Take Home Message
Our study shows that prostate cancer patients with metastases to different organs have different survival rates. Patients whose cancer spreads to the lungs first tend to live longer than those whose cancer spreads to the brain or liver first.
Keywords: Prostate cancer, Metastatic disease, Overall survival
Abstract
Background and objective
Visceral metastatic disease in prostate cancer patients conveys a poor prognosis. Using advanced imaging techniques, studies have demonstrated increasing detection rates of visceral metastasis. Visceral metastases are now seen in up to 30–60% of prostate cancer patients. Survival patterns of site-specific visceral metastasis are described poorly in the literature. Here, we sought to investigate survival patterns in prostate cancer patients according to their first detected site of visceral metastasis.
Methods
Retrospectively, we identified 203 prostate cancer patients with visceral metastases from the Mayo Clinic Advanced Prostate Cancer Registry. Patients were divided into three groups according to the first site of visceral metastases detected: lung, brain, or liver. Visceral metastases were detected primarily on either metabolic imaging (C-11 choline) or prostate-specific membrane antigen positron emission tomography computed tomography (CT) scan. Confirmation of visceral metastasis diagnosis was established with either biopsy when feasible or focused conventional imaging, including focused CT or magnetic resonance imaging. Overall survival and cancer-specific survival were estimated using the Kaplan-Meier method. Univariate and multivariate Cox regression model was conducted to assess different variables that affect overall and cancer-specific survival.
Key findings and limitations
Over a median (interquartile range) follow-up duration of 16.2 (3.9–49.8) mo, the overall and cancer-specific survival of the entire cohort suggests better survival patterns in patients with first-site lung metastases than in patients with first-site brain or liver metastases (p < 0.0001). In univariate and multivariate analyses of factors impacting patients’ overall and cancer-specific survival, a high prostate-specific antigen level at diagnosis of visceral metastasis, concomitant bone and lymph node disease, and more than four visceral metastases were associated with poor overall and cancer-specific survival (p < 0.05). On the contrary, first-site lung metastasis was associated with improved overall and cancer-specific survival, compared with first-site liver and brain metastases (p < 0.001).
Conclusions and clinical implications
These data suggest that prostate cancer patients with visceral metastatic disease have varying survival patterns according to first-site detected visceral metastasis. In our cohort, patients with first-site lung metastasis demonstrated better survival outcomes than patients with first-site brain or liver metastasis.
Patient summary
Our study explored the survival outcomes among patients with visceral metastatic prostate cancer employing cutting-edge imaging methods. Prostate cancer patients with metastases to different organs have different survival rates. Patients with cancer spreading to the lungs first showed better survival than those with cancer spreading to the brain or liver first.
1. Introduction
Prostate cancer (PCa) stands out as one of the most common malignancies in men in the USA, ranking second only to lung cancer in cancer-related mortality at 7.1% [1], [2]. The majority of patients with PCa initially present with localized disease, wherein radical prostatectomy and radiation therapy serve as the cornerstone of treatment. Of the men with PCa, 5% present with de novo metastatic disease, and approximately 17% of those diagnosed with localized PCa will eventually develop metastases [3], [4], [5]. In the context of metastatic disease, systemic treatment emerges as fundamental for patients, whether in a hormone-sensitive or a hormone-resistant state, showing reported enhancements in survival outcomes [6].
The advent of advanced imaging techniques, such as C-11 choline positron emission tomography (PET) and prostate-specific membrane antigen (PSMA) PET, has notably enhanced our ability to detect the sites of disease relapse [4], [5]. Most extraprostatic tumor spread predominantly involves lymph nodes and bones, with studies indicating a relatively favorable prognosis for individuals with lymph node and bone-only metastatic PCa compared with those with visceral metastases [7]. Historically, visceral metastasis affects up to 15% of metastatic PCa cases, commonly spreading to the liver, brain, and lungs [8], [9], with lung metastasis being most frequent, followed by the liver, while brain metastases are less prevalent [10], [11].
Visceral metastases are consistently linked to poor survival outcomes, emphasizing the significance of early detection [12], [13]. Early detection enables the prompt initiation of systemic treatment strategies, including androgen deprivation therapy (ADT), chemotherapy, and/or androgen receptor signaling inhibitors, resulting in improved overall survival (OS) and better quality of life for PCa patients [14], [15]. While the current body of literature emphasizes the generally poor prognosis linked to visceral metastasis, the available data heavily depend on national databases that lack specificity regarding the site of the initial visceral metastasis. Additionally, reliance on conventional imaging for diagnosis often results in delayed detection and the emergence of multiple visceral metastasis sites [7], [8]. As a result, there exists a significant lack of evidence regarding the influence of organ-specific first-site visceral metastasis on the prognosis and OS outcomes of patients.
Consequently, this study endeavors to analyze a cohort of PCa patients with visceral metastases, detected using either C-11 choline PET/computed tomography (CT) or PSMA PET/CT, aiming to unravel the correlation between the initial site of visceral metastatic disease and its implications for oncological outcomes.
2. Patients and methods
2.1. Patient selection
Research approval was obtained from the institutional review board under reference number IRB 16-003907. After approval, a thorough search was conducted within our prospectively maintained Mayo Clinic Advanced Prostate Cancer registry of 5575 patients. We focused our study on patients seen in the past 10 yr, from years 2012 to 2022. A total of 203 PCa patients with visceral metastases, identified through C-11 choline PET/CT or PSMA PET/CT scans, and possessing complete data in accordance with our inclusion and exclusion criteria, were included in the study. The confirmation of visceral metastasis diagnosis was established through either biopsy when feasible or focused conventional imaging methods, including CT and magnetic resonance imaging. Notably, the study encompassed patients with single-site visceral metastasis only, excluding those with multiple-site visceral metastases.
Extensive chart reviews were conducted to extract detailed demographic and clinical data, encompassing various aspects such as age at primary PCa diagnosis, age at visceral metastasis diagnosis, race, intervals between diagnoses, tumor stage, levels of prostate-specific antigen (PSA), Gleason scores, primary treatments, castrate status at visceral metastasis diagnosis, count of visceral metastases, simultaneous presence of lymph node or bone metastases, as well as the duration of follow-up.
Patients were categorized into three distinct groups based on the primary site of their visceral metastasis: group 1, consisting of patients with initial visceral metastasis to the lung (first-site lung metastases; n = 92); group 2, comprising patients with initial visceral metastasis to the brain (first-site brain metastases; n = 26); and group 3, including patients with initial visceral metastasis to the liver (first-site liver metastases; n = 85).
2.2. Primary outcome
Our study aims primarily to explore the influence of first-site visceral metastasis on patients' overall survival (OS) and cancer-specific survival (CSS).
2.3. Statistical analysis
Continuous variables were summarized by calculating means and standard deviations. Comparisons between the three groups were completed using a one-way analysis of variance. Categorical variables were presented as frequencies and percentages, and comparisons between groups were made using appropriate statistical tests such as χ2 and Fisher's exact tests. Survival analysis and event-free survival estimation were conducted utilizing the Kaplan-Meier product-limit method. To compare survival experiences across groups, the log-rank test was utilized. Furthermore, the Cox proportional hazard model was employed to establish associations, reporting relative hazards (hazard ratio [HR]) with corresponding 95% confidence intervals (CIs), between various risk factors and the time to the event of interest. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA) and R (version R-4.1; R Foundation for Statistical Computing, Vienna, Austria), with a significance level set at p < 0.05 (two sided) to denote statistical significance.
3. Results
3.1. Patients’ demographics
A total of 203 PCa patients with visceral metastasis met the inclusion and exclusion criteria and were included in the study. Table 1 demonstrates patients’ baseline characteristics. Regarding primary tumor Gleason grade, patients with first-site liver metastasis exhibited the highest median Gleason scores (median 9), followed by patients with first-site brain metastasis (median 8), and, lastly, patients with first-site lung metastasis (median 7; p < 0.0001).
Table 1.
Clinicopathological variables of study groups
| Feature | Lung Mets (n = 92) |
Brain Mets (n = 26) |
Liver Mets (n = 85) |
p value |
|---|---|---|---|---|
| Age at diagnosis of PCa (yr), median (IQR) | 63.2 (57.5–67.9) | 60.9 (55.7–69.1) | 59.9 (55.5–67.9) | 0.344 |
| Race, n (%) | 0.909 | |||
| Caucasian | 87 (94.6) | 24 (92.4) | 80 (94.1) | |
| African American | 2 (2.2) | 1 (3.8) | 1 (1.2) | |
| Other | 3 (3.3) | 1 (3.8) | 4 (4.7) | |
| Primary Gleason score, median (IQR) | 7 (7–9) | 8 (7–9) | 9 (7–9) | <0.0001* |
| Tumor staging, n (%) | 0.319 | |||
| T1 | 1 (1.1) | 2 (7.7) | 1 (1.2) | |
| T2 | 27 29.3) | 6 (23.1) | 16 (18.8) | |
| T3 | 33 (35.9) | 3 (11.5) | 30 (35.3) | |
| T4 | 3 (3.3) | 1 (3.8) | 8 (9.4) | |
| Tx | 28 (30.4) | 14 (53.8) | 30 (35.3) | |
| Primary treatment of PCa, n (%) | 0.0006* | |||
| RP | 63 (68.5) | 13 (50) | 47 (55.3) | |
| RT | 26 (28.3) | 7 (26.9) | 16 (18.8) | |
| Other | 3 (3.3) | 6 (23.1) | 22 (25.9) | |
| Treatments prior to visceral Mets Dx | <0.0001* | |||
| ADT | 69 (75) | 25 (96.2) | 85 (100) | |
| APRI | 14 (15.2) | 14 (53.8) | 58 (68.2) | |
| Chemotherapy | 15 (16.3) | 15 (57.7) | 60 (70.6) | |
| Time from PCa Dx to visceral Mets Dx (yr), median (IQR) | 73 (47.4–113.2) | 55.5 (26.9–125.8) | 77 (28.9–132.7) | 0.709 |
| Age at diagnosis of visceral Mets (yr), median (IQR) | 69.3 (65.8–75.4) | 68.7 (61.2–76.8) | 69.4 (62.7–75.8) | 0.702 |
| PSA at the time of visceral Mets Dx, median (IQR) | 4.4 (1.3–10.9) | 13.1 (0.33–59.1) | 44.9 (2.6–163.5) | 0.002* |
| Castrate status at the time of visceral Mets, n (%) | <0.0001* | |||
| Hormone sensitive | 30 (32.6) | 2 (7.7) | 1 (1.2) | |
| Hormone resistant | 62 (67.4) | 24 (92.3) | 84 (98.8) | |
| Imaging technique, n (%) | 0.063 | |||
| C-11 choline PET | 70 (76.1) | 20 (76.9) | 77 (90.6) | |
| PSMA PET | 22 (23.9) | 6 (23.1) | 8 (9.4) | |
| Conventional imaging | 69 (75) | 26 (100) | 82 (96.5) | |
| Number of Mets, n (%) | <0.0001* | |||
| 1 | 18 (19.6) | 11 (42.3) | 8 (9.4) | |
| 2–4 | 28 (30.4) | 9 (34.6) | 10 (11.8) | |
| >4 | 46 (50) | 6 (23.1) | 69 (81.2) | |
| Concomitant Mets, n (%) | <0.0001* | |||
| Lymph node | 34 (37) | 2 (7.7) | 14 (16.5) | |
| Bone | 13 (14.1) | 12 (46.2) | 33 (38.3) | |
| Both | 19 (20.7) | 9 (34.6) | 32 (37.6) | |
| Follow-up time, median (IQR) | 50.8 (28.3–80.8) | 6.1 (1.2–25.9) | 6 (1.7–14.2) | <0.0001* |
ADT = androgen deprivation therapy; ARPI = androgen receptor pathway inhibitor; Dx = diagnosis; IQR = interquartile range; Mets = metastases; PCa = prostate cancer; PET = positron emission tomography; PSA = prostate-specific antigen; PSMA = prostate-specific membrane antigen; RP = radical prostatectomy; RT = radiotherapy. "*" Indicates statistically significant p-value <0.05.
Radical prostatectomy was the predominant primary treatment for most patients regardless of the initial site of visceral metastasis (lung, liver, or brain). De novo visceral metastases were initial presentation in 25% of patients with first-site liver metastases, 23% of patients with first-site brain metastases, and 3% of patients with first-site lung metastases. Consequently, prior treatment with ADT was highly prevalent among patients with first-site liver and brain metastases compared with patients with first-site lung metastases; prior ADT was reported in 100% and 96% of patients with, respectively, first-site liver and brain metastases versus 75% of patients with first-site lung metastases (p < 0.0001).
Liver metastasis patients had the highest PSA levels at diagnosis, and castration-resistant PCa was more common in the liver and brain metastasis groups. Liver metastasis patients also tended to have a higher number of metastatic sites and more concomitant lymph node and bone metastases than lung and brain metastasis patients (p < 0.0001).
3.2. Primary outcomes
We examined disparities in OS and CSS among our groups over a 50-mo follow-up period.
3.3. Patients’ OS
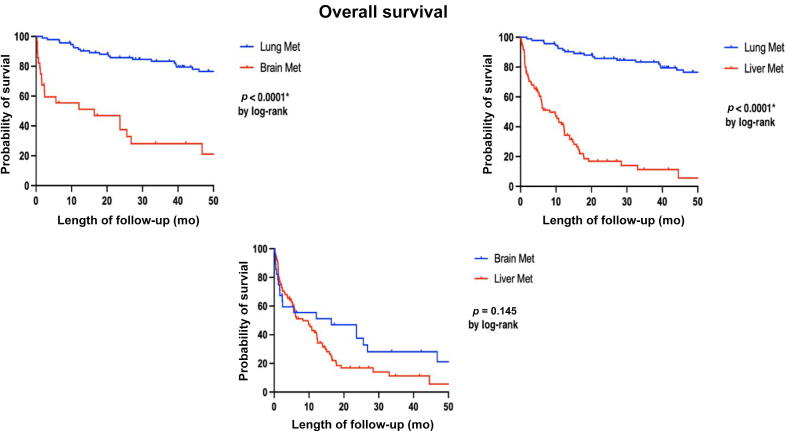
Over a 50-mo follow-up period, the study observed that among 92 patients with first-site lung metastases, 41 died. Similarly, 22/26 patients with first-site brain metastases and 83/85 patients with first-site liver metastases died. The 50-mo OS rates were 55% for those with first-site lung metastases, 15% for first-site brain metastases, and 2% for first-site liver metastases, as depicted in Figure 1 (log-rank <0.0001).
Fig. 1.
K-M curve for overall survival outcomes between our three groups according to first-site visceral metastasis. K-M = Kaplan-Meier; Met = metastasis. "*" Indicates statistically significant p-value <0.05.
Table 2 demonstrates univariable and multivariable analyses of factors influencing patients' OS. The study identified prior treatment with ADT, high PSA value at the time of visceral metastasis diagnosis, presence of more than four metastases, and concurrent lymph nodes or both bone and lymph node metastases as factors associated with poor survival outcomes. The HRs for these factors in multivariable analyses were 4.9 (1.14–21.74), 1.1 (1.00–1.21), 1.9 (1.15–3.16), 2.1 (1.10–4.84), and 2.5 (1.17–5.31), respectively (p < 0.05). Furthermore, the study observed that first-site lung metastasis predicted improved OS compared with first-site brain or liver metastasis, with an HR of 0.3 (0.15–0.50; p < 0.001).
Table 2.
Uni- and multivariate analyses of factors associated with overall survival
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Primary Gleason score | 1.5 (1.25–1.82) | <0.001 * | 1.1 (0.93–1.35) | 0.218 |
| Prior treatment | ||||
| ADT | 14.9 (3.66–60.95) | <0.001 * | 4.9 (1.14–21.74) | 0.033 * |
| ARPI | 4.9 (3.25–7.39) | <0.001 * | 1.6 (0.95–2.73) | 0.078 |
| Chemo | 3.9 (2.66–5.81) | <0.001 * | 1.4 (0.87–2.38) | 0.154 |
| Age at visceral Mets Dx | 1.1 (0.98–1.02) | 0.859 | – | – |
| PSA at visceral Mets Dx | 1.1 (1.01–1.30) | <0.001 * | 1.1 (1.00–1.21) | 0.037 * |
| CRPC status | 8.1 (2.95–21.57) | <0.001 * | 1.76 (0.61–5.11) | 0.296 |
| Number of Mets | 1.8 (1.25–2.74) | 0.002 * | 1.9 (1.15–3.16) | 0.012 * |
| Concomitant Mets | ||||
| Lymph node | 0.61 (0.38–0.96) | 0.033 * | 2.1 (1.1–4.84) | 0.047 * |
| Bone | 2.16 (1.74–3.18) | <0.001 * | 2.1 (0.99–4.86) | 0.050 |
| Both | 1.62 (1.09–2.39) | 0.016 * | 2.5 (1.17–5.31) | 0.018 * |
| First site of visceral Mets | ||||
| Lung | 0.1 (0.07–0.18) | <0.001 * | 0.3 (0.15–0.50) | <0.001 * |
| Brain | 1.9 (1.18–3.19) | 0.009 * | – | – |
| Liver | 6.4 (4.07–9.93) | <0.001 * | 1.1 (0.54–2.19) | 0.807 |
ADT = androgen deprivation therapy; ARPI = androgen receptor pathway inhibitor; CI = confidence interval; CRPC = castrate-resistant prostate cancer; Dx = diagnosis; Mets = metastases; PSA = prostate-specific antigen. "*" Indicates statistically significant p-value <0.05.
3.4. Patients’ CSS
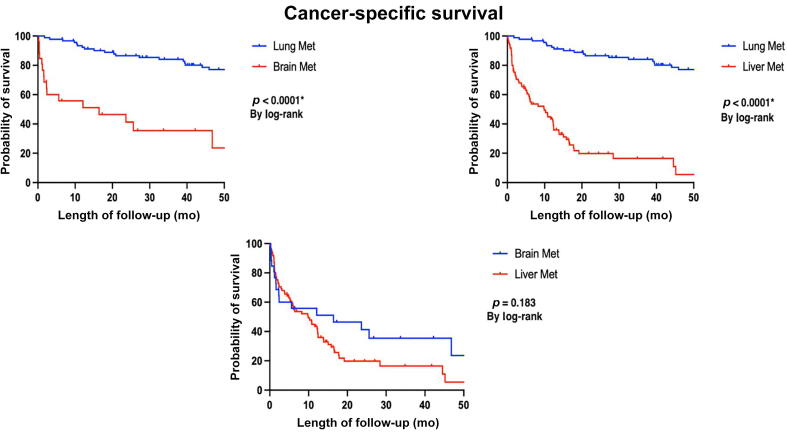
During a 50-mo follow-up period, we noted that 41/92 patients with first-site lung metastases succumbed secondary to their disease. Likewise, 23/26 patients with primary brain metastases and 83/85 patients with initial liver metastases died. This translates to CSS rates at 50 mo of 55% for individuals with first-site lung metastases, 11% for those with first-site brain metastases, and 2% for those with first-site liver metastases, as illustrated in Figure 2 (log-rank <0.0001).
Fig. 2.
K-M curve for cancer-specific survival outcomes between our three groups according to first-site visceral metastasis. K-M = Kaplan-Meier; Met = metastasis. "*" Indicates statistically significant p-value <0.05.
Table 3 provides a comprehensive analysis of both univariable and multivariable assessments focusing on the factors that impact CSS of patients. The investigation revealed that certain variables, including prior ADT or androgen receptor pathway inhibitors, high PSA values at the time of visceral metastasis diagnosis, presence of more than four metastases, and the concurrent existence of bone metastases or both bone and lymph node metastases, were linked with unfavorable outcomes in terms of survival. The HRs associated with these factors in the multivariable analyses were 9.48 (1.25–32.3), 1.9 (1.1–3.36), 1.1 (1.00–1.30), 1.9 (1.17–3.38), 2.4 (1.07–5.36), and 2.8 (1.26–6.24), respectively (p < 0.05). Again, first-site lung metastasis demonstrated a positive correlation with improved OS compared with first-site brain or liver metastasis, with an HR of 0.3 (0.21–0.55; p < 0.001).
Table 3.
Uni- and multivariate analyses of factors associated with cancer-specific survival
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Primary Gleason score | 1.2 (1.28–1.88) | <0.001 * | 1.1 (0.94–1.38) | 0.195 |
| Prior treatment | ||||
| ADT | 27.7 (3.85–53.32) | <0.001 * | 9.48 (1.25–32.3) | 0.029 * |
| ARPI | 5.4 (3.49–8.28) | <0.001 * | 1.9 (1.1–3.36) | 0.022 * |
| Chemo | 3.9 (2.65–5.99) | <0.001 * | 1.3 (0.78–2.19) | 0.316 |
| Age at visceral Mets Dx | 1.1 (0.98–1.03) | 0.695 | – | – |
| PSA at visceral Mets Dx | 1.1 (1.01–1.30) | <0.001 * | 1.1 (1.00–1.30) | 0.029 * |
| CRPC status | 7.3 (2.68–19.83) | <0.001 * | 1.5 (0.52–4.15) | 0.446 |
| Number of Mets | 1.9 (1.29–2.92) | 0.002 * | 1.9 (1.17–3.38) | 0.011 * |
| Concomitant Mets | ||||
| Lymph node | 0.6 (0.32–0.87) | 0.012 * | 2.2 (0.94–5.11) | 0.068 |
| Bone | 2.3 (1.56–3.45) | <0.001 * | 2.4 (1.07–5.36) | 0.034 * |
| Both | 1.7 (1.15–2.57) | 0.008 * | 2.8 (1.26–6.24) | 0.011 * |
| First site of visceral Mets | ||||
| Lung | 0.1 (0.07–0.19) | <0.001 * | 0.3 (0.21–0.55) | <0.001 * |
| Brain | 1.2 (1.84–3.11) | 0.022 * | – | – |
| Liver | 6.3 (3.94–9.93) | <0.001 * | 0.8 (0.41–1.74) | 0.651 |
ADT = androgen deprivation therapy; ARPI = androgen receptor pathway inhibitor; CI = confidence interval; CRPC = castrate-resistant prostate cancer; Dx = diagnosis; Mets = metastases; PSA = prostate-specific antigen. "*" Indicates statistically significant p-value <0.05.
It is noteworthy that all patients who succumbed to first-site lung and liver metastases did so due to their disease. Among those with first-site brain metastases, the majority died due to the metastatic disease, except for one individual who died from reasons unrelated to their metastatic condition.
4. Discussion
Despite recent advances in PCa management and a wide variety of treatment options, visceral metastatic disease represents a challenge. The reported incidence of visceral metastasis displays a wide spectrum across existing literature, varying between 15% and 20% across a patient’s life span. Additionally, Pezaro et al [16] reported visceral metastasis, detected on CT scans, in 32% of patients within 3 mo of death. In an autopsy study of 1500 PCa patients, Bubendorf et al [17] reported visceral metastatic disease to the lung in 46% of patients and to the liver in 25% of patients. This is consistent with our experience that the lung is the most common site of visceral metastasis followed by the liver and brain.
The presence of visceral metastasis often signals an unfavorable prognosis and late complication of the disease, often intertwined with hormonal resistance and castration-resistant PCa [18], [19], [20]. However, a comprehensive understanding of survival patterns specifically linked to site-specific visceral metastasis remains somewhat limited.
A compelling finding arising from our study revealed a significant divergence in OS and CSS of PCa patients according to site-specific visceral metastasis. Patients with lung metastases exhibited the highest rates of 50-mo OS and CSS at 55%, followed by brain with OS rates at 15% and CSS rates at 11%, and then liver metastases at 2% for both OS and CSS (log-rank <0.05). This is consistent with the findings reported by Cui et al [10]; in a population-based study investing the impact of site-specific visceral metastasis on patients’ OS, they reported better OS for patients with lung metastasis, and comparable inferior survival outcomes for distant metastases to the liver and brain. Additionally, in the setting of metastatic castration-resistant PCa (mCRPC) with visceral metastasis, Halabi et al [11] reported inferior survival outcomes for patients with liver metastasis compared with patients with lung metastasis. Moreover, in a contemporary prognostic nomogram for patients with mCRPC, Armstrong et al [21] reported poor survival outcomes of patients with liver metastasis.
Furthermore, we extended our analysis to investigate other independent predictors of poor survival than site-specific visceral metastasis. Our study underscored the pivotal role of the number of metastatic deposits as a prognostic marker. In our experience, presence of more than four visceral metastatic deposits was associated with an HR of death of 1.9 (95% CI: 1.15–3.16 and p = 0.012). This is consistent with the findings of Cui et al [10] reporting better OS in patients with single-site visceral metastasis than in those with multiple-site visceral metastasis. Notably, most patients with first-site liver metastasis presented with more than four metastatic deposits, which could contribute to their notably reduced survival, while fewer patients in first-site lung and brain metastases displayed such extensive metastatic burdens, emphasizing a significant difference among the groups (p < 0.0001).
Additionally, we investigated the impact of concomitant metastatic disease to lymph node and bone on patients’ OS and CSS. We noted that concomitant lymph node–only disease or concomitant lymph node and bone disease were associated with poor OS, with HRs of 2.1 and 2.5, respectively (p = 0.047 and 0.018, respectively; Table 2). Using the Surveillance, Epidemiology, and End Results database, Tappero et al [22] reported better survival rates in patients with lung-only visceral metastasis than in patients with lung metastases and concomitant nonvisceral metastases, specifically lymph node and/or bone disease. Interestingly, their observations were limited to only visceral metastasis to the lung. The authors noted that visceral metastases to sites other than the lung were associated with poor survival outcomes regardless of their status of concomitant disease. Moreover, we reported poor CSS in patients with visceral metastasis and concomitant bone-only disease or concomitant bone and lymph node disease, with HRs of 2.4 and 2.8, respectively (p = 0.034 and 0.011, respectively).
We observed a correlation between elevated PSA levels at the time of diagnosing visceral metastasis and unfavorable outcomes in both OS and CSS, with an HR of 1.1 (p = 0.037 and 0.029, respectively). While the adverse prognostic significance of high PSA levels is not a novel finding and has been documented in the literature across various stages of the disease process [23], [24], [25], our study is the first to specifically highlight the influence of PSA on OS and CSS in the context of visceral metastatic disease. The retrospective investigation presented herein offers valuable insights regarding the influence of first-site visceral metastasis on patients’ OS and CSS. Our study demonstrated multiple strength points. Our study represents the first to describe the impact of site-specific visceral metastasis on patients’ CSS. In addition, all visceral metastasis diagnoses were based on advanced imaging using C-11 choline PET/CT or PSMA PET/CT, and confirmed with either biopsy or focused conventional imaging.
However, it is crucial to acknowledge the inherent limitations of this study. The retrospective design of the study precludes the establishment of direct causal relationships between the identified factors and survival outcomes. Additionally, as this study's findings stem from a specific patient cohort within a single institution, their applicability to broader populations or diverse health care settings might be limited. Moreover, the majority of patients enrolled in our study underwent assessment using C-11 choline PET/CT scans. While our study benefits from the utilization of choline PET/CT scans instead of conventional imaging methods, it is essential to acknowledge the inherent shortcomings of choline PET/CT scans in accurately identifying metastatic disease when compared with PSMA PET/CT scans [26]. Despite these constraints, this study contributes significantly to the understanding of the impact of site-specific visceral metastasis on PCa patient outcomes, offering meaningful insights that can guide future research and clinical practice.
5. Conclusions
Our findings indicate distinct survival trends among PCa patients with visceral metastatic disease, contingent upon the initial site of visceral metastasis. Notably, within our cohort, patients displaying first-site lung metastasis exhibited comparatively more favorable survival outcomes than those with initial metastases in the brain or liver. These survival patterns underscore the importance of tailored counseling and the exploration of more aggressive treatment strategies for affected patients. To delve deeper into this realm, cohort analyses from prospective trials are imperative to comprehensively investigate optimal treatment approaches tailored to the varying sites and number of metastases in PCa patients.
Author contributions: Mohamed E. Ahmed had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ahmed, Mahmoud, Andrews, Karnes, Kwon.
Acquisition of data: Zeina, Day, Reitano, Lehner.
Analysis and interpretation of data: Ahmed, Mahmoud, Andrews, Kwon, Karnes, Kendi, Johnson, Orme, Childs, Riaz.
Drafting of the manuscript: Ahmed, Mahmoud, Reitano.
Critical revision of the manuscript for important intellectual content: Andrews, Kendi, Johnson, Kwon, Karnes, Childs, Orme, Riaz.
Statistical analysis: Mahmoud, Ahmed, Zeina, Day, Lehner.
Obtaining funding: None.
Administrative, technical, or material support: Day, Zeina.
Supervision: Andrews, Kwon, Karnes, Kendi, Johnson, Orme, Childs, Riaz.
Other: None.
Financial disclosures: Mohamed E. Ahmed certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Roderick van den Bergh
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Scosyrev E., Messing E.M., Mohile S., Golijanin D., Wu G. Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118:3062–3070. doi: 10.1002/cncr.26392. [DOI] [PubMed] [Google Scholar]
- 5.Mahmoud A.M., Gao R.W., Ahmed M.E., et al. Metastasis-directed therapy for metachronous lung metastases in prostate cancer. JU Open Plus. 2023;1:e00050. [Google Scholar]
- 6.Yanagisawa T., Rajwa P., Kawada T., et al. Efficacy of systemic treatment in prostate cancer patients with visceral metastasis: a systematic review, meta-analysis, and network meta-analysis. J Urol. 2023;210:416–429. doi: 10.1097/JU.0000000000003594. [DOI] [PubMed] [Google Scholar]
- 7.Gandaglia G., Abdollah F., Schiffmann J., et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74:210–216. doi: 10.1002/pros.22742. [DOI] [PubMed] [Google Scholar]
- 8.Svensson E., Christiansen C.F., Ulrichsen S.P., Rorth M.R., Sorensen H.T. Survival after bone metastasis by primary cancer type: a Danish population-based cohort study. BMJ Open. 2017;7:e016022. doi: 10.1136/bmjopen-2017-016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinjamoori A.H., Jagannathan J.P., Shinagare A.B., et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199:367–372. doi: 10.2214/AJR.11.7533. [DOI] [PubMed] [Google Scholar]
- 10.Cui P.F., Cong X.F., Gao F., et al. Prognostic factors for overall survival in prostate cancer patients with different site-specific visceral metastases: a study of 1358 patients. World J Clin Cases. 2020;8:54–67. doi: 10.12998/wjcc.v8.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halabi S., Kelly W.K., Ma H., et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34:1652–1659. doi: 10.1200/JCO.2015.65.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandaglia G., Karakiewicz P.I., Briganti A., et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol. 2015;68:325–334. doi: 10.1016/j.eururo.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Scher H.I., Halabi S., Tannock I., et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Body J.J., Casimiro S., Costa L. Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol. 2015;12:340–356. doi: 10.1038/nrurol.2015.90. [DOI] [PubMed] [Google Scholar]
- 15.Goodman O.B., Jr, Flaig T.W., Molina A., et al. Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:34–39. doi: 10.1038/pcan.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pezaro C., Omlin A., Lorente D., et al. Visceral disease in castration-resistant prostate cancer. Eur Urol. 2014;65:270–273. doi: 10.1016/j.eururo.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bubendorf L., Schopfer A., Wagner U., et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 18.Pond G.R., Sonpavde G., de Wit R., Eisenberger M.A., Tannock I.F., Armstrong A.J. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:3–6. doi: 10.1016/j.eururo.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 19.McCutcheon I.E., Eng D.Y., Logothetis C.J. Brain metastasis from prostate carcinoma: antemortem recognition and outcome after treatment. Cancer. 1999;86:2301–2311. doi: 10.1002/(sici)1097-0142(19991201)86:11<2301::aid-cncr18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Pouessel D., Gallet B., Bibeau F., et al. Liver metastases in prostate carcinoma: clinical characteristics and outcome. BJU Int. 2007;99:807–811. doi: 10.1111/j.1464-410X.2006.06663.x. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong A.J., Garrett-Mayer E.S., Yang Y.C., de Wit R., Tannock I.F., Eisenberger M. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396–6403. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 22.Tappero S., Piccinelli M.L., Incesu R.B., et al. Overall survival of metastatic prostate cancer patients according to location of visceral metastatic sites. Clin Genitourin Cancer. 2024;22:47–55.e2. doi: 10.1016/j.clgc.2023.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Matsubara N., Chi K.N., Özgüroğlu M., et al. Correlation of prostate-specific antigen kinetics with overall survival and radiological progression-free survival in metastatic castration-sensitive prostate cancer treated with abiraterone acetate plus prednisone or placebos added to androgen deprivation therapy: post hoc analysis of phase 3 LATITUDE study. Eur Urol. 2020;77:494–500. doi: 10.1016/j.eururo.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen-Nielsen M., Liede A., Maegbaek M.L., et al. Survival and PSA-markers for mortality and metastasis in nonmetastatic prostate cancer treated with androgen deprivation therapy. Cancer Epidemiol. 2015;39:623–632. doi: 10.1016/j.canep.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Komori T., Matsumoto K., Kosaka T., et al. Long-term prognosis and treatment strategy of persistent PSA after radical prostatectomy. Ann Surg Oncol. 2023;30:6936–6942. doi: 10.1245/s10434-023-13780-1. [DOI] [PubMed] [Google Scholar]
- 26.Jilg C.A., Drendel V., Rischke H.C., et al. Detection rate of (18)F-choline PET/CT and (68)Ga-PSMA-HBED-CC PET/CT for prostate cancer lymph node metastases with direct link from PET to histopathology: dependence on the size of tumor deposits in lymph nodes. J Nucl Med. 2019;60:971–977. doi: 10.2967/jnumed.118.220541. [DOI] [PMC free article] [PubMed] [Google Scholar]