Abstract
The incidence of postoperative pancreatic fistula is influenced by the effectiveness of the pancreaticojejunostomy, and the most suitable pancreaticojejunostomy for pancreaticoduodenectomy remains uncertain. Since grade A postoperative pancreatic fistula is no longer considered a true fistula, the purpose of this meta-analysis was to compare the effectiveness of duct-to-mucosa anastomosis and invagination anastomosis in reducing the incidence of grade B/C postoperative pancreatic fistula. The meta-analysis was conducted using software Review Manager 5.3, and the fixed-effect model was employed for pooled statistic calculations. The Cochrane Collaboration Risk of Bias Tool was utilized for quality assessment. Ten randomized controlled trials from Embase, Web of Science, MEDLINE, and the Cochrane Library (1990.01–2022.10) including 1471 patients, met the inclusion criteria. This meta-analysis has been registered on PROSPERO with the registration number CRD42023491673. The incidence of grade B/C fistula was significantly lower in the invagination group (7.7 %) compared to the duct-to-mucosa group (12.8 %, mostly Cattell manner)(RR = 1.65, 95%CI: 1.14–2.39, P = 0.008; heterogeneity: P = 0.008, I2 = 68 %),heterogeneity among the results was addressed through sensitivity analysis. In patients with a soft pancreas, the incidence of grade B/C fistula was significantly lower in those who underwent invagination anastomosis (10 %) compared to those who underwent duct-to-mucosa anastomosis (41.9 %)(RR = 4.19, 95%CI: 1.33–13.25, P = 0.01).No significant differences were observed in terms of the occurrence of grade B/C fistula in firm pancreas, postoperative mortality, other major postoperative complications, anastomosis time, and postoperative bile leak. Therefore, we concluded that invagination anastomosis is significantly superior to duct-to-mucosa anastomosis in reducing the incidence of grade B/C fistula, especially in patients with a soft pancreas.
Keywords: Duct-to-mucosa, Invagination, Pancreaticojejunostomy, Postoperative pancreatic fistula, Pancreaticoduodenectomy
Highlights
-
•
Invagination anastomosis is considerably more effective than duct-to-mucosa anastomosis in decreasing the occurrence of grade B/C (clinically significant) postoperative pancreatic fistula after pancreaticoduodenectomy.
-
•
Invagination anastomosis is highly recommended for patients with a soft pancreas following pancreaticoduodenectomy.
-
•
In patients with a firm pancreas, both invagination anastomosis and duct-to-mucosa anastomosis can be performed following pancreaticoduodenectomy, as there is no significant difference in the incidence of grade B/C postoperative pancreatic fistula between these two methods.
-
•
Although invagination anastomosis is less complex than duct-to-mucosa anastomosis, it does not significantly reduce the anastomosis time in open surgery.
1. Introduction
Pancreaticoduodenectomy is a viable surgical procedure for suitable patients with a resectable tumor in the pancreatic head or periampullary region, as well as certain benign diseases presenting with distinct clinical manifestations, such as mass-chronic pancreatitis of the pancreatic head. Nevertheless, owing to the extensive resection and the utilization of multiple methods for anastomosis in alimentary tract reconstruction, pancreaticoduodenectomy often results in a significant occurrence of postoperative morbidity, which can reach up to 50 % [1,2].
Currently, two reconstruction methods are commonly used for the pancreatic stump and alimentary tract: pancreaticogastrostomy and pancreaticojejunostomy. Among these, pancreaticojejunostomy is preferred because it has a lower incidence of post-pancreatectomy hemorrhage and better long-term outcomes in terms of pancreatic exocrine function and patient nutrition [3,4]. The use of pancreaticogastrostomy has become less common, pancreaticojejunostomy now being the primary method for pancreato-digestive tract reconstruction [5,6].
Postoperative pancreatic fistula (POPF) is a severe complication that can occur after pancreaticoduodenectomy and has the potential to be life-threatening due to subsequent abdominal hemorrhage or sepsis. POPF usually arises as a result of dehiscence in the pancreatic-intestinal anastomosis [7], making it imperative to effectively manage the pancreatic remnant following pancreaticoduodenectomy. The effectiveness of different methods of pancreaticojejunostomy in preventing POPF has been extensively studied, with one of the key areas of research being the need for duct-to-mucosa anastomosis in pancreaticojejunostomy [8,9].
Varco (1945) was the pioneer in introducing the concept of duct-to-mucosa anastomosis, as described in his groundbreaking case report [10]. This technique ensures that the main pancreatic duct directly drains into the intestine, thereby maintaining the continuity of the pancreatic duct and jejunal mucosa. This facilitates tissue healing and is consistent with physiological processes. The invagination anastomosis method is a straightforward and easily executed procedure. It involves fully inserting the pancreatic stump into the lumen of the jejunum, thereby enabling drainage of both the cut surface of the pancreatic stump and the main pancreatic duct [11]. Both methods have their advantages in pancreatic stump drainage. However, it remains to be determined which technique has a lower incidence of POPF.
Several meta-analyses have been conducted to compare the benefits of duct-to-mucosa anastomosis and invagination anastomosis in reducing the incidence of POPF. However, no definitive conclusions have been reached. The latest meta-analysis conducted by Hai et al. was unable to ascertain the superior approach for reducing the incidence of POPF [12]. Cao et al. conducted a meta-analysis that included seven randomized controlled trials (RCTs) and concluded that invagination anastomosis is more advantageous than duct-to-mucosa anastomosis in reducing the incidence of grade B/C POPF after pancreaticoduodenectomy [13]. However, while the authors claimed to have used the newest International Study Group Definition and Grading of POPF (ISGPF Definition) in 2016 for comparison [14], all the included studies in their articles still adhered to the ISGPF Definition of 2005 [15]. Therefore, this indiscriminate direct application approach appears to lack rationality. Zhang et al. and Sun et al. found no significant difference in the prevention of overall and grade B/C POPF between duct-to-mucosa anastomosis and invagination anastomosis [16,17]. However, Hua et al. concluded that invagination anastomosis was not superior to duct-to-mucosa anastomosis in terms of overall POPF but appeared to reduce grade B/C POPF [18]. As a result, there is still ongoing controversy regarding which pancreaticojejunostomy technique is more advantageous in reducing the incidence of POPF after pancreaticoduodenectomy.
Since grade A POPF is no longer considered a true pancreatic fistula according to the ISGPF Definition 2016, we conducted a meta-analysis based on 10 RCTs to investigate the safety and efficacy of duct-to-mucosa anastomosis and invagination anastomosis in reducing the incidence of grade B/C POPF (clinically relevant POPF). Additionally, we aimed to determine the superiority in terms of time efficiency between the two anastomosis methods. Subgroup analysis was conducted to investigate the effect of the two anastomosis methods on the incidence of grade B/C POPF in patients with different pancreatic textures, furthermore, the impact of the two methods on grade B/C POPF was examined when pancreaticojejunostomy was not accompanied by either internal or external stent drainage.
2. Materials and methods
2.1. Methods
This meta-analysis adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 statement: An updated guideline for reporting systematic reviews [19]. This meta-analysis has been registered on PROSPERO, the registration number is CRD42023491673, and the registration title is ‘Is Duct-to-Mucosa Pancreaticojejunostomy Necessary after Pancreaticoduodenectomy: A meta-analysis of randomized controlled trials’.
2.2. Data sources and search Strategies
The meta-analysis utilized several electronic databases as retrieval tools: Embase, Web of Science, MEDLINE (Pubmed and Ovid), and the Cochrane Library (CENTRAL). A comprehensive literature search was conducted to identify relevant studies comparing duct-to-mucosa pancreaticojejunostomy with alternative types of pancreaticojejunostomy following pancreaticoduodenectomy from January 1990 to October 2022. The keywords employed for retrieval included 'pancreaticoduodenectomy', 'duct-to-mucosa', 'invagination', 'anastomosis', 'pancreaticojejunostomy', and 'pancreatic fistula'. The search strategy was established as follows: (“pancreaticoduodenectomy” OR “pancreatoduodenectomy”) AND “duct-to-mucosa” AND (“invagination” OR “invaginate” OR “invaginating” OR “invaginated”) AND (“anastomosis” OR “pancreaticojejunostomy”) AND “pancreatic fistula”. Relevant studies from reference lists and recommended studies from databases were also manually retrieved.
2.3. Inclusion and exclusion criteria
During the initial selection process and full-text assessment stages, two independent reviewers (X.F. Hao and Y. Li) evaluated the eligibility of the retrieved studies. In order to ensure a robust research direction and minimize confounding bias in the current meta-analysis, the studies were required to meet the following criteria.
-
1)
Only RCT literature was considered for inclusion;
-
2)
Patients (age≥18y) with a diagnosis of malignant or benign pancreatic, periampullary, duodenal, or billiary disease that required surgical intervention;
-
3)
Only patients who had undergone pancreaticoduodenectomy were included. Pancreaticoduodenectomy encompassed standard pancreaticoduodenectomy, pyloric-preserving/resecting pancreaticoduodenectomy, or subtotal stomach-preserving pancreaticoduodenectomy;
-
4)
The reconstruction method for the pancreatic stump and alimentary tract was limited to pancreaticojejunostomy. Studies that reported pancreaticogastrostomy were excluded;
-
5)
Studies comparing duct-to-mucosa anastomosis with non-duct-to-mucosa anastomosis, specifically invagination anastomosis, were included;
-
6)
There were no language restrictions for the included studies;
-
7)
The selected studies must have reported the incidence of POPF after pancreaticoduodenectomy at least.
2.4. Outcomes of interest
Based on the primary objective of this meta-analysis, the incidence of grade B/C postoperative POPF was identified as the primary outcome. The secondary outcomes were established to assess the potential superiority of duct-to-mucosa anastomosis in terms of safety and operative efficiency.
2.4.1. Primary outcomes
-
1)
The incidence of grade B/C POPF (clinically relevant POPF), as assessed by the ISGPF Definition in 2005 or 2016, was examined;
2.4.2. Secondary outcomes
-
1)
Postoperative mortality;
-
2)
The incidence of other major postoperative complications (excluding POPF, with a Clavien-Dindo grade Ⅲ or higher);
-
3)
Incidence of postoperative bile leak;
-
4)
The duration of the anastomosis procedure for pancreaticojejunostomy.
2.5. Study selection
The study selection process followed the PICOS principle (Participants, Interventions, Comparisons, Outcomes, Study types). Two review authors, X.F. Hao and Y. Li, independently screened the titles and abstracts of the preliminarily retrieved studies during the initial selection process. In this process, non-RCTs and irrelevant studies were excluded by applying the predetermined inclusion and exclusion criteria. This step was taken to identify studies that potentially met the eligibility criteria. During the full-text assessment, both authors thoroughly read the full text of the potentially eligible studies and evaluated them based on the inclusion and exclusion criteria. This process determined which studies were suitable for quantitative synthesis (meta-analysis). Any disagreements in the study selection process were resolved through discussion among the work group.
2.6. Data extraction
The relevant information regarding the study characteristics and outcomes from the included studies was independently extracted by the two review authors, X.F. Hao and Y. Li.
-
·
Study characteristics: study design, publication date, country, sample size, randomization scheme;
-
·
Participants: number, sex, age, diagnosis;
-
·
Types of interventions: operation methods, anastomosis methods;
-
·
Outcome measures: primary and secondary outcomes.
The accuracy and correctness of the extracted data were subsequently verified by the authors, X.F. Hao and Y. Li. In cases of disagreement, resolutions were reached through group discussions.
2.7. Study quality assessment
The quality assessment of the included RCTs was conducted using the Cochrane Collaboration Risk of Bias Tool [20], which primarily emphasizes the analysis of methodological quality and risk of bias in the included studies. The assessment items were as follows.
-
·
Selection bias: random sequence generation and allocation concealment;
-
·
Performance bias: blinding of participants and personnel;
-
·
Detection bias: blinding of outcome assessment;
-
·
Attrition bias: incomplete outcome data;
-
·
Reporting bias: selective reporting;
-
·
Other potential sources of bias.
2.8. Statistical analysis
The present meta-analysis was conducted using software Review Manager 5.3(RevMan 5.3) obtained from Cochrane Collaboration. Risk ratios (RRs) were employed to calculate dichotomous variables, while weighted mean differences (WMDs) were used for continuous variables. The heterogeneity was evaluated using the Chi [2] test. If no significant heterogeneity was observed in the results of the included studies (P > 0.10), a fixed-effect model (Mantel-Haenszel method) was selected for pooled statistic calculations. However, if the P value of heterogeneity was≤0.10, it suggested the presence of substantial heterogeneity in the results of the included studies, which prompted either the use of a random effects model for pooled statistic calculations or the performance of a subgroup analysis. For the measuring heterogeneity, if I2 was ≤50 %, the heterogeneity was considered acceptable. Finally, a funnel plot was utilized for assessing publication bias.
3. Results
3.1. Included studies and study characteristics
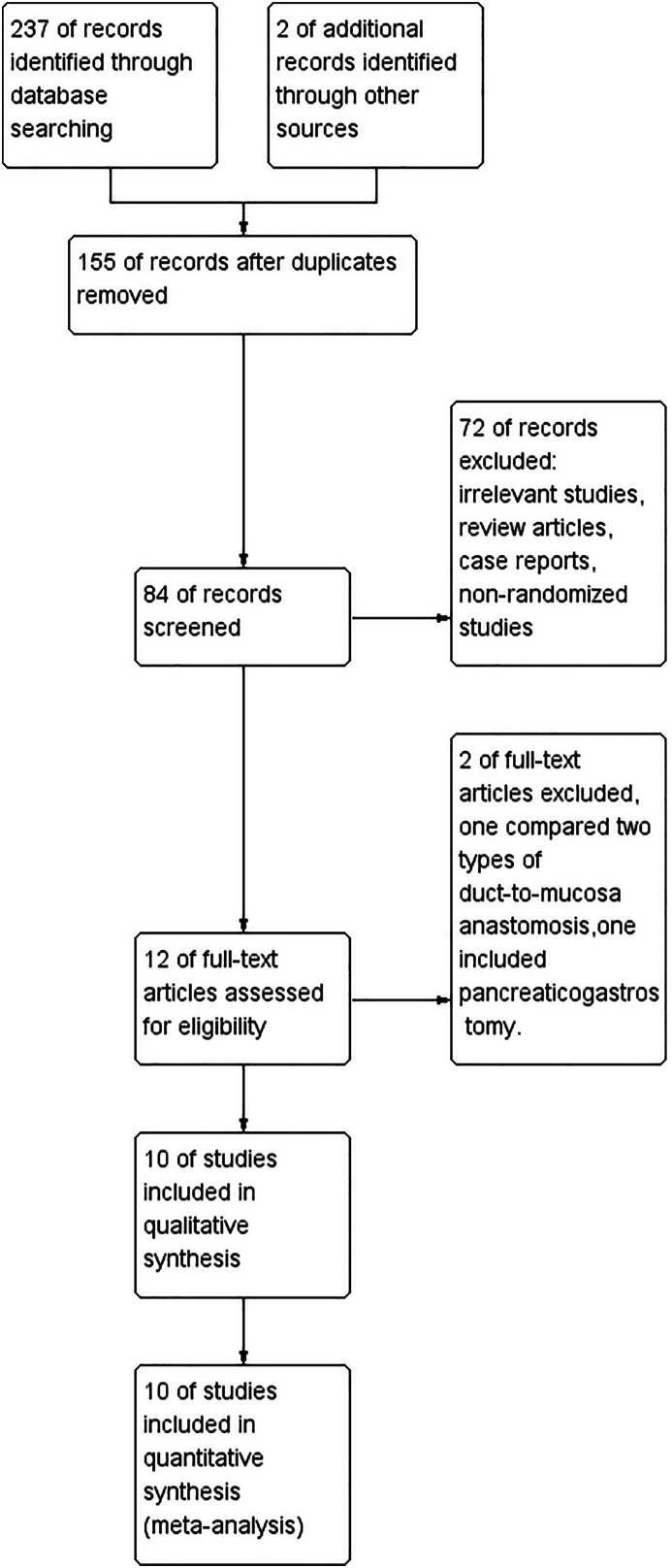
A total of 239 original records were initially retrieved through database searching (n = 237) and other sources (n = 2). After removing duplicates, a total of 84 records were subjected to the initial selection process. Subsequently, 72 records were excluded due to their classification as irrelevant studies, review articles, case reports, or non-randomized studies. The remaining 12 records underwent full-text assessment, resulting in the exclusion of two records. One record was excluded due to its comparison of different types of duct-to-mucosa anastomosis, while the other record involved pancreaticogastrostomy. Ultimately, 10 RCTs were considered eligible for inclusion in this meta-analysis [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30]]. All assessments were conducted based on the predefined inclusion and exclusion criteria outlined in the protocol. A flow diagram illustrating the study selection process is presented in Fig. 1.
Fig. 1.
Flow diagram of study selection.
A total of 1471 patients, comprising 853 men and 618 women, were included from the 10 RCTs. Among these patients, 732 were in the duct-to-mucosa pancreaticojejunostomy group, while 739 were in the invagination pancreaticojejunostomy group. Out of the included RCTs, seven used the ISGPF Definition 2005 to define POPF [[24], [25], [26], [27], [28], [29], [30]], and six of them also assessed the severity of POPF based on this definition [24,[26], [27], [28], [29], [30]]. The characteristics of the included studies are summarized in Table 1, while Table 2 presents the operation methods, description of pancreaticojejunostomy methods, and other relevant procedures.
Table 1.
Characteristics of the included studies.
| Study | Date | Country | Group | Patient | Sex (M/F) | Age (range or SD) | Pancreatic Texture (S/F) | Primary Outcome | Definition |
|---|---|---|---|---|---|---|---|---|---|
| Chou et al. [21] | 1996 | China | D-M | 47 | 23/24 | 60 ± 11 | NM | NM | Non-ISGPF |
| Invagination | 46 | 27/19 | 56 ± 12 | ||||||
| Bassi et al. [22] | 2003 | Italy | D-M | 72 | 40/32 | 62 ± 10 | 72/0 | NM | Non-ISGPF |
| Invagination | 72 | 46/26 | 61 ± 12 | 72/0 | |||||
| Langrehr et al. [23] | 2005 | Germany | D-M | 56 | 34/22 | 59 (28–86) | NM | NM | Non-ISGPF |
| Invagination | 57 | 32/25 | 60 (35–79) | ||||||
| Beger et al. [24] | 2009 | America | D-M | 97 | 45/52 | 68 (32–84) | 50/47 | Incidence of POPF | ISGPF 2005 |
| Invagination | 100 | 54/46 | 68 (41–90) | 51/49 | |||||
| Han et al. [25] | 2009 | China | D-M | 32 | 20/12 | 59 ± 11 | 32/0 | Incidence of POPF | ISGPF 2005 |
| Invagination | 32 | 24/8 | 56 ± 11 | 32/0 | |||||
| Nakeeb et al. [26] | 2015 | Egypt | D-M | 53 | 34/19 | 54 (12–73) | 25/28 | Incidence of POPF | ISGPF 2005 |
| Invagination | 54 | 33/21 | 54 (20–75) | 27/27 | |||||
| Xu et al. [27] | 2015 | China | D-M | 153 | 82/71 | 58.17 ± 11.72 | 95/58 | Incidence of POPF | ISGPF 2005 |
| Invagination | 155 | 84/71 | 58.19 ± 10.7 | 104/51 | |||||
| Bai et al. [28] | 2016 | China | D-M | 64 | 38/26 | 62 (32–79) | 36/28 | Incidence of POPF | ISGPF 2005 |
| Invagination | 68 | 39/29 | 64 (21–78) | 44/24 | |||||
| Senda et al. [29] | 2018 | Japan | D-M | 61 | 36/25 | 66(36–84) | 31/30 | Grade B/C POPF | ISGPF 2005 |
| Invagination | 59 | 36/23 | 68(22–81) | 30/29 | |||||
| Singh et al. [30] | 2018 | India | D-M | 97 | 63/34 | 53.4 (12.1) | 42/55 | Incidence of POPF | ISGPF 2005 |
| Dunking | 96 | 63/33 | 51.5 (14.2) | 48/48 |
D-M, duct-to-mucosa; POPF, postoperative pancreatic fistula; ISGPF, International Study Group definition and grading of postoperative pancreatic fistula; NM, not mentioned; S, soft; F, firm; M, male; F, female; SD, standard deviation.
Table 2.
Descriptions of operation methods and perioperative measures.
| Study | Group | Operation | Anastomosis Techniques | Pancreatic Duct Stent | Somatostatin Analogues |
|---|---|---|---|---|---|
| Chou et al. [21] | D-M | CPD,PPPD | End-to-side manner with 2 layers suture. | External drainage for D-M | NM |
| Invagination | End-to side manner with 1 layer suture: Pan-capsule to jejunum. | ||||
| Bassi et al. [22] | D-M | PPPD mostly | End-to-side manner with 2 layers suture, similar to the Cattell manner. | Depend on the surgeon | Yes |
| Invagination | End-to-side manner with 1 layer suture. | ||||
| Langrehr et al. [23] | D-M | CPD,PPPD | End-to-side manner with 2 layers suture, the Cattell manner. | External drainage | Most patients |
| Invagination | The pancreatic remnant totally enclosed by the jejunal loop, sutures brought through the pancreatic remnant. | ||||
| Beger et al. [24] | D-M | CPD | End-to-side manner with 2 layers suture, similar to the Cattell manner. | No | No |
| Invagination | End-to-side manner with 2 layers suture: Full thickness jejunum to pan-remnant for the inner; seromuscular layer to pan-capsule for the outer. | ||||
| Han et al. [25] | D-M | CPD | End-to-side manner with 2 layers suture. | Internal or external drainage | No |
| Invagination | End-to-side manner in 20 cases, end-to-end manner in 12 cases. | ||||
| Nakeeb et al. [26] | D-M | PPPD mostly | End-to-side manner with 2 layers suture, similar to the Cattell manner. | No | NM |
| Invagination | End-to-side manner with 2 layers suture: Jejunum mucosa to pan-parenchyma for the inner; jejunum serosa to pan-capsule for the outer. | ||||
| Xu et al. [27] | D-M | CPD,PPPD | End-to-side manner with 2 layers suture | No | Yes |
| Invagination | End-to-side with 2 layers suture: Full thickness jejunum to “periductal” parenchyma for the inner; seromuscular layer to pan-capsule for the outer. | ||||
| Bai et al. [28] | D-M | PD | End-to-side manner with 2 layers suture, similar to the Cattell manner. | For MPD<5 mm | For grade B/C POPF |
| Invagination | End-to-side manner with 2 layers suture: Full thickness jejunum to pan-remnant for the inner; seromuscular layer to pan-capsule for the outer. | ||||
| Senda et al. [29] | D-M | SSPPD | End-to-side manner with 2 layers suture, according to the Kakita manner. | External drainage | No |
| Invagination | End-to-side manner with 2 layers suture: Full thickness jejunum to pan-remnant for the inner. | ||||
| Singh et al. [30] | D-M | CPD,PPPD, PRPD |
End-to-side manner with 2 layers suture, similar to the Cattell manner. | Depend on the surgeon | Depend on the surgeon |
| Dunking | End-to-end manner with continuous non-absorbable suture. |
D-M, duct-to-mucosa; POPF, postoperative pancreatic fistula; CPD, classical pancreaticoduodenectomy; PPPD, pylorus-preserving pancreaticoduodenectomy; PRPD, pylorus-resecting pancreaticoduodenectomy; SSPPD, subtotal stomach-preserving pancreaticoduodenectomy; MPD, main pancreatic duct; NM, not mentioned; pan-, pancreatic.
3.2. Quality assessment of the included studies
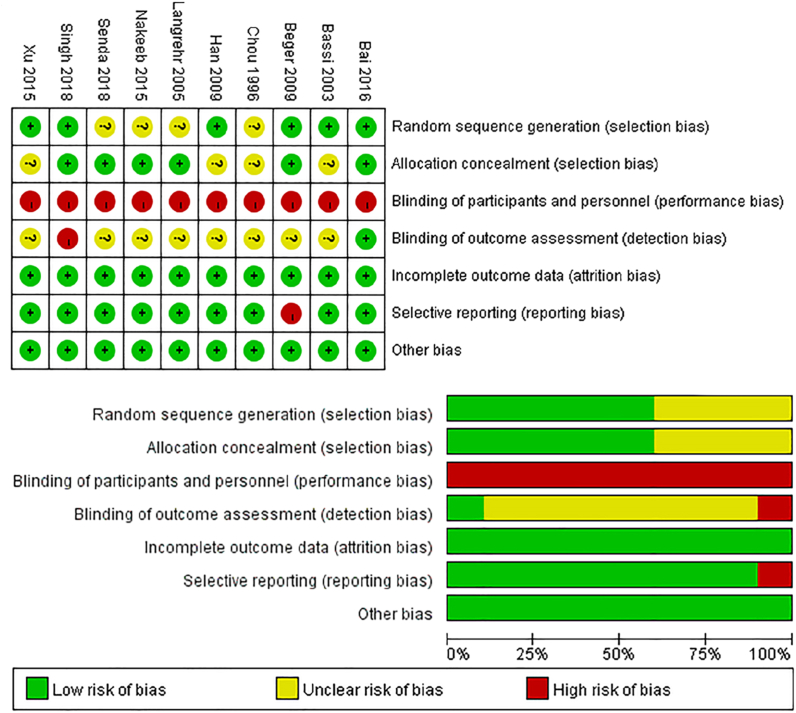
We employed the Cochrane Collaboration Risk of Bias Tool to evaluate the quality of the RCTs included in our study. Detailed descriptions of 'random sequence generation' were not provided in four studies [21,23,26,29], hence, we assessed these studies as having an 'unclear risk of bias' in this item. Four studies did not provide information regarding the 'allocation concealment' and were deemed to have an “unclear risk of bias” in relation to this aspect [21,22,25,27]. Due to the inability of the surgeons to be blinded during the operations, all the studies included in the analysis were deemed to have a “high risk of bias” in terms of ‘blinding of participants and personnel’. In one study, which was conducted as an open-label trial, the 'blinding of outcome assessment' item was deemed to have a “high risk of bias” [30]. However, in another study, an appropriate method was employed to assess the outcome, resulting in a judgment of “low risk of bias” [28]. The remaining studies lacked sufficient information on 'blinding of outcome assessment', leading to an “unclear risk of bias” judgment. All the included studies were assessed to have a “low risk of bias” in terms of ‘incomplete outcome data’. One study did not report on specific types of complications that were pre-determined in the study protocol [24]; therefore, it was deemed to have a “high risk of bias” in the 'selective reporting' item. There were no other sources of bias. The authors' assessment of each risk of bias item for every included study is presented in Fig. 2.
Fig. 2.
The authors' assessment of the risk of bias for all the included studies and the risk of bias graph.
3.3. Primary outcome
3.3.1. Grade B/C POPF (clinically relevant POPF)
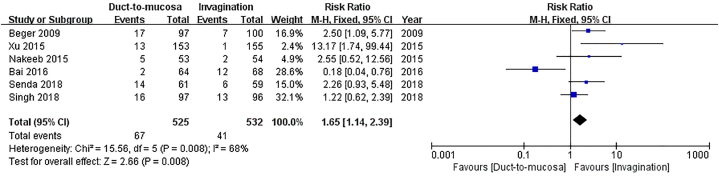
Six studies, involving a total of 1057 patients, reported the occurrence rate of grade B/C POPF according to the ISGPF Definition 2005 [24,[26], [27], [28], [29], [30]]. Although Han et al. also utilized ISGPF Definition 2005 to define POPF [25], the grading criteria for POPF were different, thus it was excluded from the pooled analysis. A total of 67 patients (12.8 %) in the duct-to-mucosa group and 41 patients (7.7 %) in the invagination group experienced grade B/C POPF. The invagination group had a significantly lower incidence of grade B/C POPF compared to the duct-to-mucosa group (RR = 1.65, 95%CI: 1.14–2.39, P = 0.008; substantial heterogeneity existed: P = 0.008, I2 = 68 %) Fig. 3.
Fig. 3.
Comparison of incidence of grade B/C postoperative pancreatic fistula (POPF). The invagination group exhibited a significantly lower incidence of grade B/C POPF in comparison to the duct-to-mucosa group.
3.4. Secondary outcomes
3.4.1. Postoperative morality
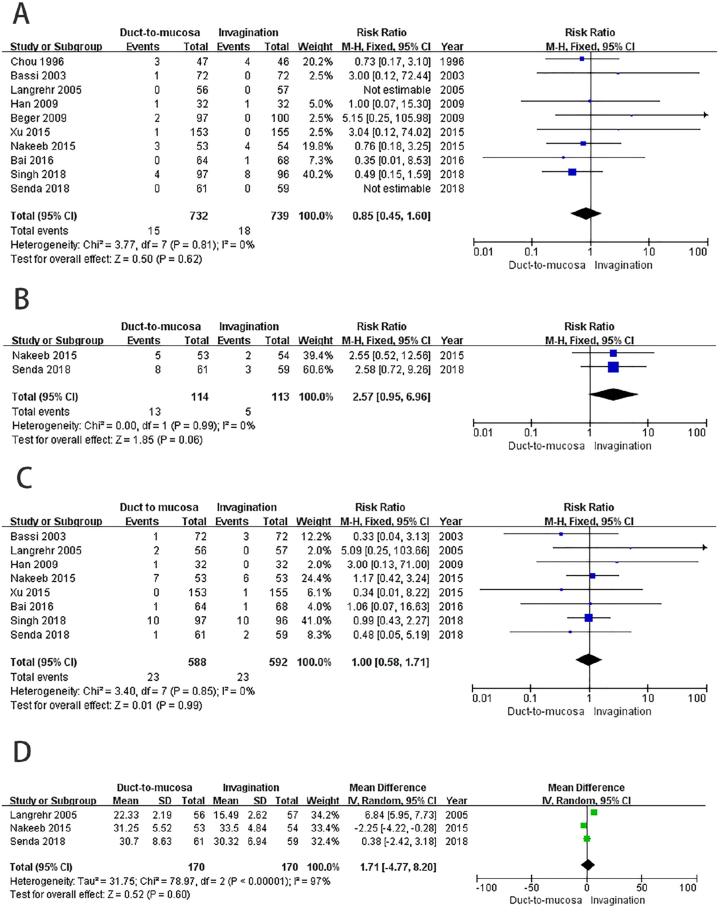
Ten studies included in the analysis reported postoperative mortality. Out of these studies, 15 patients (2 %) in the duct-to-mucosa group and 18 patients (2.4 %) in the invagination group experienced postoperative mortality. The findings revealed no significant difference in mortality rates between the two groups (RR = 0.85, 95%CI: 0.45–1.60, P = 0.62; no obvious heterogeneity: P = 0.81, I2 = 0 %) Fig. 4A.
Fig. 4.
Comparison of the secondary outcomes. A) No statistically significant difference in postoperative mortality was observed between the two groups. B) The results indicate that there was no statistically significant difference in the incidence of other major postoperative complications between the two groups. C) No significant difference was observed in the incidence of postoperative bile leak between the two groups. D) No statistically significant difference was found in anastomosis time between the two groups.
3.4.2. Other major postoperative complications (excluding POPF, with a Clavien-Dindo grade Ⅲ or higher)
Two studies [26,29] reported the occurrence of other major postoperative complications using the Clavien-Dindo classification [31]. Among the patients included in these studies, 13 (11 %) in the duct-to-mucosa group and five (4 %) in the invagination group encountered other major postoperative complications. While the outcome favored the invagination group, the observed difference was not statistically significant (RR = 2.57, 95%CI: 0.95–6.96, P = 0.06; no obvious heterogeneity: P = 0.99, I2 = 0 %) Fig. 4B.
3.4.3. Postoperative Bile leak
Eight studies reported the incidence of postoperative bile leak [22,23,[25], [26], [27], [28], [29], [30]]. Bile leak occurred in 23 patients in both the duct-to-mucosa group (3.9 %) and the invagination group (3.9 %). There was no significant difference in the incidence of postoperative bile leak between the two groups (RR = 1.00, 95%CI: 0.58–1.71, P = 0.99; no obvious heterogeneity: P = 0.85, I2 = 0 %) Fig. 4C.
3.4.4. Anastomosis time of pancreaticojejunostomy
Three studies reported the anastomosis time of pancreaticojejunostomy [23,26,29]. Since all of the studies described the data using median and range, it was necessary to transform the data into sample mean and standard deviation. The equation for calculating the mean was as follows [32]:
The equation for calculating the standard deviation was as follows [33]:
In the above two equations, a = the minimum value, m = the median, b = the maximum value, n = the sample size.
We can also utilize the online calculator developed by Tong et al. based on the above calculation principles [34]. The calculator can be accessed at the following link: https://www.math.hkbu.edu.hk/∼tongt/papers/median2mean.html. The meta-analysis did not show a statistically significant difference in the observed anastomosis time between the two groups (RR = 1.71, 95%CI: 4.77-8.20, P = 0.60; substantial heterogeneity existed: P<0.00001, I2 = 97 %, random effects model)Fig. 4D.
3.5. Subgroup analysis
Soft pancreatic tissue has been identified as one of the most significant risk factors for POPF following pancreaticoduodenectomy. Therefore, we performed a subgroup analysis to assess the occurrence of grade B/C POPF in patients with soft and firm pancreatic tissue who underwent either duct-to-mucosa anastomosis or invagination anastomosis. Additionally, our study aims to investigate the impact of two different anastomosis methods on the occurrence of grade B/C POPF in patients who did not undergo internal or external stent drainage for pancreaticojejunostomy.
3.5.1. Grade B/C POPF in soft pancreas
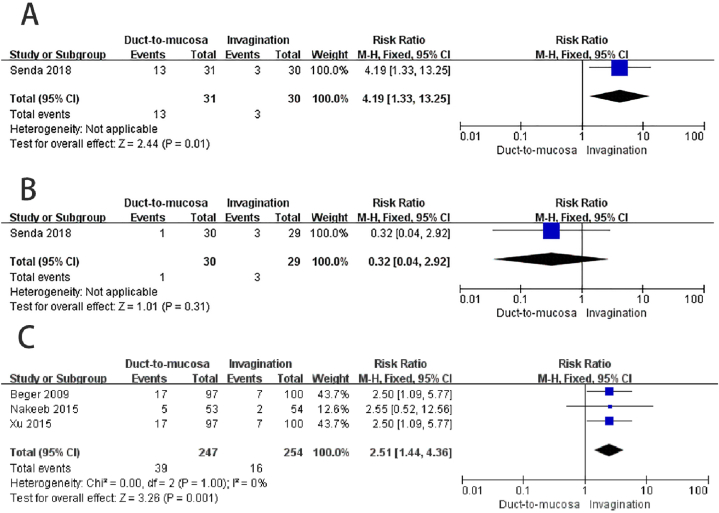
One study reported the incidence grade B/C POPF in patients with soft pancreas based on ISGPF definition 2005 [29]. The results showed that the invagination group (10 %) had a significantly lower incidence of POPF compared to the duct-to-mucosa group (41.9 %) (RR = 4.19, 95%CI: 1.33–13.25, P = 0.01; heterogeneity not applicable) Fig. 5A.
Fig. 5.
Subgroup analysis. A) The invagination group exhibited a significantly lower occurrence of grade B/C postoperative pancreatic fistula (POPF) when compared to the duct-to-mucosa group among patients with a soft pancreas. B) In patients with firm pancreas, there was no significant difference in the incidence of grade B/C POPF between the two groups. C) In patients who did not undergo internal or external stent drainage placement, the invagination group exhibited a substantially lower occurrence of grade B/C POPF in comparison to the duct-to-mucosa group.
3.5.2. Grade B/C POPF in firm pancreas
One study reported the incidence of grade B/C POPF in patients with a firm pancreas [29]. The results indicated that there was no significant difference between the duct-to-mucosa group (3.3 %) and the invagination group (10.3 %) (RR = 0.32, 95%CI: 0.04–2.92, P = 0.31; heterogeneity not applicable) Fig. 5B.
3.5.3. Grade B/C POPF without stent drainage
Three studies did not utilize the stent drainage (internal or external) following pancreaticojejunostomy [24,26,27]. The results showed that the incidence of grade B/C POPF was significantly lower in the invagination group (6.3 %) compared to the duct-to-mucosa group (15.8 %) (RR = 2.51, 95%CI: 1.44–4.36, P = 0.001; no obvious heterogeneity: P = 1.00, I2 = 0 %) Fig. 5C.
3.6. Sensitivity analysis
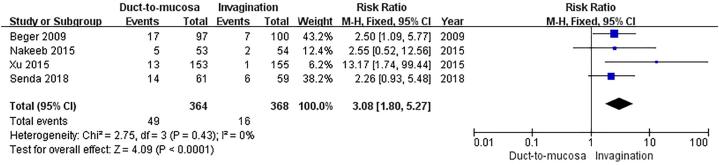
Significant heterogeneity was observed in the outcome of grade B/C POPF (primary outcome). Despite employing the random effects model for pooled statistic calculation, the heterogeneity remained high (P = 0.008, I2 = 68 % for grade B/C POPF). After thoroughly reviewing the full-texts of the studies that reported relevant outcomes, we identified that one of the included studies excluded patients if the anastomotic method was deemed difficult to perform based on subjective judgment prior to randomization [28]. Additionally, another study excluded patients with a main pancreatic duct diameter less than 2 mm prior to randomization [30]. The two studies exhibited a preference for selecting study participants with specific characteristics, particularly those directly associated with the efficiency of anastomosis. This approach effectively minimized the challenges and risks associated with duct-to-mucosa anastomosis or invagination anastomosis, which could potentially skew the study results and introduce selection bias [35,36]. Therefore, we excluded these two studies and recalculated the pooled statistics on the incidence of grade B/C POPF.
Subsequently, we incorporated the remaining four studies to assess the incidence of grade B/C POPF [24,26,27,29]. The results indicated that invagination anastomosis had a significantly lower incidence of grade B/C POPF compared to duct-to-mucosa anastomosis (RR = 3.08, 95%CI: 1.80–5.27, P<0.0001; no obvious heterogeneity: P = 0.43, I2 = 0 %)Fig. 6. The sensitivity analysis corroborated the findings of the primary outcome comparison.
Fig. 6.
Sensitivity analysis. The incidence of grade B/C postoperative pancreatic fistula was significantly lower in the invagination anastomosis compared to the duct-to-mucosa anastomosis.
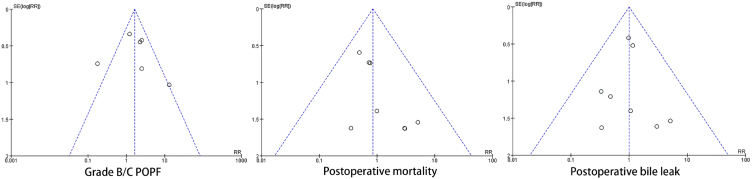
3.7. Publication bias
The funnel plots for the outcomes of grade B/C POPF, postoperative mortality, and postoperative bile leak are displayed in Fig. 7. In the funnel plot of the results for grade B/C POPF, one study exceeded the 95 % confidence interval for the effect size [28], this outlier was further analyzed in the sensitivity analysis. There was no apparent asymmetry in the distribution of the three funnel plots, suggesting the absence of significant publication bias in the included studies.
Fig. 7.
Funnel plot based on the results of grade B/C postoperative pancreatic fistula, postoperative morality and postoperative bile leak.
4. Discussion
In this meta-analysis, we have demonstrated that invagination pancreaticojejunostomy has a significantly lower incidence of grade B/C POPF compared to duct-to-mucosa pancreaticojejunostomy based on the ISGPF Definition 2005 after pancreaticoduodenectomy. Through sensitivity analysis, we effectively addressed the heterogeneity present in the primary outcome results. Additionally, this is the first study to examine the anastomosis time in pancreaticojejunostomy, and unexpectedly, we found that the anastomosis time of invagination pancreaticojejunostomy was not significantly shorter than that of duct-to-mucosa pancreaticojejunostomy. In subgroup analysis, we confirmed that invagination pancreaticojejunostomy was significantly superior to duct-to-mucosa pancreaticojejunostomy in terms of grade B/C POPF in patients with a soft pancreas. Furthermore, even without internal or external stent drainage of pancreaticojejunostomy, the incidence of grade B/C POPF in the invagination group was still significantly lower than that in the duct-to-mucosa group. There was no statistically significant difference between the two types of pancreaticojejunostomy in terms of postoperative mortality, other major postoperative complications (excluding POPF, ≥Clavien-Dindo grade Ⅲ), postoperative bile leak, and the incidence of grade B/C POPF in patients with a firm pancreas.
POPF continues to be a significant and detrimental complication following pancreaticoduodenectomy [37,38]. Our findings indicate that invagination anastomosis has a significantly lower incidence of grade B/C POPF compared to duct-to-mucosa anastomosis (12.8 % vs. 7.7 %, RR = 1.65, 95%CI: 1.14–2.39, P = 0.008). This result is further supported by sensitivity analysis. Currently, the ISGPF classification is considered the standard for defining POPF in clinical practice. Some evidence suggests that clinical outcomes of grade A POPF are comparable to the absence of a fistula and have no predictors of established risk scores assessment [[39], [40], [41]]. Therefore, in the latest version of the ISGPF Definition 2016, grade A POPF is no longer classified as a true fistula or an actual complication, but rather as a “biochemical fistula”. We focused solely on comparing grade B/C POPF, which is clinically relevant, and selected it as the primary outcome for our meta-analysis. However, the studies included in our analysis adhered to the definition and classification criteria of the ISGPF Definition 2005. Consequently, it is unclear whether this version demonstrates the same diagnostic and grading efficiency as the 2016 version. In comparison to the ISGPF 2005, the ISGPF 2016 has made two significant revisions: 1) The designation of “grade A POPF” in the ISGPF 2005 has now been changed to “biochemical fistula”, and it is no longer classified as a true pancreatic fistula or an actual complication; 2) The diagnostic criteria for grade C POPF have been modified. However, both the ISGPF 2005 and ISGPF 2016 indicate that a change in the management of the clinical or expected postoperative pathway is a critical characteristic of grade B POPF and above, which has remained unchanged. Thus, grade B and grade C POPF can be analyzed as a whole. Despite the fact that the studies included in the present meta-analysis employed the ISGPF Definition 2005 for POPF definition and classification, the results of this analysis remain compelling, particularly when examining the comparison of grade B/C POPF. However, the ISGPF Definition of 2016 has updated the demarcation point distinguishing between grade B and grade C POPF, highlighting the features associated with grade C POPF, including re-operation, organ failure, and mortality. Consequently, we did not analyze grade B and grade C POPF separately.
Although duct-to-mucosa pancreaticojejunostomy aligns more closely with normal anatomy, our findings did not establish its superiority in reducing the incidence of POPF. On the one hand, duct-to-mucosa anastomosis entails a direct connection between the main pancreatic duct and the mucosal layer of the intestine. This procedure demands considerable technical proficiency, particularly when the main pancreatic duct is not dilated and a suitable stent is unavailable. Consequently, there may be variations in the quality of pancreaticojejunostomy performed by surgeons with different levels of expertise. On the other hand, the pancreatic parenchyma is delicate, which makes sutures difficult to secure and increases the likelihood of leakage [42]. Furthermore, we observed that out of the six studies included in the analysis that reported primary outcomes, the majority of studies utilized the Cattell-Warren method for duct-to-mucosa pancreaticojejunostomy, with the exception of Senda's study, which employed the Kakita method. Both methods carry the potential risk of generating shear forces on the pancreatic parenchyma, potentially resulting in pancreatic tissue laceration [43,44]. The Cattell method generally employs a significant number of needles and sutures, which can potentially negatively impact the local blood supply and hinder the healing process of pancreaticojejunostomy [45]. Additionally, the examination of histological sections of a healthy pancreas revealed that the branch ducts on the incised surface occasionally exhibited a larger diameter than the main pancreatic duct, and numerous pancreatic acinar cells were observable. This observation indicates that a significant amount of pancreatic juice flows out from the cut surface, making it challenging to perform conventional compression by attaching the pancreatic cut surface to the jejunal serosa [46]. Consequently, the healing process of the adhesive bond between the cut surface of the pancreatic stump and the jejunal serosa could be compromised. The invagination anastomosis, also referred to as the “dunking anastomosis”, facilitates effective drainage of the main pancreatic duct and numerous small pancreatic branch ducts situated on the cut surface of the pancreatic stump. Additionally, the invagination technique is less challenging to perform compared to duct-to-mucosa anastomosis. This is because invagination anastomosis does not require a more elaborate connection between the main pancreatic duct and the intestinal mucosa. Therefore, invagination anastomosis is more suitable for narrow pancreatic duct diameters. However, invagination anastomosis has certain limitations, when the pancreatic stump is excessively large, invaginating it into the jejunum becomes challenging. Moreover, the pancreatic stump is prone to corrosion and bleeding as a result of prolonged exposure to pancreatic juice activated by digestive fluids in the intestinal lumen.
In the subgroup of soft pancreas, there was a significant increase in the incidence of grade B/C POPF in the duct-to-mucosa group compared to the invagination group (41.9 % vs. 10 %, RR = 4.19, 95%CI: 1.33–13.25, P = 0.01). In the subgroup of firm pancreas, no significant difference was observed in the incidence of grade B/C POPF between the two groups (3.3 % vs. 10.3 %, RR = 0.32, 95 %: 0.04–2.92, P = 0.31). The texture of the pancreas was assessed through palpation of the gland, either alone or in conjunction with postoperative pathological findings in the studies included. Soft pancreas, often accompanied by a non-dilated pancreatic duct, is considered a significant risk factor for POPF [47,48], increasing the probability of its occurrence. In contrast, the firm or hard pancreas often exhibits pathological features such as reduced acinar cells, fibrosis, and degeneration of branch pancreatic ducts due to prolonged malignant obstruction and pancreatitis. Consequently, the firm pancreatic parenchyma tends to be thin, accompanied by dilatation of the main pancreatic duct [46], resulting in a reduction in exocrine function and a low incidence of POPF [49,50]. Several RCTs have examined the short-term outcomes of patients who undergo pancreaticojejunostomy with or without external or internal stent drainage after pancreaticoduodenectomy [[51], [52], [53]], however, the conclusions regarding the protective effect of stent drainage on the incidence of POPF or other complications have been inconsistent. Therefore, the application of stent drainage for pancreaticojejunostomy without strict guidelines may influence the outcome. To further investigate the impact of the two anastomosis methods on the occurrence of grade B/C POPF in the absence of stent drainage, we conducted a subgroup analysis. The results demonstrated that the invagination group (6.3 %) had a significantly lower incidence of grade B/C POPF compared to the duct-to-mucosa group (15.8 %) (RR = 2.51, 95%CI: 1.44–4.36, P = 0.001; no obvious heterogeneity: P = 1.00, I2 = 0 %).
The term “major postoperative complications” primarily refers to Clavien-Dindo grade Ⅲ to Ⅴ complications [54,55]. We conducted an analysis of major complications other than POPF, only two studies furnished data regarding these outcomes. There was no statistically significant difference observed between the duct-to-mucosa and invagination groups (RR = 2.57, 95%CI: 0.95–6.96, P = 0.06). Unfortunately, the specific classifications of other major postoperative complications were unavailable. There was no significant difference in the incidence of postoperative bile leak between the duct-to-mucosa group and the invagination group (3.9 % vs. 3.9 %, RR = 1.00, 95%CI: 0.58–1.71, P = 0.99). Bile leaks typically occur due to inadequate healing of the biliary-jejunal anastomosis. It is important to note that bile can also escape from a pancreaticojejunal anastomosis with inadequate healing, even if the biliary-jejunal anastomosis is intact. Additionally, it should be considered that fluid draining from a pancreatic fistula may resemble green bilious fluid [15], thus leading to potential misdiagnosis of a bile leak. Notably, invagination anastomosis did not demonstrate any advantages in terms of reducing the duration of the operation. This could be attributed to the fact that the operational benefits of invagination anastomosis are not effectively demonstrated in open surgery. Hence, it is necessary to verify this in laparoscopic surgery. The significant heterogeneity in the meta-analysis of anastomosis time may arise from variations in surgical techniques and practices employed by different surgeons. We utilized an online calculator, which was developed based on the theories presented by Wan et al. and Luo et al., to compute the sample means and standard deviations using statistical data on medians and ranges [33,34]. Hozo et al.'s method of estimating the median and range is widely recognized for accurately estimating the mean and standard deviation of a sample [56]. However, this approach has certain limitations. One significant limitation is the failure to incorporate information on sample size, which ultimately reduces the practical effectiveness of their method.
During the quality assessment, all included studies were considered to have a high risk of bias in the item of 'blinding of participants and personnel'. This is because it was impossible to blind participants to the surgical procedures. However, the outcome measures of a clinical trial are inherently objective and not easily influenced by subjective factors, an unblinded evaluation can still provide reasonably satisfactory results [57].
The two most recent meta-analyses, which examined this specific topic, were published by Hai et al., in 2022 and Cao et al., in 2020 [12,13]. These studies conducted a thorough literature search in this specific field. Cao et al. demonstrated the effectiveness of invagination anastomosis in reducing the incidence of grade B/C POPF, which further supports our conclusion. Hai et al. published their article in Cochrane Library. In their meta-analysis, factors contributing to confusion in the included studies were identified by the authors, including a high risk of bias in blinding of participants and personnel, substantial heterogeneity in the results, varied use of pancreatic duct stent and somatostatin or its analogues among patients, and differences in the levels of surgeon experience. However, further analysis on these factors was lacking. The first three factors have been discussed in the present meta-analysis. Several studies have confirmed that the use of somatostatin analogues as a perioperative measure has no significant effect on preventing POPF [58,59]. Nevertheless, we acknowledge that the findings could potentially be influenced by variations in surgeons' levels of experience or skill.
There are several limitations associated with this meta-analysis. Firstly, the majority of the studies included in this analysis utilized the ISGPS Definition 2005, this limits our capability to thoroughly analyze the outcomes in accordance with the updated version. Secondly, the number of studies included in this meta-analysis that reported primary and secondary outcomes, as well as data from subgroup analyses, was relatively limited, this limitation might impact the robustness of the conclusions. Thirdly, both a smaller diameter of the main pancreatic duct (<5 mm) and a larger amount of intraoperative blood loss (>1000 ml) are identified as independent risk factors for POPF [60]. Unfortunately, we were unable to compare the effectiveness of the two anastomosis methods in preventing POPF in relation to different main pancreatic duct diameters and blood loss due to the absence of reported outcomes and limited data validity. Finally, the included studies were deficient in long-term follow-up data, specifically regarding the occurrence of pancreatic duct stenosis or the assessment of pancreatic exocrine function.
5. Conclusions
In summary, our study revealed that patients who underwent pancreaticoduodenectomy with invagination anastomosis exhibited a significantly lower incidence of grade B/C POPF (clinically relevant POPF) compared to those who underwent duct-to-mucosa anastomosis, particularly in cases of a soft pancreas. However, there was no notable disparity in the incidence of grade B/C POPF between the two techniques in patients with a firm pancreas. Additionally, there was no significant distinction in postoperative mortality, other major complications, or postoperative bile leak between the two types of pancreaticojejunostomy.
Therefore, invagination anastomosis is recommended for patients with a soft pancreas, while either method can be used for those with a firm pancreas. Performing invagination anastomosis may not result in superior time efficiency compared to duct-to-mucosa anastomosis in open surgery.
Funding
This study is supported by the Key Research and Development Project of Science and Technology Department of Sichuan Province (grant number: 2021YFS0101).
Data availability statement
The data used in this meta-analysis came from published studies and literature. No additional unpublished data were collected or used for this meta-analysis. The relevant data can be obtained in the following ways.
-
1.
The data used in this study are all from published literature, which can be obtained through the reference list. The available databases for searching the literature in the reference list are: Embase, Web of Science, Pubmed, Ovid, and the Cochrane Library (CENTRAL).
-
2.
If some data is not directly published publicly, interested researchers can request access to the data by contacting the authors of the original study directly through the information in the references.
For further information or access to specific datasets, please contact the corresponding author.
CRediT authorship contribution statement
Xiaofei Hao: Writing – original draft, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Yi Li: Validation, Software, Methodology, Investigation, Formal analysis, Data curation. Lin Liu: Investigation. Jian Bai: Resources. Jia Liu: Methodology. Cuinan Jiang: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. Lu Zheng: Writing – review & editing, Supervision, Project administration, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Contributor Information
Cuinan Jiang, Email: 591670443@qq.com.
Lu Zheng, Email: zhenglu@tmmu.edu.cn.
References
- 1.Miedema B.W., Sarr M.G., van Heerden J.A., et al. Complications following pancreaticoduodenectomy. Current management. Arch. Surg. 1992;127:945–949. doi: 10.1001/archsurg.1992.01420080079012. [DOI] [PubMed] [Google Scholar]
- 2.Cameron J.L., Riall T.S., Coleman J., Belcher K.A. One thousand consecutive pancreaticoduodenectomies. Ann. Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe M., Usui S., Kajiwara H., et al. Current pancreatogastrointestinal anastomotic methods: results of a Japanese survey of 3109 patients. J Hepatobiliary Pancreat Surg. 2004;11:25–33. doi: 10.1007/s00534-003-0863-6. [DOI] [PubMed] [Google Scholar]
- 4.Kleespies A., Albertsmeier M., Obeidat F., Seeliger H., Jauch K.W., Bruns C.J. The challenge of pancreatic anastomosis. Langenbeck's Arch. Surg. 2008;393:459–471. doi: 10.1007/s00423-008-0324-4. [DOI] [PubMed] [Google Scholar]
- 5.Ricci C., Casadei R., Taffurelli G., Pacilio C.A., Beltrami D., Minni F. Is pancreaticogastrostomy safer than pancreaticojejunostomy after pancreaticoduodenectomy? A meta-regression analysis of randomized clinical trials. Pancreatology. 2017;17:805–813. doi: 10.1016/j.pan.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Mcmillan M.T., Malleo G., Bassi C., Sprys M.H., Vollmer C.J. Defining the practice of pancreatoduodenectomy around the world. Hpb. 2015;17:1145–1154. doi: 10.1111/hpb.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackert T., Werner J., Buchler M.W. Postoperative pancreatic fistula. Surg. J. R. Coll. Surg. Edinb. Irel. 2011;9:211–217. doi: 10.1016/j.surge.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S., Lan Z., Zhang J., et al. Duct-to-mucosa versus invagination pancreaticojejunostomy after pancreaticoduodenectomy: a meta-analysis. Oncotarget. 2017;8:46449–46460. doi: 10.18632/oncotarget.17503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua J., He Z., Qian D., Meng H., Zhou B., Song Z. Duct-to-mucosa versus invagination pancreaticojejunostomy following pancreaticoduodenectomy: a systematic review and meta-analysis. J. Gastrointest. Surg. 2015;19:1900–1909. doi: 10.1007/s11605-015-2913-1. [DOI] [PubMed] [Google Scholar]
- 10.Varco R.L. A method of implanting the pancreatic duct into the jejunum in the whipple operation for carcinoma of the pancreas. Case report. Surgery. 1945;18:569–573. [PubMed] [Google Scholar]
- 11.Lyu Y., Li T., Wang B., Cheng Y., Zhao S. Selection of pancreaticojejunostomy technique after pancreaticoduodenectomy: duct-to-mucosa anastomosis is not better than invagination anastomosis. Medicine (Baltim.) 2018;97 doi: 10.1097/MD.0000000000012621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hai H., Li Z., Zhang Z., et al. Duct-to-mucosa versus other types of pancreaticojejunostomy for the prevention of postoperative pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst. Rev. 2022;3 doi: 10.1002/14651858.CD013462.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Z., Luo W., Qiu J., Liu Y., Zheng L., Zhang T. Is invagination anastomosis more effective in reducing clinically relevant pancreatic fistula for soft pancreas after pancreaticoduodenectomy under novel fistula criteria: a systematic review and meta-analysis. Front. Oncol. 2020;10:1637. doi: 10.3389/fonc.2020.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassi C., Marchegiani G., Dervenis C., et al. The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–591. doi: 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Bassi C., Dervenis C., Butturini G., et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S., Lan Z., Zhang J., et al. Duct-to-mucosa versus invagination pancreaticojejunostomy after pancreaticoduodenectomy: a meta-analysis. Oncotarget. 2017;8:46449–46460. doi: 10.18632/oncotarget.17503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X., Zhang Q., Zhang J., et al. Meta-analysis of invagination and duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy: an update. Int. J. Surg. 2016;36:240–247. doi: 10.1016/j.ijsu.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Hua J., He Z., Qian D., Meng H., Zhou B., Song Z. Duct-to-mucosa versus invagination pancreaticojejunostomy following pancreaticoduodenectomy: a systematic review and meta-analysis. J. Gastrointest. Surg. 2015;19:1900–1909. doi: 10.1007/s11605-015-2913-1. [DOI] [PubMed] [Google Scholar]
- 19.Page M.J., Mckenzie J.E., Bossuyt P.M., et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ Br. Med. J. (Clin. Res. Ed.) 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins Jpt, Thomas J., Chandler J., et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane. 2022 www.training.Cochrane.org/handbook Available from: [Google Scholar]
- 21.Chou F.F., Sheen-Chen S.M., Chen Y.S., Chen M.C., Chen C.L. Postoperative morbidity and mortality of pancreaticoduodenectomy for periampullary cancer. Eur. J. Surg. 1996;162:477–481. [PubMed] [Google Scholar]
- 22.Bassi C., Falconi M., Molinari E., et al. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134:766–771. doi: 10.1016/s0039-6060(03)00345-3. [DOI] [PubMed] [Google Scholar]
- 23.Langrehr J.M., Bahra M., Jacob D., Glanemann M., Neuhaus P. Prospective randomized comparison between a new mattress technique and cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J. Surg. 2005;29:1111–1119. doi: 10.1007/s00268-005-7875-0. [DOI] [PubMed] [Google Scholar]
- 24.Berger A.C., Howard T.J., Kennedy E.P., et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized prospective dual-institution trial. J. Am. Coll. Surg. 2009;208:738–747. doi: 10.1016/j.jamcollsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Han J.M., Wang X.B., Quan Z.F., Zhu W.M. Duct-to-mucosa anastomosis and incidence of pancreatic fistula following pancreaticoduodenectomy. Journal of Medical Postgraduates. 2003;22(9):961–964. [In Chinese, English abstract] [Google Scholar]
- 26.El Nakeeb A., El Hemaly M., Askr W., et al. Comparative study between duct to mucosa and invagination pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized study. Int. J. Surg. 2015;16:1–6. doi: 10.1016/j.ijsu.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Xu J., Zhang B., Shi S., et al. Papillary-like main pancreatic duct invaginated pancreaticojejunostomy versus duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Surgery. 2015;158:1211–1218. doi: 10.1016/j.surg.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Bai X., Zhang Q., Gao S., et al. Duct-to-mucosa vs invagination for pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized controlled trial from a single surgeon. J. Am. Coll. Surg. 2016;222:10–18. doi: 10.1016/j.jamcollsurg.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Senda Y., Shimizu Y., Natsume S., et al. Randomized clinical trial of duct‐to‐mucosa versus invagination pancreaticojejunostomy after pancreatoduodenectomy. Br. J. Surg. 2018;105:48–57. doi: 10.1002/bjs.10727. [DOI] [PubMed] [Google Scholar]
- 30.Singh A.N., Pal S., Mangla V., et al. Pancreaticojejunostomy: does the technique matter? A randomized trial. J. Surg. Oncol. 2018;117:389–396. doi: 10.1002/jso.24873. [DOI] [PubMed] [Google Scholar]
- 31.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size median mid-range and/or mid-quartile range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 33.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size median range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi J., Luo D., Wan X., Liu Y., Liu J., Tong T. Detecting the skewness of data from the sample size and the five-number summary. arXiv preprint arXiv. 2020 doi: 10.1177/09622802231172043. [DOI] [PubMed] [Google Scholar]
- 35.Salas M., Hofman A., Stricker B.H. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am. J. Epidemiol. 1999;149:981–983. doi: 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 36.Vetter T.R., Mascha E.J. Bias confounding and interaction: lions and tigers and bears oh my. Anesth. Analg. 2017;125:1042–1048. doi: 10.1213/ANE.0000000000002332. [DOI] [PubMed] [Google Scholar]
- 37.Miller B.C., Christein J.D., Behrman S.W., et al. Assessing the impact of a fistula after a pancreaticoduodenectomy using the post-operative morbidity index. Hpb. 2013;15:781–788. doi: 10.1111/hpb.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallance A.E., Young A.L., Macutkiewicz C., Roberts K.J., Smith A.M. Calculating the risk of a pancreatic fistula after a pancreaticoduodenectomy: a systematic review. Hpb. 2015;17:1040–1048. doi: 10.1111/hpb.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratt W.B., Maithel S.K., Vanounou T., Huang Z.S., Callery M.P., Vollmer C.J. Clinical and economic validation of the international study group of pancreatic fistula(ISGPF) classification scheme. Ann. Surg. 2007;245:443–451. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pratt W.B., Callery M.P., Vollmer C.J. Risk prediction for development of pancreatic fistula using the isgpf classification scheme. World J. Surg. 2008;32:419–428. doi: 10.1007/s00268-007-9388-5. [DOI] [PubMed] [Google Scholar]
- 41.Kim W.S., Choi D.W., Choi S.H., et al. Clinical validation of the isgpf classification and the risk factors of pancreatic fistula formation following duct-to-mucosa pancreaticojejunostomy by one surgeon at a single center. J. Gastrointest. Surg. 2011;15:2187–2192. doi: 10.1007/s11605-011-1726-0. [DOI] [PubMed] [Google Scholar]
- 42.Simon R. Complications after pancreaticoduodenectomy. Surg. Clin.-North Am. 2021;101:865–874. doi: 10.1016/j.suc.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Kleespies A., Rentsch M., Seeliger H., Albertsmeier M., Jauch K.W., Bruns C.J. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br. J. Surg. 2009;96:741–750. doi: 10.1002/bjs.6634. [DOI] [PubMed] [Google Scholar]
- 44.Kawakatsu S., Inoue Y., Mise Y., et al. Comparison of pancreatojejunostomy techniques in patients with a soft pancreas: kakita anastomosis and blumgart anastomosis. BMC Surg. 2018;18 doi: 10.1186/s12893-018-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z., Wang X., Ke N. A different suturing method of the duct-to-mucosa pancreaticojejunostomy for the normal pancreatic duct in laparoscopic pancreaticoduodenectomy. J. Minimal Access Surg. 2021;17:412. doi: 10.4103/jmas.JMAS_298_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitsuyoshi A., Hamada S., Ohe H., Fujita H., Okabe H., Inoguchi K. Proposal for a safe and functional pancreaticojejunostomy technique from a histopathological perspective. World J. Surg. 2018;42:4090–4096. doi: 10.1007/s00268-018-4718-3. [DOI] [PubMed] [Google Scholar]
- 47.Schuh F., Mihaljevic A.L., Probst P., et al. A Simple classification of pancreatic duct size and texture predicts postoperative pancreatic fistula: a classification of the international study group of pancreatic surgery. Ann. Surg. 2023;277:e597–e608. doi: 10.1097/SLA.0000000000004855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mungroop T.H., van Rijssen L.B., van Klaveren D., et al. Alternative fistula risk score for pancreatoduodenectomy (A-FRS): design and international external validation. Ann. Surg. 2019;269:937–943. doi: 10.1097/SLA.0000000000002620. [DOI] [PubMed] [Google Scholar]
- 49.Semb B.K., Bjerkeset T. Modified gastrointestinal reconstruction after pancreaticoduodenal resection with particular reference to the prevention of postoperative biliary and pancreatic fistulas. Acta Chir. Scand. 1981;147:685–691. [PubMed] [Google Scholar]
- 50.Papachristou D.N., Fortner J.G. Pancreatic fistula complicating pancreatectomy for malignant disease. Br. J. Surg. 1981;68:238–240. doi: 10.1002/bjs.1800680406. [DOI] [PubMed] [Google Scholar]
- 51.Motoi F., Egawa S., Rikiyama T., et al. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br. J. Surg. 2012;99:524–531. doi: 10.1002/bjs.8654. [DOI] [PubMed] [Google Scholar]
- 52.Bin X., Lian B., Jianping G., et al. Comparison of patient outcomes with and without stenting tube in pancreaticoduodenectomy. J. Int. Med. Res. 2018;46:403–410. doi: 10.1177/0300060517717400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuroki T., Tajima Y., Kitasato A., et al. Stenting versus non-stenting in pancreaticojejunostomy: a prospective study limited to a normal pancreas without fibrosis sorted by using dynamic MRI. Pancreas. 2011;40:25–29. doi: 10.1097/MPA.0b013e3181e861fa. [DOI] [PubMed] [Google Scholar]
- 54.Mckay A., Sutherland F.R., Bathe O.F., Dixon E. Morbidity and mortality following multivisceral resections in complex hepatic and pancreatic surgery. J. Gastrointest. Surg. 2008;12:86–90. doi: 10.1007/s11605-007-0273-1. [DOI] [PubMed] [Google Scholar]
- 55.Arru M., Pulitano C., Aldrighetti L., Catena M., Finazzi R., Ferla G. A prospective evaluation of ultrasonic dissector plus harmonic scalpel in liver resection. Am. Surg. 2007;73:256–260. [PubMed] [Google Scholar]
- 56.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median range and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao Y., Hao X., Yang Q., Li M., Wen J., Jiang C. Effect of billroth‐ii versus roux‐en‐y reconstruction for gastrojejunostomy after pancreaticoduodenectomy on delayed gastric emptying: a meta‐analysis of randomized controlled trials. J. Hepato-Biliary-Pancreat. Sci. 2021;28:397–408. doi: 10.1002/jhbp.828. [DOI] [PubMed] [Google Scholar]
- 58.Gans S.L., van Westreenen H.L., Kiewiet J.J., Rauws E.A., Gouma D.J., Boermeester M.A. Systematic review and meta-analysis of somatostatin analogues for the treatment of pancreatic fistula. Br. J. Surg. 2012;99:754–760. doi: 10.1002/bjs.8709. [DOI] [PubMed] [Google Scholar]
- 59.Schorn S., Vogel T., Demir I.E., et al. Do somatostatin-analogues have the same impact on postoperative morbidity and pancreatic fistula in patients after pancreaticoduodenectomy and distal pancreatectomy? – a systematic review with meta-analysis of randomized-controlled trials. Pancreatology. 2020;20:1770–1778. doi: 10.1016/j.pan.2020.10.043. [DOI] [PubMed] [Google Scholar]
- 60.Callery M.P., Pratt W.B., Kent T.S., et al. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J. Am. Coll. Surg. 2013;216:1–14. doi: 10.1016/j.jamcollsurg.2012.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this meta-analysis came from published studies and literature. No additional unpublished data were collected or used for this meta-analysis. The relevant data can be obtained in the following ways.
-
1.
The data used in this study are all from published literature, which can be obtained through the reference list. The available databases for searching the literature in the reference list are: Embase, Web of Science, Pubmed, Ovid, and the Cochrane Library (CENTRAL).
-
2.
If some data is not directly published publicly, interested researchers can request access to the data by contacting the authors of the original study directly through the information in the references.
For further information or access to specific datasets, please contact the corresponding author.