Abstract
Background
Systemic inflammation is believed to contribute to small cell lung cancer (SCLC) progression, but the underlying relationship remains unclear. Lipocalin‐2, a potential biomarker of inflammation, has been implicated in various cancers but its prognostic value in SCLC is underexplored.
Methods
We retrospectively analyzed 191 patients with SCLC (72 with limited‐stage [LD] and 119 with extensive‐stage) treated using platinum‐based chemotherapy. Lipocalin‐2 expression was evaluated using immunohistochemistry. Optimal cutoff values for lipocalin‐2 and neutrophil‐to‐lymphocyte ratio (NLR) were determined using time‐dependent receiver operating characteristic curve analysis. The pectoralis muscle index was used to assess sarcopenia.
Results
In LD‐SCLC, high lipocalin‐2 expression was associated with worse progression‐free survival (PFS; median: 7.0 vs. 15.9 months, p = 0.015) and overall survival (OS; median: 12.9 vs. 30.3 months, p = 0.035) compared with low lipocalin‐2 expression. Patients were stratified into three prognostic groups by combining lipocalin‐2 with NLR: low lipocalin‐2/low NLR, high lipocalin‐2/low NLR or low lipocalin‐2/high NLR, and high lipocalin‐2/high NLR (median PFS: 17.3 vs. 11.0 vs. 6.3 months, p = 0.004; median OS: 30.5 vs. 17.3 vs. 8.6 months, p = 0.002). Similar trends were observed when combining lipocalin‐2 with the pectoralis muscle index. High lipocalin‐2 expression was also associated with lower complete response rates (18.9% vs. 34.3%, p = 0.035). No significant prognostic implications were found for lipocalin‐2 in extensive‐stage SCLC.
Conclusions
High lipocalin‐2 expression is potentially associated with poorer survival in LD‐SCLC. Combining lipocalin‐2 with other inflammation‐related markers could improve prognostic stratification.
Keywords: biomarkers, inflammation, lipocalin‐2, sarcopenia, small cell lung carcinoma
High lipocalin‐2 expression is associated with poorer progression‐free and overall survival in limited‐stage small cell lung cancer (LD‐SCLC). Combining lipocalin‐2 with neutrophil‐to‐lymphocyte ratio or pectoralis muscle index enhances prognostic stratification, suggesting its potential utility as a biomarker in LD‐SCLC. No significant prognostic implications were found for lipocalin‐2 in extensive‐stage SCLC.
INTRODUCTION
Small cell lung cancer (SCLC) is a rapidly progressing malignancy with a poor prognosis. 1 Smoking, the primary risk factor for SCLC, introduces carcinogenic compounds in the body and triggers systemic inflammation, which may contribute to cancer progression. 1 , 2 Recent studies suggested the clinical relevance of inflammatory markers in SCLC, including the neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio, systemic immune‐inflammation index, and skeletal muscle index (SMI), which is associated with sarcopenia. 3 , 4 , 5 , 6 However, direct experimental evidence supporting the relationship between systemic inflammation and SCLC progression is limited, possibly due to the heterogeneity and complexity of SCLC biology. 7 , 8
Lipocalin‐2, which is also known as neutrophil gelatinase‐associated lipocalin, is emerging as a potential biomarker in various diseases. It plays essential roles in iron transport, immune response regulation, and cellular homeostasis. 9 , 10 , 11 It is expressed by various cell types, including neutrophils, epithelial cells, and some cancer cells. 12 Beyond its basic biological functions, lipocalin‐2 acts as an acute phase protein and is involved in inflammation and injury response processes. 13 , 14 In addition, lipocalin‐2 has been suggested to be related to tumor progression, metastasis, and treatment response in various types of cancer. 15 , 16 , 17 , 18 However, the clinical implication of lipocalin‐2 in SCLC is unclear, particularly regarding other potential biomarkers for systemic inflammation. This study was performed to investigate the prognostic value of lipocalin‐2 and assess its relationship with other inflammation‐related markers, such as NLR and SMI, in patients with SCLC.
METHODS
Patient selection
We retrospectively reviewed the records of all consecutive patients with histologically confirmed SCLC who were treated at the Gyeongsang National University Hospital between July 2006 and March 2020. Patients were included in the study using the following criteria: (1) platinum‐based chemotherapy as the first‐line treatment, (2) available archival tumor samples or recent biopsy specimens suitable for immunohistochemistry (IHC) analysis of lipocalin‐2, and (3) available clinical information to assess the SMI and NLR, as well as key endpoints, such as progression‐free survival (PFS), overall survival (OS), and treatment response. Patients were excluded from the study if they had a concurrent malignancy or history of another cancer within the previous 5 years, or if they experienced a histological transformation from non‐small cell lung cancer to SCLC. The study was conducted in accordance with the principles of the Declaration of Helsinki (1964) and its latest amendments. The Institutional Review Board of the Gyeongsang National University Hospital approved this study (GNUH 2023‐12‐002). The requirement for written informed consent was waived owing to the retrospective nature of the study.
IHC analysis for lipocalin‐2
IHC was performed on 4‐μm‐thick sections from formalin‐fixed paraffin‐embedded specimens (biopsy or cellblock). The tissue sections were attached to glass slides, deparaffinized, rehydrated, and incubated in 3% hydrogen peroxide for 10 min to block the endogenous peroxidase activity. The slides were subsequently heated for 20 min in 10 mmol/L sodium citrate buffer (pH 6.0, homemade) in a microwave oven (700 W) and incubated with Ultra V Block (Lab Vision; Thermo Fisher Scientific, Inc.) for 7 min at room temperature (20–25°C) to block background staining. The slides were then incubated with a monoclonal anti‐lipocalin‐2 antibody (dilution, 1:200; Clone #220310, MAB1757; R&D Systems) for 1 h at room temperature. UltraVision LP Detection System HRP DAB (Thermo Fisher Scientific) was used to visualize antigens, and the sections were counterstained using Mayer's hematoxylin.
Lipocalin‐2 was expressed in the cytoplasm. Staining intensity was scored as 0 (negative), 1 (mild), 2 (moderate), or 3 (high). The proportional score of stained tumor cells was scored as a percentage (0%–100%). The H‐score was calculated by multiplying the intensity score by the proportional score (0–300; Figure 1).
FIGURE 1.
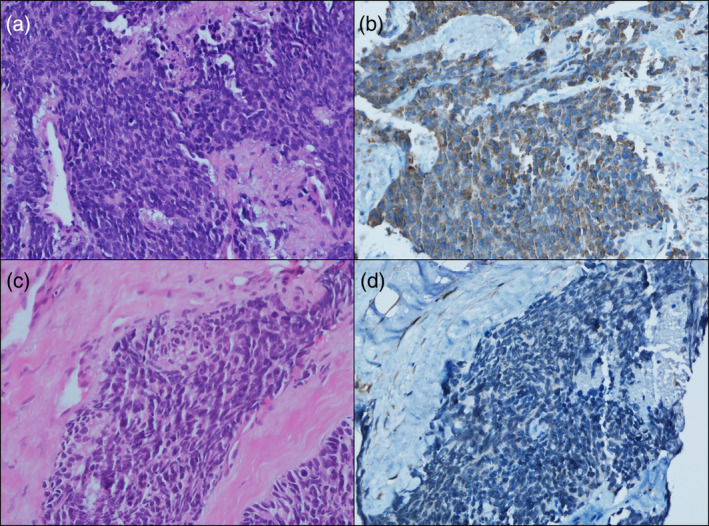
Representative images of immunohistochemical staining for lipocalin‐2. (a, hematoxylin–eosin stain) small cell carcinoma with (b) lipocalin‐2 high expression showing cytoplasmic staining and (c, hematoxylin–eosin stain) small cell carcinoma with (d) lipocalin‐2 low expression.
Assessments
The pectoralis muscle index (PMI), calculated using the cross‐sectional area of the pectoralis major and minor muscles, was used as a surrogate for the SMI in this study. 6 The bilateral pectoralis muscle area was measured by manually outlining the outermost part of the pectoralis muscles at the T4 level with a range of −29 to 100 HU, through computed tomography histogram analysis using the “X section” analysis tool (Advantage Window 4.4; GE Healthcare). The mean value of the bilateral pectoralis muscle area was then adjusted for height (m2), resulting in the PMI being expressed in cm2/m2. The sex‐specific cutoff values of PMI to indicate sarcopenia and categorize into low and high PMI groups were 4.4 cm2/m2 and 3.1 cm2/m2 for male and female patients, respectively. 19 The NLR was determined by dividing the total number of neutrophils by the total number of lymphocytes. 20 We collected baseline characteristics, including age at diagnosis, Eastern Cooperative Oncology Group performance status, and tumor stage at diagnosis as either limited‐stage (LD) or extensive‐stage (ED), from the electronic medical records of the patients. In addition, details on the types of treatments, chemotherapy protocols, number of treatment cycles, intensity of doses, and administration of concurrent chemoradiotherapy and prophylactic cranial irradiation were documented. Follow‐up evaluations were performed every 6–12 weeks or when necessary to monitor disease progression, assess treatment response, and identify any adverse event. The assessment of treatment response was based on the Response Evaluation Criteria in Solid Tumors 1.1 criteria. Early treatment discontinuation, which was defined as stopping treatment early for reasons other than cancer progression before completing the scheduled cycles of platinum‐based chemotherapy, and treatment‐related mortality, which was defined as death within 30 days after the last dose of initial chemotherapy not caused by cancer progression, were also recorded. The relative dose intensity was calculated by dividing the actual dose received per cycle by the standard dose per cycle, presented as a percentage.
Statistical analysis
Time‐dependent receiver operating characteristic curve analysis was conducted to assess the predictive validity of lipocalin‐2 and the NLR at various time points, aiming to identify the optimal cutoff values for these biomarkers. This analysis involved plotting the sensitivity (true positive rate) against 1‐specificity (false positive rate) across a range of potential cutoff values for each marker. The optimal cutoff was determined by the point on the curve that maximized the Youden index (sensitivity + specificity −1), aiming to identify patients with survival times greater than the median OS. The analysis was planned to be conducted separately for patients with LD and ED, because of anticipated differences in survival patterns and the potential variability in the prognostic value of lipocalin‐2 and NLR across stages.
Descriptive statistics, including the chi‐square, Fisher's exact, Mann–Whitney U, and Cuzick's trend tests, and the Cochran–Armitage trend test were used to analyze the baseline characteristics, treatment‐related factors, and treatment response of the study population. Kaplan–Meier survival analysis with log‐rank tests was conducted to evaluate PFS and OS. Variables with p < 0.1 in univariate analyses were subsequently included in the multivariate Cox proportional hazards models to evaluate the prognostic significance of covariates. Two‐sided p < 0.05 was considered statistically significant for all tests. All statistical analyses were conducted using STATA version 16.1 (StataCorp LLC).
RESULTS
Determination of lipocalin‐2 cutoff value in patients with LD and ED
In this study involving 191 patients (72 with LD and 119 with ED), we established a cutoff value of H‐score 50 for lipocalin‐2 in patients with LD cancer. This threshold, which was used to differentiate patients into low and high lipocalin‐2 groups, demonstrated a sensitivity of 69.4% and a specificity of 66.7% for predicting survival beyond 18.9 months (the median OS for patients with LD cancer in the study cohort; Figure S1a). For patients with ED cancer, no significant cutoff value for lipocalin‐2 was found to predict survival beyond 11.5 months (the median OS for patients with ED cancer) as indicated by the low area under the curve value and the receiver operating characteristic curve's proximity to the 45‐degree diagonal line (Figure S1b). Consequently, further analysis focused on the LD cohort.
Baseline and treatment characteristics
Table 1 describes the baseline characteristics of the LD cohort, categorized by lipocalin‐2 expression levels. The high lipocalin‐2 group exhibited a higher frequency of poor performance status and elevated lactate dehydrogenase levels. Patients with low PMI tended to be more common in the high lipocalin‐2 group, although this difference did not reach statistical significance. Otherwise, no significant differences were found in baseline characteristics between the low and high lipocalin‐2 groups. Regarding treatment characteristics (Table S1), the etoposide plus carboplatin regimen was administered more frequently to patients in the high lipocalin‐2 group than to those in the low lipocalin‐2 group. However, this imbalance was likely due to chance rather than a direct relationship with lipocalin‐2 expression, as the choice between cisplatin and carboplatin in the chemotherapy regimen was based on the physician's assessment of the patient's clinical factors, such as renal function, age, and performance status, and was made independently of the lipocalin‐2 expression levels, which were not available at the time of treatment selection. No other significant differences were observed between the groups regarding relative dose intensity, the use of concurrent chemoradiotherapy and prophylactic cranial irradiation, early discontinuation of treatment, or treatment‐related mortality.
TABLE 1.
Baseline characteristics of patients with limited stage.
| Characteristics | Low lipocalin‐2 (n = 35) | High lipocalin‐2 (n = 37) | p‐value |
|---|---|---|---|
| Median age, years (IQR) | 66 (59–70) | 70 (62–75) | 0.120 |
| Sex | 0.377 | ||
| Male | 31 (88.6) | 30 (81.1) | |
| Female | 4 (11.4) | 7 (18.9) | |
| ECOG PS | 0.042 | ||
| 0–1 | 32 (91.4) | 27 (73.0) | |
| 2–3 | 3 (8.6) | 10 (27.0) | |
| Smoking | >0.998 | ||
| Never‐smoker | 2 (5.7) | 2 (5.4) | |
| Current/former smoker | 33 (94.3) | 35 (94.6) | |
| Lactate dehydrogenase a | 0.031 | ||
| Normal | 13 (65.0) | 11 (34.4) | |
| Elevated | 7 (35.0) | 21 (65.6) | |
| PMI | 0.084 | ||
| High | 32 (91.4) | 28 (75.7) | |
| Low | 3 (8.6) | 9 (24.3) | |
| Mean (SD), cm2/m2 | 5.8 (1.3) | 5.2 (1.3) | 0.084 |
| NLR | 0.404 | ||
| Low | 25 (71.4) | 23 (62.2) | |
| High | 10 (28.6) | 14 (37.8) | |
| Mean (SD) | 2.6 (1.5) | 3.0 (2.4) | 0.714 |
Note: The variables are presented as number (%) or median (IQR).
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; NLR, neutrophil‐lymphocyte ratio; PMI, pectoralis muscle index; SD, standard deviation.
LDH levels were measured in 52 patients.
Prognostic implications of lipocalin‐2 expression
This study had a median follow‐up duration of 93 months for the LD cohort. Figure 2 presents the PFS and OS rates in the LD cohort, stratified by lipocalin‐2 expression. The high lipocalin‐2 group had significantly worse PFS (median PFS: 7.0 vs. 15.9 months; 95% confidence interval: 6.3–12.8 vs. 13.2–26.7; p = 0.015) and OS (median OS: 12.9 vs. 30.3 months; 95% confidence interval: 20.6–39.9 vs. 7.8–18.9; p = 0.035) compared with the low lipocalin‐2 group. However, lipocalin‐2 expression lost statistical significance when combined with other clinical factors in multivariate analysis (Table 2).
FIGURE 2.
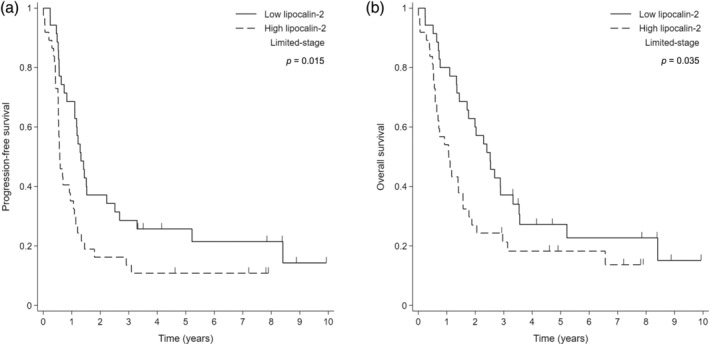
Kaplan–Meier survival curves according to lipocalin‐2 expression in patients with limited‐stage small cell lung cancer. (a) Progression‐free survival (PFS) and (b) overall survival (OS).
TABLE 2.
Cox regression for overall survival of patients with limited stage.
| Univariate | Multivariate (1) | Multivariate (2) | Multivariate (3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p‐value | HR | 95% CI | p‐value | HR | 95% CI | p‐value | HR | 95% CI | p‐value | |
| Age | 1.062 | 1.028–1.096 | < 0.001 | 1.047 | 1.004–1.092 | 0.034 | 1.051 | 1.008–1.097 | 0.021 | 1.048 | 1.007–1.091 | 0.021 |
| Sex | ||||||||||||
| Male | Ref. | |||||||||||
| Female | 0.582 | 0.273–1.242 | 0.162 | |||||||||
| ECOG PS | ||||||||||||
| 0–1 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 2–3 | 4.587 | 2.291–9.185 | < 0.001 | 3.280 | 1.560–6.895 | 0.002 | 2.904 | 1.409–5.983 | 0.004 | 2.482 | 1.176–5.236 | 0.017 |
| Chemotherapy regimen | ||||||||||||
| Etoposide/carboplatin | Ref. | Ref. | Ref. | Ref. | ||||||||
| Etoposide/cisplatin | 0.464 | 0.270–0.799 | 0.006 | 0.853 | 0.414–1.760 | 0.667 | 1.148 | 0.572–2.304 | 0.697 | 0.966 | 0.503–1.853 | 0.917 |
| Dose reduction since the first cycle | ||||||||||||
| No | Ref. | Ref. | Ref. | Ref. | ||||||||
| Yes | 2.201 | 1.175–4.120 | 0.014 | 1.022 | 0.495–2.112 | 0.952 | 0.972 | 0.468–2.017 | 0.939 | 1.152 | 0.561–2.367 | 0.699 |
| Lipocalin‐2 | ||||||||||||
| Low | Ref. | Ref. | ||||||||||
| High | 1.747 | 1.034–2.954 | 0.037 | 1.193 | 0.630–2.258 | 0.588 | ||||||
| Lipocalin‐2 + PMI | 2.369 | 1.513–3.709 | < 0.001 | 2.013 | 1.210–3.351 | 0.007 | ||||||
| Lipocalin‐2 + NLR | 1.894 | 1.297–2.765 | 0.001 | 1.708 | 1.081–2.701 | 0.022 | ||||||
Note: Since only one factor among lipocalin‐2, lipocalin‐2 + PMI, and lipocalin‐2 + NLR should be selected as a variable in each analysis, used gray shading to indicate ‘blank’.
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NLR, neutrophil‐lymphocyte ratio; PMI, pectoralis muscle index.
Prognostic implications of combining lipocalin‐2, neutrophil‐lymphocyte ratio, and pectoralis muscle index
Further analyses were conducted to assess the predictive impact of lipocalin‐2 in conjunction with the PMI and NLR, which are both indicative of systemic inflammation. In the LD cohort, the cutoff value of NLR was determined as 3 to differentiate patients into low and high NLR groups (Figure S1c). Patients characterized by low lipocalin‐2 expression and NLR exhibited the most favorable PFS and OS. Conversely, those with high levels of lipocalin‐2 expression and NLR had the worst survival outcomes (Figure 3a,b). Further stratification into three distinct cohorts based on the combined status of lipocalin‐2 expression and NLR more clearly delineated these observations, as depicted in Figure 3c (median PFS: 17.3 vs. 11.0 vs. 6.3 months in groups 1, 2, and 3, respectively; p = 0.004) and Figure 3d (median OS: 30.5 vs. 17.3 vs. 8.6 months in groups 1, 2, and 3, respectively; p = 0.002). Similar patterns were observed (Figure 4) when evaluating the combined status of lipocalin‐2 expression and PMI. The median PFS was 15.9, 11.0, and 3.7 months for group 1 (characterized by low lipocalin‐2 expression and high PMI; indicative of nonsarcopenic status), group 2 (neither group 1 nor group 3 lipocalin‐2 expression and PMI status), and group 3 (characterized by high lipocalin‐2 and low PMI; indicative of sarcopenic status), respectively (p < 0.001; Figure 4c). Median OS followed a similar trend, with values of 30.5, 16.9, and 4.8 months for groups 1, 2, and 3, respectively (p < 0.001; Figure 4d). Multivariate analysis confirmed the significant survival benefit associated with the combination of lipocalin‐2 expression and NLR, or lipocalin‐2 expression and PMI. The type of chemotherapy regimen did not significantly impact survival outcomes (Table 2), consistent with previous literature. 21
FIGURE 3.
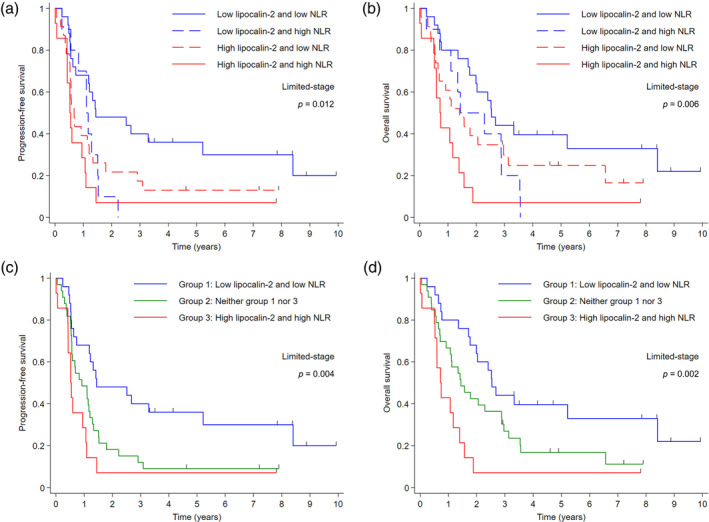
Kaplan–Meier survival curves according to the combined status of lipocalin‐2 expression and neutrophil‐lymphocyte ratio (NLR) in patients with limited‐stage small cell lung cancer. (a) Progression‐free survival (PFS) and (b) overall survival (OS) for patients stratified by the combination of lipocalin‐2 and NLR. (c) PFS and (d) OS for patients stratified into three groups based on the combined status of lipocalin‐2 and NLR.
FIGURE 4.
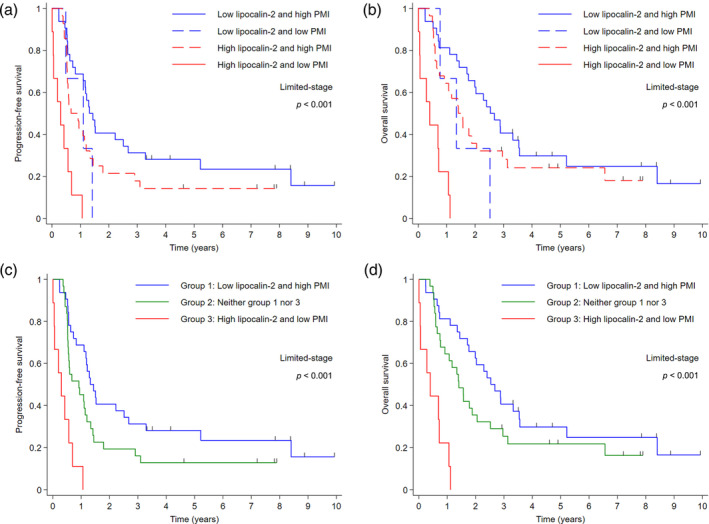
Kaplan–Meier survival curves according to the combined status of lipocalin‐2 expression and pectoralis muscle index (PMI) in patients with limited‐stage small cell lung cancer. (a) Progression‐free survival (PFS) and (b) overall survival (OS) for patients stratified by the combination of lipocalin‐2 and PMI. (c) PFS and (d) OS for patients stratified into three groups based on the combined status of lipocalin‐2 and PMI.
Association of lipocalin‐2 expression with treatment response
Table 3 presents the relationship between treatment response and the status of lipocalin‐2, NLR, and PMI in patients with LD. Patients exhibiting high lipocalin‐2 expression were less likely to achieve a complete response (CR; rate of 18.9%) compared with those with low lipocalin‐2 levels (CR rate of 34.3%; p = 0.035). However, the difference between the high and low lipocalin‐2 groups was not statistically significant (83.8% vs. 97.1%, p = 0.108) when considering the objective response rate (ORR), which encompasses both complete and partial responses.
TABLE 3.
Treatment response of patients with limited stage by lipocalin‐2, NLR, and PMI.
| Treatment response | Lipocalin‐2 alone | Lipocalin‐2 and NLR | Lipocalin‐2 and PMI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low lipocalin‐2 (n = 35) | High lipocalin‐2 (n = 37) | p‐value | Group 1 (n = 25) | Group 2 (n = 33) | Group 3 (n = 14) | p‐value | Group 1 (n = 32) | Group 2 (n = 31) | Group 3 (n = 9) | p‐value | |
| CR | 12 (34.3) | 7 (18.9) | 0.035 a | 12 (48.0) | 6 (18.2) | 1 (7.1) | 0.001 b | 11 (34.4) | 8 (25.8) | 0 | 0.002 b |
| PR | 22 (62.9) | 24 (64.9) | 12 (48.0) | 24 (72.7) | 10 (71.4) | 20 (62.5) | 22 (71.0) | 4 (44.4) | |||
| SD, PD, or not evaluated | 1 (2.9) | 6 (16.2) | 1 (4.0) | 3 (9.1) | 3 (21.4) | 1 (3.1) | 1 (3.2) | 5 (55.6) | |||
| Objective response rate (CR + PR) | 34 (97.1) | 31 (83.8) | 0.108 | 24 (96.0) | 30 (90.9) | 11 (78.6) | 0.090 a | 31 (96.9) | 30 (96.8) | 4 (44.4) | <0.001 a |
Note: The variables are presented as numbers (%).
Abbreviations: CR, complete response; NLR, neutrophil‐lymphocyte ratio; PD, progressive disease; PMI, pectoralis muscle index; PR, partial response; SD, stable disease.
Cochrane‐Armitage trend test.
Cuzick's trend test.
Further analysis incorporating the NLR and lipocalin‐2 expression revealed a stronger association with treatment response. Group 1 exhibited the highest CR rate of 48.0%, which was markedly above those of groups 2 (18.2%) and 3 (7.1%; p = 0.001). Despite the ORR differences not reaching statistical significance across groups (p = 0.090), the trend from groups 1 to 3 suggests that the combination of lipocalin‐2 expression and NLR might provide a more reliable predictive tool for assessing treatment response. The treatment response analysis relative to lipocalin‐2 expression and PMI further supports the efficacy of using a combination of markers. Groups 1 and 2 displayed high ORRs (96.9% and 96.8%, respectively) compared with a significantly lower rate in group 3 (44.4%, p < 0.001). In addition, group 1 showed a higher CR rate (34.4%) than group 2 (25.8%), and group 3 did not achieve CR (p = 0.002).
DISCUSSION
In this study, we investigated the prognostic value of lipocalin‐2 in patients with SCLC, revealing significant associations between lipocalin‐2 expression and survival outcomes. We found that high lipocalin‐2 expression was potentially associated with poorer PFS and OS in the LD cohort. In addition, our analysis demonstrated that when lipocalin‐2 is considered with other markers of systemic inflammation, such as NLR and PMI, it provides an enhanced prediction of clinical outcomes. Specifically, patients with low lipocalin‐2 expression and favorable inflammatory profiles had the best survival outcomes, while those with both high lipocalin‐2 expression and adverse inflammatory profiles had the worst survival outcomes. These findings underscore the potential of lipocalin‐2, in conjunction with other inflammation‐related markers, to serve as a valuable prognostic tool in managing patients with SCLC.
Given the absence of direct experimental studies on lipocalin‐2 in SCLC, we can infer its potential mechanisms of action from findings in other types of cancer. For instance, murine breast cancer cells with high lipocalin‐2 levels exhibit increased lung metastasis, due to the ability of lipocalin‐2 to suppress the phosphoinositide 3‐kinase (PI3K)/Akt pathway. 22 In addition, lipocalin‐2 promotes tumor progression by angiogenesis through the upregulation of vascular endothelial growth factor and the activation of the mitogen‐activated protein kinase/extracellular‐signal‐regulated kinase pathway. 23 , 24 Moreover, lipocalin‐2 inhibits ferroptosis, resulting in resistance to chemotherapy in colorectal cancer. 17 It also plays a role in systemic, cellular, and mucosal hypoferremia during inflammation, affecting the tumor microenvironment and potentially promoting tumor progression. 11 In pancreatic ductal adenocarcinoma, lipocalin‐2 promotes tumor progression by modulating the secretion of proinflammatory cytokines within the tumor microenvironment. 25 These conditions and pathways—PI3K/Akt signaling, angiogenesis, ferroptosis, and systemic inflammation—are similarly implicated in the pathogenesis of SCLC. 3 , 4 , 5 , 6 , 26 , 27 , 28 , 29 Inflammation acts as a critical regulator, activating and modulating the PI3K/Akt pathway, angiogenesis, and ferroptosis within the tumor microenvironment. 30 , 31 , 32 In addition, cancer‐related cachexia may be another factor explaining why lipocalin‐2 is associated with poor prognosis in SCLC. The plasma progastrin‐releasing peptide level has been reported to be associated with weight loss and increased in nonresponders to chemotherapy. 33 Lipocalin‐2 is known for its role in appetite suppression during cancer‐related cachexia, 34 and its expression level was inversely related to PMI level (Table 1), which is associated with sarcopenia and cachexia, in our study. These findings indicate that the lipocalin‐2 may affect SCLC progression through both direct and inflammation‐mediated mechanisms.
The significant prognostic value of lipocalin‐2 was valid only in the LD cohort in this study. In contrast to our study findings, limited evidence specifically identifying lipocalin‐2 as a prognostic biomarker in early‐stage cancer has been available until now. 23 Additionally, more evidence supporting the prognostic implications of lipocalin‐2 in late‐stage cancer and its association with more aggressive histological features has been available. 35 , 36 , 37 , 38 The unique findings observed in our study might be attributed to several factors, including differences in methodological approaches and patient cohorts across studies, which could affect the expression level and prognostic significance of lipocalin‐2. In addition, the inherent heterogeneity of SCLC, especially in the ED cohort, may further complicate the interpretation of the prognostic value of lipocalin‐2. ED‐SCLC is characterized by a wider range of genetic mutations, diverse tumor microenvironments due to metastasis, and the development of treatment resistance through clonal evolution. 39 , 40 This heterogeneity in ED‐SCLC may overshadow the prognostic significance of single biomarkers, such as lipocalin‐2, as the effect of multiple interacting biological processes becomes more pronounced. Consequently, the utility of lipocalin‐2 in ED‐SCLC may require further investigation to elucidate its role amidst the complex interplay of methodological and biological factors.
The combined model incorporating lipocalin‐2 with NLR or PMI demonstrated superior prognostic value compared with lipocalin‐2 alone. The rationale for developing the combined model lies in the complex interplay between systemic inflammation and tumor biology. Systemic inflammation promotes tumor progression through diverse mechanisms, such as angiogenesis, immune response suppression, and epithelial‐mesenchymal transition. 41 , 42 , 43 NLR and PMI each may reflect different aspects of the inflammatory process and have been suggested to be prognostic factors in SCLC. 3 , 6 , 20 , 44 , 45 , 46 The combined model integrating novel and conventional markers related to systemic inflammation may provide a more comprehensive assessment of the inflammatory status and its potential impact on tumor progression. This approach is also supported by previous studies that have demonstrated the value of combining multiple inflammatory markers to improve prognostic stratification in patients with SCLC. 6 , 47 , 48 , 49
One of the main limitations of the present study was that lipocalin‐2 alone did not demonstrate independent prognostic value in the multivariate analysis. This finding suggests that the prognostic impact of lipocalin‐2 may be confounded by other clinical factors. Although the combined model incorporating lipocalin‐2 with NLR or PMI showed improved performance, it may only partially explain the complex interplay between lipocalin‐2 and other prognostic factors. Moreover, functional translational studies are necessary to elucidate the precise mechanisms by which lipocalin‐2 affects SCLC progression. Other study limitations included its retrospective nature, relatively small sample size, missing values for certain variables such as LDH, lack of a significant cutoff value for lipocalin‐2 in the ED cohort, and scarcity of patients treated with immunotherapy in our cohort. Consequently, the present study findings may have limited applicability to future research involving patients treated using immunotherapy.
In conclusion, this study suggests that high lipocalin‐2 expression is potentially associated with poorer survival outcomes in patients with LD‐SCLC. The combined model incorporating lipocalin‐2 with inflammation‐related markers demonstrated improved prognostic performance, highlighting the potential of integrating multiple biomarkers in SCLC prognostication. Nevertheless, further research is needed to validate these findings in a larger number of prospective cohorts and explore the potential of lipocalin‐2 as a therapeutic target. Functional studies are also warranted to elucidate the precise mechanisms by which lipocalin‐2 affects SCLC progression.
AUTHOR CONTRIBUTIONS
Conceptualization: Jung Wook Yang and Gyeong‐Won Lee. Data curation: Se‐Il Go, Jung Wook Yang, Woo Je Lee, Eun Jeong Jeong, Sungwoo Park and Gyeong‐Won Lee. Formal analysis: Se‐Il Go, Jung Wook Yang and Gyeong‐Won Lee. Investigation: Se‐Il Go, Jung Wook Yang, Woo Je Lee, Eun Jeong Jeong, Sungwoo Park and Gyeong‐Won Lee. Methodology: Jung Wook Yang and Gyeong‐Won Lee. Project administration: Gyeong‐Won Lee. Resources: Jung Wook Yang, Woo Je Lee and Gyeong‐Won Lee. Supervision: Gyeong‐Won Lee. Visualization: Se‐Il Go. Writing–original draft: Se‐Il Go, Jung Wook Yang, Eun Jeong Jeong and Gyeong‐Won Lee. Writing–review and editing: Se‐Il Go, Jung Wook Yang, Woo Je Lee, Eun Jeong Jeong, Sungwoo Park and Gyeong‐Won Lee. Final approval of manuscript: All authors.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGMENTS
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (no. RS‐2023‐00219399).
Go S‐I, Yang JW, Lee WJ, Jeong EJ, Park S, Lee G‐W. Lipocalin‐2 as a prognostic biomarker and its association with systemic inflammation in small cell lung cancer. Thorac Cancer. 2024;15(21):1646–1655. 10.1111/1759-7714.15389
Se‐Il Go and Jung Wook Yang contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analyzed during this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Rudin CM, Brambilla E, Faivre‐Finn C, Sage J. Small‐cell lung cancer. Nat Rev Dis Primers. 2021;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walser T, Cui X, Yanagawa J, Lee JM, Heinrich E, Lee G, et al. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc. 2008;5:811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiong Q, Huang Z, Xin L, Qin B, Zhao X, Zhang J, et al. Post‐treatment neutrophil‐to‐lymphocyte ratio (NLR) predicts response to anti‐PD‐1/PD‐L1 antibody in SCLC patients at early phase. Cancer Immunol Immunother. 2021;70:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou H, Li J, Zhang Y, Chen Z, Chen Y, Ye S. Platelet‐lymphocyte ratio is a prognostic marker in small cell lung cancer‐a systemic review and meta‐analysis. Front Oncol. 2022;12:1086742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Y, Dai M, Zhang Z. Prognostic significance of the systemic immune‐inflammation index (SII) in patients with small cell lung cancer: a meta‐analysis. Front Oncol. 2022;12:814727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Go SI, Park MJ, Song HN, Kang MH, Park HJ, Jeon KN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer. 2016;24:2075–2084. [DOI] [PubMed] [Google Scholar]
- 7. Tian Y, Li Q, Yang Z, Zhang S, Xu J, Wang Z, et al. Single‐cell transcriptomic profiling reveals the tumor heterogeneity of small‐cell lung cancer. Signal Transduct Target Ther. 2022;7:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, et al. SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol. 2020;15:1823–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiao X, Yeoh BS, Saha P, Olvera RA, Singh V, Vijay‐Kumar M. Lipocalin 2 alleviates iron toxicity by facilitating hypoferremia of inflammation and limiting catalytic iron generation. Biometals. 2016;29:451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nairz M, Schroll A, Haschka D, Dichtl S, Sonnweber T, Theurl I, et al. Lipocalin‐2 ensures host defense against salmonella typhimurium by controlling macrophage iron homeostasis and immune response. Eur J Immunol. 2015;45:3073–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao X, Yeoh BS, Vijay‐Kumar M. Lipocalin 2: an emerging player in iron homeostasis and inflammation. Annu Rev Nutr. 2017;37:103–130. [DOI] [PubMed] [Google Scholar]
- 12. Jaberi SA, Cohen A, D'Souza C, Abdulrazzaq YM, Ojha S, Bastaki S, et al. Lipocalin‐2: structure, function, distribution and role in metabolic disorders. Biomed Pharmacother. 2021;142:112002. [DOI] [PubMed] [Google Scholar]
- 13. Sultan S, Pascucci M, Ahmad S, Malik IA, Bianchi A, Ramadori P, et al. LIPOCALIN‐2 is a major acute‐phase protein in a rat and mouse model of sterile abscess. Shock. 2012;37:191–196. [DOI] [PubMed] [Google Scholar]
- 14. Guardado S, Ojeda‐Juarez D, Kaul M, Nordgren TM. Comprehensive review of lipocalin 2‐mediated effects in lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2021;321:L726–L733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu C, Yang K, Li M, Huang W, Zhang F, Wang H. Lipocalin 2: a potential therapeutic target for breast cancer metastasis. Onco Targets Ther. 2018;11:8099–8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santiago‐Sanchez GS, Pita‐Grisanti V, Quinones‐Diaz B, Gumpper K, Cruz‐Monserrate Z, Vivas‐Mejia PE. Biological functions and therapeutic potential of lipocalin 2 in cancer. Int J Mol Sci. 2020;21:5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaudhary N, Choudhary BS, Shah SG, Khapare N, Dwivedi N, Gaikwad A, et al. Lipocalin 2 expression promotes tumor progression and therapy resistance by inhibiting ferroptosis in colorectal cancer. Int J Cancer. 2021;149:1495–1511. [DOI] [PubMed] [Google Scholar]
- 18. Ören B, Urosevic J, Mertens C, Mora J, Guiu M, Gomis RR, et al. Tumour stroma‐derived lipocalin‐2 promotes breast cancer metastasis. J Pathol. 2016;239:274–285. [DOI] [PubMed] [Google Scholar]
- 19. Go SI, Park MJ, Song HN, Kim HG, Kang MH, Lee HR, et al. Prognostic impact of sarcopenia in patients with diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle. 2016;7:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kang MH, Go SI, Song HN, Lee A, Kim SH, Kang JH, et al. The prognostic impact of the neutrophil‐to‐lymphocyte ratio in patients with small‐cell lung cancer. Br J Cancer. 2014;111:452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rossi A, di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, et al. Carboplatin‐ or cisplatin‐based chemotherapy in first‐line treatment of small‐cell lung cancer: the COCIS meta‐analysis of individual patient data. J Clin Oncol. 2012;30:1692–1698. [DOI] [PubMed] [Google Scholar]
- 22. Shi H, Gu Y, Yang J, Xu L, Mi W, Yu W. Lipocalin 2 promotes lung metastasis of murine breast cancer cells. J Exp Clin Cancer Res. 2008;27:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang J, McNeish B, Butterfield C, Moses MA. Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. FASEB J. 2013;27:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Du H, Liang L, Li J, Xiong Q, Yu X, Yu H. Lipocalin‐2 alleviates LPS‐induced inflammation through alteration of macrophage properties. J Inflamm Res. 2021;14:4189–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gomez‐Chou SB, Swidnicka‐Siergiejko AK, Badi N, Chavez‐Tomar M, Lesinski GB, Bekaii‐Saab T, et al. Lipocalin‐2 promotes pancreatic ductal adenocarcinoma by regulating inflammation in the tumor microenvironment. Cancer Res. 2017;77:2647–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin Y, Chen Y, Tang H, Hu X, Hubert SM, Li Q, et al. Activation of PI3K/AKT pathway is a potential mechanism of treatment resistance in small cell lung cancer. Clin Cancer Res. 2022;28:526–539. [DOI] [PubMed] [Google Scholar]
- 27. Montanino A, Manzo A, Carillio G, Palumbo G, Esposito G, Sforza V, et al. Angiogenesis inhibitors in small cell lung cancer. Front Oncol. 2021;11:655316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bebber CM, Thomas ES, Stroh J, Chen Z, Androulidaki A, Schmitt A, et al. Ferroptosis response segregates small cell lung cancer (SCLC) neuroendocrine subtypes. Nat Commun. 2021;12:2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cristea S, Sage J. Is the canonical RAF/MEK/ERK signaling pathway a therapeutic target in SCLC? J Thorac Oncol. 2016;11:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–296. [DOI] [PubMed] [Google Scholar]
- 31. Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. [DOI] [PubMed] [Google Scholar]
- 33. Nisman B, Nechushtan H, Biran H, Peled N, Gantz‐Sorotsky H, Doviner V, et al. New ARCHITECT plasma pro‐gastrin‐releasing peptide assay for diagnosing and monitoring small‐cell lung cancer. Br J Cancer. 2016;114:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olson B, Zhu X, Norgard MA, Levasseur PR, Butler JT, Buenafe A, et al. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat Commun. 2021;12:2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leng X, Ding T, Lin H, Wang Y, Hu L, Hu J, et al. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res. 2009;69:8579–8584. [DOI] [PubMed] [Google Scholar]
- 36. Ulusoy MH, Cirak Y, Adali Y. Predictive and prognostic role of Lipocalin‐2 expression in prostate cancer and its association with Gleason score. Prostate Cancer. 2021;2021:8836043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villodre ES, Hu X, Larson R, Finetti P, Gomez K, Balema W, et al. Lipocalin 2 promotes inflammatory breast cancer tumorigenesis and skin invasion. Mol Oncol. 2021;15:2752–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang M, Zhao X, Deng Y, Tang B, Sun Q, Zhang Q, et al. Neutrophil gelatinase associated lipocalin is an independent predictor of poor prognosis in cases of papillary renal cell carcinoma. J Urol. 2015;194:647–652. [DOI] [PubMed] [Google Scholar]
- 39. Denninghoff V, Russo A, de Miguel‐Pérez D, Malapelle U, Benyounes A, Gittens A, et al. Small cell lung cancer: state of the art of the molecular and genetic landscape and novel perspective. Cancers (Basel). 2021;13:1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stovold R, Blackhall F, Meredith S, Hou J, Dive C, White A. Biomarkers for small cell lung cancer: neuroendocrine, epithelial and circulating tumour cells. Lung Cancer. 2012;76:263–268. [DOI] [PubMed] [Google Scholar]
- 41. Dominguez C, David JM, Palena C. Epithelial‐mesenchymal transition and inflammation at the site of the primary tumor. Semin Cancer Biol. 2017;47:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rossi JF, Lu ZY, Massart C, Levon K. Dynamic immune/inflammation precision medicine: the good and the bad inflammation in infection and cancer. Front Immunol. 2021;12:595722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stockmann C, Schadendorf D, Klose R, Helfrich I. The impact of the immune system on tumor: angiogenesis and vascular remodeling. Front Oncol. 2014;4:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Go SI, Park MJ, Lee GW. Clinical significance of the cachexia index in patients with small cell lung cancer. BMC Cancer. 2021;21:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mirili C, Guney IB, Paydas S, Seydaoglu G, Kapukaya TK, Ogul A, et al. Prognostic significance of neutrophil/lymphocyte ratio (NLR) and correlation with PET‐CT metabolic parameters in small cell lung cancer (SCLC). Int J Clin Oncol. 2019;24:168–178. [DOI] [PubMed] [Google Scholar]
- 46. Wang K, Long W, Sima X, Zhao Y, Xiao B, Gulizeba H, et al. Sarcopenia defined by skeletal muscle mass index at the third lumbar vertebra is a prognostic factor for extensive‐stage small cell lung cancer patients: a retrospective study. J Thorac Dis. 2022;14:2645–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic immune‐inflammation index, based on platelet counts and neutrophil‐lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236:297–304. [DOI] [PubMed] [Google Scholar]
- 48. Kutlu Y, Aydin SG, Bilici A, Oven BB, Olmez OF, Acikgoz O, et al. Neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio as prognostic markers in patients with extensive‐stage small cell lung cancer treated with atezolizumab in combination with chemotherapy. Medicine (Baltimore). 2023;102:e33432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou T, Hong S, Hu Z, Hou X, Huang Y, Zhao H, et al. A systemic inflammation‐based prognostic scores (mGPS) predicts overall survival of patients with small‐cell lung cancer. Tumour Biol. 2015;36:337–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The data sets generated during and/or analyzed during this study are available from the corresponding author upon reasonable request.


