HIGHLIGHTS
-
•
The study identified 7 geographic hot spots of cervical cancer incidence in Texas.
-
•
Hot spots had higher proportions of Hispanic and socioeconomically disadvantaged individuals.
-
•
Identified clusters can be targeted for human papillomavirus vaccination and screening interventions.
Keywords: Cervical cancer, cancer incidence, cervical cancer prevention, cancer hot spots, cluster analysis
Abstract
Introduction
Despite being almost entirely preventable, cervical cancer is the fourth most frequently diagnosed cancer among women worldwide. Cervical cancer incidence suggests missed opportunities for prevention. Geospatial analysis could strategically guide public health interventions. This study aimed to identify geographic clusters of cervical cancer incidence in Texas, a state with higher than national rates of cervical cancer incidence and mortality.
Methods
In this population-based cross-sectional study, the authors analyzed incident cervical cancer data among Texas women aged 30–64 years, from 2014 to 2018. The authors conducted a purely spatial Poisson-based analysis function in SaTScan to examine geographic clusters of higher-than-expected proportions of cervical cancer incidence (i.e., hot spots) and adjusted for age.
Results
A total of 5,060 women aged 30–64 years with incident cervical cancer diagnosis (mean age: 45.7 years, SD=9.6), including 1,840 (36.4%) Hispanic, 591 (11.7%) non-Hispanic Black, 2,397 (47.4%) non-Hispanic White, and 232 (4.6%) other races, were analyzed. Spatial scan analysis detected 7 significant hot spots of cervical cancer incidence. Hot spots were identified in the South Texas Plains (near Mexico border), Gulf Coast (Houston), Prairies and Lakes (North Texas), Panhandle Plains (Northwest Texas), and Piney Woods (Southeast Texas) regions of Texas. Hot spots, compared with the rest of Texas, had higher proportions of Hispanic population and individuals with socioeconomic disadvantages.
Conclusions
This study found spatial variation in cervical cancer incidence in Texas. The hot spot areas can benefit from targeted, novel, scalable, and cost-effective interventions to increase human papillomavirus vaccination and screening and early detection and treatment of precancerous cervical lesions.
INTRODUCTION
Texas is one of the geographically largest and demographically heterogeneous states in the U.S. and had one of the highest cervical cancer (CC) incidence rates nationally (9.2 cases per 100,000 vs the U.S. rate of 7.6 cases per 100,000 women) from 2012 to 2016.1 In addition, Texas had lower than the national rates of CC screening (up-to-date Papanicolaou [Pap]/HPV test, women 25–65 years) (82% in Texas vs 87% U.S.) in 20202 and up-to-date human papillomavirus (HPV) vaccination among eligible females (54.8% in Texas vs 63.8% U.S.) in 2021.3 CC is almost entirely preventable4 through vaccination against HPV and screening, early detection, and treatment of precancerous lesions.5,6 The 2020 updated American Cancer Society guidelines for CC prevention recommends a primary HPV test for women ages 25 to 65 years every 5 years as the preferred method of CC screening, an HPV/Pap co-test every 5 years, or a Pap test alone every 3 years, if primary HPV screening is not available.7
Additionally, the Centers for Disease Control and Prevention (CDC) recommends HPV vaccination of children starting at 9 years and routinely at 11 or 12 years.8 The CDC's Advisory Committee on Immunization Practices also recommends HPV catch-up vaccination for older individuals through age 26 if they are not adequately vaccinated.8 However, studies have shown that the uptake of these effective preventive measures remains suboptimal across the U.S.9 Texas has lower rates of CC screening2 and up-to-date HPV vaccination than the national average rates.10 Some studies have suggested that disparities in CC have geographic and racial–ethnic variation,11,12 but specific information on geographic clustering of the disease incidence in Texas is scarce.13
There is a need to target interventions for precision public health geographically.14 Previous studies globally have demonstrated the effectiveness of spatial scan statistics in detecting hot spots of diseases such as cancers, drug poisoning, and infectious diseases (e.g., COVID-19).13,15, 16, 17 Spatial cluster analysis could identify areas with higher-than-expected CC incidence to inform interventions to address the issue. Given the disproportionately high CC incidence in Texas and suboptimal uptake of HPV vaccination and CC screening, this study aimed to examine spatial clusters of CC incidence in Texas among women aged 30–64 years.
METHODS
Study Population
The Texas Cancer Registry (TCR) served as the source for the CC cases diagnosed in Texas from 2014 to 2018. The TCR is a population-based cancer registry that collects data on all cancers diagnosed in the state of Texas. The TCR18 is a part of the U.S. National Cancer Institute Surveillance, Epidemiology and End Results and has a case-completion rate above 95%. TCR data meet high-quality standards set by the CDC and the North American Association of Central Cancer Registries. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.19
The incident CC cases analyzed in the current study comprised women aged 30–64 years with primary CC from January 1, 2014, to December 31, 2018 (n=5,124). Primary CC was ascertained based on the following International Classification of Diseases for Oncology, 3rd Edition codes: C530, C531, C538, and C539. Cases retrieved from death certificates (n=40) or autopsy (n=0), with missing or nonvalid geographic identification (n=22), and duplicate (n=2) were excluded; the final set of analyses was performed with 5,060 cases of incident CC. The study was restricted to a subgroup of CC screening-eligible women (30–64 years) for multiple reasons:
-
1.
There have been major medical advancements in CC prevention in recent years, including the introduction of HPV vaccination, HPV-based screening for high-risk HPV subtypes (recognized carcinogens)20 and precancerous lesions, and treatments.
-
2.
Some women older than 65 years may not have had the opportunity to utilize some of these interventions for prevention compared with younger women.
-
3.
Investigating CC diagnosis among screening-eligible women may highlight missed opportunities and the need for a more aggressive approach to CC prevention in this population.
Measures
The outcome of interest was primary CC incidence, and the exposure was census tract of residence at diagnosis. For the geospatial analysis, the authors created 3 input files:21
-
1.
Case file – The authors summed the counts of all CC cases among women aged 30–64 years from 2014 to 2018 in each census tract by geocoding the TCR-provided patient's address longitude (X) and latitude (Y) at the time of diagnosis.
-
2.
Population file – At-risk population data (women aged 30–64 years) were retrieved from the 2014–2018 American Community Survey (ACS) 5-year estimates and matched to CC cases in the 5,265 census tracts in Texas.
-
3.
Coordinate file – The census tract location identification numbers, longitude (X), and latitude (Y) for CC cases and at-risk population were specified in this file. All individual-level variables were accessed from the 2014–2018 TCR data, and population-level variables were retrieved from the U.S. Census Bureau's 2014–2018 ACS 5-year estimates.22
This study was reviewed and approved by the IRBs of Baylor College of Medicine and Texas Department of State Health Services.
Statistical Analysis
First, descriptive analyses of incident CC cases were conducted. Based on the Surveillance, Epidemiology, and End Results summary stage at diagnosis, the authors categorized cases into early-stage, late-stage, and unstaged CC. Early-stage CC was defined as in situ or localized; late-stage CC comprised CC with regional or distant spread; and unstaged CC included those with missing or unknown stage. The cases were also described in terms of age at diagnosis, race and ethnicity, payer at diagnosis, and year of diagnosis.
Second, the authors performed a purely spatial cluster analysis of all primary incident CC cases reported to TCR from 2014 to 2018 using the Poisson-based model in SaTScan software version 10.1.21 The Poisson-based spatial analytic model uses a systematically moving spatial circular window to identify statistically significant clusters of higher-than-expected proportions of cases (hot spots).21 The model is based on the null hypothesis that cases are independent of each other and that disease risk is the same inside and outside the spatial scanning window. The alternative hypothesis is that the disease risk within the scanning window is different from that outside the window. The model uses indirect standardization to calculate the expected number of cases in each geographic location under the null hypothesis. The SaTScan's default is to set the maximum clusters to cover half of the population at risk (50%), but because the study's area of coverage is large (the state of Texas), using a 50% maximum cluster setting can cover a large area and lead to huge clusters. Thus, the maximum cluster size was set at 25% of the at-risk population (recommended for large areas)21 and the authors conducted a sensitivity analysis using the maximum cluster setting of 50% of the at-risk population. The results for both settings were similar, that is, the authors observed equal clusters at the same locations. The spatial analysis was conducted at the census tract level and used the log-likelihood ratio test to examine the alternate hypothesis that there were greater or lower proportions of cases in the detected clusters compared with nonclusters. The authors used 999 Monte Carlo replications to ensure the stability of the estimates and report the RRs of clusters based on a 2-sided p<0.05. An initial unadjusted analysis followed by a model that adjusted for age at diagnosis was conducted. The authors defined significant clusters as those very unlikely to occur due to chance (α=0.05).
Third, to assess demographic differences between the hot spot and non-hot spot census tracts in the state, the Wilcoxon rank-sum test was used. The test compares medians of selected features between nonparametric independent groups. Neighborhood-level variables from the 2014 to 2018 ACS 5-year estimates used in the hot versus non-hot spots comparison included median age, race and ethnicity, nativity, English language proficiency, educational attainment, employment status, median household income, and health insurance coverage.
RESULTS
The descriptive analysis results are shown in Table 1. Data from 5,060 women aged 30–64 years with primary CC incidence in Texas within the study period of 2014–2018 was included. The mean age was 45.7 years (SD=9.6), and median age was 45 years (IQR=38–54); 1,840 (36.4%) were Hispanic, 591 (11.7%) non-Hispanic Black, 2,397 (47.4%) non-Hispanic White, and 232 (4.6%) identified as other races. Of the 5,060 women, 2,208 (43.6%) were diagnosed with early-stage CC (mean age was 43.6 years [SD=9.3], and the median age was 42 years [IQR=36–51]), 2,241 (44.3%) had late-stage CC (mean age was 47.8 years [SD=9.3], and the median age was 48 years [IQR=40–55]), and 611 (12.1%) were unstaged CC (mean age was 45.6 years, [SD=9.9] and median age was 45 years [IQR=37–54]). Early-stage CC and late-stage CC differed by age, race and ethnicity, and payer at diagnosis. The late-stage group had significantly (p<0.05) higher proportions of older, racially minoritized, and uninsured women than the early-stage group (Table 1).
Table 1.
Characteristics of Women Aged 30–64 Years With Cervical Cancer in Texas, 2014–2018a by Stage of Diagnosisb
| Characteristics | All CC (n=5,060; 100%) patients, No. (%) | Early-stage CC (n=2,208; 43.6%) patients, No. (%) | Late-stage CC (n=2,241; 44.3%) patient, No. (%) | Unstaged CC (n=611; 12.1%) patient, No. (%) | p-valuec |
|---|---|---|---|---|---|
| Age, mean (SD) | 45.7 (9.6) | 43.6 (9.3) | 47.8 (9.3) | 45.6 (9.9) | <0.001 |
| Age, median (IQR) | 45 (38–54) | 42 (36–51) | 48 (40–55) | 45 (37–54) | <0.001 |
| Age, years | <0.001 | ||||
| 30–44 | 2,482 (41.1) | 1,303 (52.6) | 881 (31.7) | 298 (37.9) | |
| 45–54 | 1,443 (23.9) | 537 (21.7) | 740 (26.6) | 166 (21.1) | |
| 55–64 | 1,135 (18.8) | 368 (14.9) | 620 (22.3) | 147 (18.7) | |
| Race/ethnicity | <0.001 | ||||
| Hispanic | 1,840 (36.4) | 788 (35.7) | 851 (38.0) | 201 (32.9) | |
| NHB | 591 (11.7) | 204 (9.2) | 315 (14.1) | 72 (11.8) | |
| NHW | 2,397 (47.4) | 1,099 (49.8) | 999 (44.6) | 299 (48.9) | |
| Otherd | 232 (4.6) | 117 (5.3) | 76 (3.4) | 39 (6.4) | |
| Payer at diagnosise | <0.001 | ||||
| Private | 2,128 (42.1) | 1,176 (53.3) | 762 (34.0) | 190 (31.1) | |
| Public | 1,198 (23.7) | 417 (18.9) | 650 (29.0) | 131 (21.4) | |
| Uninsured | 1,080 (21.3) | 354 (16.0) | 598 (26.7) | 128 (21.0) | |
| Unknown | 654 (12.9) | 261 (11.8) | 231 (10.3) | 162 (26.5) | |
| Diagnosis year | |||||
| 2014 | 1,031 (20.4) | 491 (22.2) | 442 (19.7) | 98 (16.0) | 0.303 |
| 2015 | 976 (19.3) | 394 (17.8) | 428 (19.1) | 154 (25.2) | |
| 2016 | 1,027 (20.3) | 431 (19.5) | 458 (20.4) | 138 (22.6) | |
| 2017 | 980 (19.4) | 434 (19.7) | 449 (20.0) | 97 (15.9) | |
| 2018 | 1,046 (20.7) | 458 (20.7) | 464 (20.7) | 124 (20.3) |
Note: Boldface indicates statistical significance (p<0.05).
Data retrieved from incident cervical cancer data provided by Texas Cancer Registry, 2014–2018.
Cervical cancer staging definition: Early-stage (SEER summary stage 0 [in situ, intraepithelial, noninvasive] and 1 [localized]); late-stage (SEER summary stage 2–4 [regional spread] and 7 [distant spread]); unstaged (SEER summary stage 9 [missing or unknown stage]) cervical cancer at diagnosis.
p-value derived from chi-square tests comparing characteristics of ECC and LCC groups for categorical variables and t-test of difference in means for the continuous variable.
Patients of non-White and non-Black races were recoded into the Other category.
Payer at diagnosis: private, types of private insurance; public/government, includes Medicare, Medicaid, TRICARE, military, Veterans Affairs, Indian/Public Health Service; uninsured, includes not insured, self-pay; unknown, insurance not otherwise specified, insurance status unknown.
CC, incident cervical cancer; ECC, Early-stage cervical cancer; LCC, Late-stage cervical cancer; NHB, non-Hispanic Black; NHW, non-Hispanic White; SEER, Surveillance, Epidemiology, and End Results.
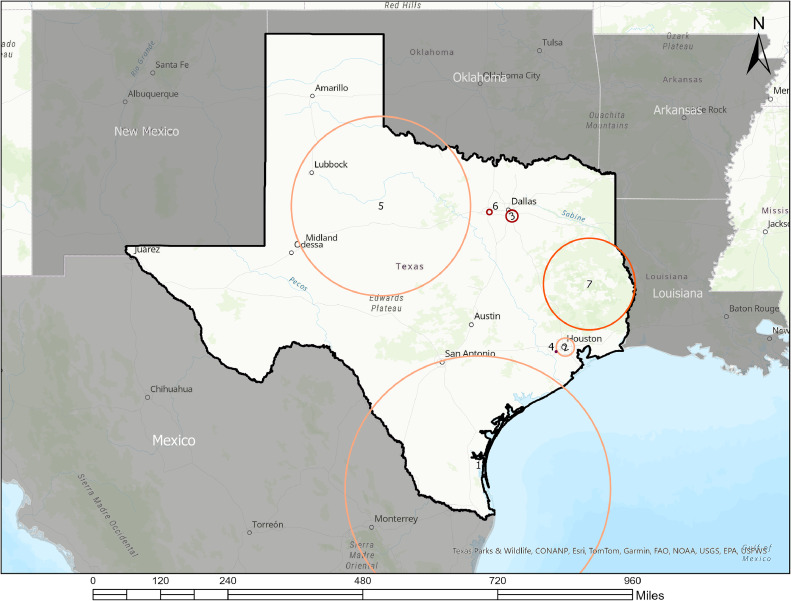
The spatial analysis detected 7 significant hot spots of CC incidence, comprising 1,755 (33.3%) of Texas's 5,265 census tracts (Table 2 and Figure 1). Hot spots were in the following Texas regions: South Texas Plains (near Mexico border), Gulf Coast (Houston), Prairies and Lakes (North Texas), Panhandle Plains (Northwest Texas), Piney Woods (Southeast Texas). Additional cluster information is provided in Appendix Table 1 and Appendix Figures 1–7. Table 3 shows the comparison of hot spot and non-hot spot demographics, suggesting statistically significant differences by age, race and ethnicity, English language proficiency, educational attainment, employment status, median household income, and health insurance coverage, but not by nativity. Specifically, a significantly higher proportion of the Hispanic population, individuals with low English proficiency, unemployed individuals, and uninsured individuals were in hot spots compared with the rest of Texas. Hot spots had lower median household income and lower proportions of non-Hispanic Black, non-Hispanic White, and individuals with some college educational attainment.
Table 2.
Age-adjusted Purely Spatial Scan Statistics Output Showing Hot Spots of Cervical Cancer Incidence in Texas, 2014–2018a
| Clusterb | Coordinate/radius | Observed cases | Expected cases | RR | LLR | p-value |
|---|---|---|---|---|---|---|
| 1 | 26.515432 N, 97.579083 W / 339.23 km | 766 | 566.1 | 1.42 | 36.27 | <0.001 |
| 2 | 29.765751 N, 95.328238 W / 22.44 km | 538 | 402.2 | 1.38 | 22.71 | <0.001 |
| 3 | 32.646998 N, 96.700372 W / 14.33 km | 132 | 145.2 | 1.81 | 19.06 | <0.001 |
| 4 | 29.664167 N, 95.565104 W / 1.98 km | 22 | 5.9 | 3.73 | 12.83 | 0.017 |
| 5 | 32.841051 N, 100.070721 W / 215.33 km | 299 | 222.4 | 1.37 | 12.50 | 0.022 |
| 6 | 32.733049 N, 97.281788 W / 6.27 km | 55 | 26.2 | 2.11 | 12.06 | 0.028 |
| 7 | 31.157482 N, 94.713113 W / 112.04 km | 164 | 110.0 | 1.51 | 11.79 | 0.036 |
| 8 | 32.758616 N, 96.966177 W / 10.86 km | 118 | 74.3 | 1.60 | 11.09 | 0.058 |
| 9 | 29.550648 N, 98.420767 W / 0 km | 6 | 0.6 | 9.60 | 8.19 | 0.576 |
| 10 | 32.938731 N, 96.795192 W / 0 km | 5 | 0.4 | 11.78 | 7.75 | 0.728 |
| 11 | 32.769046 N, 97.386474 W / 0 km | 6 | 0.8 | 7.59 | 6.95 | 0.918 |
| 12 | 32.655616 N, 97.340516 W / 0 km | 5 | 0.6 | 8.34 | 6.20 | 0.988 |
| 13 | 32.324804 N, 96.011901 W / 47.00 km | 52 | 31.3 | 1.67 | 5.74 | 0.998 |
| 14 | 31.542402 N, 97.162452 W / 1.32 km | 8 | 1.8 | 4.43 | 5.71 | 0.999 |
Note: Boldface indicates statistical significance (p<0.05).
Poisson-based purely spatial scan statistic was used to identify areas with significantly higher than expected proportion (hot spots) of primary cervical cancer incidence in Texas. Maximum spatial cluster size was set at 25% of the population at risk, using circular scan window and Replication 999.
Additional cluster information: Cluster 1 – South Texas Plains/South Texas; Cluster 2 – Gulf Coast/Upper Gulf Coast; Cluster 3 – Prairies and Lakes/North Texas; Cluster 4 – Gulf Coast/Upper Gulf Coast; Cluster 5 – Panhandle Plains/West Texas; Cluster 6 – Prairies and Lakes/North Texas; Cluster 7 – Piney Woods/East Texas; Cluster 8 – Prairies and Lakes/North Texas; Cluster 9 – South Texas Plains/South Texas; Cluster 10 – Prairies and Lakes/North Texas; Cluster 11 – Prairies and Lakes/North Texas; Cluster 12 – Prairies and Lakes/North Texas; Cluster 13 – Prairies and Lakes/North Texas; and Cluster 14 – Prairies and Lakes/North Texas; LLR, log likelihood ratio.
Figure 1.
Hot spots of cervical cancer incidence among women aged 30–64 years in Texas, 2014–2018.
Geospatial map showing clusters of cervical cancer incidence in Texas from 2014 to 2018.
Red or orange circles represent clusters with higher-than-expected proportions of cervical cancer incidence (hot spots, RR>1.00).
Greyed area did not contribute data to the cluster.
Clusters were determined using the age-adjusted Poisson-based model of the SaTScan spatial analysis. Maximum spatial cluster size was set at 25% of population at risk using circular scan window. Statistical significance was set at 0.05 and Monte Carlo replication at 999.
Table 3.
Comparing Census Tract-Level Characteristics by Cluster Classification (Texas Census Tract N=5,265)
| Census tract characteristicsa | Incident cervical cancer census tracts |
||
|---|---|---|---|
| Hot spots (n=1,755; 33.3%) median (IQR) | Rest of Texas (n=3,510; 66.7%) median (IQR) | p-valueb | |
| Demographics | |||
| Median age, female | 35.0 (30.9–40.6) | 36.9 (32.5–42.2) | <0.001 |
| % Hispanic | 52.0 (23.9–83.8) | 25.1 (14.2–45.6) | <0.001 |
| % Non-Hispanic Black | 3.5 (0.5–13.3) | 7.0 (2.3–15.6) | <0.001 |
| % Non-Hispanic White | 21.1 (5.8–58.6) | 50.8 (27.4–70.1) | <0.001 |
| % Foreign born | 14.0 (6.0–26.0) | 12.7 (7.0–22.1) | 0.053 |
| % Household with limited English proficiency, Spanish | 6.8 (1.9–17.2) | 2.5 (0.6–7.11) | <0.001 |
| Socioeconomic status | |||
| % Some college education, female ≥25 years | 45.6 (33.2–58.7) | 62.2 (48.6–76.1) | <0.001 |
| % Unemployed ≥16 years | 5.7 (3.5–8.5) | 4.7 (3.1–6.7) | <0.001 |
| Median household income ($) | 43,610 (33,208–57,230) | 60,539 (45,368–81,321) | <0.001 |
| Healthcare access | |||
| % Uninsured, female ≥18 years | 23.2 (14.6–33.3) | 15.4 (9.0–23.5) | <0.001 |
Note: Boldface indicates statistical significance (p<0.05).
Statistical significance (p<0.05, 2-sided) determined using Wilcoxon rank-sum test comparing medians of nonparametric independent groups.
Census tract-level data retrieved from the American Community Survey 5-year estimate 2014–2018 data.
p-value comparing hot spots versus rest of Texas.
DISCUSSION
Results from this study affirm geographic information systems (GIS) as an effective tool for precision public health surveillance. The data exhibited spatial variation in primary CC incidence among women aged 30–64 years in Texas. Specifically, the study found 7 hot spots of CC incidence, with significantly higher-than-expected proportions of CC cases. The largest hot spots were in the South Texas Plains (near the Mexico border) and the Texas Panhandle Plains (Northwest Texas). Other hot spots were in the Gulf Coast (Houston), Piney Woods (Southeast Texas), and Prairies and Lakes (North Texas). In alignment with other studies highlighting geographic disparities in CC in Texas,13,23 this investigation substantiates these trends at the census tract level. Compared to the 2 previous studies that investigated late-stage CC clusters in Texas,13,23 the present study examined spatial clusters of all CC diagnoses regardless of stage among a subgroup of screening-eligible women (30–64) who ideally should have had at least 1 CC screening encounter and possible exposures to HPV vaccination, or the newer and more sensitive early detection screening strategies, that is, HPV-based screening (HPV test or HPV/Pap cotest), unlike older women who may have been exposed to cytology-based screening (Pap test) only. This heightened spatial resolution allows for a more nuanced understanding of CC disparities, providing a foundation for targeted interventions at localized levels.
Moreover, some of the regions identified as hotspots in this study have also been identified in other studies as regions with poor HPV vaccination, cancer screening behaviors, and late-stage CC diagnosis.13,24,25 This alignment underscores a concerning correlation established in the broader context of cancer prevention, where populations with suboptimal uptake of preventive measures tend to experience poorer cancer outcomes.26,27 Thus, this study contributes to the growing body of evidence emphasizing the geographic dimension of CC and sheds light on the interconnectedness of prevention behaviors and health outcomes.
The regions identified as having elevated CC incidence in this study consist of disproportionately racially minoritized populations, notably Hispanic individuals. This observation highlights the pivotal role of understanding and addressing cancer health disparities within racially minoritized communities. Furthermore, the study found hot spots of CC incidence in both urban and rural Texas. Perhaps most striking are the similarities in hot spots identified in urban and rural areas of Texas, including higher than national and state poverty levels and racially minoritized populations.28 This finding contrasts with previous studies that have suggested that rural populations have disproportionately higher cancer incidence than their urban counterparts.29 Compared with urban hot spots, possible explanations for rural hot spots include availability issues, greater travel distances to preventive services, lack of insurance, and lower health literacy in rural communities.30 The availability of preventive services does not guarantee equitable access. Urban hot spots suggest that socioeconomically deprived and racially minoritized communities may have poorer access to CC prevention compared to affluent neighborhoods in urban areas. Barriers to CC prevention among urban women include costs, lack of childcare, appointment scheduling issues, and lack of insurance.31
The findings of this study underscore suboptimal CC intervention uptake in both urban and rural Texas.32 In 2021, Texas ranked 48th nationally in HPV vaccination rate,33 which may be further worsened by accentuated vaccine hesitancy during the COVID-19 pandemic. Moreover, CC screening rates in the hot spots fall below the CDC's Healthy People 2030 target of 84.3%.34 Longtime barriers to HPV vaccine uptake in Texas include parental lack of awareness, beliefs about the need for vaccination, safety concerns, and perceived low susceptibility.35 Furthermore, Texas has a higher proportion of uninsured individuals compared to the U.S. rate due to the lack of Medicaid expansion. Past studies have reported the lack of health insurance, cost, and low health literacy as hindrances to guideline-concordant CC screening in Texas.36,37 The findings indicate that hot spots tended to have higher rates of individuals without health insurance, college education, and low income, further supporting the previously reported barriers.
Cervical cancer is preventable. Hot spots of CC incidence suggest missed opportunities for prevention, including HPV vaccination and screening, and early detection and treatment of precancerous lesions in the cervix. In addition, this study underscores the documented utility of GIS in pinpointing areas requiring intervention and facilitating the development of targeted strategies. The spatial insights provided by GIS emerge as a valuable resource for precision public health planning to address and alleviate CC health disparities effectively. There is a need to develop and scale up innovative, targeted, and effective interventions to expand equitable access to CC primary prevention in rural and urban communities. The results of this study could strategically guide CC prevention to reduce the disease health disparities in affected communities.
Limitations
This study has some limitations. First, TCR does not collect data on previous cancer screenings. Thus, the authors could not assess CC screening utilization, which is critical in CC prevention. However, it is well recognized that most CCs in the U.S. occurs among underscreened women. This spatial analysis could not be conducted at a more granular area level than the census tract level because CC is relatively rare. These results may not be attributable to individuals within hot spots due to the possibility of ecological fallacy. Lastly, the current spatial analysis focused on all CC among women aged 30–64 years. This restriction may exclude potential CC clusters among women outside of this age range who were excluded from this study.
CONCLUSIONS
In this population-based study, 7 geographic clusters with higher-than-expected CC incidence in Texas from 2014 to 2018 were identified. These hot spots consisted of rural and urban census tracts. Compared with the rest of Texas, they had significantly greater proportions of Hispanic, uninsured, low-income individuals with lower rates of college education. These areas can benefit from novel, scalable, cost-effective, community-engaging interventions to ramp up HPV vaccination and screening, early detection, and treatment of precancerous lesions, particularly for affected communities.
CRediT authorship contribution statement
Itunu O. Sokale: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Visualization, Validation. Aaron P. Thrift: Funding acquisition, Investigation, Writing – review & editing. Jane R. Montealegre: Writing – review & editing. Onyema G. Chido-Amajuoyi: Writing – review & editing. Victor T. Adekanmbi: Writing – review & editing. Abiodun O. Oluyomi: Methodology, Software, Investigation, Visualization, Writing – review & editing, Supervision.
Acknowledgments
ACKNOWLEDGMENTS
Funding: This work was funded by a research training award from the Cancer Prevention & Research Institute of Texas (CPRIT) for the Systems Epidemiology of Cancer Training (SECT) Program (RP210037; PI: APT). This research was also supported by a grant from the National Institute on Minority Health and Health Disparities (NIHMD, R01MD01375, PI: JRM). This study was also supported by the facilities and resources of the Dan L Duncan Comprehensive Cancer Center P30 CA125123. AOOs effort was supported in part by the facilities and resources of the Dan L Duncan Comprehensive Cancer Center P30 CA125123 and the Gulf Coast Center for Precision and Environmental Health P30ES030285. VTA is supported by a research career development award (K12HD052023: Building Interdisciplinary Research Careers in Women's Health Program-BIRCWH) from the National Institutes of Health/Office of the Director (OD). The funders had no role in the design of the study, the collection, analyses or interpretation of the data, the writing of the manuscript or the decision to publish the results. No financial disclosures were reported by the authors of this paper.
Declaration of interest: none.
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request and with permission of the Texas Department of State Health Services.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.focus.2024.100247.
Appendix. Supplementary materials
REFERENCES
- 1.Texas Department of State Health Services. Texas Cancer Registry – Cervical Cancer in Texas Web Report. https://www.dshs.state.tx.us/tcr/data/screening/Cervical-Cancer-in-Texas.pdf. Accessed October 30, 2023.
- 2.American Cancer Society. Texas: Cancer Statistics Center. https://cancerstatisticscenter.cancer.org/#. Accessed October 30, 2023.
- 3.Texas Department of State Health Services. National immunization survey-teen (NIS-teen).https://www.dshs.texas.gov/immunization-unit/immunization-coverage-levels/national-immunization-survey/national-immunization-survey-teen-0. Updated November 16, 2021. Accessed October 30, 2023.
- 4.CDC vital signs. Cervical cancer is preventable.https://www.cdc.gov/vitalsigns/cervical-cancer/index.html#:∼:text=More%20than%204%2C000%20women%20die%20of%20cervical %20cancer%20each%20year.&text=As%20many%20as%2093%25%20of,HPV %20. Accessed October 30, 2023.
- 5.CDC National Center for Chronic Disease Prevention, Health Promotion (NCCDPHP). Power of prevention. Health and economic benefits of cervical cancer interventions. https://www.cdc.gov/cancer/cervical/statistics/index.htm#:∼:text=Each%20year%20in%20the%20United,women%20die%20of%20this%20cancer. Accessed October 30, 2023.
- 6.World Health Organization. Cervical cancer. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer. Accessed October 30, 2023.
- 7.American Cancer Society. The American Cancer Society guidelines for the prevention and early detection of cervical cancer.https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/cervical-cancer-screening-guidelines.html. Accessed October 30, 2023.
- 8.CDC vaccines and preventable diseases home. HPV vaccination recommendations. https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed October 30, 2023.
- 9.Sokale IO, Montealegre JR, Oluyomi AO, Thrift AP. Trends and racial/ethnic differences in predictors of cervical cancer screening among US women ages 30–64 years. Cancer Epidemiol Biomarkers Prev. 2023;32(1):82–90. doi: 10.1158/1055-9965.EPI-22-0970. https://DOI.ORG/10.1158/1055-9965.EPI-22-0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Texas Department of State Health Services. National immunization survey-teen (NIS-teen), Texas; 2021. Updated November 16, 2021.https://www.dshs.texas.gov/immunization-unit/immunization-coverage-levels/national-immunization-survey/national-immunization-survey-teen-0. Accessed October 30, 2023.
- 11.Cohen CM, Wentzensen N, Castle PE, et al. Racial and ethnic disparities in cervical cancer incidence, survival, and mortality by histologic subtype. J Clin Oncol. 2023;41(5):1059–1068. doi: 10.1200/JCO.22.01424. https://DOI.ORG/10.1200/JCO.22.01424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo W, Kim S, Huh WK, et al. Recent trends in racial and regional disparities in cervical cancer incidence and mortality in United States. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0172548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokale IO, Thrift AP, Montealegre J, et al. Geographic variation in late-stage cervical cancer diagnosis. JAMA Netw Open. 2023;6(11) doi: 10.1001/jamanetworkopen.2023.43152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoury MJ, Iademarco MF, Riley WT. Precision public health for the era of precision medicine. Am J Prev Med. 2016;50(3):398–401. doi: 10.1016/j.amepre.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossen LM, Khan D, Warner M. Hot spots in mortality from drug poisoning in the United States, 2007–2009. Health Place. 2014;26:14–20. doi: 10.1016/j.healthplace.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominguez RL, Cherry CB, Estevez-Ordonez D, et al. Geospatial analyses identify regional hot spots of diffuse gastric cancer in rural Central America. BMC Cancer. 2019;19(1):545. doi: 10.1186/s12885-019-5726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Cos Guerra O, Castillo Salcines V, Cantarero Prieto D. Are spatial patterns of Covid-19 changing? Spatiotemporal analysis over four waves in the region of Cantabria, Spain. Trans GIS. 2022;26(4):1981–2003. doi: 10.1111/tgis.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch . Austin. Texas Department of State Health Services; TX: 2021. Cancer in Texas 2021. [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 20.International Agency for Research on Cancer. Lyon: IARC. Agents classified by the IARC monographs. http://monographs.iarc.fr/ENG/Classification/index.php. Accessed March 28, 2024.
- 21.Kulldorff M. SaTScan User Guide for Version 10.1, 2022. http://www.satscan.org/. Accessed October 10, 2022.
- 22.U.S. Census Bureau. American Community Survey Information. 2017. https://www.census.gov/content/dam/Census/programs-surveys/acs/about/ACS_Information_Guide.pdf. Accessed November 18, 2023.
- 23.Zhan FB, Lin Y. Racial/Ethnic, socioeconomic, and geographic disparities of cervical cancer advanced-stage diagnosis in Texas. Womens Health Issues. 2014;24(5):519–527. doi: 10.1016/j.whi.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Kish JK, Rolin AI, Zou Z, et al. Prioritizing US cervical cancer prevention with results from a geospatial model. J Glob Oncol. 2016;2(5):275–283. doi: 10.1200/JGO.2015.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan FB, Liu Y, Yang M, et al. Using GIS to identify priority sites for colorectal cancer screening programs in Texas health centers. Prev Chronic Dis. 2023;20:E10. doi: 10.5888/pcd20.220205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Cancer Society. Cancer prevention and early detection continues to be suboptimal in the United States. https://pressroom.cancer.org/CPEDFF2021. Accessed October 10, 2023.
- 27.Lin Y, Zhan FB. Geographic variations of racial/ethnic disparities in cervical cancer mortality in Texas. South Med J. 2014;107(5):281–288. doi: 10.1097/SMJ.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Census Bureau. Quick facts United States. https://www.census.gov/quickfacts/US. Accessed July 5, 2023.
- 29.Zahnd WE, James AS, Jenkins WD, et al. Rural-urban differences in cancer incidence and trends in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27(11):1265–1274. doi: 10.1158/1055-9965.EPI-17-0430. https://DOI.ORG/10.1158/1055-9965.EPI-17-0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doescher MP, Jackson JE. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. J Public Health Manag Pract. 2009;15(3):200–209. doi: 10.1097/PHH.0b013e3181a117da. [DOI] [PubMed] [Google Scholar]
- 31.Hanna K, Arredondo BL, Chavez MN, et al. Cancer screening among rural and urban clinics during COVID-19: a multistate qualitative study. JCO Oncol Pract. 2022;18(6):e1045–e1055. doi: 10.1200/OP.21.00658. https://DOI.ORG/10.1200/OP.21.00658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conrey R, Valencia V, Cioletti A, Williams-Brown MY. Regional variation in human papillomavirus vaccination uptake and completion among adolescents 13–17 in the state of Texas. Vaccine. 2020;38(25):4119–4124. doi: 10.1016/j.vaccine.2020.03.059. [DOI] [PubMed] [Google Scholar]
- 33.American Cancer Society. HPV vaccination is cancer prevention in Texas. https://www.cancer.org/cancer/risk-prevention/hpv/hpv-vaccine/hpv-texas.html. Accessed November 27, 2023.
- 34.Office of Disease Prevention and Health Promotion. Healthy people 2030. Cancer: Overview and Objectives.https://health.gov/healthypeople/objectives-and-data/browse-objectives/cancer. Accessed November 27, 2023.
- 35.Javaid M, Ashrawi D, Landgren R, et al. Human papillomavirus vaccine uptake in Texas pediatric care settings: a statewide survey of healthcare professionals. J Community Health. 2017;42(1):58–65. doi: 10.1007/s10900-016-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen B, Khan H, Layeequr Rahman R. Sociodemographic determinants in cervical cancer screening among the underserved West Texas women. Womens Health Rep (New Rochelle) 2023;4(1):191–201. doi: 10.1089/whr.2022.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boom K, Lopez M, Daheri M, et al. Perspectives on cervical cancer screening and prevention: challenges faced by providers and patients along the Texas-Mexico border. Perspect Public Health. 2019;139(4):199–205. doi: 10.1177/1757913918793443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request and with permission of the Texas Department of State Health Services.