Abstract
Background
Male breast cancer (MBC) is a rare disease. Although several large-scale studies have investigated MBC patients in other countries, the features of MBC patients in China have not been fully explored. This study aims to explore the features of Chinese MBC patients comprehensively.
Methods
We retrospectively collected data of MBC patients from 36 centers in China. Overall survival (OS) was evaluated by the Kaplan-Meier method, log-rank test, and Cox regression analyses. Multivariate Cox analyses were used to identify independent prognostic factors of the patients.
Results
In total, 1119 patients were included. The mean age at diagnosis was 60.9 years, and a significant extension over time was observed (P < 0.001). The majority of the patients (89.1 %) received mastectomy. Sentinel lymph node biopsy was performed in 7.8 % of the patients diagnosed in 2009 or earlier, and this percentage increased significantly to 38.8 % in 2020 or later (P < 0.001). The five-year OS rate for the population was 85.5 % [95 % confidence interval (CI), 82.8 %–88.4 %]. Multivariate Cox analysis identified taxane-based [T-based, hazard ratio (HR) = 0.32, 95 % CI, 0.13 to 0.78, P = 0.012] and anthracycline plus taxane-based (A + T-based, HR = 0.47, 95 % CI, 0.23 to 0.96, P = 0.037) regimens as independent protective factors for OS. However, the anthracycline-based regimen showed no significance in outcome (P = 0.175).
Conclusion
As the most extensive MBC study in China, we described the characteristics, treatment and prognosis of Chinese MBC population comprehensively. T-based and A + T-based regimens were protective factors for OS in these patients. More research is required for this population.
Keywords: Male breast cancer, China, Characteristics, Treatment, Prognosis
Highlights
-
•
This is the largest-scale study reporting Chinese MBC to date, involving 1119 Chinese MBC patients from 36 centers.
-
•
The characteristics, treatment patterns and prognosis of Chinese MBC population have been fully investigated.
-
•
An optimization of treatments given to the patients over time is observed.
-
•
The efficacy of taxane-based and anthracycline plus taxane-based regimens was first indicated in Chinese MBC patients.
1. Introduction
Breast cancer has emerged as the most prevalent cancer worldwide, although male breast cancer (MBC) represents only approximately 0.6 % of all breast cancer cases globally [1,2]. Owing its rarity, limited prospective studies have been conducted [3], resulting in the extrapolation of management from guidelines for females [4,5] and a heavy reliance on retrospective studies [6].
To date, the largest-scale MBC studies have originated from American national clinical databases, which provide extensive coverage of the population. For instance, based on 8481 MBC cases from the Surveillance, Epidemiology, and End Results (SEER) Program, it is reported that outcomes for MBC in the US are improving, owing to advances in therapy [7]. Apart from the US, the broadest study was conducted in Japan, involving 3780 MBC cases. However, the study compared their characteristics and treatments with those of female patients only and lacked a prognosis report [8]. The prognosis of patients with MBC in other specific countries, including Sweden, Singapore and South Korea, has also been reported, yet these studies consistently limited by small sample sizes [[9], [10], [11]]. Furthermore, most of the present studies focused on the differences between MBC patients and their female counterparts, with relatively few examining the effectiveness of treatment regimens for MBC patients or the changes over time.
In 2019, the incident cases of MBC in China reached 7,110, with 2810 deaths, representing an increase of 1193 % and 659 % compared to the cases in 1990, respectively. Moreover, these incident cases are predicted to increase substantially from 2019 to 2034 [12]. However, despite diligent efforts to investigate the characteristics and prognosis of MBC in China, these retrospective studies are constrained by limited cohort sizes [[13], [14], [15], [16]]. Among them, the research with maximum sample size was conducted by Shang et al. They retrospectively analyzed 220 patients from 4 centers and found no significance in outcome between sentinel lymph node biopsy (SLNB) and axillary lymph node dissection (ALND) [16]. Currently, there exists only one guideline introducing the management of MBC in China, which was released in Feb. 2023 [17]. Owing to the lack of robust evidence on Chinese MBC, the guideline heavily relies on studies conducted in other countries, and aligns with other authoritative MBC guidelines [4,5].
We conducted a retrospective analysis including 1119 patients with MBC from 36 centers across China. With the maximal Chinese MBC cohort so far, we aimed to provide a comprehensive understanding of the characteristics, treatment patterns, and prognostic outcomes among patients with MBC in China.
2. Material and methods
2.1. Patient selection
We retrospectively collected MBC patients from 36 centers in China, 13 of which were from the Chinese Society of Clinical Oncology Breast Cancer Database (CSCO BC RWS 2305).
Patients who were biologically male and had been histologically diagnosed with primary breast cancer were included in the study. Exclusion criteria included the presence of skin-origin malignancy on the breast. We had no limitation to the year of diagnosis.
This study was approved by Clinical Research and Laboratory Animal Ethics Committee, the First Affiliated Hospital of Sun Yat-sen University. The consents were waived owing to the retrospective design of the study and the lack of sensitive information.
2.2. Data collection
Variables we collected include the clinicopathological characteristics, surgeries, adjuvant therapies and survival outcomes. Variable definition and classification rules are detailed in Supplementary Table S1.
Luminal-type was defined as estrogen receptor (ER) and/or progesterone receptor (PR) positive and human epidermal growth factor receptor 2 (HER-2) negative. HER-2 positive subtype was defined as HER-2 positive, regardless of ER and PR status. Triple negative subtype was defined as ER, PR, and HER-2 negative.
2.3. Statistical analysis
The primary outcome was overall survival (OS), defined as the duration between the date of the first pathologic diagnosis and death from any cause or last follow-up. Continuous variables were presented as mean and standard deviation and categorical variables were presented as percentages. Statistical comparisons were conducted using Chi-Square tests for categorical variables or independent t-tests for continuous variables.
We used Kaplan-Meier method and Cox analyses to evaluate the survival of the patients. For the multivariate Cox model, we included variables with P < 0.050 in the univariate Cox analysis, but excluded grade and axillary surgery as grade might introduce unacceptable bias owing to the substantial proportion of missing data and showed no significant correlation to OS in MBC [18]. Moreover, axillary surgery is considered more related to quality of life rather than survival [19,20].
Patients with unknown data for corresponding variables were excluded when performing statistical analyses. Two-tailed P < 0.05 was considered statistically significant. All statistical analyses were conducted using R version 4.2.2.
3. Results
3.1. Characteristics
We investigated 1124 Chinese patients diagnosed with MBC from January 1988 to February 2023. Finally, 1119 patients were included in the study (Supplementary Fig. S1). The characteristics of patients are presented in Table 1.
Table 1.
Characteristics of Chinese male breast cancer patients by period of diagnosis.
| Variables | Period of diagnosis |
Totala (N = 1119) N (%) |
P value of test for trend over time | ||
|---|---|---|---|---|---|
| Dec. 2009 or earlier (N = 128) N (%) |
Jan. 2010 to Dec. 2019 (N = 722) N (%) |
Jan. 2020 or later (N = 258) N (%) |
|||
| Age (y) | <0.001 | ||||
| Mean SD | 56.7 12.7 | 61.0 12.7 | 62.6 12.4 | 60.9 12.7 | |
| 65 | 87(68.0) | 421(58.3) | 137(53.1) | 645 (57.6) | |
| 65 | 34(26.6) | 300(41.6) | 121(46.9) | 455 (40.7) | |
| Unknown | 7(5.5) | 1(0.1) | 0 | 19(1.7) | |
| T stage | 0.032 | ||||
| 0/is | 1(0.8) | 20(2.8) | 11(4.3) | 32(2.9) | |
| 1 | 35(27.3) | 247(34.2) | 67(26.0) | 352(31.5) | |
| 2 | 53(41.4) | 258(35.7) | 123(47.7) | 438(39.1) | |
| 3 | 5(3.9) | 25(3.5) | 8(3.1) | 39(3.5) | |
| 4 | 13(10.2) | 51(7.1) | 17(6.6) | 82(7.3) | |
| Unknown | 21(16.4) | 121(16.8) | 32(12.4) | 176(15.7) | |
| N stage | 0.949 | ||||
| 0 | 69(53.9) | 384(53.2) | 143(55.4) | 601(53.7) | |
| 1 | 32(25.0) | 174(24.1) | 56(21.7) | 264(23.6) | |
| 2 | 16(12.5) | 78(10.8) | 31(12.0) | 127(11.3) | |
| 3 | 7(5.5) | 52(7.2) | 19(7.4) | 80(7.1) | |
| Unknown | 4(3.1) | 34(4.7) | 9(3.5) | 47(4.2) | |
| Stage | 0.203 | ||||
| 0 | 1(0.8) | 22(3.0) | 16(6.2) | 39(3.5) | |
| I | 24(18.8) | 143(19.8) | 46(17.8) | 213(19.0) | |
| II | 52(40.6) | 263(36.4) | 105(40.7) | 424(37.9) | |
| III | 27(21.1) | 137(19.0) | 48(18.6) | 216(19.3) | |
| IV | 6(4.7) | 52(7.2) | 16(6.2) | 75(6.7) | |
| Unknown | 18(14.1) | 105(14.5) | 27(10.5) | 152(13.6) | |
| Histology | 0.032 | ||||
| Ductal | 106(82.8) | 577(79.9) | 199(77.1) | 890(79.5) | |
| Other | 16(12.5) | 116(16.1) | 59(22.9) | 189(16.9) | |
| Unknown | 6(4.7) | 29(4.0) | 5(1.9) | 40(3.6) | |
| Grade | 0.211 | ||||
| I | 2(1.6) | 26(3.6) | 17(6.6) | 45(4.0) | |
| II | 37(28.9) | 329(45.6) | 125(48.4) | 496(44.3) | |
| III/IV | 18(14.1) | 113(15.7) | 39(15.1) | 170(15.2) | |
| Unknown | 71(55.5) | 254(35.2) | 77(29.8) | 408(36.5) | |
| ER status | 0.972 | ||||
| Positive | 111(86.7) | 628(87.0) | 234(90.7) | 981(87.7) | |
| Negative | 6(4.7) | 37(5.1) | 13(5.0) | 57(5.1) | |
| Unknown | 11(8.6) | 57(7.9) | 11(4.3) | 81(7.2) | |
| PR status | 0.553 | ||||
| Positive | 103(80.5) | 592(82.0) | 225(87.2) | 927(82.8) | |
| Negative | 14(10.9) | 69(9.6) | 21(8.1) | 106(9.5) | |
| Unknown | 11(8.6) | 61(8.4) | 12(4.7) | 86(7.7) | |
| HER-2 status | 0.007 | ||||
| Positive | 7(5.5) | 82(11.4) | 44(17.1) | 134(12.0) | |
| Negative | 101(78.9) | 512(70.9) | 185(71.7) | 803(71.8) | |
| Unknown | 20(15.6) | 128(17.7) | 29(11.2) | 182(16.3) | |
| Ki-67 index (%) | 0.797 | ||||
| Mean SD | 27.4 20.7 | 27.8 18.9 | 27.1 18.1 | 27.5 18.8 | |
| Unknown | 59(46.1) | 89(12.3) | 15(5.8) | 170(15.2) | |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; SD, standard deviation.
The exact year of diagnosis of 11 cases were unknown. These cases were thus recorded in the column Total only.
Most patients received their diagnosis between 2010 and 2019 (64.5 %, 722/1119). The mean age at diagnosis for Chinese patients was 60.9 years [standard deviation (SD): 12.7 years]. Moreover, age at diagnosis increased significantly over time (P < 0.001). In terms of the stages, most patients exhibited stage II disease (37.9 %, 424/1119). The percentages of stage I and III were 19.0 % (213/1119) and 19.3 % (216/1119) for the patients, respectively. Approximately 6.7 % (75/1119) of the patients were diagnosed as stage IV disease. The distribution of T stage by period of diagnosis was significantly different (P = 0.032), whereas that of N stage showed no significance (P = 0.949).
Ductal carcinoma (79.5 %, 890/1119) and grade II (69.8 %, 496/711, using patients with non-missing information as the denominator) were predominant in the Chinese cohort. Regarding biological markers, most of the patients were ER positive (87.7 %, 981/1119), PR positive (82.8 %, 927/1119), and HER-2 negative (71.8 %, 803/1119). The mean Ki-67 index was 27.5 % (SD: 18.8 %).
3.2. Treatments
The choices of treatments for Chinese MBC patients were also investigated (Table 2). The vast majority of the patients received mastectomy (89.1 %, 1000/1119). In addition, 63.0 % (705/1119) of the patients underwent ALND. Although the rates of mastectomy exhibited statistically significant differences among the three time periods (P = 0.041), the absolute rates remained comparable. In contrast, we observed a significant reduction in the proportion of ALND over time (P < 0.001). The percentage of patients who underwent ALND in 2009 or earlier was 87.5 % (112/128), which decreased to 48.4 % (125/258) in 2020 or later. SLNB was performed on 35.1 % (211/601) of the Chinese patients with N0 stage and 16.3 % (43/264) of the N1 patients (Supplementary Table S2). Radiotherapy was applied to 23.6 % (264/1119) of the patients.
Table 2.
Treatments for Chinese male breast cancer patients by period of diagnosis.
| Variables | Period of diagnosis |
Totala |
P value of test for trend over time | ||
|---|---|---|---|---|---|
| Dec. 2009 or earlier (N = 128) N (%) |
Jan. 2010 to Dec. 2019 (N = 722) N (%) |
Jan. 2020 or later (N = 258) N (%) |
(N = 1119) N (%) |
||
| Breast surgery | 0.041 | ||||
| Mastectomy | 119(93.0) | 641(88.8) | 230(89.1) | 1000(89.1) | |
| Partial mastectomy | 7(5.5) | 31(4.3) | 5(1.9) | 43(3.8) | |
| No | 1(0.8) | 34(4.7) | 17(6.6) | 53(4.7) | |
| Unknown | 1(0.8) | 16(2.2) | 6(2.3) | 23(2.1) | |
| Axillary surgery | <0.001 | ||||
| SLNB | 10(7.8) | 154(21.3) | 100(38.8) | 265(23.7) | |
| ALND | 112(87.5) | 459(63.6) | 125(48.4) | 705(63.0) | |
| No | 4(3.1) | 81(11.2) | 25(9.7) | 110(9.8) | |
| Unknown | 2(1.6) | 28(3.9) | 8(3.1) | 39(3.5) | |
| Radiotherapy | 0.520 | ||||
| Yes | 25(19.5) | 178(24.7) | 60(23.3) | 264(23.6) | |
| No | 60(46.9) | 424(58.7) | 174(67.4) | 658(58.8) | |
| Unknown | 43(33.6) | 120(16.6) | 24(9.3) | 197(17.6) | |
| Chemotherapy | 0.002 | ||||
| Yes | 71(55.5) | 376(52.1) | 136(52.7) | 588(52.5) | |
| No | 17(13.3) | 234(32.4) | 89(34.5) | 339(30.3) | |
| Unknown | 40(31.3) | 112(15.5) | 33(12.8) | 192(17.2) | |
| Endocrine therapy (HR positive cases) | 0.165 | ||||
| Yes | 89(78.1) | 458(72.2) | 168(71.5) | 716(72.3) | |
| No | 9(7.9) | 92(14.5) | 32(13.6) | 133(13.4) | |
| Unknown | 16(14.0) | 84(13.2) | 35(14.9) | 142(14.3) | |
| Total | 114(100) | 634(100) | 235(100) | 991(100) | |
| Anti-HER-2 therapy (HER-2 positive cases) | 0.010 | ||||
| Yes | 1(14.3) | 33(40.2) | 32(72.7) | 63(47.0) | |
| No | 1(14.3) | 30(36.6) | 7(15.9) | 40(29.9) | |
| Unknown | 5(71.4) | 19(23.2) | 5(11.4) | 31(23.1) | |
| Total | 7(100) | 82(100) | 44(100) | 134(100) | |
Abbreviations: SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; HR, hormone receptor; HER-2, human epidermal growth factor receptor 2.
The exact year of diagnosis of 11 cases were unknown. These cases were thus recorded in the column Total only.
In terms of systemic therapies, 52.5 % (588/1119) of the patients received chemotherapy. Before 2009, the proportion of patients with no chemotherapy use was significantly lower (13.3 %, 17/128, P = 0.002). In the two groups from 2010 onwards, the percentages increased and became comparable [32.4 % (234/722) and 34.5 % (89/258), respectively]. Most of the hormone receptor-positive patients were given endocrine therapy (72.3 %, 716/991). Although anti-HER-2 therapy was administered to only 47.0 % (63/134) of HER-2 positive patients, a significant increase of its administration over time was observed (P = 0.010).
The regimens of each systemic therapy of Chinese patients were also investigated (Supplementary Table S3). The most common chemotherapy regimen for Chinese patients was the anthracycline plus taxane-based (A + T-based) regimen (42.9 %, 252/588). For endocrine therapy, selective estrogen receptor modulators (SERM) were predominantly used (80.4 %, 576/716). Additionally, 14.9 % (107/716) of the patients were administered aromatase inhibitor (AI). However, only 10.3 % (11/107) of these patients received AI combined with gonadotropin-releasing hormone agonists (GnRHa), whereas the GnRHa status of 57.9 % (62/107) of the patients was unknown.
6.3. Survival.
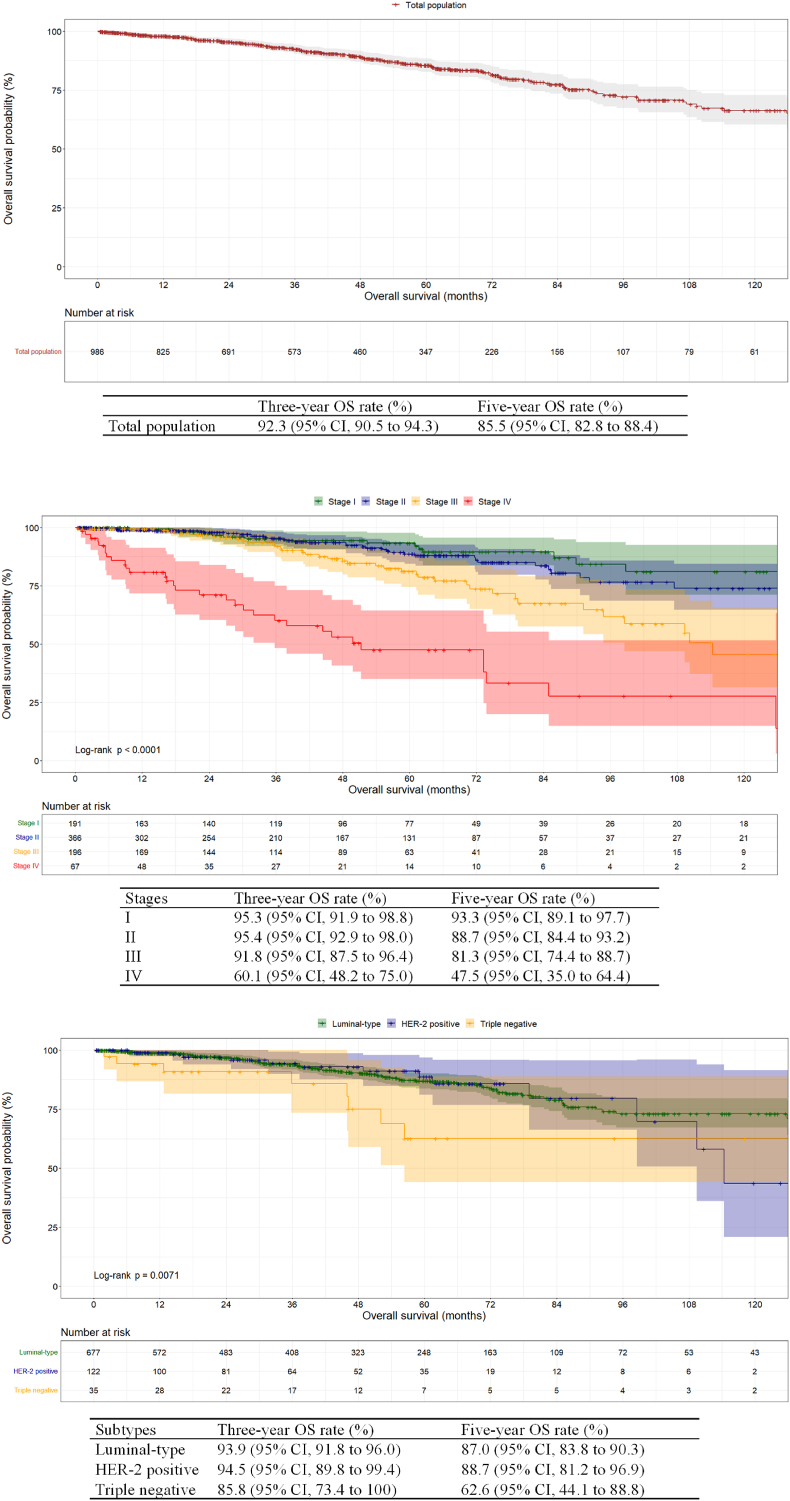
In total, 986 Chinese MBC patients were included in the survival analysis. Of these, 141 patients succumbed to any causes. The median follow-up time was 44.4 months. The 3-year and 5-year OS rates of the Chinese MBC population were 92.3 % [95 % confidence interval (CI), 90.5 %–94.3 %] and 85.5 % (95 % CI, 82.8 %–88.4 %), respectively (Fig. 1A).
Fig. 1.
Kaplan-Meier curves for overall survival for Chinese male patients with breast cancer. (a) Depicts the total population. (b) Depicts patients with stage I to IV diseases. The log-rank P < 0.0001. (c) Depicts patients with different subtypes of the tumor. The log-rank P = 0.0071.
Abbreviations: OS, overall survival; CI, confidence interval; HER-2, human epidermal growth factor receptor 2.
Moreover, the patients in different stages exhibited significantly different OS (P < 0.0001, Fig. 1B). Notably, no deaths were observed among the 33 Chinese patients with stage 0, resulting in a constant OS rate of 100 % (data not shown). The most favorable outcomes were observed in patients with stage I and II diseases. Specifically, the 5-year OS rate for patients with stage I disease was 93.3 % (95 % CI, 89.1 %–97.7 %), respectively. Comparatively, it was 88.7 % (95 % CI, 84.4 %–93.2 %) for patients in stage II. For patients with stage III and IV diseases, the 5-year OS rates were reduced to 81.3 % (95 % CI, 74.4 %–88.7 %) and 47.5 % (95 % CI, 35.0 %–64.4 %), respectively.
Significant differences in OS were also detected when patients were categorized by molecular subtypes (P = 0.0071, Fig. 1C). Patients with luminal-type or HER-2 positive tumors exhibited similar outcomes. The 5-year OS rates for these two subgroups were 87.0 % (95 % CI, 83.8 %–90.3 %) and 88.7 % (95 % CI, 81.2 %–96.9 %), respectively. However, patients with triple-negative tumors had a considerably lower 5-year OS rate of only 62.6 % (95 % CI, 44.1 %–88.8 %).
Furthermore, we attempted to identify independent prognostic factors in Chinese patients. The results of the univariate Cox regression analysis are presented in Supplementary Table S4. Finally, age, stage, ER and PR status, Ki-67 index, breast surgery, chemotherapy and endocrine therapy were included in the multivariate Cox model.
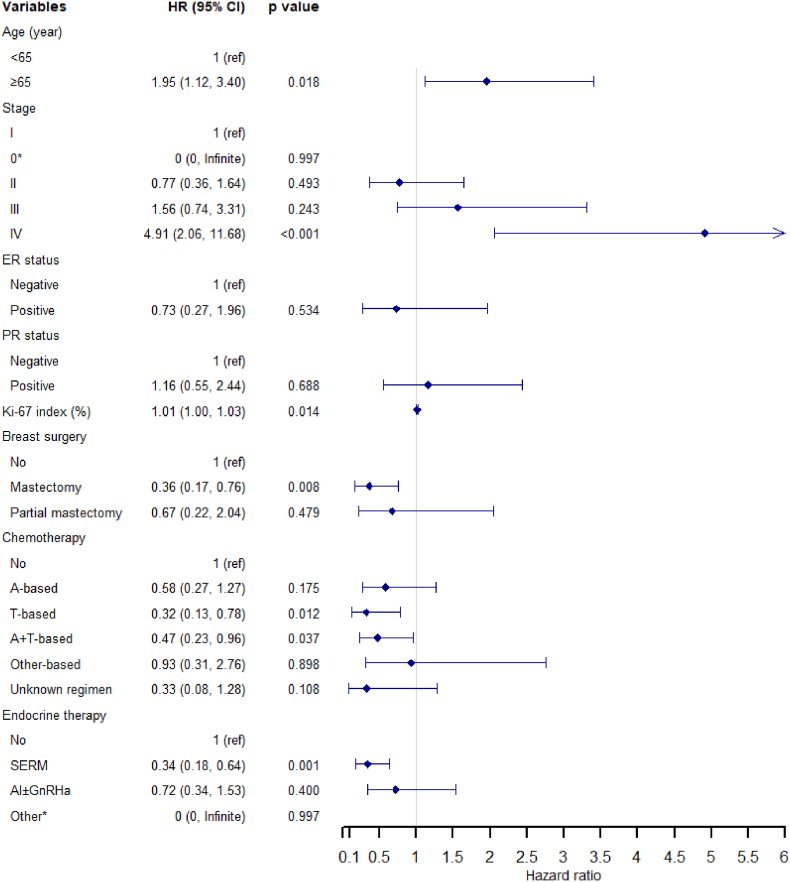
A total of 631 Chinese MBC patients were included in the multivariate Cox model, of which 78 patients died (Fig. 2). Several factors were identified as independent risk factors for OS, including age 65 years (compared to <65 years, HR = 1.95, 95 % CI, 1.12 to 3.40, P = 0.018), stage IV (compared to stage I, HR = 4.91, 95 % CI, 2.06 to 11.68, P < 0.001), and high Ki-67 index (HR = 1.01, 95 % CI, 1.00 to 1.03, P = 0.014). Mastectomy (HR = 0.36, 95 % CI, 0.17 to 0.76, P = 0.008), T-based (HR = 0.32, 95 % CI, 0.13 to 0.78, P = 0.012), A + T-based (HR = 0.47, 95 % CI, 0.23 to 0.96, P = 0.037) chemotherapy regimens, and SERM (HR = 0.34, 95 % CI, 0.18 to 0.64, P = 0.001) were associated with superior OS.
Fig. 2.
Multivariate Cox regression analysis of overall survival in Chinese male breast cancer patients.
* No death occurred among patients with stage 0 or “other” endocrine therapy administration, leading to the failure of calculating the exact hazard ratio of the two variables.
Abbreviations: HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; A-based, anthracycline-based; T-based, taxane-based; A + T-based, anthracycline + taxane-based; SERM, selective estrogen receptor modulator; AI, aromatase inhibitor; GnRHa, gonadotropin-releasing hormone agonists.
Furthermore, anti-HER-2 therapy was further examined within patients with positive HER-2. No significance was detected in univariate or multivariate analysis (data not shown). Additionally, we evaluated the safety of SLNB performed in the Chinese cohort. No significant difference was found in the impact of ALND and SLNB on OS in patients with N0 or N1 stage (Supplementary Fig. S2. P = 0.380 and P = 0.770, respectively). The results were similar in the multivariate analyses (Supplementary Table S5. P = 0.093 and P = 0.561, respectively).
4. Discussion
In this study, we comprehensively described the characteristics, treatment patterns and prognosis of Chinese patients with MBC. We further identified that age, stage IV disease, Ki-67 index, mastectomy, and several systemic therapy regimens were significantly associated with OS in Chinese MBC patients.
We observed that, in comparison to American patients, Chinese MBC patients were comparable in age and the proportion of stage IV, but presented with more locally advanced diseases. The median age of American MBC population was 63 years according to a study analyzing 16,498 cases from National Cancer Database (NCDB). Among these patients, 14.7 % were diagnosed as stage III disease and 6.6 % in stage IV [21]. Our findings regarding age also align with several small-scale Chinese studies and a Korean study on MBC, where the mean (or median) age was approximately 60 (56.4–64.5) years [11,[13], [14], [15],22]. We also observed that, with the passage of periods, the age of Chinese patients is gradually increasing, displaying a trend approaching the data in the US. This may be related to the aging population in China. Similarly, the mean age of 3780 MBC patients in Japan, a country where the overall population is experiencing severe aging, was 71 years [8]. The differences in stages between Chinese and American patients might be attributed to two main factors. First, breast cancer education and screening for Chinese males are notably insufficient, potentially contributing to more locally advanced stages. It was only in 2008 that China initiated a nationwide breast cancer screening for females [23]. Additionally, our analysis revealed no significant changes in the distribution of stages over time among Chinese MBC patients. This finding suggested that there was no notable improvement in breast cancer education for Chinese males, necessitating further enhancement. Second, considering the significantly lower body mass index of Chinese individuals compared to Americans [[24], [25], [26], [27]], we speculated that the smaller breast size in Chinese males may facilitate the detection of the mass in the breast, to some extent restricting the progression to advanced disease, resulting in the similar proportions of stage IV.
In addition, Chinese MBC patients underwent more aggressive treatment. The proportion of Chinese patients receiving mastectomy and ALND was higher than that of American patients. Leone et al. reported that 10.1 % of MBC patients received partial mastectomy, based on an analysis of 8481 MBC cases from SEER [28]. Another study based on SEER revealed that 61.1 % (735/1203) of MBC patients with cT1-2N0M0 stage who underwent mastectomy also received SLNB [29]. Smaller breast size might be one of the reasons why Chinese patients rarely undergo partial mastectomy. However, among Chinese patients with N0 and N1 stages, there was a considerably higher proportion for ALND. Other small-scale Chinese studies on MBC also reported that mastectomy and ALND as the predominant surgeries [14,22]. The preference for more aggressive surgeries requires further optimization. Fortunately, we observed a significant decrease over time in the proportion of patients who undergo ALND, suggesting such optimization for Chinese MBC patients may be underway. Furthermore, we observed a significant rise in the utilization of anti-HER-2 therapy over time. According to the work by Li et al. the utilization of trastuzumab significantly increased among Chinese female patients after its inclusion in China's reimbursement drug list for HER-2 positive breast cancer in July 2017, reaching 88.9 % by 2021 [30]. Our results indicated that Chinese MBC patients have also benefited from this policy. Nevertheless, the percentage of MBC patients receiving these treatments remained markedly lower than that observed among female patients. More efforts are needed to identify and diminish the improper sex disparities in breast cancer management in China.
Our results also revealed that higher proportions of Chinese patients received chemotherapy and endocrine therapy, respectively. Wang et al. analyzed 16,025 MBC patients documented in NCDB, revealing that 37.0 % of the patients received chemotherapy, and 57.9 % of the patients with positive hormone receptors received endocrine therapy [20]. Another study reported that 50.9 % (363/713) of the American patients with MBC received endocrine therapy through the SEER-Medicare database [31]. In South Korea, it was reported that 40.1 % of MBC patients received chemotherapy [11], a proportion that fell between the rates observed in the US and our study. Additionally, the proportion of endocrine therapy administration in South Korea is comparable to that observed in our cohort [32]. Compared to MBC patients in the US and South Korea [11,20], Chinese patients demonstrated superior OS rates. One possible reason could be the aforementioned differences in the administration of chemotherapy and endocrine therapy. However, such comparisons were unadjusted and insufficient, necessitating further investigation.
The importance of the corresponding regimens of chemotherapy and endocrine therapy was indicated by the multivariate Cox analysis in Chinese MBC patients. At present, chemotherapy is recommended for MBC according to the guidelines for females with breast cancer [4,5]. Currently, guidelines recommend considering the omission of chemotherapy for female patients with low-risk luminal-type breast cancer [33]. This recommendation primarily stems from clinical trials conducted during the 2010s, focusing on female patients with high-risk luminal-type early breast cancer [34,35]. The significant reduction in chemotherapy usage among Chinese MBC patients after the 2010s could potentially be linked to these studies and guidelines.
However, the safety of reducing chemotherapy remains uncertain for MBC patients. Despite the majority of cases being luminal-type, Yu et al. retrospectively investigated 134 Chinese MBC patients from 1990 to 2008 at a single center and found that chemotherapy was associated with better OS [22]. We further identified that T-based and A + T-based regimens, which were basically the most frequently used regimens among Chinese patients, were protective factors for OS in the Chinese MBC cohort. Additionally, we did not find a significant association between A-based regimen and OS. To date, only one study has investigated the efficacy of anthracyclines in treatment for MBC and supported our findings. Lauro et al. retrospectively analyzed 50 metastatic MBC patients and found that anthracycline-containing and anthracycline-free regimens showed no statistical significance in the outcome [36]. Our results suggested that chemotherapy, especially T-based and A + T-based regimens, need to be more actively considered for high-risk MBC patients. However, inconsistent results were reported regarding the safety of omitting anthracyclines even in female patients [34,35]. Considering the lack of prospective studies on chemotherapy regimens for MBC and studies based on SEER indicating varied chemotherapy benefits for MBC patients based on different stages or PR statuses [37,38], further research is required to determine which subgroups of MBC may benefit from which chemotherapy regimen.
For endocrine therapy, we found that SERM was the only effective regimen. These findings were consistent with the results of Eggemann et al. [39,40]. However, the use of GnRHa, as recommended for combining with AI in MBC patients with tamoxifen contraindication [5], was not taken into account in the survival analysis because of the substantial proportion of unknown GnRHa status. Furthermore, adherence to endocrine therapy, as highlighted in previous studies [32,41], was neglected.
Finally, age and stage IV were recognized as independent risk factors, whereas mastectomy was identified as a protective factor for OS. These results were supported by several studies based on SEER [[42], [43], [44]]. However, we observed that partial mastectomy was not significantly associated with OS, which might be attributed to much fewer Chinese patients choosing partial mastectomy, resulting in bias. In terms of axillary surgeries, our results revealed that there was no difference in OS between SLNB and ALND in Chinese patients with N0 and N1 stage. Similarly, Shang et al. retrospectively analyzed 92 Chinese MBC patients with cN0 stage, revealing no significance in outcomes among patients who received SLNB, ALND, or SLNB + ALND [16]. The safety of partial mastectomy and SLNB for patients with MBC has also been reported in studies from other countries [[45], [46], [47], [48]]. Our work preliminarily indicated that SLNB is safe in Chinese patients with N0 and N1 stage, providing a possibility for reducing the proportion of aggressive surgeries. Furthermore, we did not detect the association between the year of diagnosis and OS, which is contrary to other studies [18,28]. This might be related to the differences in age between the groups or bias caused by insufficient sample size. Further research is needed.
There are several limitations to this study. First, its retrospective nature restricted the reliability. Second, the sample size remains inadequate compared to the MBC studies conducted in the US. Third, socioeconomical factors such as marital status, education level, and income, as well as outcomes like recurrence and metastasis, were not studied. Finally, the coverage of this study was limited, as rural and community medical institutions were not included, which may have led to selection bias. We urge more research with larger cohorts, particularly prospective, to further investigate the safety of partial mastectomy and SLNB and the indications of chemotherapy for Chinese MBC patients. Moreover, further studies are required to explore the differences in survival between MBC patients from China and other countries.
5. Conclusions
In this largest-scale Chinese study on MBC, we comprehensively reported the characteristics, treatment patterns and prognosis of the patients. The treatments given to these patients were aggressive but likely optimized over time. T-based and A + T-based regimens, along with SERM, were protective factors for OS in Chinese patients. More studies and further treatment optimization are required for MBC patients in China.
Presentation
The results have been published in part as an e-abstract at the 2023 American Society of Clinical Oncology Annual Meeting. https://doi.org/10.1200/JCO.2023.41.16_suppl.e13543.
Data statement
A part of data that support the findings of this study are available from Chinese Society of Clinical Oncology Breast Cancer Database but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. The dataset is however available from the corresponding authors upon reasonable request and with permission of Chinese Society of Clinical Oncology Breast Cancer Database.
Ethical approval
This study was approved by Clinical Research and Laboratory Animal Ethics Committee, the First Affiliated Hospital of Sun Yat-sen University (grant number [2021]516) on June 30, 2021. The consents were waived owing to the retrospective design of the study and the lack of sensitive information.
CRediT authorship contribution statement
Yuxuan Gao: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Mengmeng Zhang: Writing – review & editing, Writing – original draft, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Gang Sun: Writing – review & editing, Writing – original draft, Investigation. Li Ma: Writing – review & editing, Writing – original draft, Investigation. Jianyun Nie: Writing – review & editing, Writing – original draft, Investigation. Zhongyu Yuan: Writing – review & editing, Writing – original draft, Investigation. Zhenzhen Liu: Writing – review & editing, Writing – original draft, Investigation. Yali Cao: Writing – review & editing, Writing – original draft, Investigation. Jianbin Li: Writing – review & editing, Writing – original draft, Resources, Investigation. Qiang Liu: Writing – review & editing, Writing – original draft, Investigation. Songqing Ye: Writing – review & editing, Writing – original draft, Investigation. Bo Chen: Writing – review & editing, Writing – original draft, Investigation. Yuhua Song: Writing – review & editing, Writing – original draft, Investigation. Kun Wang: Writing – review & editing, Writing – original draft, Investigation. Yu Ren: Writing – review & editing, Writing – original draft, Investigation. Guolin Ye: Writing – review & editing, Writing – original draft, Investigation. Ling Xu: Writing – review & editing, Writing – original draft, Investigation. Shu Liu: Writing – review & editing, Writing – original draft, Investigation. Qianjun Chen: Writing – review & editing, Writing – original draft, Investigation. Weiwen Li: Writing – review & editing, Writing – original draft, Investigation. Xinxin Chen: Writing – review & editing, Writing – original draft, Investigation. Peifen Fu: Writing – review & editing, Writing – original draft, Investigation. Wei Wei: Writing – review & editing, Writing – original draft, Investigation. Baoliang Guo: Writing – review & editing, Writing – original draft, Investigation. Hebing Wang: Writing – review & editing, Writing – original draft, Investigation. Zhenhai Cai: Writing – review & editing, Writing – original draft, Investigation. Caiwen Du: Writing – review & editing, Writing – original draft, Investigation. Zhiyong Wu: Writing – review & editing, Writing – original draft, Investigation. Xiaoming Zha: Writing – review & editing, Writing – original draft, Investigation. Heng Huang: Writing – review & editing, Writing – original draft, Investigation. Juan Xu: Writing – review & editing, Writing – original draft, Investigation. Chenglei Zhang: Writing – review & editing, Writing – original draft, Investigation. Yingying Shi: Writing – review & editing, Writing – original draft, Investigation. Ting Liu: Writing – review & editing, Writing – original draft, Methodology. Sihua Liu: Writing – review & editing, Writing – original draft, Investigation. Zefei Jiang: Writing – review & editing, Writing – original draft, Supervision, Resources, Conceptualization. Ying Lin: Writing – review & editing, Writing – original draft, Supervision, Resources, Investigation, Conceptualization.
Declaration of competing interest
None.
Acknowledgements
This work was financially supported by Beijing Medical Award Foundation (grant number YXJL-2020-0941-0753); and the “Sun Yat-Sen University Clinical Research 5010 Program” (grant number 2016007). The sponsors had no involvement in the design, data collection, analysis or any other aspects of the study.
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2024.103762.
Contributor Information
Yuxuan Gao, Email: gaoyx9@mail2.sysu.edu.cn.
Mengmeng Zhang, Email: zhangmm59@mail2.sysu.edu.cn.
Gang Sun, Email: sung853219@126.com.
Li Ma, Email: mali1021@126.com.
Jianyun Nie, Email: kmmutfh@sina.com.
Zhongyu Yuan, Email: yuanzhy@sysucc.org.cn.
Zhenzhen Liu, Email: zlyyliuzhenzhen0800@zzu.edu.cn.
Yali Cao, Email: caoyali1964@126.com.
Jianbin Li, Email: lijianbin@csco.org.cn.
Qiang Liu, Email: liuq77@mail.sysu.edu.cn.
Songqing Ye, Email: 1797017169@qq.com.
Bo Chen, Email: bochen@cmu.edu.cn.
Yuhua Song, Email: qdsongyh@126.com.
Kun Wang, Email: gzwangkun@126.com.
Yu Ren, Email: renyyyyy@126.com.
Guolin Ye, Email: yglin@fsyyy.com.
Ling Xu, Email: Ling.xu@pkufh.com.
Shu Liu, Email: 308659546@qq.com.
Qianjun Chen, Email: cqj55@163.com.
Weiwen Li, Email: liweiwen19670804@163.com.
Xinxin Chen, Email: janechew@163.com.
Peifen Fu, Email: fupeifen@zju.edu.cn.
Wei Wei, Email: weiwei@pkuszh.com.
Baoliang Guo, Email: baoliangguo2013@163.com.
Hebing Wang, Email: whb1568@fjmu.edu.cn.
Zhenhai Cai, Email: 462975632@qq.com.
Caiwen Du, Email: dusumc@aliyun.com.
Zhiyong Wu, Email: stwuzy@163.com.
Xiaoming Zha, Email: njzhaxm@njmu.edu.cn.
Heng Huang, Email: hh664693@qq.com.
Juan Xu, Email: xj999@hotmail.com.
Chenglei Zhang, Email: zcl213@163.com.
Yingying Shi, Email: 64864125@qq.com.
Ting Liu, Email: liut65@mail2.sysu.edu.cn.
Sihua Liu, Email: sihualiu0606@163.com.
Zefei Jiang, Email: jiangzefei@csco.org.cn.
Ying Lin, Email: linying3@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Miao H., Verkooijen H.M., Chia K.S., et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. Nov 20 2011;29(33):4381–4386. doi: 10.1200/jco.2011.36.8902. [DOI] [PubMed] [Google Scholar]
- 2.Latest global cancer data: Cancer burden rises to 19.3 million new cases and 10.0 million cancer deaths in 2020 December 15, 2020, 2020. https://www.iarc.who.int/wp-content/uploads/2020/12/pr292_E.pdf
- 3.Miglietta F., Pontolillo L., De Angelis C., et al. Gender minorities in breast cancer - clinical trials enrollment disparities: Focus on male, transgender and gender diverse patients. Breast. Jun 2024;75 doi: 10.1016/j.breast.2024.103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassett M.J., Somerfield M.R., Baker E.R., et al. Management of male breast cancer: ASCO guideline. J Clin Oncol. Jun 1 2020;38(16):1849–1863. doi: 10.1200/jco.19.03120. [DOI] [PubMed] [Google Scholar]
- 5.NCCN . 2023. NCCN Clinical Practice Guidelines in Oncology - Breast Cancer; p. 2023.https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Pages BINV-J 1-2. March 23. [Google Scholar]
- 6.Giordano S.H. Breast cancer in men. N Engl J Med. Jun 14 2018;378(24):2311–2320. doi: 10.1056/NEJMra1707939. [DOI] [PubMed] [Google Scholar]
- 7.Fields E.C., DeWitt P., Fisher C.M., Rabinovitch R. Management of male breast cancer in the United States: a surveillance, epidemiology and end results analysis. Int J Radiat Oncol Biol Phys. Nov 15 2013;87(4):747–752. doi: 10.1016/j.ijrobp.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Shimomura A., Nagahashi M., Kumamaru H., et al. Clinicopathological features of male patients with breast cancer based on a nationwide registry database in Japan. Breast Cancer. Jun 22 2022 doi: 10.1007/s12282-022-01378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiza A., Fredriksson I., Sund M., Valachis A. De novo metastatic breast cancer in men vs women: a Swedish population-based cohort study. JNCI Cancer Spectr. Jul 3 2023;7(4) doi: 10.1093/jncics/pkad050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim J.S.H., Sim Y., Ngeow J., et al. Male breast cancer: a Singapore perspective. ANZ J Surg. Jun 2022;92(6):1440–1446. doi: 10.1111/ans.17737. [DOI] [PubMed] [Google Scholar]
- 11.Lee E.G., Jung S.Y., Lim M.C., et al. Comparing the characteristics and outcomes of male and female breast cancer patients in Korea: Korea central cancer registry. Cancer Res Treat. Jul 2020;52(3):739–746. doi: 10.4143/crt.2019.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N., Yang D.W., Wu Y.X., et al. Burden, trends, and risk factors for breast cancer in China from 1990 to 2019 and its predictions until 2034: an up-to-date overview and comparison with those in Japan and South Korea. BMC Cancer. Jul 29 2022;22(1):826. doi: 10.1186/s12885-022-09923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W., Xu X., Tian B., et al. Clinical features of patients with male breast cancer in Shanxi province of China from 2007 to 2016. J Invest Med. Mar 2019;67(3):699–705. doi: 10.1136/jim-2018-000823. [DOI] [PubMed] [Google Scholar]
- 14.Kwong A., Chau W.W., Mang O.W., et al. Male breast cancer: a population-based comparison with female breast cancer in Hong Kong, Southern China: 1997-2006. Ann Surg Oncol. Apr 2014;21(4):1246–1253. doi: 10.1245/s10434-013-3377-8. [DOI] [PubMed] [Google Scholar]
- 15.Chen X., Liu X., Zhang L., Li S., Shi Y., Tong Z. Poorer survival of male breast cancer compared with female breast cancer patients may be due to biological differences. Jpn J Clin Oncol. Oct 2013;43(10):954–963. doi: 10.1093/jjco/hyt116. [DOI] [PubMed] [Google Scholar]
- 16.Shang Q., Feng K., Wei Y., et al. Evaluation of male breast cancer and the application of sentinel lymph node biopsy: a multicenter retrospective study. Oncol. Dec 11 2023;28(12):e1170–e1178. doi: 10.1093/oncolo/oyad189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.[Chinese Society of Breast Surgery CSoS, Chinese Medical Association] [Clinical practice China guidelines for male breast cancer (2023 edition)]. 中国男性乳腺癌临床诊治实践指南(2023版) CHINESE JOURNAL OF PRACTICAL SURGERY] 2023;43(2):139–143. doi: 10.19538/j.cjps.issn1005-2208.2023.02.04. [DOI] [Google Scholar]
- 18.Cardoso F., Bartlett J.M.S., Slaets L., et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer Program. Ann Oncol. Feb 1 2018;29(2):405–417. doi: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocco D., Shah C., Wei W., Wilkerson A., Grobmyer S.R., Al-Hilli Z. Axillary lymph node dissection can be omitted in patients with limited clinically node-positive breast cancer: a National Cancer Database analysis. Br J Surg. Nov 22 2022;109(12):1293–1299. doi: 10.1093/bjs/znac305. [DOI] [PubMed] [Google Scholar]
- 20.Wang F., Shu X., Meszoely I., et al. Overall mortality after diagnosis of breast cancer in men vs women. JAMA Oncol. Nov 1 2019;5(11):1589–1596. doi: 10.1001/jamaoncol.2019.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarmiento S., McColl M., Musavi L., et al. Male breast cancer: a closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Res Treat. Apr 2020;180(2):471–479. doi: 10.1007/s10549-020-05556-y. [DOI] [PubMed] [Google Scholar]
- 22.Yu X.F., Wang C., Chen B., et al. The effect of adjuvant chemotherapy in male breast cancer: 134 cases from a retrospective study. ESMO Open. 2017;2(2) doi: 10.1136/esmoopen-2016-000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breast cancer screening guideline for Chinese women. Cancer Biol Med. Nov 2019;16(4):822–824. doi: 10.20892/j.issn.2095-3941.2019.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz E.G., Stevens J., Truesdale K.P., Cai J., North K.E., Steffen L.M. Associations of body mass index with incident hypertension in American white, American black and Chinese Asian adults in early and middle adulthood: the Coronary Artery Risk Development in Young Adults (CARDIA) study, the Atherosclerosis Risk in Communities (ARIC) study and the People's Republic of China (PRC) study. Asia Pac J Clin Nutr. 2013;22(4):626–634. doi: 10.6133/apjcn.2013.22.4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Z., Blake G.M., Graffy P.M., et al. Hepatic steatosis: CT-based prevalence in adults in China and the United States and associations with age, sex, and body mass index. AJR Am J Roentgenol. May 2022;218(5):846–857. doi: 10.2214/ajr.21.26728. [DOI] [PubMed] [Google Scholar]
- 26.Truesdale K.P., Stevens J., Cai J. Impact of body mass index levels on lipid abnormalities in Chinese asians, American blacks and American whites: the people's Republic of China (PRC) and atherosclerosis risk in communities (ARIC) studies. Atherosclerosis. Oct 2011;218(2):517–523. doi: 10.1016/j.atherosclerosis.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C., Ahn J., Tarpey T., Yi S.S., Hayes R.B., Li H. A microbial causal mediation analytic tool for health disparity and applications in body mass index. Microbiome. Jul 27 2023;11(1):164. doi: 10.1186/s40168-023-01608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leone J.P., Freedman R.A., Leone J., et al. Survival in male breast cancer over the past 3 decades. J Natl Cancer Inst. Apr 11 2023;115(4):421–428. doi: 10.1093/jnci/djac241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu P., He D., Zhu S., et al. The role of postoperative radiation therapy in stage I-III male breast cancer: a population-based study from the surveillance, epidemiology, and End Results database. Breast. Oct 2022;65:41–48. doi: 10.1016/j.breast.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J., Zhou J., Wang H., et al. Trends in disparities and transitions of treatment in patients with early breast cancer in China and the US, 2011 to 2021. JAMA Netw Open. Jun 1 2023;6(6) doi: 10.1001/jamanetworkopen.2023.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali A., Xie Z., Stanko L., et al. Endocrine adherence in male versus female breast cancer: a seer-medicare review. Breast Cancer Res Treat. Apr 2022;192(3):491–499. doi: 10.1007/s10549-022-06536-0. [DOI] [PubMed] [Google Scholar]
- 32.Lee J., Lee K.S., Sim S.H., et al. Impacts of subtype on clinical feature and outcome of male breast cancer: multicenter study in Korea (KCSG BR16-09) Cancer Res Treat. Mar 24 2022 doi: 10.4143/crt.2021.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Z., Li J., Chen J., et al. Chinese society of clinical oncology (CSCO) breast cancer guidelines 2022. Translational Breast Cancer Research. 2022;3 doi: 10.21037/tbcr-22-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blum J.L., Flynn P.J., Yothers G., et al. Anthracyclines in early breast cancer: the ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG oncology) J Clin Oncol. Aug 10 2017;35(23):2647–2655. doi: 10.1200/jco.2016.71.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nitz U., Gluz O., Clemens M., et al. West German study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J Clin Oncol. Apr 1 2019;37(10):799–808. doi: 10.1200/jco.18.00028. [DOI] [PubMed] [Google Scholar]
- 36.Di Lauro L., Pizzuti L., Barba M., et al. Efficacy of chemotherapy in metastatic male breast cancer patients: a retrospective study. J Exp Clin Cancer Res. Mar 21 2015;34(1):26. doi: 10.1186/s13046-015-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan H., Zhang K., Wang M., Ling L., Wang S., Zhou W. The effect of chemotherapy on survival in patients with nonmetastatic male breast cancer: a population-based observational study. Cancer. Aug 15 2020;126(Suppl 16):3830–3836. doi: 10.1002/cncr.32829. [DOI] [PubMed] [Google Scholar]
- 38.Li W.P., Gao H.F., Ji F., et al. The role of adjuvant chemotherapy in stage I-III male breast cancer: a SEER-based analysis. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920958358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggemann H., Ignatov A., Smith B.J., et al. Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Res Treat. Jan 2013;137(2):465–470. doi: 10.1007/s10549-012-2355-3. [DOI] [PubMed] [Google Scholar]
- 40.Eggemann H., Brucker C., Schrauder M., et al. Survival benefit of tamoxifen in male breast cancer: prospective cohort analysis. Br J Cancer. Jul 2020;123(1):33–37. doi: 10.1038/s41416-020-0857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu S., Yang Y., Tao W., et al. Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Res Treat. Nov 2012;136(2):495–502. doi: 10.1007/s10549-012-2286-z. [DOI] [PubMed] [Google Scholar]
- 42.Yao N., Shi W., Liu T., et al. Clinicopathologic characteristics and prognosis for male breast cancer compared to female breast cancer. Sci Rep. Jan 7 2022;12(1):220. doi: 10.1038/s41598-021-04342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leone J., Freedman R.A., Lin N.U., et al. Tumor subtypes and survival in male breast cancer. Breast Cancer Res Treat. Aug 2021;188(3):695–702. doi: 10.1007/s10549-021-06182-y. [DOI] [PubMed] [Google Scholar]
- 44.Chen S., Liu Y., Yang J., et al. Development and validation of a nomogram for predicting survival in male patients with breast cancer. Front Oncol. 2019;9:361. doi: 10.3389/fonc.2019.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bateni S.B., Perry L.M., Zhao X., et al. The role of radiation therapy in addition to lumpectomy and hormone therapy in men 70 Years of age and older with early breast cancer: a NCDB analysis. Ann Surg Oncol. May 2021;28(5):2463–2471. doi: 10.1245/s10434-020-09242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav S., Karam D., Bin Riaz I., et al. Male breast cancer in the United States: treatment patterns and prognostic factors in the 21st century. Cancer. Jan 1 2020;126(1):26–36. doi: 10.1002/cncr.32472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin A.P., Huang T.W., Tam K.W. Treatment of male breast cancer: meta-analysis of real-world evidence. Br J Surg. Sep 27 2021;108(9):1034–1042. doi: 10.1093/bjs/znab279. [DOI] [PubMed] [Google Scholar]
- 48.Parpex G., Ottaviani M., Lorphelin H., et al. Accuracy of sentinel lymph node biopsy in male breast cancer: systematic review and meta-analysis. Breast. Jun 2024;75 doi: 10.1016/j.breast.2024.103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.