The chief medical officer has recently recommended that all children in the United Kingdom aged 6 months to 4 years should receive a single dose of Haemophilus influenzae type b vaccine. This is in response to a rise in the incidence of Hib disease over the past four years. The reasons for the increase are complex, but evidence points strongly to an effect of a temporary change to the use of a Hib vaccine combined with diphtheria, tetanus, and acellular pertussis (DTaP).
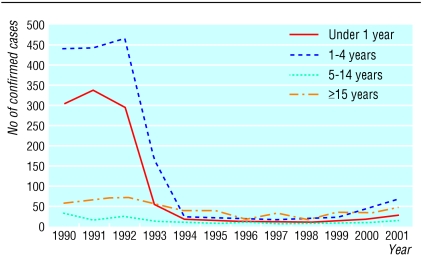
Before the Hib conjugate vaccine was introduced in 1992, Hib was the most common cause of bacterial meningitis in children. Rapid control of Hib disease was achieved by combining routine infant immunisation at 2, 3, and 4 months of age with a catch-up programme for children aged up to 4 years. Initially Hib vaccine was given as a separate injection, but in 1996 a combined DTP-Hib vaccine was introduced. Uptake of the vaccine was high, and the number of confirmed cases of type b infection fell from 855 in 1992 to 37 in 1998, but since 1999 a steady increase in cases has been seen (figure).
Figure 1.
Invasive Hib infections by age group, 1990-2001
An analysis of Hib disease over the past 10 years shows a significant decline in protection from the first two years after vaccination in infancy.1 On the basis of this observation an increase in cases might be expected in older children, but in 2002 the major increase was in vaccinated children between 1 and 2 years of age.2 The excess of younger cases in 2002 is consistent with the observation of a lower level of protection for the 2000-1 birth cohort.1 A shortage of DTP-Hib containing whole cell pertussis in late 1999 meant that another vaccine was required to ensure supply during 2000 and 2001. The vaccine used was a Hib conjugate combined with DTP containing acellular pertussis which, like the whole cell preparation, was given as a single injection. Previous studies with this and most other Hib combination vaccines containing acellular pertussis had shown Hib antibody concentrations that were lower than expected.3 Licensure was permitted because of laboratory evidence of high antibody quality and immunological memory,3 and these vaccines were being used successfully in a number of other countries. Further analysis of data from the United Kingdom has shown a significant association between receipt of vaccines containing acellular pertussis and invasive Hib disease.4 Our experience implies that, at least at the schedule used in the United Kingdom, the lower immunogenicity of such combinations is clinically relevant.
The new campaign should have a rapid effect on Hib disease, and the use of the whole cell pertussis DTP-Hib vaccine has been resumed. Global supplies of such vaccines have, however, fallen in recent years, and large pharmaceutical companies are expressing less enthusiasm for continuing production. How could the United Kingdom therefore use acellular pertussis vaccines without jeopardising the control of Hib?
Three approaches are possible. One would be to offer Hib as a separate injection. Although three injections at each visit may be acceptable as new vaccines—such as pneumococcal conjugates—come into routine use, novel combinations will be required. One such combination would be a meningitis vaccine, which includes Hib, MenC, and pneumococcal conjugates, but this will also have potential for immunological and chemical interactions, and studies adhering to the schedule used in the United Kingdom will be needed. All acellular pertussis vaccines do not behave in the same way, and there is evidence that a five component acellular pertussis vaccine that uses a different adjuvant may be less likely to have a suppressive effect in combination with Hib vaccine.5 Use of this particular DTaP-Hib combination vaccine may be a second option. Other conjugate vaccines given simultaneously but separately may also influence Hib antibody concentrations, depending on which carrier protein they use.6
The relatively small gap between vaccines in the schedule used in the United Kingdom results in a lesser, although usually adequate, immunological response compared with schedules with longer intervals. There is evidence that the effect of acellular pertussis on Hib combinations is less dramatic when more extended schedules are used.7 The third option therefore may be to review the schedule used in the United Kingdom. Examples of Hib schedules currently used in other industrialised countries include extended three dose schedules (for example, at 3, 5, and 12 months of age) or three dose accelerated schedules followed by a booster in late infancy or in the second year of life (www.phls.org.uk/inter/eu_ibis/hib19992000.pdf). Other relevant considerations include ensuring control of early pertussis and preservation of high vaccine coverage, both reasons for the initial adoption of the current schedule used in the United Kingdom.
A different approach includes the one currently being used—a catch-up campaign. In many developing countries regular mass campaigns have been effective in controlling poliomyelitis and have also been used for measles.8 Mathematical models of Hib transmission may help in deciding whether this option is feasible. Our experience emphasises the importance of continued high quality surveillance for vaccine preventable diseases even long after their apparent control. Such surveillance is increasingly critical after the introduction of new vaccines, vaccine combinations, or new formulations and will help to inform the best future strategy for the control of vaccine preventable diseases.
We wish to acknowledge the contributions of colleagues to this editorial through a recent meeting held under the auspices of the Royal College of Paediatrics and Child Health Immunisation and Infectious Disease Standing Committee.
Competing interests: PH and MR have received research grants and reimbursements for attending symposiums from vaccine manufacturers including Aventis Pasteur and Glaxo SmithKline. PH has received fees for consulting from Glaxo SmithKline.
References
- 1.Ramsay ME, McVernon J, Andrews N, Heath PT, Slack MPE. Estimating Hib vaccine effectiveness in England and Wales using the screening method. J Infect Dis (in press). [DOI] [PubMed]
- 2.Trotter CL, Ramsay ME, Slack MPE. The epidemiology of Haemophilus influenzae type b disease in England and Wales, 1990-2002. Commun Dis Public Health 2003;6: 55-8. [PubMed] [Google Scholar]
- 3.Eskola J, Ward J, Dagan R, Goldblatt D, Zepp F, Siegrist C. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 1999;354: 2063-8. [DOI] [PubMed] [Google Scholar]
- 4.McVernon J, Andrews N, Slack MPE, Ramsay ME. Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet 2003;361: 1521-3. [DOI] [PubMed] [Google Scholar]
- 5.Mills E, Gold R, Thipphawong J, Barreto L, Guasparini R, Meekison W, et al. Safety and immunogenicity of a combined five-component pertussis-diphtheria-tetanus-inactivated poliomyelitis-Haemophilus b conjugate vaccine administered to infants at two, four and six months of age. Vaccine 1998;16: 576-85. [DOI] [PubMed] [Google Scholar]
- 6.Dagan R, Eskola J, Leclerc C, Leroy O. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect Immun 1998;66: 2093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidor E, Hoffenbach A, Fletcher MA. Haemophilus influenzae type b vaccine: reconstitution of lyophilised PRP-T vaccine with a pertussis-containing paediatric combination vaccine, or a change in the primary series immunisation schedule, may modify the serum anti-PRP antibody responses. Curr Med Res Opin 2001;17: 197-209. [DOI] [PubMed] [Google Scholar]
- 8.Global measles mortality reduction and regional elimination. 2000-1. Wkly Epidemiol Rec 2002;77: 50-5. [PubMed] [Google Scholar]