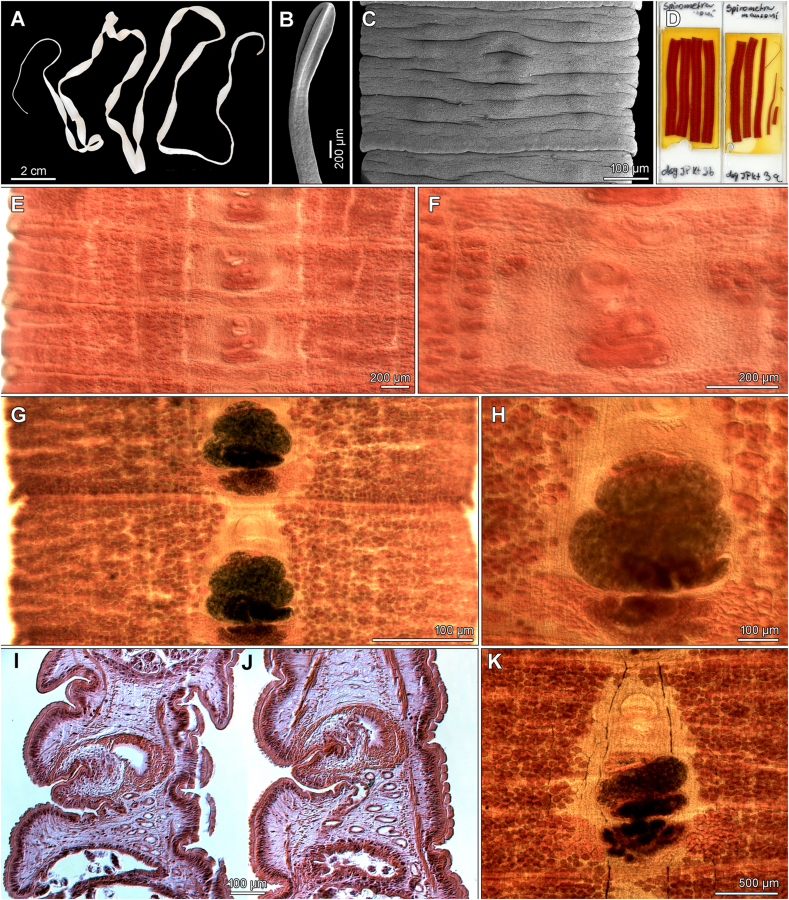
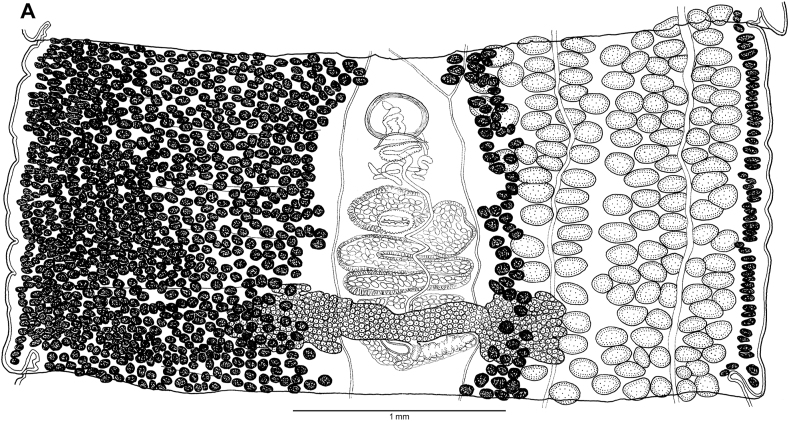
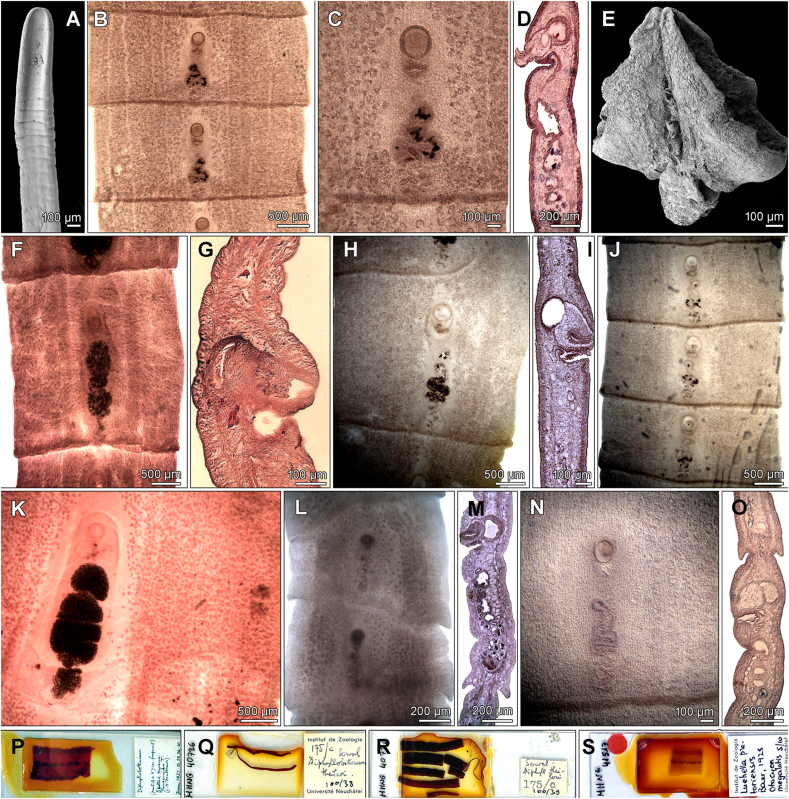
Abstract
Tapeworms of the genus Spirometra Faust, Campbell et Kellogg, 1929 have long been known as intestinal parasites of carnivores and their larvae (spargana) have been found in various vertebrates. Nevertheless, their species diversity, host associations and geographical distribution remain poorly understood. Molecular data clearly confirm the validity of the genus, which has been synonymised by several authors with Diphyllobothrium Cobbold, 1858. Despite morphological similarities between the species of the two genera, they are not closely related and also differ in their life cycle. The present review provides a list of the species recognised as valid and additional genotypes that may represent other species, with a basic characterisation of each taxon and comments on their validity, the probable range of definitive and intermediate hosts, and their distribution. The existing taxonomic problems and the insufficient knowledge of the host specificity and distribution of Spirometra tapeworms can only be solved by combining molecular and morphological data, i.e. by comparing genetically characterised specimens with corresponding morphological vouchers (hologenophores). Further targeted sampling and surveys are required to clarify the distribution and host associations.
Keywords: Broad tapeworms, Sparganosis, Diversity, Host range, Geographical distribution, Molecular phylogeny, Zoonosis
Graphical abstract
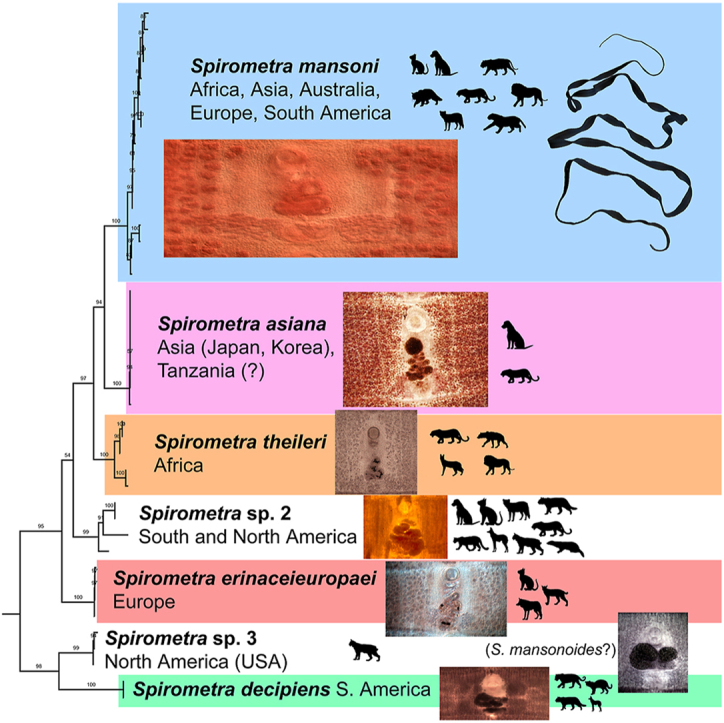
Highlights
-
•
Spirometra tapeworms (Cestoda), causative agents of human sparganosis, are reviewed.
-
•
Taxonomic problems and species diversity are discussed.
-
•
A list of species/lineages recognised as valid is presented.
-
•
The range of hosts of Spirometra and their distribution are summarised.
-
•
Further progress requires the combination of molecular and morphological data.
1. Introduction
Diphyllobothriidean tapeworms are well-known parasites of wild animals, and their adults parasitise all groups of tetrapod vertebrates, including frogs (members of the family Cephalochlamydidae), reptiles (Scyphocephalidae), birds and mammals (Diphyllobothriidae). Most species are reported from marine mammals (pinnipeds and cetaceans), but some members of the genera Adenocephalus Nybelin, 1931, Dibothriocephalus Lühe, 1899, Diphyllobothrium Cobbold, 1883, and Spirometra Faust, Campbell et Kellogg, 1929 have zoonotic potential and are the main cause of widespread food-borne cestodoses (diphyllobothriosis and sparganosis), with an estimated 20 million infected people worldwide. Mapping human hosts onto a phylogeny of broad tapeworms has shown that humans have been acquired several times as accidental hosts of broad tapeworms (Waeschenbach et al., 2017).
A better understanding of the epidemiology and distribution of broad tapeworms is hampered by difficulties in identifying both the adult tapeworms and the metacestodes (plerocercoids). Identification by morphology is problematic for several reasons, mainly because of the high intraspecific variation in most morphological characters, the different fixation methods and the usually poor quality of available material. Host type and intensity of infection (parasite abundance) may also play an important role in the intraspecific variability of individual taxa (Andersen, 1972, 1973). As a result, a large number of taxa from the same hosts and regions have been described, but their validity is questionable (Kuchta and Scholz, 2017).
Scholz and Kuchta (2016) reviewed zoonotic broad tapeworms (members of Diphyllobothrium and relatives) that use fish as intermediate hosts, while Kuchta et al. (2021) presented the first molecular phylogenetic hypothesis on the relationship of the species of Spirometra, whose members can cause life-threatening sparganosis. Species of Spirometra use terrestrial, mainly carnivorous mammals as definitive hosts and amphibians (frogs), reptiles (snakes) and mammals, rarely birds, but almost never fish (see Vettorazzi et al., 2023 and below), as their second intermediate hosts. Sparganosis is a disease of humans and animals caused by (i) ingestion of the first intermediate host (through drinking water with freshwater copepods infected with procercoids), (ii) ingestion of the second intermediate host (mainly snakes or frogs) infected with plerocercoids, or (iii) contact of an open wound with intermediate hosts (Waeschenbach et al., 2017; Kuchta et al., 2021). Occasionally, humans become infected with plerocercoids of Spirometra, which then develop into adults in the human intestine and cause spirometrosis (Kuchta et al., 2015, 2021).
Adult Spirometra tapeworms are morphologically similar to those of the genera Dibothriocephalus and Diphyllobothrium, which may even occur in the same definitive hosts (cats, dogs, foxes, etc.), making their identification even more difficult. Due to this morphological similarity and the occurrence in the same definitive hosts, some authors, including Schmidt (1986), have synonymised Spirometra with Diphyllobothrium. However, molecular data provided evidence that Spirometra is valid and not closely related to Diphyllobothrium and Dibothriocephalus (Waeschenbach et al., 2017).
In this review, we address the species diversity, distribution and biology of Spirometra species. The data are based on a critical review of the literature since 1819, when the first Spirometra species was described by Rudolphi (1819), and on the examination of extensive material of Spirometra tapeworms, including type specimens of several taxa from parasitological collections and specimens recently collected by numerous collaborators and kindly provided to the present authors (see Acknowledgements).
2. Systematics of Spirometra and species diversity
The tapeworms of the genus Spirometra are one of the most taxonomically complicated groups of tapeworms and their systematics and species identification are still a major challenge (Kuchta and Scholz, 2017). The general morphological uniformity of most taxa on the one hand and the high intraspecific, host-related variability on the other hand make it difficult to distinguish species based on morphology alone. In addition, many new species have been poorly described on the basis of limited and/or poorly fixed specimens (often from non-fresh hosts such as carcasses, roadkill and frozen hosts). These tapeworms are already decomposed, making their morphological identification impossible. Numerous authors also did not take into account the high degree of morphological variation and phenotypic plasticity of most taxa (see Iwata, 1934, 1972; Odening, 1985), and based their descriptions of ‘new’ taxa on variable morphological characters that were only examined on a few selected proglottids of an individual (Faust et al., 1929; Wardle et al., 1974).
Kuchta et al. (2021) gave a brief overview of the suitability of selected morphological and morphometric characters of the strobila, proglottids, uterine loops, ovary, testes, vitelline follicles, cirrus sac and external seminal vesicle for distinguishing individual species. However, most of these characteristics can vary even within a single specimen (Iwata, 1934, 1972). Consequently, morphologically different proglottids of the same specimen may correspond to different morphotypes (=’species’) described by Faust et al. (1929); these morphotypes are still used by some recent authors. Several authors placed great emphasis on the species of the definitive hosts, but it appears that the host specificity of Spirometra species at the definitive host level is not as strict as previously thought (Kuchta et al., 2021). In contrast, the geographical origin of individual specimens can play an important role in distinguishing some Spirometra species (Kuchta et al., 2021; Yamasaki et al., 2021, 2024).
The first tapeworm currently classified in Spirometra was Dubium erinaceieuropaei Rudolphi, 1819, which was described from metacestodes (plerocercoids) found in a hedgehog (Erinaceus europaeus L.) in Europe. The original description was very superficial and did not allow this species to be adequately characterised. The type material is available (Kuchta et al., 2021), but plerocercoid form is not suitable for morphological identification to the species level. This species name has been widely used for plerocercoids and adults of Spirometra since the study by Faust et al. (1929), including isolates from humans on all continents except Antarctica (Daly, 1981). This was apparently a mistake for cases in Asia, where this European species does not occur (Kuchta et al., 2021; Yamasaki et al., 2021, 2024).
The first Spirometra tapeworm from Asia was Ligula mansoni Cobbold, 1883 described by Cobbold (1883) in China as a plerocercoid found at autopsy of a man in Amony (now Xiamen). The first Spirometra from South America was Dibothrium decipiens Diesing, 1850 from various felids in Brazil, which was superficially described by Diesing (1850). All species described in the 19th century (see Scholz et al., 2019) were very superficially and incompletely characterised and it is impossible to compare these species with newly obtained material (Kuchta et al., 2021).
Spirometra plerocercoids with similar morphology, the adults of which were unknown for a long time, were later placed in the Sparganum Diesing, 1854 collective group proposed by Diesing (1854). The first link between plerocercoids of terrestrial vertebrates and adult tapeworms of carnivores was experimentally confirmed by Meggitt (1925) and Joyeux and Baer (1927), whereupon Faust et al. (1929) described six new species and proposed a new subgenus, Spirometra, in Diphyllobothrium, which was later elevated to the rank of a genus by Mueller (1937). Faust et al. (1929) designated S. decipiens as the type species of their new subgenus. However, when Mueller (1937) elevated this subgenus to the rank of a genus, he erroneously designated S. erinaceieuropaei as the type species. This error was then adopted by all subsequent workers, including Kuchta and Scholz (2017). Therefore, the type species of the genus is S. decipiens, although Faust et al. (1929) mistook their specimens from China (most likely S. mansoni) for S. decipiens of Diesing (1850).
Schmidt (1986) synonymised Spirometra with Diphyllobothrium, but both Bray et al. (1994) and Kamo (1999) recognised Spirometra as valid, which was fully confirmed by molecular data. These data have shown that the two genera are not closely related (Waeschenbach et al., 2017; Fraija-Fernández et al., 2021). In the key to the genera of the Diphyllobothriidae, Bray et al. (1994) separated Spirometra from other diphyllobothriid genera by the structure of the male terminal genitalia, with the internal seminal vesicle forming the posterior part of the cirrus sac, which is separated as the external seminal vesicle in other diphyllobothriid genera such as Diphyllobothrium and Dibothriocephalus. The species of these two genera also differ in the appearance of the uterus, which in Spirometra is a simple spiral of a few closely spaced coils that widen to form a uterine pore anterior to the genital pore, whereas the uterus in most genera, including Diphyllobothrium and Dibothriocephalus, consists of several loops, which may be parallel or rosette-shaped in the last gravid proglottids and occupy most of the central space of the proglottids (Bray et al., 1994; Kamo 1999).
The most detailed taxonomic reports on Spirometra tapeworms, which included an examination of freshly fixed material from experimentally infected definitive hosts, were by Iwata (1934, 1972), Dubinina (1951), Mueller (1974) and Odening (1985). These authors came to the conclusion that it is almost impossible to distinguish individual Spirometra species based on morphological characteristics alone. Scholz et al. (2019) listed 49 nominal species of Spirometra that were originally described in several different genera (Daly, 1981; Kamo, 1999; Scholz et al., 2019).
Iwata (1972) synonymised all nominal taxa of Spirometra with S. erinaceieuropaei being the only species of the genus, due to the morphological similarity of the individual taxa. Kamo (1999) recognised four species of Spirometra as valid, namely S. erinaceieuropaei, S. mansonoides (Mueller, 1935), S. pretoriensis (Baer, 1924), and S. theileri (Baer, 1924). Kuchta and Scholz (2017) accepted this taxonomic view and also recognised only four valid species. Most recently, Kuchta et al. (2021) distinguished seven lineages corresponding to different species, most of which show a strong geographic pattern (see below). One of these lineages, ‘Spirometra sp. 1’ from Asia, was recently described by Yamasaki et al. (2024) as a new species, S. asiana Yamasaki, Sugiyama et Morishima, 2024. All these lineages are listed below with notes on their morphology, host range and known distribution.
Recently, the complete mitochondrial genomes of several samples of Spirometra tapeworms have been sequenced, often with the stated aim of facilitating species identification of individual samples. Although mitogenomic data can be used for phylogenetic inference, their suitability for routine species identification is questionable, especially when considering the high cost and research effort required to obtain these data and analyse them properly, compared to the much lower cost and time required for the ‘golden standard’ for genotyping Spirometra samples, i.e., sequencing of the cox1 gene.
3. Life cycle and host associations
The life cycle of Spirometra tapeworms involves two intermediate hosts. The first host is freshwater copepods, which was first experimentally confirmed by Okumura (1919), who successfully infected Mesocyclops leuckarti (Claus) with Spirometra coradicia which contain an oncosphere (or hexacanth) and swim freely in the water. So far, planktonic copepods of the following genera of the family Cyclopidae have been confirmed as natural or experimental first intermediate hosts, namely Cyclops, Diacyclops, Ectocyclops, Leptocyclops, Macrocyclops, Megacyclops, Mesocyclops, Microcyclops, Paracyclops and Thermocyclops (Stephanson, 1985). The calanoid copepod Calamoecia tasmanica (Smith) (family Centropagidae) was also named as a suitable intermediate host (Stephanson, 1985). In contrast to the species of Dibothriocephalus, diaptomids (family Diaptomidae) are not suitable intermediate hosts for Spirometra tapeworms (Mueller, 1938).
Copepods harbour first-stage metacestodes called procercoids which have a caudal appendage (cercomer) with three pairs of embryonic hooks (Chervy, 2002). Depending on the water temperature, the development of the procercoids in the first intermediate host takes between 3 and 21 days (Okumura, 1919; Joyeux and Baer, 1927; Li, 1929; Mueller, 1938; Bearup, 1953; Opuni and Muller, 1974). The late nauplii and early copepodid stages are most susceptible to infection with coracidia, while adult copepods are rarely infected (Mueller, 1974).
The second intermediate hosts, which can include mammals, typically become infected by ingesting copepods carrying procercoids. However, direct penetration by procercoids through intact, unbroken skin, vagina or conjunctiva of mice and even human skin has been demonstrated experimentally (Li, 1929; Kobayashi, 1931; Mueller, 1966). Parenterally located plerocercoids, called spargana, develop in the second intermediate host, but can infect a wide range of paratenic hosts by predation, i.e., hosts without developing plerocercoids (Odening, 1976).
The development of plerocercoids in the second intermediate hosts can be extremely short, according to Mueller (1974) only 4.5 days, but plerocercoids can survive in these hosts for several months or even several years. Swartzwelder et al. (1964) reported a possible survival of plerocercoids in a patient from Louisiana up to 12 years and Mueller (1974) experimentally confirmed a survival of spargana transmitted between experimentally infected mice up to 16 years. Plerocercoids transplanted from mouse to mouse, or from mouse to monkey, also retained their infectivity for several years (Mueller, 1938). Successful transmission from host to host is only possible if the plerocercoids have an intact scolex (Mueller, 1938).
Transmission of spargana to other hosts is usually peroral, i.e. by predation, but direct penetration through the intact skin, vagina or eyes (conjunctiva) has also been observed in experimentally infected mice (Kobayashi, 1931). All groups of freshwater and terrestrial tetrapods, especially amphibians, reptiles and mammals, have been described as hosts of spargana, while birds are much less common. Some tetrapods such as cats, civets, dogs, foxes, hyenas, raccoon dogs, raccoons, servals and humans can serve both as second intermediate/paratenic hosts and as definitive hosts (Shipley, 1902; Baer and Fain, 1955; Harkema and Miller, 1964; Mueller, 1974).
Experimental infections of fish with procercoids injected into their body cavity failed. Small aquarium fish, catfish (Ameiurus melas [Rafinesque], Cnidoglanis macrocephalus [Valenciennes], Silurus glanis L.), mosquitofish (Gambusia affinis Baird et Girard), goldfish (Carassius auratus [L.]) and rainbow trout (Oncorhynchus mykiss [Walbaum]) were used unsuccessfully in laboratory experiments (Joyeux et al., 1934; Mueller, 1960; Odening and Bockhardt, 1982; Stephanson, 1985). However, Vettorazzi et al. (2023) reported a natural infection of the killifish Austrolebias charrua Costa et Cheffe (Cyprinodontiformes: Rivulidae) in Uruguay. The prevalence of infection was high (up to 58%), but the actual role of killifish in the life cycle of Spirometra tapeworms is not known. This recent report changes the paradigm that fish are never involved in the transmission of Spirometra tapeworms (Kuchta et al., 2015), but their role in the life cycle of Spirometra tapeworms appears to be very limited.
The definitive host becomes infected perorally by ingestion of second intermediate hosts or paratenic hosts harbouring plerocercoids (spargana). Ingested plerocercoids attach to the epithelium of the small intestine of their hosts and grow rapidly. The prepatent period is short and the first eggs have been found after two weeks in cats. Adult Spirometra tapeworms are relatively short-lived, especially compared to Dibothriocephalus species, but some adults can survive up to 3 years and 7 months, as observed by Mueller (1974) in S. mansonoides in cats. Berntzen and Mueller (1972) obtained adult Spirometra tapeworms, including egg-bearing (gravid) specimens, after culturing plerocercoids in various growth media.
The range of definitive hosts of Spirometra tapeworms is extremely broad, with representatives of the following tetrapod groups reported in the literature (see Supplementary Table S1 for a summary of host data). The most commonly reported definitive hosts of Spirometra tapeworms are cats and dogs, but no fewer than 67 mammalian species from 11 families, most commonly Felidae (28 spp.) and Canidae (19 spp.), but also Cercopithecidae (2 species in a single record), Didelphidae (2 spp.), Hominidae (1 sp.), Hyaenidae (2 spp.), Mephitidae (1 sp.), Mustelidae (4 spp.), Procyonidae (4 spp.), Sciuridae (1 species in a single record based on stool sample), and Viverridae (3 spp.) were also reported as final hosts of Spirometra spp. (Supplementary Table S1). The detection of adult Spirometra serpentis (Yamaguti, 1935) in the Chinese cobra Naja atra (Elapidae) in Taiwan by Yamaguti (1935) most likely represents postcyclic parasitism.
Mueller (1959, 1961) described in detail methods for the massive laboratory propagation of S. mansonoides from eggs to procercoids in copepods, to plerocercoids in mice and to adults in dogs, including in vitro breeding of plerocercoids. Okino et al. (2017) described a complete life cycle of S. mansoni using a triploid clone.
4. Survey of Spirometra species
This chapter contains basic information on species recognised as valid and additional genotypes representing different but not yet sufficiently characterised taxa, based on the recent studies by Kuchta et al. (2021), Yamasaki et al. (2024) and the present study (Fig. 1; Supplementary Fig. S1). For the list of nominal species, see Scholz et al. (2019) and for the invalidity of some recently used names, see below and supplementary data in Kuchta et al. (2021). For each taxon, data on definitive, second intermediate and paratenic hosts, geographical distribution and taxonomy as well as selected morphological characters are given. The taxa are listed in the chronological order of their first description. The acronyms of the parasitological collections are as follows: CMNPA – Canadian Museum of Nature Parasite Collection, Ottawa, Canada; IPCAS – Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic; NHMUK – Natural History Museum, London, UK; MHNG-PLAT – Natural History Museum, Geneva, Switzerland; MPM – Meguro Parasitological Museum, Tokyo, Japan; RMCA – Royal Museum of Central Africa, Tervuren, Belgium; USNM – Smithsonian National Museum of Natural History, Washington, D.C., USA; NMW – Natural History Museum, Vienna, Austria; ZMB – Zoological Museum, Berlin, Germany.
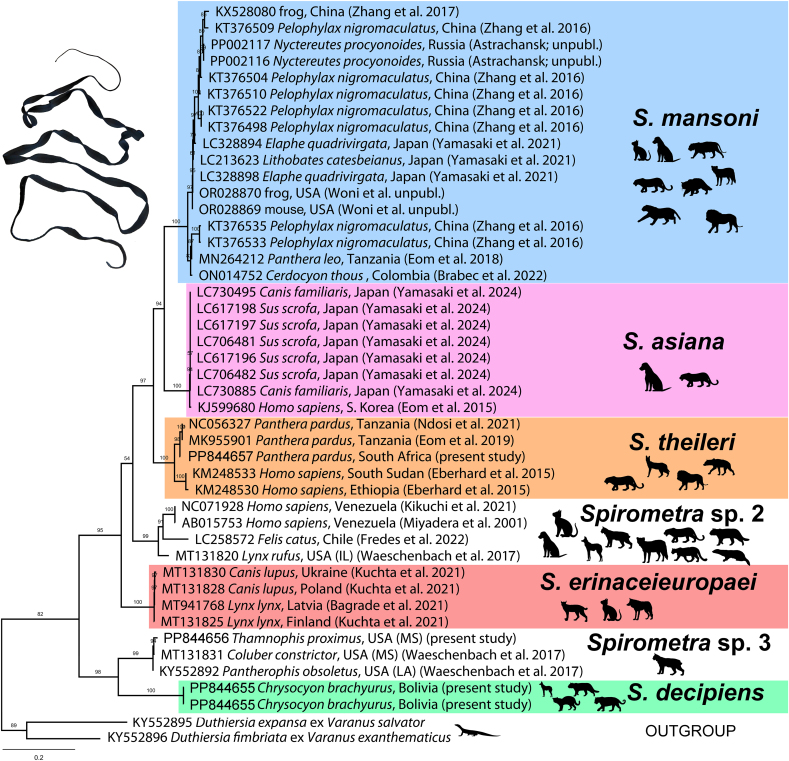
Fig. 1.
Phylogenetic tree of the interrelationships of the genus Spirometra based on selected cytochrome c oxidase subunit I (cox1) gene data (long sequences), maximum likelihood. The branch length scale bar indicates number of substitutions per site. Colours highlight specimens of different species; samples without colour represent undescribed species. See Supplementary Fig. 1 for more data based on larger dataset, but based on shorter sequences. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.1. Species or lineages recognised as valid based on molecular data
Spirometra erinaceieuropaei (Rudolphi, 1819) Faust, Campbell et Kellogg, 1929 – ‘European lineage’ of Kuchta et al. (2021) Fig. 2, Fig. 3.
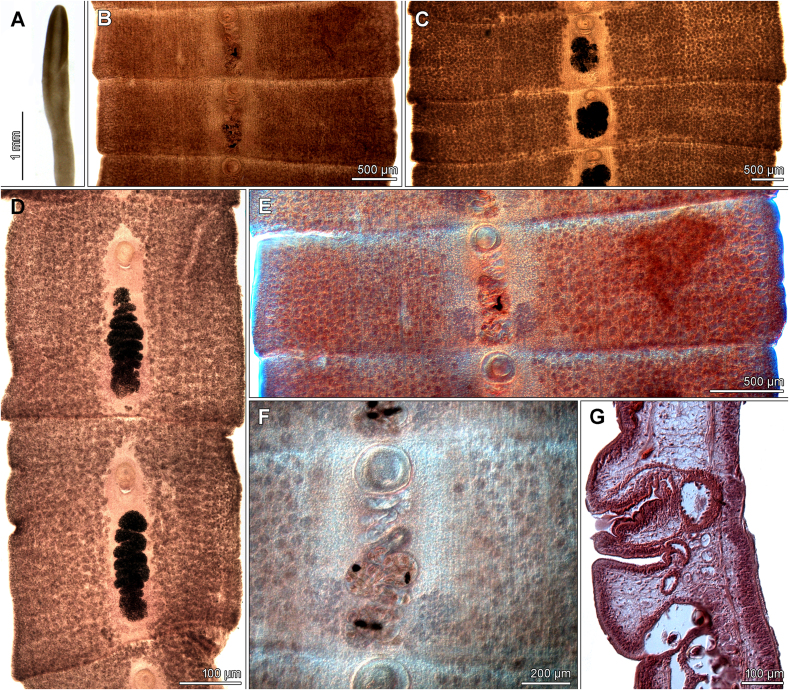
Fig. 2.
Microphotographs of Spirometra erinaceieuropaei from Canis lupus. A – Scolex from Ukraine. B–F – Gravid and mature proglottids from Poland. G – Sagittal section of gravid proglottid from Poland.
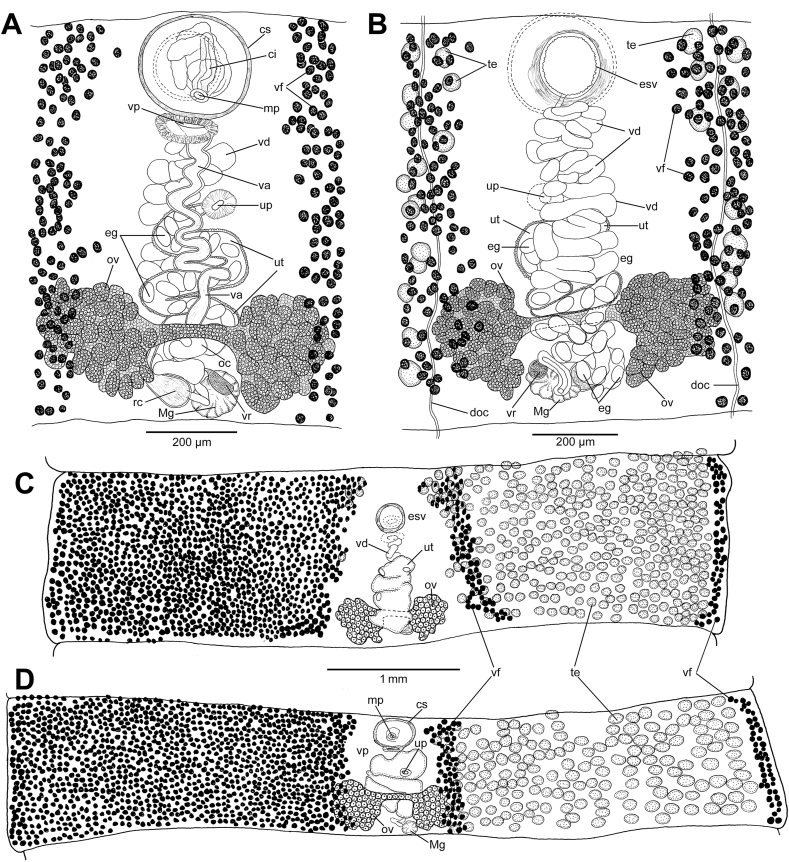
Fig. 3.
Line drawings of Spirometra erinaceieuropaei from Canis lupus, Poland. A, B – ovarian region and terminal genitalia; ventral (A) and dorsal (B) view of the same proglottid. C, D – gravid proglottids; dorsal (C) and ventral (D) view; note the shape of the ovary, which is short, but with wide lateral wings. Vitelline follicles and testes illustrated in one side of proglottids only, except for lateral-most and median-most vitelline follicles. Abbreviations: ci – cirrus; cs – cirrus sac; doc – dorsal osmoregulatory canal; eg – eggs; esv – external seminal vesicle; mp – male genital pore; Mg – Mehlis' gland; oc – oocapt; ov – ovary; rc – receptaculum seminis; te – testes; up – uterine pore; ut – uterus; va – vagina; vd – vas deferens; vf – vitelline follicles; vp – vaginal pore; vr – vitelline reservoir.
Synonyms: Dubium erinaceieuropaei Rudolphi, 1819; Spirometra janickii Furmaga, 1953; for other synonyms – see Caira et al. (2024).
Material examined: Specimens from Canis lupus L., Lubaczów, Poland, and Chernobyl, Ukraine (IPCAS C-110/1), Lynx lynx (L.), Kallasti, Finland (IPCAS C-110/2), Felis catus L. – experimental infection in Bologna, Italy (MHNG-PLAT-0045128); plerocercoids from Erinaceus europaeus L. (syntypes; ZMB-2246).
Type-host: European hedgehog Erinaceus europaeus (Eulipotyphla: Erinaceidae) – host of plerocercoids.
Definitive hosts (molecularly confirmed): wolf Canis lupus (Carnivora: Canidae); Eurasian lynx Lynx lynx; domestic cat Felis catus (both Carnivora: Felidae).
Intermediate (or paratenic) hosts (molecularly confirmed): Common racoon dog Nyctereutes procyonoides (Gray) (Carnivora: Canidae); European badger Meles meles (L.); Eurasian otter Lutra lutra (L.) (both Carnivora: Mustelidae); grass snake Natrix natrix (L.) (Squamata: Colubridae).
Type-locality: Europe (not specified more precisely — see Remarks).
Geographical distribution (molecularly confirmed): Europe (Belarus, Finland, Latvia, Poland, Ukraine).
Type-material: ZMB-2246 (see Fig. 2D in Scholz et al., 2021).
Representative DNA sequences: More than 300 sequences (mostly cox1) (Kołodziej-Sobocińska et al., 2018, 2019; Kuchta et al., 2021; Čisovská Bazsalovicsová et al., 2022; Piekarska et al. unpublished; GenBank).
Selected morphological features: All specimens examined are fragmented, only specimens from the wolf and cat are suitable for morphological study. They are relatively small and about 5 mm wide. The uterus forms numerous loops without conspicuous enlargement of the last (distal) coils as in S. decipiens and the ovary is narrow in relation to the proglottid width (ratio <0.23; Fig. 3) compared to other species (ratio >0.24). Spirometra erinaceieuropaei also differs from S. mansoni in the ratio of the length to the width of the ovary (0.32–0.48 in the wolf tapeworm compared to 0.10–0.22 in the neotype of S. mansoni).
Remarks
This is the oldest available species name accepted by most authors (Schmidt, 1986; Bray et al., 1994; Kamo 1999; Kuchta et al., 2021; Yamasaki et al., 2024), but not the type species of the genus, as erroneously mentioned by most of the authors (see above). The original description of S. erinaeieuropaei was based on plerocercoids of the European hedgehog from an unspecified location in Europe, most likely in the northern part of Central Europe (since 1810, C. A. Rudolphi worked in Berlin, the capital of the then province of Brandenburg-Prussia, later the Kingdom of Prussia, which extended to present-day Poland, Baltic states and Kaliningrad Oblast in Russia). Therefore, the Poland-Ukraine-Finland-Latvia strain/genotype was considered by Kuchta et al. (2021) to be the true S. erinaceieuropaei.
Another species, Bothriocephalus felis Creplin, 1825, was described from a cat in Greifswald (Germany), but due to the very superficial description based on two immature specimens, it is not possible to distinguish this worm from members of the genera Dibothriocephalus. Finally, Spirometra janickii Furmaga, 1953 was described by Furmaga (1953) from adult tapeworms of wolf and lynx in the Polish Białowieża Primeval Forest and from plerocercoids of the common shrew Sorex araneus L. and the fox Vulpes vulpes. The type material has been lost (R. Sałamatin, pers. comm.), but the presence of material of S. erinaceieuropaei in the same area and in the same host allows us to synonymise S. janickii with this species (see Kuchta et al., 2021).
Spirometra tapeworms are also known from Italy and southern France, but no molecular data are available from this area. A re-examination of extensive material of Joyeux and Baer (1927) (deposited in the MHNG), who analysed material from cats experimentally infected with plerocercoids of the grass snake in Bologna (Italy), suggests a possible conspecificity with S. erinaceieuropaei based on the relative size of the ovary. It appears that Spirometra ranarum (Gastaldi, 1854), described in an Italian green frog (Pelophylax esculentus [L.]) in Turin (Italy), may also be conspecific with S. erinaceieuropaei, but this should be verified by molecular data.
Molecular data confirm that S. erinaceieuropaei occurs only in Central and Eastern Europe and has a relatively low genetic variability (Čisovská Bazsalovicsová et al., 2022). However, this species name has often been erroneously applied to specimens of Spirometra from all over the world (Kamo 1999). The recently described species S. asiatica from Korea and Japan was also misidentified as S. erinaceieuropaei by Korean authors (see Kuchta et al., 2021; Yamasaki et al., 2021, 2024).
Spirometra decipiens (Diesing, 1850) Faust, Campbell et Kellogg, 1929 – type species Fig. 4.
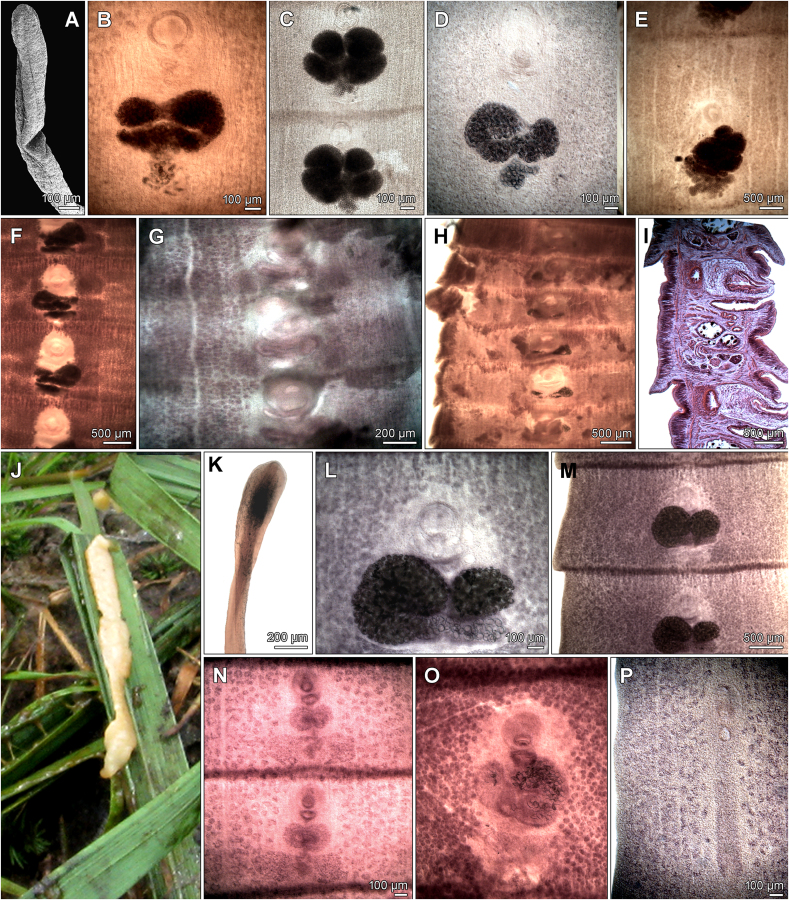
Fig. 4.
Microphotographs of Spirometra spp. from North and South America. A–C – Scolex and gravid proglottids of S. decipiens syntype from Puma concolor, Brazil (NMW 2682, 2699). D, E – gravid proglottids of S. decipiens syntype from Herpailurus yagouaroundi (NMW 12781). F–J – Gravid proglottids and sagittal section of S. decipiens hologenophore from Chrysocyon brachyurus, Bolivia (USNM 1233899). K–O – Scolex and proglottids of S. mansonoides syntype from Felis catus, USA (USNM 1333923). P – Immature proglottid of Spirometra sp. 2 hologenophore from Lynx rufus, USA Illinois (IPCAS C-987).
Synonyms: Dibothrium decipiens Diesing, 1850; Sparganum proliferum of Miyadera et al. (2001), Schauer et al. (2016), Arrabal et al. (2020); Spirometra decipiens species complex 2 (South American lineage) of Kuchta et al. (2021).
Material examined: Type specimens (syntypes) of Diesing (1850) from Panthera onca (L.) (NMW 2681, USNM 1368664), Puma concolor (L.) (NMW 2682, 2699) and Herpailurus yagouaroundi (Geoffroy) (NMW 12781), Brazil; vouchers from Chrysocyon brachyurus (Illiger), Bolivia (USNM 1233899).
Type-host: Not explicitly stated in the original description by Diesing (1850), but certainly a South American felid (syntypes are from big cats); the first listed was the puma P. concolor (see Diesing, 1850, 1856; Caira et al., 2024 for a complete list of original hosts).
Definitive hosts (confirmed molecularly): ocelot Leopardus pardalis (L.); Geoffroy’s cat Leopardus geoffroyi (d’Orbigny et Gervais) in Brazil (Carnivora: Felidae); maned wolf Chrysocyon brachyurus (Carnivora: Canidae) in Bolivia (USNM 1233899; examined by the present authors).
Intermediate (or paratenic) hosts (confirmed molecularly): Patagonia green racer Philodryas patagoniensis (Girard) (Squamata: Colubridae) in Uruguay.
Type-locality: Brazil (not further specified, but refers to the expeditions of the Austrian naturalist Johann Natterer into the rainforests of Brazil between 1817 and 1835).
Distribution and records: South America (Bolivia, Brazil, Uruguay).
Type-material: NMW 2621, 2622, 2624, 2681, 2682, 2699, 12779–12781, 12783, 12801, 12841, USNM 1368664.
Representative DNA sequences: Molecular data are available for several isolates from South American felids, a canid and a snake (Almeida et al., 2016; Armúa-Fernández et al., 2021; Trindade et al. unpublished; GenBank; present study). Škeříková et al. (2006) obtained an ITS2 sequence (DQ386133) allegedly from the type material of S. decipiens, but this sequence was not of a Spirometra species nad probably represents contamination.
Selected morphological features: All the specimens examined, including the type specimens, were decomposed because they came from dead hosts or from fresh faeces, as in the case of the specimen from the maned wolf (USNM 1233899). The tapeworms are relatively large and wide (up to 6 mm). The most characteristic feature of S. decipiens observed in the type specimens is the shape of the uterus, which consists of a few loops, with the conspicuous terminal (distalmost) C-shaped loop filled with numerous eggs (Fig. 4). The same feature was used by Mueller (1935, 1974) to distinguish S. mansonoides, described from North America, from other Spirometra species. The ovary of S. decipiens is relatively wider in relation to the proglottid width than in S. erinaceieuropaei, but similar to that of S. mansoni.
Remarks
This is the type species of Spirometra (see Faust et al., 1929) and the first species described from the New World (Brazil). Diesing (1850) briefly described Spirometra decipiens as Dibothrium decipiens from several South American felids from Brazil and the Vienna zoo. The same author (Diesing, 1856) provided further data on this species, including colour illustrations (see Caira et al., 2024). Diesing (1850) listed Bothriocephalus felis from a cat in Germany as a senior synonym of D. decipiens, but the tapeworms used for the description of D. decipiens were most likely only from large Brazilian felids collected by J. Natterer between 1817 and 1835; this material is still deposited in NMW. Therefore, S. decipiens is considered a typical species of these large cats in South America, which was also assumed by Ariola (1900) or Schmidt and Martin (1978).
A unique form of the uterus of S. decipiens was first described by Lühe (1899) and later by Schmidt and Martin (1978), who examined the type material of Diesing. Schmidt and Martin (1978) considered this shape of the uterine coils to be a useful feature for recognising S. decipiens. The present study based on Diesing’s type material (1850) confirmed that the uterus forms a conspicuous, C-shaped terminal loop (Fig. 4B, C). A similar shape of the uterus was found by Mueller (1935) in specimens from a cat in the United States, which he described as the new species S. mansonoides (Fig. 4L, M).
Kuchta et al. (2021) recognised two lineages of Spirometra tapeworms from South America, designated as S. decipiens species complex 1 and 2. The molecularly characterised specimens of complex 1 appear to have a ‘normal’ shape of the uterus consisting of several loops of similar size (Arrabal et al., 2020; Fredes et al., 2022). Therefore, they are not considered conspecific with the ‘true’ S. decipiens, although they occur in the same big cat species that harbour S. decipiens, such as ocelot and puma.
Molecular data also suggest that complex 2 may consist of two different lineages: (i) tapeworms from North America (morphological data for the only confirmed specimen from Lynx rufus [Schreber] in Wisconsin are not available) (see below), and (ii) specimens from South America, including the definitive hosts reported by Diesing (1850), i.e. ‘true’ S. decipiens; unfortunately, the available material for morphological studies is very limited and of poor quality.
The tapeworms of Chrysocyon brachyurus, which were found as worm fragments on grass from the faeces of a maned wolf on October 15, 2013 in Los Fierros, Noel Kampff National Park, Bolivia, collected by Louise Emmons, were examined by the present authors. The incomplete tapeworm of only gravid proglottids is contracted, but the enlarged terminal loops typical of S. decipiens are present (Fig. 4F, G). In addition, unpublished sequences of tapeworms from Geoffroy’s cat in Brazil (OR351540, OR351541) are designated in GenBank as S. mansonoides, possibly reflecting the shape of the uterus typical of this species. Based on the data discussed above, the tapeworms of Spirometra decipiens complex 2 (South American lineage) are considered to represent S. decipiens.
In addition, there are eight other nominal species that have been described from South America and still need to be verified (Table 1). They have been described mainly from Brazil, but the descriptions are incomplete and type material is not available or is of low quality (only histological sections and dark pieces of strobila are available), such as S. bresslaui and S. gracilis (MHNG-PLAT Nos. 56105; 56129) or S. trinitatis and S. urichi (CMNPA 1995-0151/1995-0160; 1995-0143/1995-0150).
Table 1.
List of nominal species of Spirometra described from South America.
| Species | Authority | Type host or first listed host | Distribution (country) |
|---|---|---|---|
| Dibothrium decipiens | Diesing, 1850 | Felis catusa | Brazil |
| Ligula reptans | Diesing, 1850 | Saimiri sciureus (plerocercoids)b | Brazil |
| Dibothrium serratum | Diesing, 1850 | Cerdocyon thousc | Brazil |
| Canis familiaris | |||
| Bothriocephalus didelphidisg | Ariola, 1900 | Didelphis auritad | Brazil (São Paulo) |
| Bothriocephalus longicollis | Parodi et Widakowich, 1917 | Panthera onca, | Argentina (ZOO) |
| Puma yagouaroundie | |||
| Diphyllobothrium bresslaui | Baer, 1927 | Didelphis aurita | Brazil (Rio de Janeiro) |
| Diphyllobothrium gracile | Baer, 1927 | Leopardus wiediif | Brazil (Teresópolis) |
| Diphyllobothrium trinitatie | Cameron, 1936 | Procyon cancrivorag | Suriname, Trinidad |
| Diphyllobothrium urichi | Cameron, 1936 | Leopardus pardalish | Trinidad |
First listed host (as synonym of Bothriocephalus felis Creplin 1825); first listed South American host is Felis onca (= Panthera onca).
First listed host (as Callithrix sciurea), subcutaneous follicle.
First listed host (as Canis azarae), intestine.
Reported as Didelphys azarae collected by Lutz; later misspelled as "didelphydis" by Ariola (1900).
Reported as Felis onca and Felis yaguarandi, respectively.
Reported as Felis (Oncoides) macrura which is most probably misspelled Felis macroura, a synonym of Leopardus wiedii.
Reported as Procyon carnivora.
Reported as Felis pardalis.
Spirometra mansoni (Cobbold, 1882) Faust, Campbell et Kellogg, 1929 Fig. 5, Fig. 6.
Fig. 5.
Microphotographs of Spirometra mansoni neotype from experimentally infected dog Canis familiaris, Japan. A – Whole unstained neotype. B, C – SEM photo of scolex and gravid proglottid. D – Whole mounts with part of neotype. E, F – Mature proglottids. G, H, K – Gravid proglottids. I, J – Sagittal section of gravid proglottid.
Fig. 6.
Line drawing of gravid proglottid of the neotype of Spirometra mansoni from Canis familiaris, Japan (IPCAS C-988), ventral view; note the shape of the ovary, which is long, with narrow lateral wings. Vitelline follicles and testes illustrated in one side of proglottids only, except for lateral-most and median-most vitelline follicles.
Synonyms: Bothriocephalus marginatus Krefft, 1871; Bothriocephalus liguloides Leuckart, 1886; Dibothrium tangalongi MacCallum, 1921; Sparganum phillipiensis Tubangui, 1924; Diphyllobothrium (Spirometra) houghtoni Faust, Campbell et Kellogg, 1929; Diphyllobothrium (Spirometra) okumurai Faust, Campbell et Kellogg, 1929; Diphyllobothrium fausti Vialli, 1931; Ligula ranarum of Meggitt (1924); Diphyllobothrium (Spirometra) decipiens of Faust et al. (1929); Diphyllobothrium (Spirometra) erinacei of Faust et al. (1929); Spirometra decipiens of Jeon et al. (2015); Spirometra ranarum of Eom et al. (2018) and Jeon et al. (2018).
Material examined: Neotype from experimentally infected Canis familiaris in Japan by T. Okino (IPCAS C-988; vouchers IPCAS C-242; Fig. 6); vouchers from Felis catus in Laos (IPCAS C-950/1).
Type-host: Human Homo sapiens L. (Primates: Hominidae).
Additional definitive hosts (molecularly confirmed): Domestic cat Felis catus in Australia, Laos and South Korea; jungle cat Felis chaus Schreber in Iran; Amur leopard Panthera pardus orientalis (Schreber) in Russian Far East; tiger Panthera tigris (L.) in China; leopard cat Prionailurus bengalensis Kerr in China; wild cat Felis silvestris Schreber in Iran; lion Pantera leo (L.) in Tanzania (all Carnivora: Felidae); crab-eating fox Cerdocyon thous (L.) in Colombia; domestic dog Canis familiaris in Australia, India, Indonesia, Japan, Laos (both Carnivora: Canidae).
Intermediate (or paratenic) hosts (confirmed molecularly): Many amphibians, reptiles and mammals, including humans (see Kuchta et al., 2021).
Type-locality: Amoy (now Xiamen), China.
Geographical distribution and records: Worldwide distribution, with most records from Asia (China, India, Indonesia, Iran, Japan, Korea, Laos, Myanmar, Thailand, Vietnam) and Australia, rarely from Africa (Tanzania, Mauritius), Europe (Romania), Oceania (New Zealand), North and South America (USA, Colombia – Jeon et al., 2016 (see Fig. 1).
Type-material: Does not exist; therefore, neotype of S. mansoni is designated (IPCAS C-988).
Representative DNA sequences: Too numerous to list separately (see Kuchta et al., 2021; Brabec et al., 2022; Yamasaki et al., 2024; present study).
Selected morphological features: Heat-fixed specimens are relatively small, up to 5 mm wide (Fig. 5A). The uterus consists of several loops. Compared to S. erinaceieuropaei (17–23% and 0.32–0.42) and S. asiana (24–32% and 0.42–0.59), the ovary of S. mansoni is relatively wider (about 40–50% of the proglottid width) and narrower (ratio length/width of ovary 0.10–0.26) (Fig. 6). The eggs of S. mansoni are smaller (55–72 × 32–42 μm; mean 58 × 36 μm of the neotype) than those of S. asiana (74–80 μm × 33–41 μm wide), but similar to those of S. erinaceieuropaei (54–59 × 38–43 μm) (see Yamasaki et al., 2024; present study).
Remarks
This species was described as a larva found at autopsy of a man in Amoy (now Xiamen, China), and it is the first taxon described in Asia. Almost all specimens from China and other Asian countries are considered conspecific with S. mansoni based on molecular data. This is by far the most common and widespread species of Spirometra, which has probably also been introduced to Africa, Australia and Oceania, south-eastern Europe (Romania) and North and South America (Jeon et al., 2016; Kuchta et al., 2021; Brabec et al. 2023). It is also the most important pathogen causing sparganosis and spirometrosis in humans (Kuchta et al., 2021). Jeon et al. (2015) misidentified specimens of S. mansoni from Korea as S. decipiens, which is endemic to the Americas (see above). Jeon et al. (2016) and Eom et al. (2018) also misidentified S. mansoni from Myanmar and Tanzania as S. ranarum, a species of uncertain taxonomic status known only from Europe (see below).
In general, high genetic diversity, moderate genetic differentiation and low genetic exchange between geographical populations in China have been observed (Xu et al., 2022).
Spirometra theileri (Baer, 1924); Opuni et Muller, 1974 Fig. 7.
Fig. 7.
Microphotographs of Spirometra spp. and Dibothrium folium from Africa. A–D – Scolex, proglottids and sagittal section of S. theileri from Panthera leo, DR Congo material of Baer (1959) (RMCA 32316). E – Scolex of D. folium, type specimen (NMW No. 2616). F, G – Gravid proglottid and sagittal section of hologenophore from Panthera pardus, South Africa (IPCAS C-986). H, I – Gravid proglottid and sagittal section from P. pardus, Siera Leone (NHMUK 1924.6.12.116). J – Gravid proglottid from P. pardus, DR Congo (NHMUK 1934.12.18.51). K, P – Gravid proglottid and mounted specimen from Crocuta crocuta, Tanzania (NHMUK 1937.10.20.26–30). L, M – Gravid proglottid and sagittal section from P. leo (RMCA 32316). N, O – Gravid proglottid and sagittal section from P. pardus, DR Congo (1934.12.18.51–54). Q, R – Syntype slides of Diphyllobothrium theileri from Leptailurus serval, South Africa (MHNG-PLAT 40726). S – Syntype slide of Lueheella pretoriensis from Otocyon megalotis, South Africa (MHNG-PLAT 41517).
Synonyms: Sparganum baxteri Sambon, 1907 (?); Diphyllobothrium theileri Baer, 1924; Lueheella pretoriensis Baer, 1924 (?); Spirometra pretoriensis (Baer, 1924) Wardle, McLeod et Stewart, 1947 (?); Spirometra Africa II of Opuni and Muller, 1974); Spirometra sp. of Eberhard et al. (2015); Spirometra folium of Kuchta et al. (2021).
Material examined: Syntypes of S. theileri from Leptailurus serval (Schreber) and Felis lybica cafra Desmarest, South Africa (MHNG-PLAT 40724–26, 56111–12, 56218, 56143, 56145); syntypes of S. pretoriensis from Otocyon megalotis (Desmarest), South Africa (MHNG-PLAT 41517–18, 56137, 60952); hologenophore from Panthera pardus pardus, Mpumalanga Province, South Africa, collected by Sonia Dumendiak ((SAF48a – IPCAS C-986); vouchers of uncertain taxonomic position: from Crocuta crocuta (Erxleben), Tanzania (NHMUK 1937.10.20.26–30), from Panthera leo (L.), DR Congo (NHMUK 1967.11.27.51–55, RMCA 32316); from P. p. pardus, Sierra Leone (NHMUK 1924.6.12.116) and DR Congo (1934.12.18.51–54); specimens (in poor condition) from Caracal caracal (Schreber), Prague Zoo (IPCAS C-109).
Type-host: Serval Leptailurus serval (as Zibethailurus serval) – first listed, Southern African wildcat Felis lybica cafra.
Definitive hosts (molecularly confirmed): Leopard Panthera pardus; lion Panthera leo (Carnivora: Felidae); spotted hyena Crocuta crocuta (Carnivora: Hyaenidae).
Intermediate (or paratenic) hosts (molecularly confirmed): Human Homo sapiens (Primates: Hominidae) (Eberhard et al., 2015); most probably also other mammals such as desert warthog Phacochoerus aethiopicus (Pallas) or spotted hyena (see Opuni and Muller 1974).
Type-locality: Moovilei (first listed) and Bridgewater, Rustenburg, South Africa.
Distribution and records: Africa (Chad [?], DR Congo, Ethiopia, Kenya, Sierra Leone, South Africa, South Sudan, Sudan, Tanzania) (Eberhard et al., 2015; Kuchta et al., 2021); potentially also in other African countries (see Supplementary Table S1).
Type-material: Syntypes in MHNG (see above for collection numbers).
Representative DNA sequences: Eberhard et al. (2015); Eom et al. (2019); Ndosi et al. (2020, 2021); present study.
Selected morphological features: All specimens examined were decomposed because they came from dead hosts. The tapeworms of the spotted hyena are relatively large and wide (up to 13 mm), similar to the syntypes of S. pretoriensis which are up to 10 mm wide, as also reported by Graber (1981). The uterus of S. theileri forms several loops, and the ovary is relatively long and narrow, similar to the Palaearctic species.
Remarks
The first Spirometra species described from Africa is Dibothrium folium Diesing, 1850 from Ichneumia albicauda (Cuvier) (Carnivora: Herpestidae). This was the reason why Kuchta et al. (2021) referred to the African isolates of Spirometra as S. folium. However, a recent study of the type of this species (NMW 2616) and Diesing’s (1856) drawings of D. folium has shown that it is not a species of Spirometra. Although the type material is in poor condition (the worm is very dark and its anatomy is not recognisable), it has a broad, arrow-shaped scolex (Fig. 7E), which is never present in Spirometra species. In contrast, a similar lanceolate scolex is typical of species of Duthiersia Perrier (1873) from varanid lizards, which also occur in Africa, or some Diphyllobothrium species (see Kuchta and Scholz, 2017; Waeschenbach et al., 2017).
As D. folium has never been described since its first description, it is not possible to reliably identify this taxon to genus level and it is considered a nomen dubium. Based on the ITS2 sequence of the type specimen of D. folium (DQ386134) obtained by Škeříková et al. (2006), it is identical to Diphyllobothrium cordatum (Leuckart, 1863). However, later attempts to obtain other sequences from this specimen failed (unpublished data).
The second species of Spirometra described from Africa is Sparganum baxteri Sambon, 1907. A 15 cm long plerocercoid was removed from an abscess on the thigh of a Maasai in British Central Africa (now Malawi). The plerocercoid showed numerous irregular transverse folds and a pronounced longitudinal furrow on the ventral surface. The anterior end was 2–5 mm wide, and the scolex was completely invaginated (Sambon, 1907). It is possible that this plerocercoid corresponds to some adult Spirometra species described from African carnivores such as S. theileri (see Opuni and Muller 1974), but this taxon is provisionally considered to be a species inquirenda. Plerocercoids isolated from humans in South Sudan and Ethiopia were molecularly characterised by Eberhard et al. (2015) and all seven isolates are closely related to other sequenced tapeworms from Africa, here named S. theileri (Fig. 1).
Baer (1924) described two species of Diphyllobothriidea from carnivores in South Africa, namely Lueheella pretoriensis Baer, 1924 (as the type and only species of the new genus Lueheella, which was never recognised as valid) and Diphyllobothrium theileri Baer, 1924. Both species were then placed in Spirometra by Wardle et al. (1974) and Opuni and Muller (1974), respectively. However, the available molecular data from several adult tapeworms of carnivores and plerocercoids of humans throughout Africa confirmed the presence of a single genotype more similar in size to S. theileri than to S. pretoriensis.
Spirometra theileri was described from the serval and the Southern African wildcat, both hosts near Rustenburg, South Africa (Baer, 1924, 1925). Later, Baer and Fain (1955) and Baer (1959) reported S. theileri in lion, leopard and serval, both from the DR Congo. Material from lion in the DR Congo (RMCA 32316; Fig. 7L, M) was examined by the present authors, and its morphology is fully consistent with the description of S. theileri and the hologenophore of the sequenced material from leopard from South Africa (IPCAS C-986; Fig. 7F, G). Graber (1981) found S. theileri in Chad in F. lybica, Acinonyx jubatus (Schreber), Canis aureus (most likely Lupulella mesomelas [Schreber], as C. aureus does not occur in Africa) and Panthera pardus.
Spirometra pretoriensis was described from an incomplete and highly contracted specimen (no scolex present) from the bat-eared fox and then reported from spotted hyena and African wild dog in Ethiopia and Zambia (Graber, 1981). The morphology of this species is poorly known, but it has a more massive, much broader strobila (>70 cm long and up to 10 mm wide) than S. theileri (total length <35 cm, maximum width of 3.5 mm; Graber, 1981). Odening (1985) mentioned that the configuration of the uterus of S. pretoriensis is similar to that of S. mansonoides (and S. decipiens), but the type material is highly contracted and not suitable for morphological description. In contrast, the type material of S. theileri contains a scolex and the strobilar morphology corresponds to that of the sequenced specimens from leopard for which hologenophores (morphological vouchers) are available (Fig. 1). In addition, tapeworms identified as S. theileri have been repeatedly reported from African carnivores (Round, 1968; Graber, 1981).
Only recently, several tapeworms of leopard, spotted hyena and lion in Tanzania were characterised molecularly and morphologically as S. theileri (Eom et al., 2019; Ndosi et al., 2020). These tapeworms included relatively smaller tapeworms in a leopard (45 cm long and probably 6.0–6.5 mm wide; the original values, i.e. 0.60–0.65 mm, are apparently erroneous) and much larger specimens in a spotted hyena (135 cm × 10 mm), but both specimens are genetically identical and belong to the same species, for which the name S. theileri is proposed to maintain taxonomic stability.
Tapeworms from the leopard recently collected by S. Dumendiak in South Africa (present study) are genetically indistinguishable from tapeworms found in various felids in Tanzania and are tentatively identified as S. theileri. In addition, Eom et al. (2018) reported a large tapeworm (75 × 1.2 cm) from a lion; this specimen, identified as S. ranarum, is genetically identical to S. mansoni. Ndosi et al. (2020) found tapeworms in leopard that were 45 cm long and 0.9 cm wide; they were misidentified as S. erinaceieuropaei, but actually belong to S. asiana. However, this sequence is very short, which casts doubt on the correct identification.
In summary, the species identity of Spirometra tapeworms in Africa is uncertain and future research based on matching molecularly characterised specimens with their morphological vouchers (hologenophores) is highly desirable. Currently, S. theileri is thought to be the species that occurs as adults in African carnivores, while its plerocercoids can infect humans and cause sparganosis (Opuni and Muller, 1974; Eberhard et al., 2015). If future research proves the existence of other species, S. baxteri could be a candidate for the designation of tapeworms that parasitise humans.
Spirometra asianaYamasaki, Sugiyama et Morishima, 2024.
Synonyms: Spirometra erinaceieuropaei of Eom et al. (2015), Jeon et al. (2015), Kudo et al. (2017); Spirometra Type II of Yamasaki et al. (2021); Spirometra sp. 1 of Kuchta et al. (2021).
Material examined: not available.
Type-host: Domestic dog Canis familiaris (Carnivora: Canidae).
Additional definitive host (molecularly confirmed): Domestic cat Felis catus (South Korea; experimental host); leopard Panthera pardus pardus (?) (Tanzania) (Carnivora: Felidae – Ndosi et al., 2020).
Intermediate (or paratenic) hosts (molecularly confirmed): Japanese boar Sus scrofa leucomystax Temminck) (Japan) (Artiodactyla: Suidae); human Homo sapiens (Japan, Korea).
Type-locality: Yasakacho Takauchi, Hamada City, Japan.
Geographical distribution: East Asia (Japan, Korea), Africa (?) (Tanzania).
Type-material: Meguro Parasitological Museum, Tokyo, Japan (MPM 21893 – holotype, MPM 21894a, b – paratypes).
Representative DNA sequences: Jeon et al. (2015), Kudo et al. (2017), Ndosi et al. (2020) – as S. erinaceieuropaei, Yamasaki et al. (2024).
Selected morphological features: Only two adult specimens are available (Jeon et al., 2015; Yamasaki et al., 2024); they are relatively large worms (holotype 2.43 m long) and about 8 mm wide. The uterus forms numerous loops and the ovary is relatively wide (ovary length/width ratio 0.42–0.59) and narrow (ovary width is 24–32% of the proglottid width), resembling the ovary of S. erinaceieuropaei. The eggs are larger than those of S. mansoni and S. erinaceieuropaei (74–80 μm long and 33–41 μm wide; average 77 × 33 μm).
Remarks
This is the best characterised species, which is genetically distinct from the widespread S. mansoni (see Yamasaki et al., 2024). It was recently described by Yamasaki et al. (2024) using adult cestodes from dogs and plerocercoids from wild boars in Japan. These tapeworms belong to the second, rare Asian lineage recognised by Kuchta et al. (2021) and Yamasaki et al. (2021).
Plerocercoids (spargana) of this species were found by Jeon et al. (2015) and Kudo et al. (2017) in 35 individuals from Korea and one patient from Japan, respectively. All specimens were misidentified as S. erinaceieuropaei by Jeon et al. (2015) and Eom et al. (2015) as well as in other studies by these authors. Recently, Ndosi et al. (2020) confirmed a single case of S. asiana (misidentified as S. erinaceieuropaei) in a leopard in Africa. The validity of this record should be checked as the sequence is short and does not match well with the regions covered by other sequences.
Spirometra sp. 2 (American lineage)Fig. 4.
Synonym: Spirometra decipiens complex 1 of Kuchta et al. (2021) and Vettorazzi et al. (2023).
Material examined: Only one immature piece (hologenophore) from Lynx rufus, USA (Illinois) (IPCAS C-987).
Definitive host (molecularly confirmed): Domestic cat Canis familiaris in Uruguay; crab-eating fox Cerdocyon thous in Brazil and Uruguay; domestic cat Felis catus in Brazil and Chile; lesser grison Galictis cuja (Molina) in Brazil; Geoffroy’s cat Leopardus geoffroyi in Brazil; pampa cat Leopardus munoai (Ximénez) in Uruguay); ocelot Leopardus pardalis in Argentina; pampas fox Lycalopex gymnocercus (Fischer) in Argentina; hoary fox Lycalopex vetulus (Lund) in Brazil; bobcat Lynx rufus in USA; puma Puma concolor in Argentina.
Intermediate (or paratenic) hosts (molecularly confirmed): Human Homo sapiens in Venezuela; Crotalus sp. in Brazil; Philodryas patagoniensis in Uruguay; Didelphis albiventris in Brazil, Uruguay; Austrolebias charrua in Uruguay.
Geographical distribution: South America (Argentina, Brazil, Chile, Uruguay, Venezuela), North America (USA – Illinois).
Representative DNA sequences: Miyadera et al. (2001), Petrigh et al. (2015), Almeida et al. (2016), Arrabal et al. (2020), Armúa-Fernández et al. (2021), Kuchta et al. (2021), Fredes et al. (2022), Kikuchi et al. (2021), Vettorazzi et al. (2023); Trindade et al. unpublished – see the Genbank database).
Selected morphological features: The uterus is similar in appearance to the Palaearctic species, i.e. it lacks the conspicuously enlarged C-shaped distal loops typical of S. decipiens (Fig. 4).
Remarks
Kuchta et al. (2021) found two different lineages of Spirometra tapeworms in North and South America and named them S. decipiens complex 1 and S. decipiens complex 2. Tapeworms of complex 1 form a relatively well-supported lineage between the European S. erinaceieuropaei and the predominantly Palaearctic S. mansoni and S. asiana (Fig. 1). This complex 1 consists of specimens of various South American carnivores (adults) and plerocercoids of reptiles and humans, including the enigmatic Sparganum proliferum (Arrabal et al., 2020). Vettorazzi et al. (2023) have also indicated the rivulid killifish Austrolebias charrua as paratenic host of this tapeworm, which is the first report of Spirometra plerocercoids in fish.
Spirometra sp. 3 (North American lineage) Fig. 4
Synonym: Spirometra decipiens complex 2 (North American lineage) of Kuchta et al. (2021).
Possible synonym: Spirometra mansonoides (Mueller, 1935) Mueller, 1936.
Material examined: syntypes of S. mansonoides from Felis catus L., USA (USNM 1333923).
Definitive host (molecularly confirmed): Bobcat Lynx rufus in USA (Wisconsin) (Carnivora: Felidae).
Intermediate (or paratenic) hosts (molecularly confirmed): Eastern racer Coluber constrictor (L.) in USA (Mississippi); western rat snake Pantherophis obsoletus (Say in James) in USA (Louisiana); Thamnophis proximus in USA (Mississippi) (Squamata: Colubridae); meerkat Suricata suricatta (Schreber) in USA (South Carolina zoo) (Carnivora: Herpestidae).
Geographical distribution: USA (Louisiana, Mississippi, South Carolina, Wisconsin).
Representative DNA sequences: Waeschenbach et al. (2015), McHale et al. (2020).
Remarks
Tapeworms of the part of the former S. decipiens complex 2 from large cats in South America, including Brazil, where S. decipiens was described by Diesing (1850), are considered to be ‘true’ S. decipiens (see above) and the second lineage from reptiles and bobcats from North America form an independent lineage (possibly a separate species). In contrast, it remains an open question whether the tapeworms of the other North American lineage (S. decipiens complex 2) actually belong to S. mansonoides, which was described in a domestic cat in New York State (see below; Fig. 1).
4.2. Species of unclear taxonomic status
Spirometra ranarum (Gastaldi, 1854) Faust, Campbell et Kellogg, 1929.
Synonym: Ligula ranarum Gastaldi, 1854.
Remarks
This little-known species of uncertain taxonomic status was described by Gastaldi (1854) on the basis of plerocercoids found in the green frog Pelophylax esculentus (L.) from northern Italy (Turin). Meggitt (1924) reported this species, including experimentally obtained adults, from Asia (Myanmar) (MHNG-PLAT 56230; examined by the present authors). This obvious misidentification with S. mansoni was later repeated by Faust et al. (1929) in the identification of specimens from China. Molecular data on ‘S. ranarum’ were presented by Eom et al. (2018), Jeon et al. (2018) and Ndosi et al. (2020) for isolates from Korea, Myanmar and Tanzania, but all tapeworms actually belong to S. mansoni based on the molecular data (see Kuchta et al., 2021).
Molecular data on S. ranarum from southern Europe (Italy and France) are not available to confirm the validity of this species, which could be conspecific with the European S. erinaceieuropaei or with the cosmopolitan S. mansoni, which was molecularly confirmed in ranid frogs (Pelophylax spp.) from the Danube Delta in Romania (Kuchta et al., 2021).
Spirometra raillieti (Rátz, 1913) Wardle, McLeod et Stephanson, 1985
Synonym: Sparganum raillieti Rátz, 1913.
Remarks
The systematic status of this species, described from plerocercoids of domestic pigs (Sus scrofa L.) in Hungary and Serbia, is unclear as it has never been reported since its first description and no molecular data on Spirometra from Hungary are available to confirm the validity of this poorly characterised species. Kotlán (1923) obtained adult tapeworms from experimentally infected dogs, but was unable to become infected himself. The geographically closest record of Spirometra tapeworms comes from Romania, where the presence of S. mansoni in green frogs (Pelophylax spp.) was confirmed by molecular data (Kuchta et al., 2021).
Spirometra mansonoides (Mueller, 1935) Mueller, 1936 Fig. 4K–O.
Synonym: Diphyllobothrium mansonoides Mueller (1935).
Remarks
This species was described by Mueller (1935) as Diphyllobothrium mansonoides, based on adult tapeworms found in domestic cat near Syracuse, New York, USA. The type material was examined by the present authors (USNM 1333923). Morphologically, S. mansonoides differs from most Spirometra species (mainly S. asiana, S. erinaceieuropaei, S. mansoni and S. theileri) in that the uterus consists of two large terminal loops and forms a typical C-shaped terminal part of the uterus (Fig. 4). However, this type of uterus is also present in S. decipiens (see Lühe 1899; Schmidt and Martin 1978; Mueller 1935, 1974; present study). According to Marucci and Mueller (1972), the plerocercoids of S. mansonoides are more delicate and fragile than those of other Spirometra species. However, no molecular data on S. mansonoides are available, which makes a clear characterisation of this species impossible.
The available sequences of Spirometra tapeworms from the USA include two lineages designated by Kuchta et al. (2021) as ‘S. decipiens complex 1’ (=‘true’ S. decipiens; see above) from the bobcat in Illinois, and ‘S. decipiens complex 2’ from snakes in Mississippi, and the meerkat in a zoo in South Carolina. Molecular data on tapeworms from the region where the type locality is located (Syracuse, New York) are needed to clarify the taxonomic status and confirm the validity of S. mansonoides.
Bothriocephalus maculatus Leuckart, 1848.
Remarks
It is likely that this species is related to the genus Spirometra. However, it was only briefly described by Leuckart (1848), using material from an imported “leopard” (probably Panthera pardus) of unknown origin. It is therefore not possible to identify this species. Due to this incomplete description, this species is considered a species inquirenda.
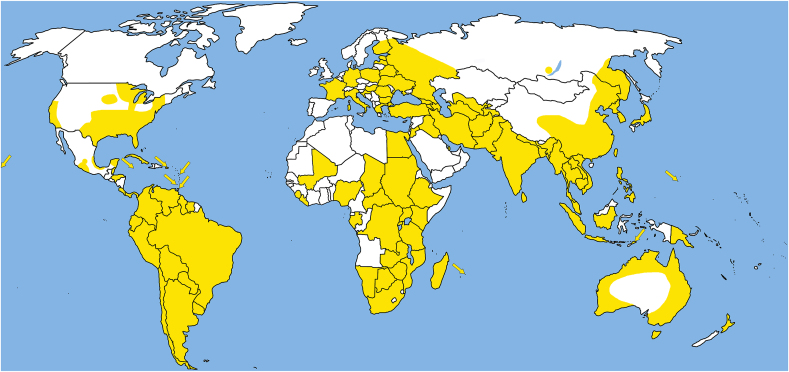
5. Geographical distribution
Spirometra tapeworms are distributed worldwide and have been reported from all continents except Antarctica, including some isolated islands such as Guam, Hawaii, Madagascar, Mauritius, New Zealand and Puerto Rico (Daly, 1981; Scholz et al., 2019) (Fig. 8; Supplementary Table S1). Most records come from South and East Asia, but relatively many also from South America. Only a few records have been published from Africa and some from the USA thanks to the numerous studies by J. F. Mueller (Supplementary Table S1). However, it is difficult to provide more precise information on the occurrence of individual species, as most published reports cannot be reliably assigned to any of these species, with the exception of specimens that have been sequenced (see Supplementary Table S1). The main reasons for the lack of reliable species identification are discussed in Chapter 2. In addition, the geographical separation of individual species may be questionable, as Spirometra tapeworms may also have been spread throughout the world by domestic dogs and cats (Mueller, 1974; Odening, 1979; Daly, 1981). For example, Spirometra may have been introduced to Australia with the arrival of placental mammals (Eutheria), possibly also through human activities (Stephanson 1985).
Fig. 8.
Map of the distribution of Spirometra spp. in the world (in yellow). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Despite the obstacles mentioned above, it is obvious that most records of Spirometra tapeworms originate from subtropical and tropical latitudes with warmer climates (Fig. 8). In this respect, Spirometra tapeworms differ from Dibothriocephalus tapeworms, which are more common in colder climates, i.e. in temperate zones, including the Arctic (Scholz et al., 2019). In addition, a certain geographical pattern can be observed in the distribution of the individual species that have been genetically characterised (Kuchta et al., 2021; Yamasaki et al., 2024). In short, most species appear to have a rather limited geographical distribution (see Fig. 3 in Kuchta et al., 2021; further details are given in a review of individual valid species – Chapter 4 – and in a review of the fauna of individual continents below): (i) S. erinaceieuropaei is possibly endemic to Europe, as there are no reliable records of this species outside Europe supported by molecular data; (ii) S. theileri is probably endemic to Africa; (iii) S. decipiens and Spirometra sp. 2 and 3 (American lineage 2) are possibly endemic to the Americas; (iv) the recently described S. asiana has been found in Japan and Korea; Ndosi et al. (2020) have reported the alleged occurrence of this species in Africa (Tanzania) but this record needs to be verified. In contrast, the most widespread S. mansoni, by far the most commonly reported in humans, has a wide geographical distribution (Eurasia, Australia and Africa), with a recent molecularly confirmed case from Colombia (Brabec et al., 2022).
5.1. Africa
The first species named from Africa is ‘Spirometra’ folium, but it is in fact not a species of Spirometra (see above). Several other species have been described from Africa (see Scholz and Kuchta, 2019), such as Sparganum baxteri, Spirometra pretoriensis and Spirometra theileri (see Sambon, 1907; Baer, 1924, 1925); the last species is the most common and considered valid (see above).
Spirometra tapeworms have been reported in about 40 records from the following countries, including human cases (Supplementary Table S1; Fig. 8): Central African Republic, Chad, DR Congo, Egypt, Ethiopia, Gabon, Kenya, Liberia, Madagascar, Mali, Mozambique, Namibia, Nigeria, Republic of Congo, Rwanda, Sierra Leone, South Africa, South Sudan, Sudan, Tanzania, Zambia and Zimbabwe. However, molecular data on Spirometra tapeworms in Africa are scarce and genetically characterised specimens are only available for two, possibly three species from the following countries: Ethiopia (S. theileri), Mauritius (S. mansoni), South Africa (S. theileri), South Sudan (S. theileri) and Tanzania (S. theileri, S. asiana (?) and S. mansoni) (Eberhard et al., 2015; Ndosi et al., 2020; Kuchta et al., 2021; present study).
The definitive host of Spiromera tapeworms in Africa is mainly the spotted hyena, but they are also frequently reported from lions and leopards (Nelson et al., 1965; Engh et al., 2003; Berentsen et al., 2012; Kavana et al., 2015; Ndosi et al., 2020). Plerocercoids are known from mammals such as desert warthog, the spotted hyena, but also from humans with sparganosis. Opuni and Muller (1974) experimentally infected dogs with plerocercoids of Phacochoerus aethiopicus and obtained adults identified as S. theileri (material not available). They maintained the entire life cycle in England, where they infected several mammals that served as a second intermediate or paratenic host (rodents, rhesus monkeys). However, they were unable to infect amphibians and reptiles, which are commonly used as second intermediate hosts of S. erinaceieuropaei or S. mansoni.
5.2. Asia
In Asia, two Spirometra species have been confirmed by molecular data, with the widespread, probably cosmopolitan S. mansoni dominating; most records come from East (China, Japan) and Southeast Asia (Laos, Thailand and Vietnam). There are reports from the following countries: Azerbaijan, Bangladesh, China, Georgia, India, Indonesia, Iran, Iraq, Japan, Jordan, Malaysia, Philippines, Russia, South Korea, Sri Lanka, Tajikistan, Thailand, Turkmenistan, Uzbekistan and Vietnam (see Supplementary Table S1).
Molecular evidence for the occurrence of S. mansoni is available from the following 11 countries (see Supplementary Table S1): Cambodia, China, India, Indonesia, Iran, Laos, Japan, Myanmar, South Korea, Thailand and Vietnam. The second Asian lineage of Spirometra (see Kuchta et al., 2021) was recently described as S. asiana by Yamasaki et al. (2024) from Japan and South Korea. The report by Ndosi et al. (2020) of S. asiana (misidentified as S. erinaceieuropaei) in a leopard in Africa should be confirmed.
5.3. Australia and Oceania
Spirometra tapeworms are widely distributed in Australia, with the exception of the central part of the country, and are common in introduced placental mammals. The prevalence of adults can be high (up to 60%) in cats and foxes, but much lower in dogs (Stephanson 1985). Spirometra tapeworms have also been reported from isolated islands such as Guam, Hawaii, New Zealand, Papua New Guinea and Tasmania (Supplementary Table S1). However, molecular data from this zoogeographical region are sparse and confirm the presence of the widespread S. mansoni in dogs and cats from Melbourne (Waeschenbach et al., 2017), a cat from Manawatu, New Zealand (Ugarte et al., 2005 – erroneously as S. erinaceieuropaei), as well as plerocercoids from the Australian green tree frog Ranoidea caerulea (White) imported to Europe (Fischer et al., 2010), and the lowland copperhead Austrelaps superba (Günther) in Victoria (Kuchta et al., 2021).
5.4. Europe
Several species have been described from Europe, but their validity is questionable. Spirometra tapeworms, especially plerocercoids from second intermediate hosts, including humans, have been reported from 17 countries (Kuchta et al., 2021, Fig. 8). Currently, the presence of only two species, S. erinaceieuropaei and S. mansoni, has been confirmed based on molecular data (Kuchta et al., 2021). Molecular data confirmed that S. erinaceieuropaei occurs in Central and Eastern Europe and has relatively low genetic variability (Čisovská Bazsalovicsová et al., 2022).
However, this species name has often been erroneously applied to specimens of Spirometra from around the world, including the recently described S. asiatica from Korea and Japan, which was also misidentified as S. erinaceieuropaei by Korean authors (see Kuchta et al., 2021; Yamasaki et al., 2021, 2024). The cosmopolitan S. mansoni was recently found as a plerocercoid in green frogs in Romania, but the species identity of Spirometra tapeworms reported from the Mediterranean and the Balkans is still unclear (Kuchta et al., 2021).
The systematic status of Spirometra ranarum, described as a larva from an Italian frog (but erroneously reported by several authors from Africa and Asia), and Spirometra raillieti (Rátz, 1913), described as a larva from domestic pigs in Hungary and Serbia, is unclear, as no molecular data are available to confirm the validity of these poorly known and insufficiently characterised species (see above).
5.5. North America (including Central America and the Carribean)
Spirometra tapeworms have been found in Belize, Canada, the Cayman Islands, Costa Rica, Cuba, Grenada, Honduras, Mexico, the Netherlands Antilles, Puerto Rico and in 24 states of the USA (Supplementary Table S1), but molecular evidence is only available for specimens from two different genetic lineages corresponding to Spirometra sp. 2 and sp. 3 (=‘S. decipiens species complex 1 and 2’). Spirometra sp. 2 occurs mainly in South America, but a single specimen from a bobcat in Illinois (USA) genetically corresponds to South American isolates of Spirometra sp. 2 (Fig. 1).
The specimens of Spirometra sp. 3 (North American lineage) could represent a separate species of the tapeworms of the same lineage (S. mansonoides?) found in North and South America, but the available data are too limited to make a reliable decision. Because S. mansonoides has been described from the northeastern United States, it is desirable to collect new material from near the type locality (Syracuse, New York) to clarify the validity and phylogenetic relationships of this previously repeatedly reported but poorly characterised species with an unknown host range and geographical distribution. For the most widespread Spirometra species, S. mansoni, there is no reliable (molecularly characterised) evidence from North America.
5.6. South America
Several species assigned to Spirometra have been described from South America, but the validity and taxonomic status of most of them are uncertain (Table 1). Spirometra tapeworms with different names have been found in Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, Panama, Paraguay, Peru, Suriname, Trinidad, Uruguay and Venezuela (Supplementary Table S1). However, only specimens from Argentina, Bolivia, Brazil, Colombia, Uruguay and Venezuela were sequenced (Vettorazzi et al., 2023).
Currently, tapeworms of three different lineages (= putative species) have been found in South America, most likely corresponding to three different species: (i) S. decipiens (=‘S. decipiens species complex 2 South American lineage’), which is a typical parasite of felids in South America and was recently also found in rivulid fish in Uruguay by Vettorazzi et al. (2023); (ii) Spirometra sp. 2 (=‘S. decipiens species complex 1’) commonly reported from a wide range of wild felids as well as domestic cats and dogs in South America, but also one isolate from bobcat from USA (Illinois); and (iii) the widespread S. mansoni, recently found in crab-eating fox from Colombia by Brabec et al. (2022) (Fig. 1; Supplementary Fig. 1).
6. Conclusions and prospects
With a little exaggeration, one can say that the taxonomy of Spirometra should start from scratch due to the lack of reliable morphological data associated with genetically characterised specimens. A radical approach would be to consider any species described from spargana as species inquirenda or even nomina dubia, unless there is irrefutable molecular or life cycle evidence that a particular sparganum is associated with a properly known/described adult tapeworm.
The recent description of S. asiana by Yamasaki et al. (2024) should serve as a guide for what future taxonomic accounts should look like, i.e. individual species should be characterised based on properly fixed fresh material of tapeworms suitable for molecular and morphological description.
Molecular data indicate the presence of at least seven different Spirometra lineages, but it is possible that additional species will be recognised based on the sequencing of new samples, especially from South America and Africa. The available data suggest broad host specificity of most species at the definitive and intermediate host level, but much more information is needed before a more reliable conclusion can be drawn. With the exception of the cosmopolitan S. mansoni, the other species appear to have a rather limited geographical distribution, mostly restricted to a single continent.
Fixation is an important factor that can significantly influence morphology of the samples. To obtain comparable samples suitable for morphological studies, heat-fixation is strongly recommended: a near-boiled 4% formaldehyde solution (formalin), provided that a piece of tissue from the same sample is simultaneously fixed in 96–99% molecular ethanol for sequencing, or killing with a near-boiled saline solution (0.9% NaCl) followed by preservation of the entire sample in 70–80% molecular ethanol (Chervy, 2024).
The traditional practice of relaxing diphyllobothriidean tapeworms in saline or even in water, or fixing them under strong pressure, must be avoided, as these methods cause artificial changes in morphology (Kuchta and Scholz, 2017; Chervy, 2024). However, most Spirometra tapeworms are obtained from dead or frozen definitive hosts, making the specimens unsuitable for reliable morphological examination. Nevertheless, molecular data can be obtained from these samples.
The morphological differences between individual Spirometra species do not appear to be very great. Therefore, genotyping will remain unavoidable for the identification of adult tapeworms and plerocercoids, including clinical samples of human sparganosis or spirometrosis. Despite the obstacles in accessing fresh material, new species should never be described on the basis of limited and/or poorly fixed specimens, as this makes it impossible to distinguish between intraspecific morphological and morphometric variation and interspecific differences (Iwata 1934, 1972; Kuchta and Scholz 2017; Kuchta et al., 2021).
In terms of nomenclature, it is advisable to use already established names of Spirometra, provided that new, properly fixed material is collected from type hosts near type localities of a given taxon. Otherwise, new names should be proposed for putatively new species when there is no link to previously described taxa, i.e. when there is no correspondence with the definitive hosts and locality of the original description.
Despite great efforts in recent decades, the most enigmatic ‘species’, Sparganum proliferum, described from Japan and causing fatal infections in humans, has still not been identified, partly because most clinical samples have been previously fixed in formalin, making their molecular characterisation impossible, with the exception of one sample from Venezuela (Miyadera et al., 2001; Kikuchi et al., 2021). All clinical staff, physicians and veterinarians are strongly advised to fix all samples of Spirometra tapeworms in molecular ethanol to allow genotyping of the samples and subsequent species identification.
CRediT authorship contribution statement
Roman Kuchta: Writing – review & editing, Visualization, Investigation, Data curation, Conceptualization. Anna J. Phillips: Writing – review & editing, Investigation. Tomáš Scholz: Writing – review & editing, Writing – original draft, Supervision, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Two reviewers provided helpful suggestions. Sonja Dumendiak, University of Hohenheim, Germany provided sequences of her samples, Caroline Kibet, Institute of Parasitology, BC CAS, České Budějovice, Czech Republic, helped with phylogenetic analyses and Tetsuya Okino, Kawasaki Medical School, Japan, provided specimens of S. mansoni from experimentally infected domestic dog. Thanks are also due to Jan Brabec, Institute of Parasitology, BC CAS, České Budějovice, Czech Republic, for providing unpublished sequences, to Kimberly M. Bates, Winona State University, USA for providing DNA of Spirometra from a bobcat in Wisconsin, Louise Emmons, USNM for providing samples from maned wolf, and to curators of parasitological collections in Natural History Museums in London (Jesus Hernández-Orts), Geneva (Jean Mariaux and Isabel Blasco-Costa) and Vienna (Helmut Sattmann) and the staff of the Royal Museum of Central Africa, Tervuren, Belgium. Research stays of R. K. in Vienna (2013) and London (2005) were supported by the EU-SYNTHESYS projects (AT-TAF 2645 and GB-TAF 735). This study was supported by the Czech Science Foundation (project No. 19-28399X) and the Institute of Parasitology, BC CAS (RVO: 60077344).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2024.100947.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1. Phylogenetic tree of the interrelationships of the genus Spirometra based on selected cytochrome c oxidase subunit I (cox1) gene data (short sequences), Bayesian inference. The branch length scale bar indicates number of substitutions per site.
References
- Almeida G.G., Coscarelli D., Melo M.N., Melo A.L., Pinto H.A. Molecular identification of Spirometra spp. (Cestoda: Diphyllobothriidae) in some wild animals from Brazil. Parasitol. Int. 2016;65:428–431. doi: 10.1016/j.parint.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Andersen K.I. Studies of the helminth fauna of Norway XXI: The influence of population size (intensity of infection) on morphological characters in Diphyllobothrium dendriticum Nitzsch in the golden hamster (Mesocrisetus auratus Waterhouse) Norw. J. Zool. 1972;20:1–7. [Google Scholar]
- Andersen K.I. Studies of the helminth fauna of Norway XXXII: The primary strobila in Diphyllobothrium Cobbold. Studies on the development of primary strobilae in D. dendriticum (Nitzsch), D. latum (L.) and D. ditremum (Creplin) Norw. J. Zool. 1973;21:341–350. [Google Scholar]
- Ariola V. Revisione della famiglia Bothriocephalidae s. str. Arch. Parasitol. 1900;3:369–484. [Google Scholar]
- Armúa-Fernández M.T., Burutarán M., Bazzano V., Félix M.L., Castro O., Venzal J.M. Molecular characterization of Spirometra decipiens complex (Eucestoda: Diphyllobothriidea) from Uruguay. Taxonomy. 2021;1:270–277. doi: 10.3390/taxonomy1030021. [DOI] [Google Scholar]
- Arrabal J.P., Pérez M.G., Arce L.F., Kamenetzky L. First identification and molecular phylogeny of Sparganum proliferum from endangered felid (Panthera onca) and other wild definitive hosts in one of the regions with highest worldwide biodiversity. Int. J. Parasitol.: Parasit. Wildlife. 2020;13:142–149. doi: 10.1016/j.ijppaw.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer J.G. Contribution à la faune helminthologique Sud-Africaine. Ann. Parasitol. Hum. Comp. 1924;2:239–247. doi: 10.1051/parasite/1924023239. [DOI] [Google Scholar]
- Baer J.G. The Government Printing and Stationery Office; Pretoria: 1925. Contributions to the helminth-fauna of South Africa; p. 79. [Google Scholar]
- Baer J.G. Helminthes Parasites. Institut des Parcs Nationaus du Congo Belge; Brussels: 1959. Exploration of national parcs of Belgium’s Congo; p. 163. [Google Scholar]
- Baer J.G., Fain A. In: Exploration du Parc National de l’Upemba. de Witte G.F., editor. Mission G. F. de Witte; Brussel: 1955. Cestodes; p. 38. [Google Scholar]
- Bearup A.J. Life-history of a spirometrid tapeworm, causing sparganosis in feral pigs. Aust. Vet. J. 1953;29:217–224. doi: 10.1111/j.1751-0813.1953.tb05274.x. [DOI] [Google Scholar]
- Berentsen A.R., Becker M.S., Stockdale-Walden H., Matandiko W., McRobb R., Dunbar M.R. Survey of gastrointestinal parasite infection in African lion (Panthera leo), African wild dog (Lycaon pictus) and spotted hyaena (Crocuta crocuta) in the Luangwa Valley, Zambia. Afr. Zool. 2012;47:363–368. [Google Scholar]
- Berntzen A.K., Mueller J.F. In vitro cultivation of Spirometra spp. (Cestoda) from the plerocercoid to the gravid adult. J. Parasitol. 1972;58:750–752. doi: 10.2307/3278305. [DOI] [PubMed] [Google Scholar]
- Brabec J., Uribe M., Chaparro-Gutiérrez J.J., Hermosilla C. Presence of Spirometra mansoni, causative agent of sparganosis, in South America. Emerg. Infect. Dis. 2022;28:2347–2350. doi: 10.3201/eid2811.220529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R.A., Jones A., Andersen K.I. In: Order Pseudophyllidea Carus, 1863. Khalil L.F., Jones A., Bray R.A., editors. CAB International; Wallingford, U.K.: 1994. pp. 205–247. [Google Scholar]
- Caira J.N., Jensen K., Barbeau E. Global cestode database. World Wide Web Electr. Publ. 2024 www.tapewormdb.uconn.edu [Google Scholar]
- Chervy L. The terminology of larval cestodes or metacestodes. Syst. Parasitol. 2002;52:1–33. doi: 10.1023/A:1015086301717. [DOI] [PubMed] [Google Scholar]
- Chervy L. Manual for the study of tapeworms (Cestoda) parasitic in ray-finned fish, amphibians and reptiles. Folia Parasitol. 2024;71:1. doi: 10.14411/fp.2024.001. [DOI] [PubMed] [Google Scholar]
- Čisovská Bazsalovicsová E., Radačovská A., Lavikainen A., Kuchta R., Králová-Hromadová I. Genetic interrelationships of Spirometra erinaceieuropaei (Cestoda: Diphyllobothriidea), the causative agent of sparganosis in Europe. Parasite. 2022;29:8. doi: 10.1051/parasite/2022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold T.S. Description of Ligula mansoni, a new human cestode. J. Linn. Soc. Lond. Zool. 1883;17:78–83. doi: 10.1111/j.1096-3642.1883.tb00237.x. [DOI] [Google Scholar]
- Daly J.J. In: CRC Handbook Series in Zoonoses. Section C: Parasitic Zoonoses. Steele J.H., Beran G.W., editors. CRC Press; Boca Raton: 1981. Sparganosis; pp. 293–312. [Google Scholar]
- Diesing K.M. W. Braumüller; Vindobonae: 1850. Systema Helminthum; p. 679. [Google Scholar]
- Diesing K.M. Über eine naturgemässe Vertheilung der Cephalocotyleen. Sitz.-Ber. K. Akad. Wiss. Math.-Naturwiss Cl. 1854;13:556–616. [Google Scholar]
- Diesing K.M. Zwanzig Arten von Cephalocotyleen. Denkschrift. Kaiser. Akad. Wissenschaften. 1856;12:23–38. [Google Scholar]
- Dubinina M.N. On the biology and distribution of Diphyllobothrium erinacei-europaei (Rud., 1819) Iwata, 1933. Zool. Zh. 1951;30:421–429. [Google Scholar]
- Eberhard M.L., Thiele E.A., Yembo G.E., Yibi M.S., Cama V.A., Ruiz-Tiben E. Thirty-seven human cases of sparganosis from Ethiopia and South Sudan caused by Spirometra spp. Am. J. Trop. Med. Hyg. 2015;93:350–355. doi: 10.4269/ajtmh.15-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh A.L., Nelson K.G., Peebles R., Hernandez A.D., Hubbard K.K., Holekamp K.E. Coprologic survey of parasites of spotted hyenas (Crocuta crocuta) in the Massai Mara National Reserve, Kenya. J. Wildl. Dis. 2003;39:224–227. doi: 10.7589/0090-3558-39.1.224. [DOI] [PubMed] [Google Scholar]
- Eom K.S., Park H., Lee D., Choe S., Kang Y., Bia M.M., Lee S.-H., Keyyu J., Fyumagwa R., Jeon H.-K. Molecular and morphologic identification of Spirometra ranarum found in the stool of African lion, Panthera leo in the Serengeti Plain of Tanzania. Kor. J. Parasitol. 2018;56:379–383. doi: 10.3347/kjp.2018.56.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom K.S., Park H., Lee D., Choe S., Kang Y., Bia M.M., Ndosi B.A., Nath T.C., Eamudomkarn C., Keyyu J., Fyumagwa R., Mduma S., Jeon H.-K. Identity of Spirometra theileri from a leopard (Panthera pardus) and spotted hyena (Crocuta crocuta) in Tanzania. Kor. J. Parasitol. 2019;57:639–645. doi: 10.3347/kjp.2019.57.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust E.C., Campbell H.E., Kellogg C.R. Morphological and biological studies on the species of Diphyllobothrium in China. Am. J. Hyg. 1929;9:560–583. [Google Scholar]
- Fischer D., Heuser W., Pantchev N., Wolken S., Neumann D., Lierz M. vol. 38. Tierarztl Prax Ausg K Kleintiere Heimtiere; 2010. Subkutane Sparganose Beim Korallenfingerfrosch (Litoria Caerulea) pp. 249–253. [PubMed] [Google Scholar]
- Fraija-Fernández N., Waeschenbach A., Briscoe A.G., Hocking S., Kuchta R., Nyman T., Littlewood D.T.J. Evolutionary transitions in broad tapeworms (Cestoda: Diphyllobothriidea) revealed by mitogenome and nuclear ribosomal operon phylogenetics. Mol. Phylogenet. Evol. 2021;163 doi: 10.1016/j.ympev.2021.107262. [DOI] [PubMed] [Google Scholar]
- Fredes F., Mercado R., Salas I.P., Sugiyama H., Kobayashi H., Yamasaki H. Morphological observation and molecular phylogeny of Spirometra decipiens complex 1 (Cestoda: Diphyllobothriidae) found in cat from Chile. Parasitol. Int. 2022;87 doi: 10.1016/j.parint.2021.102493. [DOI] [PubMed] [Google Scholar]
- Furmaga S. Spirometra janickii sp. n. (Diphyllobothriidae) Acta Parasitol. Polon. 1953;1:29–59. [Google Scholar]
- Graber M. Diphyllobothriose et sparganose en Afrique tropicale. Rev. d’Élevage Méd. Vét. Pays Trop. 1981;34:303–311. [PubMed] [Google Scholar]
- Harkema R., Miller G.C. Helminth parasites of the raccoon, Procyon lotor in the Southeastern United States. J. Parasitol. 1964;50:60–66. doi: 10.2307/3276029. [DOI] [PubMed] [Google Scholar]
- Iwata S. Some experimental studies on the regeneration of the plerocercoid of Mansonʼs tapeworm, Diphyllobothrium erinacei (Rudolphi), Sparganum proliferum Ijima. Jpn. J. Zool. 1934;6:209–247. [Google Scholar]
- Iwata S. Experimental and morphological studies of Mansonʼs tapeworm Diphyllobothrium erinacei (Rudolphi) – special reference with its scientific name and relationship with Sparganum proliferum Ijima. Prog. Med. Parasitol. Jpn. 1972;4:535–590. [Google Scholar]
- Jeon H.-K., Park H., Lee D., Choe S., Kang Y., Bia M.M., Lee S.-H., Sohn W.-M., Hong S.-J., Chai J.-Y., Eom K.S. Genetic and morphologic identification of Spirometra ranarum in Myanmar. Kor. J. Parasitol. 2018;56:275–280. doi: 10.3347/kjp.2018.56.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H.-K., Park H., Lee D., Choe S., Kim K.-H., Huh S., Sohn W.-M., Chai J.-Y., Eom K.S. Human infections with Spirometra decipiens plerocercoids identified by morphologic and genetic analyses in Korea. Kor. J. Parasitol. 2015;53:299–305. doi: 10.3347/kjp.2015.53.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H.-K., Park H., Lee D., Choe S., Sohn W.-M., Eom K.S. Molecular detection of Spirometra decipiens in the United States. Kor. J. Parasitol. 2016;54:503–507. doi: 10.3347/kjp.2016.54.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyeux C., Baer J.G. Sur quelques larves de Bothriocéphales. Bull. Soc. Pathol. Exot. 1927;20:921–936. [Google Scholar]
- Joyeux C., Houdemer E., Baer J.G. Recherches sur la biologie des sparganum et l’étiologie de la sparganose oculaire. Bull. Soc. Pathol. Exot. 1934;27:70–78. [Google Scholar]
- Kamo H. Gendaikikaku; Tokyo, Japan: 1999. Guide to Identification of Diphyllobothriid Cestodes; p. 146. [Google Scholar]
- Kavana N.J., Kassuku A.A., Kasanga C.J. Prevalence of Spirometra species and other gastrointestinal helminths in wild lions (Panthera leo) in Tarangire National Park, northern Tanzania. Int. J. Microbiol. Immunol. Res. 2015;4:10–14. [Google Scholar]
- Kikuchi T., Dayi M., Hunt V.L., Ishiwata K., Toyoda A., Kounosu A., Sun S., Maeda Y., Kondo Y., de Noya B.A., Noya O., Kojima S., Kuramochi T., Maruyama H. Genome of the fatal tapeworm Sparganum proliferum uncovers mechanisms for cryptic life cycle and aberrant larval proliferation. Commun. Biol. 2021;4:649. doi: 10.1038/s42003-021-02160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. Studies in the development of Diphyllobothrium mansoni (Cobbold, 1882) Joyeux, 1928. III. Experimental studies on the infection by the mature procercoid. Taiwan Igakkai Zasshi (J. Med. Asso. Formosa) 1931;30:16–39. [Google Scholar]
- Kołodziej-Sobocińska M., Stojak J., Kondzior E., Ruczyńska I., Wójcik J.M. Genetic diversity of two mitochondrial DNA genes in Spirometra erinaceieuropaei (Cestoda: Diphyllobothriidae) from Poland. J. Zool. Syst. Evol. Res. 2019;57:764–777. doi: 10.1111/jzs.12319. [DOI] [Google Scholar]
- Kołodziej-Sobocińska M., Yakovlev Y., Schmidt K., Hurníková Z., Ruczyńska I., Bednarski M., Tokarska M. Update of the helminth fauna in Eurasian lynx (Lynx lynx) in Poland. Parasitol. Res. 2018;117:2613–2621. doi: 10.1007/s00436-018-5953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlán A. Über Sparganum raillieti Ratz und den zugehörigen geschlechtsreifen Bandwurm Dibothriocephalus rallieti Ratz. Centralbl. Bakteriol. Parasitenkd. 1923;90:277–285. [Google Scholar]
- Kudo T., Fujioka A., Korenaga M., Yamasaki H., Morishima Y., Sugiyama H., Nakajima H., Sano S. Molecular identification of intramuscular and subcutaneous Spirometra erinaceiuropaei sparganosis in a Japanese patient. J. Dermatol. 2017;44:e138–e139. doi: 10.1111/1346-8138.13739. [DOI] [PubMed] [Google Scholar]
- Kuchta R., Kołodziej-Sobocińska M., Brabec J., Młocicki D., Sałamatin R., Scholz T. Sparganosis (Spirometra) in Europe in the molecular era. Clin. Infect. Dis. 2021;72:882–890. doi: 10.1093/cid/ciaa1036. [DOI] [PubMed] [Google Scholar]
- Kuchta R., Scholz T. In: Planetary Biodiversity Inventory (2008–2017): Tapeworms from Vertebrate Bowels of the Earth. Caira J.N., Jensen K., editors. University of Kansas; Lawrence, KS, USA: 2017. Diphyllobothriidea; pp. 167–189. Natural History Museum, Special Publication No. 25. [Google Scholar]
- Kuchta R., Scholz T., Brabec J., Narduzzi-Wicht B. In: Biology of Foodborne Parasites. Section III. Important Foodborne Helminths. Xiao L., Ryan U., Feng F., editors. CRC Press; Boca Raton, FL: 2015. Chapter 17. Diphyllobothrium, Diplogonoporus and Spirometra; pp. 299–326. [Google Scholar]
- Leuckart R. Beschreibung zweier neuen Helminthen. Arch. Naturgesch. 1848;14:26–29. [Google Scholar]
- Li H.C. Life histories of Diphyllobothrium decipiens and D. erinacei. Am. J. Hyg. 1929;10:527–550. [Google Scholar]
- Lühe M. Zur Anatomie und Systematik der Bothriocephaliden. Verh. Dtsch. Zool. Ges. 1899;9:30–55. [Google Scholar]
- Marucci A.A., Mueller J.F. Comparative antigenic constitution of Spirometra spp. J. Parasitol. 1972;58:1211–1213. doi: 10.2307/3278172. [DOI] [PubMed] [Google Scholar]
- Meggitt F.J. On the occurrence of Ligula ranarum in a frog. J. Nat. Hist. Series. 1924;9(13):216–219. doi: 10.1080/00222932408633030. [DOI] [Google Scholar]
- Meggitt F.J. On the life-histroy of an amphibian tapeworm (Diphyllobothrium ranarum, Gastaldi) J. Nat. Hist. Series. 1925;9(16):654–656. [Google Scholar]
- McHale B., Callahan R.T., Paras K.L., Weber M., Kimbrell L., Velázquez-Jiménez Y., McManamon R., Howerth E.W., Verocai G.G. Sparganosis due to Spirometra sp. (Cestoda; Diphyllobothriidae) in captive meerkats (Suricata suricatta) Int. J. Parasitol.: Parasit. Wildlife. 2020;13:186–190. doi: 10.1016/j.ijppaw.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyadera H., Kokaze A., Kuramochi T., Kita K., Machinami R., Noya O., Alarcón de Noya B., Okamoto M., Kojima S. Phylogenetic identification of Sparganum proliferum as a pseudophyllidean cestode by the sequence analyses on mitochondrial COI and nuclear sdhB genes. Parasitol. Int. 2001;50:93–104. doi: 10.1016/S1383-5769(01)00071-X. [DOI] [PubMed] [Google Scholar]
- Mueller J.F. A Diphyllobothrium from cats and dogs in the Syracuse region. J. Parasitol. 1935;21:114–121. doi: 10.2307/3271757. [DOI] [Google Scholar]
- Mueller J.F. A repartition of the genus Diphyllobothrium. J. Parasitol. 1937;23:308–310. doi: 10.2307/3272427. [DOI] [Google Scholar]
- Mueller J.F. The life history of Diphyllobothrium mansonoides Mueller, 1935, and some considerations with regard to sparganosis in the United States. Am. J. Trop. Med. Hyg.ene. 1938;18:41–66. [Google Scholar]
- Mueller J.F. The laboratory propagation of Spirometra mansonoides as an experimental tool. I. Collecting, incubation and hatching of the eggs. J. Parasitol. 1959;45:353–361. doi: 10.2307/3274380. [DOI] [PubMed] [Google Scholar]
- Mueller J.F. Libro Homenaje Al Doctor Eduardo Caballero Y Caballero. Editorial Politécnica. 1960. The immunologic basis of host specificity in the sparganum larva of Spirometra mansonoides; pp. 435–442. Mexico. [Google Scholar]
- Mueller J.F. The laboratory propagation of Spirometra mansonoides as an experimental tool. IV. Experimental inversion of the primary axis in the developing egg. Exp. Parasitol. 1961;11:311–318. doi: 10.1016/0014-4894(61)90037-6. [DOI] [PubMed] [Google Scholar]
- Mueller J.F. In: Host-parasite Relationships: Proceedings of the 26th Annual Biology Colloquium. McCauley J.E., editor. Oregon State University Press; Corvallis: 1966. Host-parasite relationships as illustrated by the cestode Spirometra mansonoides; pp. 15–58. [Google Scholar]
- Mueller J.F. The biology of Spirometra. J. Parasitol. 1974;60:3–14. doi: 10.2307/3278670. [DOI] [PubMed] [Google Scholar]
- Ndosi B.A., Park H., Lee D., Choe S., Kang Y., Nath T.C., Bia M.M., Eamudomkarn C., Jeon H.-K., Eom K.S. Morphological and molecular identification of Spirometra tapeworms (Cestoda: Diphyllobothriidae) from carnivorous mammals in the Serengeti and Selous ecosystems of Tanzania. Kor. J. Parasitol. 2020;58:653–660. doi: 10.3347/kjp.2020.58.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndosi B.A., Park H., Lee D., Choe S., Kang Y., Nath T.C., Bia M.M., Eamudomkarn C., Jeon H.-K., Eom K.S. Mitochondrial genome of Spirometra theileri compared with other Spirometra species. Kor. J. Parasitol. 2021;59:139–148. doi: 10.3347/kjp.2021.59.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G.S., Pester F.R.N., Rickman R. The significance of wild animals in the transmission of cestodes of medical importance in Kenya. Trans. Roy. Soc. Trop. Med. Hyg. 1965;59:507–524. doi: 10.1016/0035-9203(65)90153-7. [DOI] [PubMed] [Google Scholar]
- Odening K. Conception and terminology of hosts in parasitology. Adv. Parasitol. 1976;14:1–93. doi: 10.1016/S0065-308X(08)60513-8. [DOI] [PubMed] [Google Scholar]
- Odening K. Zum Erforschungsstand des "Sparganum Growth Factor" von Spirometra. Angew. Parasitol. 1979;20:185–192. [PubMed] [Google Scholar]
- Odening K. Neue Erkenntnisse zu Ökographie, Bionomie und Taxonomie von Spirometra (Cestoda: Pseudophyllidea) MILU: Wissenschaft. Kultur. Mitt. Tierpark. 1985;6:277–294. [Google Scholar]
- Odening K., Bockhardt I. Two European Spirometra forms (Cestoidea: Diphyllobothriidae) with different sparganum growth factors. Angew. Parasitol. 1982;23:15–27. [PubMed] [Google Scholar]
- Okino T., Ushirogawa H., Matoba K., Nishimatsu S.-i., Saito M. Establishment of the complete life cycle of Spirometra (Cestoda: Diphyllobothriidae) in the laboratory using a newly isolated triploid clone. Parasitol. Int. 2017;66:116–118. doi: 10.1016/j.parint.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Okumura T. An experimental study of the life-history of Sparganum mansoni Cobbold. Kitasato Arch. Exp. Med. 1919;3:190–197. [Google Scholar]
- Opuni E.K., Muller R. Studies on Spirometra theileri (Baer, 1925) n. comb. 1. Identification and biology in the laboratory. J. Helminthol. 1974;48:15–23. doi: 10.1017/s0022149x00022550. [DOI] [PubMed] [Google Scholar]
- Petrigh R.S., Scioscia N.P., Denegri G.M., Fugassa M.H. Research note. Cox-1 gene sequence of Spirometra in Pampas foxes from Argentina. Helminthologia. 2015;52:355–359. doi: 10.1515/helmin-2015-0056. [DOI] [Google Scholar]
- Round M.C. Checklist of the helminth parasites of African mammals of the orders Carnivora, Tubulidentata, Proboscidea, Hyracoidea, Artiodactyla and Perissodactyla. Commonwealth Agricult. Bureaux, St. Albans. 1968 [Google Scholar]
- Rudolphi C.A. S. A. Rücker; Berolini (Berlin): 1819. Entozoorum synopsis cui accident mantissa duplex et indices locupletissimi. [Google Scholar]
- Sambon L.L. Descriptions of some new species of animal parasites. Proc. Zool. Soc., London. 1907;77:282–283. [Google Scholar]
- Schmidt G.D. CRC Press; Boca Raton: 1986. CRC Handbook of Tapeworm Identification; p. 675. [Google Scholar]
- Schmidt G.D., Martin R.L. Tapeworms of the Chaco Boreal, Paraguay, with two new species. J. Helminthol. 1978;52:205–209. doi: 10.1017/S0022149X00005381. [DOI] [PubMed] [Google Scholar]
- Scholz T., Kuchta R. Fish-borne, zoonotic cestodes (Diphyllobothrium and relatives) in cold climates: a never-ending story of neglected and (re)-emergent parasites. Food Waterbor. Parasitol. 2016;4:23–38. doi: 10.1016/j.fawpar.2016.07.002. [DOI] [Google Scholar]
- Scholz T., Kuchta R., Brabec J. Broad tapeworms (Diphyllobothriidae), parasites of wildlife and humans: recent progress and future challenges. Int. J. Parasitol.: Parasit. Wildlife. 2019;9:359–369. doi: 10.1016/j.ijppaw.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanson J. Murdoch University; 1985. Biology and Immunology of Spirometra in Western Australia; p. 247. [Google Scholar]
- Shipley A. On a collection of parasites from the Soudan. Arch. Parasitol. 1902;6:604–612. [Google Scholar]
- Škeříková A., Brabec J., Kuchta R., Jiménez J.A., García H.H., Scholz T. Is the human-infecting Diphyllobothrium pacificum a valid species or just a South American population of the holarctic fish broad tapeworm, D. latum? Am. J. Trop. Med. Hyg. 2006;75:307–310. doi: 10.4269/ajtmh.2006.75.307. [DOI] [PubMed] [Google Scholar]
- Swartzwelder J.C., Beaver P.C., Hood M.W. Sparganosis in southern United States. Am. J. Trop. Med. Hyg. 1964;13:43–47. doi: 10.4269/ajtmh.1964.13.43. [DOI] [PubMed] [Google Scholar]
- Ugarte C.E., Thomas D.G., Gasser R.B., Hu M., Scott I., Collett M.G. Spirometra erinacei/S. erinaceieuropaei in a feral cat in Manawatu with chronic intermittent diarrhoea. N. Z. Vet. J. 2005;53:347–351. doi: 10.1080/00480169.2005.36573. [DOI] [PubMed] [Google Scholar]
- Vettorazzi R., Norbis W., Martorelli S.R., García G., Rios N. First report of Spirometra (Eucestoda; Diphyllobothriidae) naturally occurring in a fish host. Folia Parasitol. 2023;70:8. doi: 10.14411/fp.2023.008. [DOI] [PubMed] [Google Scholar]
- Waeschenbach A., Brabec J., Scholz T., Littlewood D.T.J., Kuchta R. The catholic taste of broad tapeworms – multiple routes to human infection. Int. J. Parasitol. 2017;47:831–843. doi: 10.1016/j.ijpara.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Wardle R.A., McLeod J.A., Radinovsky S. University of Minnesota Press; 1974. Advances in the Zoology of Tapeworms, 1950–1970; p. 300. [Google Scholar]
- Xu F.F., Chen W.Q., Liu W., Liu S.S., Wang Y.X., Chen J., Cui J., Zhang X. Genetic structure of Spirometra mansoni (Cestoda: Diphyllobothriidae) populations in China revealed by a Target SSR-seq method. Parasites Vectors. 2022;15:485. doi: 10.1186/s13071-022-05568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguti S. Studies on the helminth fauna of Japan Part 7. Cestodes of mammals and snakes. Jpn. J. Zool. 1935;6:233–246. [Google Scholar]
- Yamasaki H., Sanpool O., Rodpai R., Sadaow L., Laummaunwai P., Un M., Thanchomnang T., Laymanivong S., Aung W.P.P., Intapan P.M., Maleewong W. Spirometra species from Asia: genetic diversity and taxonomic challenges. Parasitol. Int. 2021;80 doi: 10.1016/j.parint.2020.102181. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Sugiyama H., Morishima Y., Kobayashi H. Description of Spirometra asiana sp. nov. (Cestoda: Diphyllobothriidae) found in wild boars and hound dogs in Japan. Parasitol. Int. 2024;98 doi: 10.1016/j.parint.2023.102798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Phylogenetic tree of the interrelationships of the genus Spirometra based on selected cytochrome c oxidase subunit I (cox1) gene data (short sequences), Bayesian inference. The branch length scale bar indicates number of substitutions per site.