Highlights
-
•
Despite its effectiveness, young adults are hesitant to take the COVID-19 vaccine.
-
•
We examined adverse events and potential risk factors after COVID-19 vaccination.
-
•
Higher number of vaccinations is associated with adverse events post vaccination.
-
•
Female sex, and low body mass index are associated with adverse events.
-
•
Regular breakfast and sufficient sleep were associated with fewer adverse events.
-
•
Healthy lifestyle may reduce incidence of adverse events in Japanese adults.
Keywords: Adverse events, Body mass index, COVID-19, Vaccinations
Abstract
Introduction
Young adults are hesitant to receive the coronavirus disease 2019 (COVID-19) vaccination owing to concerns regarding adverse events despite the effectiveness of vaccines in preventing SARS-CoV-2 infection-associated serious illness, hospitalization, and death.
Methods
A retrospective cohort study was conducted in Gifu University students receiving the mRNA-1273 vaccine and boosters to elucidate the real incidence of adverse events and factors that prevent them. We examined the adverse events and identified potential risk factors through a self-administered questionnaire on the participants’ physical condition after COVID-19 vaccination.
Results
Focal/systemic adverse events were highly frequent among university students after receiving the COVID-19 vaccine; however, there were no life-threatening cases or hospitalizations over two years. A higher number of vaccinations (p < 0.001), female sex (p < 0.001), and lower body mass index (BMI) (p = 0.002) were associated with an increased incidence of adverse events on the day of COVID-19 vaccination or the day after vaccination. Regular breakfast consumption was significantly associated with a decreased incidence of post-vaccination itching (p = 0.019) and abdominal pain and diarrhea (p = 0.042). Sufficient sleep duration was significantly associated with a decreased incidence of post-vaccination abdominal pain and diarrhea (p = 0.042).
Conclusions
High frequency of adverse events of COVID-19 mRNA-1273 among Japanese university students was reported. A higher number of shots, female sex, and lower BMI were associated with a higher incidence of adverse events. Regular breakfast and sufficient sleep were associated with fewer adverse events. This study may provide a possible solution to the worldwide problem of vaccine hesitancy.
Introduction
The clinical vaccine for coronavirus disease 2019 (COVID-19) was developed after the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in December 2019, which caused global devastation. One of the COVID-19 vaccines, mRNA-1273 (Moderna), exhibited 94.1 % efficacy in preventing COVID-19 illness [1] for over 5 months, without safety concerns [2]. However, frequent local and systemic reactions were reported in surveillance conducted by the Centers for Disease Control and Prevention after the vaccine received the Emergency Use Authorization by the US Food and Drug Administration as a two-dose series [3]. Frequent injection site reactions, including pain and systemic reactions such as headache and fatigue, were reported among adolescents (12–17 years of age). In particular, injection-site pain reaching grade 3 severity has been reported in approximately 5 % of individuals who received the COVID-19 vaccine [4].
Although the efficacy, safety, and immunogenicity of the COVID-19 and booster vaccines are established [5], [6], a substantial percentage of people worldwide have concerns [7], hesitation [8], and fear [9]. Moreover, concerns regarding adverse events may become barriers and decrease the COVID-19 vaccine/booster shot rate in young adults since the younger generation is more apprehensive and hesitant. Therefore, real data on adverse events, with frequency and life-threatening levels and their associated factors should be provided to the young population to reduce these unfavorable feelings. However, little is known about the factors associated with adverse events associated with the SARS-CoV-2 vaccine among young adults.
We aimed to analyze the adverse event surveillance data of Japanese university students who received the first shot and second/third booster shots of the mRNA-1273 SARS-CoV-2 vaccine to assess the real reactogenicity among young adults. Additionally, we aimed to analyze the relationship between adverse events and health background information, including lifestyle factors, retrieved from the annual health check-up data and identify factors that should be considered before vaccination. The study results will provide a scientific basis for lifestyle recommendations regarding the vaccination of students and young adults in the context of health education and vaccination promotion.
Material and Methods
All study procedures complied with the ethical requirements of the national and institutional committees that oversee human studies and with the 1964 Declaration of Helsinki and its later revisions. The study design was reviewed and approved by the Ethical Review Committee of the Graduate School of Medicine, Gifu University, Japan (no. 2021-B160), and the requirement for obtaining informed consent was waived owing to the retrospective nature of the study. The research details were made accessible to the participants, and they were provided with the opportunity to decline participation in this study. They were notified that their responses would remain confidential and would have no effect on their academic performance. There was no compensation for participants.
Participants and study procedures
This study was conducted on all students who had COVID-19 vaccine from July 12, 2021 to March 23, 2022, at Gifu University – a national general university in Japan. The STROBE guidelines were followed in the preparation of this study [10]. Vaccine shot service with mRNA-1273 (Moderna) was provided for Gifu University students in three rounds: the first from July 12, 2021 to July 21, 2021; second, August 9, 2021 to August 20, 2021; and third, March 15, 2022 to March 23, 2022. Participants were invited to complete a questionnaire (Supplemental File: Post-vaccination Questionnaires) related to the post-vaccination physical condition using Microsoft Forms on the day of vaccination and the following day. The first, second, and third post-vaccination questionnaires were administered from July 12, 2021, to July 30, 2021; August 9, 2021, to August 27, 2021; and March 14, 2022, to March 31, 2022; respectively. All students who had vaccine shots were invited to complete an online self-answered questionnaire on the post-vaccination physical condition immediately after the first, second, and third shots. The web-based post-vaccination questionnaires sites were opened from July 12 to 30, 2021; August 9 to 27, 2021; and March 14 to 31, 2022 for the first, second, and third shots recipients, respectively. The participants answered the questions about symptoms after vaccination; local reactions including redness / swelling / induration / pain / heat sensation / itch and systemic reactions including headache / general fatigue / fever over 37.5 °C / nausea / joint or muscle pain / abdominal pain or diarrhea on the vaccination day and the next day in a digital form (Appendix) according to a systematic review of adverse events reports [11]. The responses of students whose health checkup records could be verified were selected for the study among the students who responded to the questionnaire.
The following inclusion criteria were used to select the participants included the final analysis: a student who had at least the first shot of the mRNA-1273 SARS-CoV-2 vaccine at Gifu University in July 2021, answered the post-vaccination questionnaire at least once, and completed all the examination items of the annual health checkup in 2021–2022.
Participants’ health background
Participants’ health backgrounds (self-reported sex, age, and past/present illness) and body mass index (BMI) calculated as measured body weight (kg) ÷ [measured height (m)]2 were collected during the annual health check-up, which is mandated by the Japanese School Health and Safety Act for all students in Japan. Information on lifestyle, including exercise/breakfast habits, sleeping duration, and allergy, were also recorded at the annual health checkup as responses to the following questions: “how often did you exercise till you got sweaty, including walking for approximately > 30 min, per week in the previous month?” (choices: 0 day, 1 day, 2–5 days, 6–7 days = almost every day), “how often did you have breakfast per week in the previous month?” (choices: < 1 day, 2–3 days, 4–5 days, 6–7 days = almost every day), “how long did you typically sleep on weekdays in the previous month?” (choices: < 5 h, 6 h, 7 h, 8 h, 9 h, 10 h, > 11 h), and “have you ever had food/drug allergies or anaphylaxis?” (choices: no, yes; if yes (description)).
Statistical analyses
The characteristics of the participants are summarized using the median and interquartile ranges (IQR) for continuous variables. Categorical variables are summarized by frequency. Generalized estimating equations (GEE) were used to confirm the association of risk factors, such as the number of vaccinations, sex, BMI, exercise habits, frequency of breakfast consumption per week, history of allergies and anaphylaxis, and hours of sleep per day, with the self-reported presence of at least one symptom related to the vaccination site and systemic symptoms on the day of or the day after vaccination. These variables were simultaneously included in the model. Statistical significance was defined as two-sided P < 0.05. All statistical analyses were performed using R software (version 4.2.1; https://www.r-project.org, The R Project for Statistical Computing).
Results
The COVID-19 vaccination program at Gifu University and the participants
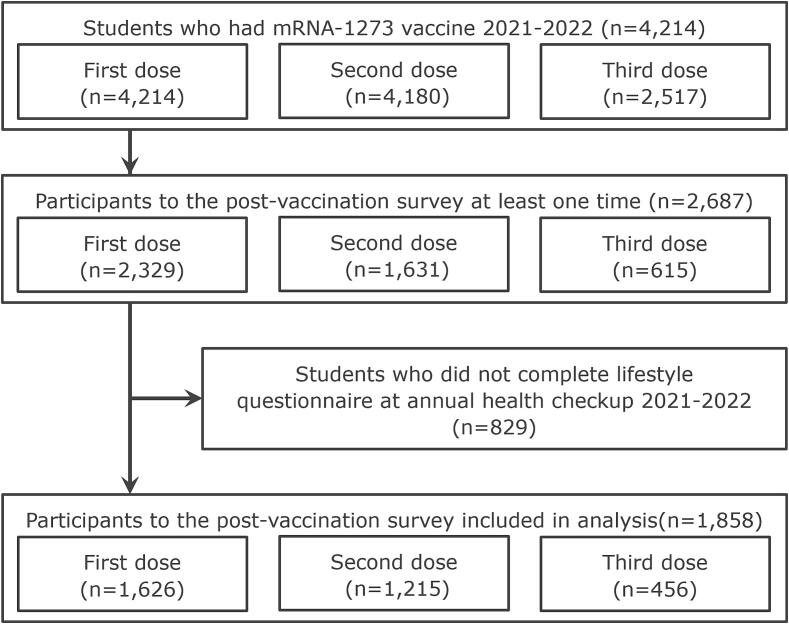
The COVID-19 vaccination program at Gifu University involved three rounds of vaccination: 4,214, 4,180, and 2,517 individuals underwent the first, second, and third rounds, respectively. Among these individuals, 2,329 (55.3 %), 1,631 (39.0 %), and 615 (24.4 %) responded for the post-first, post-second, and post-third vaccination questionnaire, respectively. In total, 2,687 individuals responded to the post-vaccination survey at least one time. Eight hundred and twenty-nine individuals had missing data of annual health checkups among a total of 2,687 individuals; therefore, 1,858 (1,004 males and 854 females, median age 20 years) met the inclusion criteria. The charts of 1,626 participants (38.6 %) from the first round, 1,215 (29.1 %) from the second, and 456 (18.1 %) from the third were included in the analysis (Figure 1).
Fig. 1.
Study flow chart. Students were administered the mRNA-1273 vaccine in the period 2021–2022 (n = 4,214). Participants that completed the post-vaccination survey at least once were enrolled. A total of 2,687 students who received COVID-19 vaccination at Gifu University responded to the questionnaire. Among them, 829 students did not complete the lifestyle questionnaire at the annual health checkup and were excluded, resulting in a final sample of 1,858 students who participated in the post-vaccination survey. Of these, there were 1,626, 1,215, and 456 responses after the first, second, and third doses, respectively.
Background characteristics of the participants
The background characteristics of the participants are listed in Table 1. The median age was 20 years (IQR: 18–22), 854 (46.0 %) participants were women, and the median BMI was 20.3 (IQR: 18.8–22.2) kg/m2. A history of allergies was reported by 110 participants (5.9 %). The median sleep duration was 7 h (IQR: 6.0–7.0). Exercise frequency was reported as zero in 552 (29.7 %) participants; once a week in 539 (29.0 %) participants; 2–5 times a week in 669 (36.0 %) participants; and 6–7 times a week in 97 (5.2 %) participants. The frequency of consuming breakfast was less than once a week in 165 participants (8.9 %); 2–3 times a week in 194 participants (10.4 %); 4–5 times a week in 375 participants (20.2 %); and daily in 1,123 participants (60.5 %). The background characteristics of the group of students at Gifu University who underwent health checkups during the 2021–2022 season but did not respond to the questionnaire are presented in Table 2. There were no significant differences in the background characteristics between the participants who responded to the questionnaire and those who did not.
Table 1.
Participant characteristics.
| Characteristics | First dose, N = 1,626 |
Second dose, N = 1,215 |
Third dose, N = 456 |
Total, N = 1,858 |
|---|---|---|---|---|
| Age, year | 20 (18, 22) | 20 (18, 22) | 20 (18, 22) | 20 (18, 22) |
| Female | 739 (45.4 %) | 557 (45.8 %) | 228 (50.0 %) | 854 (46.0 %) |
| BMI | 20.3 (18.8, 22.2) | 20.3 (18.7, 22.2) | 20.3 (18.7, 22.0) | 20.3 (18.8, 22.2) |
| BMI category | ||||
| <18.5 | 338 (20.8 %) | 260 (21.4 %) | 96 (21.1 %) | 384 (20.7 %) |
| 18.5–24.9 | 1,161 (71.4 %) | 859 (70.7 %) | 324 (71.1 %) | 1,332 (71.7 %) |
| >25.0 | 127 (7.8 %) | 96 (7.9 %) | 36 (7.9 %) | 142 (7.6 %) |
| History of allergy | 89 (5.5 %) | 73 (6.0 %) | 25 (5.5 %) | 110 (5.9 %) |
| Hours of sleep | 7.0 (6.0, 7.0) | 7.0 (6.0, 7.0) | 6.0 (6.0, 7.0) | 7.0 (6.0, 7.0) |
| Exercise habits | ||||
| None | 484 (29.8 %) | 366 (30.1 %) | 151 (33.1 %) | 552 (29.7 %) |
| Once a week | 482 (29.7 %) | 360 (29.7 %) | 114 (25.0 %) | 539 (29.0 %) |
| 2–5 times a week | 573 (35.3 %) | 422 (34.8 %) | 165 (36.2 %) | 669 (36.0 %) |
| 6–7 times a week | 86 (5.3 %) | 66 (5.4 %) | 26 (5.7 %) | 97 (5.2 %) |
| Habit of consuming breakfast | ||||
| Less than once a week | 148 (9.1 %) | 92 (7.6 %) | 42 (9.2 %) | 165 (8.9 %) |
| 2–3 times a week | 158 (9.7 %) | 125 (10.3 %) | 41 (9.0 %) | 194 (10.4 %) |
| 4–5 times a week | 327 (20.1 %) | 242 (19.9 %) | 96 (21.1 %) | 375 (20.2 %) |
| Every day | 992 (61.0 %) | 756 (62.2 %) | 277 (60.7 %) | 1,123 (60.5 %) |
Values are presented as numbers (percentage) or median (IQR; Interquartile range); BMI, body mass index
Table 2.
Health checkup data of 1,858 student’s health checkup data who were administered the COVID-19 vaccine.
| Characteristics | n | |
|---|---|---|
| Age, year | 3,281 | 20 (19–22) |
| Sex | 3,281 | |
| Female | 1,340 (40.8 %) | |
| Male | 1,941 (59.2 %) | |
| BMI | 3,281 | 20.4 (18.9–22.4) |
| History of allergy | 2,239 | 155 (6.9 %) |
| Hours of sleep | 2,230 | 7.0 (6.0–7.0) |
| Exercise habits | 2,239 | |
| None | 610 (27.2 %) | |
| Once a week | 643 (28.7 %) | |
| 2–5 times a week | 840 (37.5 %) | |
| 6–7 times a week | 146 (6.5 %) | |
| Habit of consuming breakfast | 2,240 | |
| Less than once a week | 279 (12.5 %) | |
| 2–3 times a week | 276 (12.3 %) | |
| 4–5 times a week | 429 (19.2 %) | |
| Every day | 1,256 (56.1 %) |
Values are presented as numbers (percentage) or median (IQR; Interquartile range); BMI, body mass index; COVID-19, coronavirus disease 2019
The incidence of local and systemic symptoms on the day of COVID-19 vaccination and the next day after vaccination
The symptoms observed on the day of vaccination or the next day after vaccination are summarized in Table 3. Among all the respondents, local and systemic symptoms were observed in 1,666 (89.7 %) and 1,195 (64.3 %) participants, respectively, on the day of vaccination and/or the next day after vaccination. On the day of vaccination, local and systemic symptoms were observed in 1,534 (82.6 %) and 816 participants (43.9 %), respectively. Overall, no life-threatening cases or hospitalizations, which could suggest delayed adverse events, were reported for two years since March 23, 2022.
Table 3.
Symptoms on the day of COVID-19 vaccination or the next day after vaccination.
| Symptoms | First dose, N = 1,626 |
Second dose, N = 1,215 |
Third dose, N = 456 |
Total, N = 1,858 |
|---|---|---|---|---|
| The day of vaccination or the next day after vaccination | ||||
| Any vaccination site symptoms | 1,458 (89.7 %) | 1,118 (92.0 %) | 432 (94.7 %) | 1,666 (89.7 %) |
| Redness | 145 (8.9 %) | 342 (28.1 %) | 82 (18.0 %) | 198 (10.7 %) |
| Swelling | 344 (21.2 %) | 523 (43.0 %) | 189 (41.4 %) | 437 (23.5 %) |
| Induration | 160 (9.8 %) | 138 (11.4 %) | 62 (13.6 %) | 188 (10.1 %) |
| Pain | 1,400 (86.1 %) | 1,025 (84.4 %) | 409 (89.7 %) | 1,582 (85.1 %) |
| Heat sensation | 288 (17.7 %) | 390 (32.1 %) | 103 (22.6 %) | 359 (19.3 %) |
| Itch | 91 (5.6 %) | 138 (11.4 %) | 37 (8.1 %) | 116 (6.2 %) |
| Any systemic symptoms | 989 (60.8 %) | 1,133 (93.3 %) | 399 (87.5 %) | 1,195 (64.3 %) |
| Headache | 433 (26.6 %) | 857 (70.5 %) | 297 (65.1 %) | 588 (31.6 %) |
| General fatigue | 612 (37.6 %) | 919 (75.6 %) | 327 (71.7 %) | 786 (42.3 %) |
| Fever | 272 (16.7 %) | 970 (79.8 %) | 256 (56.1 %) | 435 (23.4 %) |
| Nausea | 66 (4.1 %) | 142 (11.7 %) | 46 (10.1 %) | 92 (5.0 %) |
| Joint and muscle pain | 595 (36.6 %) | 705 (58.0 %) | 239 (52.4 %) | 728 (39.2 %) |
| Abdominal pain and diarrhea | 43 (2.6 %) | 93 (7.7 %) | 20 (4.4 %) | 58 (3.1 %) |
| The day of vaccination | ||||
| Vaccination site symptoms | 1,344 (82.7 %) | 1,032 (84.9 %) | 409 (89.7 %) | 1,534 (82.6 %) |
| Systemic symptoms | 665 (40.9 %) | 802 (66.0 %) | 290 (63.6 %) | 816 (43.9 %) |
Values are presented as numbers (percentages)
Factors associated with post-vaccination local and systemic symptoms on the day of COVID-19 vaccination or the next day after vaccination
The incidence of local symptoms on the day of vaccination or the next day after vaccination was significantly higher among those who received multiple doses (odds ratio [OR]: 1.355; 95 % confidence interval [CI]: 1.153–1.592; P < 0.001) and among women (OR: 2.436; 95 % CI: 1.797–3.302; P < 0.001) (Table 4). Similarly, receiving multiple doses (OR: 4.046; 95 % CI: 3.321–4.928; P < 0.001) and being female (OR: 1.610; 95 % CI: 1.340–1.935; P < 0.001) were associated with significantly higher incidence of systemic symptoms. However, increased BMI (OR: 0.956; 95 % CI: 0.929–0.984; P < 0.001) was associated with significantly lower incidence of systemic symptoms (Table 4).
Table 4.
Factors associated with symptoms on the day of COVID-19 vaccination or the next day after vaccination.
| OR | (95 % CI) | P values | |
|---|---|---|---|
| Vaccination site symptoms | |||
| Number of vaccinations | 1.355 | (1.153–1.592) | <0.001 |
| Female | 2.436 | (1.797–3.302) | <0.001 |
| BMI | 0.994 | (0.946–1.045) | 0.814 |
| Exercise habits | |||
| Once a week | 0.824 | (0.575–1.181) | 0.291 |
| 2–5 times a week | 0.919 | (0.641–1.317) | 0.644 |
| 6–7 times a week | 0.853 | (0.456–1.596) | 0.619 |
| Habit of consuming breakfast | |||
| 2–3 times a week | 1.724 | (0.856–3.470) | 0.127 |
| 4–5 times a week | 1.039 | (0.605–1.784) | 0.890 |
| Every day | 0.962 | (0.594–1.558) | 0.874 |
| History of allergy | 1.133 | (0.623–2.058) | 0.683 |
| Hours of sleep | 0.944 | (0.799–1.115) | 0.495 |
| Systemic symptoms | |||
| Number of vaccinations | 4.046 | (3.321–4.928) | <0.001 |
| Female | 1.610 | (1.340–1.935) | <0.001 |
| BMI | 0.956 | (0.929–0.984) | 0.002 |
| Exercise habits | |||
| Once a week | 1.022 | (0.810–1.290) | 0.852 |
| 2–5 times a week | 0.938 | (0.748–1.175) | 0.576 |
| 6–7 times a week | 1.193 | (0.784–1.817) | 0.410 |
| Habit of consuming breakfast | |||
| 2–3 times a week | 1.218 | (0.808–1.836) | 0.346 |
| 4–5 times a week | 1.012 | (0.708–1.447) | 0.947 |
| Every day | 0.896 | (0.652–1.232) | 0.499 |
| History of allergy | 0.877 | (0.609–1.264) | 0.482 |
| Hours of sleep | 1.026 | (0.929–1.133) | 0.611 |
Analyses were performed using generalized estimating equations; Bold text indicates statistically significant results (p < 0.05); BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio
Factors associated with post-vaccination local and systemic symptoms on the day of COVID-19 vaccination
The incidence of local symptoms on the day of vaccination was significantly higher among those who received multiple doses (OR: 1.264; 95 % CI: 1.119–1.427; P < 0.001) and among women (OR: 2.269; 95 % CI: 1.803–2.857; P < 0.001). Similarly, receiving multiple doses (OR: 1.923; 95 % CI: 1.737–2.129; P < 0.001) and being female (OR: 1.656; 95 % CI: 1.412–1.943; P < 0.001) were significantly associated with the incidence of systemic symptoms. However, increased BMI (OR: 0.965; 95 % CI: 0.940–0.991; P < 0.008) and a higher frequency of breakfast consumption were significantly associated with a lower incidence of systemic symptoms on the day of vaccination (Table 5).
Table 5.
Factors associated with symptoms on the day of COVID-19 vaccination.
| OR | (95 % CI) | P values | |
|---|---|---|---|
| Vaccination site symptoms | |||
| Number of vaccinations | 1.264 | (1.119–1.427) | <0.001 |
| Female | 2.269 | (1.803–2.857) | <0.001 |
| BMI | 0.999 | (0.962–1.038) | 0.963 |
| Exercise habits | |||
| Once a week | 0.756 | (0.568–1.005) | 0.054 |
| 2–5 times a week | 0.902 | (0.680–1.196) | 0.475 |
| 6–7 times a week | 0.961 | (0.571–1.617) | 0.881 |
| Habit of consuming breakfast | |||
| 2–3 times a week | 1.132 | (0.683–1.876) | 0.631 |
| 4–5 times a week | 0.955 | (0.615–1.484) | 0.838 |
| Every day | 0.823 | (0.559–1.213) | 0.325 |
| History of allergy | 1.120 | (0.710–1.765) | 0.627 |
| Hours of sleep | 0.948 | (0.837–1.073) | 0.397 |
| Systemic symptoms | |||
| Number of vaccinations | 1.923 | (1.737–2.129) | <0.001 |
| Female | 1.656 | (1.412–1.943) | <0.001 |
| BMI | 0.965 | (0.940–0.991) | 0.008 |
| Exercise habits | |||
| Once a week | 1.053 | (0.862–1.287) | 0.612 |
| 2–5 times a week | 0.976 | (0.802–1.189) | 0.812 |
| 6–7 times a week | 1.096 | (0.766–1.568) | 0.618 |
| Habit of consuming breakfast | |||
| 2–3 times a week | 0.941 | (0.660–1.343) | 0.739 |
| 4–5 times a week | 0.742 | (0.544–1.012) | 0.059 |
| Every day | 0.687 | (0.520–0.906) | 0.008 |
| History of allergy | 1.006 | (0.731–1.385) | 0.969 |
| Hours of sleep | 0.920 | (0.844–1.002) | 0.056 |
Analyses were performed using generalized estimating equations; Bold text indicates statistically significant results (p < 0.05); BMI, body mass index; CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio
Body mass index, breakfast, sleep, and history of allergy associated with each local and systemic symptom after vaccination
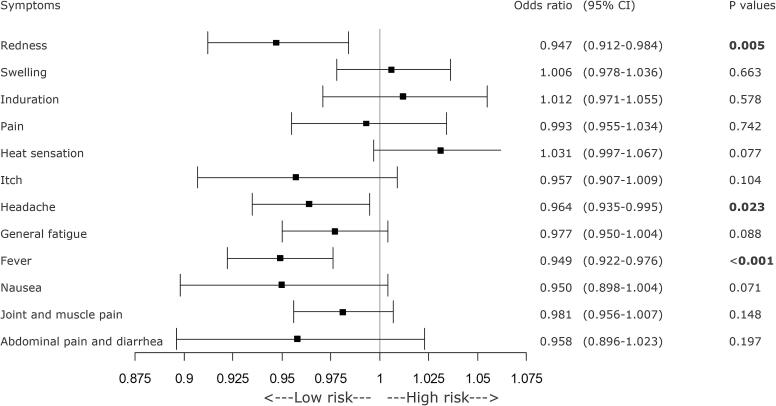
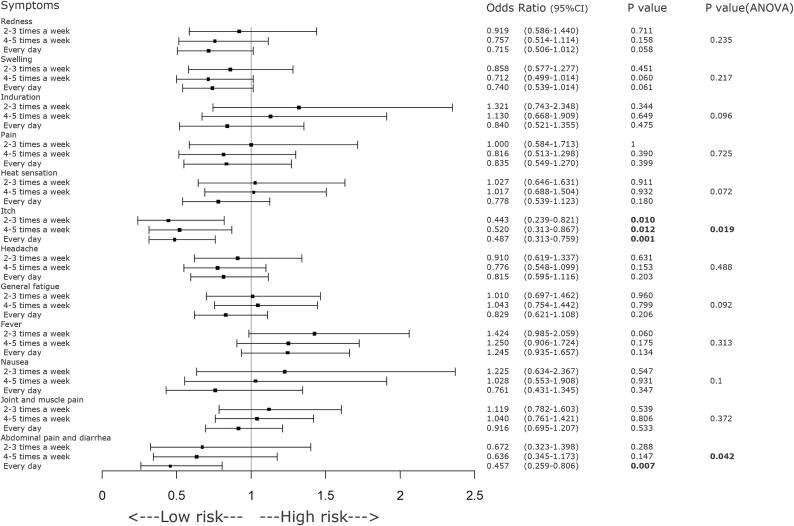
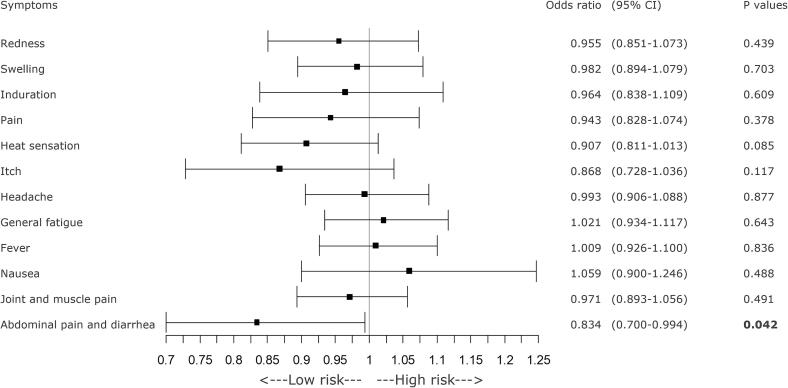
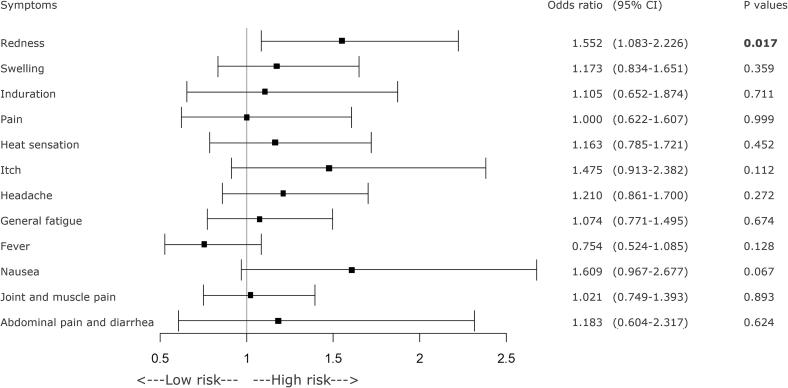
GEE analysis revealed significant decreases in the incidence of local redness at the injection site, headache, and fever as BMI increased (Figure 2). Consuming breakfast at least twice a week was significantly associated with a reduced incidence of pruritus (itch sensation) at the injection site. Additionally, daily breakfast regime was associated with a greater reduction in the incidence of abdominal pain and diarrhea (Figure 3). A longer sleep duration was significantly associated with a lower incidence of abdominal pain and diarrhea (Figure 4). However, a history of allergic symptoms was significantly associated with increased occurrence of focal redness as a local symptom, but not with the incidence of systemic symptoms (Figure 5).
Fig. 2.
Forest plot of various symptoms and BMI on the day of COVID-19 vaccination or the day after the vaccination. Participants with higher BMI showed significantly reduced occurrences of redness at the injection site and post-vaccination headache or fever. BMI = body mass index, CI = confidence interval, COVID-19 = coronavirus disease 2019.
Fig. 3.
Forest plot of various symptoms on the day of COVID-19 vaccination or the day after vaccination and frequency of consuming breakfast. Regular consumption of breakfast (at least 2 days per week) was significantly associated with a decrease in the incidence of injection site pruritus following COVID-19 vaccination. As the frequency of breakfast intake increased, the incidence of post-vaccination symptoms such as headache, abdominal pain, and diarrhea significantly decreased. CI = confidence interval, COVID-19 = coronavirus disease 2019.
Fig. 4.
Forest plot of various symptoms on the day of COVID-19 vaccination or the day after vaccination and sleep duration. A longer sleep duration was significantly associated with a decrease in the frequency of injection site warmth and post-vaccination abdominal pain and diarrhea. CI = confidence interval, COVID-19 = coronavirus disease 2019.
Fig. 5.
A forest plot of various symptoms on the day of COVID-19 vaccination or the day after vaccination and history of allergies. If there was a history of allergies, the incidence of local redness was significantly lower, as was the incidence of fever. CI = confidence interval, COVID-19 = coronavirus disease 2019.
Discussion
This is the first study to precisely analyze the occurrence of COVID-19 vaccine adverse events and identify the related factors, including shot times, sex, BMI, allergic history, and lifestyle factors, including breakfast habit and sleeping time, among Japanese university students.
Although a few reports worldwide have demonstrated the adverse events following mRNA-based COVID-19 vaccinations [4], [5], [6], [11] in a wide variety of populations, three reports analyzed adverse events among university students or staff/students. One study was conducted among various universities in the Czech Republic, which demonstrated that most of the 539 students (18–30 years old) reported at least one side effect, including injection site pain (91.8 %), fatigue (62.5 %), headache (36.4 %), and muscle pain (34.9 %) [12]. The second study on 144 medical clerkship students in Indonesia showed that 45 % and 67 % of the participants reported localized pain, and 36 % and 41 % of the participants reported malaise after the first and booster doses, respectively [13]. The third study was conducted among 6059 staff and 15,347 students at Hiroshima University and Hiroshima University Hospital, Japan. They demonstrated the incidence of adverse reactions after the second dose of mRNA-1273 in the 20 s generation as injection site pain (94.7 %), fever (75.0 %), fatigue (83.3 %), and headache (62.4 %) [14]. The frequency and characteristics of the adverse events among university students showed the same trend.
In our study, 89.7 % and 64.3 % of the students had local and systemic symptoms, respectively, within 2 days of vaccination. Although these incidence rates were likely among adolescents [4] and university students in the Czech Republic [12], they were relatively higher than those in other previous reports [3], [15], [16]. According to the Hiroshima University data, the mRNA-1237 vaccine was more likely to generate systemic reactions than the BNT16b2 vaccine [14]. Since our study participants had received mRNA-1237, our data are specific for mRNA-1237. The incidence of non-life-threatening adverse events increased in our study because the participants comprised the younger generation, as demonstrated in several previous studies [14], [17], [18], [19], [20], [21], [22].
Moreover, shot dose and sex were significantly associated with local and systemic adverse events in this study. The risk of adverse events was significantly higher in the second dose than in the first dose and in women than in men, as reported previously among university students [12], [14]. Although a previous study demonstrated the adverse events in the first and second doses, our results showed that the third dose significantly increased the risk of adverse events. Similarly, several studies demonstrate increased incidence of adverse events with an increase in the number of doses of mRNA-based vaccines [3], [12], [19], [20], [23], [24], [25]. Our study also showed that women may experience more adverse events than men, which correlated with previous surveys [12], [18], [19], [20], [21], [22], [24], [26].
Furthermore, we demonstrated that a decreased BMI increased the risk of systemic adverse events, especially headache/fever and local symptoms of redness. The mean BMI in our study was 20 kg/m2, and the distribution of lean (less than 18.5 kg/m2), normal (18.5–24.9 kg/m2), and obese (over 25.0 kg/m2) subjects was 20.7 %, 71.7 %, and 7.6 %, respectively. Therefore, “decreased BMI increased the risk” might imply that adverse events were less likely to occur as BMI increased from the lean to the normal BMI group. The lean status may be associated with a higher risk of adverse events in young Japanese adults. There was no significant association between adverse events and BMI in a Spanish population [22]. However, the distribution of lean, normal, and obese subjects in their study was 1.9 %, 58.0 %, and 36.8 %, respectively, in the target group aged 18 to > 65 years. To the best of our knowledge, this is the first report of the relationship between BMI and adverse events of vaccination in young Japanese adults.
Association between lifestyle factors and adverse events following COVID-19 vaccination
Breakfast consumption significantly decreased the risk of systemic adverse events including stomach pain, diarrhea, and local symptoms of itchiness. Furthermore, sufficient sleep duration significantly suppressed stomach symptoms. Although allergy history was associated with an increased risk of local adverse events such as redness, a favorable lifestyle that includes breakfast/good sleep and education regarding the possibility of local reactions (especially in people with an allergy history) might decrease the frequency and level of adverse events.
These results imply that a good lifestyle contributes to maintenance of healthy body weight while avoiding a decrease in BMI. Therefore, health education to maintain a healthy lifestyle is important to reduce the level and frequency of adverse events of RNA-based COVID-19 vaccines. However, few studies have examined the relationship between lifestyle or other physiological factors and the occurrence of adverse events; therefore, further in-depth studies are warranted.
Many reports, including clinical trials [1], [2], [3], [4], [5], [6] revealed that most of the adverse events are not serious and resolve within 3 days [12], [19]. Several researchers have also reported significant factors associated with adverse events, such as history of allergic reactions or anaphylaxis [19], vaccine dose [12], [19], [20], [23], [24], [25], vaccine brand [3], [19], age [17], [18], [19], [20], [21], [22], female sex [12], [18], [19], [20], [21], [22], [24], [26], and COVID-19 diagnosis before vaccination [19]. These findings support our data and provide crucial information to medical professionals who administer COVID-19 vaccines [27].
This study had several limitations. First, this study only considered adverse events that occurred on the day of COVID-19 vaccination and the next day after vaccination. We were unable to investigate the association between adverse events occurring on the third day of vaccination or long-term adverse events. Second, we were only able to extract information on the symptoms covered by the questionnaire used in this study. Nevertheless, this allowed standardization of adverse events and ensured the quality of the study because we did not intend to find very rare events, except life-threatening events. Third, this study only included individuals who received the mRNA-1273 vaccine from Moderna. However, there are other COVID-19 vaccines (such as the BNT162b2 mRNA COVID-19 vaccine) that use the same mRNA technology and several other vaccines that use different mechanisms. Therefore, it may be necessary to conduct studies on adverse events following vaccination with different types of vaccines. Fourth, this study focused solely on the relationship between early adverse events after COVID-19 vaccination and lifestyle habits. Further research is necessary to investigate the effects of other factors, such as immune responses and psychological influences, on the adverse events of COVID-19 vaccines.
Conclusions
Here, we demonstrated a high frequency of focal/systemic adverse events after administration of the COVID-19 mRNA-1273 vaccine among university students in Japan; however, there were no life-threatening cases or hospitalizations. A higher number of shots, female sex, and decreased BMI were associated with a higher incidence of adverse events. Regular breakfast consumption and longer sleep duration were associated with a decreased incidence of adverse events. Healthy food/sleep habits and maintaining an ideal body weight may reduce adverse events after administration of mRNA-based COVID-19 vaccines among young adults.
Funding
The authors received no financial support.
CRediT authorship contribution statement
Nobuyuki Tetsuka: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Keiko Suzuki: Writing – original draft, Data curation. Kodai Suzuki: Writing – review & editing. Takuma Ishihara: Writing – original draft, Formal analysis. Takao Miwa: Writing – review & editing. Satoko Tajirika: Writing – review & editing. Miho Adachi: Writing – review & editing. Ryo Horita: Writing – review & editing. Taku Fukao: Writing – review & editing. Mayumi Yamamoto: Writing – review & editing, Writing – original draft, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The Department of Infection Control, Graduate School of Medicine, Gifu University is an endowment department supported by an unrestricted grant from the Gifu Prefecture. We thank Editage (www.editage.com) for English language editing.
Author contributions
NT and MY conceived and designed the study. NT, KS, and MY collected the data. NT and TI analyzed and interpreted the data. NT, KS, TI, and MY wrote the manuscript. KS, TM, ST, MA, RH, and TF significantly revised the manuscript. All authors approved the final manuscript.
Data availability statement
The data supporting the findings of this study are available from the corresponding author, NT, upon reasonable request.
Ethics declarations and approval for human experiments
All study procedures complied with the ethical requirements of the national and institutional committees that oversee human studies and with the 1964 Declaration of Helsinki and its later revisions. The study design was reviewed and approved by the Ethical Review Committee of the Graduate School of Medicine, Gifu University, Japan (no. 2021-B160).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2024.100516.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Sahly H.M., Baden L.R., Essink B., Doblecki-Lewis S., Martin J.M., Anderson E.J., et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapin-Bardales J., Gee J., Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325:2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 4.Ali K., Berman G., Zhou H., Deng W., Faughnan V., Coronada-Voges M., et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385:2241–2251. doi: 10.1056/NEJMoa2109522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharif N., Alzahrani K.J., Ahmed S.N., Dey S.K. Efficacy, immunogenicity and safety of COVID-19 vaccines: A systematic review and meta-analysis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.714170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386:1046–1057. doi: 10.1056/NEJMoa2116414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uehara M., Fujita S., Shimizu N., Liew K., Wakamiya S., Aramaki E. Measuring concerns about the COVID-19 vaccine among Japanese internet users through search queries. Sci Rep. 2022;12:15037. doi: 10.1038/s41598-022-18307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noh Y., Kim J.H., Yoon D., Choe Y.J., Choe S.A., Jung J., et al. Predictors of COVID-19 booster vaccine hesitancy among fully vaccinated adults in Korea: A nationwide cross-sectional survey. Epidemiol Health. 2022;44:e2022061. doi: 10.4178/epih.e2022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holzmann-Littig C., Frank T., Schmaderer C., Braunisch M.C., Renders L., Kranke P., et al. COVID-19 vaccines: Fear of side effects among German health care workers. Vaccines (Basel) 2022;10:689. doi: 10.3390/vaccines10050689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Kaur R.J., Dutta S., Bhardwaj P., Charan J., Dhingra S., Mitra P., et al. Adverse events reported from COVID-19 vaccine trials: A systematic review. Indian J Clin Biochem. 2021;36:427–439. doi: 10.1007/s12291-021-00968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riad A., Pokorná A., Klugarová J., Antalová N., Kantorová L., Koščík M., et al. Side effects of mRNA-based COVID-19 vaccines among young adults (18–30 years old): An independent post-marketing study. Pharmaceuticals (Basel) 2021;14:1049. doi: 10.3390/ph14101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Supangat S.E.N., Sakinah E.N., Nugraha M.Y., Qodar T.S., Mulyono B.W., Tohari A.I. COVID-19 vaccines Programs: Adverse events following immunization (AEFI) among medical Clerkship Student in Jember. Indonesia BMC Pharmacol Toxicol. 2021;22:58. doi: 10.1186/s40360-021-00528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitagawa H., Kaiki Y., Sugiyama A., Nagashima S., Kurisu A., Nomura T., et al. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J Infect Chemother. 2022 Apr;28(4):576–581. doi: 10.1016/j.jiac.2021.12.034. Epub 2022 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadali R.A.K., Janagama R., Peruru S., Gajula V., Madathala R.R., Chennaiahgari N., et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021;93:4420–4429. doi: 10.1002/jmv.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zewude B., Habtegiorgis T., Hizkeal A., Dela T., Siraw G. Perceptions and experiences of COVID-19 vaccine side-effects among healthcare workers in Southern Ethiopia: A cross-sectional study. Pragmat Obs Res. 2021;12:131–145. doi: 10.2147/POR.S344848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dziedzic A., Riad A., Attia S., Klugar M., Tanasiewicz M. Self-reported adverse events of COVID-19 vaccines in polish healthcare workers and medical students. Cross-sectional study and pooled analysis of CoVaST project results in Central Europe. J Clin Med. 2021;10:5338. doi: 10.3390/jcm10225338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lounis M., Rais M.A., Bencherit D., Aouissi H.A., Oudjedi A., Klugarová J., et al. Side effects of COVID-19 inactivated virus vs. adenoviral vector vaccines: Experience of Algerian healthcare workers. Front. Public Health. 2022;10 doi: 10.3389/fpubh.2022.896343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beatty A.L., Peyser N.D., Butcher X.E., Cocohoba J.M., Lin F., Olgin J.E., et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi: 10.1001/jamanetworkopen.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama A., Sawa T., Teramukai S., Katoh N. Adverse reactions to the first and second doses of Pfizer-BioNTech COVID-19 vaccine among healthcare workers. J Infect Chemother. 2022;28:934–942. doi: 10.1016/j.jiac.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alemayehu A., Demissie A., Yusuf M., Abdullahi Y., Abdulwehab R., Oljira L., et al. COVID-19 vaccine side effect: Age and gender disparity in adverse effects following the first dose of AstraZeneca COVID-19 vaccine among the vaccinated population in Eastern Ethiopia: A community-based study. SAGE Open Med. 2022;10 doi: 10.1177/20503121221108616. 20503121221108616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iguacel I., Maldonado A.L., Ruiz-Cabello A.L., Casaus M., Moreno L.A., Martínez-Jarreta B. Association between COVID-19 vaccine side effects and body mass index in Spain. Vaccines (Basel) 2021;9:1321. doi: 10.3390/vaccines9111321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desalegn M., Garoma G., Tamrat H., Desta A., Prakash A. The prevalence of AstraZeneca COVID-19 vaccine side effects among Nigist Eleni Mohammed memorial comprehensive specialized hospital health workers. Cross sectional survey PLoS ONE. 2022;17:e0265140. doi: 10.1371/journal.pone.0265140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green M.S., Peer V., Magid A., Hagani N., Anis E., Nitzan D. Gender differences in adverse events following the Pfizer-BioNTech COVID-19 vaccine. Vaccines (Basel) 2022;10:233. doi: 10.3390/vaccines10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tissot N., Brunel A.S., Bozon F., Rosolen B., Chirouze C., Bouiller K. Patients with history of Covid-19 had more side effects after the first dose of Covid-19 vaccine. Vaccine. 2021;39:5087–5090. doi: 10.1016/j.vaccine.2021.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Qazaz H.K., Al-Obaidy L.M., Attash H.M. COVID-19 vaccination, do women suffer from more side effects than men? A retrospective cross-sectional study Pharm Pract (Granada) 2022;20:2678. doi: 10.18549/PharmPract.2022.2.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto K. Adverse effects of COVID-19 vaccines and measures to prevent them. Virol J. 2022;19:100. doi: 10.1186/s12985-022-01831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.