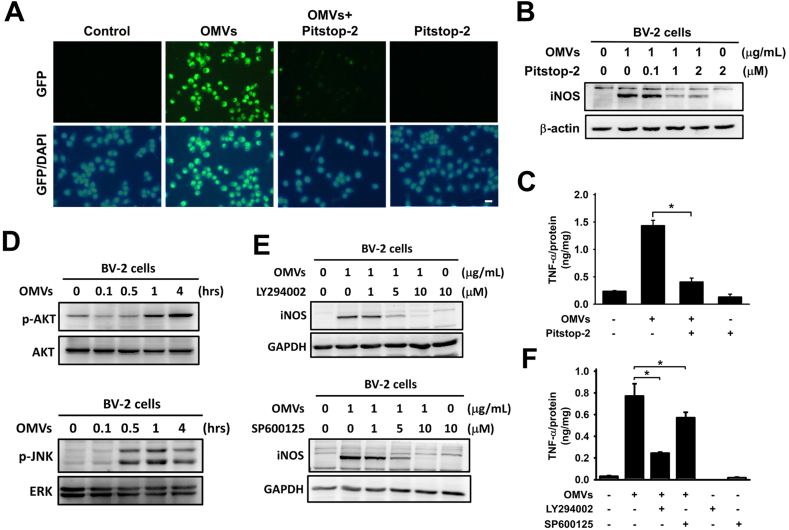
Pg-OMVs-mediated activation of microglial cells is dependent on endocytosis and AKT and JNK signaling
Fluorescence microscopy revealed the uptake of Pg-OMVs by BV-2 cells. Similarly, BV-2 cells pretreated with Pitstop-2 exhibited a lower fluorescence compared with the control group (Fig. 5A). BV-2 cells were exposed to Pg-OMVs, resulting in heightened iNOS production. Subsequently, when Pitstop-2 was introduced, a distinct reduction in iNOS and TNF-α production was observed (Fig. 5B and C), indicating that both the iNOS and TNF-α pathways were activated only upon endocytosis of Pg-OMVs by the cells. To explore the inflammatory response pathway induced by Pg-OMVs in microglial cells, we treated BV-2 microglial cell lines with Pg-OMVs, and a substantial increase in p-AKT and p-JNK levels was observed (Fig. 5D). Inhibitors targeting p-AKT and p-JNK were administered, resulting in a subsequent decrease in iNOS (Fig. 5E) and TNF-α levels (Fig. 5F). These results suggested that following the endocytosis of Pg-OMVs by BV-2 cells, an activated pathway involving p-AKT and p-JNK influenced the production of iNOS and TNF-α.
Figure 5.
Pg-OMVs-mediated microglial activation is dependent on endocytosis and AKT and JNK signaling pathways. (A) The BV-2 cells were pretreated with Pitstop (2 μM) for 30 min and then incubated with DiO3-labeled Pg-OMVs (green) for another 24 h. Nuclei were stained with DAPI (blue). Scale bar, 100 μm. (B) Western blot analysis of the levels of inducible nitric oxide synthase (iNOS) in BV-2 cells pretreated with Pitstop (2 μM) for 30 min and then incubated with Pg-OMVs (1 μg/mL) for 24 h. (C) ELISA analysis of TNF-α production in BV-2 cells exposed to Pg-OMVs and Pitstop. The levels of TNF-α were normalized to total protein. The bar represents the mean ± SEM of three different experiments (∗P < 0.05). (D) Western blot analysis of pAKT and pJNK production in BV-2 cells exposed to Pg-OMVs (1 μg/mL) for the indicated time. (E) Western blot analysis of the levels of iNOS in BV-2 cells pretreated with different concentrations of AKT inhibitor LY294002 and JNK inhibitor SP600125 for 30 min and then incubated with OMVs (1 μg/mL) for 4 h. (F) ELISA analysis of TNF-α production in BV-2 cells exposed to Pg-OMVs and AKT and JNK inhibitors. The levels of TNF-α were normalized to total protein. The bar represents the mean ± SEM of three different experiments (∗P < 0.05).
Discussion
The results of this study indicate that the neurotoxic effects induced by Pg-OMVs are primarily mediated by gingipain and the Pg-OMVs can activate microglial cells through LPS rather than gingipain. Our results revealed a cascade of events: subsequent to the endocytosis of Pg-OMVs by microglial cells, LPS can activate pathways involving p-AKT and p-JNK, thereby playing a pivotal role in influencing the production of iNOS and TNF-α. Our results provide valuable perspectives on the intricate molecular interactions governing both neurotoxicity and immune responses associated with Pg-OMVs.
Pg-OMVs serve as highly efficient vehicles for delivering virulence factors into host cells because they have the potential to migrate through the blood and affect distant organs. This facilitates the translocation of these virulence factors beyond the oral cavity, thus exhibiting a strong association with systemic diseases.12 Our results were consistent with the previous research, confirming the successful extraction of Pg-OMVs from the supernatant of a P. gingivalis culture, with the majority of vesicles falling within the diameter range of 50–150 nm. This observation is consistent with the reported size of outer membrane vesicles in the prior studies, typically ranging between 20 and 250 nm.10,29 TEM revealed that Pg-OMVs have a spherical layered structure with an average diameter of 110.6 ± 7.6 nm, a finding similar to that of a prior study.15 Our experiment, conducted using gingipain activity assays, confirms that isolated Pg-OMVs can carry active forms of Rgp and Kgp. This finding was consistent with that of a previous study in which mass spectrometry confirmed the presence of Rgp and Kgp in Pg-OMVs.10
Haditsch et al.20 demonstrated in vitro that P. gingivalis infection triggers neuropathological changes in neurons, including synaptic loss and the formation of autophagic vacuoles in neurites, primarily driven by the persistent expression of gingipains. Furthermore, results from an animal study revealed that orally administering a Kgp inhibitor to mice effectively treated brain infections caused by P. gingivalis, simultaneously preventing the loss of hippocampal Gad67+ interneurons.21 Giuseppe et al.30 found that exposing human neuroblastoma cells to Pg-OMVs led to the formation of Aβ plaques and phosphorylated Tau tangles, both of which are characteristic of neurodegeneration. However, the precise mechanism by which Pg-OMVs affect neuronal cells remained uncertain. Prompted by these findings, we investigated the relationship between the virulence factors in Pg-OMVs and neurodegenerative traits. Our results showed that Pg-OMVs could induce neuron death and neurite damage via a gingipain-dependent pathway. Importantly, the clathrin inhibitor significantly suppressed Pg-OMVs-mediated neurotoxicity. Similarly, another study suggested that intraneuronal gingipains may drive neuronal nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 1 (NLRP1) activation, resulting in pyroptosis of neurons and activation of caspase-1, leading to the release of neuroinflammatory mediators.21 Therefore, after Pg-OMVs are internalized by neurons, intracellular gingipain can contribute to toxicity toward neuronal cells.
Previous research has highlighted that both gingipains and LPS derived from P. gingivalis can play roles in activating immune cells.2,25 Recent animal studies have provided evidence that Pg-OMVs can promote the activation of microglia and tau hyperphosphorylation in Pg-OMVs-treated mouse brains, with increased proinflammatory cytokine expression in a gingipain-dependent manner.15,16 In addition, gingipain-mediated activation of protease-activated receptor-2 (PAR2) serves as a mechanism driving microglial migration and inflammatory responses through the AKT and ERK pathways.31 However, in our study, Pg-OMVs could not activate microglial cells through gingipain. The difference might be attributable to various factors such as differences in experimental conditions. For instance, experiments involving P. gingivalis typically lead to the extracellular activation of immune cells through receptors and signaling pathways,31,32 whereas Pg-OMVs require internalization into cells before initiating the activation of immune cells.12 Such variations in cellular interactions could account for the different outcomes observed among these studies. Differences in the cell types used in vitro experiments could also contribute to this variation.
Additional studies are necessary to investigate the signal pathways of Pg-OMVs and LPS that trigger neuroinflammation. The inflammatory response triggered by Pg-OMVs induced significant production of inflammatory mediators such as NO, IL-6, IL-10, interferon-beta, interferon-gamma, and matrix metalloproteinase-9.12 Moreover, these vesicles induced the production of TNF-α via activation of the NF-κB signaling pathway.12 The results based on the previous research indicate that P. gingivalis infection of microglia induces inflammation through the activation of PAR2 and involves phosphoinositide 3-kinase/AKT and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways,31 and our study explored the effects of Pg-OMVs on BV-2 cells. We found that Pg-OMVs activated a pathway in BV-2 cells similar to that observed in microglia during P. gingivalis infection. Specifically, upon endocytosis by BV-2 cells, Pg-OMVs triggered the activation of p-AKT and p-JNK, significantly influencing the production of iNOS and TNF-α. Regarding the involvement of JNK upon Pg-OMVs stimulation, a previous study found similar involvement in human gingival epithelial cells.33 Their findings revealed that Pg-OMVs can stimulate complex signaling pathways within cells, including Erk1/2, p38, JNK, STING, and NF-κB pathways. This cascade of activations culminates in heightened expression of pro-inflammatory cytokines.
In conclusion, our study indicated that Pg-OMVs can induce neurotoxicity in SH-SY5Y cells via gingipain. Furthermore, Pg-OMVs-associated LPS can trigger neuroinflammation via AKT and JNK signaling pathways in BV-2 cells. This study serves as evidence of a novel mechanistic pathway by which Pg-OMVs induce neurotoxicity and neuroinflammation. However, the effect of Pg-OMVs on neuronal and microglial changes in vivo was not explored. Further works will address whether Pg-OMVs can enhance neurotoxicity and neuroinflammation in the animal models. These subsequent studies may elucidate the role of Pg-OMVs in the pathogenesis of neurodegeneration.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was supported by the grants (110-2628-B-002-036 and 111-2628-B-002-037) from the Ministry of Science and Technology, Taiwan.
Contributor Information
Chun-Pin Chiang, Email: cpchiang@ntu.edu.tw.
Young-Ji Shiao, Email: yshiao@nricm.edu.tw.
Yi-Wen Chen, Email: yiwenchen@ntu.edu.tw.
References
- 1.Genco R.J., Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol 2000. 2020;83:7–13. doi: 10.1111/prd.12344. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z., Liu D., Liu S., Zhang S., Pan Y. The role of Porphyromonas gingivalis outer membrane vesicles in periodontal disease and related systemic diseases. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.585917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mei F., Xie M., Huang X., et al. Porphyromonas gingivalis and its systemic impact: current status. Pathogens. 2020;9:944. doi: 10.3390/pathogens9110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borsa L., Dubois M., Sacco G., Lupi L. Analysis the link between periodontal diseases and Alzheimer's disease: a systematic review. Int J Environ Res Publ Health. 2021;18:9312. doi: 10.3390/ijerph18179312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanagasingam S., Chukkapalli S.S., Welbury R., Singhrao S.K. Porphyromonas gingivalis is a strong risk factor for Alzheimer's Disease. J Alzheimers Dis Rep. 2020;4:501–511. doi: 10.3233/ADR-200250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorillo L., Cervino G., Laino L., et al. Porphyromonas gingivalis, periodontal and systemic implications: a systematic review. Dent J. 2019;7:114. doi: 10.3390/dj7040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elwishahy A., Antia K., Bhusari S., Ilechukwu N.C., Horstick O., Winkler V. Porphyromonas gingivalis as a risk factor to Alzheimer's Disease: a systematic review. J Alzheimers Dis Rep. 2021;5:721–732. doi: 10.3233/ADR-200237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleksijević L.H., Aleksijević M., Škrlec I., Šram M., Šram M., Talapko J. Porphyromonas gingivalis virulence factors and clinical significance in periodontal disease and coronary artery diseases. Pathogens. 2022;11:1173. doi: 10.3390/pathogens11101173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mysak J., Podzimek S., Sommerova P., et al. Porphyromonas gingivalis: major periodontopathic pathogen overview. J Immunol Res. 2014;2014 doi: 10.1155/2014/476068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakao R., Takashiba S., Kosono S., et al. Effect of Porphyromonas gingivalis outer membrane vesicles on gingipain-mediated detachment of cultured oral epithelial cells and immune responses. Microb Infect. 2014;16:6–16. doi: 10.1016/j.micinf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Li N., Collyer C.A. Gingipains from Porphyromonas gingivalis - complex domain structures confer diverse functions. Eur J Microbiol Immunol (Bp) 2011;1:41–58. doi: 10.1556/EuJMI.1.2011.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okamura H., Hirota K., Yoshida K., et al. Outer membrane vesicles of Porphyromonas gingivalis: novel communication tool and strategy. Jpn Dent Sci Rev. 2021;57:138–146. doi: 10.1016/j.jdsr.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veith P.D., Chen Y.Y., Gorasia D.G., et al. Porphyromonas gingivalis outer membrane vesicles exclusively contain outer membrane and periplasmic proteins and carry a cargo enriched with virulence factors. J Proteome Res. 2014;13:2420–2432. doi: 10.1021/pr401227e. [DOI] [PubMed] [Google Scholar]
- 14.Seyama M., Yoshida K., Yoshida K., et al. Outer membrane vesicles of Porphyromonas gingivalis attenuate insulin sensitivity by delivering gingipains to the liver. Biochim Biophys Acta, Mol Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165731. [DOI] [PubMed] [Google Scholar]
- 15.Gong T., Chen Q., Mao H., et al. Outer membrane vesicles of Porphyromonas gingivalis trigger NLRP3 inflammasome and induce neuroinflammation, tau phosphorylation, and memory dysfunction in mice. Front Cell Infect Microbiol. 2022;12 doi: 10.3389/fcimb.2022.925435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida K., Yoshida K., Seyama M., et al. Porphyromonas gingivalis outer membrane vesicles in cerebral ventricles activate microglia in mice. Oral Dis. 2023;29:3688–3697. doi: 10.1111/odi.14413. [DOI] [PubMed] [Google Scholar]
- 17.Liu S., Butler C.A., Ayton S., Reynolds E.C., Dashper S.G. Porphyromonas gingivalis and the pathogenesis of Alzheimer's disease. Crit Rev Microbiol. 2024;50:127–137. doi: 10.1080/1040841X.2022.2163613. [DOI] [PubMed] [Google Scholar]
- 18.Ryder M.I. Porphyromonas gingivalis and Alzheimer disease: recent findings and potential therapies. J Periodontol. 2020;91(Suppl 1):S45–S49. doi: 10.1002/JPER.20-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadrameli M., Bathini P., Alberi L. Linking mechanisms of periodontitis to Alzheimer's disease. Curr Opin Neurol. 2020;33:230–238. doi: 10.1097/WCO.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 20.Haditsch U., Roth T., Rodriguez L., et al. Alzheimer's disease-like neurodegeneration in Porphyromonas gingivalis infected neurons with persistent expression of active gingipains. J Alzheimers Dis. 2020;75:1361–1376. doi: 10.3233/JAD-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominy S.S., Lynch C., Ermini F., et al. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5 doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Y., Ren J., Yu H., Yu W., Zhou Y. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immun Ageing. 2018;15:6. doi: 10.1186/s12979-017-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilievski V., Zuchowska P.K., Green S.J., et al. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H.S., Kim S., Shin S.J., et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer's disease: pathologic roles and therapeutic implications. Transl Neurodegener. 2021;10:49. doi: 10.1186/s40035-021-00273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J., Yu C., Zhang X., et al. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J Neuroinflammation. 2018;15:37. doi: 10.1186/s12974-017-1052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin R., Ning X., Liu X., Zhao Y., Ye G. Porphyromonas gingivalis-induced periodontitis could contribute to cognitive impairment in Sprague-Dawley rats via the P38 MAPK signaling pathway. Front Cell Neurosci. 2023;17 doi: 10.3389/fncel.2023.1141339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsay H.J., Liu H.K., Kuo Y.H., et al. EK100 and antrodin C improve brain amyloid pathology in APP/PS1 transgenic mice by promoting nicroglial and perivascular clearance pathways. Int J Mol Sci. 2021;22 doi: 10.3390/ijms221910413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang C.N., Wu M.F., Liu C.C., et al. Differential protective effects of connective tissue growth factor against Aβ neurotoxicity on neurons and glia. Hum Mol Genet. 2017;26:3909–3921. doi: 10.1093/hmg/ddx278. [DOI] [PubMed] [Google Scholar]
- 29.Cecil J.D., Sirisaengtaksin N., O'Brien-Simpson N.M., Krachler A.M. Outer membrane vesicle-host cell interactions. Microbiol Spectr. 2019;7:1128. doi: 10.1128/microbiolspec.psib-0001-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pezzotti G., Adachi T., Imamura H., et al. In situ Raman study of neurodegenerated human neuroblastoma cells exposed to outer-membrane vesicles isolated from Porphyromonas gingivalis. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241713351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y., Wu Z., Nakanishi Y., et al. Infection of microglia with Porphyromonas gingivalis promotes cell migration and an inflammatory response through the gingipain-mediated activation of protease-activated receptor-2 in mice. Sci Rep. 2017;7 doi: 10.1038/s41598-017-12173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y., Li H., Zhang J., et al. Periodontitis induced by P. gingivalis-LPS is associated with neuroinflammation and learning and memory impairment in Sprague-Dawley rats. Front Neurosci. 2020;14:658. doi: 10.3389/fnins.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uemura Y., Hiroshima Y., Tada A., et al. Porphyromonas gingivalis outer membrane vesicles stimulate gingival epithelial cells to induce pro-inflammatory cytokines via the MAPK and STING pathways. Biomedicines. 2022;10:2643. doi: 10.3390/biomedicines10102643. [DOI] [PMC free article] [PubMed] [Google Scholar]