Summary
Ascaridia species are the most common nematodes infecting pigeons. The current study investigated specific identity of nematode parasites collected from domestic pigeons (Columba livia domestica) in Al-Qassim Region, Saudi Arabia. Out of 354 pigeons, 13.3 % were infected with nematode parasites. The morphological structure and genetic relationship of nematode worms were studied using conventional methods (Light and scanning electron microscopes) coupled with the newly introduced molecular method. Microscopical and ultrastructure observations showed that the present nematode worms belong to the genus Ascaridia and have all the characteristic features of Ascaridia columbae. Moreover, Random Amplifier morphometric (RAPD) PCR analysis revealed that the present A. columbae had a close identity of up to 98.3 % to Ascaridia columbae JX624729 for Cox-1 gene regions, and up to 98.3 % to Ascaridia nymphii LC057210, and Ascaridia galli EF180058 for ITS1-5.8s- ITS2 rDNA gene regions. Phylogenetic analysis supported the placement of this Ascaridia species within Ascaridiidae family with close relationships to other nematode species obtained from GenBank. Finally, our study recommends using molecular analysis in helminths identification as the main methodology for correct identification especially in closely related species.
Keywords: Phylogenetic, Ascaridia columbae, Cox-1, ITS1-5.8s- ITS2 rDNA
Introduction
Pigeons (Columba livia domestica) are one of bird species found in ancient times, which has evolved to dwell in both rural and urban locations even in Saudi Arabia (Natala et al., 2009). Numerous harmful and potentially fatal blood parasites and helminth parasites are thought to infect pigeons and induce disease in them, (Gilik & Arslan, 2011; Adang et al., 2008). According to Cheng (1973) and Adang et al. (2008). Helminth infections result in both major harm and financial losses to infected pigeons. They can enter the body through the mouth, skin, or respiratory system, among other routes (Assafa et al., 2006). Since the intestine is the best habitat for them, most of them choose to reside there (Matthews, 2001). They receive nourishment and secure shelter from the intestine (Matthews, 2001). Nematodes are thought to be the most significant and widely distributed group of helminth parasites that affect birds. The primary nematode genera which is most common in pigeons are Ascaridia, Heterakis, Syngamus, and Capillaria (Matur & Dawam, 2010). Ascaridia galli and Ascaridia columbae also infect pigeons (Abdel Rahman et al., 2019). However, A. columbae was frequently discovered in pigeons’ digestive systems (Tadelle & Ogle, 2001). According to Caira et al. (2014), the morphological criteria of Ascaridia species revealed a wide range of variability both within and across species, making it challenging to identify by morphology. Many molecular methods such as Random Amplifier morphometric (RAPD) PCR, and Restriction Fragment Length Polymorphism (RELF), clarified knowledge on the genus Ascaridia (Penner et al., 1993). This investigation aims to determine the morphological and genetic relationships of A. columbae infecting the domestic pigeon (C. L. domestica) in the Al-Qassim region of Saudi Arabia.
Materials and Methods
Sample collection
A total of 354 pigeons (Columba livia domestica) were collected from bird markets in Al-Qassim region, located (between 25° 48′ 22.68″ N, 42° 52′ 23.52 E) roughly 400 kilometers (250 miles) northwest of the capital, Riyadh, Saudi Arabia during the period from January to December (2021) and transferred to the health and scientific colleges research center, Majmaah University. Pigeons were humanely anesthetized with an isoflurane-soaked cotton pad and then dissected ethically according to the procedure outlined by (Al-Hussaini & Demian, 1982). All birds handling followed the Institutional Animal Ethics Committee guidelines at the Department of Biological Sciences at King Abdulaziz University. Nematode parasites were collected from infected pigeons and washed several times in saline solution to remove mucous and other host debris.
Preparation of permanent slides of Nemathelminths
After being fixed in 70 % ethanol, the recovered nematodes were cleared using lactophenol and identified using the keys of Soulsby (1982), Ruff (1984); Khalil et al. (2014). Nematode size determines how long cleaning takes.
The Scanning electron microscope
Some nematode worms were fixed with 3 % buffered glutaraldehyde in phosphate buffer (PH 7.2). Then samples were dried to CO2 critical point, and gold coated for 60 seconds using an Auto Fine Coater (JFC-1600) after the samples were then examined with an FEI Quanta FEG 450 Scanning Electron Microscope at 20 KV at King Abdulaziz University.
Molecular analysis
Before being processed, little portions from each individual worm were soaked in sterile distilled water five times. Using a DNeasy tissue kit© (Qiagen, Hilden, Germany) and the manufacturer’s instructions, genomic DNA (g DNA) was extracted. Thermo Fischer Scientific, Inc., Wilmington, DE, USA, provided the NanoDrop ND-1000 spectrophotometer, which was used to evaluate the concentration and purity of each DNA sample. Two pairs of unique primers were used in a specialized PCR technique to amplify the rDNA internal transcribed spacer (ITS1-5.8s-ITS2 rDNA) and the Cytochrome oxidase-1 (Cox1) genes (Table 1). Using the Gene JETTM PCR Purification Kit [Thermo (Fermentas)], both gene areas were amplified in a total volume of 50 μl, comprising 5 μl of 10 × buffer, 5 μl of each dNTP (10 mM), and 10 μl of each primer (1 pmol/μl), 0.3 μl of Taq polymerase (5 U/ml), 2.5 μl MgCl2 (50 mM), and 2 μl of total genomic DNA. A traditional PCR thermocycler technique was used to perform the PCR, as shown in Tables 2 and 3 for the Cox1 and ITS1-5.8s-ITS2 rDNA genes, respectively. 1.5 % agarose gel electrophoresis in 1X TAE buffer (100 mM Tris-HCl, glacial acetic acid, and 20 mM EDTA) was used to resolve reaction products (10μl). Ethidium bromide was used to stain the gel, and a digital camera was used to take pictures of it under a UV lamp. Sanger sequencing was carried out using the BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies, USA) on a 310 Automated DNA Sequencer (Applied Biosystems, Foster City, CA). The analysis of the sequences was done with Geneious Prime® 2022.1.1. For every sequence, a BLAST search was run to identify related sequences. Using the CLUSTAL-W algorithm (Thompson et al., 1994), multiple sequence alignments were produced with a penalty of 10 for opening gaps and 1 for extending them. The Tamura_Nei model, 10,000 repetitions, and the Neighbor-Joining method (Saitou & Nei, 1987) were used to generate the phylogenetic tree.
Table 1.
List of primers used for PCR amplification of Ascaridia columbae.
| Gene ID | Direction | Sequencing | References |
|---|---|---|---|
| Cox-1 | Cox1-F | 5′-TGGTGGTTTAAGTGTTTGACTG-3′ | Hamzah. et al., 2020 |
| Cox1-R | 5′-CCAACAACAAAGGCAACATT-3′ | ||
| ITS1-5.8s-ITS2 rDNA region | Physa-F | 5′-GCGAAC GGC TCA TTA TAA CA-3′ | Al Quraishy et al., 2020 |
| Physa-R | 5′-AAT TTCACC TCT CAC GCA-3′ |
Cox-1; Cytochrome oxidase-1, ITS; rDNA internal transcribed spacer (ITS1-5.8s-ITS2 rDNA)
Table 2.
Optimized cycling condition of Cox -1 gene.
| PCR program of Cox -1 | ||||||
|---|---|---|---|---|---|---|
| Hold | 1 Cycles | 35 Cycles | 1 Cycles | Cycles | ||
| Final Extention | Extention | Anneling | Denaturation | Initial Denaturation | STEP | |
| 4 C | 72 C | 72 C | 52 C | 95 C | 95 C | TEMP |
| ∞ | 7 min | 45 sec | 1 min | 30 sec | 3 min | TIME |
Table 3.
Optimized cycling condition of ITS1-5.8s-ITS2 rDNA gene.
| PCR program of ITS1-5.8s-ITS2 rDNA | ||||||
|---|---|---|---|---|---|---|
| Hold | 1 Cycles | 35 Cycles | 1 Cycles | Cycles | ||
| Final Extention | Extention | Anneling | Denaturation | Initial Denaturation | STEP | |
| 4 C | 72 C | 72 C | 54 C | 95 C | 95 C | TEMP |
| ∞ | 7 min | 1 min | 1 min | 30 sec | 3 min | TIME |
Statistical analysis
The Pearson Chi-Square test was used to compare all the qualitative variables between infected and uninfected. Statistical significance was set at “P” <0.05 (two-tailed). The Statistical Package for Social Sciences Version 25 (SPSS) was used.
Ethical Approval and/or Informed Consent
All procedures in the present study were conducted and authorized according to the King Abdulaziz University animal ethics committee (protocol no. 327-19).
Results
The nematode infection in C.L. domestica was 47 (13.3 %) of the 354 samples examined. The incidence of nematode infection varied considerably between seasons (p=0.004). Of the 67 birds examined, none of the pigeons had nematode infections throughout the winter (0 %). The prevalence of nematode infection in summer was (16.7 %), spring (14.3 %), and fall (17.7 %). Male and female infection rates were 14.7 % and 11.6 %, respectively, among the two genders. All four cities had the same infection rate: Unaizah (17.2 %), Buraydah (11.1 %), Ar-Rass (9.1 %), and Al-Bukairiyah (15.2 %). The prevalence rate of nematode infection was 13. 3 %. The body of the present nematode species of both male and female appeared cylindrical and creamy white-colored. Males ranged from (20 ± 35 mm) in length and (0.5 ± 0.9 mm) in width, while females ranged from (25 ± 45 mm) in length and (1.2 ± 1.8 mm) in width. The mouth is surrounded by three globular, trilobed, and equal lips. The esophagus is cylindrical without a posterior bulb and slightly extended towards the posterior end (Fig.1 A&B). The posterior end of male worms showed two strong and equal spicules protruded out from the cloacal opening and measures about (1.1 – 1.4 mm) long. Pre-cloacal sucker is located a short distance before the cloacal opening and measures about (0.18 – 0.20 mm) in diameter. It is circular to oval in shape with a strong chitinous-rimmed wall. There are thirteen pairs of caudal papillae, including (8 pairs postcloacal and 5 pairs precloacal) (Fig. 1 C). The female worms have a long, pointed tail with an anus (Fig. 1 D). The middle part of female shows a uterus filled with eggs (Fig. 1 E). The egg is oval to circular in shape and enveloped by a thick and smooth shell measured about (58 × 30μ) (Fig. 1 F). The scanning electron microscope (SEM) of the anterior end of nematode worms showed three large trilobed lips, and the inner surface of each lip carried two triangular teeth (spoon-like). Behind each lip, two cervical papillae and amphidial pores were also seen. Two cephalic alae extending from the anterior end on both lateral sides of the body surface were also observed. The cuticular surface is wrapped with faint transverse striations and lacks any cuticular vesicles (Fig. 2).
Fig. 1.
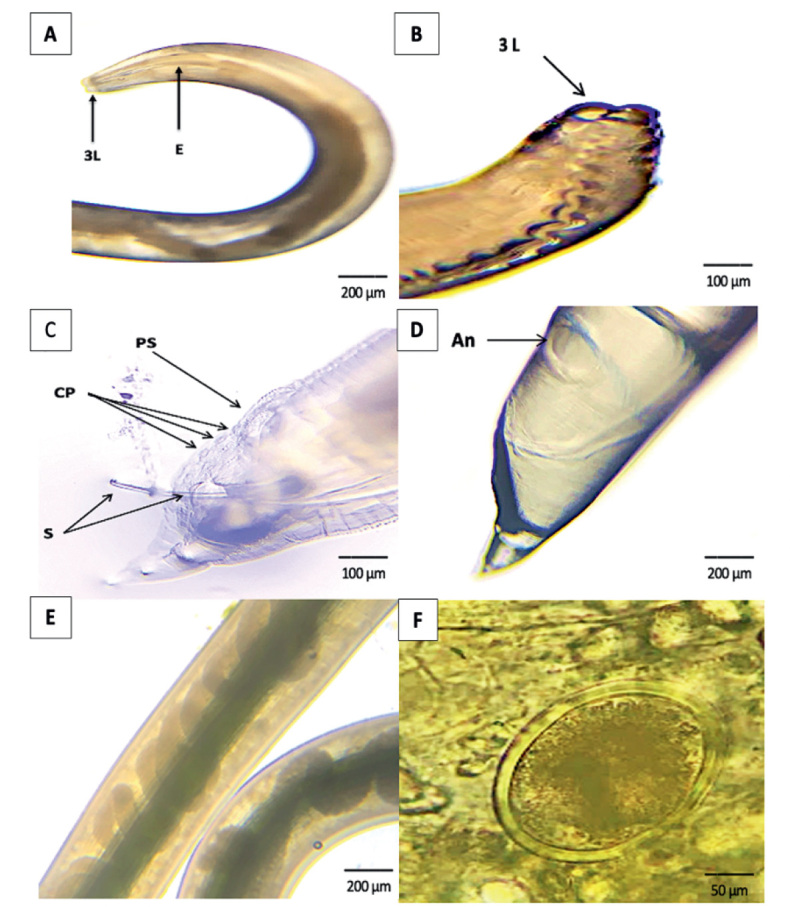
(A & B) The anterior end of A. columbae showing a mouth with three trilobed lips (L) and a cylindrical esophagus (E) (Lactophenol) (Scale bar=200 µm) (Scale bar=100 µm).(C) The posterior end of A. columbae male showing 2 spicules (S), precloacal suker (PS), and caudal papillae (CP) (Lactophenol) (Scale bar=100 µm). (D) The posterior end of A. columbae female with anus (An) (Lactophenol) (Scale bar=200 µm). (E) The middle part of A. columbae female showing the uterus with eggs (Lactophenol) (Scale bar=200 µm). (F) The egg of A. columbae in feces of infected pigeon without stain (Scale bar=50 µm)
Fig. 2.
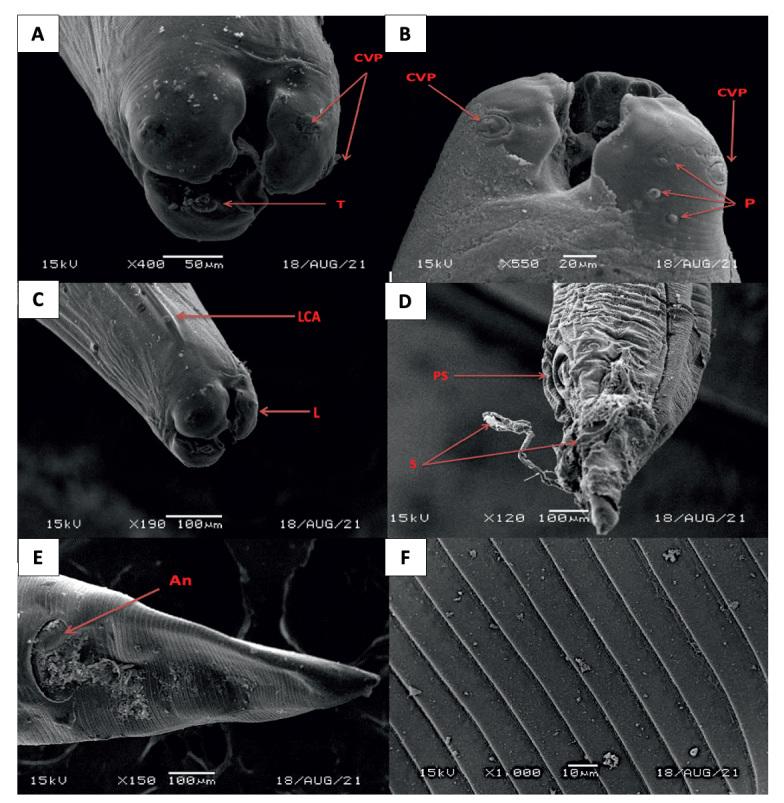
SEM micrographs of A. columbae; (A). The anterior end showing three lips with teeth (T) and two cervical papillae behind each lip (CVP). (B). The anterior end showing cervical papillae (CVP) and amphidial pores (P). (C). The anterior end showing three lips (L) and lateral cephalic alae (LCA). (D). The posterior end of the male showing precloacal sucker (PS) and two spicules (S). (E). The posterior end of the female showing anus (An). (F). The body surface is ornamented with cuticular transverse striations.
PCR amplification, Sequencing, and phylogenetic relationships of Ascaridia columbae based on Cox-1 gene
The gel electrophoresis revealed that the molecular size of the PCR product for samples number (1, 2, 3, and 4) of the Cox-1 gene was (240 bp) (Fig. 3), with Guanine-Citosine (GC) content of 34.1 – 35.6 % and pairwise identity of 99.1 %. The BLAST search showed that the four samples had similarities to Ascaridia columbae (accession number JX624729) hosted in pigeons from China, with 98.3 % pairwise identity and 99.4 % coverage. The multiple sequence alignment of the four sequences of the Cox-1 gene with (JX624729) is shown in (Fig. 5). The four sequences of the Cox-1 gene from the current study were deposited successfully in GenBank under accession numbers (ON844188, ON844189, ON844190, and ON844191) for the first time from Saudi Arabia. Using related sequences that are accessible on GenBank, a phylogenetic tree was built using the data sequence from this study. The phylogenetic tree of the four-sequence of the Cox-1 gene sequences in this study with other species retrieved from GenBank is shown in (Fig. 6), and Thelazia callipaeda (accession number AB538282) was used as an outgroup. The tree showed the present Ascaridia species are also related to Ascaris suum (accession number HQ704901) and Ascaris ovis (accession number KU522453) from China. The Ascaridia group was monophyletic with strong nodal support for grouping.
Fig. 3.
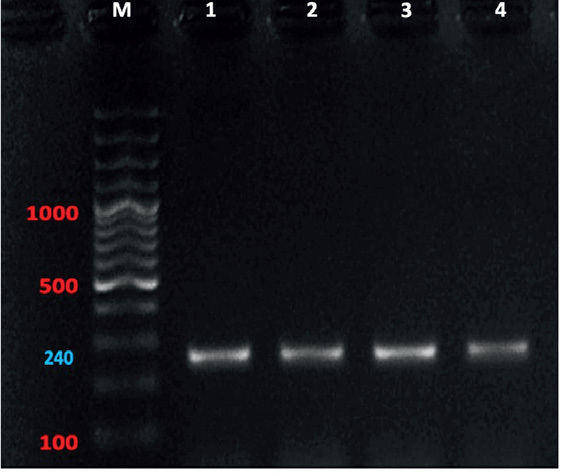
Gel electrophoresis of PCR products of Cox-1 gene for Ascaridia columbae (n=4) on 1.5 agarose gel. The molecular size of the ladder is 1500 bp (M)
Fig. 5.
Multiple sequence alignment of the four sequences of the Cox-1 gene in this study aligned with Ascaridia columbae (JX624729)
Fig. 6.
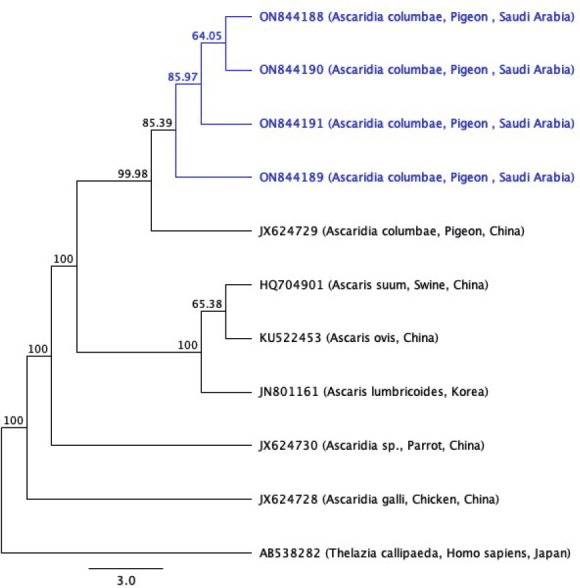
Maximum likelihood tree of the four sequences of the Cox-1 gene in the current study with other species downloaded from GenBank.
PCR amplification, Sequencing, and phylogenetic relationships of Ascaridia columbae based on ITS1-5.8s- ITS2 rDNA gene
The gel electrophoresis revealed that the molecular size of the PCR product for samples number (5, 6, 7, and 8) of the ITS1-5.8s-ITS2 rDNA gene was (800 – 810 bp) (Fig. 4), with GC content of 45.8 – 46.2 % and pairwise identity of 97.8 %. The BLAST search showed that the samples have similarity to Ascaridia nymphii (accession number LC057210) from Japan, with 98.3 % pairwise identity and 100 % coverage. The results also showed similarity to Ascaridia galli (accession number EF180058) from California with 98.3 % pairwise identity and 100 % coverage. For sample 8, no significant similarity was found. The multiple sequence alignment of the three sequences with (LC057210) and (EF180058), respectively are shown in (Figs. 7 and 8). The three-sequences of the ITS1-5.8s- ITS2 rDNA gene from the current study were deposited successfully in GenBank under accession numbers (ON855017, ON855018, and ON855019). Using similar sequences that are accessible on GenBank, a phylogenetic tree was constructed using the data sequence from this study. The phylogenetic tree of the three ITS1-5.8s- ITS2 rDNA sequences in this study with other species retrieved from GenBank is shown in (Fig. 9), and Thelazia callipaeda (accession number AB538282) was used as an outgroup. The tree showed the present Ascaridia species are also related to Ascaridia galli (accession number OK501308) from Israel and a close association between the present A. columbae and Heterakis dispar (accession number MG 763171) from Poland. The Ascaridia group was monophyletic with moderate nodal support for grouping.
Fig. 4.
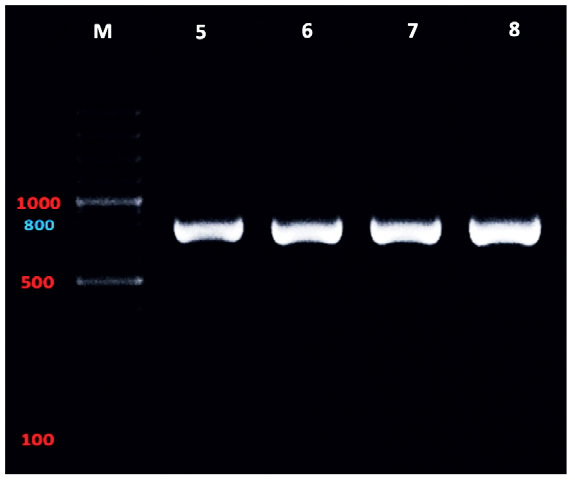
Gel electrophoresis of PCR products of ITS1-5.8s- ITS2 rDNA gene for Ascaridia columbae (n=4) on 1.5 agarose gel. The molecular size of the ladder is 1500 bp (M)
Fig. 7.
Multiple sequence alignment of the three sequences of the ITS1-5.8s- ITS2 rDNA gene in this study aligned with Ascaridia nymphii (LC057210)
Fig. 8.
Multiple sequence alignment of the three sequences of the ITS1-5.8s- ITS2 rDNA gene in this study aligned with Ascaridia galli (EF180058)
Fig. 9.
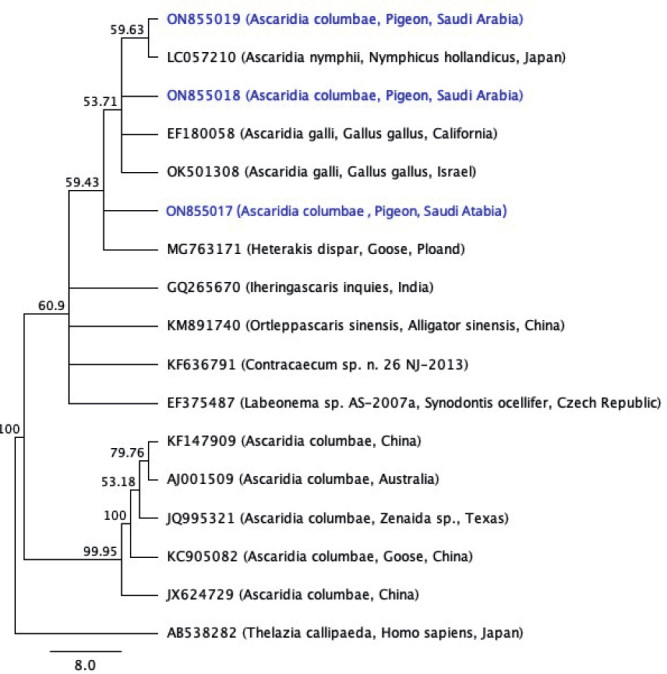
Maximum likelihood tree of the three sequences of the ITS1-5.8s- ITS2 rDNA gene in the current study with other species downloaded from GenBank.
Discussion
According to Lichtenfels et al. (1997), helminth parasites are typically identified through the examination of their morphological features, pathogenic potential, or modes of transmission. But frequently, these standards fall short of allowing for precise identification (Chilton, 1999). The variation in infection rates of helminth species associated with habitat alteration explain such changes in relation to parasite, host, and environmental features (Carrera-Játiva & Acosta-Jamett, 2023) Molecular diagnostics has emerged as the most sensitive and reliable identifying method. According to Ibrahim et al. (2018a); Safi-Eldin et al. 2019; Al Quraishy et al. (2020), it improves knowledge and data for recognition, identification, and phylogenetic relationships among the species. The physical traits of the nematode worms in this study verified that they are all members of the Ascaridiidae family, which is subdivided into the genus Ascaridia, as proposed by Dujardin (1845) the presence of two cephalic alae on opposite sides of the body, a club-shaped esophagus without a posterior bulb, and three globular, trilobed, identically sized lips in the mouth; Two spicules, a pre-cloacal sucker with a chitinous rim, and the quantity and arrangement of caudal papillae are present in males; a pointed tail and thick-shelled eggs are present in females. These results corroborated those reported by Ibrahim et al. (2018a), Abdel Rahman et al. (2019), Salem et al. (2022), Banaja et al. (2013), and Al Quraishy et al. (2020). The Ascaridia species examined in this study were contrasted with other Ascaridia species found in various bird hosts in various parts of the world. Our findings verified that all the differentiating features and host-specific species of the present Ascaridia species were highly comparable to those of the previously identified Ascaridia columbae. The most prevalent nematode found in domestic pigeons is A. columbae, according to reports from various researchers across the globe: Bangladesh (Begum & Shaikh, 1987); Brussels (Bernard & Biesman, 1987); Spain (Martinez et al., 1989); Yugoslavia (Kulisic, 1989); Italy (Tacconi et al., 1993); Egypt (Ibrahim et al., 1995; Ibrahim et al., 2018a; Salem et al., 2022); Pakistan (Hayat et al., 1999); Tanzania (Msoffe et al., 2010); Saudi Arabia (Banaja et al., 2013; Ali et al., 2020; Al Quraishy et al., 2020). Furthermore, the present study’s description of A. columbae ultrastructure features is consistent with that provided by Banaja et al. (2013), Abdel Rahman et al. (2019), and Al Quraishy et al. (2020). Among the most variable characteristics of the male Ascaridia species is the quantity and orientation of caudal papillae. There were thirteen pairs of caudal papillae on the current A. columbae. This finding is consistent with other research from Egypt (Abdel Rahman et al., 2019) and Ibrahim et al., 2018a), which discovered 13 pairs of caudal papillae grouped as 5 precloacal and 8 postcloacal. In contrast, 10 pairs of papillae were observed in the prior Saudi Arabian study (Al Quraishy et al., 2020), which were divided into 3 pairs of pre-anal, 1 pair of ad-anal, 3 pairs of post-anal, and 3 pairs of sub-terminal caudal papillae. It also differs from A. platyceri (Mines, 1979), A. dissimilis and A. nicobarensis (Soota et al., 1971), and A. galli (Ramadan & Abou Znada, 1992) in terms of the quantity and arrangement of caudal papillae, with the latter species having 10 pairs. Furthermore, other investigations have documented differences in the number of caudal papillae in other Ascaridia species, such as A. galli (5 – 10) (Dehlawi, 2007), A. amblymoria (Von Drasche, 1883), and others, A. francolina (Von Linstow, 1899), A. cordata (Von Linstow, 1901), A. dolichocerca (Stossich, 1902), (9) in A. longecirrata (Von Linstow, 1879), A. cristata (Von Linstow, 1901), A. magnipapilla (Barus, 1966), A. compar (Barus, 1966), (12) in A. orthocerca (Stossich, 1902), A. magalhaesi (Travassos, 1913), (12 – 13) in A. sergiomeirai (Pereira, 1933), (13 – 16) in A. hermaphrodita (Travassos, 1913), 13 in A. australis (Von Linstow, 1898), and (18) in A. catheturina (Johnston, 1912). The variation seen in the quantity and location of papillae among Ascaridia species could potentially be linked to the characteristics of the insemination process within the Ascaridia genus. Furthermore, using the Cox-1 and ITS1-5.8s-ITS2 rDNA genes, we examined the sequencing and phylogenetic connections of Ascaridia columbae and other related species in this work. Saudi Arabia deposited the Cox-1 gene’s four sequences into GenBank for the first time. The Cox-1 gene’s phylogenetic tree revealed that the four Ascaridia columbae sequences under investigation were only clustered with their closely related species in GenBank, with a high bootstrap of 99.98 %. This finding was corroborated by Berry and Gascuel’s (1996) assertion that high bootstrap values near 100 % indicate uniform support, if the bootstrap value for a certain clade is close to 100 %, it means that nearly all the species of this clade have uniform characters and considered as a group.
Furthermore, the identification of the present Ascaridia species was validated by Physa-F/Physa-R primer-based PCR amplification and sequencing of the ITS1-5.8s-ITS2 rDNA gene region. Gomes et al. (2015) used a similar primer to classify and differentiate three different nematode species that infect pigeons (C. L. domestica) from Brazilian Pantanal Wetlands: Ancylostoma buckleyi, Pterigodermatites pluripectinata, and Ascaridia galli. Additionally, Al Quraishy et al. (2020) employed a similar gene region to perform molecular phylogenetic analysis to clarify the taxonomic status of Ascaridia species that infect C. L. domestica, adopting a comparable strategy for the first time in Saudi Arabia. The current A. columbae is firmly buried in the Ascaridia genus and highly linked to A. nymphii and A. galli, according to the phylogenetic tree of the ITS1-5.8s-ITS2 rDNA gene. Bootstrapping revealed moderate nodal support for grouping. In line with previous research by Kim et al. (2014) and Šnábel et al. (2014), the tree also revealed a close association between the current A. columbae and Heterakis dispar (MG 763171) from Poland. This suggests that the Ascaridia genus is a sister genus of the Heterakis genus and supports the theory that the Heterakidae and Ascaridiidae families are closely linked to the Ascaridomorpha suborder. Our research shows that the ITS1-5.8s-ITS2 rDNA gene is a highly variable area that may particularly discriminate between closely related species. It also verified that molecular identification of nematode species is a very successful method in separating morphologically similar species. The findings we obtained corroborated the statements made by Park et al. (2007) and Engelmann et al. (2009) that ITS spacers are thought to be the most variable and informative region. It can describe various closely related species. Nonetheless, every primer utilized in this research was helpful and unique to the species being examined.
In the present study, the pigeons were collected from one region. As mentioned earlier, Saudi Arabia has several areas that significantly differ in climate conditions and geographical landforms. This can substantially impact the intermediate host and the prevalence of infection as a result. A national study with pigeons collected from different regions is required to accurately assess the prevalence of infection in Saudi Arabia. Further investigations should focus on the analysis of different genes to clarify the phylogenetic relationships of Ascaridiidae
Conclusion
One extremely effective method for distinguishing physically similar species from one another has been the molecular identification of species. Consequently, the primary methodological tool for precise helminth identification is advised to be molecular methods.
The PCR primer used in the molecular approach is very unique to the Ascaridia columbae species being studied, making it more accurate in identifying hybrid and cryptic species.
Acknowledgments
We would like to acknowledge the Department of Biological Science at King Abdulaziz University, for their supporting and facilitating carried out this study.
Footnotes
Conflict of interest
The authors have indicated that they have no conflict of interest regarding the content of this article.
Contributor Information
M. A. Aldamigh, Email: ma.aldamigh@mu.edu.sa.
A. A. Alahmadi, Email: aaalahmadi1@kau.edu.sa.
I. M. Al-Turaiki, Email: ialturaiki@ksu.edu.sa.
A. H. Hassan, Email: ahhassan1@kau.edu.sa.
References
- Abdel Rahman M.M.I.A., Tolba H.M.N., Abdel-Ghany H.M.. Ultrastructure, morphological differentiation and pathological changes of Ascaridia species in pigeons. Adv Anim Vet Sci. 2019;7(2):66–72. doi: 10.17582/journal.aavs/2019/7.2.66.72. [DOI] [Google Scholar]
- Adang K.L., Oniye S.J., Ajanusi O.J., Ezealor A.U., Abdu P.A.. Gastrointstinal helminth of the domestic pigeons (Columba livia domestica Gmelin, 1789 Aves: Columbidae) in Zaria, Northern Nigeria. Sci World J. 2008;3:33–7. doi: 10.4314/swj.v3i1.51769. [DOI] [Google Scholar]
- Al-Hussaini A.H., Demian E.S. Practical Animal Biology. 11th ed. II. Dar AL-Maaref; 1982. pp. 283–299. [Google Scholar]
- Ali M., Ibrahim R., Alahmadi S., Elshazly H.. Ectoparasites and Intestinal Helminthes of Pigeons in Medina, Saudi Arabia. J Parasitol. 2020;106(6):721–729. doi: 10.1645/20-64. [DOI] [PubMed] [Google Scholar]
- Al-Quraishy S., Abdel-Gaber R., Dkhil M.A., Alzuabi K.. Morphological and molecular characteristics of the gastro-intestinal nematode parasite Ascaridia columbae infecting the domestic pigeon Columba livia domestica in Saudi Arabia. Acta Parasitol. 2020;65(1):208–224. doi: 10.2478/s11686-019-00151-8. [DOI] [PubMed] [Google Scholar]
- Assafa D., Kibru E., Nagesh S., Gebreselassie S., Deribe F., Ali J.. Ethiopia Public Health Training Initiative. Med Parasitol. 2006:139. [Google Scholar]
- Banaja A.E., Ashour A.A., Awad N.S., Al-Jody M.H., El-Tarras A.E.. Ultrastructural and genetic characterization of the two Ascaridia galli and A. columbae from birds in Taif, Saudi Arabia. Life Sci J. 2013;10(2):1794–1800. [Google Scholar]
- Barus V.. Parasitic nematodes of Birds in Czechoslovakia: Columbiformes, Piciformes, Falconiformes and Strigiformes. Folia Parasitol. 1966;13:7–27. [Google Scholar]
- Begum N.J., Shaikh H.. Prevalence of helminth parasites of pigeons (Columba livia) Bangladesh J. Vet. Med. 1987;21:89–93. [Google Scholar]
- Bernard J., Bieseman W.. Endoparasitic helminths of pigeons from the city of Brussels. Bull. Rech. Agron. Gembloux. 1987;22:81–85. [Google Scholar]
- Berry V., Gascuel O.. On the interpretation of bootstrap trees: appropriate threshold of clade selection and induced gain. Mol Biol Evol. 1996;13(7):999–1011. doi: 10.1093/molbev/13.7.999. [DOI] [Google Scholar]
- Caira J.N., Jensen K., Waeschenbach A., Olson P.D., Littlewood D.T.. Orders out of chaos-molecular phylogenetics reveals the complexity of shark and stingray tapeworm relationships. Int J Parasitol. 2014;44(1):55–73. doi: 10.1016/j.ijpara.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera-Játiva P., Acosta-Jamett G.. Influence of habitat alteration on the structure of helminth communities in small mammals: a systematic review and critical appraisal of theory and current evidence. Parasitol Res. 2023;122(5):1053–1070. doi: 10.1007/s00436-023-07804-8. [DOI] [PubMed] [Google Scholar]
- Cheng T. General parasitology. Academic Press; New York, San Francisco, and London: 1973. [Google Scholar]
- Chilton N.B.. Multilocus enzyme electrophoresis: a valuable technique for providing answers to problems in parasite systematics. Int J Parasitol. 1999;29(2):213–253. doi: 10.1016/s0020-7519(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Dehlawi M.S.. The occurance of nematodes in the intestine of local (Baladi) chicken (Gallus gallus domesticus) in Jeddah Provience-Saudi Arabia. Sci. J. King Faisal Univ. 2007;8(2):61–71. [Google Scholar]
- Dujardin F. Librairie Encyclopédique de Roret. Paris: 1845. Historie naturelle des helminths ou vers intestinaux [Natural history of helminths or intestinal worms] (In French) [Google Scholar]
- Engelmann J.C., Rahmann S., Wolf M., Schultz J., Fritzilas E., Kneitz S., Müller T.. Modelling cross-hybridization on phylogenetic DNA microarrays increases the detection power of closely related species. Mol Ecol Resour. 2009;9(1):83–93. doi: 10.1111/j.1755-0998.2008.02199.x. [DOI] [PubMed] [Google Scholar]
- Foronda P., Casanova J. C., Valladares B., Martinez E., Feliu C.. Molecular systematics of several cyclophyllid families (Cestoda) based on the analysis of 18S ribosomal DNA gene sequences. Parasitol Res. 2004;93(4):279–282. doi: 10.1007/s00436-004-1130-8. [DOI] [PubMed] [Google Scholar]
- Gomes A.P., Olifiers N., Santos M.M., Simões Rde O., Maldonado Júnior A.. New records of three species of nematodes in Cerdocyon thous from the Brazilian Pantanal wetlands. Rev Bras Parasitol Vet. 2015;24(3):324–330. doi: 10.1590/S1984-29612015061. [DOI] [PubMed] [Google Scholar]
- Gilik Y., Arslan M.O.. Blood parasites of wild Pigeon in Ankara District. Turkish J Vet Anim Sci. 2011;25:169–172. [Google Scholar]
- Hamzah D.J., Muhammed H.A., AlAli F.. Molecular identification of Ascaridia columbae in the Local Healthy Pigeon (Columba livia domestica, Gmelin, 1780) in Karbala Province. Indian J Forensic Med Toxicol. 2020;14(1):1008–1012. doi: 10.37506/ijfmt.v14i1.186. [DOI] [Google Scholar]
- Hayat C.S., Maqbool A., Hayat B., Badar N., Ayub S.. Prevalence of various endoparasites of domestic pigeons. Indian Vet Med J. 1999;29:55–56. [Google Scholar]
- Ibrahim A.I., Hassanin H.H., Aly S.E.M., Abdelaal A.A.. A study on some parasitic infections in domestic pigeons in Ismailia province. Assiut Vet Med J. 1995;38:84–88. [Google Scholar]
- Ibrahim N., Hassan E., Moawad T., Ghobashy M.. Morphological and Molecular Identification of Some Intestinal Helminthes Infesting the Domestic Pigeon (Columba livia domestica) at Ismailia, Egypt. Catrina J. 2018a;17(1):61–70. doi: 10.21608/cat.2018.14312. [DOI] [Google Scholar]
- Johnston T.H.. Notes on some Metazoa. The Proceedings of the Royal Society of Queensland’. 1912;24:63–91. [Google Scholar]
- Kim T., Kim J., Cho S., Min G.S., Park C., Carreno R.A., Nadler S.A., Park J.K.. Phylogeny of Rhigonematomorpha based on the complete mitochondrial genome of Rhigonema thysanophora (Nematoda: chromadorea) Zool. Scr. 2014;43:289–303. doi: 10.1111/zsc.12047. [DOI] [Google Scholar]
- Khalil A.I., Lashein G.H., Morsy G.H., Abd El-Mottaleb D.I.. Oxyurids of wild and laboratory rodents from Egypt. Life Sci J. 2014;11(3):94–107. [Google Scholar]
- Kulisic Z.. Parasitical infection among pigeons (Columba livia) of different ages in the area of Belgrade. Acta Vet (Beograd) 1989;39:155–162. [Google Scholar]
- Lichtenfels J.R., Hoberg E.P., Zarlenga D.S.. Systematics of gastrointestinal nematodes of domestic ruminants: advances between 1992 and 1995 and proposals for future research. Vet Parasitol. 1997;72(3-4):225–245. doi: 10.1016/s0304-4017(97)00099-x. [DOI] [PubMed] [Google Scholar]
- Martinez-Moreno F.J., Martinez-Moreno A., Becerra-Martell C., Martinez-Cruz M.S.. Parasite fauna of pigeons in Cordoba province, Spain. Rev Iberica Parasitol. 1989;49:279–281. [Google Scholar]
- Matthews B.E. An introduction to parasitology. Cambridge University Press; 2001. Getting settled; p. 81. [Google Scholar]
- Mattiucci S., Cipriani P., Webb S.C., Paoletti M., Marcer F., Bellisario B., Gibson D.I., Nascetti G.. Genetic and morphological approaches distinguish the three sibling species of the Anisakis simplex species complex, with a species designation as Anisakis berlandi n. sp. for A. simplex sp. C (Nematoda: Anisakidae) J Parasitol. 2014;100:199–214. doi: 10.1645/12-120.1. [DOI] [PubMed] [Google Scholar]
- Matur B.M., Dawam N.N., Malann Y.D.. Gastrointestinal helminth parasites of local and exotic chickens slaughtered in Gwagwalada, Abuja (FCT) NY Sci J. 2010;3(5):96–99. [Google Scholar]
- Mines J.J.. Ascaridia sprenti, a new species of nematode in Australian parrots. Int J Parasitol. 1979;9:371–379. [Google Scholar]
- Msoffe P.L.M., Muhairwa A.P., Chiwanga G.H., Kassuku A.A.. A study of ecto-and endoparasites of domestic pigeons in Morogoro Municipality, Tanzania. Afr J Agric. Res. 2010;5:264–267. [Google Scholar]
- Natala A.J., Asemadahun N.D., Okubanjo O.O., Ulayi B.M., Owolabi Y.H., Jato I.D., Yusuf K.H.. A Survey of Parasites of Domesticated Pigeon (Columba livia domestica) in Zaria, Nigeria. Int. J. Soft Comput. 2009;4(4):148–150. [Google Scholar]
- Penner G.A., Bush A., Wise R., Kim W., Domier L., Kasha K., Laroche A., Scoles G., Molnar S.J., Fedak G.. Reproducibility of random amplified polymorphic DNA (RAPD) analysis among laboratories. PCR Methods Appl. 1993;2(4):341–345. doi: 10.1101/gr.2.4.341. [DOI] [PubMed] [Google Scholar]
- Park M.H., Sim C.J., Baek J., Min G.S.. Identification of genes suitable for DNA barcoding of morphologically indistinguishable Korean Halichondriidae sponges. Mol Cells. 2007;23:220–227. [PubMed] [Google Scholar]
- Pereira C.. Novo nematoide parasito de psitacideos. Rev Med Cirurg Brasil. 1933;41:7–10. [Google Scholar]
- Ramadan H., Abou Znada N.Y.. Morphology and life history of Ascaridia galli in the domestic fowl that are raised in Jeddah. J. King Abdulaziz Univ. Sci. 1992;4:87–99. [Google Scholar]
- Ruff M. D. Hofstad M.S., Barnes H.J., Calneck B.W., Reeid W.M., Yonder H.W. , Jr. Diseases of poultry. Iowa State Univ. Press; Ames; 1984. Nematodes and acanthocephalans; pp. 614–628. (Eds) [Google Scholar]
- Safi-Eldin M.A.R.W.A., Taha H.A., Ashour A.A.. Description of cestodes infecting domesticated pigeon (columba livia domestica) in Egypt with special reference to the molecular characterization of Raillietina spp. J. Egypt. Soc. Parasitol. 2019;49(3):493–504. [Google Scholar]
- Saitou N., Nei M.. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salem H.M., Khattab M.S., Yehia N., Abd El-Hack M.E., El-Saadony M.T., Alhimaidi A.R., Attia M.M.. Morphological and molecular characterization of Ascaridia columbae in the domestic pigeon (Columba livia domestica) and the assessment of its immunological responses. Poult Sci. 2022;101(2):101596. doi: 10.1016/j.psj.2021.101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šnábel V, Utsuki D., Kato T., Sunaga F., Ooi H. K., Gambetta B., Taira K.. Molecular identification of Heterakis spumosa obtained from brown rats (Rattus norvegicus) in Japan and its infectivity in experimental mice. Parasitol Res. 2014;113:3449–3455. doi: 10.1007/s00436-014-4014-6. [DOI] [PubMed] [Google Scholar]
- Soota T.D., Srivastava C.B., Ghosh R.K.. Studies on the helminth fauna of the Great Nicobar Island. Proc. Indian Acad. Sci. 1971;73:20–22. [Google Scholar]
- Soulsby E. J. L. Helminths, arthropods, and protozoa of domesticated animals. Seventh Edition. Lea & Febiger; Philadelphia: 1982. pp. 630–637. [Google Scholar]
- Stossich M.. Sopra aleuni nematodi delin eollezione elmintologiea del Prof. Dott. Corrado Parona [Some nematodes from the helminthology collection of Prof. Dr. Corrado Parona], vol 116. Boll. Mus. di Zool., Genova. 1902;116:16. (In Italian) [Google Scholar]
- Tacconi G., Moretti A., Piergili F.D., Latini M.. Endoparasitoses of pigeons (Columba livia, Gmelin 1789): epidemiological survey in the city of Terni. Zootec. Int. 1993;4:83–85. [Google Scholar]
- Tadelle D., Ogle B.. Village poultry production systems in central high lands of Ethiopia. Trop Anim Health Prod. 2001;33:521–537. doi: 10.1023/a:1012740832558. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J.. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties, and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travassos L.. Sobre as especies brazileiras da subfamilia Heterakinae Railliet and Henry [About the Brazilian species of the subfamily Heterakinae Railliet and Henry] Mem. Inst. Oswaldo Cruz. 1913:1–33. (In Portuguese) [Google Scholar]
- Von Drasche R.. Revision der in der Nematoden-Sammlung des K. K. zoologischen Hofcabinets befindlichen Original-Exemplare Diesing’s und Molin’s [Revision of the original specimens of Diesing and Molin in the nematode collection of the K. K. zoological court cabinet] Verh. Zool.-Bot. Ges. Wien. 1883;32:117–138. (In German) [Google Scholar]
- Von Linstow O.. Helminthologische Untersuchungen. [Helminthological studies] Jahreshefte des Vereins für vaterländische Naturkunde in Württemberg. 1879;35:313–342. In. (In German) [Google Scholar]
- Von Linstow O.. Nemathelminthen gesammelt von Herrn Prof. Dr. F. Dahl in Bismarck-Archipel [Nemathelminths collected by Prof. Dr. F. Dahl in Bismarck Archipelago] Arch. Naturgesch., Berlin. 1898;63:281–291. (In German) [Google Scholar]
- Von Linstow O.. Nematoden aus der Berliner zoologischen Sammlung [Nematodes from the Berlin zoological collection] Mitt A D Zoologischen Sammlung. D Mus F Naturk Berlin. 1899;1:3–28. (In German) [Google Scholar]
- Von Linstow O.. Helminthen von den Ufern des Nyassa-Sees, ein Beitrag zur Hel minthen-Fauna von Süd-Afrika [Helminths from the shores of Lake Nyassa, a contribution to the helminth fauna of South Africa] Jenaische Ztschr F Naturw Jena. 1901;35:409–428. (In German) [Google Scholar]
- Yamaguti S. Systema Helminthum. Vol. III. The Nematodes of Vertebrates. Inter science Publishers, Inc.; New York: 1961. 1261 pp. [Google Scholar]





