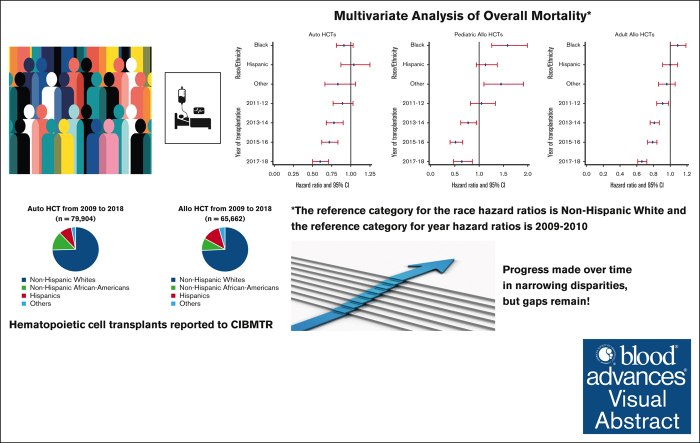
Key Points
-
•
The number of autoHCT and alloHCT grew faster in NHAAs and Hispanics than in NHWs.
-
•
Survival after autoHCT and alloHCT improved over time for all racial/ ethnic groups, though African Americans have worse outcomes.
Visual Abstract
Abstract
There has been an increase in volume as well as an improvement in overall survival (OS) after hematopoietic cell transplantation (HCT) for hematologic disorders. It is unknown if these changes have affected racial/ethnic minorities equally. In this observational study from the Center for International Blood and Marrow Transplant Research of 79 904 autologous (auto) and 65 662 allogeneic (allo) HCTs, we examined the volume and rates of change of autoHCT and alloHCT over time and trends in OS in 4 racial/ethnic groups: non-Hispanic Whites (NHWs), non-Hispanic African Americans (NHAAs), and Hispanics across 5 2-year cohorts from 2009 to 2018. Rates of change were compared using Poisson model. Adjusted and unadjusted Cox proportional hazards models examined trends in mortality in the 4 racial/ethnic groups over 5 study time periods. The rates of increase in volume were significantly higher for Hispanics and NHAAs vs NHW for both autoHCT and alloHCT. Adjusted overall mortality after autoHCT was comparable across all racial/ethnic groups. NHAA adults (hazard ratio [HR] 1.13; 95% confidence interval [CI] 1.04-1.22; P = .004) and pediatric patients (HR 1.62; 95% CI 1.3-2.03; P < .001) had a higher risk of mortality after alloHCT than NHWs. Improvement in OS over time was seen in all 4 groups after both autoHCT and alloHCT. Our study shows the rate of change for the use of autoHCT and alloHCT is higher in NHAAs and Hispanics than in NHWs. Survival after autoHCT and alloHCT improved over time; however, NHAAs have worse OS after alloHCT, which has persisted. Continued efforts are needed to mitigate disparities for patients requiring alloHCT.
Introduction
The number of autologous (auto) and allogeneic (allo) hematopoietic cell transplantations (HCTs) continues to increase.1 Outcomes after autoHCT and alloHCT have improved over time, likely because of improved transplantation techniques and supportive care.2, 3, 4, 5, 6, 7, 8 Racial/ethnic disparities in access to and outcomes of HCT are well documented.9, 10, 11, 12, 13, 14, 15, 16 The origin of these inequities is complex and multifactorial: medical (higher comorbidities, lack of optimum donor sources, aggressive biology) and nonmedical (socioeconomic and health system/payer–related) barriers. The increasing diversity of the US population has increased attention to and investment in ensuring equitable access to optimal cancer care.17 Systematic efforts to reduce gaps in health insurance and eliminate discrimination and bias include national initiatives and policies such as the Affordable Care Act to increase access to medical care. The transplant community has made efforts to mitigate disparities by providing education for improved patient referral and selection, better understanding of financial barriers to transplant, and expanding the donor pool through public investment in donor recruitment and the use of alternative donor sources.8,18,19
It is not known if these efforts have decreased the gap in HCT use between different racial/ethnic groups. It is also not known if the improved transplantation techniques and supportive care have benefited racial/ethnic minorities equally in terms of survival. In other cancers, significant advancements in treatment and outcomes have occurred over time, but racial/ethnic and socioeconomic disparities in outcomes persist.20, 21, 22, 23, 24
The purpose of this study was to analyze trends over time for the volume and rate of change for autoHCT and alloHCT performed in the US for all diseases by racial/ethnic group from 2009 to 2018. We also report trends in survival for those who underwent transplantation for specific diseases by racial/ethnic group, adjusting for clinical and sociodemographic factors. The results provide a start to understanding gaps in real-world access to and outcomes of HCT in the modern era to continue to design future efforts to reduce disparities.
Materials and methods
About the CIBMTR
Data were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR), which collects patient data on 90% of autoHCT and nearly all alloHCT recipients in the United States. The CIBMTR is a research collaboration between the Medical College of Wisconsin and the National Marrow Donor Program/Be The Match. Two hundred six US transplant centers contribute data on consecutive HCTs to the CIBMTR. Observational studies by CIBMTR are compliant with the privacy rule as a public health authority and with all applicable federal regulations for the protection of human research participants as determined by continuous review of the National Marrow Donor Program Institutional Review Board.
Study population
For this study, we included all autoHCTs (adults) and alloHCTs (adults and children) reported to CIBMTR between 2009 and 2018. Patient selection criteria included first HCTs performed in the United States, consented for research use of observational data, and excluded those with missing race/ethnicity information and multirace individuals. We compared the volume of autoHCT and alloHCT in 4 different racial/ethnic groups: non-Hispanic Whites (NHWs), non-Hispanic African Americans (NHAAs), Hispanics, and “Others” across 5 2-year cohorts from 2009 to 2018. “Others” included American Indians or Alaska Natives, Asians, and Native Hawaiians, or Other Pacific Islanders because the numbers in individual categories were too low to be meaningful. Asians formed >80% of the “Others” category (supplemental Table 1).
Data collection
The CIBMTR collects data at 2 levels: transplant-essential data (TED) and comprehensive report form (CRF) data before HCT, 100 days and 6 months after HCT, and annually thereafter until death. TED includes disease type, age, sex, pre-HCT disease status, diagnosis date, graft type, conditioning regimen, relapse, and survival. All centers reporting to CIBMTR submit TED-level data. More detailed clinical information is collected for a subset of patients selected for the CRF track by a weighted randomization schema. CRF data include detailed clinical characteristics (disease risk index, Karnofsky performance score, and HCT-comorbidity index [HCT-CI]) and detailed sociodemographic variables, including insurance, education, marital status, and 5-digit zip code. The zip code was used to determine the geographic region per CIBMTR classification, the distance from the transplant center, and calculate the proportion of the population below the national poverty level.25
Outcomes
The volume of transplants was assessed using TED forms to get the broadest representation of HCT use. CRF level data were used to enable adjustment for detailed patient level variables for survival analysis for specific diseases: multiple myeloma (MM), non-Hodgkin lymphoma (NHL), and Hodgkin lymphoma (HL) for autoHCT (these 3 diseases account for 90% of autoHCTs) and acute myeloid and lymphoblastic leukemia (AML and ALL), lymphoma (NHL, including chronic lymphocytic leukemia and HL), and myelodysplastic syndrome/myeloproliferative neoplasms (MDS/MPN) for alloHCT (these diseases account for >80% of alloHCTs).
Statistical analysis
Volume and rates of change for autoHCT and alloHCT for all diagnoses were reported. Descriptive statistics, including proportions, medians, and ranges, were calculated for baseline characteristics in all groups. Trends in proportions of racial/ethnic groups over time were assessed using the generalization of the Cochran-Armitage test. Poisson model was used to model the overall and race/ethnicity specific rate of change in the number of transplants over time.
The rate of change was calculated as the year-over-year (for each combined 2-year cohort) increase/decrease in volume and was compared between the racial/ethnic groups. Graphical diagnostic tools indicated that the rate of change was constant from 1 2-year period to another. A P value < .001 was considered statistically significant for the rate of change in the number of transplants. Specific patterns of transplant volumes were examined by age, disease type for both autoHCT and alloHCT, and donor type for alloHCT. Overall survival (OS) estimates were calculated using the Kaplan-Meier method. Cox proportional hazards models examined differences in risk of mortality after autoHCT in adult patients (for NHL, HL, and MM) and adult and pediatric patients (<18 years of age) who underwent alloHCT (for AML, ALL, lymphoma, and MDS/MPN) in the 4 racial/ethnic groups over 5 study time periods. Models were adjusted for age, sex, Karnofsky performance score (KPS)/Lansky performance score, HCT-CI, disease, disease status, geographic region, insurance, marital status, education, distance to the transplant center, and median income. Graft type, donor, conditioning, graft vs host disease prophylaxis, donor-recipient cytomegalovirus match, and donor-recipient sex match were considered in alloHCT models. Time from diagnosis was not included in the multivariable analysis because of the heterogeneity of diagnoses. In all models, the center effect was accounted for via a random effect with a log-normal distribution. After stepwise model selection, only variables significant at .01 level were retained. The primary variables of interest, race/ethnicity, and year of transplantation, were included in all models. The interaction between race/ethnicity and year was not significant in any model. Interaction between race and donor type was found to be significant in the Cox model for mortality among adult patients who underwent alloHCT; therefore, a stratified analysis was performed by donor type in this patient group. Analyses were performed using SAS statistical software (SAS Institute, Cary, NC).
Results
Volumes and rate of change by race/ethnicity
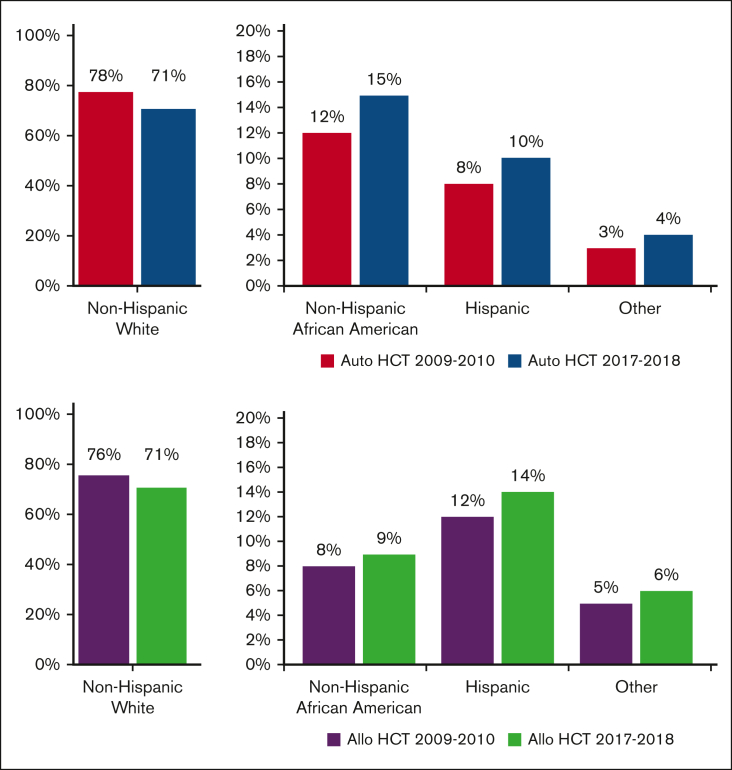
From 2009 to 2018, a total of 79 904 autoHCTs and 65 662 alloHCTs were reported to CIBMTR. The volume of autoHCT and alloHCT performed each year increased overall and within each race/ethnicity group from 2009 to 2018. Figure 1 shows the proportion of autoHCT and alloHCT recipients in each of the 4 racial/ethnic groups in the terminal time cohorts (2009-2010 and 2017-2018), demonstrating an increase in the relative proportion of racial/ethnic minorities and a corresponding decrease in the relative proportion of NHWs in both autoHCT and alloHCT. The rate of change was 2 to 3 times higher in racial/ethnic minorities than in NHWs for both autoHCT and alloHCT (Table 1).
Figure 1.
Proportion of HCT by race/ethnicity in 2009 to 2010 and 2017 to 2018 using data from TED forms.
Table 1.
Rate of change for autoHCT and alloHCT in different racial/ethnic groups from 2009 to 2018 using data from TED forms
| Race/ethnicity | Rate of change of autoHCT from 1 time period to another (95% CI) | P value for trend | Rate of change of alloHCT from 1 time period to another (95% CI) | P value for trend |
|---|---|---|---|---|
| NHWs | 1.06 (1.05-1.08)∗ | <.0001 | 1.04 (1.02-1.05) | <.0001 |
| NHAAs | 1.14 (1.10-1.17) | <.0001 | 1.11 (1.07-1.16) | <.0001 |
| Hispanics | 1.18 (1.13-1.22) | <.0001 | 1.11 (1.07-1.14) | <.0001 |
| Others | 1.17 (1.09-1.25) | <.0001 | 1.09 (1.04-1.15) | .0009 |
| Total (adjusted for race) | 1.08 (1.06-1.11) | <.0001 | 1.06 (1.04-1.07) | <.0001 |
A rate of change of 1.06 means that the number of transplants grew on average 6% from 1 2-year time period to another.
The increased rate of change in volume of autoHCTs per time period was significantly higher for Hispanics (P < .001) and NHAAs (P = .0001) than NHWs, but not significantly different between NHWs and Others (P = .005) or between NHAAs and Hispanics (P = .19). Similarly, the increased rate of change for alloHCT was significantly higher for Hispanics (P = .0002) and NHAAs (P = .0006) than NHWs, but not significantly different between NHWs and Others (P = .07) or between NHAAs and Hispanics (P = .78).
Baseline characteristics by race/ethnicity
For autoHCT, MM was more common in NHAAs as compared with all other groups (77% in NHAAs vs 55% in Hispanics, 58% in NHWs, and 53% in Others). Most autoHCTs in Hispanics, NHAAs, and Others were reported from the West or South regions of the United States, whereas NHWs had a more even geographic distribution. Private insurance was more common in NHWs and Others compared with Hispanics and NHAAs. A higher proportion of NHWs and Others were married than Hispanics and NHAAs (supplemental Tables 2 and 3).
For alloHCT in adult patients, median age at HCT was higher for NHWs than all other groups (55 years in NHWs vs 31 in Hispanics, 37 in NHAAs, and 41 in Others; P < .01). A higher proportion of NHWs who underwent alloHCT had a poor KPS <80 (12% in NHWs vs 8% in Hispanics, 10% in NHAAs and Others). A higher proportion of Hispanics received myeloablative conditioning than other groups (61% vs 47% in NHWs, 47% in NHAAs, and 52% in Others). Unrelated donor HCTs (both HLA-matched and mismatched) were more common in NHWs than all groups (54% in NHWs vs 33% in Hispanics, 26% in NHAAs, and 35% in Others). Cord blood (16% in Hispanics, 17% in NHAAs, 15% in Others, and 7% in NHWs) and haploidentical donors (14% in Hispanics, 25% in NHAAs, 14% in Others, and 9% in NHWs) were more common in minority groups compared with NHWs. The geographic distribution was relatively equal among US regions for NHWs, with a higher proportion of Hispanics and Others, in the West and NHAAs in the South. Private insurance was more common in NHWs and Others (57% in NHWs, 60% in Others vs 46% and 51%, respectively, in Hispanics and NHAAs). A higher distance from the transplant center was seen in NHWs than other groups (median 42 miles vs 23 miles in Hispanics, 19 miles in NHAAs, and 21 miles in Others; supplemental Tables 4 and 5).
In pediatric patients, ALL was the indication for alloHCT in a higher proportion of Hispanics (56% in Hispanics vs 35% in NHWs, 39% in NHAAs, and 37% in Others). Cord blood and haploidentical donors were the least common in NHWs. Geographic distribution showed a higher proportion of Hispanics and NHAAs in the West and the South, respectively. The distance from the transplant center was higher for NHWs (median 47 miles) than Hispanics (26 miles), NHAAs (20 miles), and Others (22 miles). Private insurance was more common in NHWs (54%) and Others (48%) as compared with Hispanics (23%) and NHAAs (25%; supplemental Table 6).
Subgroup analysis for volume or rate of change
For autoHCT, NHWs experienced lower volumes between 2009 and 2010 and 2017 and 2018 for patients under 40 years, with stable volume in 40 to 59 years and a 72% increase in autoHCTs for patients aged >60 years. AutoHCT for MM increased by 62%, whereas lymphoma volumes were stable and those of other diseases decreased.
In contrast to NHW, Hispanics experienced modest increases in volumes for patients under 40 years, whereas autoHCT for 40 to 59 years increased by 67% and >60 years increased almost threefold. AutoHCT volume more than doubled for MM and increased 83% for lymphomas, with other diseases staying stable.
In addition, in contrast to NHWs, NHAAs experienced modest increases in volumes for patients under 40 years, whereas autoHCT for 40 to 59 years increased by 48% and more than doubled for >60 years. AutoHCT volume almost doubled for MM and increased 23% for lymphomas, with other diseases staying stable.
In the case of alloHCT, NHWs had the largest volume increases between 2009 and 2010 and 2017 and 2018 for MDS/MPN (70% increase) and acute leukemias (29% increase) but had a 40% decrease in the volume of alloHCT for lymphomas and a 12% decrease for other diseases. NHW saw lower volumes in every age group under 60 years; however, the >60 years group increased by 74%. The use of HLA-identical siblings decreased by 19%, whereas non-HLA–identical sibling donors (including haploidentical and other related HLA-mismatched donors) increased by almost fourfold. Well-matched (HLA 8/8) unrelated donors increased by 49%, whereas mismatched unrelated and cord blood transplants all decreased by at least 25%.
Hispanics had larger volume increases than NHWs for MDS/MPN (89% increase) and acute leukemias (65% increase), whereas other diseases had modest increases (<10%). In contrast to NHWs, Hispanics demonstrated increases in volume for all age groups (2.6-fold increase in patients >60 years, 1.7-fold in 40 to 59 years, 1.5-fold in 20 to 39 years, and 1.1-fold increase in <20 years). Most of the volume increases for Hispanics were due to a 7.3-fold increase in the use of non-HLA–identical sibling donors (including haploidentical and other related HLA-mismatched donors) and more than doubling the volume of 8/8 HLA-matched unrelated donors.
NHAAs had larger volume increases than NHWs for MDS/MPN (81% increase) and acute leukemias (67% increase) and also had a 26% increase in lymphomas and a 40% increase in other diseases. In contrast to NHW, NHB saw increases in every age group (2.5-fold increase in patients >60 years, 1.4-fold in 40 to 59 years, 1.5-fold 20 to 39 years, and 1.4-fold increase in <20 years). Most volume increases for NHAAs were due to a 4.3-fold increase in the use of non-HLA–identical sibling donors (including haploidentical and other related HLA-mismatched donors) and almost doubling the volume of 8/8 HLA-matched unrelated donors.
Mortality/survival after autoHCT using data from patients included in CRFs
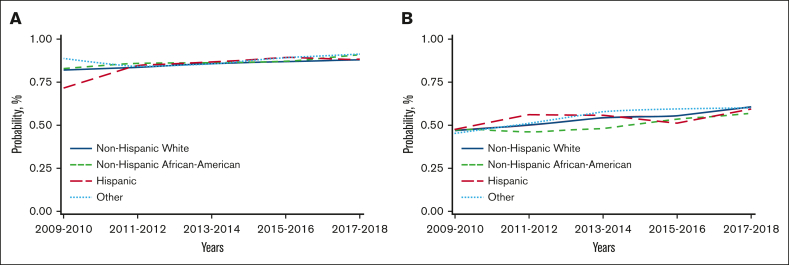
Unadjusted analysis showed a significantly lower risk of overall mortality in NHAAs; however, there was no significant difference in mortality risk by race/ethnicity after multivariable adjustment. Unadjusted analysis also showed a significantly lower risk of mortality in the more recent cohorts, which remained significant after adjustment for other significant covariates (Table 2). Other factors associated with higher risk of mortality included older age, male, NHL, not in complete remission at HCT, high HCT-CI, KPS <80, and being single/divorced or widowed (supplemental Table 7). The adjusted 2-year OS improved over time in all 4 racial/ethnic groups, as depicted in Figure 2A.
Table 2.
Unadjusted and adjusted models for overall mortality (using data from CRF)
|
Variable |
AutoHCT (n = 7888) |
Adult alloHCT (n = 16 558) |
Pediatric alloHCT (n = 1820) |
|||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI); P value | Adjusted HR∗ (95% CI); P value | Unadjusted HR (95% CI); P value | Adjusted HR† (95% CI); P value | Unadjusted HR (95% CI); P value | Adjusted HR‡ (95% CI); P value | |
| Race | ||||||
| NHWs | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| NHAAs | 0.85 (0.76-0.95); .004 | 0.91 (0.81-1.03); .12 | 1.12 (1.03-1.21); .005 | 1.13 (1.04-1.22); .004 | 1.76 (1.4-2.2); <.001 | 1.62 (1.3-2.03); <.001 |
| Hispanics | 0.93 (0.77-1.11); .41 | 1.04 (0.87-1.25); .66 | 0.91 (0.84-0.99); .03 | 1.01 (0.92-1.1); .89 | 1.22 (1.02-1.46); .03 | 1.17 (0.97-1.41); .10 |
| Others | 0.81 (0.64-1.030); .09 | 0.83 (0.65-1.05); .13 | 0.91 (0.82-1.01); .08 | 0.97 (0.87-1.07); .50 | 1.52 (1.15-2.02); .004 | 1.47 (1.11-1.96); .008 |
| Year of transplant | ||||||
| 2009-2010 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2011-2012 | 0.78 (0.68-0.9); .0006 | 0.89 (0.77-1.03); .13 | 1.01 (0.94-1.08); .84 | 0.91 (0.85-0.98); .014 | 1.00 (0.79-1.26); .99 | 1.05 (0.83-1.32); .71 |
| 2013-2014 | 0.77 (0.67-0.87); <.001 | 0.78 (0.69-0.9); .0004 | 0.93 (0.87-0.99); .015 | 0.81 (0.76-0.86); <.001 | 0.77 (0.63-0.94); .01 | 0.75 (0.6-0.93); .008 |
| 2015-2016 | 0.75 (0.65-0.86); <.001 | 0.72 (0.62-0.83); <.001 | 0.91 (0.85-0.97); .003 | 0.78 (0.73-0.83); <.001 | 0.56 (0.44-0.7); <.001 | 0.53 (0.42-0.68); <.001 |
| 2017-2018 | 0.60 (0.5-0.72); <.001 | 0.60 (0.5-0.72); <.001 | 0.75 (0.7-0.81); <.001 | 0.66 (0.61-0.71); <.001 | 0.74 (0.57-0.96); .03 | 0.71 (0.54-0.92); .01 |
CI, confidence interval; HR, hazard ratio.
Other significant variables include patient age, sex, diagnosis, disease status, HCT-CI, KPS, and marital status.
Other significant variables include patient age, diagnosis, disease risk, HCT-CI, KPS, insurance, and donor type.
Other significant variables include disease status at HCT, HCT-CI, and recipient cytomegalovirus status.
Figure 2.
Temporal trends in adjusted OS at 2 years after HCT in different racial/ethnic groups (using data from CRF). (A) Temporal trends in adjusted OS at 2 years after autoHCT in different racial/ethnic groups. (B) Temporal trends in adjusted OS at 2 years after alloHCT in adults in different racial/ethnic groups. (C) Temporal trends in adjusted OS at 2 years after alloHCT in pediatric patients in different racial/ethnic groups.
Mortality/survival after alloHCT in adults using data from patients included in CRFs
Both unadjusted and adjusted analyses showed a significantly higher risk of overall mortality in NHAAs vs NHWs. There was a significant decrease in the risk of overall mortality over time for all alloHCT recipients (Table 2). Other significant factors in the multivariable analysis include age, diagnosis, disease risk, HCT-CI, KPS, donor type, and insurance (supplemental Table 8). Temporal patterns in the adjusted 2-year OS in all racial/ethnic groups showed NHAAs having worse survival than all the other groups (Figure 2B). In the stratified analysis, there was no significant difference in risk of mortality by race/ethnicity for HLA-identical siblings or other related donor alloHCTs. NHAAs had a significantly higher risk of mortality after well-matched and partially matched unrelated donor alloHCT but not after cord blood transplants. Hispanics had a higher risk of mortality only after cord blood transplants (supplemental Table 9).
Mortality/survival after alloHCT in pediatric patients using data from patients included in CRFs
In pediatric patients, there was a significantly higher risk of overall mortality in NHAAs and Others than NHWs in unadjusted and multivariable analyses (Table 2). In the adjusted analysis, there was a significantly lower risk of overall mortality in the most recent 3 time periods. Other significant factors in the multivariable analysis include relapsed/refractory disease, recipient cytomegalovirus serostatus, and an HCT-CI >3 (supplemental Table 10). The gaps in OS were more evident, with NHAAs and Others doing worse (Figure 2C).
Discussion
Our large, population-based national cohort study, comprising of over 145 000 patients, shows an increase in the rates of change in volume and improved survival after autoHCT and alloHCT in adults and children from 2009 to 2018 for all racial/ethnic groups, despite sociodemographic differences such as age, geographic region, and private vs public insurance between the groups. Progress is reflected in higher rates of increase in volume for both autoHCT and alloHCT in racial/ethnic minority groups than NHWs, aligned with changes in the proportions of these demographic groups in the US population. There was some variation in patterns of rate of change across the subgroups based on age, disease type, and donor type across race/ethnicity. Comparable survival was seen across all racial/ethnic groups for autoHCTs. The higher risk of mortality remains a challenge for NHAAs and Others undergoing unrelated donor alloHCT, despite improvements in OS over time. Hispanic adults had a comparable risk of overall mortality with NHWs, unlike previous CIBMTR studies.13,26
Racial/ethnic barriers to access and outcomes in autoHCT have been explored in previous studies.12,27, 28, 29, 30, 31, 32, 33 In addition, studies report improved survival for the more recently treated patients undergoing autoHCT for MM, although not for specific racial/ethnic groups.34 In this study, the rate of change for the increase in autoHCTs over time was significantly higher for NHAAs, Hispanics, and Others than NHWs. Although some of the increase in autoHCT volume may be attributed to increases in the population of these racial/ethnic groups, other factors such as an increase in the number of HCT centers and policy changes such as Medicaid expansion may have contributed. Medicaid expansion is associated with improvements in access and health status/outcomes.35,36
MM was a common indication for autoHCT in a higher proportion of NHAAs than other groups, likely due to its higher incidence in NHAAs than in NHWs.37 Mortality after autoHCT decreased over time and was comparable for all racial/ethnic groups. Prior studies report that race/ethnicity do not affect autoHCT outcomes, especially with access to timely transplants.27,28
In addition to socioeconomic barriers, a lack of optimal donors may affect access to alloHCT for minority patients, with a 75% probability of finding an 8/8 HLA-matched donor for patients of European descent, 16% to 19% for NHAAs, and between 27% and 52% for Hispanic, Asian, Pacific Islander, and Native American groups.11,16,32,38,39 Use of HLA–mismatched unrelated donors, cord blood, and haploidentical donors has helped widen the donor pool, leading to a significantly higher rate of alloHCT in NHAAs and Hispanics.1,8,40 In our study, a higher proportion of Hispanics and NHAAs received alternative donor sources (cord blood and haploidentical HCT) than NHWs receiving more HLA-matched sibling and unrelated transplants. The younger age of Hispanics undergoing HCT reflects the overall population demographic. A higher incidence of ALL in Hispanics vs all other groups (age-adjusted rate of 2.9 per 100 000 persons in Hispanics vs 1.8 in NHWs and 1.1 in NHAAs) is a possible reason for ALL being a more common indication for alloHCT in Hispanic children.41
Outcomes continue to improve over time for most patients undergoing HCT, similar to Hahn et al.3 Mortality risk was comparable between NHWs and Hispanics, but higher for NHAAs for unrelated alloHCT only. There are similarities and differences between our results and those of previous studies.13, 14, 15,42 Minority patients have worse socioeconomic parameters, reflected by a higher proportion of those below the national poverty level, lower education, and a higher proportion of those with public insurance in Hispanics and NHAAs than NHWs in our study. The term Hispanic Paradox is not well understood but is observed when mortality risk is similar to or better than NHW despite the unfavorable socioeconomic profile of Hispanics.43 In contrast, NHAAs with a similar socioeconomic profile to Hispanics have a considerably higher mortality risk than NHWs.44 Disease biology may have affected outcomes. Insurance may play a role as well, with poor outcomes reported in pediatric patients with Medicaid.45 Poor survival after alloHCT, but not autoHCT for NHAAs may be due to higher mortality for this group with unrelated donors, but it is also likely due to the more complicated and prolonged post-HCT recovery period after alloHCT, which is prone to disparities in quality of care and difficulty participating in follow-up (distance, financial toxicity, availability of caregiver support, etc).
There are certain limitations to our study. The numbers of HCT in Asian, Native American, and Pacific Islander groups were not enough to provide meaningful results, so they were combined as an Others category. The CIBMTR collects data only for patients who undergo transplants with no information about the denominator as to how many patients would be optimal candidates for transplant. Hence, we report only on rate of change rather than true use, which we are investigating in a separate ongoing study using data from the Surveillance, Epidemiology, and End Results program and the US Census to calculate the denominator. Attribution of patient race/ethnicity in the CIBMTR provided by the transplant centers is self-reported, and centers are regularly audited on this critical data field. We did not have the detailed sociodemographic data in the TED forms to adjust the rate of change analysis. Finally, we acknowledge that this is a quantitative study describing the change in volume and outcomes over time. But our results can help us understand gaps that drive disparities and provide a foundation for qualitative work to understand the impact of implicit bias and quality of care on post-HCT survival when treating minority groups, specifically NHAAs.46 We hope that this study will be pivotal in distributing resources and/or strategizing future actions to improve on the progress already made.
The US population has become more diverse, with the proportion of NHW decreasing from 64% in 2010 to 58% in 2020.47 The increasing diversity compels us to strengthen our efforts to improve access to and outcomes after HCT, both in routine practice and clinical trials. Disparities in access will be more complicated with highly expensive and complex cellular therapies; thus, efforts will be needed to narrow the gap for patients requiring these treatments, irrespective of their socioeconomic characteristics.48,49 Leveraging advanced genomics and molecular technologies and computational tools to better understand the interaction of biological factors and social determinants of health can help decrease disparities in outcomes. The recent ACCESS initiative is a timely multistakeholder initiative to reduce barriers and outcome disparities in HCT/cellular therapy.50
Our study highlights the progress in increasing the rate of change in autoHCT and alloHCT for everyone and in narrowing the gap in survival between race/ethnicity groups for certain diseases. However, the risk of overall mortality for NHAAs remains high, indicating the need for investment in research, training, practice, and community engagement to address the remaining disparities and enable everyone to enjoy the benefits of scientific advances.
Conflict-of-interest disclosure: S.A. reports consultancy for GlaxoSmithKline (GSK), Sanofi, Bristol Myers Squibb (BMS), Takeda, BeiGene, Janssen, Regeneron, Cellectar, and Pfizer, and research funding from GSK, BMS, Pharmacyclics, Amgen, Janssen, Cellectar Biosciences, AbbVie, Ascentage, and Sanofi. C.U. reports honoraria (speakers’ bureau) from Takeda. S.C. reports advisory board participation for Hansa Therapeutics, CareDx, Spectrum/Acrotech Biopharma, Kiadis Pharma, Allogene, Celularity, MolMed, and Pharmacyclics, and research funding from Miltenyi Biotech and Kiadis Pharma. B.N.S. reports a government conflict-of-interest. A. Sharma reports compensation (consulting) for Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, Sangamo Therapeutics, and Editas Medicine; serving as a medical monitor for RCI BMT CSIDE clinical trial that receives financial compensation; research funding from CRISPR Therapeutics; honoraria from Vindico Medical Education; serving as the St. Jude Children’s Research Hospital site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287), Novartis Pharmaceuticals (NCT04443907), and Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trial, which includes salary support paid to A. Sharma’s institution. A. Sharma has no direct financial interest in these therapies. J.C. reports being a data safety and monitoring board member for ICON-AlloVir Inc, and ICON-Prolacta; being an advisory board member for MERIT CRO, and BMS; and stocks for Actinium Pharmaceuticals Inc, bluebird bio, Cellectar Biosciences, Dynavax Technologies, Gamida Cell, aTyr Pharma, Novavax, Ovid Therapeutics, Sorrento Therapeutics, VERY Inc, Viridian Therapeutics, Vaxart Inc, and 2seventy bio Inc, and Reg SHS. A.H.K. reports research funding from CareDx. T.K.-K. reports consulting fees (data safety monitoring committee) from Orca Bio. A. Steinberg reports speakers’ bureau participation for Jazz Pharmaceuticals, and advisory board participation for MorphoSys. W.A.W. reports research funding (to institution) from Pfizer and Genentech; serves as an adviser for Koneksa Health; and consulting for Teladoc. H.G.R. reports being a medical monitor for the CD33 chimeric antigen receptor study (unpaid), and having a contract with National Marrow Donor Program (unpaid). The remaining authors declare no competing financial interests.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported primarily by the Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID), 75R60222C00011 from the Health Resources and Services Administration (HRSA), and N00014-23-1-2057 and N00014-24-1-2057 from the Office of Naval Research.
The support is also provided by the Medical College of Wisconsin, National Marrow Donor Program, Gateway for Cancer Research, Pediatric Transplantation and Cellular Therapy Consortium and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Alexion; Allogene; AlloVir, Inc; Amgen, Inc; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; BioLineRX; Blue Spark Technologies; bluebird bio, inc; Blueprint Medicines; BMS; CareDx Inc; CSL Behring; CytoSen Therapeutics, Inc; DKMS; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd; Gift of Life Biologics; Gift of Life Marrow Registry; GSK; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miller Pharmacal Group, Inc; Miltenyi Biotec, Inc; MorphoSys; MSA-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; OriGen BioMedical; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie company; PPD Development, LP; REGiMMUNE; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc; Sobi, Inc; Stemcell Technologies; Stemline Technologies; STEMSOFT; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; WellSky; and Xenikos BV.
Authorship
Contribution: N.K., S.A., R.B., J.P., B.J., W.S., and T.H. were responsible for conception, design, and analysis; R.B., J.P., B.J., and W.S. were responsible for acquisition of data, administrative, technical, or material support; and all authors were responsible for the interpretation of data and the writing, review, and/or revision of the manuscript.
Footnotes
Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accordance with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The final analysis data set will be posted to the CIBMTR website at https://cibmtr.org/CIBMTR/Resources/Publicly-Available-Datasets#.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Auletta JJ KJ, Chen M, Shaw BE. Current Use and Outcome of Hematopoietic Stem Cell Transplantation: CIBMTR US Summary Slides. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.asp CIBMTR.
- 2.Penack O, Peczynski C, Mohty M, et al. How much has allogeneic stem cell transplant–related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020;4(24):6283–6290. doi: 10.1182/bloodadvances.2020003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hahn T, McCarthy PL, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31(19):2437–2449. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy PL, Jr., Hahn T, Hassebroek A, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995-2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013;19(7):1116–1123. doi: 10.1016/j.bbmt.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald GB, Sandmaier BM, Mielcarek M, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003-2007 versus 2013-2017 cohorts. Ann Intern Med. 2020;172(4):229–239. doi: 10.7326/M19-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedell PA, Hamadani M, Ahn KW, et al. Outcomes and utilization trends of front-line autologous hematopoietic cell transplantation for mantle cell lymphoma. Transplant Cell Ther. 2021;27(11):911.e1–911.e7. doi: 10.1016/j.jtct.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auletta JJ, Kou J, Chen M, et al. Real-world data showing trends and outcomes by race and ethnicity in allogeneic hematopoietic cell transplantation: a report from the Center for International Blood and Marrow Transplant Research. Transplant Cell Ther. 2023;29(6):346.e1–346.e10. doi: 10.1016/j.jtct.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham IE, Rauscher GH, Patel AA, et al. Structural racism is a mediator of disparities in acute myeloid leukemia outcomes. Blood. 2022;139(14):2212–2226. doi: 10.1182/blood.2021012830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirtane K, Lee SJ. Racial and ethnic disparities in hematologic malignancies. Blood. 2017;130(15):1699–1705. doi: 10.1182/blood-2017-04-778225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell JM, Meehan KR, Kong J, Schulman KA. Access to bone marrow transplantation for leukemia and lymphoma: the role of sociodemographic factors. J Clin Oncol. 1997;15(7):2644–2651. doi: 10.1200/JCO.1997.15.7.2644. [DOI] [PubMed] [Google Scholar]
- 12.Costa LJ, Huang J-X, Hari PN. Disparities in utilization of autologous hematopoietic cell transplantation for treatment of multiple myeloma. Biol Blood Marrow Transplant. 2015;21(4):701–706. doi: 10.1016/j.bbmt.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker KS, Loberiza FR, Jr., Yu H, et al. Outcome of ethnic minorities with acute or chronic leukemia treated with hematopoietic stem-cell transplantation in the United States. J Clin Oncol. 2005;23(28):7032–7042. doi: 10.1200/JCO.2005.01.7269. [DOI] [PubMed] [Google Scholar]
- 14.Ballen KK, Klein JP, Pedersen TL, et al. Relationship of race/ethnicity and survival after single umbilical cord blood transplantation for adults and children with leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2012;18(6):903–912. doi: 10.1016/j.bbmt.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mielcarek M, Gooley T, Martin PJ, et al. Effects of race on survival after stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(3):231–239. doi: 10.1016/j.bbmt.2004.12.327. [DOI] [PubMed] [Google Scholar]
- 16.Pulte D, Redaniel MT, Jansen L, Brenner H, Jeffreys M. Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica. 2013;98(2):222–229. doi: 10.3324/haematol.2012.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Census Bureau 2020 US population more racially and ethnically diverse than measured in 2010. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- 18.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. Registry. N Engl J Med. 2014;371(4):339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Dept. of Health and Human Services OotS, Office of the Assistant Secretary for Planning and Evaluation and Office of Minority Health. HHS Action Plan to Reduce Racial and Ethnic Health Disparities Implementation Progress Report. Office of the Assistant Secretary for Planning and Evaluation; 2015. [Google Scholar]
- 20.Tai EW, Ward KC, Bonaventure A, Siegel DA, Coleman MP. Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):5178–5189. doi: 10.1002/cncr.30899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):5014–5036. doi: 10.1002/cncr.31076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 23.Tong M HL, Artiga S. Racial Disparities in Cancer Outcomes, Screening, and Treatment. https://www.kff.org/racial-equity-and-health-policy/issue-brief/racial-disparities-in-cancer-outcomes-screening-and-treatment/ KFF.
- 24.Jatoi I, Sung H, Jemal A. The emergence of the racial disparity in U.S. breast-cancer mortality. N Engl J Med. 2022;386(25):2349–2352. doi: 10.1056/NEJMp2200244. [DOI] [PubMed] [Google Scholar]
- 25.US Census Bureau Explore Census Data. https://data.census.gov/
- 26.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(12):1543–1554. doi: 10.1016/j.bbmt.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatnagar V, Wu Y, Goloubeva OG, et al. Disparities in black and white patients with multiple myeloma referred for autologous hematopoietic transplantation: a single center study. Cancer. 2015;121(7):1064–1070. doi: 10.1002/cncr.29160. [DOI] [PubMed] [Google Scholar]
- 28.Schriber JR, Hari PN, Ahn KW, et al. Hispanics have the lowest stem cell transplant utilization rate for autologous hematopoietic cell transplantation for multiple myeloma in the United States: a CIBMTR report. Cancer. 2017;123(16):3141–3149. doi: 10.1002/cncr.30747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Hamadani M, Hashmi SK, Go RS. Use of autologous hematopoietic cell transplantation as initial therapy in multiple myeloma and the impact of socio-geo-demographic factors in the era of novel agents. Am J Hematol. 2014;89(8):825–830. doi: 10.1002/ajh.23753. [DOI] [PubMed] [Google Scholar]
- 30.Hari PN, Majhail NS, Zhang MJ, et al. Race and outcomes of autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2010;16(3):395–402. doi: 10.1016/j.bbmt.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaughn JL, Soroka O, Epperla N, Safford M, Pinheiro LC. Racial and ethnic differences in the utilization of autologous transplantation for lymphoma in the United States. Cancer Med. 2021;10(20):7330–7338. doi: 10.1002/cam4.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshua TV, Rizzo JD, Zhang MJ, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. 2010;116(14):3469–3476. doi: 10.1002/cncr.25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ailawadhi S, Parikh K, Abouzaid S, et al. Racial disparities in treatment patterns and outcomes among patients with multiple myeloma: a SEER-Medicare analysis. Blood Adv. 2019;3(20):2986–2994. doi: 10.1182/bloodadvances.2019000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa LJ, Zhang M-J, Zhong X, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19(11):1615–1624. doi: 10.1016/j.bbmt.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madeline Guth MA. Building on the Evidence Base: Studies on the Effects of Medicaid Expansion, February 2020 to March 2021. https://www.kff.org/report-section/building-on-the-evidence-base-studies-on-the-effects-of-medicaid-expansion-february-2020-to-march-2021-appendix-a/ KFF.
- 36.Han X, Zhao J, Yabroff KR, Johnson CJ, Jemal A. Association between Medicaid expansion under the Affordable Care Act and survival among newly diagnosed cancer patients. J Natl Cancer Inst. 2022;114(8):1176–1185. doi: 10.1093/jnci/djac077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood. 2010;116(25):5501–5506. doi: 10.1182/blood-2010-07-298760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jabo B, Morgan JW, Martinez ME, Ghamsary M, Wieduwilt MJ. Sociodemographic disparities in chemotherapy and hematopoietic cell transplantation utilization among adult acute lymphoblastic and acute myeloid leukemia patients. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang JP, Lam TP, Cohen DS, Donato ML, Geraci JM. Hematopoietic stem cell transplantation among patients with leukemia of all ages in Texas. Cancer. 2004;101(10):2230–2238. doi: 10.1002/cncr.20628. [DOI] [PubMed] [Google Scholar]
- 40.Gyurkocza B, Rezvani A, Storb RF. Allogeneic hematopoietic cell transplantation: the state of the art. Expert Rev Hematol. 2010;3(3):285–299. doi: 10.1586/ehm.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.SEER Cancer Stat Facts: Leukemia — Acute Lymphocytic Leukemia (ALL) https://seer.cancer.gov/statfacts/html/alyl.html#:∼:text=Acute%20lymphocytic%20leukemia%20is%20most%20common%20in%20children%2C,women%20per%20year%20based%20on%202014%E2%80%932018%20cases%2C%20age-adjusted
- 42.Serna DS, Lee SJ, Zhang MJ, et al. Trends in survival rates after allogeneic hematopoietic stem-cell transplantation for acute and chronic leukemia by ethnicity in the United States and Canada. J Clin Oncol. 2003;21(20):3754–3760. doi: 10.1200/JCO.2003.03.133. [DOI] [PubMed] [Google Scholar]
- 43.Markides KS, Eschbach K. Aging, migration, and mortality: current status of research on the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. 2005;60(spec no 2):68–75. doi: 10.1093/geronb/60.special_issue_2.s68. [DOI] [PubMed] [Google Scholar]
- 44.National Center for Health Statistics Mortality trends by race and ethnicity among adults aged 25 and over: United States, 2000–2017. https://www.cdc.gov/nchs/products/databriefs/db342.htm
- 45.Bona K, Brazauskas R, He N, et al. Neighborhood poverty and pediatric allogeneic hematopoietic cell transplantation outcomes: a CIBMTR analysis. Blood. 2021;137(4):556–568. doi: 10.1182/blood.2020006252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):19. doi: 10.1186/s12910-017-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Census Bureau 2020 U.S. Population More Racially, Ethnically Diverse Than in 2010. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- 48.Alqazaqi R, Schinke C, Thanendrarajan S, et al. Geographic and racial disparities in access to chimeric antigen receptor–T cells and bispecific antibodies trials for multiple myeloma. JAMA Netw Open. 2022;5(8) doi: 10.1001/jamanetworkopen.2022.28877. e2228877-e2228877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed N, Shahzad M, Shippey E, et al. Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transplant Cell Ther. 2022;28(7):358–364. doi: 10.1016/j.jtct.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Auletta JJ, Sandmaier BM, Jensen E, et al. The ASTCT-NMDP ACCESS initiative: a collaboration to address and sustain equal outcomes for all across the hematopoietic cell transplantation and cellular therapy ecosystem. Transplant Cell Ther. 2022;28(12):802–809. doi: 10.1016/j.jtct.2022.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.