Abstract
To assess the role of naturally occurring basic amino acid substitutions in the V3 loop of human immunodeficiency virus type 1 (HIV-1) subtype E on viral coreceptor usage and cell tropism, we have constructed a panel of chimeric viruses with mutant V3 loops of HIV-1 subtype E in the genetic background of HIV-1LAI. The arginine substitutions naturally occurring at positions 8, 11, and 18 of the V3 loop in an HIV-1 subtype E X4 strain were systematically introduced into that of an R5 strain to generate a series of V3 loop mutant chimera. These chimeric viruses were employed in virus infectivity assays using HOS-CD4 cells expressing either CCR5 or CXCR4, peripheral blood mononuclear cells, T-cell lines, or macrophages. The arginine substitution at position 11 of the V3 loop uniformly caused the loss of infectivity in HOS-CD4-CCR5 cells, indicating that position 11 is critical for utilization of CCR5. CXCR4 usage was conferred by a minimum of two arginine substitutions, regardless of combination, whereas arginine substitutions at position 8 and 11 were required for T-cell line tropism. Nonetheless, macrophage tropism was not conferred by the V3 loop of subtype E R5 strain per se. We found that the specific combinations of amino acid changes in HIV-1 subtype E env V3 loop are critical for determining viral coreceptor usage and cell tropism. However, the ability to infect HOS-CD4 cells through either CXCR4 or CCR5 is not necessarily correlated with T-cell or macrophage tropism, suggesting that cellular tropism is not dictated solely by viral coreceptor utilization.
CD4-positive T lymphocytes and cells of monocyte-macrophage lineages are the primary targets of human immunodeficiency virus type 1 (HIV-1) in vivo (22, 34). During the early phase of infection, non-syncytium-inducing (NSI) and macrophage (M)-tropic HIV-1 strains are predominant (27, 34, 41). T-cell (T)-tropic, syncytium-inducing (SI) isolates emerge in the late phase of infection (6, 26). Changes in cellular tropism by HIV-1 strains in vivo seem to be a key event in the pathogenesis of HIV-1 disease (11, 16, 31, 32), whereby the transition from M tropism to T tropism occurs in association with rapid progression to AIDS (6).
It has been reported that the third variable (V3) region of the HIV-1 envelope glycoprotein gp120 is the most critical determinant for cellular tropism (7, 14) or coreceptor use (3–5, 30, 39), although other regions of envelope glycoprotein are suggested to have some role in conjunction with the V3 loop sequence contexts (4, 12, 15, 29). Specific amino acid variations in the V3 loop, especially distribution of charged amino acids, have been shown to correlate with viral phenotype (2, 7, 8, 10) and coreceptor usage (30, 39). The V3 loop amino acid sequences of HIV-1 SI isolates are more positively charged than those of NSI isolates (2, 10, 19, 20). The basic amino acids at positions 11 and 25 (numbering from the amino-terminal cysteine residue) of the V3 loop were reported to confer an SI phenotype on an NSI virus in the genetic background of HXB2 recombined with a patient-derived V3 loop (7). However, most of these studies were carried out in the genetic background of subtype B isolates, and therefore it is not known whether the V3 regions of other subtypes determine such viral characteristics.
In a previous study, we identified an epidemiologically linked case of intrafamilial infection of HIV-1 subtype E and isolated genetically closely related HIV-1 subtype E strains from each family member (24, 25). The X4 virus (HIV-1NH1) was isolated from the father (NH1; the index case), who had developed AIDS. The R5 virus (HIV-1NH2) was isolated from mother (NH2), who was an asymptomatic carrier at the time of virus isolation. Both isolates were phylogenetically closely related, indicating that the virus was transmitted directly from NH1 to NH2 sexually (24, 25).
Close comparison between V3 loop amino acid sequences of these nearly isogenic X4 and R5 isolates implied the importance of the basic amino acid residues at positions 8, 11, and 18 in the V3 loop for phenotypic transition from R5 (NSI) to X4 (SI) (23). Furthermore, we showed that the V3 loop of HIV-1 subtype E envelope glycoproteins can specify viral coreceptor usage and MT2 cell tropism in chimeric viruses in the genetic background of HIV-1LAI (23). In this study, we investigated the role of the naturally occurring basic amino acid substitutions on the HIV-1 subtype E V3 loop by using chimeric viruses with mutated V3 loop in the genetic background of HIV-1LAI. We found that specific combinations of these amino acid changes at specific loci in the HIV-1 subtype E env V3 loop were indeed critical for determining viral coreceptor usage and cell tropism.
MATERIALS AND METHODS
Construction of V3 loop recombinant DNA clones.
For systematic generation of chimeric viruses with various types of V3 loop mutations, pLAI (21)-derived subclones constructed previously (23) were used. These include (i) pUC-LAI/SB, where the 2.7-kb SalI-BamHI fragment harboring the env region of LAI is cloned into pUC18, and (ii) pUC-LAI/NH2V3, constructed by replacing the V3 loop gene of pUC-LAI/SB with that of the HIV-1 subtype E R5 strain HIV-1NH2 (23) (Fig. 1).
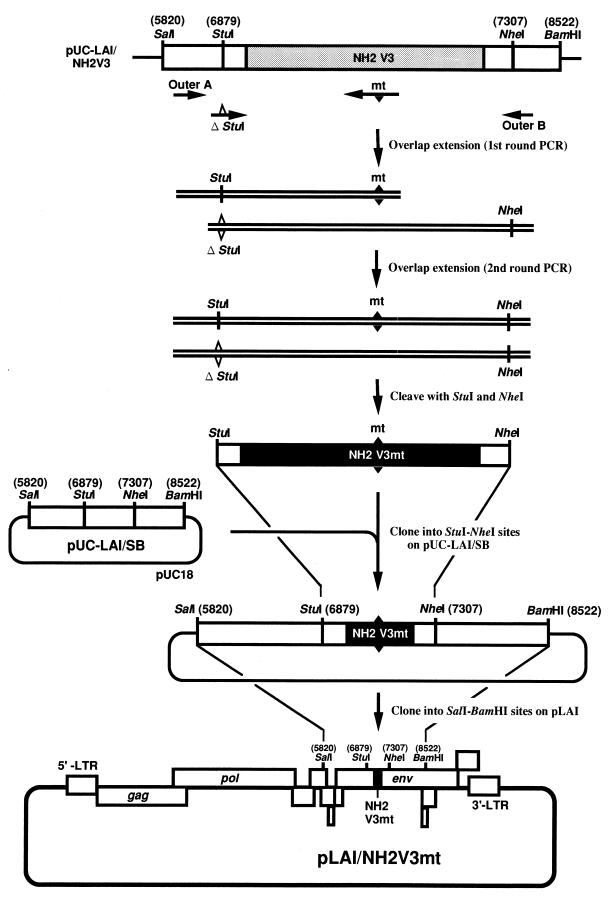
FIG. 1.
Schematic representation of the construction of LAI-based infectious molecular clones with mutated HIV-1 subtype E V3 loops. Plasmid pUC-LAI/NH2V3 (23) was used as the template for PCR to generate DNA fragments containing the mutation(s) of interest. The PCR primers are listed in Table 1. The mt primers contain a series of mutations (closed triangle) to introduce the amino acid substitution(s). The native StuI site was mutated (open triangle) in the ΔStuI primer. Outer A and outer B primers have the original sequence of the LAI env gene. The mixture of the first-round PCR products generated by either primer pair, Outer A and an mt primer or ΔStuI and Outer B, is subsequently amplified with outer A and outer B primers. Two forms of amplified products are generated. The upper form containing the mutation(s) of interest is readily cleaved with the restriction enzymes, StuI and NheI and thus selectively cloned into StuI-NheI-cleaved pUC-LAI/SB (23). Subsequently, the SalI-BamHI fragment of the resultant plasmid is cloned into SalI-BamHI-cleaved pLAI to reconstitute the LAI-based chimeric molecular clones with mutated V3 loops of HIV-1 subtype E.
StuI-NheI fragments with the V3 loop mutations were generated by the overlap extension method (13), using a primer (mt series) with the point mutation(s) of interest listed in Table 1. In the ΔStuI primer, the StuI site present in pLAI at nucleotide position 6879 (21) was mutated (23). Outer A and Outer B primers have the original sequences flanking the V3 loop region of the LAI env gene (Table 1; Fig. 1). For introduction of mutations, pUC-LAI/NH2V3 was PCR amplified with two sets of primer pairs, either Outer A and an mt primer or ΔStuI and Outer B (Fig. 1). The PCR products were then subjected to a second round of PCR with Outer A and Outer B primers, yielding two forms of PCR products; one retains the StuI site and the other does not. PCR product with a mutated V3 loop is readily cleaved with restriction enzymes StuI and NheI and thereby selectively cloned into StuI-NheI-cleaved pUC-LAI/SB (Fig. 1). The SalI-BamHI fragment of the resultant plasmid was inserted into SalI-BamHI-cleaved pLAI to reconstitute the V3 loop mutant chimeric molecular clones. The nucleotide sequences of the PCR fragments and sequences around the cloning sites of each V3 mutant were confirmed with an ABI PRISM 310 automated sequencer (Perkin-Elmer, Norwalk, Conn.).
TABLE 1.
PCR primers for introduction of V3 loop mutations by the overlap extension method
| Primer | Sequence (5′→3′)a | Positionb |
|---|---|---|
| Outer A | 5′-ACG TTG ACA AGT TGT AAC ACC-3′ | 6845–6865 |
| Outer B | 5′-CTT AAT TTG CTA GCT ATC TGT-3′ | 7320–7300 |
| ΔStuI | 5′-GTC ATT ACA CtG GCg TGT CCA-3′ | 6869–6889 |
| mt1 | 5′-ACT TGT TCT TGc ATT GTT GG-3′ | 33–14 |
| mt2 | 5′-ACT TGT TCT TcT ATT GTT GG-3′ | 33–14 |
| mt3 | 5′-CCT ATA GTT ATA CgT GTT CTT G-3′ | 50–29 |
| mt4 | 5′-ATA CCA TAC TcG CCC TGG TC-3′ | 40–21 |
| mt5 | 5′-Ctc GCC CTG GTC CTA TAG TTA TAC gTG TTC-3′ | 47–18 |
| mt6 | 5′-CTc GCC CTG GTC CTA TAG TTA TAC TTG TTC TTc TAT TG-3′ | 55–18 |
| mt7 | 5′-GTT ATA CgT GTT CTT cTA TTG-3′ | 55–35 |
| mt8 | 5′-CTc GCC CTG GTC CTA TAG TTA TAC gTG TTC TTc TAT TG-3′ | 55–18 |
Preparation of cell-free virus stocks.
HeLa cells (5 × 105 cells) were grown in Dulbecco’s modified Eagle medium with 10% (vol/vol) heat-inactivated fetal calf serum (FCS) in a T25 flask for 1 day and transfected with 30 μg of plasmid DNA by the calcium phosphate coprecipitation method (GIBCO, Grand Island, N.Y.). The culture supernatants were collected at 48 and 72 h after transfection, filtered through 0.45-μm-pore-size filters, and subjected to virion-associated reverse transcriptase (RT) assay (37). The supernatants were kept at −152°C before use.
Cell culture and virus infections.
Peripheral blood mononuclear cells (PBMCs) and CD8-depleted PBMCs were prepared from whole blood of HIV-1-seronegative donors by Ficoll-Hypaque (Pharmacia LKB) density centrifugation. Before use, they were stimulated with 1 μg of phytohemagglutinin (PHA) per ml for 3 days and grown in RPMI 1640 with 10% (vol/vol) heat-inactivated FCS and 20 U of recombinant human interleukin-2 (a kind gift from Shionogi Pharmaceutical Co.) per ml. The human CD4+ T-lymphocyte cell lines MT2, H9, M8166, Molt4, and PM1 were grown in RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated FCS. CD4+ human osteosarcoma (HOS-CD4) cell lines expressing either human CCR5 or CXCR4 (9) were grown in Dulbecco’s modified Eagle medium DMEM with 10% FCS and 1.0 μg of puromycin per ml. Monocyte-derived macrophage cultures were prepared as previously described (18).
Infectivity assay.
PHA-stimulated PBMCs (2 × 106) and macrophages (105) were incubated in 0.1 ml of cell-free supernatant containing each recombinant virus (2 × 105 to 5 × 105 32P cpm of RT activity) (37) for 2 to 16 h at 37°C, washed once with phosphate-buffered saline, and grown in 2 and 0.5 ml of the growth medium in 24- and 48-well plates, respectively. The CD4+ human T-cell lines MT2, H9, M8166, Molt4, and PM1 (5 × 104 cells) were incubated in 50 μl of cell-free supernatant containing one of the recombinant viruses (4 × 105 32P cpm of RT activity) (37) for 2 h at 37°C and grown in 0.2 ml of medium in 96-well plates. Half of the volume of the culture medium was replaced by fresh medium every 2 to 3 days for either 25 (PBMCs) or 16 (macrophages and T-cell lines) days after infection. In all infections, a portion of culture supernatant was collected every 2 to 3 days, stored at −80°C until all samples were collected at the indicated time points, and analyzed for virion-associated RT activities. The results were shown as a average of duplicated or triplicated experiments; variations were within 10% of the average.
Virus infectivity on the HOS cell lines was determined by endpoint dilution of virus stocks. The infections were done in triplicate in 96-well plates. HOS-CD4 cells (6 × 103 in 200 μl/well) expressing either CCR5 or CXCR4 were incubated in medium containing serially diluted (fivefold) recombinant virus stocks corresponding to 1 × 106 to 8 × 103 cpm of virion-associated RT activity, washed once with phosphate-buffered saline, fed with 0.2 ml of culture medium, and monitored for RT production in the culture supernatants. The highest dilution required to produce an RT-positive culture was taken as the endpoint, and HIV-1 titers of infectious recombinants on the HOS-CD4 cells were expressed as 2, 10, 50, or 250 tissue culture infective doses (TCID) per 5 × 105 32P cpm of RT activity.
RESULTS
Construction of pLAI-based infectious molecular clones with a mutated V3 loop derived from HIV-1 subtype E.
To assess the contributions of the naturally occurring basic amino acid substitutions found at positions 8, 11, and 18 of the V3 loop of HIV-1 subtype E on viral coreceptor usage and cell tropism, we have constructed a panel of V3 loop mutant chimeras in the genetic background of HIV-1LAI (21) by the overlap extension method (13) (Fig. 1). The V3 loop substitutions and mutagenesis were designed so that only sequences between two cysteine residues flanking the V3 loop were altered. The predicted amino acid sequences of the V3 loops of the mutants generated in this study are listed in Fig. 2. In mt1, the threonine residue at position 8 in the V3 loop of the R5 strain HIV-1NH2, which constitutes a highly conserved potential N-glycosylation site, was replaced with alanine. In mutants mt2 through mt8, various combinations of arginine substitutions at position 8, 11, or 18 were introduced into the V3 loop of HIV-1NH2.
FIG. 2.
Amino acid sequences of the V3 loops of chimeric molecular clones. The positions (shadowed) of the amino acid substitutions of V3 loops are indicated by the numbers (with arrow) starting from the N-terminal cysteine residue of the loop. The potential N-linked glycosylation site (box) and the net charge in each V3 loop amino acid sequence are shown.
Coreceptor usage of V3 loop mutant chimeras in the HOS-CD4 cell assay.
To assess the contributions of V3 loop mutations in coreceptor usage, infectivities of mutant viruses on HOS-CD4 cell lines expressing either CCR5 or CXCR4 (9) were assayed by endpoint dilution infection (23). The chimeric viruses generated in this study were infective in HOS-CD4 cells expressing either CCR5 or CXCR4, except for mt3, in which serine at position 11 was replaced with arginine (Fig. 3). Since the protein profiles of the virions produced by transfection of the mt3 recombinant DNA clone appeared to be normal according to Western blot analysis (data not shown), the loss in infectivity of mt3 is not due to incorrect or inefficient processing during viral production. The mutant chimeras which retain a serine residue at position 11 (mt1, mt2, mt4, and mt6) could grow in HOS-CD4-CCR5 cells, whereas the mutants in which a serine residue at position 11 was replaced with arginine uniformly lost the ability to utilize CCR5 (Fig. 3). It is noted that the infectivity to HOS-CCR5 cells was reduced fivefold in mt1, where the threonine residue at position 8, which constitutes a potential N-glycosylation site, was changed to alanine (Fig. 3).
FIG. 3.
Coreceptor usage of mutant V3 loop chimeric virus in the HOS-CD4 cell infectivity assay. Virus stocks were generated by transfecting HeLa cells with each recombinant plasmid DNA. HOS-CD4 cell lines expressing either CCR5 or CXCR4 were seeded in a 96-well plate 1 day prior to infection and were infected with 1:5 serially diluted virus inocula (ranging from 1 × 106 to 8 × 103 cpm of virion-associated RT activity). After overnight incubation, the inoculum was removed and 200 μl of culture medium was added. Medium was replaced on day 2 postinfection, and virus replication was monitored by virion-associated RT activity on day 5 postinfection. TCID normalized by RT activity of the virus inoculum is plotted. Assays were done in triplicate. Since all assays gave identical TCIDs, error bars are not shown. Each amino acid substitution (amino acid position in the V3 loop with the substituting amino acid residue denoted in one-letter code) introduced into the V3 loop of HIV-1 subtype E R5 strain HIV-1NH2 is shown at the top.
The mutants with two or three arginine substitutions (mt5 through mt8) were able to replicate in HOS-CD4-CXCR4 cells, whereas mutants with only one arginine substitution (mt2 to mt4) were not (Fig. 3). This result indicates that a minimum of two basic substitutions are required to confer CXCR4 usage. In addition, mt5 was less infective than mt6, mt7, and mt8 (Fig. 3), suggesting that a basic substitution at position 8 may be required for efficient utilization of CXCR4.
mt6, in which the residues at both position 8 and position 18 were replaced with arginine but the serine residue at position 11 was retained, showed usage of both CXCR4 and CCR5 (Fig. 3). This dual coreceptor usage is consistent with our observation that the serine residue at position 11 is critical for CCR5 usage and that the minimal two arginine substitutions are required for the acquisition of CXCR4 utilization (Fig. 3).
Infectivities of the mutant V3 loop chimeric viruses in PBMCs, T-cell lines, and macrophages.
The tropic properties of the mutant viruses were examined by infectivity assay in three human cell types: PBMCs, macrophages, and T-cell lines MT2, H9, M8166, Molt4, and PM1 cells. Mutant viruses replicated on PBMCs to various extents (Fig. 4A). We detected no difference in the pattern of replication kinetics of each chimeric virus between PBMCs and CD8-depleted PBMCs. As shown in Fig. 4A, mt2, mt4, mt6, and mt8 grew well, but mt1 and mt7 replicated very poorly in PBMCs. Notably, mt3 was not able to establish infection in PBMCs, consisting with its lack of usage of both CCR5 and CXCR4 in the HOS-CD4 cell assay (Fig. 3). In most cases, the degree of infectivity on PBMCs paralleled that on HOS-CD4 cell lines expressing either CCR5 or CXCR4, except for mt7, which replicated efficiently in HOS-CXCR4 cells but poorly in PBMCs (Fig. 4A). mt7 showed delayed replication kinetics in most T-cell lines (Fig. 4C; Table 2).
FIG. 4.
Replication kinetics of chimeric viruses in PBMCs, macrophages, and T-cell lines. Virus stocks used to infect the indicated cell types were generated by transfecting HeLa cells with recombinant plasmid DNAs. The CD8-depleted PBMCs stimulated with PHA (A), macrophages (B), and T-cell lines MT2, H9, M8166, Molt4, and PM1 (C) were infected with each virus stock containing equal amounts of RT activity, and progeny virus production was monitored by RT activity released into the culture medium at the indicated time points. Each plot represents the average of duplicated or triplicated assays.
TABLE 2.
Infectivities of HIV-1 subtype E V3 loop mutant chimeras in various cell types
| Virus | Replicationa
|
||||||
|---|---|---|---|---|---|---|---|
| PBMCs | Macro-phages | T-cell lines
|
|||||
| MT2 | H9 | M8166 | Molt4 | PM1 | |||
| LAI-NH2V3 mutants | |||||||
| mt1 | (+) | − | − | − | − | − | − |
| mt2 | + | − | − | − | − | − | − |
| mt3 | − | − | − | − | − | − | − |
| mt4 | + | − | − | − | − | − | + |
| mt5 | + | − | − | − | (+)d | − | +d |
| mt6 | + | − | − | − | − | − | +d |
| mt7 | (+) | − | +d | (+) | +d | (+)d | +d |
| mt8 | + | − | + | + | + | (+)d | + |
| LAIV3 chimeras | |||||||
| LAIV3 | + | − | + | + | + | + | + |
| NH1V3 | + | − | + | + | + | (+)d | + |
| NH2V3 | + | − | − | − | − | − | +d |
| AD8V3 | + | − | − | − | − | − | + |
| Parental strains | |||||||
| LAI | + | − | + | + | + | + | + |
| AD8 | + | + | − | − | − | − | +d |
| NH1 | + | (+) | + | ND | + | ND | + |
| NH2 | +d | (+) | − | ND | − | ND | + |
Results are qualitatively scored according to the replication kinetics shown in Fig. 4. +, good growth; (+), poor growth; +d and (+)d, delayed replication kinetics; −, no growth; ND, not determined. The growth properties of parental HIV-1NH1 and HIV-1NH2 were assessed in separate experiments.
The chimeric virus LAI-NH1V3, harboring the V3 loop from the HIV-1 subtype E X4 strain, replicated efficiently in all T-cell lines, while LAI-NH2V3, with the V3 loop from the HIV-1 subtype E R5 strain, did not replicate in any of T-cell lines except PM1 (Table 2; Fig. 4C). The PM1 cells were permissive to both X4- and R5-type recombinants, including LAI-NH1V3, LAI-NH2V3, and LAI-AD8V3 as well as parental LAI and AD8, and to broader ranges of HIV-1 subtype E V3 loop chimeras, including mt4 through mt8 (Table 2; Fig. 4C). This finding is consistent with the observation that PM1 cells, a derivative of HUT78, are known to be permissive to certain NSI-type HIV-1 isolates, presumably because of the presence of CCR5 as well as CXCR4 on the cell surface (5, 17). In contrast, other T-cell lines, including MT2, H9, M8166, and Molt4, were permissive only to mt8 and mt7 (Table 2; Fig. 4C). The V3 loop mutant chimeras mt5 and mt6, which utilize CXCR4 in HOS cell infectivity (Fig. 3), showed no or little infectivity in T-cell lines except for PM1 (Table 2; Fig. 4C), indicating that not all CXCR4-utilizing viruses are T tropic (3).
To assess infectivities to primary macrophages, we prepared monocyte-derived macrophages as described previously (18). None of the V3 loop chimeras generated in this study were infective to macrophages (Fig. 4B). Moreover, the chimeric virus LAI-AD8V3, harboring the V3 loop of M-tropic HIV-1 strain AD8 in a genetic background of HIV-1LAI (23), also could not establish an infection on macrophages (Fig. 4B). These results indicate that M tropism could not be conferred by replacement of the V3 loop per se, at least in a genetic background of HIV-1LAI (29, 33).
DISCUSSION
From this study using a pLAI-based chimeric virus with a mutated V3 loop derived from HIV-1 subtype E, we have determined the following structure-function relationships.
First, CXCR4 usage was conferred by any combination of two arginine substitutions at either position 8, 11, or 18 of the V3 loop. This finding is consistent with the previous observation that the overall charge around the tip of the V3 loop appeared to be responsible for viral syncytium-inducing ability, cell tropism, and coreceptor usage (2, 28). This may imply that the V3 region, independent of the backbone, directly interacts with CXCR4 during virus entry. Alternatively, the presence of two or more positively charged amino acids at those critical sites in the V3 loop may alter the conformation of the chimeric gp120 and thereby enable interaction with CXCR4.
The data seemed to support a central role of the overall increase in charge of the V3 loop in determining the interaction with CXCR4. However, the structure of the V3 loop could affect the strength of this interaction, as observed in mt5, where infectivity was fivefold lower than that for mt6 and mt7, although the net charges of the V3 loops of mt5, mt6, and mt7 are the same (Fig. 2 and 3).
The arginine substitution at position 8 appeared to be required for efficient use of CXCR4 (Fig. 3, mt6 through mt8). The residues at position 8 in the HIV-1 subtype E V3 region, which constitute a highly conserved potential N-glycosylation site in NSI variants of HIV-1 subtype E, are uniformly lost in all SI viruses (40). It has been reported that the N-glycan at this site interferes the viral binding of neutralizing antibodies and thereby facilitates viral escape from host immunity (1). Our results suggest that the loss of this potential N-glycosylation site and the acquisition of a basic amino acid at position 8 may provide a structural advantage for efficient interaction of X4 viruses with CXCR4.
Earlier studies of HIV-1 subtype B indicated that basic changes at position 25 in the V3 loop were required for MT2 cell tropism (7) or CXCR4 utilization (30). In contrast, we found that CXCR4 usage by HIV-1 subtype E was conferred by two positively charged amino acid substitutions without changing the negatively charged residue (aspartic acid) at position 25 in the V3 loop. Therefore, the determinants for CXCR4 utilization may differ based on the genetic background of Env proteins.
Among the mutant chimeras constructed in the present study, mt4, mt5, mt6, and mt8 carry a GPGR motif at the tip of the V3 loop, which is typical of HIV-1 subtype B (Fig. 2). Since mt5 grew poorly in the HOS cell assay compared with other mutant chimeras carrying a GPGR motif (Fig. 3), the GPGR motif does not necessarily make the virus more robust like subtype B viruses.
Second, CCR5 usage was abrogated by the arginine substitution at position 11 (Fig. 3, mt3, mt5, mt7, and mt8). Residues at positions 11 and 25 in the V3 loop have been implicated in determining the usage for CCR5 or other coreceptors, since the most viral isolates of various subtypes which exclusively use CCR5 had uncharged amino acids at position 11 and negatively charged amino acids at position 25, in addition to the consensus GPG motif at the tip of the V3 loop (39). This finding is consistent with our observations (Fig. 3, mt1, mt2, mt4, and mt6). However, the site-specific basic substitution at position 11 (from serine to arginine) in an HIV-1 subtype B R5 strain did not alter CCR5 usage (39). Taken together, the results indicate that position 11 of the V3 loop may be critical for modulating viral CCR5 usage in a context-specific manner. Furthermore, this study demonstrates that the structural balance is very delicate, being restrained in a very narrow range of genetic variation for efficient transmission of infection even though the virus is rapidly changing.
Third, T tropism was conferred only when positions 8 and 11 were changed to arginine (Table 2, mt7 and mt8). It has been reported that the introduction of positively charged amino acid residues at position 11 and/or 25 was required for acquisition of the SI phenotype in the HIV-1 subtype B V3 loop, where arginine is present at position 18 (7). Thus, the T tropism of HIV-1 is conferred by positively charged amino acids in the V3 loop, but the pattern of changes responsible for T tropism varies depending on the viral genetic background.
Fourth, replacement of the V3 loop with that of M-tropic or R5 virus and any of the V3 loop mutations could not confer M tropism to LAI (Fig. 4B, LAI-AD8V3, LAI-NH2V3, and LAI-NH2V3mt1-8). This finding is consistent with the observation that the dualtropic 89.6 env V3 domain conferred the ability to use CCR5 for viral entry but not the ability to establish productive infection in macrophages in the genetic background of HXB2 (29). Similarly, the V1 and V2 envelope sequences of the M-tropic Ba-L strain were shown to enhance the ability to spread in macrophage cultures (33). Taken together with our observations on HIV-1 subtype E, these data show that sequences other than the env V3 loop are involved in productive macrophage infection (2).
Finally, some chimeric viruses utilized CXCR4 to infect HOS-CD4 cells but showed no or little infectivity in T-cell lines (Fig. 3 and Table 2, mt5 and mt6). However, our recent study with patient-derived V3 loop sequences of HIV-1 subtype E revealed that CXCR4 usage perfectly correlated with MT2 cell tropism (27a). Since the site-directed mutations introduced in this study created nonnatural sequences, it is possible that the mutations which we introduced could render Env glycoproteins defective in certain properties such as affinity for coreceptors. Moreover, the infectivity of HIV-1 is known to be highly dependent on cell surface concentrations of CCR5 and CD4 (38). Therefore, the discrepancy between infectivity in the HOS-CD4 cell assay and cellular tropism may be explained in part by the possibility that HOS-CD4 cells are excessively permissive to viral infections (e.g., because of overexpression of the chemokine receptors). Alternatively, the cell-type-specific differences in certain postentry mechanisms governed by the V3 loop sequences might influence the infection and replication processes.
In the present study, we have demonstrated that the naturally occurring basic amino acid substitutions found at specific residues in the V3 loop of HIV-1 subtype E in fact influence coreceptor utilization and cellular tropism. Since infectious molecular clones of HIV-1 subtype E are presently not available, additional studies are required for a better understanding of coreceptor utilization and cellular tropism of HIV-1 subtype E, one of the major strains causing the current epidemic of HIV-1 infection throughout southeast Asia (35, 36).
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank Kiyoko Akagawa for preparation of monocyte-derived primary macrophage cultures and Keith Peden for providing pLAI. We also thank Roger Pomerantz and Yoshiyuki Nagai for critical reading of the manuscript and Teiichiro Shiino, Tatsuo Shioda, and Hiroshi Yoshikura for stimulating discussions.
This study was supported by grants from the Ministry of Health and Welfare, Ministry of Education, Science and Culture, and the Science Technology Agency of Japan. Kayoko Kato is a recipient of research resident fellowship from the Japanese Foundation for AIDS Prevention.
REFERENCES
- 1.Back N K, Smit L, De Jong J J, Keulen W, Schutten M, Goudsmit J, Tersmette M. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology. 1994;199:431–438. doi: 10.1006/viro.1994.1141. [DOI] [PubMed] [Google Scholar]
- 2.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho M, Lee M, Carney M, Berson J, Doms R, Martin M. Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4. J Virol. 1998;72:2509–2515. doi: 10.1128/jvi.72.3.2509-2515.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 5.Cocchi F, DeVico A, Garzino-Demo A, Cara A, Gallo R, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 6.Connor R I, Ho D D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Jong J-J, de Ronde A, Keulen W, Tersmette M, Goudsmit J. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitution. J Virol. 1992;66:6777–6780. doi: 10.1128/jvi.66.11.6777-6780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong J-J, Goudsmit J, Keulen W, Klaver B, Krone W, Tersmette M, de Ronde A. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992;66:757–765. doi: 10.1128/jvi.66.2.757-765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng H, Liu R, Wilfried E, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R, Hill M, Davis C, Peiper S, Schall T, Littman D, Landau N. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 10.Fouchier R A M, Groenink M, Kootstra N A, Tersmette M, Huisman H G, Miedema F, Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992;66:3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goudsmit J. The role of viral diversity in HIV pathogenesis. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;10(Suppl. 1):S15–S19. [PubMed] [Google Scholar]
- 12.Groenink M, Fouchier R A, Broersen S, Baker C H, Koot M, van’t Wout A B, Huisman H G, Miedema F, Tersmette M, Schuitemaker H. Relation of phenotype evolution of HIV-1 to envelope V2 configuration [see comments] Science. 1993;260:1513–1516. doi: 10.1126/science.8502996. [DOI] [PubMed] [Google Scholar]
- 13.Ho S, Hunt H, Horton R, Pullen J, Pease L. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 14.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 15.Koito A, Stamatatos L, Cheng-Mayer C. Small amino acid sequence changes within the V2 domain can affect the function of a T-cell line-tropic human immunodeficiency virus type 1 envelope gp120. Virology. 1995;206:878–884. doi: 10.1006/viro.1995.1010. [DOI] [PubMed] [Google Scholar]
- 16.Koot M, van’t Wout A B, Kootstra N A, de Goede R E, Tersmette M, Schuitemaker H. Relation between changes in cellular load, evolution of viral phenotype, and the clonal composition of virus populations in the course of human immunodeficiency virus type 1 infection. J Infect Dis. 1996;173:349–354. doi: 10.1093/infdis/173.2.349. [DOI] [PubMed] [Google Scholar]
- 17.Lusso P, Cocchi F, Balotta C, Markham P D, Louie A, Farci P, Pal R, Gallo R C, Reitz M S., Jr Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1. J Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda S, Akagawa K, Honda M, Yokota Y, Takebe Y, Takemori T. Suppression of HIV replication in human monocyte-derived macrophages induced by granulocyte/macrophage colony-stimulating factor. AIDS Res Hum Retroviruses. 1995;11:1031–1038. doi: 10.1089/aid.1995.11.1031. [DOI] [PubMed] [Google Scholar]
- 19.Milich L, Margolin B, Swanstrom R. Patterns of amino acid variability in NSI-like and SI-like V3 sequences and a linked change in the CD4-binding domain of the HIV-1 Env protein. Virology. 1997;239:108–118. doi: 10.1006/viro.1997.8821. [DOI] [PubMed] [Google Scholar]
- 20.Milich L, Margolin B, Swanstrom R. V3 loop of the human immunodeficiency virus type 1 Env protein: interpreting sequence variability. J Virol. 1993;67:5623–5634. doi: 10.1128/jvi.67.9.5623-5634.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peden K, Emerman M, Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology. 1991;185:661–672. doi: 10.1016/0042-6822(91)90537-l. [DOI] [PubMed] [Google Scholar]
- 22.Roos M T L, Lange J M A, Goede R E Y, Coutinho R A, Schellekens P T A, Miedema F, Tersmette M. Viral phenotype and immune response in primary immunodeficiency virus type 1 infection. J Infect Dis. 1992;165:427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- 23.Sato H, Kato K, Takebe Y. Functional complementation of the envelope hypervariable V3 loop of human immunodeficiency virus type 1 subtype B by subtype E V3 loop. Virology. 1999;257:491–501. doi: 10.1006/viro.1999.9670. [DOI] [PubMed] [Google Scholar]
- 24.Sato H, Shiino T, Kodaka N, Taniguchi K, Tomita Y, Kato K, Miyakuni T, Takebe Y. Evolution and biological characterization of human immunodeficiency virus type 1 subtype E gp120 V3 sequences following horizontal and vertical virus transmission in a single family. J Virol. 1999;73:3551–3559. doi: 10.1128/jvi.73.5.3551-3559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato H, Taniguchi K, Tomita Y, Shiino T, Miyakuni T, Takebe Y. Evidence for the selective pressure to reduce heterogeneity of HIV-1 subtype E envelope V3-loop sequences in an intrafamilial infection case. AIDS. 1997;11:396–397. [PubMed] [Google Scholar]
- 26.Schuitemaker H, Koot M, Kootsra N A, Wouter-Dercksen M, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuitemaker H, Kootstra N A, de Goede R E, de Wolf F, Miedema F, Tersmette M. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991;65:356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Shiino, T., et al. Unpublished data.
- 28.Shioda T, Levy J A, Cheng-Mayer C. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell-line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth R J, Yi Y, Singh A, Collman R G. Determinants of entry cofactor utilization and tropism in a dualtropic human immunodeficiency virus type 1 primary isolate. J Virol. 1998;72:4478–4484. doi: 10.1128/jvi.72.5.4478-4484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speck R, Wehrly K, Platt E, Atchison R, Charo I, Kabat D, Chesebro B, Goldsmith M. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tersmette M, de Goede R E Y, Al B J M, Winkel I N, Gruters R A, Cuypers H T, Huisman H G, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tersmette M, Lange J M A, de Goede R E, de Wolf F, Eeftink-Schattenkerk J K, Schellenkens P T, Coutinho R A, Huisman J G, Goudsmit J, Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;i:983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 33.Toohey K, Wehrly K, Nishio J, Perryman S, Chesebro B. Human immunodeficiency virus envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology. 1995;213:70–79. doi: 10.1006/viro.1995.1547. [DOI] [PubMed] [Google Scholar]
- 34.Valentin A, Albert J, Fenyo E M, Asjo B. Dual tropism for macrophages and lymphocytes is a common feature of primary human immunodeficiency virus type 1 and 2 isolates. J Virol. 1994;68:6684–6689. doi: 10.1128/jvi.68.10.6684-6689.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weniger B G, Brown T. The march of AIDS through Asia. N Engl J Med. 1996;335:343–345. doi: 10.1056/NEJM199608013350510. . (Editorial; comment.) [DOI] [PubMed] [Google Scholar]
- 36.Weniger B G, Takebe Y, Ou C-Y, Yamazaki S. The molecular epidemiology of HIV in Asia. AIDS. 1994;8(Suppl. 2):S13–S28. [PubMed] [Google Scholar]
- 37.Willey R L, Smith D H, Lasky L A, Theodore T S, Earl P L, Moss B, Capon D J, Martin M A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, LaRosa G, Kassam N, Gordon C, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore J, Mackay C. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao L, Owen S, Goldman I, Lal A, deJong J, Goudsmit J, Lal R. CCR5 coreceptor usage of non-syncytium-inducing primary HIV-1 is independent of phylogenetically distinct global HIV-1 isolates: delineation of consensus motif in the V3 domain that predicts CCR-5 usage. Virology. 1998;240:83–92. doi: 10.1006/viro.1997.8924. [DOI] [PubMed] [Google Scholar]
- 40.Yu X-F, Wang Z, Beyrer C, Celentano D D, Khamboonruang C, Allen E, Nelson K. Phenotypic and genotypic characteristics of human immunodeficiency virus type 1 from patients with AIDS in northern Thailand. J Virol. 1995;69:4649–4655. doi: 10.1128/jvi.69.8.4649-4655.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]