Highlights
-
•
Age ≥65 and dependence are predictors of worse general self-rated health (SRH) 3 months post-stroke.
-
•
Motor impairment is a predictor of worse time-comparative SRH 3 months post-stroke.
-
•
Being female is a predictor of worse time-comparative SRH 12 months post-stroke.
-
•
Dependence is a predictor of worse time-comparative SRH 12 months post-stroke.
-
•
Age and motor impairments were the strongest SRH predictors.
Keywords: Cerebrovascular disorders, Health, Health status, Longitudinal studies, Perceived health, Predictors, Rehabilitation
Abstract
Background
Self-rated health (SRH) is the perception of an individual regarding their health and an indicator of health status. Identifying predictors of SRH allows the selection of evidence-based interventions that mitigate factors leading to poor SRH and the identification of individuals at risk of worse SRH.
Objective
To determine the acute predictors of general and time-comparative SRH of individuals with stroke at 3 and 12 months after hospital discharge, considering personal, physical, and mental functions.
Methods
A prospective study was developed to assess general and time-comparative SRH at 3 and 12 months after hospital discharge according to 2 questions (“In general, how would you say your health is?” and “Compared to a year ago, how would you rate your general health now?”). Potential acute predictors analyzed were personal (age, sex, comorbidities, socioeconomic status, and family arrangement), physical (stroke severity, motor impairment, and independence for basic activities of daily living [ADLs]), and mental (cognitive) functions.
Results
Age (adjusted odds ratio [aOR]=2.10) and independence in basic ADLs (aOR=0.29) were significant predictors of SRH at 3 months; at 12 months, no significant predictor was found. Motor impairment (aOR=3.90) was a significant predictor of time-comparative SRH at 3 months; at 12 months, sex (aOR=0.36) and independence in basic ADLs (aOR=0.32) were significant predictors.
Conclusions
At 3 months, individuals with stroke who were ≥65 years old and dependent on basic ADLs were more likely to have worse general SRH, while those with higher motor impairments were more likely to have worse time-comparative SRH. At 12 months, women and individuals dependent on basic ADLs were more likely to have worse time-comparative SRH.
Introduction
Stroke is a condition that causes serious disability in adults, with 12 million cases registered in the world in 2019.1 Most survivors remain with disabilities,2 which largely compromise their health and quality of life.3 In this context, several aspects regarding functioning and health should be investigated, including the self-perception of health, named self-rated health (SRH).4
SRH is an indicator of health status of the World Health Organization (WHO) that assesses the perception of an individual regarding their health.4,5 SRH is commonly assessed using simple questions that are widely used in clinical and research contexts with various objectives and populations.4,7, 8, 9 SRH encompasses relevant information related to the personal, physical, and mental functions of the individual.4,10 Identifying factors related to these functions that could be associated with better or worse SRH provides useful information to health professionals in their clinical practice, focusing on measures of health that consider the individual perspective and follow the evolution of the individual's health over time. Therefore, clinical decision-making and planned actions can be based on factors associated with a health indicator that is recommended by the WHO and is a patient-centered measure.
Only three studies11, 12, 13 investigating variables associated with better or worse SRH in individuals with stroke were found in the literature.8 Larsen et al.11 reported that a more severe stroke, comorbidities, smoking, worse education level, and high age were associated with worse general SRH in the subacute phase (three to six months after stroke). Mavaddat et al.12 reported that physical impairments, comorbidities, depression, and being of lower social class were associated with worse general SRH in the chronic phase (> six months after stroke). Finally, Araújo et al.13 reported that only depression was associated with worse general SRH in the chronic phase. Despite the important results, these studies used a cross-sectional design. Therefore, these variables cannot be considered predictors of SRH.
Identifying well-established predictors of SRH that rehabilitation strategies can modify may allow the selection of evidence-based interventions that mitigate factors leading to poor SRH. Furthermore, identifying acute predictors of SRH may help target effective treatment by defining risk groups for adverse outcomes.5,8,13,14 Finally, a complete SRH assessment for individuals with stroke requires the use of general and time-comparative questions.5 Therefore, the present study aimed to determine the acute predictors of general and time-comparative SRH of individuals with stroke at 3 and 12 months after hospital discharge, considering personal (age, sex, comorbidities, socioeconomic status, and family arrangement), physical (stroke severity, motor impairment, and independence for basic activities of daily living [ADLs]), and mental (cognitive) functions. Our findings may assist in developing new treatment strategies and public policies to improve SRH throughout the subacute (3 months) and chronic (12 months) phases following hospital discharge.
Methods
Design
This longitudinal, observational, study was conducted in a public hospital of Belo Horizonte (Minas Gerais state, Brazil). This metropolis has the third largest urban agglomeration in Brazil. This study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and was approved by the research ethics committees of the Universidade Federal de Minas Gerais and the Hospital Risoleta Tolentino Neves (CAAE: #26431319.6.0000.5149). All individuals who agreed to participate signed the informed consent form.
Participants
Individuals ≥ 20 years old, diagnosed with primary stroke confirmed by neuroimaging, and admitted to the stroke unit of the hospital between February 2020 and February 2021 were invited to participate in this study. We only included individuals without previous disabilities as defined by a Barthel Index score > 1714 and without cognitive deficits as determined by the Cognition Hetero-Anamnesis List.15 For the initial assessment, family and caregivers answered both questionnaires referring to activity performance and cognitive function of the participants immediately before the stroke, following established procedures and recommendations.14 SRH evaluation was performed by telephone at 3 and 12 months after hospital discharge. Individuals with cognitive impairments (assessed using the 22-item of the Mini-Mental State Examination, cut-off score < 1516) or aphasia (identified by the subtest of sentence comprehension of the Quick Aphasia Battery17) at 3 and 12 months were excluded.
Procedures
Within 72 h after hospital admission, individuals or their caregivers answered a semi-structured questionnaire regarding sociodemographic data (age, sex, civil status, schooling, comorbidities, socioeconomic status, and family arrangement). Clinical-functional assessments (stroke type and severity, motor impairment, independence for basic ADLs, and cognitive function) were performed using instruments applied by trained examiners and following recommended and standardized procedures.18, 19, 20, 21, 22
At 3 and 12 months after hospital discharge, individuals were contacted by telephone, and those without cognitive impairments or aphasia had their SRH assessed. These criteria were adopted because the SRH is self-reported, thus requiring adequate cognitive and language functions to ensure data reliability.
The dependent variable was general and time-comparative SRH, being evaluated by two questions of the Short- Form-36 questionnaire (“In general, how would you say your health is?” and “Compared to a year ago, how would you rate your general health now?”).18 The general SRH question was dichotomized into good and poor, with the responses "excellent," "very good," and "good" considered as "good SRH," and the responses "poor" and "very poor" as "poor SRH."5 The time-comparative SRH question was dichotomized into better and worse, with the responses "much better," "little better," and "almost the same" considered as "better SRH," and the responses "little worse" and "much worse" as "worse SRH."5 In the time-comparative SRH, for both periods (3 and 12 months), the comparison period to be considered by the patient was before the stroke.
The independent variables and potential predictors of SRH were organized according to personal, physical, and mental functions:
- Personal: 1) age (adult < 64 years, or older adults ≥ 65 years); 2) sex (male or female); 3) comorbidities (present or absent); 4) socioeconomic status according to the economic classification criteria of the Brazilian Association of Research Companies (ABEP), classified as high (A, B, and C ABEP classes: monthly family income ≥ R$1024.00 / ∼$200.00) or low (D and E ABEP classes: monthly family income < R$1024.00 / ∼$200.00)19; and 5) family arrangement (alone or accompanied).
- Physical: 1) stroke severity assessed by the National Institute Health Stroke Scale, classified as mild (≤ 3 points) or moderate to severe (4 to 42 points)20; 2) motor impairment assessed by the Fugl-Meyer Scale (FMS), classified as mild (> 79 points) or moderate to severe ≤ (79 points) motor impairment21, 22, 23; and 3) independence in basic ADLs assessed by the Modified Barthel Index, classified as full to moderate dependence (≤ 45 points) or slight dependence to total independence (46 to 50 points).24
-Mental: cognitive function assessed by the MMSE,16 classified as high (≤ 23 points) or moderate (24 to 30 points).
Sample size
For the sample size calculation, nine independent variables were considered as possible predictors for general and time-comparative SRH, including five personal (age, sex, comorbidities, socioeconomic status, and family arrangement), three physical (stroke severity, motor impairment, and independence in basic ADLs), and one mental (cognitive function). The formula used was P = (n + 1)*10, where "n" is the number of independent variables inserted in the model. At least 100 individuals were estimated to be evaluated at 3 and 12 months after hospital discharge. However, a loss of follow-up of 50% was considered, totaling 150 individuals to be recruited and included in this study.
Statistical analysis
Descriptive statistics and normality tests (Kolmogorov-Smirnov) were conducted for all variables. Binary logistic regression (stepwise method) was used to identify the general and time comparative SRH predictors at 3 and 12 months after hospital discharge, resulting in four regression models, all including the nine independent variables. Multicollinearity was verified considering tolerance value > 0.1 and variance inflation factors (VIF) < 10. The association between the dependent and independent variables was adjusted considering χ2, p, R2 Nagelkerke, and Hosmer–Lemeshow. The results were presented in odds ratio (OR) with a confidence interval of 95% (95% CI). All statistical analyses were performed using the SPSS software (SPSS Inc., Chicago, IL, USA), version 20, considering a significance level of α = 0.05.
Results
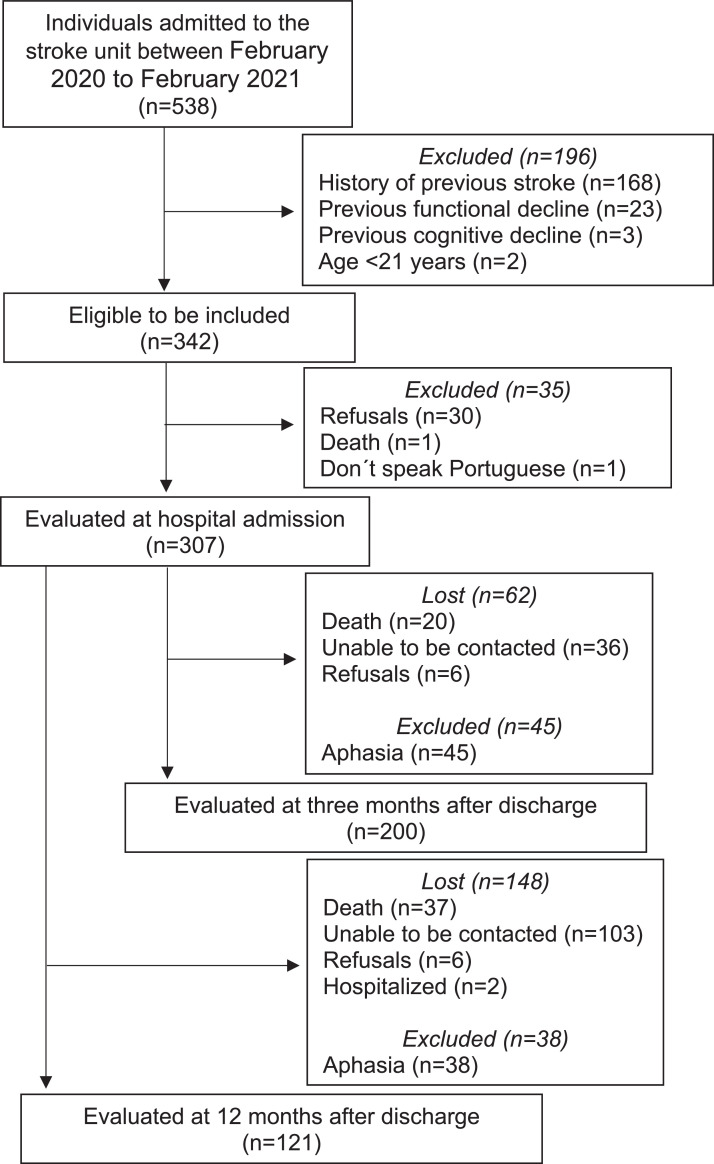
Of the 538 individuals admitted to the stroke unit during the recruitment period, 342 met the eligibility criteria, and 307 were evaluated. General and time-comparative SRH were assessed in 200 and 121 individuals at 3 and 12 months, respectively. Of the 107 participants who were not assessed at the first follow-up, 62 were lost and 45 excluded. Of the 186 participants who were not evaluated in the second follow-up, 148 were lost and 38 were excluded. The detailed reasons and respective values are shown in Fig. 1. No statistical differences were found in the baseline characteristics between individuals who dropped out and those who remained in the study during the follow-up. The sociodemographic and clinical-functional characteristics of the individuals included in the study are described in Table 1.
Fig. 1.
Flow of the participants thorughout the study.
Table 1.
Sociodemographic and clinical-functional characteristics of the sample.
| Variables | At 3 months (n = 200) | At 12 months (n = 121) | |
|---|---|---|---|
| Age (years), mean (SD) | 61.1 (14.8) | 59.6 (14.9) | |
| Sex | Men, n (%) | 107 (53.8%) | 53 (43.8%) |
| Women, n (%) | 93 (46.2%) | 68 (56.2%) | |
| Civil status, n (%) | Married | 101 (50.8%) | 67 (55.3%) |
| Single | 35 (17.6%) | 18 (14.9%) | |
| Widower | 34 (17.1%) | 18 (14.9%) | |
| Separated | 30 (14.5%) | 18 (14.9%) | |
| Schooling, n (%) | Illiterate | 24 (11.5%) | 14 (11.6%) |
| From 1 to 4 years | 87 (43.7%) | 47 (38.8%) | |
| From 5 to 7 years | 33 (16.6%) | 25 (20.7%) | |
| From 8 to 10 years | 29 (14.6%) | 22 (18.2%) | |
| 11 or more years | 27 (13.6%) | 13 (10.7%) | |
| Stroke type, n (%) | Ischemic | 173 (86.9%) | 103 (85.1%) |
| Hemorrhagic | 27 (13.1%) | 18 (14.9%) | |
| Stroke severity (NIHSS), n (%) | Mild (≤3) | 120 (60.0%) | 74 (61.2%) |
| Moderate to severe (4–42) | 80 (30.0%) | 47 (38.8%) | |
| General SRH, n (%) | Good | 149 (74.5%) | 102 (84.2%) |
| Bad | 51 (25.5%) | 19 (15.8%) | |
| Time comparative SRH, n (%) | Better | 109 (54.5%) | 77 (63.7%) |
| Worse | 91 (45.5%) | 44 (36.3%) | |
NIHSS, National Institute Health Stroke Scale; SD, standard deviation; SRH, self-rated health.
The four regression models met the assumptions of error independence, linearity, and absence of multicollinearity (VIF values ranging from 1.0 to 1.2). At 3 months, age and independence in basic ADLs were significant predictors of general SRH (X2 = 18.78; p < 0.001; R² Nagelkerke = 0.146; Hosmer–Lemeshow test = 0.979). Individuals ≥ 65 years had a greater chance of having worse general SRH (adjusted OR = 2.10), whereas individuals independent in basic ADLs had a lesser chance of having worse general SRH (adjusted OR = 0.29). No significant predictor was found for general SRH at 12 months (Table 2).
Table 2.
Results of binary logistic regression models.
| SRH | Time after hospital discharge | Predictors | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| General SRH | 3 months | Age | 2.10 (1.04, 4.26) | 0.04 |
| Independence for basic activities of daily living | 0.29 (0.14, 0.58) | 0.001 | ||
| 12 months | -* | – | – | |
| Time-comparative SRH | 3 months | Motor impairment | 3.90 (2.01, 7.60) | 0.001 |
| 12 months | Sex | 0.36 (0.14, 0.93) | 0.030 | |
| Independence for basic activities of daily living | 0.32 (0.13, 0.82) | 0.020 |
95% CI, 95% confidence interval; OR, odds ratio; SRH, self-rated health.
No significant predictor was found.
Motor impairment was a significant predictor of time-comparative SRH at 3 months, (X2 = 17.50; p < 0.001; R² Nagelkerke = 0.124; Teste de Hosmer–Lemeshow = 0.979). Individuals with higher motor impairments were more likely to have worse time-comparative SRH (adjusted OR = 3.90). Sex and independence in basic ADLs were significant predictors of time comparative SRH at 12 months (X2 = 12.06; p = 0.002; R² Nagelkerke = 0.166; Teste de Hosmer–Lemeshow = 0.995). Women (adjusted OR = 0.36) and individuals dependent on basic ADLs (adjusted OR = 0.32) were more likely to have worse time-comparative SRH.
Discussion
The present study aimed to determine the acute predictors of general and time-comparative SRH of individuals with stroke at 3 and 12 months after hospital discharge, considering personal, physical, and mental functions. Regarding the general SRH, age and independence in basic ADLs were significant predictors at 3 months. No significant predictors were found for general SRH at 12 months. Regarding the time-comparative SRH, motor impairment was a significant predictor at 3 months, and sex and independence in basic ADLs were at 12 months.
At 3 months, age was a significant and strong (aOR=2.10) predictor of general SRH, indicating that individuals ≥ 65 years old are more likely to have a worse SRH. One previous study also found age as predictor of general SRH in individuals with chronic stroke (aOR=0.99),6 indicating that older age seems to contribute to a worse general SRH. A possible explanation is that older adults, especially in Brazil, are often exposed to different types of violence, lack of specialized medical care, low retirement and pension incomes, and few leisure opportunities.25 These factors can lead to a negative self-perception of health, especially for those with chronic diseases, such as stroke. In addition, older adults are also more vulnerable to developing other diseases, which can generate more disabilities, limitations, and restrictions that impact their general SRH.26
Thus, although age is not a modifiable variable, public policies aimed at preventing and promoting health throughout the ageing process should be increasingly encouraged and valued.
Independence in basic ADLs was also a significant predictor of general SRH at 3 months and for time-comparative SRH at 12 months, indicating that independent individuals are less likely to have a worse general SRH. One previous study also found a significant association between independence and general SRH in individuals with chronic stroke,6 indicating that dependence level also contributes to worse general SRH. This may occur because more dependent individuals commonly have limitations in activities performed in and outside the home environment, and restrictions on social participation.27 These limitations can generate disabilities and affect personal, physical, and mental functions,27 which are part of the health concept according to the WHO.
At 12 months, no significant predictors were identified for general SRH. A possible explanation is that the time-comparative SRH question is specific, and the general SRH question is broad. Thus, other variables of the acute phase not considered in the present study may be significant predictors, such as depression. Furthermore, the recovery of individuals after the stroke varies according to each phase (hyperacute, acute, early subacute, late subacute, and chronic).28 Consequently, it is possible that the general SRH at 12 months is predicted by variables obtained in more advanced stages of the condition (3 or 6 months after the stroke).
At 3 months, motor impairment was a significant and strong (aOR=3.90) predictor of time-comparative SRH, indicating that individuals with greater motor impairments are more likely to have worse SRH. No previous study investigated associations between motor impairment and SRH after stroke. However, individuals after stroke commonly present motor impairments, which limit their ADLs, restrict their social participation, and may be associated with a worse perception of health.27 Thus, interventions focused on these impairments need to be prioritized in the acute period of the stroke.
Finally, at 12 months, sex was a significant predictor of time-comparative SRH, indicating that women are more likely to have worse SRH. This result differs from the findings of Bjälkefur et al.,6 probably because these authors evaluated general SRH, while the present study rated the time-comparative SRH. Furthermore, cultural differences may also explain this variation. According to the National Health Survey of 2019, in Brazil, women seek health services more than men (22.1% versus 14.8%).29 Therefore, women possibly tend to self-evaluate health more carefully than men. Lastly, women have higher rates of comorbidities after a stroke when compared to men, which may also justify worsened SRH perception among this group.30 This reasoning is corroborated by a previous Brazilian study reporting that men tend to worry less about their health31 and may reflect less on their time-comparative SRH.
Among the acute predictors of SRH identified in this study, independence in basic ADLs and motor impairment are aspects modifiable by health professionals over time. Previous studies have shown that muscle strength training32 positively affects these outcomes (independence in basic ADLs and motor impairment) in individuals with stroke. Therefore, if implemented immediately after stroke, it may positively impact general and time-comparative SRH at 3 and 12 months after hospital discharge. Future studies should investigate the effectiveness of these interventions to improve the general and time-comparative SRH in individuals with stroke.
Limitation
To our knowledge, this study was the first to investigate the acute predictors of SRH in individuals with stroke in the subacute and chronic phases, considering personal, physical, and mental functions and using two questions of the SRH. The first limitation of this study was the large drop-out rate of participants mainly due to the difficulty of contacting individuals by telephone, which limits the generalization of the results. Also, other variables not evaluated by this study, such as depression, may be predictors of general and time-comparative SRH. Finally, the individuals were recruited in only one hospital. Although this was a reference hospital for urgent and emergency care offering a stroke unit and specialized care to this population, future studies could purposedly collect data from various health settings relevant to neurologic care.
Conclusions
Individuals ≥ 65 years old and dependent on basic ADLs were more likely to have a worse general SRH at 3 months. Individuals with higher motor impairments were more likely to have worse time-comparative SRH at 3 months, while women and individuals dependent on basic ADLs were more likely to have worse time-comparative SRH at 12 months. The strongest predictors were age for general SRH at 3 months and motor impairments for time-comparative SRH at 3 months. Therefore, actions directed at elderly people and for motor impairments must be implemented immediately after the stroke.
Conflicts of interest
Nothing to declare.
Acknowledgements
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [Code #001]; Fundação de Amparo à Pesquisa do Estado de Minas Gerais [grants PPM-00496-17 and APQ-00736-20]; Conselho Nacional de Desenvolvimento Científico e Tecnológico [grant # 308516/2021-4]; and Pró-reitoria de Pesquisa da Universidade Federal de Minas Gerais [grant #05/2021 and #09/2021].
References
- 1.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick P.B. The global burden of stroke: persistent and disabling. Lancet Neurol. 2019;18:417–418. doi: 10.1016/S1474-4422(19)30030-4. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho-Pinto Bárbara P.B., Faria Christina D.C.M. Health, function and disability in stroke patients in the community. Braz J Phys Ther. 2016;20:355–366. doi: 10.1590/bjpt-rbf.2014.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med. 2009;69:307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Viana R.T., de Freitas Araújo É., Lima L.A.O., Teixeira-Salmela LF, de Morais Faria CDC. General and comparative self-rated health in chronic stroke: an important outcome measure for health professionals. BMC Neurol. 2022;22:78. doi: 10.1186/s12883-022-02592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjälkefur K., Nasic S., Bertholds E., Jood K., Rejnö Å. Self-rated health over the first five years after stroke. BMC Neurol. 2020;20:389. doi: 10.1186/s12883-020-01956-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagotto V., Bachion M.M., Silveira E.A. Self-assessment of health by older Brazilians: systematic review of the literature. Rev Panam Salud Publica. 2013;33:302–310. doi: 10.1590/s1020-49892013000400010. [DOI] [PubMed] [Google Scholar]
- 8.Araújo É.F., Viana R.T., Teixeira-Salmela L.F., Lima L.A.O., Faria C.D.C.M. Self-rated health after stroke: a systematic review of the literature. BMC Neurol. 2019;19:221. doi: 10.1186/s12883-019-1448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomioka K., Kurumatani N., Hosoi H. Self-rated health predicts decline in instrumental activities of daily living among high-functioning community-dwelling older people. Age Ageing. 2017;46:265–270. doi: 10.1093/ageing/afw164. [DOI] [PubMed] [Google Scholar]
- 10.Bailis D.S., Segall A., Chipperfield J.G. Two views of self-rated general health status. Soc Sci Med. 2003;56:203–217. doi: 10.1016/s0277-9536(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 11.Larsen L.P., Johnsen S.P., Andersen G., Hjollund N.H. Determinants of self-rated health three months after stroke. J Stroke Cerebrovasc Dis. 2016;25:1027–1034. doi: 10.1016/j.jstrokecerebrovasdis.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Mavaddat N., Van der Linde R., Savva G.M., Brayne C., Mant J. What determines the self-rated health of older individuals with stroke compared to other older individuals? A cross-sectional analysis of the Medical Research Council Cognitive Function and Aging Study. BMC Geriatr. 2013;13:85. doi: 10.1186/1471-2318-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araújo É., Viana R.T., da Cruz C.F., Ferreira de Brito S.A., Ferreira Dos Reis M.T., de Morais Faria C.D. Self-rated health determinants in post-stroke individuals. J Rehabil Med. 2020;52 doi: 10.2340/16501977-2712. jrm00080. [DOI] [PubMed] [Google Scholar]
- 14.van Mierlo M.L., van Heugten C.M., Post M.W., Lindeman E., de Kort P.L., Visser-Meily J.M. A longitudinal cohort study on quality of life in stroke patients and their partners: restore4Stroke Cohort. Int J Stroke. 2014;9:148–154. doi: 10.1111/j.1747-4949.2012.00882.x. [DOI] [PubMed] [Google Scholar]
- 15.Meijer R., van Limbeek J., de Haan R. Development of the Stroke-unit Discharge Guideline: choice of assessment instruments for prediction in the subacute phase post-stroke. Int J Rehabil Res. 2006;29:1–8. doi: 10.1097/01.mrr.0000175269.59788.41. [DOI] [PubMed] [Google Scholar]
- 16.Camozzato A.L., Kochhann R., Godinho C., Costa A., Chaves M.L. Validation of a telephone screening test for Alzheimer's disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2011;18:180–194. doi: 10.1080/13825585.2010.521814. [DOI] [PubMed] [Google Scholar]
- 17.Wilson S.M., Eriksson D.K., Schneck S.M., Lucanie J.M. A quick aphasia battery for efficient, reliable, and multidimensional assessment of language function. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciconelli R.M., Ferraz M.B., Santos W., Meinão I., Quaresma M.R. Brazilian-Portuguese version of the SF-36. A reliable and valid quality of life outcome measure. Rev Bras Reumatol. 1999;39:143–150. [Google Scholar]
- 19.Associação Brasileira de Empresa e Pesquisa. Critério Brasil - ABEP 2021. Retrieved January 7, 2022, from: http://www.abep.org/criterio-brasil.
- 20.Cincura C., Pontes-Neto O.M., Neville I.S., et al. J. Validation of the National Institutes of Health Stroke Scale, modified Rankin Scale and Barthel Index in Brazil: the role of cultural adaptation and structured interviewing. Cerebrovasc Dis. 2009;27:119–122. doi: 10.1159/000177918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maki T., Quagliato E., Cacho E., et al. Reliability study on the application of the Fugl-Meyer scale in Brazil. Braz J Phys Ther. 2006;10:177–183. [Google Scholar]
- 22.Hernández E.D., Forero S.M., Galeano C.P., Barbosa N.E., Sunnerhagen K.S. Alt Murphy M. Intra- and inter-rater reliability of Fugl-Meyer Assessment of Lower Extremity early after stroke. Braz J Phys Ther. 2021;25:709–718. doi: 10.1016/j.bjpt.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaelsen S.M., Rocha A.S., Knabben R.J., Rodrigues L.P., Fernandes C.G. Translation, adaptation and inter-rater reliability of the administration manual for the Fugl-Meyer assessment. Rev Bras Fisioter. 2011;15:80–88. [PubMed] [Google Scholar]
- 24.Guimarães R.B., Guimarães R.B. Brazilian versions of stroke scales and clinical assessment tools: a standardization attempt plus improvement of the quality of life. Rev Bras Neurol. 2004;40:5–13. [Google Scholar]
- 25.Veras R. Population aging today: demands, challenges and innovations. Rev Saúde Pública. 2009;43:548–554. doi: 10.1590/s0034-89102009000300020. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain A.M., Rutten L.J.F., Jacobson D.J., et al. Multimorbidity, functional limitations, and outcomes: interactions in a population-based cohort of older adults. J Comorb. 2019;9 doi: 10.1177/2235042X19873486. 2235042×19873486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wurzinger H., Abzhandadze T., Rafsten L., Sunnerhagen K.S. Dependency in activities of daily living during the first year after stroke. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.736684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernhardt J., Hayward K.S., Kwakkel G., et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Recovery and Rehabilitation Roundtable taskforce. Int J Stroke. 2017;12:444–450. doi: 10.1177/1747493017711816. [DOI] [PubMed] [Google Scholar]
- 29.IBGE B. Coordenação de Trabalho e Rendimento. Rio de Janeiro: IBGE; 2020. Pesquisa Nacional De saúde: 2019; Informações Sobre domicílios, Acesso e Utilização Dos Serviços De saúde: Brasil, Grandes Regiões e Unidades Da federação/IBGE. [Google Scholar]
- 30.Rathfoot C., Edrissi C., Sanders C.B., Knisely K., Poupore N., Nathaniel T. Gender differences in comorbidities and risk factors in ischemic stroke patients with a history of atrial fibrillation. BMC Neurol. 2021;21:209. doi: 10.1186/s12883-021-02214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinheiro R.S., Viacava F., Travassos C., Brito A.D.S. Gender, morbidity, access and utilization of health services in Brazil. Ciênc Saúde Coletiva. 2002;7:687–707. [Google Scholar]
- 32.Ada L., Dorsch S., Canning C.G. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust J Physiother. 2006;52:241–248. doi: 10.1016/s0004-9514(06)70003-4. [DOI] [PubMed] [Google Scholar]