Abstract (301 words)
Introduction
The effects of low energy availability (LEA) on the immune system are poorly understood. This study examined the effects of 14 days of LEA on immune cell redox balance and inflammation at rest and in response to acute exercise, and exercise performance in female athletes.
Methods
Twelve female endurance athletes (age: 26.8 ± 3.4 yrs, maximum oxygen uptake (O2max): 55.2 ± 5.1 mL × min−1 × kg−1) were included in a randomized, single-blinded crossover study. They were allocated to begin with either 14 days of optimal energy availability diet (OEA, 52 ± 2 kcal × kg fat free mass (FFM)−1 × day−1) or LEA diet (22 ± 2 kcal × kg FFM−1 × day−1), followed by 3 days of refueling (OEA) with maintained training volume. Peripheral blood mononuclear cells (PBMCs) were isolated, and plasma obtained at rest before and after each dietary period. The PBMCs were used for analysis of mitochondrial respiration and H2O2 emission and specific proteins. Exercise performance was assessed on cycle by a 20-min time trial and time to exhaustion at an intensity corresponding to ∼110 % O2max).
Results
LEA was associated with a 94 % (P = 0.003) increase in PBMC NADPH oxidase 2 protein content, and a 22 % (P = 0.013) increase in systemic cortisol. LEA also caused an alteration of several inflammatory related proteins (P < 0.05). Acute exercise augmented H2O2 emission in PBMCs (P < 0.001) following both OEA and LEA, but to a greater extent following LEA. LEA also reduced the mobilization of white blood cells with acute exercise. After LEA, performance was reduced in both exercise tests (P < 0.001), and the reduced time trial performance remained after the 3 days of refueling (P < 0.001).
Conclusion
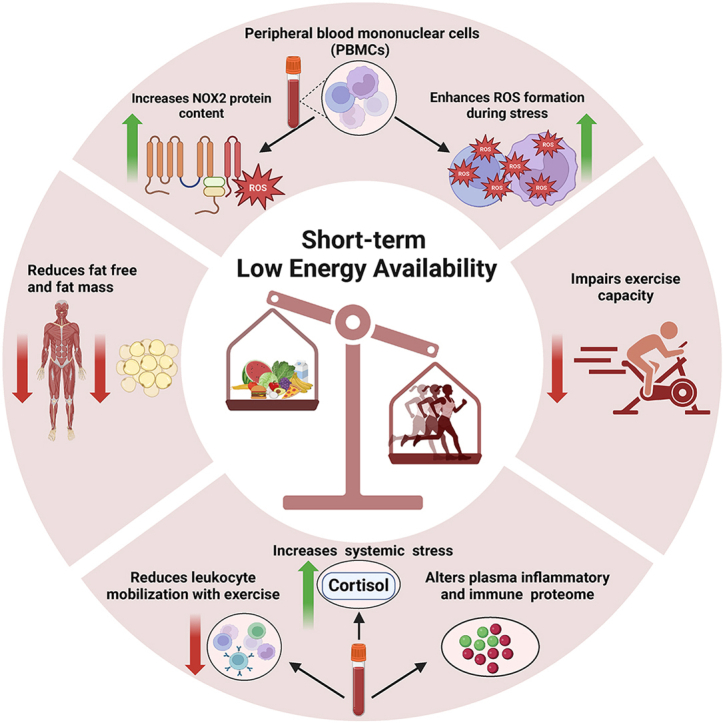
14 days of LEA in female athletes increased cortisol levels and had a pronounced effect on the immune system, including increased capacity for ROS production, altered plasma inflammatory proteome and lowered exercise induced mobilization of leukocytes. Furthermore, LEA resulted in a sustained impairment in exercise performance.
Keywords: Low energy availability, Peripheral blood mononuclear cell, Oxidative stress, Exercise performance, Proteomics, Immune function
Graphical abstract
Highlights
-
•
Increases immune cell oxidative stress but not inflammatory markers.
-
•
Increases resting systemic cortisol and alters the plasma immune proteome.
-
•
Impairs endurance and intense exercise performance.
1. Introduction
While nutrition and exercise have been shown to individually impact the immune system [1,2], less is known of how a combination of inadequate dietary intake and acute and excessive exercise affects the immune system. Low energy availability (LEA) is defined as a mismatch between dietary energy intake (EI) and exercise energy expenditure (EEE) that leads to inadequate energy [3,4] to support bodily functions required to maintain optimal health and performance [5]. LEA is highly prevalent, particularly among female endurance athletes [6,7] where short-term LEA (<14 days) is reportedly associated with impairments in endocrine, metabolic and muscular functions [8,9]. If sustained over longer periods of time, LEA has been found to have potential detrimental effects in female athletes with signs and symptoms such as functional hypothalamic amenorrhea and impaired bone health [6,10]. However, despite strong indications of negative effects of LEA, there are very few well controlled studies to date on the influence of LEA on the physiology of athletes in general and in particular on the effect of LEA on inflammation and the immune system.
Peripheral blood mononuclear cells (PBMC), which encompass lymphocytes and monocytes, play a vital role in the immune system. PBMCs offer valuable insights into immune function and have served as surrogate tissue for monitoring nutritional and metabolic responses [[11], [12], [13]]. As such, PBMCs are highly dynamic cells which can alter their bioenergetics and redox state in response to various conditions, including acute and exercise training [[14], [15], [16]] and nutrition [17,18]. Impaired mitochondrial respiratory capacity and excessive production of reactive oxygen species (ROS) in PBMCs are directly linked to immune function [19,20], making them excellent markers of immediate immune status. Immune cell ROS has pleiotropic actions; on one hand ROS are necessary for immune function, contributing to oxidative pathogen killing, inflammation, signal transduction, differentiation, and proliferation [21]. On the other hand, excessive ROS can lead to hyperactivation of inflammatory responses, resulting in tissue damage and pathology [[21], [22], [23], [24]]. It is currently unclear how LEA impacts the immune system by disrupting PBMC bioenergetics and redox homeostasis and thereby also potentially increasing susceptibility to e.g. infections. Although valuable information on the immune system can be obtained at rest, assessments in response to acute exercise can provide additional valuable insights into how energy availability influences the response of the immune system to acute stress and furthermore, whether such changes are affected by LEA. Acute exercise provokes the release of a cascade of cytokines [25,26] – in particular interleukin-6, which is a pleiotropic cytokine with important actions to regulate energy status and inflammation [[27], [28], [29]]. Further, exercise provokes mobilization of leukocytes (including alterations in the distribution of specific immune cell types) [[30], [31], [32]]. Importantly, an immunosuppressive effect of strenuous endurance exercise [33,34] during LEA could heighten the risk of infection/illness and injuries, which might explain the high prevalence observed among elite athletes [[35], [36], [37], [38], [39]]. Furthermore, given the sensitivity of the immune system to changes in nutritional statuses, the potential energetic stress caused by short-term LEA exposure may be transient and reversed by restoring energetic conditions within days.
The present study assessed the impact of 14 days of LEA on PBMC bioenergetics, redox state, systemic inflammation, stress markers, and the plasma inflammatory proteome. Measurements were made both at rest and following acute exercise as exercise is known to induce a transient inflammatory response [25]. We hypothesized that LEA would affect PBMC bioenergetic and ROS formation and further, cause an increase in systemic stress. We furthermore, hypothesized that LEA would acutely impair short-intense and endurance exercise performance; and to assess whether these immunological and performance-related effects were sustained, we included a subsequent three-day refueling period.
2. Methods
2.1. Participants
The experimental trials were conducted from January 2022 to January 2023. In total, 12 endurance trained eumenorrheic females were recruited. Inclusion criteria were females aged 18–40 years, body mass index 18–23 kg × m−2, regular normal menstrual cycle or hormonal contraceptive users, maintained body mass (±3 kg) and >6 h of endurance exercise every week for the past six months. Exclusion criteria included a ratio between measured and predicted resting metabolic rate of <0.90 [40] (Cunningham equation [41]), smoking, chronic use of prescription medication, chronic illness or history of eating disorders. Participants were fully informed of experimental procedures and written consent was obtained prior to participation. The study was approved by the Health Research Ethics Committee of the Capital Region of Denmark (H-21032399) and was performed in accordance with the Declaration of Helsinki.
2.2. Assessment of eligibility criteria
Prior to inclusion, eligibility criteria were assessed during a screening where participants reported to the lab following 12 h of fasting. Following 10-min of supine rest, participants underwent a dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare) scan to assess body composition. Next, an anamnesis regarding history of participants health, diseases, menstrual cycle, and training was performed. Participants answered the Low Energy in Females Questionnaire (LEAF-Q, cutoff: ≥8 indicative of risk of LEA [42]), and the Eating Disorder Examination Questionnaire (EDE-Q, cutoff: global score <2.3 indicative of disordered eating behavior) [43] followed by a 25-min measurement of resting metabolic rate by indirect calorimetry (Vyntus™ CPX, Vyarire Medical INC.). Next, participants completed a ramp test on an electronically braked cycle ergometer (Excalibur Sport, Lode B.V., Groningen, The Netherlands) for determination of maximal oxygen consumption (O2max) by indirect calorimetry. The exercise protocol was initiated by warm-up stages of 4 min at 100 W, 125 W, and 150 W, respectively followed by an incremental graded test with increments of 25 W × min−1 until voluntary exhaustion. Following exhaustion, participants rested for 2 min, then performed 1 min at 100 W before completing a constant power output test at 110 % of the incremental exercise performance in the ramp test to exhaustion as a verification of VO2max achieved during the ramp test. After 15 min of rest, participants were familiarized to the 20-min time trial performance test used during the study.
2.3. Experimental design
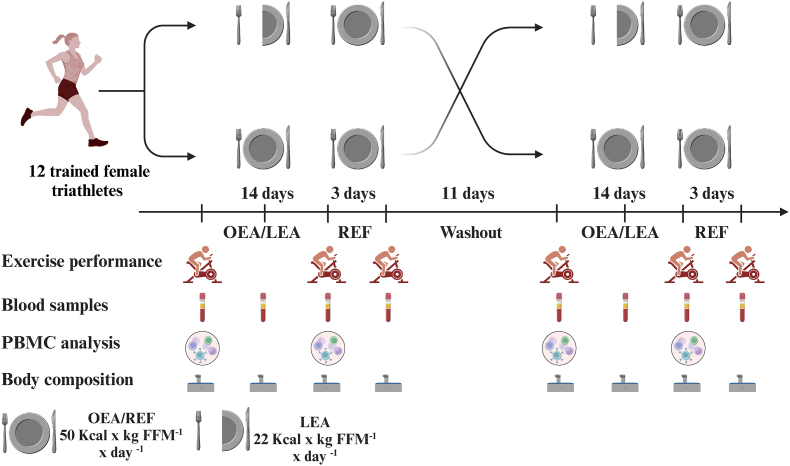
The study was performed as a randomized, single-blinded, placebo-controlled crossover design (Fig. 1). Upon inclusion, participants completed two 14-day dietary periods receiving 1) optimal energy availability (OEA) (50 kcal × kg FFM−1 × day−1) and 2) LEA (22 kcal × kg FFM−1 × day−1). Following OEA and LEA, participants underwent three days of refueling receiving OEA (50 kcal × kg FFM−1 × day−1). The two dietary periods were separated by 11 days.
Fig. 1.
Study design. Randomized single-blinded crossover design with 14 days of optimal (OEA) or low energy availability (LEA) followed by three days of refueling (REF).
Participants underwent a total of 8 experimental days during the study (Fig. 1); before, seven days into, after 14 days OEA and LEA, and following the subsequent three days of refueling. For all test days, participants reported to the lab at the same time in the morning (±1 h) fasted and refrained from caffeine, nicotine, and alcohol for 12 h and from strenuous physical activity for 24 h.
2.4. Dietary intervention
During OEA, LEA, and the refueling phase, participants were provided with all food based on an individualized meal plan. The food provided contained breakfast, pre-prepared lunch, and dinner (Getfitfood.dk, Skovlunde, Denmark) and pre-packed (1 g accuracy) snacks and drinks. Participants were blinded to the intervention by providing a large proportion of the snacks as either high carbohydrate (CHO) concentrated or non-calorie juice or CHO and protein rich powder. Participants were instructed to adhere to their usual daily timing of the three main meals while distributing the provided snacks throughout the day. EEE was calculated based on the scheduled daily training volume and intensity (via a linear regression between EEE and HR rate measured during the last 60-s of the three 4-min steady-state submaximal warmup intensities performed on the initial screening visit) and resting metabolic rate was subtracted. Daily physical activity (non-exercise activity thermogenesis; NEAT) was not measured and assumed to be relatively consistent between the two dietary interventions.
2.5. Exercise training
Two weeks prior to inclusion, participants registered their exercise training (type, duration and HR) on an online platform which was used to structure individual training programs during the study and to calculate daily EEE. The participants registered all their training though an online training platform (Strava or Garmin Connect) during the OEA, LEA, and refueling periods.
3. Experimental trials
3.1. Pre- and post-intervention trials
Before and after OEA and LEA, participants reported to the lab overnight fasted, and following 10 min of supine rest, body composition was assessed by DXA. Next, a standardized breakfast (454 kcal, 72 g CHO, 19 g protein, 19 g fat) was provided. Sixty min following breakfast, participants completed the cycling exercise tests (see below).
3.2. Mid-intervention trial
After seven days of OEA or LEA (MID), body composition was assessed (DXA), and a blood sample was collected.
3.3. Refueling trial
Following three days of refueling after OEA and LEA (REF), body composition (DXA) was assessed. Next, participants had a standardized breakfast and following 60 min of rest, participants completed the cycling exercise tests.
4. Testing procedures and equipment
4.1. Cycling exercise tests
All cycling tests were performed on the same electronically braked cycle ergometer (Excalibur Sport, Lode B.V., Groningen, The Netherlands) with specific individualized geometric setup used at each test. HR was continuously recorded (Suunto HR-monitor, Smart Belt, Vaanta, Finland) throughout all tests.
Participants performed a standardized warm up consisting of three 4-min stages at 50 %, 60 % and 70 % of VO2max, respectively. Following 5 min of rest, participants completed a 20-min time trial performed as an isokinetic test with an individually determined fixed cadence. Standardized verbal encouragement was provided by blinded researchers, and the subject was blinded (HR and power output) apart from time. After the test, participants rested for 7 min before completing a time to exhaustion test consisting of 3 min at an intensity corresponding to 50 % of VO2max followed by a constant load at 100 % of VO2max.
4.2. Blood samples
At every experimental day (PRE, POST, and REF), blood samples were collected at rest and immediately following the cycling exercise test from an antecubital vein in vacutainers containing 3.5 mL LH lithium heparin and 4 mL EDTA. The LH lithium heparinized samples were analyzed for cortisol, creatine kinase and high sensitivity C-reactive protein at the department of Clinical Biochemistry, Rigshospitalet, Copenhagen. The two EDTA samples were analyzed in duplicates for leukocytes (Sysmex XN-450, Sysmex, Kobe, Japan). An additional blood sample (3.5 mL LH lithium heparinized) was collected before and after OEA and LEA and was analyzed for sex hormones (estrogen, follicle stimulating hormone (FSH), luteinizing hormone (LH), progesterone, and testosterone) at the department of Clinical Biochemistry, Rigshospitalet. Before and after OEA and LEA at rest and immediately following the cycling exercise test, 12 mL EDTA whole blood was collected for isolation of PBMCs.
4.3. Isolation of peripheral blood mononuclear cells
PBMCs were isolated according to Ficoll's protocol [44]. Briefly, 12 mL of whole blood was mixed with Dulbecco's Phosphate Buffered Saline (DPBS) (1:1) and carefully added on top of 18 mL Ficoll density gradient (Ficoll® Paque Plus, Merck). The samples were centrifuged for 30-min at 400g with no braking. The PBMC layer was then collected and washed twice in 40 mL DPBS for 10-min at 80g without applying braking and then resuspended in 1 mL DPBS for immediate use or stored at −80 °C for later analysis. Total PBMCs were counted using an automated hematology analyzer (Sysmex XN-450, Sysmex, Kobe, Japan).
5. Analysis
5.1. Mitochondrial respiratory capacity and hydrogen peroxide emission of peripheral blood mononuclear cells
Mitochondrial respiratory capacity and hydrogen peroxide (H2O2) emission of 1.5–3 million PBMCs were measured simultaneously using a two-chamber high-resolution respirometry (Oxygraph-2k; Oroboros Instruments, Innsbruck, Austria) in MIR06 (0.5 mM EGTA, 3 mM MgCl2.6H2O, 60 mM K+-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 Mm HEPES, 110 mM d-sucrose and 1 g × l−1 of fatty acid free BSA, 280 U × ml−1 catalase, pH 7.4) at 37 °C with O2 maintained between 100 and 200 nmol O2. Calibration was conducted according to manufacturers’ instructions. Initially, superoxide dismutase (5U × L−1), amplex ultra red (10 μM) and horse radish peroxidase (1 U × L−1) were added for fluorometric measurements. 1.5–3 million PBMCs were added to each chamber. Respiration and H2O2 emission were measured in intact and permeabilized cells (digitonin, 8 μg × 106 cells) and were assessed across different states using combinations of different substrates including malate (2 mM), glutamate (10 mM), ADP (5 mM) with magnesium (5 mM), succinate (10 mM), rotenone (0.5 μM) and H2O2 (0.1 μM). Data were collected and analyzed in Datlab v.6.1 software (Oroboros Instruments).
5.2. Protein expression
Protein expression was determined using Western blotting as described in detail previously [45]. Briefly, 3 million PBMCs were homogenized (see suppl. methods). Total protein concentration in each sample was determined in triplicates by a BCA standard kit (Thermo Scientific), according to manufactures’ protocol. Equal amounts of total protein of PBMCs were loaded in each well of self-casted 10 % gels. All samples from each subject were loaded on the same gel. The bands were visualized with ECL (Millipore) and recorded with a digital camera (ChemiDoc MP Imaging System, Bio-Rad Laboratories). Densitometry quantification of the Western blot band intensity was done using Image Lab version 6.0 (Bio-Rad Laboratories) and determined as the total band intensity adjusted for background intensity. Primary antibodies used are shown in Suppl. Table 1.
5.3. Protein biomarkers
Lithium heparinized plasma samples were analyzed for inflammatory and immune protein biomarkers (Olink Proteomics, Uppsala, Sweden, Target 96 Inflammation Panel, https://olink.com/products-services/target/inflammation/) using Proximity Extension Assay (PEA). A protein was excluded from the analysis if the concentrations was undetectable in >25 % of the measured samples.
5.4. Statistics
SPSS was used for statistical analysis (IBM SPSS Statistics Corp, New York, USA, version 28). Normal distribution was confirmed using Q-Q plots. A linear mixed-effects model for repeated measurements [46] was used to estimate within- and between-period effects. Fixed factors were time (PRE, MID, POST, REF), treatment (OEA vs. LEA), and time × treatment. The absolute delta changes from rest and with acute exercise were used to compare the acute effects of exercise. Subject ID was included as a random effect to account for repeated measures between participants. Model reduction was not performed to avoid potential selective inference, meaning that post hoc tests were performed despite non-significant interactions [47]. Outcome statistics are presented as percentage or absolute change with delta changes and 95 % confidence interval (CI) or ± SD with the corresponding p-value. The level of significance was set to P ≤ 0.05. For protein biomarkers, P ≤ 0.05 was used as the level of significance after adjusting for false discovery rate (FDR) using the Benjamini-Hochberg procedure.
6. Results
6.1. Participant flow and diet
Of 17 healthy well-trained female endurance athletes, 13 met the inclusion criteria and were included. One was excluded as she did not adhere to the training schedule and the prescribed food. All 12 included participants (Table 1), of which seven were using hormonal contraceptives, completed the LEA and the subsequent refueling period, whereas one participant was not able to adhere to the prescribed food and training program during OEA and data from that period and the subsequent refueling was excluded from the analysis. Half of the participants (n = 6) were randomly allocated to begin with LEA. Energy availability and total energy and macronutrient intake is presented in Supplementary Table 1. Two weeks leading up the study, weekly training duration and average HR were 08:39 ± 01:40 h:min × week−1 and 80 ± 5 % of HRmax, respectively. These training parameters were maintained throughout both dietary intervention periods (08:34 ± 01:30 h × week−1 and 79 ± 6 % of HRmax and 08:36 ± 01:30 h:min × week−1 and 78 ± 5 % of HRmax for OEA and LEA, respectively).
Table 1.
Participant characteristics, n = 12.
| Age (years) | 26.8 ± 3.4 |
|---|---|
| Height (cm) | 169.8 ± 7.1 |
| Weight (kg) | 62.2 ± 8.8 |
| BMI (kg/m2) | 21.7 ± 2.2 |
| Fat free mass (kg) | 48.4 ± 5.7 |
| VO2max (mL × min−1 × kg−1) | 55.2 ± 5.1 |
Data are presented as mean ± SD.
6.2. Bioenergetics and redox state in isolated PBMCs at rest and following acute exercise
Assessment of isolated intact and permeabilized PBMCs revealed that mitochondrial respiration (Table 2) and H2O2 emission (Fig. 2) remained similar at rest with OEA and LEA. Mitochondrial respiration in isolated intact and permeabilized PBMCs was not changed with acute exercise (Table 2).
Table 2.
Mitochondrial respiratory capacity in intact and permeabilized PBMCs obtained at rest (Rest) following an acute exercise bout (EX) before (PRE) and after (POST) 14 days of optimal (OEA) (n = 11) and low energy availability (LEA) (n = 12).
| Optimal energy availability (OEA) |
low energy availability (LEA) |
Interaction Time x treatment |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PRERest | PREEX | POSTRest | POSTEX | PRERest | PREEX | POSTRest | POSTEX | ||
| Intact cells (pmol/(s × 106 cell−1) | 6 ± 1 | 6 ± 2 | 6 ± 3 | 5 ± 1 | 7 ± 2 | 5 ± 2 | 8 ± 3 | 7 ± 2 | 0.334 |
| Leak (pmol/(s × 106 cell−1) | 2 ± 1 | 2 ± 1 | 2 ± 2 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 3 ± 2 | 3 ± 2 | 0.247 |
| Complex I (pmol/(s × 106 cell−1) | 5 ± 2 | 4 ± 2 | 6 ± 4 | 3 ± 1 | 5 ± 2 | 4 ± 2 | 5 ± 3 | 4 ± 2 | 0.572 |
| Complex II (pmol/(s × 106 cell−1) | 10 ± 4 | 9 ± 4 | 11 ± 6 | 11 ± 3 | 12 ± 5 | 11 ± 3 | 14 ± 4 | 12 ± 4 | 0.785 |
| Complex I + II (pmol/(s × 106 cell−1) | 15 ± 5 | 13 ± 5 | 16 ± 8 | 13 ± 3 | 16 ± 5 | 14 ± 4 | 18 ± 6 | 14 ± 5 | 0.999 |
Data are presented as mean ± SD.
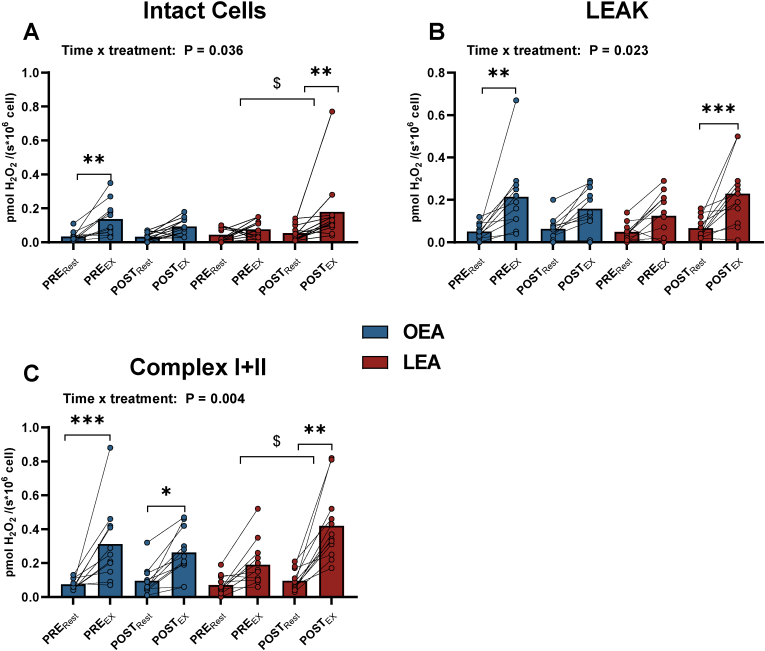
Fig. 2.
Hydrogen peroxide (H2O2) emission in intact (A) and permeabilized peripheral blood mononuclear cells (PBMC) at LEAK state (B), and Complex I + II (C) before (PRE) and after (POST) 14 days of optimal (OEA) (n = 11) or low energy availability (LEA) (n = 12) at rest (Rest) and following a 45-min intense exercise bout (EX). * Denotes differences from rest within diet. $ denotes differences in changes (Δ) from rest to exercise within group. Significance code: * <0.05, **P < 0.01, ***P < 0.01, $ P < 0.01.
Before OEA, H2O2 emission was higher after acute exercise in intact (Δ 0.10 pmol H2O2 × s−1 × 106 cell−1, 95 % CI: 0.01 to 0.27, P = 0.027) and permeabilized PBMCs (leak; Δ 0.17 pmol H2O2 × s−1 × 106 cell−1, 95 % CI: 0.07 to 0.26, P < 0.001 and Complex I + II; Δ 0.24 pmol H2O2 × s−1 × 106 cell−1, 95 % CI: 0.09 to 0.38, P < 0.001). The same patterns were observed for Complex I and Complex II. After OEA, H2O2 emission was higher after acute exercise only in permeabilized PBMCs (Complex I + II; Δ 0.17 pmol H2O2 × s−1 × 106 cell−1, 95 % CI: 0.08 to 0.31, P = 0.014, Fig. 2C). Before LEA, there was no change in H2O2 emission in the PBMCs with acute exercise, whereas after LEA it was higher after exercise in intact (+238 %, Δ 0.13 pmol H2O2 × s−1 × 106 cell−1, 95 % CI: 0.03 to 0.22, P = 0.002) and in permeabilized PBMCs (leak by 242 % Δ 0.16 pmol H2O2 × s−1 × 106 cell−1, 95 % CI: 0.07 to 0.26, P < 0.001, and Complex I + II by 341 %, Δ 0.32 pmol H2O2 × s−1 × 106 cell−1, 95 % CI: 0.19 to 0.46, P < 0.001). The exercise-induced increases in H2O2 emission after LEA were 313 %, (P = 0.041) and 135 % (P = 0.045) higher in intact cells and permeabilized (Complex I + II) PBMCs than before LEA (Fig. 2 A/C).
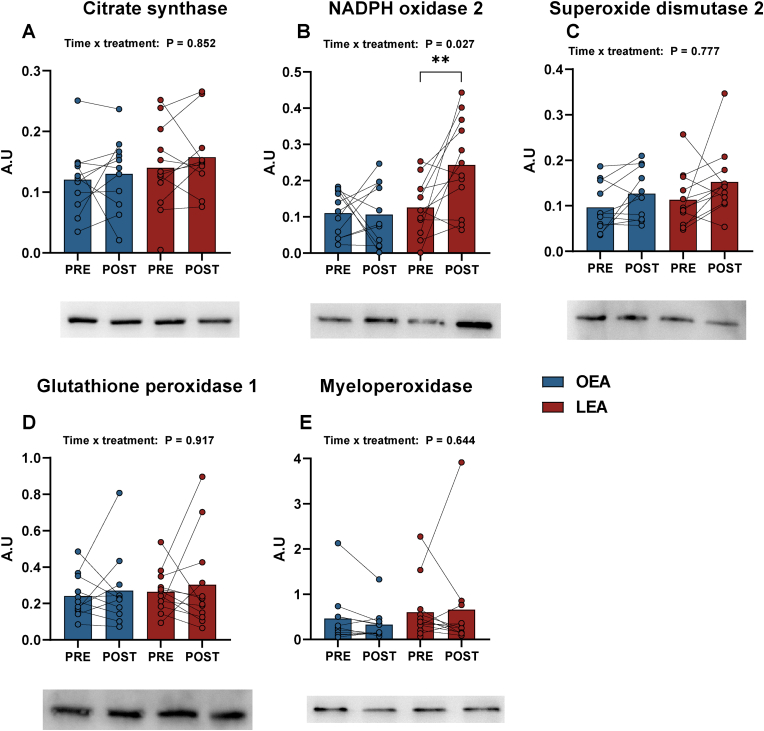
Assessment of isolated PBMCs (Fig. 3) collected at rest showed increases in NADPH oxidase 2 (NOX2, Gp91PHOX) by 93 % (Δ 0.11 a.u, 95 % CI: 0.04 to 0.19, P = 0.003) following LEA, whereas CS, SOD2, GPX1 and MPO were unaffected by LEA. There were no changes in CS, HK, NOX2, SOD2, GPX1 or MPO with OEA (all P > 0.05).
Fig. 3.
Peripheral blood mononuclear cell (PBMC) protein abundance of citrate synthase (CS) (A), NADPH oxidase 2 (NOX2, Gp91PHOX) (B), superoxide dismutase 2 (SOD2) (C), glutathione peroxidase 1 (GPX1) (D), and myeloperoxidase (MPO) (E) before (PRE) and after (POST) 14 days of optimal (OEA) (n = 11) or low energy availability (LEA) (n = 12). * Denotes differences from PRE within group. Significance code: * <0.05 and **P < 0.01.
6.3. Systemic cortisol, sex hormones and plasma proteome
The plasma cortisol concentration was 22.3 % (Δ 155 nmol × L−1, 95 % CI: 56 to 254, P < 0.001; Table 3) higher after compared to before LEA. Three days of refueling decreased cortisol levels by 17.4 % (Δ -142 nmol × L−1, 95 % CI: −241 to −143, P = 0.001; Table 3), which were not different from pre-LEA levels (P = 1.000). The plasma testosterone concentration was 22.1 % (Δ 0.14, 95 % CI: 0.25 to 0.32 nmol × L−1; P < 0.001, Table 3) higher after compared to before LEA. Plasma cortisol and testosterone levels were not different pre vs. post OEA. Plasma creatine kinase, hsCRP, estradiol, FSH, LH and progesterone were not different within OEA and LEA interventions. Plasma cortisol, hsCRP, and creatine kinase did not change with acute exercise (Suppl. Table 2).
Table 3.
Plasma concentrations at rest before (PRE), after 7 (MID) and 14 days (POST) following optimal (OEA) (n = 11) and low (LEA) energy availability (n = 12) and after three days of refueling (REF). * Denotes differences from PRE within diet. # Denotes differences from POST within diet. Significance code: *P < 0.05, ***P < 0.001, ##P < 0.01.
| Optimal energy availability (OEA) |
Low energy availability (LEA) |
Interaction Time x treatment |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PRE | MID | POST | REF | PRE | MID | POST | REF | ||
| Cortisol (nmol × L−1) | 719 ± 258 | 757 ± 265 | 676 ± 244 | 677 ± 276 | 666 ± 245 | 738 ± 251 |
821 ± 318 *** |
679 ± 240 ## | 0.002 |
| hsCRP (mg × L−1) | 1.3 ± 1.0 | 1.2 ± 1.3 | 0.9 ± 0.8 | 0.7 ± 0.4 | 1.1 ± 0.8 | 1.2 ± 1.0 | 0.9 ± 0.8 | 0.6 ± 0.3 | 0.523 |
| Creatine Kinase (U × L−1) | 136 ± 82 | 143 ± 67 | 115 ± 53 | 152 ± 69 | 126 ± 67 | 149 ± 98 | 169 ± 126 | 169 ± 128 | 0.212 |
| Estradiol (nmol × L−1) | 0.3 ± 0.3 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.2 ± 0.1 | 0.429 | ||||
| Follicle stimulating hormone (FSH) (IU × L−1) | 4.8 ± 5.1 | 4.7 ± 3.0 | 4.8 ± 3.3 | 5.2 ± 3.4 | 0.706 | ||||
| Luteinizing hormone (LH) (IU × L−1) | 4.6 ± 4.5 | 3.3 ± 2.4 | 6.0 ± 7.9 | 3.8 ± 3.3 | 0.685 | ||||
| Progesterone (nmol × L−1) | 1.7 ± 2.5 | 6.3 ± 12.1 | 1.3 ± 1.1 | 6.3 ± 13 | 0.928 | ||||
| Testosterone (nmol × L−1) | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.6 ± 0.2 |
0.8 ± 0.3 * |
0.097 | ||||
Data are presented as mean ± SD.
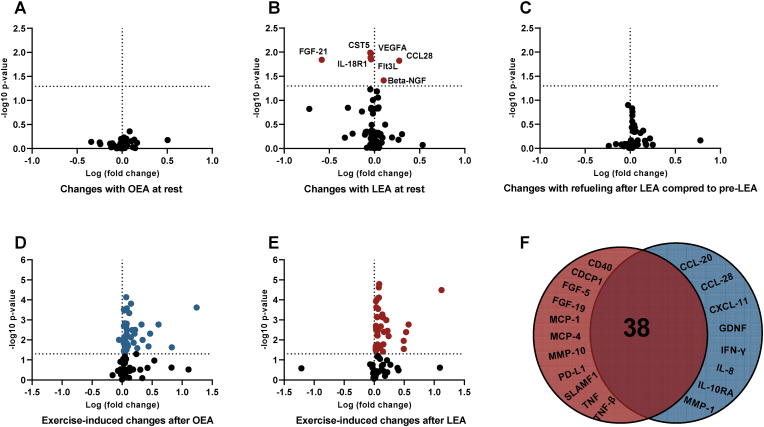
A targeted analysis of plasma proteins related to immediate inflammatory and immune status were assessed and out of 96 measured proteins, 78 proteins were quantifiable. Out of the 78 proteins analyzed, 5 (CST5, FGF-21, Flt3L, IL-18R1, and VEGFA) were downregulated and 2 (beta-NGF and CCL28) were upregulated with LEA (FDR < 0.05). Following three days of refueling these 7 proteins were not different from pre- or post-LEA. (Fig. 4A/B/C, suppl results). Following OEA and LEA, acute exercise altered 45 (44 upregulated and 1 downregulated) and 49 (49 upregulated) out of 78 proteins, respectively, with 8 and 11 of the upregulated proteins being specific to OEA (CCL-20, CCL-28, CXCL-11, GDNF, IFN-y, IL-8, IL-10RA, MMP-1) and LEA (CD40, CDCP1, FGF-19, FGF-5, MCP-1, MCP-4, MMP-10, PD-L1, SLAMF1, TNF, TNF-β), respectively (Fig. 4D/E, suppl results).
Fig. 4.
Volcano plot displaying changes out of 78 plasma proteins after 14 days of optimal (OEA) (A) (n = 11) and low energy availability (LEA) (B) (n = 12), 3 days of refueling following LEA (n = 12) (C), and the changes with acute exercise following 14 days of OEA (D) (n = 11) and LEA (E) (n = 12) (e.g., rest vs. exercise on the post-diet test days). D; Venn diagram of the proteins commonly regulated (mid) and uniquely regulated by LEA (left) and OEA (right) with exercise. Adj. p < 0.05 indicated by dashed line on the y-axis. Abbreviations: Beta-NGF: Beta-nerve growth factor, CCL-20: Chemokine (C–C motif) ligand 20, CCL-28: Chemokine (C–C motif) ligand 28, CCL28: Chemokine (C–C motif) ligand 28, CDCP1: CUB domain-containing protein 1, CD40: Cluster of Differentiation 40, CST5: Cystatin D, CXCL-11: Chemokine (C-X-C motif) ligand 11, FGF-5: Fibroblast Growth Factor 5, FGF-19: Fibroblast Growth Factor 19, FGF-21: Fibroblast Growth Factor 21, GDNF: Glial cell line-derived neurotrophic factor, IFN-γ: Interferon- γ, IL-8: Interleukin-8, IL-10RA: Interleukin-10 receptor subunit alpha, IL-18R1: Interleukin-18 receptor subunit alpha, MCP-1: Monocyte chemoattractant protein-1, MCP-4: Monocyte chemoattractant protein-4, MMP-1: Matrix metalloproteinase-1, MMP-10: Matrix metalloproteinase-10, PD-L1: Programmed death-ligand 1, SLAMF1: Signaling lymphocytic activation molecule family member 1, TNF: Tumor necrosis factor, TNF-β: Tumor necrosis factor-β, VEGFA: Vascular endothelial growth factor A.
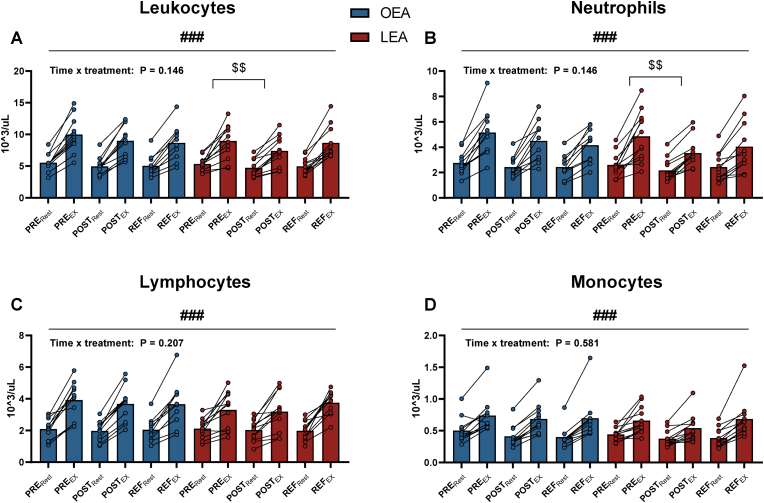
6.4. Leukocyte type distribution and mobilization following acute exercise
There were no differences in leukocyte, neutrophil, lymphocyte, and monocyte counts between OEA, LEA, or refueling at rest. Both before and after OEA, LEA and refueling, leukocytes, neutrophils, lymphocytes, and monocytes increased with acute exercise (all P < 0.001, Fig. 5). After LEA, the acute exercise-induced increase in leukocytes was 14.2 % (Δ −1.6 103 × uL−1, 95 % CI: −3.0 to −0.2, P = 0.017) lower than before LEA which was mainly driven by a 24.8 % (Δ −1.3 103 × uL−1, 95 % CI: −2.4 to −0.3, P = 0.003) lower increase in neutrophils with acute exercise.
Fig. 5.
Concentration of leukocytes (A), neutrophils (B), lymphocytes (C), and monocytes (D) before (PRE) and after (POST) 14 days of optimal (OEA) (n = 11) or low energy availability (LEA) (n = 12), and after three days of refueling (REF) at rest (Rest) and following an acute exercise bout (EX). # denotes differences from rest to following acute exercise. $ denotes differences in changes (Δ) from rest to exercise within group. Significance code: $$ P < 0.01, ###P < 0.001.
6.5. Body composition
Body weight, fat mass, and fat free mass were all significantly (all P < 0.05) lowered after LEA. Body weight and fat mass were unaffected by three days of refueling. Fat free mass was enhanced by three days of refueling (P < 0.037). Data is presented in Suppl. Table 3.
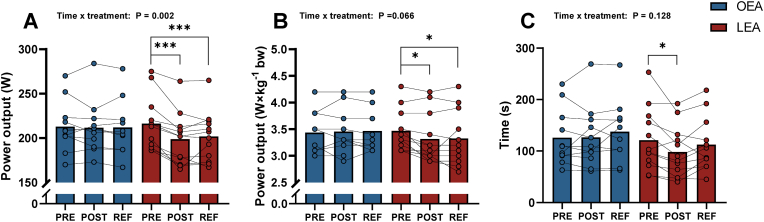
6.6. Time trial and time to exhaustion performance
Power output during the 20-min time trial was 7.8 % (Δ −17.5 W, 95 % CI: −25.2 to −9.8, P < 0.001) lower after LEA and remained 6.7 % lower (Δ −14.5 W, 95 % CI: −22.2 to −6.8, P < 0.001) after refueling, compared to before LEA (Fig. 6A). Power output relative to body mass was lower by 4.3 % (Δ −0.15 W × kg bw−1, 95 % CI: −0.27 to −0.03, P = 0.012) after compared to before LEA and remained 4.1 % lower (Δ −0.14 W × kg bw−1, 95 % CI: −0.28 to −0.02, P = 0.020) after refueling, compared to before LEA (Fig. 6B).
Fig. 6.
Absolute power output (A), relative power output to body weight (B) during the 20-min time trial and time to exhaustion (TTE) (C) before (PRE), after (POST) 14 days with optimal (OEA) (n = 11) or low energy availability (LEA) (n = 12), and after three days of refueling (REF). Two data points are missing in REF with OEA. * Denotes differences from PRE within group. Significance code: * <0.05 and ***P < 0.001.
Time to exhaustion during constant load at 100% VO2max after LEA was 18.9 % lower (Δ −22.9 s, 95 % CI: −43.9 to −2.9, P = 0.020) after compared to before LEA (Fig. 6C). After three days of refueling, time to exhaustion was not different compared to pre- LEA (P = 0.634). Exercise performance was unaffected by OEA (Fig. 6C).
7. Discussion
The most important findings of the present study were that 14 days of LEA: 1) increased the capacity of PBMCs to produce ROS as evidenced by an increased level of NADPH oxidase, and an amplified ROS production in PBMCs in response to acute exercise despite lower total work performed during the exercise test, as indicated by impaired exercise performance, 2) increased resting cortisol and altered the plasma inflammatory proteome at rest and following acute exercise, and 3) reduced the exercise induced mobilization of leukocytes. Moreover, three days of refueling led to restoration of cortisol levels and inflammatory proteins to initial pre-LEA levels, whereas it did not restore the LEA induced impairment in performance (20- min time trial).
7.1. Low energy availability enhances the peripheral blood mononuclear cell redox capacity and formation
To evaluate how LEA may influence the immune system we determined the redox capacity in PBMC's. Our results showed that 14 days of LEA led to an increase in systemic stress, as indicated by elevated plasma cortisol levels, an increased capacity for ROS production in PBMCs, as evidenced by an upregulation of NOX2 content, and increased level of stress induced ROS formation with intense exercise. The content of MPO and the protective antioxidants enzymes GPX1 and SOD2, remained unaltered; together with the upregulation of NOX2 protein and increase in stress-induced ROS formation, these data demonstrate that short-term energy deprivation apparently increase the potential for ROS formation to enable them to rapidly increase ROS production when required, such as observed following acute exercise.
Although acute exercise caused an increase in ROS production in PBMCs in both OEA and LEA, the increase was greater following LEA. The higher NOX2 content in PBMCs after LEA likely contributed to the higher level of ROS; however, mitochondrial ROS formation could also have contributed. As such, when mitochondrial respiration in post exercise isolated PBMC's was increased by substrate addition (LEAK, Complex I, and I + II), ROS production was further enhanced. Notably, the larger increase in ROS formation following acute exercise with LEA was apparent despite the lower total amount of work performed after LEA during the cycling test, as evident by lower power output and shortened time to exhaustion following LEA. This fact, strengthens the observation of a higher exercise induced ROS production in PBMCs after LEA. The larger increase in ROS formation by PBMC's following LEA was associated with an increase in plasma levels of MCP-1 (monocyte chemoattractant protein-1), a chemokine released in response to ROS formation in PBMC's [48]. The pleiotropic actions of ROS in leukocytes make it challenging to ascertain whether the increase observed with LEA would be beneficial or harmful. It may be that the amplified ROS with LEA is a compensatory mechanism for lower mobilization of leukocytes following acute exercise. On the other hand, increased ROS production in PBMCs has been associated with pathology [22,49,50] and as excessive oxidative stress in general is associated with destruction of biological molecules such as DNA and proteins [51], the increase in ROS may be harmful and directly impair immune responses. It is worth noting that LEA was only sustained for 14 days in this experiment and therefore the rise in exercise-induced ROS with LEA may contribute to immunosuppression in long-term LEA.
As the short-term LEA exposure did not influence ROS production in PBMCs obtained at rest, despite elevated cortisol levels, this observation implies that although LEA increases systemic stress, it does not increase basal oxidative stress in PBMCs nor their respiratory capacity.
7.2. LEA does not increase the pro-inflammatory effects of exercise
Elevated ROS in leukocytes have the capability of activating a pro-inflammatory response, as commonly observed with acute exercise [52]. In the present study we measured an array of inflammatory proteins before and after acute exercise in OEA and LEA to determine the pro-inflammatory response to exercise in the two dietary conditions. While we did observe an overall pro-inflammatory response to acute exercise, as evidenced by an increase IL-6 and TNF-α, we found that LEA did not further amplify this pro-inflammatory response, despite the enhanced ROS production in PBMCs after LEA. This suggests that the altered redox balance in PBMCs in LEA was not associated with the extent of exercise-induced pro-inflammatory changes. Interestingly, after LEA, acute exercise led to a reduced post-exercise neutrophil count. The decreased neutrophil count in response to exercise could be related to the lower total work performed after LEA (impaired performance) or to a reduced cortisol response [53] following acute exercise after LEA as indicated by a non-significant (15 %) reduction in cortisol levels with acute exercise (Suppl. Table 3).
7.3. LEA alters systemic stress and inflammatory proteins at rest
While LEA did not affect the basal inflammatory state at rest - as evidenced by maintained protein levels such as hsCRP, IL-6, and TNF-α, and as none of the uniquely changed plasma proteins with LEA were specific to inflammation – the rise in cortisol levels suggests that short-term LEA is associated with enhanced systemic stress. This is in accordance with previous studies [54,55], which have shown that even short-term LEA directly impacts the hypothalamic-pituitary-adrenal axis and increases cortisol levels. Even though LEA was not associated with alterations in peripheral leukocyte composition, it is noteworthy that the absolute neutrophil count following LEA was approaching levels below normal reference values (<2.0 × 103 cells × μL) [56]. Clinically low levels in neutrophil count may be evident in situations with long-term LEA and could increase the risk of infection and partly explain the high prevalence of upper respiratory tract infections in elite athletes [35,37]. However, it is worth noting that both cortisol and neutrophils were reversed with only three days of refueling and therefore may only be useful markers of the immediate energetic state.
7.4. Low energy availability impairs exercise performance
To evaluate how female athletes are functionally influenced by LEA, we also determined exercise performance capacity. Performance was determined by a 20-min time trial and by a subsequent time to exhaustion test, where the tests were performed before and after the dietary intervention periods as well as after three days refueling. The 14 days of LEA was found to have a detrimental effect on exercise performance in both tests, and the reduced performance for the 20 min time-trial was observed even after the three days refueling. It is noteworthy, that, even when considering the 4 % reduction in body mass and expressing exercise performance relative to body mass (power to weight ratio), performance was impaired. Our findings are in agreement with one previous study in trained females [57] but contrast those of a recent study, in which highly-trained race-walkers presented enhanced performance after only 24 h refueling following nine days of LEA (10 males and 2 females) [58]. The discrepancy may be explained by sex-specific consequences of LEA, as males have been suggested to be more resilient to LEA [59]. However, given the differences in both the larger decrease in LEA, difference in carbohydrate intake, and the shorter duration of refueling, we find it unlikely that sex-differences fully can account for the disparity.
7.5. Summary
Our study illustrates a substantial impact of short-term LEA on the immune system, as evidenced by altered redox balance in PBMC, altered immune/inflammatory proteome and a reduced exercise-induced mobilization of PBMCs. This finding suggests that LEA may heighten the susceptibility to infections and disease. Additionally, our study shows that, at a functional level, LEA reduces endurance exercise performance, an effect which was not reversible with three days of refueling. Taken together, these findings underscore the critical importance of limiting LEA exposure for female athletes.
CRediT authorship contribution statement
Jan S. Jeppesen: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. Hannah G. Caldwell: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. Lone O. Lossius: Data curation, Investigation, Methodology. Anna K. Melin: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Lasse Gliemann: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. Jens Bangsbo: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. Ylva Hellsten: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by The Danish Ministry of Culture Funding for Sports Research, Frimodt-Heineke Fonden, and as part of the Novo Nordisk Foundation grant to Team Denmark to the research network “Training strategies and competition preparation”. Jens Jung Nielsen and Martin Thomassen are acknowledged for their skillful contribution throughout the study. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103250.
Appendix ASupplementary data
The following are the Supplementary data to this article.
References
- 1.Campbell J.P., Turner J.E. Debunking the Myth of exercise-induced immune Suppression: Redefining the impact of exercise on immunological health across the Lifespan. Front. Immunol. 2018;9:648. doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Childs C.E., Calder P.C., Miles E.A. Diet and immune function. Nutrients. 2019;11 doi: 10.3390/nu11081933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ihle R., Loucks A.B. Dose-response relationships between energy availability and bone turnover in young exercising women. J. Bone Miner. Res. 2004;19:1231–1240. doi: 10.1359/JBMR.040410. [DOI] [PubMed] [Google Scholar]
- 4.Loucks A.B., Thuma J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003;88:297–311. doi: 10.1210/jc.2002-020369. [DOI] [PubMed] [Google Scholar]
- 5.Mountjoy M., et al. International Olympic Committee's (IOC) consensus statement on relative energy deficiency in Sport (REDs) Br. J. Sports Med. 2023;57:1073–1097. doi: 10.1136/bjsports-2023-106994. [DOI] [PubMed] [Google Scholar]
- 6.Heikura I.A., et al. Low energy availability is Difficult to assess but Outcomes have large impact on bone Injury rates in elite distance athletes. Int J Sport Nutr Exerc Metab. 2018;28:403–411. doi: 10.1123/ijsnem.2017-0313. [DOI] [PubMed] [Google Scholar]
- 7.Melin A., et al. Energy availability and the female athlete triad in elite endurance athletes. Scand. J. Med. Sci. Sports. 2015;25:610–622. doi: 10.1111/sms.12261. [DOI] [PubMed] [Google Scholar]
- 8.Areta J.L., Taylor H.L., Koehler K. Low energy availability: history, definition and evidence of its endocrine, metabolic and physiological effects in prospective studies in females and males. Eur. J. Appl. Physiol. 2021;121:1–21. doi: 10.1007/s00421-020-04516-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxfeldt M., et al. Low energy availability reduces myofibrillar and sarcoplasmic muscle protein synthesis in trained females. J Physiol. 2023;601:3481–3497. doi: 10.1113/JP284967. [DOI] [PubMed] [Google Scholar]
- 10.Koehler K., Achtzehn S., Braun H., Mester J., Schaenzer W. Comparison of self-reported energy availability and metabolic hormones to assess adequacy of dietary energy intake in young elite athletes. Appl Physiol Nutr Metab. 2013;38:725–733. doi: 10.1139/apnm-2012-0373. [DOI] [PubMed] [Google Scholar]
- 11.Reynes B., Priego T., Cifre M., Oliver P., Palou A. Peripheral blood cells, a Transcriptomic tool in Nutrigenomic and Obesity studies: Current state of the Art. Compr. Rev. Food Sci. Food Saf. 2018;17:1006–1020. doi: 10.1111/1541-4337.12363. [DOI] [PubMed] [Google Scholar]
- 12.Caimari A., Oliver P., Keijer J., Palou A. Peripheral blood mononuclear cells as a model to study the response of energy homeostasis-related genes to acute changes in feeding conditions. OMICS. 2010;14:129–141. doi: 10.1089/omi.2009.0092. [DOI] [PubMed] [Google Scholar]
- 13.Caimari A., Oliver P., Rodenburg W., Keijer J., Palou A. Feeding conditions control the expression of genes involved in sterol metabolism in peripheral blood mononuclear cells of normoweight and diet-induced (cafeteria) obese rats. J. Nutr. Biochem. 2010;21:1127–1133. doi: 10.1016/j.jnutbio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Janssen J.J.E., et al. Extracellular flux analyses reveal differences in mitochondrial PBMC metabolism between high-fit and low-fit females. Am. J. Physiol. Endocrinol. Metab. 2022;322:E141–E153. doi: 10.1152/ajpendo.00365.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai H.H., et al. Exercise training Alleviates Hypoxia-induced mitochondrial Dysfunction in the lymphocytes of Sedentary males. Sci. Rep. 2016;6 doi: 10.1038/srep35170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busquets-Cortes C., et al. Training and acute exercise modulates mitochondrial dynamics in football players' blood mononuclear cells. Eur. J. Appl. Physiol. 2017;117:1977–1987. doi: 10.1007/s00421-017-3684-z. [DOI] [PubMed] [Google Scholar]
- 17.Calton E.K., Keane K.N., Soares M.J., Rowlands J., Newsholme P. Prevailing vitamin D status influences mitochondrial and glycolytic bioenergetics in peripheral blood mononuclear cells obtained from adults. Redox Biol. 2016;10:243–250. doi: 10.1016/j.redox.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calton E.K., et al. Winter to summer change in vitamin D status reduces systemic inflammation and bioenergetic activity of human peripheral blood mononuclear cells. Redox Biol. 2017;12:814–820. doi: 10.1016/j.redox.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartman M.L., et al. Relation of mitochondrial oxygen consumption in peripheral blood mononuclear cells to vascular function in type 2 diabetes mellitus. Vasc. Med. 2014;19:67–74. doi: 10.1177/1358863X14521315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P., et al. Mitochondrial respiratory dysfunctions of blood mononuclear cells link with cardiac disturbance in patients with early-stage heart failure. Sci. Rep. 2015;5 doi: 10.1038/srep10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinvalet D., Walch M. Editorial: the role of reactive oxygen species in protective immunity. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.832946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ederle C., et al. Mitochondrial function in peripheral blood mononuclear cells (PBMC) is enhanced, together with increased reactive oxygen species, in Severe Asthmatic patients in Exacerbation. J. Clin. Med. 2019;8 doi: 10.3390/jcm8101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiku V., Tan M.W., Dikic I. Mitochondrial functions in infection and immunity. Trends Cell Biol. 2020;30:263–275. doi: 10.1016/j.tcb.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen B.K., Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol. Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 26.Ostrowski K., Rohde T., Asp S., Schjerling P., Pedersen B.K. Chemokines are elevated in plasma after strenuous exercise in humans. Eur. J. Appl. Physiol. 2001;84:244–245. doi: 10.1007/s004210170012. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen B.K., Steensberg A., Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536:329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Febbraio M.A., Pedersen B.K. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. Faseb j. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen B.K., et al. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. 2003;446:9–16. doi: 10.1007/s00424-002-0981-z. [DOI] [PubMed] [Google Scholar]
- 30.Gabriel H., Schwarz L., Steffens G., Kindermann W. Immunoregulatory hormones, circulating leucocyte and lymphocyte subpopulations before and after endurance exercise of different intensities. Int. J. Sports Med. 1992;13:359–366. doi: 10.1055/s-2007-1021281. [DOI] [PubMed] [Google Scholar]
- 31.Ronsen O., Pedersen B.K., Øritsland T.R., Bahr R., Kjeldsen-Kragh J. Leukocyte counts and lymphocyte responsiveness associated with repeated bouts of strenuous endurance exercise. J. Appl. Physiol. 2001;91:425–434. doi: 10.1152/jappl.2001.91.1.425. [DOI] [PubMed] [Google Scholar]
- 32.Peake J.M., Neubauer O., Walsh N.P., Simpson R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017;122:1077–1087. doi: 10.1152/japplphysiol.00622.2016. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K., et al. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur. J. Appl. Physiol. 2000;81:281–287. doi: 10.1007/s004210050044. [DOI] [PubMed] [Google Scholar]
- 34.Shaw D.M., Merien F., Braakhuis A., Dulson D. T-cells and their cytokine production: the anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine. 2018;104:136–142. doi: 10.1016/j.cyto.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Peters E.M., Bateman E.D. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S. Afr. Med. J. 1983;64:582–584. [PubMed] [Google Scholar]
- 36.Gleeson M., et al. The effect on immunity of long-term intensive training in elite swimmers. Clin. Exp. Immunol. 1995;102:210–216. doi: 10.1111/j.1365-2249.1995.tb06658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spence L., et al. Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med. Sci. Sports Exerc. 2007;39:577–586. doi: 10.1249/mss.0b013e31802e851a. [DOI] [PubMed] [Google Scholar]
- 38.Cox A.J., et al. Clinical and laboratory evaluation of upper respiratory symptoms in elite athletes. Clin. J. Sport Med. 2008;18:438–445. doi: 10.1097/JSM.0b013e318181e501. [DOI] [PubMed] [Google Scholar]
- 39.Bermon S., et al. Consensus statement Immunonutrition and exercise. Exerc. Immunol. Rev. 2017;23:8–50. [PubMed] [Google Scholar]
- 40.De Souza M.J., Hontscharuk R., Olmsted M., Kerr G., Williams N.I. Drive for thinness score is a proxy indicator of energy deficiency in exercising women. Appetite. 2007;48:359–367. doi: 10.1016/j.appet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham J.J. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am. J. Clin. Nutr. 1980;33:2372–2374. doi: 10.1093/ajcn/33.11.2372. [DOI] [PubMed] [Google Scholar]
- 42.Melin A., et al. The LEAF questionnaire: a screening tool for the identification of female athletes at risk for the female athlete triad. Br. J. Sports Med. 2014;48:540–545. doi: 10.1136/bjsports-2013-093240. [DOI] [PubMed] [Google Scholar]
- 43.Fairburn C.G., Beglin S.J. Assessment of eating disorders: interview or self-report questionnaire? Int. J. Eat. Disord. 1994;16:363–370. [PubMed] [Google Scholar]
- 44.Riedhammer C., Halbritter D., Weissert R. Peripheral blood mononuclear cells: isolation, Freezing, Thawing, and Culture. Methods Mol. Biol. 2016;1304:53–61. doi: 10.1007/7651_2014_99. [DOI] [PubMed] [Google Scholar]
- 45.Thomassen M., Bangsbo J., Hostrup M. Effect of sample fractionation and normalization when immunoblotting for human muscle Na(+)/K(+)-ATPase subunits and glycogen synthase. Anal. Biochem. 2023;666 doi: 10.1016/j.ab.2023.115071. [DOI] [PubMed] [Google Scholar]
- 46.Cnaan A., Laird N.M., Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 47.Taylor J., Tibshirani R.J. Statistical learning and selective inference. Proc Natl Acad Sci U S A. 2015;112:7629–7634. doi: 10.1073/pnas.1507583112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh S., Anshita D., Ravichandiran V. MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101 doi: 10.1016/j.intimp.2021.107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coluccia R., et al. Chronic heart failure is characterized by altered mitochondrial function and structure in circulating leucocytes. Oncotarget. 2018;9:35028–35040. doi: 10.18632/oncotarget.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alfatni A., et al. Peripheral blood mononuclear cells and Platelets mitochondrial Dysfunction, oxidative stress, and circulating mtDNA in Cardiovascular diseases. J. Clin. Med. 2020;9 doi: 10.3390/jcm9020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bassoy E.Y., Walch M., Martinvalet D. Reactive oxygen species: Do they play a role in Adaptive immunity? Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.755856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naik E., Dixit V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki K., et al. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. 2003;35:348–355. doi: 10.1249/01.Mss.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- 54.De Souza M.J., et al. Adrenal activation and the prolactin response to exercise in eumenorrheic and amenorrheic runners. J. Appl. Physiol. 1991;70:2378–2387. doi: 10.1152/jappl.1991.70.6.2378. [DOI] [PubMed] [Google Scholar]
- 55.Loucks A.B., Mortola J.F., Girton L., Yen S.S. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J. Clin. Endocrinol. Metab. 1989;68:402–411. doi: 10.1210/jcem-68-2-402. [DOI] [PubMed] [Google Scholar]
- 56.Coates S., et al. Time- and race-specific Haematological reference intervals for healthy Volunteer trials: a Retrospective analysis of Pooled data from Multiple phase I trials. Front. Pharmacol. 2020;11:314. doi: 10.3389/fphar.2020.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oxfeldt M.M.D., Christensen P.M., Andersen O.E., Johansen F.T., Bangshaab M., Risikesan J., Jeppesen J.S., Hellsten Y., Phillips S.M., Melin A.K., Ørtenblad N., Hansen M. Low energy availability followed by optimal energy availability does not benefit performance in trained females. Med. Sci. Sports Exerc. 2023 doi: 10.1249/MSS.0000000000003370. Accepted 2023, Dec 6. [DOI] [PubMed] [Google Scholar]
- 58.Burke L.M., et al. Short Severe energy Restriction with refueling reduces body mass without altering training-associated performance Improvement. Med. Sci. Sports Exerc. 2023;55:1487–1498. doi: 10.1249/MSS.0000000000003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papageorgiou M., et al. Effects of reduced energy availability on bone metabolism in women and men. Bone. 2017;105:191–199. doi: 10.1016/j.bone.2017.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.