Abstract
Background
The 300IR house dust mite (HDM) sublingual immunotherapy (SLIT) tablet is approved for treatment of HDM-induced allergic rhinitis (AR). To provide a comprehensive review of the 300IR HDM-SLIT tablet safety profile based on randomized controlled trial (RCT) pooled data and post-marketing (PM) pharmacovigilance data.
Methods
Subjects (5–65 years) with confirmed HDM-AR with or without controlled asthma were treated with 300IR or placebo in 8 RCTs. Reported treatment-emergent adverse events (TEAEs) were pooled and analyzed descriptively in subsets of adults/adolescents and children. Adverse reactions (ADRs) collected from spontaneous reporting and PM studies through a pharmacovigilance system since the first marketing authorization were also analyzed.
Results
Across RCTs, 1853 subjects were treated with the 300IR HDM-SLIT tablet and 1846 with placebo. In both subsets of adults/adolescents and children whichever their asthma status, treatment-related TEAEs of higher incidence in active groups vs placebo were mostly consistent with mild or moderate local application-site reactions. They were mainly reported on the first days of treatment and decreased over time. 4 severe laryngopharyngeal reactions (2 requiring adrenaline/epinephrine) and 1 moderate eczema considered serious rapidly resolved with medications; no anaphylaxis was reported. In PM settings, ADRs reported in more than 235,000 patients were in line with RCT findings. Severe systemic reactions occurred rarely; 12 anaphylactic reactions resolved safely (5 with adrenaline). No new safety signal was raised.
Conclusion
Safety data from RCTs and more than 7 years of real-life experience confirmed the favorable safety profile of 300IR HDM-SLIT tablet in patients across different regions, regardless of age and asthma status.
Clinical trial registrations
NCT00674700; Retrospectively registered 06 May 2008.
NCT01199133; Retrospectively registered 09 September 2010.
NCT01527188; Retrospectively registered 01 February 2012.
NCT02443805; Registered 29 April 2015/EudraCT 2014-004223-46; Registered 16 September 2015.
jRCT2080221872/JapicCTI-121917; Registered 01 August 2012.
jRCT2080222929/JapicCTI-15298; Registered 04 August 2015.
Keywords: Antigens, Dermatophagoides, Asthma, Dust mite allergy, Rhinitis, Allergic, Perennial, Sublingual immunotherapy/adverse effects
Introduction
House dust mite (HDM) is one of the most prevalent allergens responsible of allergic rhinitis with or without conjunctivitis (AR/C), affecting millions of people worldwide.1,2 Importantly, HDM-sensitized patients frequently experience AR with comorbid asthma. Beyond the clinical burden, HDM-AR considerably impacts quality of life, social interactions, and productivity in untreated or inadequately treated patients.3, 4, 5 Allergen immunotherapy (AIT) administered subcutaneously (SCIT) or sublingually (SLIT) represents a potentially disease-modifying alternative to symptom-relieving medications for patients with AR not sufficiently controlled or wishing to reduce long-term use of those medications and unacceptable adverse effects.6, 7, 8, 9, 10
Since 2015, the 300IR SLIT tablet of Dermatophagoides pteronyssinus and D. farinae allergen extracts (Actair®/Orylmyte®/Aitmyte®, Stallergenes Greer, Antony, France/Shionogi & Co. Ltd., Osaka, Japan; equivalent to 57,000 Japanese allergy units [JAU]) has been approved for treatment of patients with HDM-AR/C in Europe and Asia-Pacific (including for children aged 5–12 years in Japan, South Korea and Australia).11, 12, 13
The efficacy and safety of the 300IR HDM-SLIT tablet have been established throughout a large clinical development program including randomized, double-blind, placebo-controlled trials (RDBPCTs) conducted worldwide. After demonstrating the tolerance of different doses in Phase (Ph)I trials, the dose of 300IR was selected based on the results of a dose-ranging trial in an environmental exposure chamber (EEC) in Canada and 2 PhII/III trials in Europe and Japan.14, 15, 16 The efficacy and safety of the 300IR HDM-SLIT tablet in HDM-AR adults and adolescents were confirmed in a large global PhIII study.17 The positive results from a Japanese pediatric PhIII trial supported the benefit of this treatment in children.18 Noteworthy, the European PhII/III trial showed the beneficial effect of HDM tablet was maintained over a treatment-free follow-up year.14 In a post-marketing observational study in Japan, the 300IR HDM-SLIT tablet was confirmed safe and effective for up to 4 years in routine practice.19,20
Considering the widespread use of this product, the purpose of this article is to provide a comprehensive and thorough review of safety data from the clinical and post-marketing experiences, confirming the consistency with the well-characterized safety profile of SLIT.
Patients and methods
Clinical development program
Trial designs
The overall clinical safety experience with the 300IR HDM-SLIT tablet is based on pooled data from HDM-AR subjects treated with this product or placebo in one of 8 RDBPCTs. These 8 trials were 2 PhI studies conducted in adults in France (VO36.04F)21 or Japan (1109D1711), one PhII study in adults in a Canadian EEC (VO67.10; NCT01527188),16 2 PhII/III studies in adults in Europe (VO57.07; NCT00674700)14 or in adults/adolescents in Japan (1207D1731; JapicCTI-121917),15 1 global PhIII study in adults/adolescents in Europe, United States, Canada, Israel, and Russia (SL75.14; NCT02443805/EudraCT 2014-004223-46)17 and 2 PhIII studies in adolescents/children in Europe (VO64.08; NCT01199133)22 or Japan (1501D1732; JapicCTI-152981).18 A tabulated summary of the clinical trials is presented in Supplemental Table S1. All studies were carried out between 2004 and 2018. The RDBPCTs were performed in accordance with good clinical practice defined by the International Council for Harmonization and the principles that have their origin in the Declaration of Helsinki and local laws and regulations. Ethics committees or institutional review boards approved all study protocols and all participants or parents or legal representatives (for participants 17 years or younger) gave their written consent to participation before any study procedure was performed.
Settings and participants
All enrolled subjects (5–65 years old) had at least a 1-year clinical history of HDM-AR/C confirmed by a positive skin prick test (SPT) or nasal provocation test and HDM-specific serum IgE level of 0.7 kU/L or more (≥3.5 kU/L in SL75.14 and 1501D1732). Subjects had to have moderate or severe symptoms self-rated on a 4-point or 5-point scale23 and evaluated through a baseline total rhinitis symptom score adjusted for or combined with rescue medication use of at least 5 or 6 on a scale from 0 to 12 or 15, depending on the trials. Polysensitized subjects ie, demonstrating antigen-specific IgE to 2 or more allergens by SPT or in vitro testing,24 could be enrolled provided that there were no significant symptoms of AR/C due to allergens other than HDM during the trial. Subjects with concomitant asthma controlled with GINA treatment Step 1 or 2 therapies could participate. Those with standard contra-indications of AIT such as severe or poorly controlled asthma, active autoimmune diseases, immunodeficiencies, malignant neoplasias, significant cardiovascular diseases, receiving β-blockers or monoamine oxidase inhibitors, pregnancy or breast-feeding were excluded. Nasal or oral conditions which could have interfered with the study outcome assessments were also part of exclusion criteria.
Randomization and intervention
In all studies, subjects were randomized to receive either active treatment or placebo through a computer-generated scheme with a ratio (1:1, 1:1:1 or 1:1:1:1) leading to groups of equal size. Randomization by blocks or minimization (in Japanese studies) ensured balance in the treatment assignments.
Active treatment consisted of sublingual tablets containing a 1:1 mixture of both D. pteronyssinus and D. farinae allergen extracts providing coverage to the major and most potent group 1 and group 2 allergens including Der p 1, Der p 2, Der f 1, Der f 2, as well as Der p 23.14,16,17,25 HDM extract allergenic activity was quantified based on in-house reference standard established from titrated SPT of allergic subjects, the Index of Reactivity or “IR” being the potency unit.26,27 Depending on the studies, tablets at doses of 100IR, 300IR and 500IR were administered. Since 300IR was the optimal selected dose in the indication,28 the 100IR and 500IR doses will not be further described in this article. Subjects in the active group received the 300IR maintenance dose following a 3-day dose escalation (Day 1–100IR, Day 2–200IR and Day 3–300IR) except for those included in one of the treatment groups of study VO36.04F (300IR directly taken). Active and corresponding placebo tablets were matched in packaging, shape, taste, color, and appearance to ensure blinding. The treatment period lasted from 10 to 14 days in PhI studies, 6 months in EEC study, to approximately 12 months in PhII/III natural field studies. In the latter, antihistamines (oral/ocular), intranasal or oral corticosteroids were provided to participants to manage severe or intolerable AR/C symptoms according to a stepwise regimen. Additionally, participants in the global study received an adrenaline autoinjector for use in the event of a severe allergic reaction.
Outcomes and follow-up
Safety was documented by adverse event (AE) reporting, laboratory data, spirometry and physical examination (including vital signs). AE characteristics included onset and resolution dates, severity (ie, intensity of reaction), seriousness, action taken with the investigational product (IP), medication, hospitalization, procedures, outcome and investigator's causality assessment. According to Medical Dictionary for Regulatory Activities (MedDRA) definition, serious AEs were events immediately life-threatening or resulting in hospitalization or prolongation of existing hospitalization, persistent or significant disability or incapacity, congenital anomalies/birth defects or death. They included medically significant AEs that might jeopardize the subject or require medical/surgical intervention to prevent above-listed outcomes.
In the global study, any symptoms evoking one the 4 following clinical pictures were considered an AE of Special Interest (AESI) in the context of SLIT: severe anaphylactic reactions, severe laryngopharyngeal reactions, eosinophilic esophagitis (EoE) and autoimmune disorders. The integrated safety database was retrospectively reviewed to identify and medically assess all AESIs using Standardized MedDRA Queries (SMQ) and pre-defined lists of relevant High Level Group Terms (HLGT), High Level Terms (HLT) and Preferred Terms (PT).29,30
Subject withdrawal from the study was at the investigator's discretion in case of drug intolerability, and systematic in case of anaphylaxis, severe laryngopharyngeal reaction or EoE. Serious AEs or AEs leading to discontinuation were followed up until resolution or stabilization of the subject's condition.
Statistical analysis
The Safety Analysis Set comprised all randomized subjects who received at least one dose of IP (active or placebo).
To harmonize the safety data from all studies, all AEs were re-coded using MedDRA version 18.1 and the rules for missing data imputation and derived variables calculation (eg, TEAE onset or duration, treatment duration) were homogenized. Treatment-emergent AEs (TEAEs) were defined as any AE that started on or after the day the first dose of IP and up to the thirtieth day (inclusive) after the last administration of IP. All other AEs were classified as non-TEAEs.
TEAEs were analyzed descriptively, and data expressed in numbers and/or percentages of subjects reporting TEAEs. When a subject had experienced multiple episodes of any AE, the AE was counted once, or once by severity grade (if different severity grades were assigned).
The following pooled analyses were performed: i) all adults and adolescents (12–65 years old at entry) randomized and actually treated with 300IR or placebo in VO36.04F-group 3, 1109D1711-group 2, VO67.10, VO57.07, SL75.14, 1207D1731, VO64.08 and 1501D1732, referred to as “Adult/Adolescent pool”; ii) all children (5–11 years old at entry) randomized and actually treated with 300IR or placebo in VO64.08 and 1501D1732, referred to as “Children pool”; iii) population subsets according to asthma status at baseline.
Post-marketing experience
Safety data consisting of adverse reaction (ADR) reports were collected from spontaneous reporting and post-marketing studies and registered in the Global Safety Database owned by the Company since the marketing authorization of 300IR HDM-SLIT tablet in Japan in 2015. The regular and periodic reviews of solicited and unsolicited ADR reports contribute to early signal detection by means of medical evaluation and reporting rate (RR) calculation (number of case reports/total number of patients exposed). In the Global Safety Database, the closely monitored safety concerns in the context of SLIT (i.e., anaphylactic reactions, severe laryngopharyngeal reactions, EoE, and autoimmune disorders) were medically reviewed and assessed using SMQs and pre-defined lists of relevant HLGT, HLT and PT. More specifically, identification of cases of anaphylactic reaction or anaphylactic shock involved the use of SMQs “Anaphylactic reaction”, “Hypersensitivity”, “Angioedema” and “Anaphylactic/anaphylactoid shock conditions”, clinical criteria for diagnosing anaphylaxis31 and the selection of patients treated with interventions proven effective in the acute management of anaphylaxis, (eg, adrenaline, intravenous fluids, parenteral corticosteroids or antihistamines). To detect severe laryngopharyngeal reactions, the SMQ “Oropharyngeal allergic conditions” plus a list of appropriate terms were used together with an evaluation of the seriousness of ADRs, associated respiratory symptoms and defined treatment given (eg, adrenaline, intravenous fluids, parenteral corticosteroids or antihistamines, inhaled beta agonists, oxygenotherapy).
Results
Safety of 300IR HDM-SLIT tablet in clinical trials
Safety population
Of 4446 HDM-AR subjects exposed to the IP in RCTs, 1853 received the 300IR HDM tablet. Of these, 1583 adults and adolescents (1155 and 428, respectively) comprised the Adult/Adolescent pool (plus 1588 placebo-recipients) and 270 children comprised the Children pool (plus 258 placebo-recipients).
Demographics and baseline characteristics
At study entry, no notable differences in demographics and disease characteristics were observed in either adults/adolescents or children whether receiving active treatment or placebo (Supplemental Table S2). Noteworthy, the safety population included a majority of White participants and one third of Asian (28.4% and 54.0% in the Adult/Adolescent and Children pools, respectively). Subjects in Adult/Adolescent pool had a history of HDM-AR for about 11 years on average and more than one-half were polysensitized (ie, positive SPT to HDM and at least 1 other allergen). Children had a history of HDM-AR for about 3 years on average and about two thirds were polysensitized. About one-third of all subjects had concomitant asthma controlled with GINA treatment Step 1 or Step 2 therapies at baseline.
Duration of exposure
The majority of adults/adolescents and children were treated with 300IR HDM-SLIT tablet or placebo for longer than 6 months (Supplemental Table S3).
Safety in adults, adolescents and children
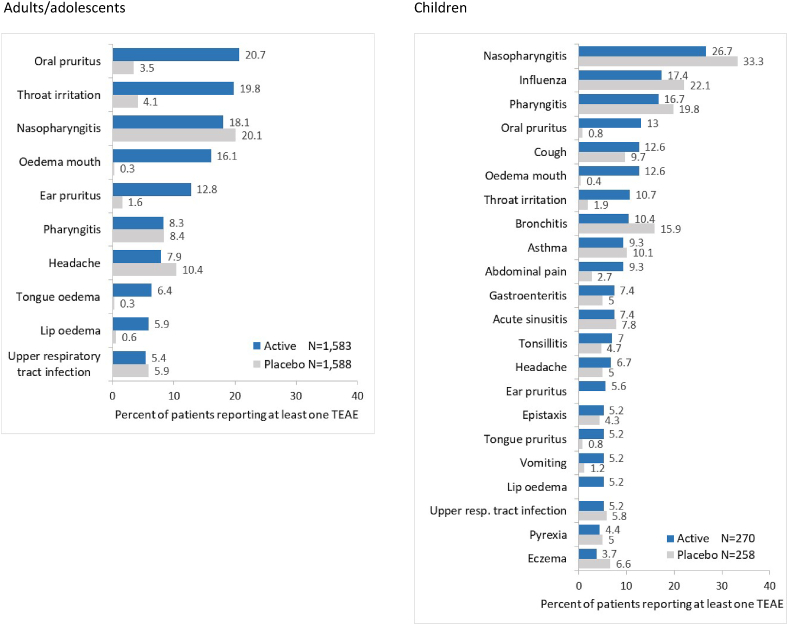
In both adult/adolescent and child populations, TEAEs were reported with similar frequencies in subjects receiving 300IR HDM-SLIT tablet and in placebo-recipients (Table 1) and were most commonly oropharyngeal in nature (Fig. 1). Severe TEAEs occurred in less than 5% of actively-treated adults/adolescents or children (less than 3% in the placebo groups).
Table 1.
Overview of TEAEs in the pooled populations - Safety Set.
| Subjects reporting at least 1 TEAE n (%) | Adults and adolescents |
Children |
||
|---|---|---|---|---|
| 300IR HDM-SLIT tablet (N = 1583) | Placebo (N = 1588) | 300IR HDM-SLIT tablet (N = 270) | Placebo (N = 258) | |
| TEAE | 1238 (78.2) | 1036 (65.2) | 240 (88.9) | 224 (86.8) |
| →Mild | 1129 (71.3) | 886 (55.8) | 231 (85.6) | 216 (83.7) |
| →Moderate | 527 (33.3) | 442 (27.8) | 70 (25.9) | 63 (24.4) |
| →Severe | 71 (4.5) | 39 (2.5) | 8 (3.0) | 4 (1.6) |
| Suspected to be drug-related | 909 (57.4) | 285 (17.9) | 135 (50.0) | 34 (13.2) |
| Serious | 39 (2.5) | 18 (1.1) | 7 (2.6) | 4 (1.6) |
| Serious suspected to be drug-related | 4 (0.3) | 1 (<0.1) | 1 (0.4) | 0 |
| Leading to premature withdrawal | 147 (9.3) | 41 (2.6) | 22 (8.1) | 2 (0.8) |
| Suspected to be drug-related & leading to premature withdrawal | 122 (7.7) | 14 (0.9) | 19 (7.0) | 0 |
Abbreviations: HDM, house dust mite; IR, index of reactivity; N, number of subjects per treatment group; n, number of subjects with data; SLIT, sublingual immunotherapy; TEAE, treatment-emergent adverse event
Fig. 1.
TEAEs occurring in ≥5% of subjects - Safety Set. TEAE, treatment-emergent adverse event
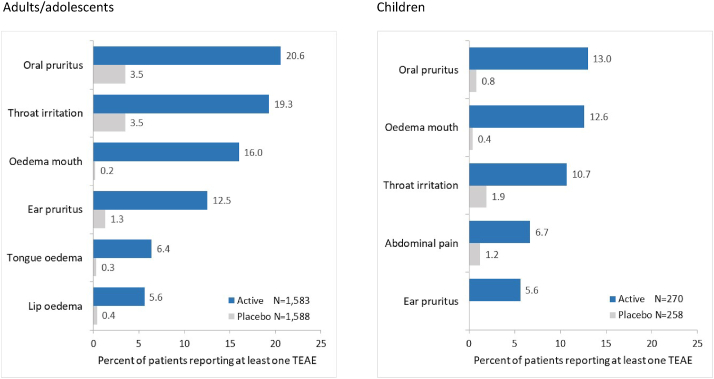
The incidences of treatment-related TEAEs were higher in active groups vs placebo (Table 1). As expected with SLIT, such events were mostly mild or moderate application-site reactions (eg, oral pruritus, throat irritation or mouth oedema) (Fig. 2). Frequencies, nature and severity of 300IR HDM-SLIT tablet-related events were generally consistent in all age groups.
Fig. 2.
Treatment-related TEAEs occurring in ≥5% of subjects - Safety Set. TEAE, treatment-emergent adverse event
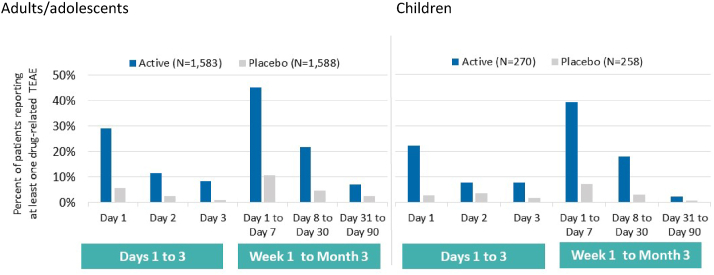
Time to onset of treatment-related TEAEs
In both age groups, treatment-related TEAEs occurred more frequently during the first days of treatment and decreased over the following 3 months (Fig. 3). In all subjects receiving the 300IR HDM-SLIT tablet, the median time to onset of very common application-site reactions (in at least 10% of subjects) ranged from 1 to 8 days after treatment initiation. The median duration of all occurrences of those events ranged from 6 to 9 days in adults/adolescents and from 22 to 30 days in children (Table 2).
Fig. 3.
Time to onset of treatment-related TEAEs (first occurrence) - Safety Set. TEAE, treatment-emergent adverse event
Table 2.
Time to onset and duration of treatment-related TEAEs in the pooled populations - Safety Set.
| Adults and adolescents |
Children |
||||
|---|---|---|---|---|---|
| 300IR HDM-SLIT tablet (N = 1583) | Placebo (N = 1588) | 300IR HDM-SLIT tablet (N = 270) | Placebo (N = 258) | ||
| Time to onset of first occurrence of very common treatment-related TEAEsa | |||||
| Oral pruritus | median days (range) | 2 (1–292) | 1 (1–309) | 3 (1–281) | 23 (17–29) |
| number of events | 325 | 55 | 35 | 2 | |
| Throat irritation | median days (range) | 1 (1–367) | 2 (1–171) | 1 (1–185) | 2 (1–8) |
| number of events | 306 | 55 | 29 | 5 | |
| Oedema mouth | median days (range) | 8 (1–328) | 3 (1–7) | 8 (1–106) | 14 (14–14) |
| number of events | 253 | 3 | 34 | 1 | |
| Ear pruritus | median days (range) | 1 (1–101) | 9 (1–177) | NAb | NAb |
| number of events | 198 | 20 | |||
| Duration of all occurrences of very common treatment-related TEAEsa | |||||
| Oral pruritus | median days (range) | 6 (1–375) | 2 (1–365) | 22 (1–366) | 56 (1–111) |
| number of events | 478 | 62 | 36 | 2 | |
| Throat irritation | median days (range) | 7 (1–446) | 3 (1–614) | 30 (1–379) | 4 (1–135) |
| number of events | 456 | 69 | 32 | 5 | |
| Oedema mouth | median days (range) | 9 (1–446) | 4 (3–21) | 22 (1–367) | 125 (71–179) |
| number of events | 372 | 3 | 38 | 2 | |
| Ear pruritus | median days (range) | 6 (1–446) | 3 (1–370) | NAb | NAb |
| number of events | 295 | 24 | |||
Abbreviations: HDM, house dust mite; IR, index of reactivity; N, number of subjects per treatment group; NA, not applicable; SLIT, sublingual immunotherapy; TEAE, treatment-emergent adverse event.
Reported in equal to or greater than 10% of subjects.
Ear pruritus was reported in less than 10% of children
Serious TEAEs
Serious TEAEs were reported in less than 3% of actively-treated subjects regardless of the age category. In adults/adolescents, 4 serious TEAEs were suspected to be drug-related. Three were severe application-site reactions which resolved rapidly under corrective treatment: (1) a laryngopharyngeal reaction in an adolescent after the first dose intake, treated with intramuscular adrenaline/epinephrine, salbutamol and cetirizine; (2) a pharyngeal reaction in an adult after the second dose intake, treated with cetirizine; (3) a pharyngeal oedema in another adult on Day 11, treated with cetirizine and intramuscular methylprednisolone. Those 3 subjects were withdrawn. The fourth event, a moderate eczema in an adult on Day 107 resolved with topical medications and the subject continued the SLIT treatment. Besides, a 5-year-old child reported 1 serious and suspected to be drug-related TEAE of pseudocroup (subglottic laryngitis) on Day 22. The event resolved within one day with inhaled adrenaline and inhaled/oral corticosteroids; the subject was withdrawn.
Other significant TEAEs
Across the studies, there were no reports of death, severe anaphylactic reactions or anaphylactic shock in any adult, adolescent or child. Besides the above-described serious cases of severe laryngopharyngeal reactions with the 300IR HDM-SLIT tablet, a non-serious case of pharyngeal oedema was reported on Day 3 in an adult (spontaneously resolved; SLIT continued). There were 2 non-serious cases of EoE suspected to be related to the HDM tablet (both subjects were withdrawn). One report of autoimmune disorder (coeliac disease) in an actively-treated adult was not serious and not suspected to be drug-related (SLIT continued). TEAE of asthma and related events were reported at lower, similar or higher incidences in the active groups vs placebo. In adults/adolescents, incidences were 2.6% of actively-treated subjects vs. 3.1% placebo recipients for asthma, 4.0% vs. 4.5% for cough, 1.2% vs. 1.7% for bronchospasm, 0.4% vs. 0.6% for wheezing, 1.8% vs. 1.4% for dyspnoea, 1.6% vs. 0.6% for chest pain and 1.3% vs. 0.5% for chest discomfort. In children, incidences were 9.3% vs. 10.1% for asthma, 0.7% vs. 0.0% for chest pain, 0.4% vs. 0.0% for wheezing, 0.0% vs. 0.4% for chest discomfort, 12.6% vs. 9.7% for cough and 1.8% vs. 1.4% for dyspnoea. Very few cases were severe and suspected to be drug-related in actively-treated adults (two chest pain and one chest discomfort); none in adolescents nor in children.
In both adult/adolescent and child populations, a similar percentage of subjects discontinued due to treatment-related TEAEs (less than 8% in active groups vs less than 1% in placebo groups; Table 1). Mild or moderate application-site reactions such as oral pruritus, mouth/tongue oedema, throat irritation, mostly reported within the first weeks of treatment, were the main reasons for discontinuation regardless of the age category.
Safety in subpopulations by asthma status
In HDM-AR adults, adolescents and children, no notable differences in terms of incidences, nature and severity of TEAEs and drug-related TEAEs were observed according to their asthma status (Table 3). Across RCTs, the 300IR HDM-SLIT tablet showed an acceptable safety profile and was well tolerated in HDM-AR subjects with or without asthma requiring therapies consistent with GINA treatment Step 1 or 2.
Table 3.
Overview of TEAEs in the pooled populations by asthma status - Safety Set.
| Subjects reporting at least 1 TEAE n (%) | Adults and adolescents |
Children |
||||||
|---|---|---|---|---|---|---|---|---|
| With asthma (N = 982) |
Without asthma (N = 2175) |
With asthma (N = 196) |
Without asthma (N = 332) |
|||||
| 300IR HDM-SLIT tablet (N = 505) | Placebo (N = 477) | 300IR HDM-SLIT tablet (N = 1072) | Placebo (N = 1103) | 300IR HDM-SLIT tablet (N = 103) | Placebo (N = 93) | 300IR HDM-SLIT tablet (N = 167) | Placebo (N = 165) | |
| TEAE | 379 (75.0) | 312 (65.4) | 853 (79.6) | 720 (65.3) | 89 (86.4) | 83 (89.2) | 151 (90.4) | 141 (85.5) |
| Suspected to be drug-related | 283 (55.4) | 83 (17.4) | 623 (58.1) | 200 (18.1) | 42 (40.8) | 11 (11.8) | 93 (55.7) | 23 (13.9) |
| Serious | 13 (2.6) | 9 (1.9) | 26 (2.4) | 9 (0.8) | 4 (3.9) | 2 (2.2) | 3 (1.8) | 2 (1.2) |
| Serious suspected to be drug-related | 1 (0.2) | 0 | 3 (0.3) | 1 (<0.1) | 0 | 0 | 1 (0.6) | 0 |
| Leading to premature withdrawal | 58 (11.5) | 15 (3.1) | 89 (8.3) | 23 (2.1) | 7 (6.8) | 0 | 15 (9.0) | 2 (1.2) |
| Suspected to be drug-related & leading to premature withdrawal | 51 (10.1) | 6 (1.3) | 71 (6.6) | 6 (0.5) | 6 (5.8) | 0 | 13 (7.8) | 0 |
Abbreviations: HDM, house dust mite; IR, index of reactivity; N, number of subjects per treatment group; n, number of subjects with data; SLIT, sublingual immunotherapy; TEAE, treatment-emergent adverse event
Safety of 300IR HDM-SLIT tablet in post-marketing experience
Between 2015 and 2022, more than 235,000 patients received the 300IR HDM-SLIT tablet in post-marketing settings. A total of 1646 spontaneous ADR reports from health care professionals or patients were collected worldwide at the Marketing Authorization Holder (MAH)'s pharmacovigilance department, which corresponded to a reporting rate (RR) of 7/1000 patients. Of these ADRs, 71 (4%) were serious, corresponding to a RR of 0.3/1000. There were no reports of death or any long-term sequelae. Reactions were mostly gastrointestinal or respiratory disorders, mainly consistent with application-site reactions: mouth oedema, oral pruritus and throat irritation, and to a lesser extent skin or general disorders.
Cumulatively, 12 cases of anaphylactic reactions were identified in the Global Safety Database (RR 0.05/10,000). The event occurred on the first or third day of treatment (4 cases), after more than a week of treatment (7–9 days, 5 cases), ≥2.5 years or unspecified onset for the remaining 3 cases. In 6 cases, the anaphylactic reaction developed shortly (<30 min after the last HDM tablet intake). Adrenaline was administered in 5 patients aged 10–19 years. Of the 12 cases, 5 patients were hospitalized of whom 4 recovered and resolution was unknown for the remaining patient (this patient was discharged after 4 h at the hospital). All 7 patients not hospitalized recovered. No case of anaphylactic shock meeting the pre-specified clinical criteria for diagnosis was reported to the product MAH's pharmacovigilance department over these 7 years.
Five cases of severe laryngopharyngeal reactions were reported (RR 0.2/10,000). Symptoms occurred after the first HDM tablet intake (one case), the fourth HDM tablet intake (2 cases), unspecified onset for the remaining 2 cases. Adrenaline was administered in one 14-year-old patient. All patients recovered within 48 h.
Five non-serious cases of EoE were reported (RR 0.2/10,000). It should be noted that for all cases, no gastroscopy or biopsy results were provided.
Two cases of autoimmune disorders were identified (RR 0.2/10,000). The first case, an unspecified autoimmune disorder diagnosed from a skin biopsy in a patient of unspecified age, was considered unlikely related to the HDM tablet. The second case, an IgA nephropathy in an adolescent was considered possibly related to the HDM tablet.
The post-marketing experience over 7 years in more than 235,000 patients is in concordance with the clinical trial safety data; no new safety signal was raised.
Discussion
The analysis of pooled safety data from RCTs showed the 300IR HDM-SLIT tablet was well tolerated by a broad population of HDM-AR adults, adolescents and children (more than 1800 actively-treated subjects) from different regions, including one third of Asian subjects. As expected with SLIT, the most common treatment-related TEAEs were mild to moderate application-site reactions (e.g., oral pruritus, throat irritation, mouth oedema), early occurring during treatment, primarily on the first week, and decreasing over time. Few serious TEAEs were related to the drug and rapidly resolved with medications. The safety profile of the 300IR HDM-SLIT tablet was similar in adults, adolescents and children, and between subjects with or without asthma. Noteworthy, the longer duration of treatment-related TEAEs observed in children, compared to adults, may have been influenced by the emotional perceptions and reporting tendencies of parents, who may be more attuned to their child's discomfort and influenced by concern about their well-being, potentially leading to an overestimation of the duration of adverse events.32,33
In more than 235,000 patients treated in real-life settings, the nature and frequencies of ADRs reported with the 300IR HDM-SLIT tablet were consistent with those in RCTs. In addition, a consistent safety profile was apparent across various populations from RCTs and real-life including a substantial proportion of Asian. Consistent with this, a post-marketing survey on the use of this tablet in routine practice in Japan confirmed its effectiveness for up to 4 years with no new safety signal raised.19,20
The safety profile of the 300IR HDM-SLIT tablet is in agreement with the well-characterized profile of SLIT as published by the scientific societies in the field9,34, 35, 36 and globally consistent with that observed with another HDM SLIT tablet (SQ-HDM, ALK-Abelló, Hørsholm, Danemark).37, 38, 39
Given the route of administration, the spectrum of application-site reactions can range from mild oral pruritus to severe laryngopharyngeal disorders. With the 300IR HDM-SLIT tablet, the rates of severe laryngopharyngeal reactions represented about 0.3% of treated subjects in the clinical data and remained very rare since commercialization (0.2/10,000 patients). There were no reports of deaths or anaphylactic shock, confirming the favorable safety profile of SLIT over SCIT recognized in the literature.10,34,37,40, 41, 42, 43 Systemic allergic reactions such as anaphylactic reactions are reported sporadically with SLIT.10,42,44 Accordingly, rare cases of anaphylactic reactions have been reported with the 300IR HDM-SLIT tablet (0.05/10,000 patients) or SQ-HDM tablet in real-life conditions.45,46 Severe events requiring the use of adrenaline were reported in clinical trials with both HDM tablets.17,47 Such cases were scarce and never resulted in fatal outcome. Severe laryngopharyngeal reactions which may compromise patient airways, and anaphylactic reactions remain important identified risks with SLIT.44 To prevent and mitigate those risks in routine practice, safe use measures are described in the labelling.28 In addition, a robust worldwide pharmacovigilance system holding in the use of 2 complementary sources of safety information (ie, spontaneous and post-marketing studies reporting) contributes to signal detection and evaluation of the product safety profile under typical conditions of use.44
Considering ADRs mainly occur within the first days and the low risk of severe allergic reactions, at-home administration of the 300IR HDM-SLIT tablet can be encouraged as per other SLIT products provided the first intake under medical supervision is tolerated. Also, the recommended treatment regimen includes a 3-day dose escalation period with 100IR HDM tablets to reach the maintenance dose. Depending on the specific patient's profile eg, medical history, sensitization status or presence of any intercurrent disease, the physician may prefer adjusting the titration doses upon treatment initiation. To allow for this, the product labelling specifies the dose-escalation period could be prolonged as necessary according to the patient's condition.28 In addition, patients must be given specific instructions to identify and manage their potential allergic reactions. Notably, they may be advised to use symptomatic medications such as antihistamines when experiencing significant application-site reactions,36,48,49 when to interrupt and restart AIT, and to seek immediate medical care when necessary. It is important to note that patients' information on the side-effects they might expect and how to control them will help in improving their adherence to SLIT.9,36,50,51
In line with the class effect of SLIT products, EoE is also considered an important identified risk associated with the HDM tablets. Collected isolated cases of EoE17,28,52 substantiate several published reports suggesting a possible association with SLIT.53, 54, 55, 56, 57, 58, 59 Minimization can be achieved by educating patients about suggestive severe gastroesophageal symptoms so that they can seek appropriate medical care.
Evidence of a relationship between AIT and the incidence of autoimmune disorders is low.60,61 In RCTs with the 300IR HDM-SLIT tablet, one reported case of autoimmune disorder was not considered related to this treatment which was continued. Nevertheless, HDM tablets are contraindicated in patients with active forms of autoimmune disease and prescribers are recommended to exercise caution in those with autoimmune disease in remission.28,52
Overall, the analysis of identified and potential risks with the 300IR HDM-SLIT tablet across the development program and since its commercialization showed the product benefit-risk ratio remains favorable for the patients with HDM-AR. It is also important to note that no specific risk is expected in the pediatric population compared to adults. Since patients with HDM-AR are at higher risk of developing comorbid asthma and those with severe, uncontrolled asthma are more prone to experience severe systemic allergic reactions notably under AIT,10,62,63 it was important to assess the safety of the 300IR HDM-SLIT tablet with a specific focus in asthmatic subjects. In RCTs, in accordance with SLIT contraindications, participants had their asthma well-controlled and no notable difference in their safety profile was observed compared to non-asthmatic subjects. These findings consistent with other published trials39,64,65 support the safe use of this product in HDM-AR patients with concomitant controlled asthma.
Conclusion
Collectively, the safety data from pooled clinical trials and from more than 7 years of real-life experience indicate the 300IR HDM-SLIT tablet is well tolerated in adults, adolescents and children with HDM-AR with or without controlled asthma from different regions in Europe, Asia and North America. Results from clinical trials showed a similar safety profile whatever the age category and in patients with asthma compared to those without asthma. The post-marketing findings in more than 235,000 patients were in line with the clinical safety data; no new safety signal was raised.
Abbreviations
AE, Adverse event; ADR, Adverse drug reactions; AIT, Allergen immunotherapy; AR, Allergic rhinitis; AR/C, Allergic rhinitis with or without conjunctivitis; EEC, Environmental exposure chamber; EoE, Eosinophilic esophagitis; GINA, Global Initiative for Asthma; HDM, House dust mite; IR, Index of reactivity; MAH, Marketing Authorization Holder; SCIT, Subcutaneous immunotherapy; SLIT, Sublingual immunotherapy; TEAE, Treatment-emergent adverse event.
Funding
This work was funded by Stallergenes Greer (Antony, France) which provided support for medical writing assistance and was involved in the decision to submit the article for publication. Of the 8 clinical trials presented in this article, 5 were conducted and funded by Stallergenes Greer and 3 were conducted and funded by Shionogi & Co., Ltd., (Osaka, Japan) manufacturers and licensees of Actair® (extracts of Dermatophagoides pteronyssinus and Dermatophagoides farinae) in Japan.
Availability of data and materials
Data are available from the corresponding author on reasonable request.
Author's contributions and consent for publication
MW, PD, YO, CV, TBC and KCB significantly contributed to the conception, design, execution, or interpretation of the reported clinical trials. KD significantly contributed to the interpretation of pharmacovigilance data. All authors contributed to the interpretation of data, writing and editing of the manuscript. All authors approved the final version of this manuscript for publication.
Ethics approval and consent to participate
The double-blind, placebo-controlled, randomized clinical trials were performed in accordance with good clinical practice defined by the International Council for Harmonization and the principles that have their origin in the Declaration of Helsinki and local laws and regulations. Ethics committees or institutional review boards approved all study protocols and all participants or parents or legal representatives (for participants 17 years or younger) gave their written consent to participation before any study procedure was performed.
Declaration of competing interest
M. Worm reports consulting fees and payment or honoraria for lecture, presentations, speakers bureaus, manuscript writing or educational events from Novartis Pharma GmbH, Sanofi-Aventis Deutschland GmbH, DBV Technologies S.A, Aimmune Therapeutics UK Limited, Leo Pharma GmbH, AstraZeneca GmbH, ALK-Abelló Arzneimittel GmbH, Lilly Deutschland GmbH, Kymab Limited, Amgen GmbH, Abbvie Deutschland GmbH & Co. KG, Pfizer Pharma GmbH, Mylan Germany GmbH (a Viatris Company), Boehringer Ingelheim Pharma GmbH & Co. KG, GlaxoSmithKline GmbH & Co. KG, Almirall S. A., Pfizer Deutschland GmbH, Bristol-Myers Squibb GmbH & Co. KGaA and FomF GmbH, outside the submitted work.
P. Demoly reports grants paid to his institutions from ALK-Abelló, AstraZeneca, GlaxoSmithKline, Menarini, Puressentiel, Stallergenes Greer, ThermoFisher Scientific, Viatris, and support for attending meetings and/or travel from Stallergenes Greer outside the submitted work.
Y. Okamoto reports consulting fees and/or payment or honoraria for lecture, presentations, speakers bureaus, manuscript writing or educational events from Torii Co., Ltd., Kirin Holding Co., Ltd., Stallergenes Greer, ALK-Abelló, Shionogi Co., Ltd., Yansen Co., Ltd., Tanabe-Mitsubishi Co., Ltd., Meiji Pharma, Novartis Co., Ltd.; support for attending meetings and/or travel from Torii Co., Ltd., Stallergenes Greer; participation on Data Safety Monitoring Board or Advisory Board from Stallergenes Greer, Kirin Holding Co., Ltd., Greer outside the submitted work.
C. Vidal reports consulting fees paid to her institution from Stallergenes Greer, ALK- Abelló; honoraria from Stallergenes Greer; participation on Data Safety Monitoring Board or Advisory Board from Stallergenes Greer, Leti, ALK- Abelló, outside the submitted work.
K. Daghildjian is an employee of Stallergenes Greer.
K. Yan declares he has no conflicts of interests regarding the submitted work.
T.B. Casale reports grants paid to his institution for conduct of original study from Stallergenes Greer.
K.C. Bergmann reports payment for expert testimony, support for attending meetings and/or travel and participation on Data Safety Monitoring Board or Advisory Board from Bencard, Leti, Sanofi, GlaxoSmithKline, AstraZeneca, Novartis, Stallergenes Greer, HAL, outside the submitted work.
Acknowledgments
The authors present this publication on behalf of all the involved investigators from the trials. The authors would like to thank all investigators. The trials described in this article were designed and funded by Stallergenes Greer, and Shionogi & Co., Ltd for 3 of them. In this context, the authors would like to thank the clinical trial teams and pharmacovigilance teams at Stallergenes Greer and Shionogi for clinical project management, operational oversight, safety monitoring, data management, and statistical analyses. Dr Josiane Cognet-Sicé (Stallergenes Greer) was responsible for medical writing, editorial, and journal submission assistance for this manuscript.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2024.100924.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Calderon M.A., Linneberg A., Kleine-Tebbe J., et al. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol. 2015;136:38–48. doi: 10.1016/j.jaci.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Borges M., Fernandez-Caldas E., Thomas W.R., et al. International consensus (ICON) on: clinical consequences of mite hypersensitivity, a global problem. World Allergy Organ J. 2017;10:14. doi: 10.1186/s40413-017-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linneberg A., Dam Petersen K., Hahn-Pedersen J., Hammerby E., Serup-Hansen N., Boxall N. Burden of allergic respiratory disease: a systematic review. Clin Mol Allergy. 2016;14:12. doi: 10.1186/s12948-016-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brozek J.L., Bousquet J., Agache I., et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140:950–958. doi: 10.1016/j.jaci.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Leger D., Bonnefoy B., Pigearias B., de La Giclais B., Chartier A. Poor sleep is highly associated with house dust mite allergic rhinitis in adults and children. Allergy Asthma Clin Immunol. 2017;13:36. doi: 10.1186/s13223-017-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox L., Nelson H., Lockey R., et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–S55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Jutel M., Agache I., Bonini S., et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Pfaar O., Agache I., de Blay F., et al. Perspectives in allergen immunotherapy: 2019 and beyond. Allergy. 2019;74(Suppl 108):3–25. doi: 10.1111/all.14077. [DOI] [PubMed] [Google Scholar]
- 9.Roberts G., Pfaar O., Akdis C.A., et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73:765–798. doi: 10.1111/all.13317. [DOI] [PubMed] [Google Scholar]
- 10.Wise S.K., Damask C., Roland L.T., et al. International consensus statement on allergy and rhinology: allergic rhinitis – 2023. International Forum of Allergy & Rhinology. 2023;13:293–859. doi: 10.1002/alr.23090. [DOI] [PubMed] [Google Scholar]
- 11.Masuyama K., Matsuoka T., Kamijo A. Current status of sublingual immunotherapy for allergic rhinitis in Japan. Allergol Int. 2018;67:320–325. doi: 10.1016/j.alit.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Demoly P., Okamoto Y., Yang W.H., Devillier P., Bergmann K.C. 300 IR HDM tablet: a sublingual immunotherapy tablet for the treatment of house dust mite-associated allergic rhinitis. Expet Rev Clin Immunol. 2016:1–11. doi: 10.1080/1744666X.2016.1237288. [DOI] [PubMed] [Google Scholar]
- 13.Klimek L., Brehler R., Casper I., et al. Allergen immunotherapy in house dust mite-associated allergic rhinitis: efficacy of the 300 IR mite tablet. Allergo Journal International. 2023;32:10–17. [Google Scholar]
- 14.Bergmann K.C., Demoly P., Worm M., et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–16014 e6. doi: 10.1016/j.jaci.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto Y., Fujieda S., Okano M., Yoshida Y., Kakudo S., Masuyama K. House dust mite sublingual tablet is effective and safe in patients with allergic rhinitis. Allergy. 2017;72:435–443. doi: 10.1111/all.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roux M., Devillier P., Yang W.H., et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts: results of a dose-ranging study in an environmental exposure chamber. J Allergy Clin Immunol. 2016;138:451–458 e5. doi: 10.1016/j.jaci.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 17.Demoly P., Corren J., Creticos P., et al. A 300 IR sublingual tablet is an effective, safe treatment for house dust mite-induced allergic rhinitis: an international, double-blind, placebo-controlled, randomized phase III clinical trial. J Allergy Clin Immunol. 2021;147:1020–1030 e10. doi: 10.1016/j.jaci.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto Y., Fujieda S., Okano M., Hida H., Kakudo S., Masuyama K. Efficacy of house dust mite sublingual tablet in the treatment of allergic rhinoconjunctivitis: a randomized trial in a pediatric population. Pediatr Allergy Immunol. 2019;30:66–73. doi: 10.1111/pai.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okamoto Y., Ishii K., Kato M., Hayashi H., Hata T. Safety and effectiveness of the 300 IR sublingual house dust mite allergen immunotherapy tablet: 2-year interim analysis of a specified drug-use survey. Immunotherapy. 2021;13:1333–1343. doi: 10.2217/imt-2021-0173. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto Y., Kato M., Ishii K., Sato Y., Hata T., Asaka Y. Safety and effectiveness of a 300 IR house dust mite sublingual tablet: descriptive 4-year final analysis of a post-marketing surveillance in Japan. Immunotherapy. 2023 doi: 10.2217/imt-2023-0100. [DOI] [PubMed] [Google Scholar]
- 21.Demoly P., Meziane L., Le Gall M., Andre C., Melac M. Safety and tolerability of house dust mite tablets in sublingual immunotherapy. J Allergy Clin Immunol. 2008;121 [Google Scholar]
- 22.Halken S., Wahn U., Melac M., Nguyen H., Cadic V., Zeldin R.K. Assessment of efficacy and safety of sublingual tablets of house dust mite allergen extract in children and adolescents with allergic rhinitis. Clin Transl Allergy. 2014;4(Suppl 1):O17. [Google Scholar]
- 23.European Medicines Agency. Committee for medicinal products for human use (CHMP): guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases (CHMP/EWP/18504/2006). Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-development-products-specific-immunotherapy-treatment-allergic-diseases_en.pdf Accessed April 2024.
- 24.Pepper A.N., Calderon M.A., Casale T.B. Sublingual immunotherapy for the polyallergic patient. J Allergy Clin Immunol Pract. 2017;5:41–45. doi: 10.1016/j.jaip.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Potapova E., Bordas-Le Floch V., Schlederer T., et al. Molecular reactivity profiling upon immunotherapy with a 300 IR sublingual house dust mite tablet reveals marked humoral changes towards major allergens. Allergy. 2022;77:3084–3095. doi: 10.1111/all.15327. [DOI] [PubMed] [Google Scholar]
- 26.Demoly P., Passalacqua G., Calderon M.A., Yalaoui T. Choosing the optimal dose in sublingual immunotherapy: rationale for the 300 index of reactivity dose. Clin Transl Allergy. 2015;5:44. doi: 10.1186/s13601-015-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hrabina M., Purohit A., Oster J.P., et al. Standardization of an ash (Fraxinus excelsior) pollen allergen extract. Int Arch Allergy Immunol. 2007;142:11–18. doi: 10.1159/000095994. [DOI] [PubMed] [Google Scholar]
- 28.Actair® 100 IR & 300 IR sublingual tablets summary of product characteristics. January 2023. https://products.mhra.gov.uk/search/?search=Actair&page=1&doc=Spc&rerouteType=0
- 29.MedDRA hierarchy. Available at https://www.meddra.org/how-to-use/basics/hierarchy. Accessed April 2024.
- 30.Standardised MedDRA Queries (SMQs). Available at https://www.meddra.org/how-to-use/tools/smqs. Accessed April 2024.
- 31.Sampson H.A., Munoz-Furlong A., Campbell R.L., et al. Second symposium on the definition and management of anaphylaxis: summary report--second national institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 32.Goubert L., Vervoort T., Sullivan M.J.L., Verhoeven K., Crombez G. Parental emotional responses to their child's pain: the role of dispositional empathy and catastrophizing about their child's pain. J Pain. 2008;9:272–279. doi: 10.1016/j.jpain.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Parrella A., Gold M., Marshall H., Braunack-Mayer A., Baghurst P. Parental perspectives of vaccine safety and experience of adverse events following immunisation. Vaccine. 2013;31:2067–2074. doi: 10.1016/j.vaccine.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Canonica G.W., Cox L., Pawankar R., et al. Sublingual immunotherapy: world Allergy Organization position paper 2013 update. World Allergy Organization J. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Passalacqua G., Nowak-Wegrzyn A., Canonica G.W. Local side effects of sublingual and oral immunotherapy. J Allergy Clin Immunol Pract. 2017;5:13–21. doi: 10.1016/j.jaip.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Pfaar O., Ankermann T., Augustin M., et al. Guideline on allergen immunotherapy in IgE-mediated allergic diseases. Allergologie select. 2022;6:167–232. doi: 10.5414/ALX02331E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulalert Phinyo, Lao-Araya Efficacy and safety of house dust mite sublingual immunotherapy tablets in allergic rhinitis: a systematic review and meta-analysis. World Allergy Organization J. 2022;15 doi: 10.1016/j.waojou.2022.100691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nolte H., Bernstein D.I., Sussman G.L., et al. Impact of adverse event solicitation on the safety profile of SQ house dust mite sublingual immunotherapy tablet. J Allergy Clin Immunol Pract. 2018;6:2081–2086.e1. doi: 10.1016/j.jaip.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 39.Bernstein D.I., Kleine-Tebbe J., Nelson H.S., et al. SQ house dust mite sublingual immunotherapy tablet subgroup efficacy and local application site reaction duration. Ann Allergy Asthma Immunol. 2018;121:105–110. doi: 10.1016/j.anai.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Calderon M.A., Simons F.E., Malling H.J., Lockey R.F., Moingeon P., Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2012;67:302–311. doi: 10.1111/j.1398-9995.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 41.James C., Bernstein D.I. Allergen immunotherapy: an updated review of safety. Curr Opin Allergy Clin Immunol. 2017;17:55–59. doi: 10.1097/ACI.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Incorvaia C., Pucciarini F., Makri E., Gritti B.L., Ridolo E. Allergen immunotherapy for respiratory allergy: to what extent can the risk of systemic reactions be reduced? Expet Opin Drug Saf. 2020:1–6. doi: 10.1080/14740338.2020.1773788. [DOI] [PubMed] [Google Scholar]
- 43.Creticos P.S. New insights in mite immunotherapy – sublingual tablets. Curr Opin Allergy Clin Immunol. 2021;21:602–610. doi: 10.1097/ACI.0000000000000785. [DOI] [PubMed] [Google Scholar]
- 44.Mösges R., Passali D., Di Gioacchino M. Worldwide surveys on anaphylaxis to sublingual immunotherapy with house dust mite tablets are urgently needed. Clin Transl Allergy. 2021;11 doi: 10.1002/clt2.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssens N.S., Ouwerkerk L., Gerth van Wijk R., Karim F. Acute systemic reactions to sublingual immunotherapy for house dust mite. Allergy. 2020;75:2962–2963. doi: 10.1111/all.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiber R., Wolf H., Futschik T., et al. Safety and tolerability of the standardized quality house dust mite sublingual immunotherapy tablet in real life: a noninterventional, open-label study. J Allergy Clin Immunol Pract. 2021;9:3221. doi: 10.1016/j.jaip.2021.03.045. 3.e5. [DOI] [PubMed] [Google Scholar]
- 47.Nolte H., Casale T.B., Lockey R.F., et al. Epinephrine use in clinical trials of sublingual immunotherapy tablets. J Allergy Clin Immunol Pract. 2017;5:84–89 e3. doi: 10.1016/j.jaip.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Nelson H.S. Allergy immunotherapy for inhalant allergens: strategies to minimize adverse reactions. Allergy Asthma Proc. 2020;41:38–44. doi: 10.2500/aap.2020.41.190014. [DOI] [PubMed] [Google Scholar]
- 49.Calderon M.A., Waserman S., Bernstein D.I., et al. Clinical practice of allergen immunotherapy for allergic rhinoconjunctivitis and asthma: an expert panel report. J Allergy Clin Immunol Pract. 2020;8:2920–29236.e1. doi: 10.1016/j.jaip.2020.04.071. [DOI] [PubMed] [Google Scholar]
- 50.Scurati S., Frati F., Passalacqua G., et al. Adherence issues related to sublingual immunotherapy as perceived by allergists. Patient Prefer Adherence. 2010;4:141–145. doi: 10.2147/ppa.s10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antico A. Improving long-term adherence to sublingual immunotherapy. Results of a proactive patient-centered management planning. Eur Ann Allergy Clin Immunol. 2022;54:16. doi: 10.23822/EurAnnACI.1764-1489.197. [DOI] [PubMed] [Google Scholar]
- 52.Acarizax® 12 SQ-HDM Summary of Product Characteristics. November 2023. https://www.medicines.org.uk/emc/product/12905/smpc#gref [Google Scholar]
- 53.Antico A., Fante R. Esophageal hypereosinophilia induced by grass sublingual immunotherapy. J Allergy Clin Immunol. 2014;133:1482–1484. doi: 10.1016/j.jaci.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 54.Bene J., Ley D., Roboubi R., Gottrand F., Gautier S. Eosinophilic esophagitis after desensitization to dust mites with sublingual immunotherapy. Ann Allergy Asthma Immunol. 2016;116:583–584. doi: 10.1016/j.anai.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 55.Miehlke S., Alpan O., Schröder S., Straumann A. Induction of eosinophilic esophagitis by sublingual pollen immunotherapy. Case Reports in Gastroenterology. 2013;7:363–368. doi: 10.1159/000355161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel C. A complication of eosinophilic esophagitis from sublingual immunotherapy. J Allergy Clin Immunol. 2016;137 [Google Scholar]
- 57.Rokosz M., Bauer C., Schroeder S. Eosinophilic esophagitis induced by aeroallergen sublingual immunotherapy in an enteral feeding tube-dependent pediatric patient. Ann Allergy Asthma Immunol. 2017;119:88–89. doi: 10.1016/j.anai.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Cafone J., Capucilli P., Hill D.A., Spergel J.M. Eosinophilic esophagitis during sublingual and oral allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2019;19:350–357. doi: 10.1097/ACI.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawashima K., Ishihara S., Masuhara M., et al. Development of eosinophilic esophagitis following sublingual immunotherapy with cedar pollen extract: a case report. Allergol Int. 2018;67:515–517. doi: 10.1016/j.alit.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Pitsios C., Tsoumani M., Bilo M.B., et al. Contraindications to immunotherapy: a global approach. Clin Transl Allergy. 2019;9:45. doi: 10.1186/s13601-019-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linneberg A., Madsen F., Skaaby T. Allergen-specific immunotherapy and risk of autoimmune disease. Curr Opin Allergy Clin Immunol. 2012;12:635–639. doi: 10.1097/ACI.0b013e3283588c8d. [DOI] [PubMed] [Google Scholar]
- 62.Muraro A., Roberts G., Worm M., et al. Anaphylaxis: guidelines from the European academy of allergy and clinical immunology. Allergy. 2014;69:1026–1045. doi: 10.1111/all.12437. [DOI] [PubMed] [Google Scholar]
- 63.Pitsios C., Demoly P., Bilo M.B., et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70:897–909. doi: 10.1111/all.12638. [DOI] [PubMed] [Google Scholar]
- 64.Maloney J., Durham S., Skoner D., et al. Safety of sublingual immunotherapy Timothy grass tablet in subjects with allergic rhinitis with or without conjunctivitis and history of asthma. Allergy. 2015;70:302–309. doi: 10.1111/all.12560. [DOI] [PubMed] [Google Scholar]
- 65.Epstein T.G., Calabria C., Cox L.S., Dreborg S. Current evidence on safety and practical considerations for administration of sublingual allergen immunotherapy (SLIT) in the United States. J Allergy Clin Immunol Pract. 2017;5:34–40 e2. doi: 10.1016/j.jaip.2016.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the corresponding author on reasonable request.