Abstract
Background and Aims
Recognizing the ability to adapt coping mechanisms in response to the unique issues present in various Iranian societies underscores the importance of considering culture and religion when interacting with diverse groups of individuals. The objective of this study was to assess the reliability and validity of the fear of progression questionnaire‐short form (FoP‐Q‐SF) in Iranian breast cancer patients.
Methods
In this methodological cross‐sectional research design, 400 Iranian breast cancer patients completed the FoP‐Q‐SF in 2023. We assessed the characteristics, content, and both exploratory and confirmatory construct validity of the measures. To evaluate the reliability and construct validity of the FoP‐Q‐SF, we calculated Cronbach's α, McDonald's omega, and the Intraclass Correlation Coefficient.
Results
The average age of the patients was 49.18 (standard deviation = 16.14) years. The results of exploratory factor analysis revealed that a single‐factor structure, specifically the self‐efficacy scale, accounted for 65.045% of the total variance. The findings from the confirmatory factor analysis indicated a satisfactory model fit. The reliability analysis indicated that the internal consistency and stability of the measures were acceptable.
Conclusion
The short Persian version of the FoP‐Q‐SF exhibits satisfactory validity and reliability. Thus, we recommend using this questionnaire to assess the fear of disease progression among breast cancer patients in Iran.
Keywords: cancer, fear of progression, Iran, reliability, validity
1. INTRODUCTION
The incidence and mortality rates of breast cancer have seen a significant global increase in recent years. 1 Iran is no exception to this trend, with a concerning rise in statistics. 2 The treatment journey for this chronic disease carries profound personal, economic, familial, and psychological implications for the patient. One of the most noteworthy psychological consequences that breast cancer survivors face post‐treatment is the fear of cancer recurrence. 3
While moderate levels of this fear can serve as a motivator for adhering to treatment regimens and regular check‐ups, heightened levels of fear regarding cancer recurrence can lead to anxiety, disruptions in daily functioning, and a reduction in the quality of life for cancer survivors and even their caregivers. 3 , 4 Additionally, it can evidently give rise to a multitude of functional complications.
Numerous researchers have dedicated their efforts to studying the fear of cancer recurrence. 5 , 6 , 7 , 8 These studies have revealed varying degrees of fear of recurrence, with reported levels falling into the categories of mild (13%), moderate (49%), and severe (7%). 8 , 9 Recognizing the significance of addressing fear in breast cancer patients, a range of tools have been developed and made available for investigation, one of which is the fear of progression questionnaire‐short form (FoP‐Q‐SF), 10 the Fear of Cancer Recurrence Inventory (FCRI), 11 the Fear of Recurrence Index 12 and the Fear of Recurrence Questionnaire. 13 While extensive research has been conducted in this domain, the absence of a universally accepted definition has resulted in the creation of diverse assessment tools, prompting the need for further exploration in this area. 14
In Iran, the FCRI tool has been translated and validated for use among breast cancer patients 15 ; however, the items in the FoP‐Q are more aligned with Iranian culture and the values of Islamic societies. Additionally, in a separate study, the FoP‐Q has been validated for use in cancer patients more broadly. 16 Given that patients with different types of cancer have distinct and individualized experiences, having specific tools tailored to each patient group can furnish researchers with more reliable results in the future. Therefore, it is imperative to conduct a targeted investigation of the FoP tool within this particular population.
Cancer, like many other diseases, is subject to the influence of cultural factors. Factors such as individual personality differences, life experiences, socioeconomic status, and cultural elements all have a substantial impact on how patients and their families navigate the challenges of cancer. 17 , 18 On the contrary, it has been noted that religion places a significant emphasis on an individual's behaviors, attitudes, and coping strategies. These factors can aid long‐term adaptation by fostering emotional support, instilling hope, and providing a sense of purpose and meaning. 19 Several studies have indicated that individuals may turn to religion as a coping mechanism for the challenges presented by cancer. 20 Religious coping is a method through which individuals utilize their beliefs and religious practices, such as prayer and fostering a relationship with a higher power, to effectively navigate and cope with stressors and adversities. 21
Religious beliefs have been found to be more prevalent among Iranian cancer patients, serving as a mitigating factor. 22 Recognizing the ability to adapt coping mechanisms in response to the unique issues present in various Iranian societies underscores the importance of considering culture and religion when interacting with diverse groups of individuals. The present study was conducted with the objective of exploring the psychometric properties of the FoP‐Q‐SF in Iranian breast cancer patients.
2. METHODS
This cross‐sectional study was done with methodological design in 2023. Concentrating on patients who were referred to the Baghban Specialized Oncology Clinic and the Imam Khomeini Hospital in the city of Sari (located in Northern Iran), this study was carried out. The study was reported based on guidelines. 23 , 24
2.1. Population and sampling
Participants were selected using a random sampling technique from July 2023 to September 2023. Many experts have suggested different methods for calculating the appropriate sample size for research studies. 25 , 26 , 27 It is commonly acknowledged that psychometric studies require a minimum of 200 participants. As a result, a total of 430 patients were included in this study.
To be eligible for the study, participants had to be at least 18 years old, have the ability to read and write, and have received a confirmed breast cancer diagnosis from their physicians. Exclusion criteria encompassed physical impairments, concurrent affliction with other physical conditions such as Multiple Sclerosis, brain, and spinal tumors, and other types of cancer. Furthermore, patients with severe mental illnesses, including schizophrenia, bipolar disorder, or severe emotional disturbances, were not eligible for participation.
Then 30 patients were excluded due to Major Depressive Disorder (12 patients), concurrent with other types of cancer (14 patients), and physical impairments (4 patients). All of the included patients (N = 400) agreed to participate in this study (response rate = 100%).
2.2. Data collection process
A trained researcher collected data through a paper survey method. The study's objective was clearly communicated to the patients before starting, and they provided their consent. The consent process guaranteed that participants were aware that their involvement in the study was entirely voluntary and that they had the right to withdraw at any point. Furthermore, all participants were guaranteed that their personal information would be kept confidential, and they were informed that they could obtain the study results if they wished. The patients were guided by researchers in a private room to complete the questionnaires, which typically took around 3−5 min to finish.
2.3. Data collection scales
2.3.1. Demographic characteristics information
Table 1 contains details about the patients, including age, gender, and marital status.
Table 1.
Demographic profiles of patients.
| Variables | N (%) | Variables | N (%) |
|---|---|---|---|
| Marital status | Cancer stage | ||
| Single | 56 (14%) | Stage 1 | 4 (1%) |
| Married | 329 (82.3%) | Stage 2 | 109 (27.3%) |
| Divorced | 3 (0.70%) | Stage 3 | 243 (60.8%) |
| Widow | 12 (3%) | Stage 4 | 33 (8.3%) |
| Educational status | Not known | 11 (20.6%) | |
| Elementary | 79 (19.8%) | Job | |
| Postelementary | 36 (9%) | House keeper | 87 (21.75%) |
| High school | 44 (11%) | employee | 106 (26.5%) |
| Diploma | 63 (15.8%) | Free lance | 121 (30.25%) |
| Bachelor | 129 (32.3%) | Retirement | 86 (21.5) |
| Master of sciences and upper | 49 (12.3%) | ||
| Present economic Status | |||
| Poor | 77 (19.2%) | ||
| Average | 199 (49.8%) | ||
| Good | 124 (31%) | ||
2.3.2. FoP‐Q‐SF
The FoP‐Q‐SF is a multidimensional questionnaire that was initially developed by Herschbach, Berg 28 using a sample of patients with various conditions, including cancer, rheumatic diseases, and diabetes mellitus. The FoP‐Q‐SF serves as a self‐report questionnaire designed for use by cancer and diabetic patients, as well as those with rheumatic disorders. This questionnaire was originally formulated in Germany and the final version consists of five distinct factors and a total of 43 items. Respondents use a 5‐point Likert‐type scale to provide their responses. It's worth noting that, with the exception of the component addressing anxiety coping, the total score of the FoP‐Q‐SF is determined by summing the results of its individual components.
2.4. Translation
The developer of the FoP‐Q‐SF scale provided written consent for its use. The World Health Organization utilized the forward‐backward translation technique to convert the scale from English to Persian. 29 Two translators, one fluent in English and the other in Persian, were invited to independently translate the FoP‐Q‐SF. A group of experts, including some of the authors of this paper and two professional translators, carefully examined and combined the two translations to produce a unified persian version of the FoP‐Q‐SF. Subsequently, a translator fluent in both Persian and English was asked to translate the Persian version into English. The precision of the translation and the resemblance between the back‐translated English version of the FoP‐Q‐SF and the original were then confirmed and accepted by expert evaluation.
2.5. Normal distribution of data, outliers, and missing data
To look into the data's normal distribution and outliers, the univariate (by assessing Skewness ±3 and Kurtosis ±7) and multivariate (Mardia's test) distributions of the data were looked at one at a time. The presence of multivariate outliers was further investigated using the violated multivariate elongation and the Mahalanobis distance (Mahalanobis d‐squared) (p < 0.001). 30 The missing data were assessed using multiple imputations, and and exploratory factor analysis (EFA) used the pairwise deletion method to handle missing data. 31
2.6. Face validity
To evaluate the qualitative face validity of the FoP‐Q‐SF, 10 patients who met the inclusion criteria were asked to complete the questionnaire. Following this, experts were invited to provide feedback on the content, clarity, readability, simplicity, comprehensibility of the items, and the ease of questionnaire completion.
2.7. Content validity
2.7.1. Qualitative content validity
Twelve experts, including psychiatrists and psychologists, were invited to provide their insights following a comprehensive qualitative assessment of the FoP‐Q‐SF. Their feedback focused on assessing the accuracy of grammar, the suitability of vocabulary, the importance and pertinence of questionnaire items, their positioning, and precise scoring. Additionally, the researchers implemented minor grammatical adjustments to the questionnaire in accordance with their recommendations.
2.7.2. Quantitative content validity
The tool's quantitative content validity was evaluated using the content validity ratio (CVR) and content validity index (CVI). A team of ten experts, consisting of psychiatrists and psychologists, assessed each item using three scoring categories: (1) not required, (2) beneficial but not essential, and (3) essential. The minimum acceptable CVR, as per the lawshe table, was 0.56. 32 For the purpose of this study, experts were asked to evaluate each item using three criteria: simplicity/fluency, relevance, and clarity. Items scoring above 0.79 were deemed acceptable and kept unchanged. Items scoring between 0.70 and 0.79 required revision, while those scoring below 0.70 were considered unacceptable and required modification or removal. 33
2.8. Construct validity
In the initial phase of our study, we employed EFA in conjunction with maximum likelihood estimation (ML) to assess the construct validity. 34 To ensure the suitability of our data, we conducted Bartlett's test and the Kaiser−Meyer−Olkin (KMO) test to assess sample adequacy. KMO values greater than 0.8 were considered good, and those falling between 0.8 and 0.9 were considered high, in line with the guidelines. 35
The presence of an item in a latent factor was determined based on a factor loading of at least 0.3, which was estimated using the following formula: CV = 5.152 ÷ √(n − 2), where “n” represents the sample size. 36 Moreover, the “three indicator rule,” as suggested by Munro, necessitates that each latent factor must be represented by at least three observed variables. 37 Items with communalities values below 0.2 were also removed from the EFA. 38
Next, using AMOS version 27 software, a ML confirmatory factor analysis (CFA) was performed to evaluate and validate the discovered factor structure. Using the following goodness of fit indices, the model fit was evaluated: Chi‐square Minimum (CMIN), CMIN/degree of freedom ratio < 5, Goodness‐of‐Fit Index > 0.90, Comparative Fit Index (CFI) > 0.90, Relative Fit Index > 0.90, Incremental Fit Index > 0.90 and Tucker–Lewis Index (TLI) > 0.90, and Root Mean Square Error of Approximation (RMSEA) < 0.08. 39 Convergent and discriminant validity were both evaluated to test the construct validity. Composite reliability and Average Variance Extracted for each construct should be greater than 0.7 and 0.5, respectively, for convergent validity. 40
2.9. Reliability
We assessed internal consistency reliability using Cronbach's α. Cronbach's α coefficient values exceeding 0.7 41 were deemed acceptable, while the AIC values ranging between 0.2 and 0.4 indicated good internal consistency. 42 In the context of structural equation modeling, the Cronbach's α coefficient was replaced by the CR value, with scores exceeding 0.7 considered acceptable. 43
2.10. Statistical analysis
The SPSS‐AMOS 27, and JASP 0.18.3.0 were used to perform all of the statistical analyses. For quantitative variables, the basic descriptive statistics were presented as the mean (standard deviation [SD]), and for qualitative variables, the frequency was reported as the number (%). The scale's construct validity was assessed through EFA, with the reporting of the KMO measure of sampling adequacy and bartlett's test of sphericity. The reliability of the scale was evaluated by calculating the Cronbach's α coefficient for internal consistency and the Intraclass Correlation Coefficient (ICC) for test−retest reliability. Statistical significance was determined to be present at a two‐tailed p Value less than 0.05.
3. RESULTS
In present study, 400 patients were included in the analysis. The mean (SD) for their age was 49.18 (SD = 16.14). Most of the patients treated with surgery and chemotherapy (51.8%). Also, the mean score of fear of progression in cancer patients was 44.85 (SD = 13.62, 95% confidence interval [CI]: 43.45, 46.14). Other main demographic information was presented in Table 1.
3.1. Face validity
Quantitative face validity was evaluated for each item with a coefficient higher than 1.5.
3.2. Content validity
Following the necessary adjustments to the instrument, experts conducted a qualitative analysis to assess its content validity. The CVR values were all above 0.49, suggesting that every item should be included in the scale. The scores obtained for CVI and K coefficient for each item indicated a strong correlation with the instrument's underlying concept. The overall CVI (S‐CVI/ave) for the entire instrument was calculated to be 0.942, suggesting a high level of content validity. Moreover, all experts concurred that the instrument was thorough.
3.3. EFA
The KMO was 0.95, and the Bartlett's test of sphericity was significant (p < 0.001, χ 2 = 4201.02, df = 66) indicating that the sampling was adequate. Based on the EFA and the Eigenvalues greater than 1, as detailed in Table 2, we identified a single latent factor that explained approximately 65.04% of the total variance in the concept of fear of progression among patients with breast cancer. This conclusion is further supported by the loading strength of FoP‐Q‐SF items as illustrated in Figure 1 and Figure 2, which also present the scree‐plot for EFA, demonstrating the presence of a single latent factor for this questionnaire.
Table 2.
Maximum likelihood factor analysis in the FoP‐Q‐SF.
| Factor | Items | Factor Loading | h 2 | ʎ | % Variance |
|---|---|---|---|---|---|
| Fear of progression | 1. I become anxious if I think my disease may progress | 0.802 | 0.643 | 7.805 | 65.045 |
| 2. I am nervous before doctors’ appointments or periodic examinations | 0.806 | 0.649 | |||
| 3. I am afraid of pain | 0.854 | 0.730 | |||
| 4. I have concerns about reaching my professional goals because of my illness | 0.824 | 0.678 | |||
| 5. When I am anxious, I have physical symptoms such as a rapid heartbeat, stomach ache or agitation | 0.786 | 0.618 | |||
| 6. The possibility of my children contracting my disease disturbs me | 0.724 | 0.525 | |||
| 7. It disturbs me that I may have to rely on strangers for activities of daily living | 0.763 | 0.583 | |||
| 8. I am worried that at some point in time I will no longer be able to pursue my hobbies because of my illness | 0.824 | 0.679 | |||
| 9. I am afraid of severe medical treatments during the course of my illness | 0.891 | 0.794 | |||
| 10. I worry that my treatment could damage my body | 0.864 | 0.747 | |||
| 11. I worry about what will become of my family if something should happen to me | 0.727 | 0.528 | |||
| 12. The thought that I might not be able to work due to my illness disturbs me | 0.795 | 0.632 | |||
| Reliability measures | |||||
| Cronbach's α | 0.950 | ||||
| McDonald's Omega | 0.957 | ||||
| Average Inter‐Item Correlation | 0.649 | ||||
Abbreviations: FoP‐Q‐SF, fear of progression questionnaire‐short form; h 2, communalities; ʎ, Eigenvalue.
Figure 1.
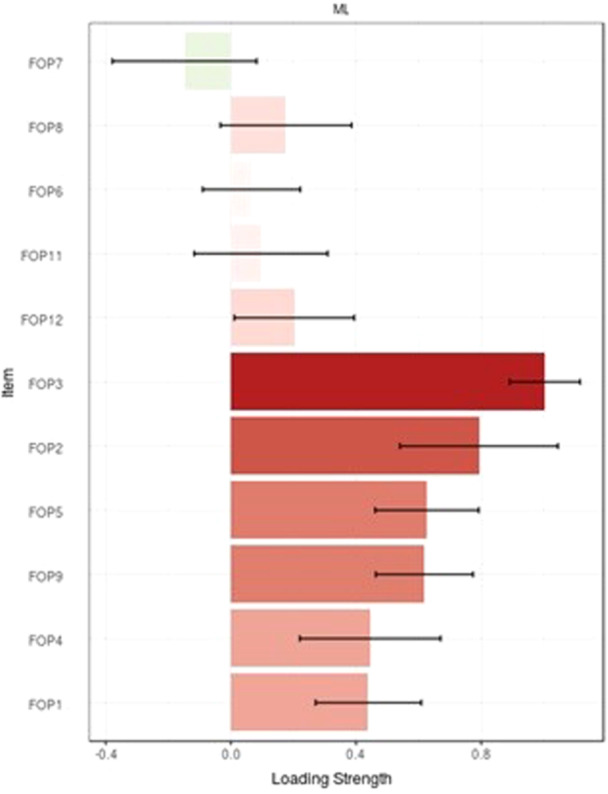
loading strength of FoP‐Q‐SF. FoP‐Q‐SF, fear of progression questionnaire‐short form.
Figure 2.
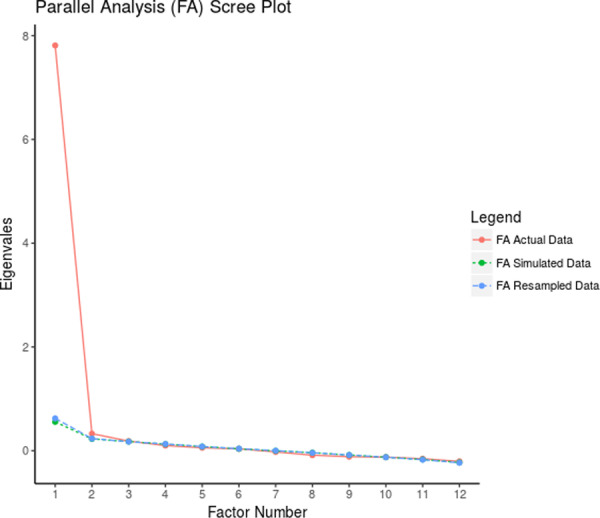
Scree‐plot for EFA of FoP‐Q‐SF. EFA, exploratory factor analysis; FoP‐Q‐SF, fear of progression questionnaire‐short form.
3.4. CFA
Results of a χ 2 goodness‐of‐fit test (p < 0.001, df = 51, χ 2 = 192.736) from CFA performed on a separate sample of 200 breast cancer patients were first obtained and compared to assess fit the model. As shown in Table 3, all fit indices were valid for testing the final model. The final FoP‐Q‐SF model is shown in Figure 3.
Table 3.
Goodness of fit indexes results from CFA of FoP‐Q‐SF.
| Indexes | Calculated | Acceptable range |
|---|---|---|
| χ 2 | 192.736 (<0.001) | >0.05 |
| RMSEA | 0.118 | good: <0.08, average: 0.08‐0.1, weak: >0.1 |
| CFI | 0.933 | >0.9 |
| NFI | 0.912 | >0.9 |
| CMIN/DF | 3.779 | good: <3, acceptable: <5 |
| PNFI | 0.705 | >0.05 |
| PCFI | 0.721 | >0.05 |
| IFI | 0.934 | >0.9 |
| TLI | 0.914 | >0.9 |
Abbreviations: CFA, confirmatory factor analysis; CFI, Comparative Fit Index; CMIN, Chi‐square Minimum; DF, degree of freedom; FoP‐Q‐SF, fear of progression questionnaire‐short form; IFI, incremental fit index; NFI, normed fit index; PCFI, parsimonious comprative fit index; PNFI, parsimonious normed fit index; RMSEA, Root Mean Square Error of Approximation; TLI, Tucker–Lewis Index.
Figure 3.
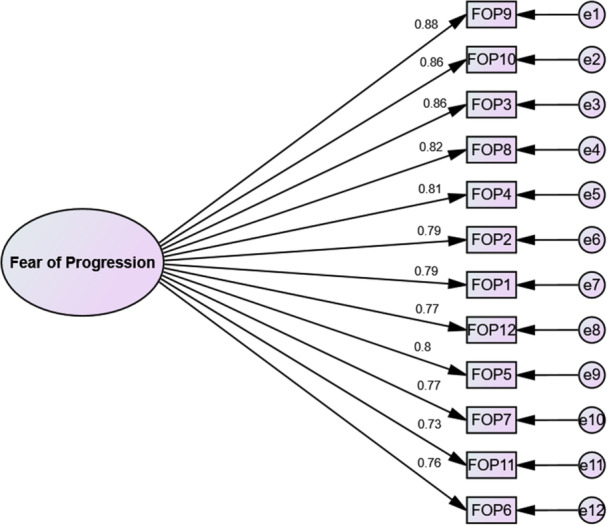
Final model of the FoP‐Q‐SF. FoP‐Q‐SF, fear of progression questionnaire‐short form.
3.5. Reliability
The internal consistency of all FoP‐Q‐SF items was assessed using Cronbach's α (α = 0.956, 95% CI: 0.95, 0.96). As indicated in Table 2, McDonald's Omega and AIC values for this scale were also deemed acceptable, further confirming its reliability. Additionally, to evaluate test‐retest reliability, a subset of 15 breast cancer patients who met various study criteria was selected. They were asked to complete the FoP‐Q‐SF twice, with a 2‐week interval between the two administrations. The ICC, a measure of consistency between the two administrations, was calculated to be 0.91 (95% CI: 0.85, 0.95). This indicates a high level of agreement between the responses in the two test sessions, suggesting that the FoP‐Q‐SF is a reliable instrument over time.
4. DISCUSSION
In a prior study conducted in Iran, the psychometric properties of the full 43‐item FoP‐Q were examined in patients with gastrointestinal cancer. 16 However, due to the advantages of using shorter questionnaires for ease of completion and the fact that the FoP‐Q‐SF had not been translated and subjected to psychometric evaluation in Iran, the primary objective of this present study is to translate and assess the psychometric properties of the FoP‐Q‐SF in Iranian patients with breast cancer.
The results of this study demonstrated that the Farsi version of the FoP‐Q‐SF exhibited strong validity and reliability when used with patients who have breast cancer. The original one‐factor model 44 was confirmed in this study and accounted for approximately 65.04% of the variance. In a separate study by Zimmermann, Herschbach, 45 they also identified a one‐factor structure in their research. However, they employed principal component analysis for factor extraction, and the one factor they identified explained 42% of the variance, which is notably lower than the variance explained in our current study. This suggests that the Farsi version of the FoP‐Q‐SF in the context of breast cancer patients may offer a more robust one‐factor model with a higher proportion of explained variance.
Indeed, it's worth noting that different studies can yield varying results when assessing the factor structure of a questionnaire. In the case of the study by Clever, Schepper, 46 they investigated partners of chronically ill patients, including those with breast cancer, prostate cancer, and diabetes mellitus. Their EFA results initially suggested the presence of two factors in the questionnaire. However, because the second factor only consisted of two items, they deemed it unstable and, after conducting additional tests and analysis, reported that the questionnaire had a single factor.
In contrast, the present study found that all items of the FoP‐Q‐SF exhibited strong factor loadings within a single factor. These variations in findings can be influenced by the specific study population, the cultural context, and the specific methodology used in each investigation, highlighting the importance of considering these factors when interpreting and comparing research results. The disparities in the variance explained by the factor structure between your study, where a one‐factor structure explained more variance, and the Clever, Schepper 46 study, which identified two factors explaining a lower percentage of variance (50.2%), may indeed be associated with differences in the research population.
It's essential to acknowledge that the concept of fear of progression can vary significantly between patients themselves and their partners or parents, as observed in studies. 45 , 46 Patients who are directly experiencing a health condition such as breast cancer might attach different levels of importance to specific items in the questionnaire, like item 6, when compared to their partners or parents. These variations emphasize the impact of perspective and the nature of the relationship with the affected individual on the interpretation of questionnaire results.
In Hinz, Mehnert, 47 EFA revealed two factor structure but because the factor two had Eigenvalues only slightly higher than 1, they accepted one factor structure, thus their results are differ from present study. One key factor contributing to these differences might be the types of cancer present in the study populations. In the Hinz et al. study, a significant portion of the patients had prostate cancer (31%), while in the present study, the focus was exclusively on breast cancer patients. The nature and experience of fear of progression can differ substantially between various cancer types, which could influence the factor structure.
Moreover, the mean age of the patients in the Hinz, Mehnert 47 study (62.4) was notably higher than in the present study (49.18). Some items in the FoP‐Q‐SF may have limited relevance or meaningfulness for older patients, as noted with item 12. Age‐related variations in the understanding and experience of fear of progression may also contribute to the differences in factor structures between the two studies
The results of the CFA in this study revealed that the one‐factor model of the FoP‐Q‐SF had good fit indices. Notably, the RMSEA in this model was comparable to the one‐factor model proposed by Kwakkenbos et al. 48 However, when considering the CMIN/DF, our model outperformed the one from Kwakkenbos, van den Hoogen. 48 Additionally, our model scored better in terms of CFI and TLI when compared to the one‐factor model from the Hinz, Mehnert 47 study.
The Farsi version of the FoP‐Q‐SF in this study exhibited a commendable level of internal consistency, with a Cronbach's α coefficient of 0.95, which is indicative of strong reliability. Comparatively, the questionnaire in the study by Hinz, Mehnert 47 and the Zimmermann, Herschbach 45 questionnaire had a Cronbach's α equal 0.90 and 0.88, respectively, which were slightly differ from our study. It's worth noting that in our study, you evaluated the Omega coefficient and stability of the questionnaire, which are valuable measures of reliability and consistency. However, since these properties were not assessed in other studies. Nevertheless, the strong Cronbach's α in all three studies suggests that the questionnaires are indeed reliable instruments for measuring fear of progression.
The findings of this study affirm the validity and reliability of the FoP‐Q‐SF as a valuable tool for assessing fear of progression in breast cancer patients, consistent with the outcomes of similar studies. The availability of a Farsi version of this questionnaire presents an important opportunity for Farsi‐speaking researchers and healthcare professionals. It enables them to effectively screen patients with breast cancer for fear of progression, facilitating the planning of preventive and care interventions to enhance the quality of life for patients and their families. This valuable resource contributes to the overall well‐being and support of breast cancer patients within Farsi‐speaking communities.
5. LIMITATIONS AND STRENGTH
This study, like any research, has its limitations and strengths. One notable limitation is the use of a convenience sampling method, which may not fully represent the broader population. Additionally, the study did not establish a cutoff value for high levels of fear of progression in Iranian breast cancer patients. Furthermore, the research did not have access to Iranian cancer patients in other countries, limiting the ability to generalize findings to a more diverse population.
Despite these limitations, the study possesses several strengths. The use of Horn's parallel analysis and the exploratory graph analysis approach to identify factors demonstrates methodological rigor and enhances the reliability of the results. Furthermore, calculating the Omega‐McDonald's coefficient in addition to Cronbach's α is strength, as it provides a more comprehensive assessment of the questionnaire's reliability. These strengths contribute to the overall robustness of the study's findings.
6. CLINICAL IMPLICATIONS
The study's findings can guide healthcare providers in developing and implementing targeted psychosocial support programs for Iranian breast cancer patients. Recognizing the validity and reliability of FoP assessments enables clinicians to identify those who may require additional emotional and psychological support to cope with their fear of progression. Oncologists and healthcare professionals can use validated tools to assess FoP, facilitating better communication with patients. By understanding a patient's level of fear of progression, clinicians can tailor their discussions and treatment recommendations, addressing patients' concerns and providing reassurance when necessary.
The study's findings can be incorporated into survivorship care plans for breast cancer patients in Iran. Survivorship care planning involves addressing not only the physical aspects of cancer care but also the psychosocial and emotional well‐being of survivors. Reliable assessments of FoP can be a valuable component of such plans. Ultimately, the study's findings can lead to reduced psychological distress and improved overall well‐being for Iranian breast cancer patients. This can have a positive impact on treatment adherence, quality of life, and patient satisfaction.
7. CONCLUSION
The short‐form Farsi version of the Fear of Progression questionnaire in this study demonstrates acceptable validity and reliability. However, it's advisable to further explore additional psychometric properties of the questionnaire. Future research should consider assessing aspects such as convergent and discriminant validity, feasibility, and responsiveness. Additionally, determining a cutoff value for high levels of fear of progression in a more representative sample of Iranian patients with breast cancer would be valuable. These efforts can enhance our understanding of fear of progression and contribute to more effective support and care interventions for breast cancer patients in Iran.
AUTHOR CONTRIBUTIONS
Hamid Sharif‐Nia: Conceptualization; writing—original draft; formal analysis; supervision; methodology; project administration; writing—review and editing. Pooria Sobhanian: Data curation; writing—review and editing; writing—original draft. Erika Sivarajan Froelicher: Writing—original draft; writing—review and editing; formal analysis. Bahar Farhadi: Methodology; validation; writing—original draft; writing—review and editing. Sima Hejazi: Writing—original draft; validation; writing—review and editing. Amir Hossein Goudarzian: Conceptualization; writing—original draft; writing—review and editing; data curation; methodology; project administration. Mobin Mohamadinezhad: Data curation; writing—original draft. Ehsan Zaboli: Writing—original draft; writing—review and editing. Mohammad Mohsen Hosseinian: Writing—original draft; data curation; writing—review and editing; resources. Maryam Hasannezhad Reskati: Writing—original draft; writing—review and editing. Seyed Hamzeh Hosseini: Data curation; writing—review and editing; writing—original draft.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The ethics committee at Mazandaran University of Medical Sciences in Sari, Iran, granted ethical approval for this study (Code: IR.MAZUMS.IMAMHOSPITAL.REC.1402.022). All methods conducted in the present study adhered to the principles outlined in the Helsinki statement, ensuring the ethical conduct of research involving human subjects. Participants in the study were fully informed that their participation was voluntary, and the objectives and procedures of the study were comprehensively explained to them. Each participant provided written, fully informed consent to participate. The privacy and confidentiality of participants' data were guaranteed, and they were assured that the study findings would be shared and published with care and respect for their privacy and confidentiality.
TRANSPARENCY STATEMENT
The lead author Amir Hossein Goudarzian affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The authors would like to extend their gratitude to the research deputy of Mazandaran University of Medical Sciences (Sari, Iran) for their invaluable support throughout the course of this study. They would also like to express their appreciation to all the patients and staff members of the oncology clinic for their kind and cooperative participation, which was instrumental in the successful completion of this research.
Sharif‐Nia H, Sobhanian P, Froelicher ES, et al. A validity and reliability evaluation of fear of progression questionnaire in Iranian breast cancer patients: a methodological study. Health Sci Rep. 2024;7:e2260. 10.1002/hsr2.2260
DATA AVAILABILITY STATEMENT
All authors have carefully examined and approved the final version of the manuscript. As the main author, Amir Hossein Goudarzian had complete access to all the data in the study and takes full responsibility for the accuracy and integrity of the data analysis.
REFERENCES
- 1. Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524‐541. [DOI] [PubMed] [Google Scholar]
- 2. Karimi B, Baneshi M, Haghdoost A, Molavi Vardanjani H, Ostovarfar J. Population‐Based prevalence of cancer family history in southeastern Iran. Shiraz E‐Med J. 2022;23:e128068. [Google Scholar]
- 3. mT B, Landrani M, Sadeghi E. Relationship between cognitive flexibility and fear of cancer recurrence in breast cancer patients. Iranian J Cancer Care. 2021;2:3‐10. [Google Scholar]
- 4. Sharif Nia H, Sharif SP, Esmaeili R, et al Factors influencing the level of death depression in patients with cancer: a path analysis. J Mazan Univ Med Sci. 2017;26:318‐331. [Google Scholar]
- 5. Crist JV, Grunfeld EA. Factors reported to influence fear of recurrence in cancer patients: a systematic review. Psycho‐Oncology. 2013;22:978‐986. [DOI] [PubMed] [Google Scholar]
- 6. Fardell JE, Thewes B, Turner J, et al. Fear of cancer recurrence: a theoretical review and novel cognitive processing formulation. J Cancer Survivor. 2016;10:663‐673. [DOI] [PubMed] [Google Scholar]
- 7. Koch L, Jansen L, Brenner H, Arndt V. Fear of recurrence and disease progression in long‐term (≥5 years) cancer survivors—a systematic review of quantitative studies. Psycho‐Oncology. 2013;22:1‐11. [DOI] [PubMed] [Google Scholar]
- 8. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Survivor. 2013;7:300‐322. [DOI] [PubMed] [Google Scholar]
- 9. Koch‐Gallenkamp L, Bertram H, Eberle A, et al. Fear of recurrence in long‐term cancer survivors—do cancer type, sex, time since diagnosis, and social support matter? Health Psychol. 2016;35:1329‐1333. [DOI] [PubMed] [Google Scholar]
- 10. Mehnert A, Berg P, Henrich G, Herschbach P. Fear of cancer progression and cancer‐related intrusive cognitions in breast cancer survivors. Psycho‐Oncology. 2009;18:1273‐1280. [DOI] [PubMed] [Google Scholar]
- 11. Simard S, Savard J. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Supp Care Cancer. 2009;17:241‐251. [DOI] [PubMed] [Google Scholar]
- 12. Lasry JCM, Margolese RG. Fear of recurrence, breast‐conserving surgery, and the trade‐off hypothesis. Cancer. 1992;69:2111‐2115. [DOI] [PubMed] [Google Scholar]
- 13. LL N. Mastectomy patients and the fear of cancer recurrence. Cancer Nurs. 1981;4:213‐220. [PubMed] [Google Scholar]
- 14. Thewes B, Butow P, Zachariae R, Christensen S, Simard S, Gotay C. Fear of cancer recurrence: a systematic literature review of self‐report measures. Psycho‐oncology. 2012;21:571‐587. 10.1002/pon.2070 [DOI] [PubMed] [Google Scholar]
- 15. Bateni FS, Rahmatian M, Kaviani A, Simard S, Soleimani M, Nejatisafa AA. The Persian version of the fear of cancer recurrence inventory (FCRI): translation and evaluation of its psychometric properties. Archi Breast Cancer. 2019;6:174‐180. [Google Scholar]
- 16. Hasannezhad Reskati M, Elyasi F, Hosseini SH, et al. The psychometric properties of the fear of progression questionnaire (FoP‐Q) for cancer patients in Iran. J Gastroint Cancer. 2022;54:855‐866. 10.1007/s12029-022-00875-3 [DOI] [PubMed] [Google Scholar]
- 17. Kim JH, McMahon BT, Hawley C, Brickham D, Gonzalez R, Lee DH. Psychosocial adaptation to chronic illness and disability: a virtue based model. J Occup Rehabil. 2016;26:45‐55. [DOI] [PubMed] [Google Scholar]
- 18. Afrooz R, Rahmani A, Zamanzadeh V, et al. The nature of hope among Iranian cancer patients. Asian Paci J Cancer Prevent. 2014;15:9307‐9312. [DOI] [PubMed] [Google Scholar]
- 19. Park CL, Masters KS, Salsman JM, et al. Advancing our understanding of religion and spirituality in the context of behavioral medicine. J Behav Med. 2017;40:39‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pahlevan Sharif S, Lehto RH, Amiri M, et al. Spirituality and quality of life in women with breast cancer: the role of hope and educational attainment. Palliat Support Care. 2021;19:55‐61. [DOI] [PubMed] [Google Scholar]
- 21. Sharif Nia H, Pahlevan Sharif S, Goudarzian AH, Allen KA, Jamali S, Heydari Gorji MA. The relationship between religious coping and self‐care behaviors in Iranian medical students. J Relig Health. 2017;56:2109‐2117. [DOI] [PubMed] [Google Scholar]
- 22. Rahnama M, Khoshknab MF, Maddah SSB, Ahmadi F, Arbabisarjou A. Religion as an alleviating factor in Iranian cancer patients: a qualitative study. Asian Pacific J Cancer Prevent. 2016;16:8519‐8524. [DOI] [PubMed] [Google Scholar]
- 23. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Assel M, Sjoberg D, Elders A, et al. Guidelines for reporting of statistics for clinical research in urology. BJU Int. 2019;123:401‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacCallum RC, Widaman KF, Zhang S, Hong S. Sample size in factor analysis. Psychol Methods. 1999;4:84‐99. [Google Scholar]
- 26. Anthoine E, Moret L, Regnault A, Sébille V, Hardouin JB. Sample size used to validate a scale: a review of publications on newly‐developed patient reported outcomes measures. Health Qual Life Outcomes. 2014;12:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gilbert GE, Prion S. Making sense of methods and measurement: sample size issues for psychometric studies. Clin Simulat Nurs. 2016;12:482‐483. [Google Scholar]
- 28. Herschbach P, Berg P, Dankert A, et al. Fear of progression in chronic diseases. J Psychosom Res. 2005;58:505‐511. [DOI] [PubMed] [Google Scholar]
- 29. Organization WH . Process of translation and adaptation of instruments. http://www.who.int/substance_abuse/research_tools/translation/en/2009
- 30. Vinzi VE, Chin WW, Henseler J, Wang H. Handbook of partial least squares: Concepts, methods and applications. Springer; 2010. [Google Scholar]
- 31. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lawshe CH. A quantitative approach to content validity. Perso psychol. 1975;28:563‐575. [Google Scholar]
- 33. Jay Lynn S, Surya Das L, Hallquist MN, Williams JC. Mindfulness, acceptance, and hypnosis: cognitive and clinical perspectives. Int J Clin Exp Hypn. 2006;54:143‐166. [DOI] [PubMed] [Google Scholar]
- 34. Sharif‐Nia H, She L, Osborne J, Gorgulu O, Khoshnavay Fomani F, Goudarzian AH. Statistical concerns, invalid construct validity, and future recommendations. Nurs Pract Today. 2024;11:16‐21. [Google Scholar]
- 35. Shrestha N. Factor analysis as a tool for survey analysis. American J Appl Mathem Stati. 2021;9:4‐11. [Google Scholar]
- 36. Fok D. Development and testing of a Low Vision Product Selection Instrument (LV‐PSI): A mixed‐methods approach. 2011.
- 37. Munro BH. Statistical methods for health care research. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 38. Samitsch C. Data quality and its impacts on decision‐making: How managers can benefit from good data. springer; 2014. [Google Scholar]
- 39. Nikkhah M, Heravi‐Karimooi M, Montazeri A, Rejeh N, Sharif Nia H. Psychometric properties the Iranian version of older people's quality of life questionnaire (OPQOL). Health Qual Life Outcomes. 2018;16:1‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharif Nia H, Pahlevan Sharif S, Lehto RH, et al. Development and psychometric evaluation of a Persian version of the death depression Scale‐Revised: a cross‐cultural adaptation for patients with advanced cancer. Jpn J Clin Oncol. 2017;47:713‐719. 10.1093/jjco/hyx065 [DOI] [PubMed] [Google Scholar]
- 41. Mayers A. Introduction to statistics and SPSS in psychology. 2013.
- 42. Hosseini L, Sharif Nia H, Ashghali Farahani M. Development and psychometric evaluation of family caregivers' hardiness scale: a sequential‐exploratory mixed‐method study. Front Psychol. 2022;13:807049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sharif Nia H, Pahlevan Sharif S, Boyle C, et al. The factor structure of the spiritual well‐being scale in veterans experienced chemical weapon exposure. J Relig Health. 2018;57:596‐608. [DOI] [PubMed] [Google Scholar]
- 44. Mehnert A, Herschbach P, Berg P, Henrich G, Koch U. Progredienzangst bei brustkrebspatientinnen ‐ validierung der kurzform des progredienzangstfragebogens PA‐F‐KF/Fear of progression in breast cancer patients – validation of the short form of the fear of progression questionnaire (FoP‐Q‐SF). Z Psychosom Med Psychother. 2006;52:274‐288. 10.13109/zptm.2006.52.3.274 [DOI] [PubMed] [Google Scholar]
- 45. Zimmermann T, Herschbach P, Wessarges M, Heinrichs N. Fear of progression in partners of chronically ill patients. Behav Med. 2011;37:95‐104. 10.1080/08964289.2011.605399 [DOI] [PubMed] [Google Scholar]
- 46. Clever K, Schepper F, Pletschko T, Herschbach P, Christiansen H, Martini J. Psychometric properties of the fear of progression questionnaire for parents of children with cancer (FoP‐Q‐SF/PR). J Psychosom Res. 2018;107:7‐13. 10.1016/j.jpsychores.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 47. Hinz A, Mehnert A, Ernst J, Herschbach P, Schulte T. Fear of progression in patients 6 months after cancer rehabilitation‐a‐ validation study of the fear of progression questionnaire FoP‐Q‐12. Supp Care Cancer. 2015;23:1579‐1587. 10.1007/s00520-014-2516-5 [DOI] [PubMed] [Google Scholar]
- 48. Kwakkenbos L, van den Hoogen FHJ, Custers J, et al. Validity of the fear of progression questionnaire‐short form in patients with systemic sclerosis. Arth Care Res. 2012;64:930‐934. 10.1002/acr.21618 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors have carefully examined and approved the final version of the manuscript. As the main author, Amir Hossein Goudarzian had complete access to all the data in the study and takes full responsibility for the accuracy and integrity of the data analysis.


