Abstract
Purpose
To describe a patient with a unique retinal phenotype of probable Susac syndrome.
Observations
A 47-year-old female who presented with bilateral tinnitus and vision changes was found to have bilateral sensorineural hearing loss and many bilateral retinal arteriolar Gass plaques. She had bilateral scotomas corresponding with temporal thinning and atrophy of the inner nuclear layer (INL) on OCT. Retinal examination and fluorescein angiography demonstrated minimal arteriolar wall hyperfluorescence with no evidence of acute branch retinal artery occlusion. She developed daily headaches. MRI of the brain was normal with no corpus callosal lesions. She was diagnosed with probable Susac syndrome based on the above findings.
Conclusions and importance
Our patient’s bilateral high frequency sensorineural hearing loss, numerous bilateral Gass plaques, and headaches are most likely attributable to Susac syndrome. While BRAO is considered a cornerstone of retinal involvement in Susac syndrome, it may only be appreciable angiographically in the acute setting, and it is important to recognize Gass plaques as a significant diagnostic marker of disease.
Keywords: Susac syndrome, Gass plaque, Arteriolar wall hyperfluorescence, Branch retinal artery occlusion
1. Introduction
Susac syndrome (SS) is a rare disease characterized by the clinical triad of encephalopathy, branch retinal artery occlusion (BRAO) and sensorineural hearing loss (SNHL). Many affected individuals typically do not exhibit the complete triad at presentation, making diagnosis a challenge. The underlying pathophysiology is presumed to be due to an autoimmune endotheliopathy resulting in arteriolar infarction in the brain, retina, and inner ear.1,2 While retinal involvement is traditionally defined by evidence of BRAO, the arteriolar wall involvement may present with Gass plaques on funduscopic exam and arteriolar wall hyperfluorescence (AWH) on fluorescein angiography (FA).3,4 The Gass plaque was described by Don Gass5 in some of his earlier writings and is caused by focal injury to an arterial wall and can be transient in nature. Thus, the Gass plaque is more commonly not found at an arteriolar bifurcation. Other authors have noted that these plaques are visible on FA. Herein, we report a patient who presented with a unique retinal appearance of a profound number of bilateral Gass plaques that resolved without treatment with minimal associated AWH.
2. Case report
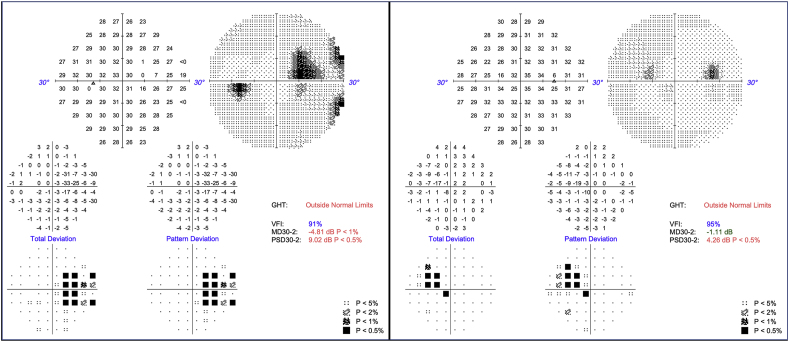
A 47-year-old female with a past medical history of osteoarthritis and neuropathic pain initially presented to her primary care physician in December of 2022 endorsing bilateral tinnitus and vision changes. She stated that the tinnitus had started about two years earlier but had progressed significantly during the prior two weeks. Her visual changes began around one month prior to presentation and were characterized by difficulty focusing, spots in her vision and speck-like floaters in both eyes. Her family history was notable for mother with breast cancer and father with colon cancer and Lewy body dementia. She was diagnosed with bilateral sensorineural hearing loss and prescribed hearing aids. Ophthalmology examination (approximately 2 months from the onset of symptoms) revealed vision of 20/20 in each eye with normal color vision. Formal visual field testing with 30-2 static perimetry revealed superonasal steps in both eyes, though more pronounced in the left than the right (Fig. 1). Ophthalmoscopic examination was notable for numerous bilateral yellow arteriolar plaques (>50 per eye) (Fig. 2). These appeared to be deposited in the vessel walls. In the right eye, there were three segments of arteriolar sheathing in the superior and inferonasal peripheries as well as two small cotton wool spots (CWS) adjacent to a flame hemorrhage nasally (Fig. 2B and C). No sheathing or CWS were seen in the left eye. There were no areas of retinal whitening, boxcarring, sclerotic vessels or collaterals in either eye. The bilateral optic nerves and maculae were healthy in appearance. There was no evidence of intraocular inflammation such as anterior chamber or vitreous cells or haze in either eye. Fluorescein angiography (FA) demonstrated arteriolar wall hyperfluorescence (AWH) corresponding to the sheathed inferonasal vessel in the right eye (Fig. 3). There was also an area of hyperfluorescence temporally in the right eye but given its far peripheral location it is unclear whether this area was pathologic. There was no hyperfluorescence corresponding to the two sheathed vessels superiorly in the right eye (Fig. 4). No areas of AWH were seen in the left eye. There were no areas of non-perfusion indicating acute vascular obstruction in either eye. Of particular note, none of the Gass plaques were visible on FA (Fig. 3, Fig. 4). OCT of the macula demonstrated mild inner retinal thinning of the temporal macula symmetrically in both eyes (Fig. 5). There was no inner retinal hyper-reflectivity to suggest edema from acute infarction. There was atrophy of the inner nuclear layer (INL) in the temporal macula bilaterally, roughly corresponding to the scotomas. OCT RNFL demonstrated normal neuro-retinal rim thickness bilaterally. GCL + IPL analysis demonstrated superotemporal thinning in the right macula but otherwise demonstrated normal thicknesses.
Fig. 1.
Visual Fields. Humphrey visual fields using a 30-2 static perimetry demonstrating nasal paracentral scotomas in both eyes, more prominent in the left.
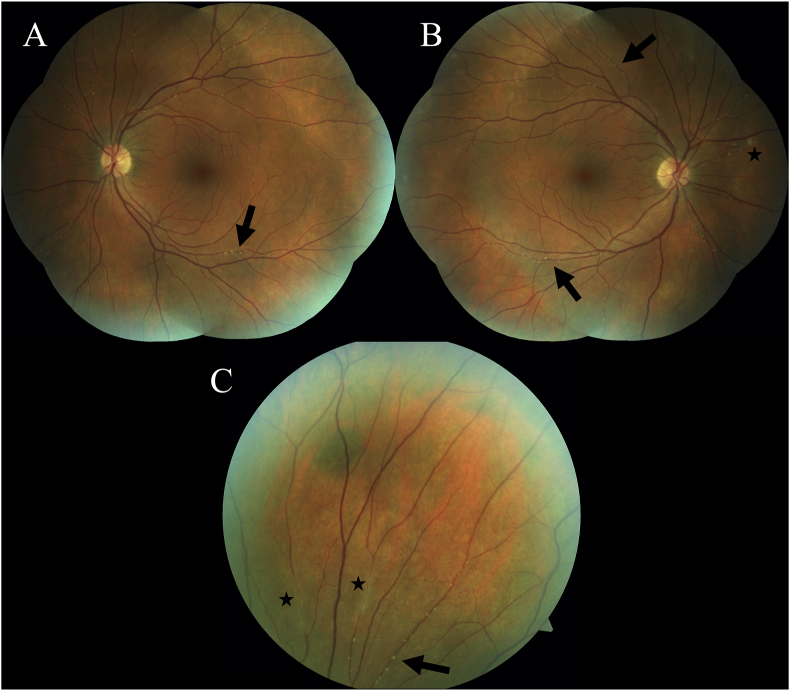
Fig. 2.
Fundus Photos. Color fundus photographs of the left (A) and right (B) eyes demonstrating numerous and diffuse Gass plaques (arrows) in all quadrants. The optic nerve heads and maculae are healthy in appearance and there is no funduscopic evidence of BRAO in either eye. The right eye shows two small cotton wool spots adjacent to a small flame hemorrhage nasal to the disc (asterisk). Panel (C) shows a magnified view of the superior periphery of the right eye demonstrating many Gass plaques along an arteriolar segment (arrow). Note that these plaques are yellow, nonrefractile and are along straight segments of vasculature, not at retinal arteriolar bifurcations. Also shown here are two areas that clinically resemble arteriolar sheathing (asterisks).
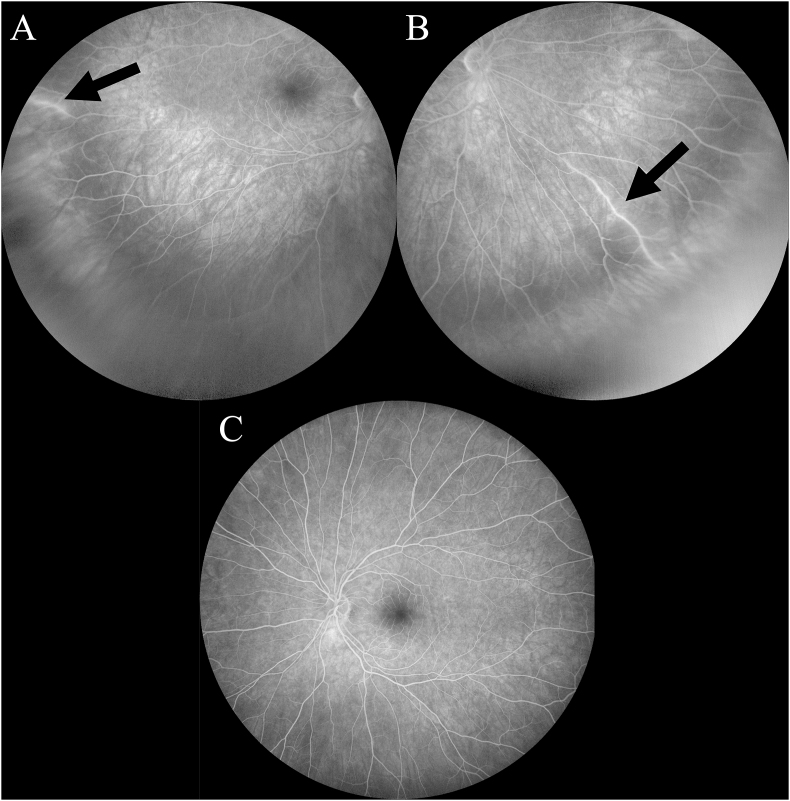
Fig. 3.
Fluorescein Angiography. Two areas of arteriolar wall hyperfluorescence (arrows) in the temporal periphery (A) and inferonasal periphery (B) of the right eye. The vessel in the temporal periphery appeared normal on funduscopic exam while the vessel in the inferonasal periphery did appear sheathed clinically. Mild vessel wall hyperfluorescence can be seen in the far temporal periphery in normal eyes, so panel (A) may or may not indicate pathologic AWH due to SS. (C) There were no such areas of AWH seen on FA of the left eye. Note that there was no evidence of acute arteriolar occlusion in either eye.
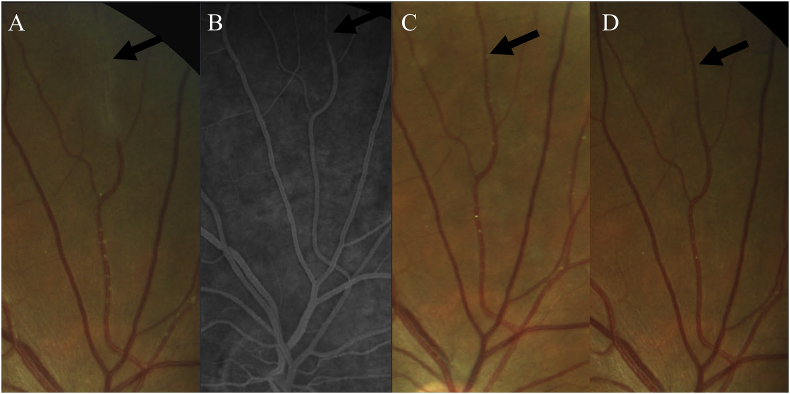
Fig. 4.
Elongated Gass Plaque. Magnified view of a superior peripheral arterial branch of the right eye which clinically resembled vascular sheathing (A), however did not stain on FA (B) and over several months of follow-up dissolved into more discrete appearing Gass plaques (C) and ultimately resolved completely. (D) Therefore, this area was likely an elongated Gass plaque representing an extended area of lipid extravasation. Gass plaques are invisible on FA.
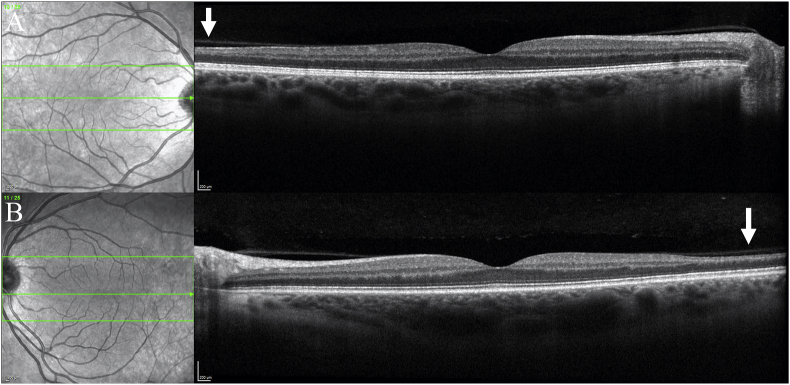
Fig. 5.
OCT Macula of the right (A) and left (B) eyes which demonstrate normal central architecture and no evidence of acute arterial obstruction, but show bilateral temporal inner retinal thinning and atrophy of INL (arrows) consistent with past occlusive events.
She underwent a comprehensive workup which included infectious/inflammatory serologies (treponema pallidum antibody, ESR, ANA, and ANCA) and a full hypercoagulability panel (Proteins C and S, Antithrombin III, Prothrombin Gene Mutation, Anticardiolipin antibodies, Lupus Anticoagulant, Beta-2 Glycoprotein 1 antibodies, Homocysteine, Factor V Leiden, D Dimer, SPEP, PTT, PT/INR) which were all unremarkable. A transthoracic echocardiogram was unrevealing. MRI/MRA of the head and neck demonstrated very few white matter lesions that looked more like ventricular capping and nonspecific and none of these lesions looked demyelinating or vascular. There were no corpus callosal lesions. Lumbar puncture demonstrated bland CSF studies with negative cytopathology and flow cytometry. She was placed on aspirin.
Her concurrent tinnitus, sensorineural hearing loss and extensive retinal arteriolar plaques, in the absence of another underlying condition, hypercoagulable predisposition or ongoing inflammation, was concerning for a diagnosis of probable Susac Syndrome.
She underwent neurological evaluation and treatment with IVIG and Rituximab was recommended with close monitoring.
A formal audiology exam was obtained which showed normal hearing sensitivity through 3k Hz with a mild sloping to moderately-severe sensorineural hearing loss from 4k-8k Hz bilaterally and no significant conductive component.
Over the course of two-three months, she developed new headaches which were frontal and bilateral in nature, occasionally associated with nausea. They were occurring daily and sometimes waking her during the night. Her tinnitus and visual symptoms were ongoing. Repeat visual field testing continued to show superonasal deficits. Her funduscopic exam two months after presentation to ophthalmology was notable for markedly reduced number of peri-arteriolar plaques in both eyes without undergoing any treatment, as she was still awaiting prior authorization to initiate the recommended IVIG and Rituximab (Figs. 4 and 6).
3. Discussion
Susac syndrome is a rare and likely underdiagnosed disease characterized by encephalopathy, BRAO and SNHL. The encephalopathy may be marked by any combination of cognitive decline, memory loss, concentration deficits, decline in executive function, confusion, emotional disturbance, behavioral changes and/or psychosis with paranoia.1,2 As of 2013, 304 cases had been reported in the literature, and of these patients, only 13 % displayed the complete triad during initial evaluation. Encephalopathy was present in 67 %, BRAO in 40 % and SNHL in 37 % of patients at initial presentation. Migraine-like headaches were present in 80 % of patients. Ultimately, 85 % of these patients did go on to exhibit the complete spectrum of disease, though the average time between symptom onset and complete triad was 21 weeks.1
Prognosis and treatment decisions are dependent on the expected clinical course which is largely determined by the symptoms manifested in the first two years of disease. If encephalopathy presents within this initial two-year period, the disease is believed to be self-limited and thus termed monocyclic although recurrences over 15 years later have been reported.1 If patients with BRAO and sensorineural hearing loss do not develop encephalopathy within the first two years of symptoms, they will most likely never develop encephalopathy, but are prone to experience recurrent BRAO and SNHL in a polycyclic fashion. Forty-two percent of patients follow this clinical course. Since our patient had essentially a normal MRI of the brain, she likely is falling into the latter category and at higher risk of retinal recurrence. Finally, a small percentage of patients (∼4 %) experience a chronic progressive and sometimes even fatal course.1 While her headaches indicate possible brain involvement, headaches are not believed to count toward classifying someone as monocyclic or encephalopathic.
According to updated diagnostic criteria by the European Susac Consortium, retinal involvement is confirmed by the presence of a BRAO.2 BRAO has traditionally been regarded as the defining clinical finding of SS and is often what distinguishes the disorder from other diseases on the differential such as acute disseminated encephalomyelitis (ADEM) and multiple sclerosis. In the aforementioned review of all cases published prior to 2013, evidence of a BRAO was seen in 99 % of patients for whom FA was available.1 Nevertheless, the acute findings of BRAO on funduscopic exam and FA may fade with time6 so angiographic evidence may be absent if a patient presents subacutely as in our case. This emphasizes the importance of other diagnostic indicators on multi-modal imaging and functional testing. Our patient demonstrated thinning of the INL in the temporal macula in both eyes on OCT, corresponding to her paracentral nasal scotomas on visual field testing, indicating past afferent retinal occlusion. This is analogous to structural findings in a prior case series of Susac patients in which a majority demonstrated inner retinal damage on OCT in the absence of findings on FA despite past angiographic evidence of BRAO.7 Therefore, while FA is a very useful tool in the acute phase, it may not be demonstrative in later or different phases of the disease.
Though our patient did not present with acute retinal arteriolar occlusion, she did have a profound number of Gass plaques in both eyes (>50 per eye) likely indicating active endotheliopathy. First described by Donald Gass in six of nine patients who at the time were diagnosed with idiopathic BRAO, Gass plaques are yellow peri-arteriolar deposits thought to be due to extravasation of blood lipid into the arteriole wall at focal sites of endothelial damage.5 As they were in this case, these lesions are frequently mistaken for retinal emboli. However, Gass plaques can be distinguished from retinal emboli based on their color, location, relation to vascular obstruction and ability to wax and wane. While platelet–fibrin emboli are usually gray/white, Hollenhorst cholesterol plaques are orange and refractile and located at retinal arteriolar bifurcations, and calcium plaques are chalk-white and typically located in an arteriole on the optic disc, Gass plaques are typically yellow and nonrefractile. While the color differences can be challenging to distinguish, the differences in location are easier to decipher as Gass plaques are normally found randomly along the middle segments of arterioles rather than at vessel bifurcations where emboli typically reside. Unlike emboli, Gass plaques are rarely found at the site of vessel occlusions when occlusions are present.3,4 Finally, Gass plaques have been noted to arise during acute stages of disease and can disappear rather quickly. In some cases, they have been shown to fluctuate along with disease activity.8 While our patient’s subjective symptoms persisted, her Gass plaques dramatically diminished in number at her most recent visit. Whether or not this reduction in plaque number may indicate a decreased level of disease activity in her case may be more clearly elucidated with time. Although Gass plaques are typically small and discrete deposits, we question whether the two areas that resembled vascular sheathing superiorly in the right eye on funduscopic exam (one example shown in Fig. 4A) were simply elongated or confluent Gass plaques given that they did not hyperfluoresce on FA (Fig. 4B), subsequently dissolved into multiple plaques (Fig. 4C), and then disappeared along with many of the other plaques (Fig. 4D). While Gass plaques are characteristic for SS, they are not pathognomonic as they have been noted in the context of other retinal vascular diseases including retinal macroaneurysms, toxoplasmosis retinitis, acute retinal necrosis, and large cell non-Hodgkin’s lymphoma.3,4
The occurrence of Gass plaques in SS fits with the leading hypothesis regarding the pathophysiology of this disease as an autoimmune inflammatory endotheliopathy. While the etiology of SS is yet to be demonstrated definitively, brain, muscle and skin biopsies demonstrate perivascular deposition of complement (C4d in particular) and infiltration of lymphocytes in pre-capillary arterioles which suggests antibody mediated injury. Histopathology has shown consequent hypertrophy of endothelial cells, vessel wall thickening and perivascular collagen deposition leading to vascular narrowing, occlusion and finally tissue microinfarction.9,10 Clinical response to immunosuppressive therapy further supports this auto-immune hypothesis.
While Gass plaques are not visible on fluorescein angiography, a pathognomonic marker of SS which can be seen on FA is multifocal arteriolar wall hyperfluorescence (AWH) which results from staining of fluorescein into the vessel wall at sites of endothelial damage. In keeping with the presumed widespread endotheliopathy that extends beyond obviously obstructed vessels in cases of SS, AWH is classically seen in normal appearing vessels remote from, or even in the absence of, frank BRAO.3 In some patients these areas have been noted to pre-date areas of eventual complete occlusion but this is certainly not always the case.11 Our case is unusual in that, despite the numerous Gass plaques seen on our patients funduscopic exam, seemingly indicating diffuse endothelial compromise, there was only one segment of definite AWH in the right eye and none in the left. The other segment resembling AWH in the right eye is so far in the temporal periphery that it may just represent the mild staining that is sometimes observed in this location in normal eyes. Regardless, we would have expected more numerous sites of AWH given that new vessel wall staining has been shown to be the most sensitive marker of active disease in SS and staining often persists on FA even during periods of disease remission.12
In this report we have classified the patient as having probable Susac, however, there is disagreement in the literature regarding the precise diagnostic classification of this patient’s particular phenotype. In 2016, a set of diagnostic criteria was published by the European Susac Consortium based on a reference group of 32 patients with an unambiguous diagnosis of Susac syndrome as assessed by all experts of the consortium. According to their criteria, to be classified as ‘definite Susac’ a patient needs to demonstrate the complete clinical triad along with the characteristic MRI findings of hyperintense snowball-like lesions in the corpus callosum.2 As a result, our patient would be classified as ‘probable Susac’ according to these diagnostic criteria given fulfillment of the retinal and vestibulocochlear criteria but lack of MRI findings. However, in 2019, Dr. Robert Egan proposed a revised classification system in which a patient is still diagnosed as having definite Susac in the absence of central callosal lesions on MRI if they demonstrate AWH on FA in normal appearing retinal arterioles that are remote from any BRAO. This is due to the fact that the presence of AWH distant from other retinal vascular injury has never been described in any other condition.8 Our patient does meet this criterion given the presence of remote AWH in the inferonasal periphery of the right eye (Fig. 3).
Regardless, our patient demonstrates a unique retinal phenotype of disease whereby the funduscopic exam demonstrated an impressive number of bilateral Gass plaques but there was no angiographic evidence of acute vascular occlusion and there was minimal AWH. While FA is considered the gold standard of retinal diagnosis, our patient’s angiogram was fairly unremarkable and poorly illustrative of her level of vasculopathy given that Gass plaques are not visible on FA, contrary to some prior beliefs. We also demonstrate the transient nature of Gass plaques in our patient due to dramatic reduction in number without any immunomodulatory therapy.
4. Conclusions
This case represents a unique retinal phenotype of probable Susac syndrome whereby a diffuse endotheliopathy is represented by numerous bilateral Gass plaques with minimal AWH and no acute branch artery occlusion. This emphasizes the importance of Gass plaques as a marker of retinal disease in Susac syndrome and likely active retinal disease. While new staining on FA is the most sensitive marker of active disease14, the Gass plaque likewise is a very sensitive indicator of activity.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
CRediT authorship contribution statement
Devin C. Cohen: Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Fawaz Naeem: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Daniel Will: Writing – review & editing, Supervision, Investigation, Formal analysis, Conceptualization. Robert A. Egan: Writing – review & editing, Supervision, Investigation, Formal analysis, Conceptualization. Madhura A. Tamhankar: Writing – review & editing, Supervision, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Dörr J., Krautwald S., Wildemann B., et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol. 2013;9(6):307–316. doi: 10.1038/nrneurol.2013.82. [DOI] [PubMed] [Google Scholar]
- 2.Kleffner I., Dörr J., Ringelstein M., et al. Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiatry. 2016;87(12):1287–1295. doi: 10.1136/jnnp-2016-314295. [DOI] [PubMed] [Google Scholar]
- 3.Egan R.A., Hills W.L., Susac J.O. Gass plaques and fluorescein leakage in Susac Syndrome. J Neurol Sci. 2010;299(1-2):97–100. doi: 10.1016/j.jns.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 4.Egan R.A., Ha Nguyen T., Gass J.D., et al. Retinal arterial wall plaques in Susac syndrome. Am J Ophthalmol. 2003;135(4):483–486. doi: 10.1016/s0002-9394(02)02085-8. [DOI] [PubMed] [Google Scholar]
- 5.Gass J.D., Tiedeman J., Thomas M.A. Idiopathic recurrent branch retinal arterial occlusion. Ophthalmology. 1986;93(9):1148–1157. doi: 10.1016/s0161-6420(86)33600-5. [DOI] [PubMed] [Google Scholar]
- 6.Martinet N., Fardeau C., Adam R., et al. Fluorescein and indocyanine green angiographies in Susac syndrome. Retina. 2007;27(9):1238–1242. doi: 10.1097/IAE.0b013e31809ff824. [DOI] [PubMed] [Google Scholar]
- 7.Ringelstein M., Albrecht P., Kleffner I., et al. Retinal pathology in Susac syndrome detected by spectral-domain optical coherence tomography. Neurology. 2015;85(7):610–618. doi: 10.1212/WNL.0000000000001852. [DOI] [PubMed] [Google Scholar]
- 8.Egan R.A. Diagnostic criteria and treatment algorithm for susac syndrome. J Neuro Ophthalmol. 2019;39(1):60–67. doi: 10.1097/WNO.0000000000000677. [DOI] [PubMed] [Google Scholar]
- 9.Sauma J., Rivera D., Wu A., et al. Susac’s syndrome: an update. Br J Ophthalmol. 2020;104(9):1190–1195. doi: 10.1136/bjophthalmol-2019-315597. [DOI] [PubMed] [Google Scholar]
- 10.Marrodan M., Fiol M.P., Correale J. Susac syndrome: challenges in the diagnosis and treatment. Brain. 2022;145(3):858–871. doi: 10.1093/brain/awab476. [DOI] [PubMed] [Google Scholar]
- 11.Notis C.M., Kitei R.A., Cafferty M.S., et al. Microangiopathy of brain, retina, and inner ear. J Neuro Ophthalmol. 1995;15(1):1–8. [PubMed] [Google Scholar]
- 12.Cohen D.A., Tajfirouz D., Vodopivec I., et al. Fluorescein angiography findings in susac syndrome: a multicenter retrospective case series. J Neuro Ophthalmol. 2023 doi: 10.1097/WNO.0000000000001826. [published online ahead of print, 2023 Apr 19] [DOI] [PubMed] [Google Scholar]