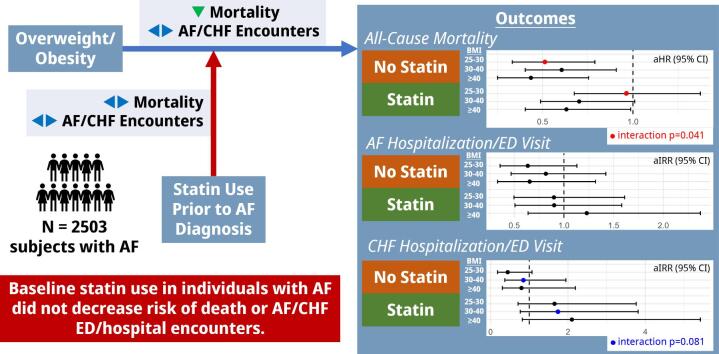
Graphical abstract
Relationship between obesity, statin use, and af outcomes. Relationship between obesity, statin use, and AF outcomes.
Keywords: Atrial fibrillation, Obesity, Mortality, Hospitalization, Statins
Abstract
Background
Obesity increases risk of atrial fibrillation (AF) at least in part due to pro-inflammatory effects, but has been paradoxically associated with improved mortality. Although statins have pleiotropic anti-inflammatory properties, their interaction with obesity and clinical outcomes in AF is unknown. We explored the relationship between BMI, statin use, and all-cause mortality and AF/congestive heart failure (CHF)-related encounters, hypothesizing that statin exposure may be differentially associated with improved outcomes in overweight/obesity.
Methods
This was a single center retrospective cohort study of adults with AF diagnosed between 2011–2018. Patients were grouped by body mass index (BMI) and statin use at time of AF diagnosis. Outcomes included all-cause mortality and ED or inpatient encounters for AF or CHF.
Results and Conclusions
A total of 2503 subjects were included (median age 66 years, 43.4 % female, median BMI 29.8 kg/m2, 54.6 % on baseline statin therapy). Increasing BMI was associated with decreased mortality hazard but not associated with AF/CHF encounter risk. Adjusting for statin-BMI interaction, demographics, and cardiovascular comorbidities, overweight non-statin users experienced improved mortality (adjusted hazard ratio [aHR] 0.55, 95 % CI 0.35–0.84) compared to statin users (aHR 0.98, 95 % CI 0.69–1.40; interaction P-value = 0.013). Mortality hazard was consistently lower in obese non-statin users than in statin users, however interaction was insignificant. No significant BMI-statin interactions were observed in AF/CHF encounter risk. In summary, statin use was not differentially associated with improved mortality or hospitalization risk in overweight/obese groups. These findings do not support statins for secondary prevention of adverse outcomes based on overweight/obesity status alone.
1. Introduction
Atrial fibrillation (AF), the most common arrhythmia in clinical practice, is responsible for over 450,000 hospitalizations and 150,000 deaths in the United States each year [1], [2], [3]. Concurrently, the worsening obesity epidemic now affects 39.6 % of the US adult population [3]. Obesity has been causally linked to the pathogenesis of AF, where inflammatory factors produced by epicardial adipose are a key driver of this process [4], [5], [6], [7]. Obesity is also associated with worsened symptom severity, quality of life, higher risk of cardiovascular-associated hospitalizations, and greater length-of-stay [8], [9]. Conversely, obesity is associated with decreased all-cause mortality, cardiovascular mortality, and stroke or systemic embolism, defining an “obesity paradox” [10], [11], [12], [13]. To target the role of inflammation on AF pathogenesis and morbidity, statins have been explored as a safe and cost-effective preventative therapy with pleiotropic anti-oxidative and anti-inflammatory effects [14], [15], [16]. Mechanisms by which statins may achieve this effect include reduction of reactive oxygen species, upregulation of endothelial nitric oxide, or protection against oxidant-induced cardiac mitochondrial dysfunction [16], [14], [17]. Randomized controlled trials studying effects of statin use on AF incidence have provided conflicting results [15], [18]. A post-hoc analysis of Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) demonstrated that statins were associated with reduced risk of incident AF in groups with higher high-sensitivity C-reactive protein (hs-CRP) [19], however, a meta-analysis of randomized trials only identified benefit in primary prevention following cardiac surgery, or secondary prevention following ablation or cardioversion [20], [21], [22]. Given the mechanistic link between inflammation and AF, there is an important knowledge gap in our understanding of the relationship between obesity, statin use and AF-related clinical outcomes. Here, we examined the role of statins in modulating the relationship between obesity and clinical outcomes in AF, hypothesizing that statins may be associated with reduced mortality or hospitalization risk in obese patients. We first characterized the independent roles of statin use and BMI on clinical outcomes in our single-center retrospective patient cohort, and then examined outcome incidence in each BMI subgroup based on statin use. Finally, we adjusted for BMI-statin interaction and potential confounders related to either BMI or statin use using multivariable regression.
2. Materials and Methods
2.1. Study design
This is a single center, observational, retrospective cohort study of patients in the University of Illinois Health (UIH, Chicago, IL) system approved by the UIH Institutional Review Board. Individuals ≥ 18 years old at time of AF diagnosis were identified from International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes across all patient encounters. Demographics, medical comorbidities, BMI, laboratory testing, and oral medication orders and prescriptions between January 1, 2010, to September 1, 2020 were captured from the electronic health record as described in Supplemental Tables 1 and 2. Incident cases were defined as those with first AF encounter occurring between January 1, 2011, and September 1, 2018, to exclude pre-existing diagnoses and allow at least 2 years of follow-up time. Cases also met one of the following criteria: 1) diagnosis code on two outpatient visits on different dates, 2) diagnosis code on one outpatient visit with an electrocardiogram confirming AF within 30 days of the visit, or 3) diagnosis code from an emergency department (ED) or inpatient encounter [1]. Individuals with mitral stenosis, congenital or rheumatic disease, prior valve replacement, BMI < 18.5 or ≥ 60 kg/m2, no recorded BMI within 1 year of first AF encounter, or death occurring within 30 days of enrollment were excluded. A breakdown of cases by inclusion criteria is contained in Supplemental Table 3. Mortality status and date of death was obtained from United States Social Security Administration Limited Access Death Master File and the UIH electronic health record and valid as of February 23, 2023. The primary outcome was all-cause mortality. Secondary outcomes included ED/inpatient encounters with primary diagnosis of AF or primary diagnosis of CHF. In secondary analyses, patients were censored on the date of the last diagnosis code or lab test available within the EHR. There were 401 subjects without encounters after the date of initial AF diagnosis who were excluded from this analysis. Individuals were separately grouped by BMI category and then by baseline statin use to evaluate their respective relationship to clinical outcomes. Further definitions are provided in the Supplemental Material.
2.2. Validation cohort
To validate the relative accuracy of AF diagnosis, demographics, and medical comorbidities in our cohort, 257 individuals were randomly selected to represent approximately 10 % of the cohort and undergo manual electronic health record review by two experienced clinical reviewers (NK and MH). Summary statistics of this validation cohort are presented in Supplemental Table 4.
2.3. Statistical analysis
Continuous variables are reported as median with interquartile range (IQR) and are compared with the Kruskal-Wallis test. Categorical variables are represented as proportion (%) and are compared with the χ2 test. Post-hoc Holm-Bonferroni correction was used where appropriate to adjust for multiple comparisons. Kaplan-Meier survival analysis and log-rank testing were used to evaluate overall survival. Univariable and multivariable Cox proportional hazard models were constructed to compare overall survival between BMI and statin groupings and derive hazard ratios (HR) with 95 % confidence intervals (CI). Counts of ED/inpatient AF and CHF encounters were compared using negative binomial regression adjusting for total length of follow-up, and these models were used to derive incidence rate ratios [IRRs] with 95 % CIs. All models included BMI category, statin use, and an interaction term between BMI category and statin use as independent variables. We additionally included covariates with established clinical relationships to either AF or statin use, or those tested previously in studies exploring obesity-AF relationships [8], [23], [24]: standard demographic parameters of age, sex, and race-ethnicity; AF risk factors of diabetes, hypertension, vascular disease (a composite of coronary artery disease, peripheral arterial disease, prior stroke/transient ischemic attack (TIA), or prior myocardial infarction), CHF, and chronic kidney disease (CKD); and AF-related treatments of rate control, rhythm control, and anticoagulation. To further examine relationships between covariates and significant BMI-statin interactions, a post-hoc sensitivity analysis was performed testing three-way interactions between BMI category, statin use, and each covariate (described further in the Supplemental Methods). All data were analyzed using R Version 4.2.1 (packages: arsenal, DiagrammeR, dplyr, dunn.test, ggplot2, ggsurvfit, gtsummary, MASS, survival) [25].
3. Results
3.1. Study characteristics and patient demographics
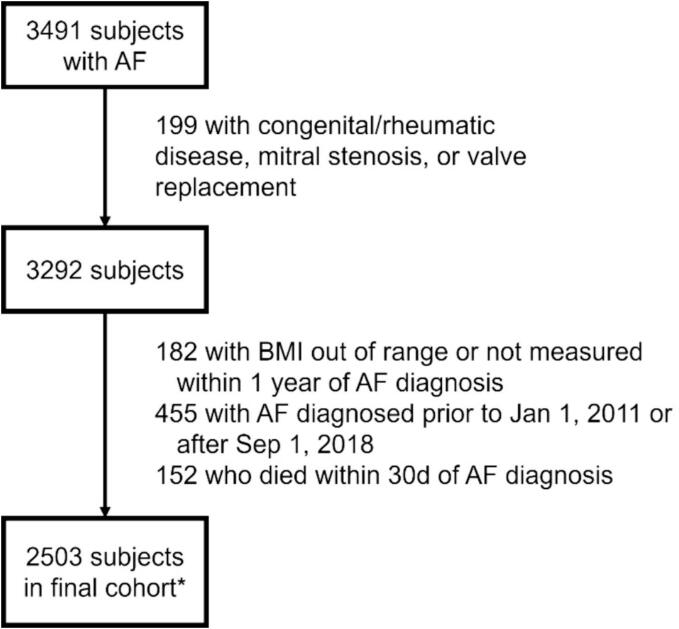
The study flowchart is presented in Fig. 1. A total of 3491 subjects with AF were identified, of whom 1020/3491 (29.2 %) met outpatient enrollment criteria only (Criteria 1 and/or 2), and 1452/3291 (41.6 %) met inpatient enrollment criteria only (Criterion 3); the remainder met both outpatient and inpatient criteria (Supplemental Table 3). After exclusion, 2503 subjects were included in the final cohort, with a median age of 66 years (interquartile range [IQR] 58.0 – 76.0), 43.4 % female, and median BMI of 29.8 (IQR 25.4 – 35.6) kg/m2 (Table 1). The race-ethnicity composition was well-balanced (42.7 % Non-Hispanic Black, 26.7 % Non-Hispanic White, 16.4 % Hispanic/Latinx). BMI was positively associated with diabetes, hypertension, CHF, chronic obstructive pulmonary disease (COPD)/asthma, obstructive sleep apnea (OSA), and prescription of statin and anticoagulation. Compared with normal weight individuals, there was higher statin use in overweight (p = 0.032), class 1 obesity (p < 0.001), and class 2 obesity (p = 0.018); other BMI group comparisons were insignificant. Compared to individuals without baseline statin therapy, statin users were more likely to have higher BMI, diabetes, hypertension, vascular disease, CHF, COPD/asthma, OSA, CKD, and tobacco use (Supplemental Table 5). Median CHA2DS2-VASc score was higher in statin users (5.0 vs. 4.0, p < 0.001), and statin users were more likely to receive aspirin, beta blocker/calcium channel blocker, antiarrhythmic, and anticoagulation.
Fig. 1.
Study flowchart. *All subjects were analyzed for all-cause mortality. For AF/CHF encounter analysis, 401 patients without longitudinal encounter data were excluded (by BMI 18.5–24.99, 25–29.99, 30–39.99, and ≥ 40 kg/m2, respectively: 100, 132, 123, 46; by statin use: 156 statin users, 245 statin non-users).
Table 1.
Baseline demographics according to body mass index (BMI) at time of enrollment. All categorical variables are reported as number of subjects (%) and continuous variables are reported as median (first quartile, third quartile).
|
BMI (kg/m2) category |
Total (N = 2503) |
P-value | ||||
|---|---|---|---|---|---|---|
|
Normal BMI (18.5–24.99) (N = 569) |
Overweight (25–29.99) (N = 720) |
Class 1–2 Obesity 30–39.99 (N = 832) |
Class 3 Obesity ≥40 (N = 382) |
|||
| Age (years) | 67.0 (59.0, 77.0) | 66.0 (58.0, 76.0) | 66.0 (57.0, 76.0) | 67.0 (58.0, 75.0) | 66.0 (58.0, 76.0) | 0.125 |
| Female sex | 246 (43.2 %) | 313 (43.5 %) | 359 (43.1 %) | 169 (44.2 %) | 1087 (43.4 %) | 0.987 |
| Race/Ethnicity | 0.644 | |||||
| Non-Hispanic White | 140 (24.6 %) | 208 (28.9 %) | 217 (26.1 %) | 103 (27.0 %) | 668 (26.7 %) | |
| Non-Hispanic Black | 264 (46.4 %) | 293 (40.7 %) | 347 (41.7 %) | 165 (43.2 %) | 1069 (42.7 %) | |
| Hispanic/Latinx | 87 (15.3 %) | 112 (15.6 %) | 149 (17.9 %) | 62 (16.2 %) | 410 (16.4 %) | |
| Asian | 18 (3.2 %) | 20 (2.8 %) | 32 (3.8 %) | 11 (2.9 %) | 81 (3.2 %) | |
| Other | 60 (10.5 %) | 87 (12.1 %) | 87 (10.5 %) | 41 (10.7 %) | 275 (11.0 %) | |
|
BMI (kg/m2) |
22.6 (21.2, 23.8) | 27.5 (26.2, 28.7) | 33.7 (31.6,36.2) | 45.3 (42.3, 49.4) | 29.8 (25.4, 35.6) | < 0.001 |
| Comorbidities | ||||||
| Diabetes | 159 (27.9 %) | 253 (35.1 %) | 388 (46.6 %) | 207 (54.2 %) | 1007 (40.2 %) | < 0.001 |
| Hypertension | 413 (72.6 %) | 568 (78.9 %) | 698 (83.9 %) | 329 (86.1 %) | 2008 (80.2 %) | < 0.001 |
| Coronary artery disease | 184 (32.3 %) | 238 (33.1 %) | 279 (33.5 %) | 110 (28.8 %) | 811 (32.4 %) | 0.408 |
| Peripheral arterial disease | 61 (10.7 %) | 54 (7.5 %) | 85 (10.2 %) | 31 (8.1 %) | 231 (9.2 %) | 0.132 |
| Prior stroke/transient ischemic attack | 104 (18.3 %) | 124 (17.2 %) | 140 (16.8 %) | 46 (12.0 %) | 414 (16.5 %) | 0.068 |
| Prior myocardial infarction | 74 (13.0 %) | 92 (12.8 %) | 98 (11.8 %) | 31 (8.1 %) | 295 (11.8 %) | 0.092 |
| Vascular disease composite | 259 (45.5 %) | 324 (45.0 %) | 386 (46.4 %) | 151 (39.5 %) | 1120 (44.7 %) | 0.153 |
| Congestive heart failure | 171 (30.1 %) | 240 (33.3 %) | 284 (34.1 %) | 162 (42.4 %) | 857 (34.2 %) | 0.001 |
| Chronic obstructive pulmonary disease /asthma | 117 (20.6 %) | 135 (18.8 %) | 160 (19.2 %) | 103 (27.0 %) | 515 (20.6 %) | 0.008 |
| Obstructive sleep apnea | 20 (3.5 %) | 47 (6.5 %) | 157 (18.9 %) | 152 (39.8 %) | 376 (15.0 %) | < 0.001 |
| Chronic kidney disease | 157 (27.6 %) | 182 (25.3 %) | 233 (28.0 %) | 115 (30.1 %) | 687 (27.4 %) | 0.363 |
| Tobacco use | 144 (25.3 %) | 183 (25.4 %) | 184 (22.1 %) | 87 (22.8 %) | 598 (23.9 %) | 0.354 |
| CHA2DS2-VASc score | 4.0 (3.0, 5.0) | 4.0 (3.0, 5.0) | 4.0 (3.0, 6.0) | 4.0 (3.0, 6.0) | 4.0 (3.0, 6.0) | 0.044 |
|
Baseline Medications |
||||||
| Statin | 267 (46.9 %) | 395 (54.9 %) | 489 (58.8 %) | 216 (56.5 %) | 1367 (54.6 %) | < 0.001 |
| Aspirin | 329 (57.8 %) | 429 (59.6 %) | 472 (56.7 %) | 218 (57.1 %) | 1448 (57.9 %) | 0.703 |
| Beta blocker/calcium channel blocker | 406 (71.4 %) | 548 (76.1 %) | 604 (72.6 %) | 285 (74.6 %) | 1843 (73.6 %) | 0.217 |
| Antiarrhythmic | 117 (20.6 %) | 155 (21.5 %) | 202 (24.3 %) | 95 (24.9 %) | 569 (22.7 %) | 0.236 |
| Anticoagulation | 278 (48.9 %) | 373 (51.8 %) | 475 (57.1 %) | 235 (61.5 %) | 1361 (54.4 %) | < 0.001 |
To assess the accuracy of AF diagnosis and relevant comorbidities, we manually reviewed approximately 10 % of patients by electronic health record. The diagnosis of AF was accurate in 88.7 % of cases, with differences observed in race/ethnicity and reported diagnoses of peripheral arterial disease, myocardial infarction, hypertension, CKD, and tobacco use (Supplemental Table 4).
3.2. Clinical outcomes stratified independently by BMI and statin use
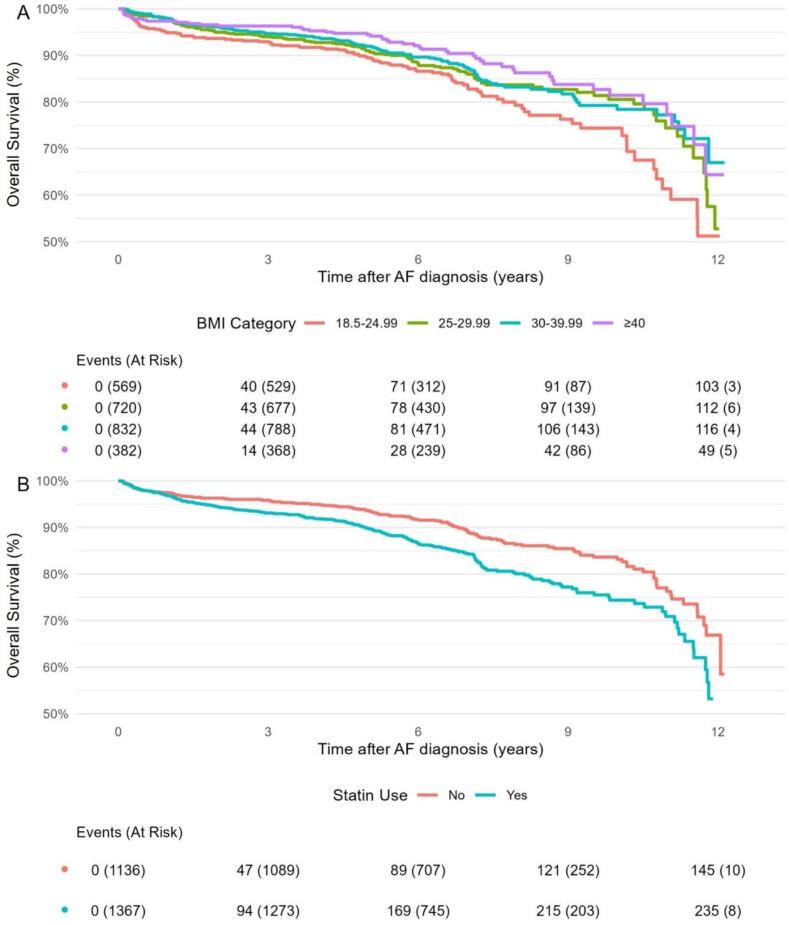
Clinical outcomes are shown in Table 2. A total of 384 subjects (15.3 %) died after a median follow-up period of 6.4 (IQR 5.2 – 8.0) years (16748 total person-years [p-y] of follow-up). Survival differed according to BMI status (log-rank p = 0.010). Relative to BMI 18.5–24.99 kg/m2, survival was significantly higher in BMI 25–29.99 (p = 0.049), BMI 30–39.99 (p = 0.011), and BMI ≥ 40 (p = 0.001) groups. Statin users had lower overall survival than non-users (10-year survival: 74.4 % vs. 83.2 %, p < 0.001). Kaplan-Meier survival curves according to BMI grouping and baseline statin use are presented in Fig. 2. Univariable Cox proportional hazard regression demonstrated reduced all-cause mortality in BMI 30–39.99 (HR 0.71 [95 % CI 0.54–0.92]) and BMI ≥ 40 (HR 0.59 [0.42–0.83]) groups compared to BMI 18.5–24.99, and increased all-cause mortality in statin users (HR 1.54 [1.25–1.89]) compared to non-users (Supplemental Table 6).
Table 2.
Clinical outcomes of overall survival and ED/inpatient encounters for AF and CHF stratified by BMI and baseline statin use. Survival data is reported as number of subjects at risk (% surviving) (p-y = person-years).
|
BMI (kg/m2) category |
Statin Use |
Total (N = 2503) | |||||
|---|---|---|---|---|---|---|---|
|
Normal BMI 18.5–24.99 (N = 569) |
Overweight 25–29.99 (N = 720) |
Class 1–2 Obesity 30–39.99 (N = 832) |
Class 3 Obesity ≥40 (N = 382) |
No (N = 1136) |
Yes (N = 1367) | ||
| All-Cause Mortality | |||||||
| Follow-up time (years, median [Q1, Q3]) | 6.25 [4.99, 7.38] | 6.51 [5.24, 8.10] | 6.33 [5.24, 7.87] | 6.76 [5.30, 8.77] | 6.66 [5.33, 8.65] | 6.21 [5.10, 7.58] | 6.42 [5.20, 8.04] |
| Total no. events | 104 | 113 | 116 | 51 | 147 | 237 | 384 |
| Events per 100p-y | 2.88 | 2.32 | 2.09 | 1.88 | 1.85 | 2.69 | 2.29 |
| 5-year survival | 424 (89.5 %) | 579(91.0 %) | 668(92.0 %) | 316(94.5 %) | 932 (93.6 %) | 1055(89.8 %) | 1987 (91.5 %) |
| 10-year survival | 47(74.4 %) | 93(80.6 %) | 91(78.4 %) | 60(81.4 %) | 169 (83.2 %) | 122 (74.4 %) | 291 (78.7 %) |
| ED/Hospital Encounters | |||||||
| Follow-up time (years, median [Q1, Q3]) | 1.53 [0.36, 3.02] | 1.71 [0.59, 3.62] | 2.11 [0.80, 3.64] | 2.44 [0.71, 4.42] | 1.83 [0.60, 3.53] | 2.05 [0.64, 3.64] | 1.94 [0.61, 3.57] |
| Atrial fibrillation | |||||||
| Total no. events | 224 | 288 | 365 | 290 | 451 | 716 | 1167 |
| Events per 10p-y | 2.31 | 2.05 | 2.03 | 3.04 | 1.90 | 2.61 | 2.28 |
| Congestive heart failure | |||||||
| Total no. events | 180 | 235 | 351 | 261 | 305 | 722 | 1027 |
| Events per 10p-y | 1.86 | 1.67 | 1.96 | 2.74 | 1.28 | 2.63 | 2.01 |
Fig. 2.
Unadjusted Kaplan-Meier survival curves representing overall survival stratified by A) BMI (kg/m2) and B) baseline statin use.
A total of 1167 ED/inpatient encounters for AF and 1027 encounters for CHF occurred after a median follow-up period of 1.9 (IQR 0.6 – 3.6) years (5120 total p-y of follow-up) (Table 2). Both AF and CHF encounters were most frequent in subjects with BMI ≥ 40 kg/m2 (AF: 3.04 events per 10p-y, CHF: 2.74 events per 10p-y). AF and CHF encounters were both more frequent in statin users compared to non-users (AF: 2.61 vs. 1.90 per 10p-y; CHF: 2.63 vs. 1.28 per 10-py). Increasing BMI was not associated with significant change in unadjusted risk of ED/inpatient AF or CHF encounters; however, statin use was associated with increased risk of both (AF: IRR 1.51 [1.14–2.01]; CHF: IRR 2.85 [1.84–4.40]) (Supplemental Table 6).
3.3. Outcomes stratified by combined BMI and statin groups
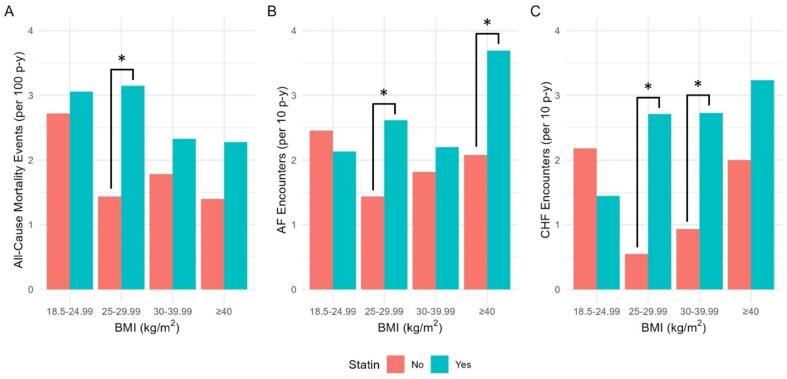
We next compared clinical outcomes stratified by statin use for each BMI subgroup (Fig. 3, Supplemental Table 7). Compared with non-statin users, all-cause mortality was higher in statin users with BMI 25–29.99 (3.15 vs. 1.44 deaths per 100p-y, log-rank p < 0.001). Risk of AF encounters was higher with statin use in BMI 25–29.99 (2.62 vs. 1.43 events per 10p-y, p = 0.005) and BMI ≥ 40 (3.69 vs. 2.08 events per 10p-y, p = 0.048) groups. Risk of CHF encounters was higher in statin users of BMI 25–29.99 (2.71 vs. 0.55 events per 10p-y, p < 0.001) and BMI 30–39.99 (2.73 vs. 0.93 events per 10p-y, p < 0.001) groups. This subgroup analysis was underpowered to detect some potentially clinically significant differences, including higher mortality and CHF encounter rates in statin users with BMI ≥ 40, and lower CHF encounter rates in statin users with BMI 18.5–24.99.
Fig. 3.
Incidence of A) all-cause mortality, B) AF encounters, and C) CHF encounters stratified by BMI and baseline statin groupings (p-y = person-years). *Denotes p < 0.05 between statin users and non-users for that BMI category by log-rank testing (A) and negative binomial regression (B, C).
3.4. Modeling BMI-statin interaction and adjusted risk of clinical outcomes
Multivariable regression models incorporating BMI, statin use, and an interaction term without additional adjustments demonstrated significant interaction for all-cause mortality at BMI 25–29.99 (interaction HR 2.03 [1.16–3.54], p = 0.013), and for risk of CHF encounter at BMI 25–29.99 (interaction HR 6.90 [1.95–24.1], p = 0.003) and BMI 30–39.99 (interaction HR 4.30 [1.29–14.0], p = 0.015) (Supplemental Table 6). Interaction terms were not significant for other BMI groups or for risk of AF encounters at any BMI group.
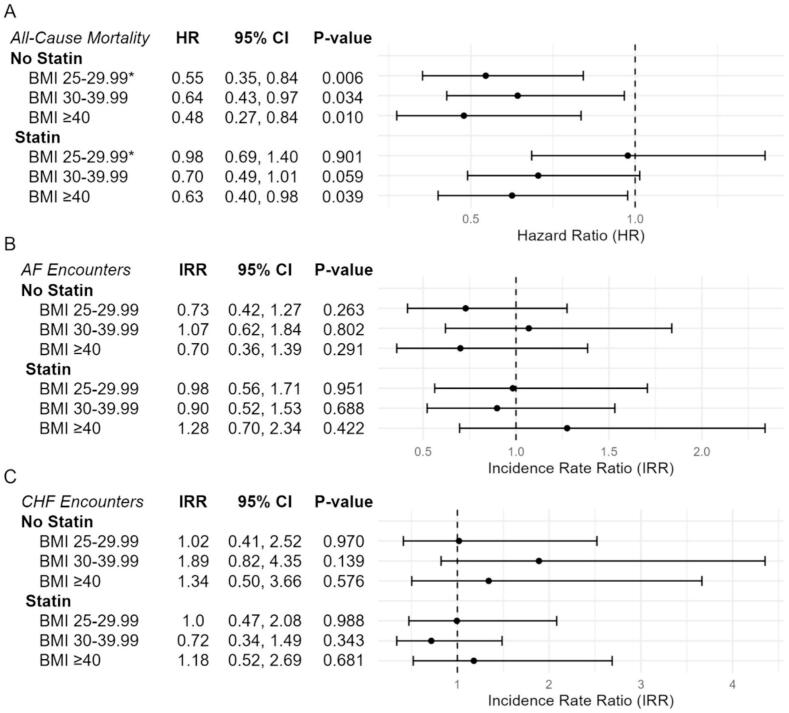
In fully adjusted models accounting for age, sex, race/ethnicity, and cardiovascular comorbidities and medications, as well as the interaction above (Fig. 4A), increasing BMI was consistently associated with reduced mortality hazard, with the largest difference in effect size at BMI 25–29.99 favoring statin non-users (statin users: HR 0.98 [0.69–1.40], non-users: HR 0.55 [0.35–0.84], interaction p = 0.041). Statin use in the overall cohort was not associated with significant mortality hazard after adjustment (HR 0.84 [0.57–1.25]). Covariates associated with increased mortality after adjustment included vascular disease (HR 1.50 [1.19–1.88]), CKD (HR 2.03 [1.63–2.54]), and CHF (HR 1.31 [1.05–1.63]), whereas anticoagulation was associated with decreased mortality (HR 0.77 [0.62–0.95]) (Supplemental Table 8). A sensitivity analysis identified CKD as having potential interaction with the BMI-statin relationship (likelihood ratio test p = 0.015; Supplemental Table 9). Statin-BMI 25–29.99 interaction remained significant in subjects without CKD (interaction HR 2.73 [1.31–5.67], p = 0.007) and was not significant in subjects with CKD (interaction HR 0.88 [0.36–2.19], p = 0.788, Supplemental Table 10).
Fig. 4.
Forest plot of multivariable model estimates for A) all-cause mortality hazard, B) AF-related ED/inpatient encounters, and C) CHF-related ED/inpatient encounters according to BMI category and statin use. All risk estimates are presented relative to subjects of the same category of baseline statin use with BMI 18.5–24.99 kg/m2. *Denotes interaction p-value < 0.05. (HR = hazard ratio, IRR = incidence rate ratio, CI = confidence interval).
Risk of AF encounters was not significantly different across BMI groups or with statin use after full adjustment (Fig. 4B, Supplemental Table 8). Individuals with BMI ≥ 40 experienced the largest change in magnitude and direction of effect with statin use (statin users: IRR 1.28 [0.70,2.34]; non-users: IRR 0.70 [0.36–1.39]), though this interaction was not statistically significant (p = 0.183). Covariates which were associated with increased risk of AF encounter included CKD, CHF, antiarrhythmic, and anticoagulation, with no significant variables associated with decreased risk.
Finally, adjusted risk of CHF encounters was also not significantly different across BMI groups or with statin use overall (Fig. 4C, Supplemental Table 8). Risk estimates for BMI 30–39.99 favored statin use most strongly (IRR 0.72 [0.34–1.49]), with opposite direction of effect compared to non-users (IRR 1.89 [0.82–4.35]), however this interaction was again not statistically significant (p = 0.081). Covariates associated with increased CHF encounter risk include non-Hispanic Black race/ethnicity, diabetes, vascular disease, CKD, CHF, antiarrhythmic, and anticoagulation use, with hypertension associated with decreased risk.
4. Discussion
In this single-center retrospective analysis, we explored the relationships between obesity, statin use, all-cause mortality, and AF- and CHF-related ED/hospital encounters, and observed the following: 1) Increasing BMI was associated with lower unadjusted mortality hazard but was not significantly associated with incidence of AF or CHF encounters, 2) statin use was associated with higher unadjusted mortality and incidence of AF and CHF encounters, which were all attenuated after multivariable adjustment, 3) the paradoxical relationship between overweight/obesity and improved mortality was stronger in non-statin users, with differential mortality reduction in overweight (BMI 25–29.99) subjects not on statin, and 4) the interaction between statin and BMI groups was otherwise not significant for rates of AF/CHF encounters after full adjustment for demographics, cardiovascular medications, and comorbidities.
4.1. Obesity and AF relationship
The association between increased BMI and decreased mortality risk has been well-described in AF and other cardiovascular diseases, termed an “obesity paradox” [10], [11], [12], [13]. Our findings expand the body of evidence describing this relationship and suggest for the first time that statin use interacts with this process. A meta-analysis comprising over 160,000 individuals with AF demonstrated a 14 % reduction in all-cause mortality per 5-unit increase in BMI, with a “U”-shaped exposure-effect relationship, where risk reduction was insignificant beyond a BMI of 35 kg/m2 [13]. Our study similarly demonstrated lower mortality hazard with higher BMI, but this relationship did not diminish or reverse in higher BMI categories. Regarding hospitalization, a prior analysis of 297 individuals demonstrated increased hospitalization rate and length of stay with increasing BMI [8]. In our analysis, although observed AF and CHF event rates were both highest in the BMI ≥ 40 kg/m2 group, increasing BMI was not significantly associated with unadjusted or adjusted AF or CHF encounter risk.
Potential biological hypotheses that may explain the obesity paradox include greater metabolic reserves, increased muscle mass or strength, and lower rates of cachexia than in normal or underweight groups; additional explanations for the obesity paradox may include confounders such as age differences or cardiovascular medication profile among BMI subgroups [26]. Importantly, observational studies of obesity-related outcomes in AF and similar conditions can be substantially affected by collider bias [27], [28], [29]. Considering the numerous comorbidities that accompany obesity, increased BMI is expected to correlate with increased mortality and hospitalization rates. As AF is among these comorbidities, it exists as a collider in ours and other similar analyses; other conditions such as diabetes or vascular disease (prompting the use of statins) have also been described as colliders between obesity and clinical outcomes. It is also possible that normal-weight subjects with AF carry alternate risk profiles, which may include autoimmune/inflammatory conditions, structural heart disease, or genetic predisposition, for these subjects to manifest with AF despite lacking obesity or associated conditions such as OSA or diabetes.
4.2. Statin and AF relationship
Among statin users, we also observed higher incidence of all-cause mortality, AF, and CHF encounters, however, after adjustment, statins were not significantly associated with risk. A recent meta-analysis of over 100,000 subjects with AF demonstrated a nearly two-fold reduction in all-cause mortality hazard associated with statin use [30]. In our cohort, we noted that statin users had an unadjusted ∼ 1.5-fold increase in mortality hazard, which was nullified upon adjustment. Comorbidities including vascular disease, diabetes, and CHF were significantly associated with mortality and are associated with statin use. A limitation of prior studies examining the statin-mortality relationship is wide variability in covariate selection and study designs, an important caveat when relating our current findings to the existing literature. Our findings extend results from a recent study examining incident CHF and mortality in individuals with AF from a territory-wide clinical database, which demonstrated that statin users had lower incidence of CHF and both all-cause and CHF-related death [31]. Notably, obesity was very rare in this cohort and its interaction with statin use was not examined. Our study demonstrated that statins were associated with increased unadjusted risk of both AF and CHF encounters, but after controlling for pertinent demographics and risk factors, these relationships were no longer significant. We note that, even after adjustment, the point estimate for CHF encounter risk remained elevated (IRR 1.70), which may be due to residual confounding from unmeasured variables. We did not explore incident CHF as a secondary outcome, but our results do not suggest that statins improve CHF disease burden in patients with AF.
4.3. Statin-obesity interaction
As inflammation is a major mechanism by which obesity increases risk of AF, we hypothesized that statin use may improve outcomes in obese subjects by attenuating this pro-inflammatory state. Our findings do not support this hypothesis. Relative to individuals with normal BMI, overweight and obese were associated with decreased adjusted risk of mortality, with significant BMI-statin interaction in the overweight group favoring non-statin users. This interaction was further mediated by CKD. In AF- and CHF-related encounters, we did not observe significant interaction. A potential explanation for reduced mortality among overweight non-statin users may involve differences in AF pathogenesis based on prior statin exposure. By the time obesity-related AF occurs, extensive oxidative stress and inflammation have likely taken place. Prolonged baseline statin use may attenuate this, so AF development or recurrence in statin users might instead result from alternate pathways that are less susceptible to the anti-inflammatory effects of statins, as has been proposed elsewhere [32]. This may also lead to more severe atrial remodeling in statin users at time of AF diagnosis, and thus higher susceptibility for adverse outcomes.
The interaction between statins and obesity has previously been examined in acute myocardial infarction, where statins were found to enhance mortality reduction with increasing BMI [33]. Our findings show some degree of interaction between statins, obesity, and mortality in AF, but did not enhance the ‘obesity paradox.’ As statins have a clear role in management of coronary artery disease, which itself is the etiology of a substantial number of CHF cases, the utility in myocardial infarction may be more intuitive, though the mechanism of differential improvement in obese patients remains to be explored. Our findings do align with a prospective observational study exploring the connection between statin use and BMI in diabetes, where paradoxically lower mortality at higher BMI was only observed in non-statin users [34]. This finding was attributed to statins reducing mortality in the BMI 18.5–24.9 subgroup, rather than an increase in mortality in overweight/obese. In our study, event rates were higher in statin users and significantly influenced by their comorbidities. As diabetes and metabolic syndrome overlap considerably with the pathogenesis of AF, further studies are needed to characterize the metabolic and inflammatory environment in patients with these conditions who have normal BMI.
4.4. Strengths and limitations
Our study has several important strengths. To our knowledge, this is the first study analyzing the relationship between obesity and statin use in the context of AF-related outcomes. Our study adds to the body of evidence describing both obesity and statin use as they relate to mortality and hospitalization in AF. Our cohort consists of a large, ethnically diverse patient sample from an academic tertiary care center; as Black and Hispanic/Latinx groups are frequently underrepresented in clinical studies, this improves the generalizability of our findings across races. Additionally, we validated AF diagnosis in a subset of patients with a case ascertainment rate that is comparable to other validation studies using claims queries [35]. There were minor differences in comorbidity profiles between the main cohort and the manually validated subset, which we do not believe were of clinically significant magnitude to affect the study results.
There are important limitations of our study. Firstly, our cohort was designed to assess AF-related clinical outcomes and not AF incidence. These are two different but equally important questions in understanding the potential role of statins in modulating the relationship between obesity and AF, and how inflammation may be involved in this process. Secondly, our analysis included only BMI and statin use at the time of AF diagnosis. Both can be considered as time-varying properties; however, in our cohort, BMI and prescription data prior to AF diagnosis was too limited to accurately quantify cumulative exposure to obesity or statin use, respectively. Third, our data were limited to explore AF-specific intermediate outcomes such as patient-reported symptoms or AF burden assessed by electrocardiogram or cardiac event monitor. Third, despite controlling for many factors that overlap with statin use, there may be residual unmeasured confounders influencing statin prescribing patterns. Additionally, statin dose and duration of exposure were not available to test for a possible dose–response relationship. Fourth, ED/hospital encounter data relied on continued follow-up within our health system, resulting in earlier censoring and shorter median follow-up duration in AF/CHF encounter analysis. This may impact statistical power to detect clinically significant obesity-statin interactions for these outcomes. Finally, we did not analyze inflammatory markers such as hs-CRP; understanding that statin therapy was associated with decreased AF incidence in high-CRP subsets of the JUPITER trial [19], these markers may be helpful in studying the obesity-statin relationship. However, in a retrospective cohort design such as ours, a significant selection bias may result from the clinical indications prompting hs-CRP assessment. A randomized trial testing statin use in individuals with AF based on hs-CRP may provide the best insight into this relationship.
5. Conclusions
In a retrospective cohort of individuals with AF, baseline statin use at time of AF diagnosis interacted with the relationship between BMI and risk of mortality, with improved mortality observed in overweight non-statin users compared to overweight statin users. However, statin use did not differentially influence AF- or CHF- related ED or inpatient events. These findings do not support the role of statins in preventing AF-related outcomes in obese individuals. Further studies in subgroups such as those with elevated inflammatory markers, and ascertainment of AF-specific intermediate measures such as symptom or arrhythmia burden, could help clarify the statin-obesity relationship, and personalize treatment for select patient groups.
Funding/Grant Support.
This work was supported by the National Institutes of Health (NIH) T32 HL139439 and American Heart Association 23POST1019044 (MCH), NIH R01 HL148444, NIH R01 HL138737, and Veterans Affairs (VA) Merit I01 BX004268 (DD), NIH R01 HL151508 and VA Merit I01 BX004918 (MDM). The UIC Center for Clinical and Translational Science is supported by NIH UL1 TR002003. (National Institutes of Health: 9000 Rockville Pike, Bethesda, Maryland 20892; American Heart Association: 7272 Greenville Avenue, Dallas, TX 75231; VA Office of Research & Development: 810 Vermont Avenue, NW Washington DC 20420).
CRediT authorship contribution statement
Michael C. Hill: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis. Noah Kim: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. William Galanter: Writing – review & editing, Writing – original draft, Project administration, Investigation, Formal analysis, Data curation. Ben S. Gerber: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Colin C. Hubbard: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Dawood Darbar: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Mark D. McCauley: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101450.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Colilla S., Crow A., Petkun W., Singer D.E., Simon T., Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Naccarelli G.V., Varker H., Lin J., Schulman K.L. Increasing prevalence of atrial fibrillation and flutter in the United States. Am. J. Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 3.E.J. Benjamin, P. Muntner, A. Alonso, M.S. Bittencourt, C.W. Callaway, A.P. Carson, A.M. Chamberlain, A.R. Chang, S. Cheng, S.R. Das, F.N. Delling, L. Djousse, M.S.V. Elkind, J.F. Ferguson, M. Fornage, L.C. Jordan, S.S. Khan, B.M. Kissela, K.L. Knutson, T.W. Kwan, D.T. Lackland, T.T. Lewis, J.H. Lichtman, C.T. Longenecker, M.S. Loop, P.L. Lutsey, S.S. Martin, K. Matsushita, A.E. Moran, M.E. Mussolino, M. O’Flaherty, A. Pandey, A.M. Perak, W.D. Rosamond, G.A. Roth, U.K.A. Sampson, G.M. Satou, E.B. Schroeder, S.H. Shah, N.L. Spartano, A. Stokes, D.L. Tirschwell, C.W. Tsao, M.P. Turakhia, L.B. VanWagner, J.T. Wilkins, S.S. Wong, S.S. Virani, E. American Heart Association Council on, C. Prevention Statistics, S. Stroke Statistics. (2019) Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association, Circulation 139 e56–e528. doi.org/10.1161/CIR.0000000000000659. [DOI] [PubMed]
- 4.Hatem S.N., Redheuil A., Gandjbakhch E. Cardiac adipose tissue and atrial fibrillation: the perils of adiposity. Cardiovasc Res. 2016;109:502–509. doi: 10.1093/cvr/cvw001. [DOI] [PubMed] [Google Scholar]
- 5.Packer M. Characterization pathogenesis, and clinical implications of inflammation-related atrial myopathy as an important cause of atrial fibrillation. J. Am. Heart Assoc. 2020;9:e015343. doi: 10.1161/JAHA.119.015343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thanassoulis G., Massaro J.M., O’Donnell C.J., Hoffmann U., Levy D., Ellinor P.T., Wang T.J., Schnabel R.B., Vasan R.S., Fox C.S., Benjamin E.J. Pericardial fat is associated with prevalent atrial fibrillation: the framingham heart study. Circ. Arrhythm Electrophysiol. 2010;3:345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCauley M.D., Hong L., Sridhar A., Menon A., Perike S., Zhang M., da Silva I.B., Yan J., Bonini M.G., Ai X., Rehman J., Darbar D. Ion channel and structural remodeling in obesity-mediated atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball J., Lochen M.L., Carrington M.J., Wiley J.F., Stewart S. Impact of body mass index on mortality and hospitalisation of patients with atrial fibrillation. Eur. J. Cardiovasc Nurs. 2018;17:627–636. doi: 10.1177/1474515118772446. [DOI] [PubMed] [Google Scholar]
- 9.Chalazan B., Dickerman D., Sridhar A., Farrell M., Gayle K., Samuels D.C., Shoemaker B., Darbar D. Relation of body mass index to symptom burden in patients with atrial fibrillation. Am. J. Cardiol. 2018;122:235–241. doi: 10.1016/j.amjcard.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badheka A.O., Rathod A., Kizilbash M.A., Garg N., Mohamad T., Afonso L., Jacob S. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J. Med. 2010;123:646–651. doi: 10.1016/j.amjmed.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Cambeiro G., Cristina M., Manero R., Moises R., Roubin A.A., Emad S., Juanatey G., Ramon J. Review of obesity and atrial fibrillation: exploring the paradox. J. Atr. Fibrillation. 2015;8:1259. doi: 10.4022/jafib.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal M.A., Garg L., Shah M., Patel B., Jain N., Jain S., Kabra R., Kovesdy C., Reed G.L., Lavie C.J. Relation of obesity to outcomes of hospitalizations for atrial fibrillation. Am. J. Cardiol. 2019;123:1448–1452. doi: 10.1016/j.amjcard.2019.01.051. [DOI] [PubMed] [Google Scholar]
- 13.Liu X., Guo L., Xiao K., Zhu W., Liu M., Wan R., Hong K. The obesity paradox for outcomes in atrial fibrillation: evidence from an exposure-effect analysis of prospective studies. Obes. Rev. 2020;21 doi: 10.1111/obr.12970. [DOI] [PubMed] [Google Scholar]
- 14.Adam O., Neuberger H.R., Bohm M., Laufs U. Prevention of atrial fibrillation with 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Circulation. 2008;118:1285–1293. doi: 10.1161/CIRCULATIONAHA.107.760892. [DOI] [PubMed] [Google Scholar]
- 15.Groves D., Mihos C.G., Larrauri-Reyes M., Santana O. The use of statins in the treatment and prevention of atrial fibrillation. Cardiol Rev. 2016;24:224–229. doi: 10.1097/CRD.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 16.Oesterle A., Liao J.K. The pleiotropic effects of statins - from coronary artery disease and stroke to atrial fibrillation and ventricular tachyarrhythmia. Curr. Vasc Pharmacol. 2019;17:222–232. doi: 10.2174/1570161116666180817155058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones S.P., Teshima Y., Akao M., Marban E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2003;93:697–699. doi: 10.1161/01.RES.0000097262.21507.DF. [DOI] [PubMed] [Google Scholar]
- 18.Oraii A., Vasheghani-Farahani A., Oraii S., Roayaei P., Balali P., Masoudkabir F. Update on the efficacy of statins in primary and secondary prevention of atrial fibrillation. Rev. Port. Cardiol. 2021;40:509–518. doi: 10.1016/j.repce.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Pena J.M., MacFadyen J., Glynn R.J., Ridker P.M. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur. Heart J. 2012;33:531–537. doi: 10.1093/eurheartj/ehr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fauchier L., Clementy N., Babuty D. Statin therapy and atrial fibrillation: systematic review and updated meta-analysis of published randomized controlled trials. Curr. Opin Cardiol. 2013;28:7–18. doi: 10.1097/HCO.0b013e32835b0956. [DOI] [PubMed] [Google Scholar]
- 21.Yan P., Dong P., Li Z., Cheng J. Statin therapy decreased the recurrence frequency of atrial fibrillation after electrical cardioversion: a meta-analysis. Med. Sci. Monit. 2014;20:2753–2758. doi: 10.12659/MSM.891049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng H., Yang Y., Zhao Y., Xiao H. The effect of statins on the recurrence rate of atrial fibrillation after catheter ablation: a meta-analysis. Pacing Clin. Electrophysiol. 2018;41:1420–1427. doi: 10.1111/pace.13485. [DOI] [PubMed] [Google Scholar]
- 23.Joglar J.A., Chung M.K., Armbruster A.L., Benjamin E.J., Chyou J.Y., Cronin E.M., Deswal A., Eckhardt L.L., Goldberger Z.D., Gopinathannair R., Gorenek B., Hess P.L., Hlatky M., Hogan G., Ibeh C., Indik J.H., Kido K., Kusumoto F., Link M.S., Linta K.T., Marcus G.M., McCarthy P.M., Patel N., Patton K.K., Perez M.V., Piccini J.P., Russo A.M., Sanders P., Streur M.M., Thomas K.L., Times S., Tisdale J.E., Valente A.M., Van Wagoner D.R. ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2023;149(2024):e1–e156. doi: 10.1161/CIR.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu W., Wan R., Liu F., Hu J., Huang L., Li J., Hong K. Relation of body mass index with adverse outcomes among patients with atrial fibrillation: a meta-analysis and systematic review. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2022. https://www.R-project.org/.
- 26.Lavie C.J., Pandey A., Lau D.H., Alpert M.A., Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70:2022–2035. doi: 10.1016/j.jacc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Burden A.F., Timpson N. Ethnicity, heart failure, atrial fibrillation and diabetes: collider bias. Heart. 2019;105:814–816. doi: 10.1136/heartjnl-2018-314467. [DOI] [PubMed] [Google Scholar]
- 28.Fekri N., Hadaegh F., Ramezankhani A., Mansournia M.A. The protective effect of obesity on mortality among those with (or without) CVD cannot be fully explained by collider-stratification bias. Int. J. Obes. 2021;45:918–919. doi: 10.1038/s41366-021-00756-y. [DOI] [PubMed] [Google Scholar]
- 29.Horita N., Kato S., Utsunomiya D. Collider bias and the obesity paradox. Nutr. Rev. 2023;81:231–232. doi: 10.1093/nutrit/nuac077. [DOI] [PubMed] [Google Scholar]
- 30.Pastori D., Baratta F., Di Rocco A., Farcomeni A., Del Ben M., Angelico F., Violi F., Pignatelli P., Lip G.Y.H. Statin use and mortality in atrial fibrillation: a systematic review and meta-analysis of 100,287 patients. Pharmacol. Res. 2021;165 doi: 10.1016/j.phrs.2021.105418. [DOI] [PubMed] [Google Scholar]
- 31.Huang J., Chan Y., Tse Y., Yu S., Li H., Chen C., Zhao C., Liu M., Wu M., Ren Q., Leung K., Hung D., Li X., Tse H., Lip G.Y.H., Yiu K. Statin therapy is associated with a lower risk of heart failure in patients with atrial fibrillation: a population-based study. J. Am. Heart Assoc. 2023;12 doi: 10.1161/JAHA.123.032378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negi S., Shukrullah I., Veledar E., Bloom H.L., Jones D.P., Dudley S.C. Statin therapy for the prevention of atrial fibrillation trial (SToP AF trial) J Cardiovasc Electrophysiol. 2011;22:414–419. doi: 10.1111/j.1540-8167.2010.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Won K.-B., Hur S.-H., Nam C.-W., Ann S.H., Park G.-M., Lee S.-G., Kim H.-E., Cho Y.-K., Yoon H.-J., Park H.-S., Kim H., Han S., Jeong M.-H., Ahn Y.-K., Rha S.-W., Kim C.-J., Cho M.-C., Kim H.-S., Chae S.-C., Kim K.-S., Kim Y.-J., Kim K.-B., Barter P. Evaluation of the impact of statin therapy on the obesity paradox in patients with acute myocardial infarction: a propensity score matching analysis from the Korea Acute myocardial infarction registry. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000007180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nylén E.S., Faselis C., Kheirbek R., Myers J., Panagiotakos D., Kokkinos P. Statins modulate the mortality risk associated with obesity and cardiorespiratory fitness in diabetics. J. Clin. Endocrinol. Metab. 2013;98:3394–3401. doi: 10.1210/jc.2013-1431. [DOI] [PubMed] [Google Scholar]
- 35.Cozzolino F., Montedori A., Abraha I., Eusebi P., Grisci C., Heymann A.J., Lombardo G., Mengoni A., Orso M., Ambrosio G. A diagnostic accuracy study validating cardiovascular ICD-9-CM codes in healthcare administrative databases. the umbria data-value project. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.