Abstract
Enamel is composed of numerous uniformly wide, well-oriented hydroxyapatite crystals. It possesses an acellular structure that cannot be repaired after undergoing damage. Therefore, remineralization after enamel defects has become a focal point of research. Hydrogels, which are materials with three-dimensional structures derived from cross-linking polymers, have garnered significant attention in recent studies. Their exceptional properties make them valuable in the application of enamel remineralization. In this review, we summarize the structure and formation of enamel, present the design considerations of hydrogels for enamel remineralization, explore diverse hydrogels types in this context, and finally, shed light on the potential future applications in this field.
Keywords: Hydrogels, Remineralization, Hydroxyapatite, Interpenetrating network hydrogels
Funding
There is no funding support this study.
1. Introduction
Tooth enamel in humans, which covers the crown of teeth, constitutes the hardest and most mineralized tissue. Comprised of highly-ordered hydroxyapatite (HAP), it is predominantly composed of 95–97 % mineral content by weight, containing less than 1 % organic material [1]. Enamel significantly differs from bone in that its composition is primarily mineral-based, lacking collagen (Col) fibers, thereby making it harder than bone. This inherent strength allows enamel to withstand substantial chewing forces without breaking. Being the outermost layer of the tooth structure, enamel is particularly vulnerable to bacterial attacks and acid erosions, leading to demineralization. Unfortunately, due to its acellular structure, it cannot be repaired effectively after damage occurs. Consequently, researchers have directed their focus towards discovering diverse materials to repair and regenerate damaged enamel. Despite the attractiveness of enamel regeneration, its practical application and engineering for future clinical purposes remain a considerable challenge.
Natural enamel initially forms in a gel-like extracellular matrix (ECM). Within this environment, ameloblasts secrete proteins, mainly amelogenin (AMEL), which interact with inorganic ions like calcium ions (Ca2+) and phosphate (PO43−), which are present in the surroundings. This interaction leads to the formation of highly-oriented and parallel enamel crystallites [2,3]. Therefore, the key to regenerate enamel is finding or designing materials to simulate the environment conducive to HAP crystal mineralization under physiological conditions.
Hydrogels, composed of three-dimensional (3D) polymer structures, exhibit exceptional swelling properties and water-retention abilities, capable of absorbing a large amount of water without dissolving [4]. These materials possess numerous desirable properties, such as biodegradability, biocompatibility, absorption, and hydrophilicity, making them pivotal in regenerative medicine applications. Hydrogels serve as attractive scaffold materials owing to their good biocompatibility, mimicking the ECM for cell adhesion. Moreover, due to their notable biodegradability, hydrogels can degrade and create space for new tissue growth [5]. Their porous structure allows water retention and simulation of the ECM, thereby offering an ideal environment for inter-cellular substance exchange. Consequently, hydrogels find extensive utility in tissue scaffold materials, drug delivery systems, and tissue barriers [[6], [7], [8]]. Recently, hydrogels have been employed in enamel remineralization due to their ability to simulate the ECM and foster an ideal mineralization environment. However, current reviews of hydrogels in stomatology primarily focus on hydrogel applications in pulp and periodontal tissue regeneration. Notably, there is a lack of reviews on hydrogels specifically addressing tooth enamel remineralization [9,10]. Therefore, this review encompasses an overview of hydrogels, natural enamel structure and formation, considerations regarding hydrogel use in enamel remineralization, and their applications, aiming to serve as a comprehensive reference for enamel remineralization research.
2. Overview of hydrogels
The hydrogel is a kind of material with the ability to assimilate a considerable amount of water without dissolving. Its polymer chains cross-link chemically via covalent bonds or physically through non-covalent interactions, resulting in a network structure [9]. The first synthesis hydrogel can be traced back to the fabrication of poly (2-hydroxymethacrylate) (PHEMA) hydrogels by Wichterle and Lim in 1960 [11]. Over six decades of research and development have seen the widespread applications of hydrogels in scaffold materials, drug delivery system, tissue barriers, and more [[6], [7], [8]]. They have emerged as the preferred material for numerous applications in regenerative medicine [12].
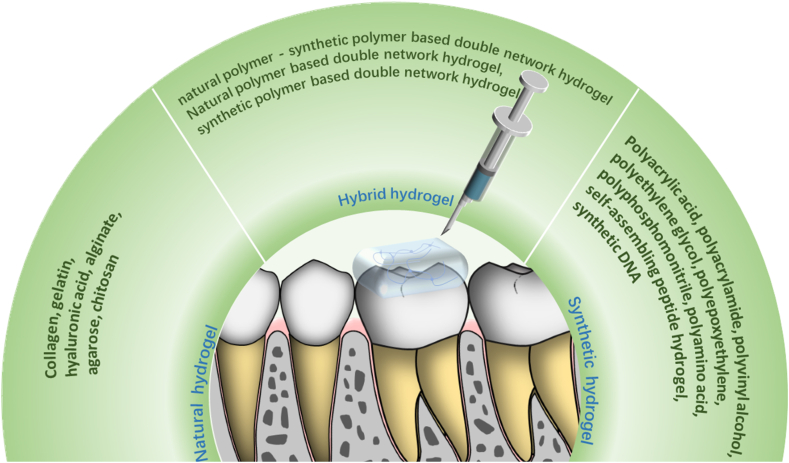
Hydrogels can be classified based on different factors, including the source of polymers, the type of crosslinking, the hydrogel's response to stimuli, or the charge properties [13]. In terms of polymer source, hydrogels can be categorized into: natural, synthetic, and hybrid (a combination of both) [9,[14], [15], [16]]. Natural hydrogels are obtained from natural polymers, such as Col, gelatin, hyaluronate, alginate, agarose, and chitosan (CS). Synthetic hydrogels are composed of synthetic polymers, including polyacrylic acid (PAA), polyacrylamide, poly(vinyl alcohol) (PVA), polyethylene glycol (PEG), polyepoxy, polyphosphazene, polyamino acids, synthetic peptides, and synthetic DNA. Hybrid hydrogels are a combination of natural and synthetic hydrogels (Fig. 1).
Fig. 1.
Shows the classification of hydrogels. Hydrogels can be divided into natural hydrogels, synthetic hydrogels and hybrid hydrogels.
Hydrogels find extensive use in biomedical research due to their smart and environmentally-sensitive nature, enabling them to change their properties in response to the surrounding stimuli, thereby regulating drug release. Based on their response to different stimuli, hydrogels can be classified into various types, such as thermoresponsive [17], pH-responsive [18], photoresponsive [19], magnetic-responsive [20]. Moreover, further hydrogel categorization is also considered via enzyme-catalyzed formation [21]. Hydrogels serve as common scaffolds in tissue engineering, maintaining distinct 3D structures that provide mechanical support for cells and mimic the natural ECM, facilitating the transfer of nutrients and cytokines [22]. Their versatile applications in tissue engineering span wound healing [23], tissue revascularization [24], nerve regeneration [25] and bone repair [26], and more. Traditional tissue engineering relies on tissue-specific cell populations, appropriate scaffolds, and cytokines. However, in enamel regeneration, challenges arise when culturing highly differentiated ameloblasts that form enamel and recreating an enamel-like mineralized structure in vitro. As a result, mineral ions cannot be transported in a specific direction [[27], [28], [29]]. Therefore, current research on enamel regeneration focuses on biomimetic mineralization of enamel without cell involvement [30,31].
3. Structure and formation of natural enamel
Dental enamel, the hardest and most mineralized human tissue, lacks cellular components in its mature form. Consequently, it cannot be effectively repaired after incurring caries, trauma, or defects, undergoing only limited passive repair through mineral deposition in the oral environment [32]. The complete absence of cellular components, predominantly inorganic substances, and minimal organic components impart physical properties akin to ceramics. The highly-ordered HAP in enamel forms enamel rods with diameters of 5–7 μm, contributing to its exceptional hardness, rendering it the hardest tissue in the human body.
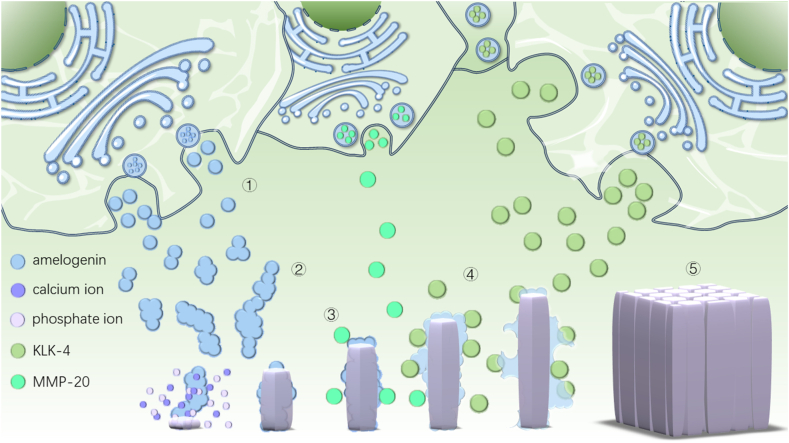
Enamel rods developed through the secretions of ameloblasts. These cells secrete a variety of proteins, mainly amelogenin (AMEL), to regulate HAP mineralization. The role of AMEL in the formation of HAP crystals includes: i) Stabilize amorphous calcium phosphate (ACP) [33], ii) Regulate the morphology and elongation of HAP crystals [34], and iii) Control the nucleation and growth of HAP [35]. Following AMEL production, self-assembly leads to the creation of a 20–50 nm spherical structure. Furthermore, a chain structure is formed, aligned along the C-axis of the HAP crystal, providing a template for HAP formation and growth control. AMEL can selectively and specifically inhibit HAP growth and precisely regulate HAP crystal mineralization. Additionally, AMEL plays a crucial role in stabilizing ACPs, preventing the rapid aggregation of Ca2+ and PO43−, and regulating both the growth direction and rate of HAP crystals [36,37]. As the process continues, the ECM proteins are degraded by enzymes like matrix metalloproteinase 20 (MMP-20) and KLK4, which are secreted by ameloblasts, leading to a significant reduction in AMEL content. Consequently, the extracellular organic matrix is replaced by which is mineral-ion-rich extracellular fluid, creating space for the growth of HAP crystals. This process finally culminates in the formation of mature enamel [38] (Fig. 2).
Fig. 2.
AMEL in enamel formation. ①AMELs are secreted to extracellular matrix; ②AMEL self-assembles into spheroids and chains; ③AMELs control the direction of hydroxyapatite mineralization; ④Proteases MMP-20 secreted during the secretory stage and KLK-4 secreted during maturation stage by ameloblasts degrade AMELs; ⑤Enamel hydroxyapatite crystals mature.
4. Considerations for the use of hydrogels in enamel remineralization
4.1. Biocompatibility
In the domain of enamel remineralization engineering, ensuring biocompatibility is the primary consideration for hydrogels. The materials used, including hydrogels and their degradation products, should not induce toxic reactions or trigger intense immune responses in the body. Although enamel is an acellular tissue, its location in the oral environment entails contact with the gingiva, tongue, and buccal mucosa. However, clinical treatment aimed at restoring enamel defects often cause inflammation and bodily damage [39]. The materials utilized for repairing enamel defects in clinical application, such as dental composite resin [40], cast alloys [41] and ceramic [42], can induce inflammation and bodily harm. Therefore, it is imperative for hydrogels utilized in enamel remineralization to demonstrate good biocompatibility.
Unreacted monomers, cross-linkers, and initiators present in hydrogel may induce toxic or deleterious immunological reactions within the body [12]. To address this problem, researchers have sought to employ materials with relatively low- or non-toxic profiles [43]. Additionally, preformed hydrogels undergo a series of purification processes, such as solvent washing and dialysis, to eliminate any residual unreacted materials [44]. In general, natural hydrogels exhibit better biocompatibility than synthetic hydrogels [12]. However, it is necessary to consider the potential toxicity stemming from crosslinking agents, including the presence of unreacted glutaraldehyde during the crosslinking process [45]. This can be realized by looking for naturally non-toxic or low-toxic crosslinkers such as polyphenols including gallic acid and ferulic acid [46], genipin [47] and dextran dialdehyde [48], or by promptly removing unreacted crosslinkers during hydrogel synthesis [49,50]. Furthermore, employing physical crosslinking methods can often mitigate or circumvent toxic reactions induced by chemical crosslinking [51].
4.2. Biodegradability
Degradability is a crucial design criterion, as the ability to specifically tailor the degradation rate of templates to that of new tissue formation significantly enhances the rate and extent of new tissue formation in tissue engineering [52]. In the context of enamel remineralization, hydrogels serve not only as an environment for mineralization but also a template for nucleation and HAP growth. Controlling the rate of hydrogel degradation offers the opportunity to synchronize HAP growth rates and ensure suitable space for its growth. An ideal template for HAP mineralization requires a controllable degradation rate. If the rate is too fast, it can impact HAP formation; conversely, if it is too slow, the organic template occupies the HAP crystal growth space, resulting in a suboptimal crystal structure. If the organic templates have not been removed effectively, the mechanical properties of remineralized layer will be inferior to intact enamel [53,54]. Interestingly, during the formation of natural enamel, if AMEL degradation is adversely affected, it leads to the creation of a poor enamel crystal structure, known as amelogenesis imperfecta, underscoring the importance of a degradable template in mineralization [55,56].
Biodegradable hydrogels can be prepared by adding biodegradable groups to the backbone or crosslinking agents of a polymer [57]. The degradation rate of the hydrogel can also be adjusted by changing the proportion of the components [58]. Other additives in the hydrogel can also be designed to be degraded by proteases to regulate hydrogel degradation [59]. Physiologically, ameloblasts secrete MMP-20 to degrade AMEL and prevent protein occlusion inside apatite crystals [60]. Prajapati et al. added MMP-20 to a CS-AMEL hydrogel and obtained HAP crystals with more uniform orientation and better mechanical properties than those without MMP-20 [61].
Notably, if the hydrogel is used in vivo to achieve enamel remineralization in situ, certain factors must be addressed. These include the degradation of the hydrogel due to swelling in the fluid environment, the potential breakdown of the hydrogel by mechanical washing by saliva in the mouth, and degradation of the hydrogel caused by enzymes present in the saliva.
4.3. Mechanical properties
Theoretically, the mechanical properties of hydrogels are strongly influenced by their degree of swelling, which can pose limitations in applying hydrogels to enamel remineralization due to reduced mechanical strength [62]. The various factors affecting the mechanical properties of conventional hydrogels include the strength of the polymer chain, type of crosslinking, crosslinking density, preparation method, among others [47,[63], [64], [65]]. Over the past decade, interpenetrating network (IPN) hydrogels have attracted considerable attention. IPN hydrogels comprise polymeric materials composed of two interpenetrating or semipenetrating polymer networks. Typically, in IPN hydrogels, the first network is brittle, rigid, and adequately cross-linked, such as in a polyelectrolyte. This network provides sacrificial bonds during deformation that dissipate considerable energy, providing IPN hydrogels with mechanical strength and rigidity. In contrast, the second network is often ductile, soft, weakly cross-linked or non-cross-linked. It resides inside the first network, absorbing external stress to make the hydrogel flexible and tough [66,67]. Moreover, the addition of tough nanoparticles (NPs) can further enhance the mechanical properties of hydrogels [68].
At present, most studies on the application of hydrogels for enamel remineralization are conducted in vitro, with few studies performed in vivo [69]. This limited in vivo exploration is primarily attributed to the low mechanical strength of conventional hydrogels. To function effectively as templates for enamel remineralization in the oral environment, hydrogels are expected to withstand the mechanical challenges posed by oral cavity.
4.4. ACP stabilization
ACP is considered a key precursor in the formation of HAP [70]. However, in aqueous solutions, ACP NPs are easily transformed into crystal phases [71], with rapid ACP aggregation negatively affecting the structure and properties of HAP crystals [72]. Therefore, stabilizing ACP NPs is critical in the remineralization process of enamel [73].
While PH and temperature play significant factors in ACP crystallization [74], studies on enamel remineralization often focuses on replicating physiological conditions. To better simulate the natural environment for enamel development, experiments are conducted at the standard PH and temperature levels found in the human body. Currently, there are several methods for stabilizing ACP NPs in hydrogels under physiological conditions.
-
1)
Addition of ions to stabilize ACP: Ca and PO43− ions interact with each other to form ACPs, which are rapidly deposited to form larger clusters. Therefore, it is not surprising that the other ions in the solution can also affect the formation and subsequent crystallization in the same way. Metal ions such as those of strontium (Sr2+) [75], magnesium (Mg2+) [76], and zinc (Zn2+) [77], along with nonmetallic ions such as citrate [78], pyrophosphate [79] and carbonate [80], have the ability to stabilize ACP. Surface-associated citrate on ACP plays a key role in controlling the nucleation of HAP by inhibiting the reaction of surface nucleation [81].
-
2)
Adding bioactive macromolecules to stabilize ACP: Leucine-rich AMEL polypeptides (LRAP) stabilize ACP during the physiological process of enamel formation and regulate the shape and orientation of growing enamel crystals [72,82]. The addition of LRAP to the hydrogel can stabilize the mineralized clusters and prevent further aggregation of ACP [83].
-
3)
Adding polymers to stabilize ACP: Apart from natural biomacromolecules, some synthetic polymers have been shown to slow the rate of ACP aggregation into HAP crystals. Wang et al. stabilized ACP with hydroxypropyl methylcellulose and polyaspartic acid, and obtained ordered HAP crystals both in vivo and in vitro, achieving a satisfactory remineralization effect on enamel [84].
4.5. Regulation of HAP orientation
Natural enamel features a highly-ordered HAP structure due to the regulation of AMEL and its differential splicing products during HAP crystal growth. Accordingly, researchers in the field of enamel remineralization aim to replicate this highly-ordered and parallel structure. Both in vivo and in vitro studies have demonstrated that AMEL and its differential splicing products are capable of achieving satisfactory mineralization effects in enamel remineralization. They successfully realized highly-ordered HAP crystals with robust mechanical properties [38,82].
In order to obtain the ability to regulate the orientation of crystal growth, researchers introduced AMEL and its splicing products into the hydrogel. For instance, Fan et al. incorporated recombinant full-length AMEL into a hydrogel for remineralizing acid-etched enamel, obtaining a remineralization layer with an ordered crystal structure [85]. However, the hydrophobic core domain and hydrophilic C-terminal of full-length AMEL facilitates its easy interaction with charged polymer chains in hydrogels such as CS and alginate, which can negatively impact its ability to regulate HAP. Therefore, uncharged polymer hydrogels might be the preferred choice for designing hydrogels with AMEL for enamel remineralization research. Additionally, splicing products of AMEL, such as LRAP, can also form dense mineralized layers of enamel rod-like HAP crystals in the hydrogel [83]. Kaushik et al. achieved densely-organized HAP crystals by adding the AMEL-derived peptides P26 and P32 to a CS hydrogel [86]. Notably, in agarose hydrogels and CS-agarose hydrogels without amelogenic protein or its splicing products, agarose is cross-linked by hydrogen bonding and interacts with Ca2+ and PO43–in the environment to form stable agarose calcium-phosphorus clusters, resulting in prism-like HAP crystals on the enamel surface [30], however, formation of oriented hydroxyapatite could be attributed to linear agarose chains, template for hydroxyapatite. The Ca2+- agarose interactions along the polymer chain leads to the oriented hydroxyapatite. In contrast, Fan et al. reported that ordered HAP crystals were not formed in agarogenic hydrogels without AMEL [85]. Therefore, addition of AMEL or other regulatory factors to hydrogels is necessary to control the orientation of HAP crystal mineralization (Table 1).
Table 1.
Summarizes the design factors that need to be considered for the use of hydrogels for enamel remineralization.
| design considerations | purpose | references |
|---|---|---|
| biocompatibility | To protect the body from injury | [47] |
| biodegradability | To keep the rate of hydrogel degradation matching the rate of tissue regeneration | [48] |
| mechanical property | To make the hydrogel resistant to the complex oral environment | [54] |
| stabilize amorphous calcium phosphate | To prevent ACP from accumulating too quickly | [64] |
| regulate the direction of crystal growth | To form directed growth, orderly arrangement of hydroxyapatite crystals | [30] |
5. Applications of hydrogels in enamel remineralization
5.1. Natural hydrogels
Most natural polymers contain numerous active sites, enabling the creation of hydrogels with specific properties based on the research requirements. Natural hydrogels offer various advantages, includng cost-effectiveness, potential biodegradability, and biocompatibility. Natural polymers are generally more biocompatible and biodegradable than synthetic polymers. Examples of natural polymers that can crosslink to form hydrogels include natural proteins, Col, gelatin, hyaluronic acid (HA), alginate, agarose, and CS.
5.1.1. Col hydrogels
Col is the most prevalent component of the ECM in mammalian tissues and is found in the skin, bone, cartilage, tendons, and ligaments. It is also present in cementum and dentin. However, mature enamel does not contain Col [38]. Furthermore, Col exhibits an elegant structural motif, with three parallel polypeptide strands adopting a left-handed polyproline II-type helical conformation coil around each other in a right-handed triple helix structure with a one-residue stagger [87]. Col-based hydrogels can simulate the interaction of the ECM with cells to promote cell adhesion, proliferation, and migration [88]. They are excellent scaffolds for tissue engineering and can be used for the biomimetic mineralization of enamel.
Col can be used as a template for HAP crystal mineralization to participate in enamel remineralization [89]. HAP crystals can grow on the surface of Col, and their c-axes are oriented along the longitudinal axes of Col and have the ability to control the size and distribution of HAP [90,91]. Col hydrogels produce HAP crystals in vitro [92]. In addition, the use of Col hydrogels in combination with other materials can promote nucleation [93] and Adhesion [94] that are beneficial for enamel remineralization. Though Col hydrogels have not been used for enamel remineralization, Col hydrogels have the potential to repair defective enamel and are promising materials for enamel remineralization.
5.1.2. Gelatin hydrogels
Gelatin is a natural polymer that is made by the hydrolytic degradation of protein from Col; therefore, some of its properties are similar to those of Col. During processing, the heat increments for gelatin will dissolve its structure into colloids, but at temperature drops below 35 °C, its state becomes gelatinous. However, if the aqueous solution of gelatin is boiled for a long time, its properties change owing to decomposition and will not be reformed after cooling. Overall, gelatin demonstrates excellent physical properties, such as jelly force, affinity, high dispersibility, low viscosity characteristics, dispersion stability, and water retention [95].
Gelatin is widely used in various fields due to its excellent biocompatibility and easy biodegradability [96]. It has potential as a remineralization template, promoting mineralization [[97], [98], [99]]. Gelatin-based hydrogels can be used as templates for remineralization by interacting effectively with Ca and PO43− ions [100], encouraging nucleation on the porous gelatin template. Moreover, gelatin stabilizes ACP and prevents its aggregation [99]. Under physiological environmental conditions, HAP crystals can be formed using a nano-composite scaffold containing gelatin and ACP [97]. Additionally, ion diffusion in gelatin can induce the development of rod-like fluorapatite on the enamel surface [101]. However, due to the low mechanical strength of gelatin, its application in the enamel remineralization is limited. Therefore, it is necessary to enhance its mechanical properties by combining with other materials [102,103]. Recently, gelatin methacryloyl (GelMA), which is obtained by the chemical modification of gelatin with methacrylic anhydride (MA), underwent photoinitiated radical polymerization to form covalently crosslinked hydrogels [104]. GelMA hydrogels, characterized by biocompatibility, adjustable mechanical properties, and other advantages, successfully induce the directed growth of HAP crystals in simulated body fluids (SBF) [105]. It is noteworthy that the gelatin hydrogel biomimetic mineralization-kit (BIMIN), developed by Guentsch et al., has successfully established a mineralized enamel-like layer in vitro. Furthermore, clinical research also confirmed that BIMIN has good remineralization effect in patients with hypersensitive teeth [[106], [107], [108]].
5.1.3. HA hydrogels
HA is a long, unbranched polysaccharide composed of repeating d-glucuronic acid and N-acetyl-d-glucosamine disaccharides with a molecular weight (MW) of up to 2 × 107 Da. Owing to its carboxyl groups, HA is negatively charged, highly hydrophilic, and forms a viscous network at high molecular weights. The physiological activity and biocompatibility of HA make it ideal for use in regenerative medicine. Due to its numerous sites for reactive group modification, HA finds extensive used in the design of hydrogels [109,110].
In recent years, increasing attention has been directed towards the use of HA in remineralization processes. HA can be used as a template for the mineralization of HAP in vitro [111]. The negatively charged groups on HA can attract Ca ions and form calcium phosphate nanospheres. When calcium phosphate begins to deposit, HA coils wrap the calcium phosphate nuclei, thereby preventing the nucleation of HAP and stabilizing ACP [111,112]. Yang et al. modified HA with bisphosphonates to improve its ability of HA to bind Ca ions and promote the formation of calcium phosphate nanospheres in the HA hydrogel system [113]. Owing to the poor mechanical strength of HA hydrogels, it is often necessary to enhance their mechanical strength by other methods, as it is difficult to control their degradation rate, which limits their application of HA hydrogels [[114], [115], [116]]. Although HA hydrogel has many advantages in the formation of hydroxyapatite and is a potential material for enamel remineralization, its research in enamel remineralization has not been reported yet.
5.1.4. Alginate hydrogels
Alginates are polysaccharides isolated from brown algae found in coastal waters, such as Laminaria hyperborea and Lessonia. They are linear unbranched copolymers that contain homopolymeric blocks of (1,4)-linked b-d-mannuronic acid (M) and a-l-guluronic acid (G) residues, respectively, which are covalently linked together in different sequences or blocks [117]. Two G blocks of adjacent polymer chains can be crosslinked with multivalent cations through interactions with the carboxylic groups of the sugars, leading to the formation of a gel network [64,118]. The overall gel stiffness depends on the polymer MW distribution composition (that is, the M/G ratio) and the stoichiometry of the alginate with the chelating cation [117].
Alginate gel serves as an effective template for mineralization. Its G blocks concentrated Ca ions, promoting hydroxyapatite mineralization [64,119]. In vitro experiments, spherical ACP can be rapidly formed in alginate hydrogel, with ACP quickly transforming into OCP, a precursor to HAP crystal, under physiological PH [120]. The advantage of alginate hydrogels for enamel remineralization is that they can regulate the mineralization rate [120]. In sodium alginate hydrogels formed by cation cross-linking, the application of Ca2+ as a cross-linking agent promotes mineralization, whereas the addition of Barium ion (Ba2+), which does not easily interact with PO43− groups, antagonizes this phenomenon. The bisphosphonate alendronate can chelate Ca2+ and strongly adhere to HAP to prevent crystal growth, thus regulating mineralization process [118]. In addition, alginate hydrogel can act as a carrier for injectable tooth epithelial cells, such as HAT-7 cells, promoting cell-assisted enamel regeneration assisted by cells [121]. Similar to other natural hydrogels, alginate hydrogels have the disadvantage of poor mechanical properties, which can be improved by designing photo-crosslinked methacrylate alginate gels to improve their mechanical strength and regulate their degradation rate [57]. Although alginate hydrogels have potential for biomimetic mineralization, their application to biomimetic mineralization of enamel alone has not been reported, and they are mostly cross-linked with other polymers to form double-network hydrogels for enamel surface remineralization [122].
5.1.5. Agarose hydrogel
Agarose is a linear polysaccharide derived from red seaweeds. Agarose is thermo-reversible and can melt or gel at various temperatures. Because of its biocompatibility, it is widely used in the field of biomedicine. Agarose hydrogels can simulate a gel-like ECM during enamel formation and induce enamel regeneration [31,123]. The abundant hydroxyl groups in the agarose hydrogel can interact with Ca2+, and the ACP NPs formed in the hydrogel are coated with organic components, which prevent nucleation and stabilize ACP [30,31]. In addition, agarose hydrogels provide mineral precursors as reservoirs and dynamically transport them to the enamel surface, thereby promoting the formation of HAP on the enamel surface [124]. In addition, the agarose hydrogel matrix can coordinate with the enamel surface to form parallel HAP crystals on the enamel surface [30]. With the progress of mineralization, the agarose hydrogel gradually retreats from the mineralized surface to provide space for the growth of HAP which is consistent with the phenomenon that AMEL degrades and forms mature enamel during the formation of enamel in the physiological process [31].
5.1.6. CS hydrogel
CS derived from deacetylated chitin, is a natural amino homogeneous linear polysaccharide composed of glucosamine and N-acetyl glucosamine units linked by β-(1–4) glycosidic bond [125]. CS is known for its biocompatibility and biodegradability, which allow it to be used in various medical applications. CS hydrogels can be crosslinked with glutaraldehyde and PO43−, and photo-crosslinked CS modified with azides can form hydrogels [126,127].
In recent years, extensive research has been directed towards the application of CS hydrogels in enamel remineralization [30,128,129]. However, CS is difficult to dissolve in aqueous solutions with pH above 6.0, whereas the PH of the physiological environment is close to 7.4, which limits its application [130]. Carboxymethyl CS can be obtained by the carboxymethylation of CS, which can solve this problem. Moreover, the introduction of carboxylic groups can improve the interaction with Ca2+ and promote mineralization in CS hydrogels [131]. Both CS and carboxymethyl CS have Ca2+ binding sites that serve as templates for mineralization and promote crystal nucleation [129]. To enhance the function of CS hydrogels in regulating mineralization orientation, AMEL [131], AMEL-derived peptide [132] and AMEL-splicing product [83] can be added to CS hydrogels to obtain parallel HAP crystal remineralization layers on the surface of enamel in situ. In addition, CS and agarose can be hydrogen-bonded to form CS-agarose hydrogels, which play the role of agarose in regulating crystal growth orientation and obtain a parallel HAP crystal structure and high hardness of the regenerated enamel [30].
5.2. Synthetic hydrogels
Most natural polymers have poor mechanical properties, and polymer chains are vulnerable to attack by enzymes, such that the rate of hydrogel degradation is unpredictable. Synthetic polymers can be used to solve this problem more effectively. Synthetic hydrogels are formed from synthetic polymers, and are generally less biocompatible than natural polymer hydrogels. Structures of synthetic hydrogels can be designed according to research requirements. They are not limited to natural factors, such as natural polymers, which endows synthetic polymer hydrogels with rich diversity in structure as well as in function and makes them ideal tissue engineering materials.
5.2.1. Polyacrylic acid (PAA) hydrogel
Acrylic, which has a carboxylic acid group, along with a carboxylic end connected to a vinyl group, can be polymerized to produce PAA, which contains a large number of carboxyl groups. Both AA and PAA exhibit strong ability to attract metal ions such as Ca2+, making them potential materials for enamel remineralization [133].
PAA exhibits a dual effect on ACP-mediated HAP crystal mineralization. Notably, an appropriate concentration of PAA promotes HAP crystallization by primarily incorporating into ACP, resulting in the formation of ACP NPs, thus providing a large specific surface area for HAP nucleation. Excessive PAA (incorporated into and adsorbed on ACP) impairs the crystallization of HAP, while surface-associated PAA prevents the nucleation of HAP on ACP [134]. Polyacrylate-based hydrogels foster the nucleation of HA crystals through two primary mechanisms, the one is that chelation of Ca2+ by the COOH groups thus, the activity of Ca ions is decreased by complex formation and other effect is that the concentration of Ca2+ by multiple COOH groups [135]. At an appropriate concentration, it has the ability inhibit the deposition of ACP and thus stabilize ACP [134]. As a template, the PAA hydrogel participates in the formation of HAP crystals, and the 3D network of organic matrices plays an important role in the superfine interaction of HAP and the hydrogel through the compartment-effect of the interior of the hydrogel; thus, nano-sized HAP particles are homogenously distributed within the organic template, and the inorganic particles are fine and uniform [136].
5.2.2. Polyacrylamide (PAM) hydrogel
PAM is a long chain polymer. Its structural units contain amide groups that easily form hydrogen bonds, and it exhibits remarkable water solubility and chemical reactivity. PAM can be cross-linked to form hydrogels in various methods [137,138].Calcium phosphate crystals can be synthesized in PAM hydrogels [139] which had a good affinity for phosphate groups in solution to provide template for nucleation and promote the synthesis of HAP. Meanwhile, linear PAM can well regulate the direction of the growth of hydroxyapatite crystals. Because of the steric hindrance produced by PAM, the dispersity of HAP was greatly strengthened, therefore, diverse morphologies of HAP crystals were successfully achieved through changing concentration of polyacrylamide in Xu et al. [140] Similar results were observed in Yokoi et al. [141]. However, the crystalline phases of the calcium phosphates formed in the PAM hydrogel depends on the calcium and phosphate ion concentrations, hydroxyapatite formed in a high ionic concentration environment [142], which was difficult to achieve in the physical environment. In addition, spherulitic porous hydroxyapatite synthesized into PAM hydrogels was very different from the parallel oriented hydroxyapatite in the enamel [140,142]. These problems need to be solved in future research.
5.2.3. PVA hydrogel
PVA is a hydrophilic polymer with abundant hydroxyl groups. PVA hydrogels can be prepared by physical crosslinking [143], chemical crosslinking [144], and radiation crosslinking [145]. They have attracted considerable attention because of their low toxicity, high water absorption, good mechanical properties (i.e., high elastic modulus and mechanical strength), and good biocompatibility [146].
In the study of enamel remineralization, PVA hydrogels can be used as templates for synthesizing HAP crystals and to regulate the orientation and rate of crystal mineralization. In PVA hydrogels, the polymer chains of PVA are rich in hydroxyl groups, which show a partial negative charge to attract Ca2+ [147]. Therefore, PVA chains have multiple nucleation sites for HAP crystals and PVA hydrogels can be used as templates for HAP crystal mineralization [[148], [149], [150]]. In the PVA hydrogels, the swelling decreased significantly with an increase in crosslinking density, thus improving the mechanical properties; however, the HAP crystal formation rate also decreased. Therefore, the rate of HAP crystal formation can be controlled by controlling the crosslinking density of PVA [151]. Under appropriate conditions, PVA hydrogels also showed the ability to regulate the mineralization direction of HAP crystals. Tatsuo et al. showed that after drawing and fixing by successive chemical cross-linking, HAP crystals were successfully mineralized onto oriented hydrogels by repeated alternate immersion in solutions of PO43− and Ca ions [152].
5.2.4. PEG hydrogel
PEG, also known as polyethylene oxide (PEO), can be synthesized via anionic polymerization of ethylene oxide with hydroxyl initiators. Researchers often refer to molecular weights below 20,000 as PEG, and those above 20,000 as PEO. PEG is a linear polymer. In aqueous solutions, the hydrophilic group interacts with water and easily forms an unfolded or folded conformation, similar to the behavior of proteins in aqueous solutions. They exhibit good biocompatibility and are widely used in biomedical applications and research [153].
PEG hydrogels can also be employed to study enamel remineralization. They can be used as a template for HA mineralization and regulates the morphology of HAP crystals [154,155]. In PEG hydrogels, PEG chains can wrap around ACP and help in stabilizing ACP [78,155]. When HAP nuclei are formed, PEG molecules adsorb on the surface of HAP through hydrogen interactions and inhibit the growth of the c-column of HAP; however, with an increase in PH, more PEG molecules interact with free hydroxyl ion (OH−) in the solution instead of HAP, which leads to the hypoactive inhibition of PEG on the c-axes growth of HAP [156]. Zn2+ and citrate can also be added to the PEG hydrogels to stabilize ACP [78]. Although PEG hydrogels have many advantages for HAP crystal growth, it cannot be ignored that PEG plays a negative role in regulating HAP morphology. With an increase in the PEG concentration, the crystal structure changed from a regular rod-like structure similar to natural enamel to an irregular structure which is not what researchers expect [157]. So PEG hydrogels are potential materials for remineralization of enamel.However, potential hypersensitivity reactions induced by PEG should be considered [158].
5.2.5. Polyphosphazene hydrogel
Polyphosphazene consists of a backbone comprising alternating phosphorus and nitrogen (N) atoms, with each phosphorus atom containing two organic side groups. The chemical and physical properties of polyphosphazene largely depend on the type of side groups attached to the polyphosphazene backbone. Their unique biodegradation, which can be altered by introducing different side groups, has generated enormous interest from researchers to study [159,160].
The advantages of polyphosphazene for enamel remineralization lie in its controlled degradation rate and ability to regulate mineralization, especially polyphosphazene with amino acid ester [[161], [162], [163]]. Tomasz et al. showed that the degradation rate of polyphosphazene and its ability to control HAP formation are both dependent on specific active side groups [162]. The degradation rate of polyphosphazene can be controlled by the type and proportion of the amino acid ester side groups [163]. The degradation of polyphosphonitrile with amino acid ester side groups is mainly due to 1) An unstable P–OH moiety formed by the cleavage of the side group from the polymer, which ultimately leads to polymer backbone cleavage, and 2) the hydrolytically unstable P–N bond linking the side group to the polymer backbone, which facilitates hydrolytic breakdown [162,164]. The mineralization ability of polyphosphonitrile is mainly realized by its acidic side groups, and the difference in both the rate and extent of mineralization can be attributed to the different types of acidic side groups present in each polymer [162]. Multiple acidic side groups in polyphosphazene concentrate Ca2+ and form nucleation sites to promote mineralization as templates [165].
5.2.6. Polypeptide hydrogel
Amino acids are natural biomolecules that can form polypeptides through dehydration condensation reactions. Peptide-based hydrogels have proven to be excellent biomedical materials because they have the advantages of nontoxicity, biodegradability, hydrophilicity, and low immunogenicity, and are extremely suitable for the preparation of remineralization hydrogels. Polypeptide hydrogels can simulate ECM and have potential biomedical applications [166,167].
Polypeptide hydrogels formed by acidic amino acids containing two carboxyl groups, such as polyglutamic acid [168] and polyaspartic acid [169], which are rich in carboxyl groups that can attract Ca2+ from the environment, act as a template for HAP mineralization and perform the function of stabilize ACP. Recently, the self-assembled peptide P11-4 hydrogel (Ace-Gln-Gln-Arg-Phe-Glu-Trp-Glu-Phe-Glu-Gln-Gln-NH2) has achieved breakthroughs in enamel remineralization. The design idea of self-assembling peptide P11-4 comes from AMEL. In the physiological state, AMEL forms a chain structure through self-assembly, interacts with HAP, and causes HAP crystals to grow along the c-axis, thus regulating enamel mineralization. Because of the difficulty and high cost of the AMEL extraction process, simulating AMEL using synthetic materials has become a solution to this problem [170]. P11-4 can self-assemble layer by layer into β-sheet structures through a mechanism similar to that of AMEL self-assembly, and further form a fibrous structure as a template for HAP crystal mineralization, regulating HAP crystal mineralization in a manner similar to the natural formation of tooth enamel, and promoting the remineralization of tooth enamel [170,171]. Several clinical studies have demonstrated the effectiveness of P11-4 hydrogels for enamel remineralization in vivo [172,173]. Although self-assembled peptide hydrogels have many advantages for enamel remineralization, their clinical efficacy is slightly inferior to traditional resin therapy [174]. Moreover, changes in the PH of the oral environment and the presence of bacteria can impair the effects of P11-4 on enamel remineralization [175]. In addition, Liu et al. designed a novel biomimetic hydrogel composite containing the QP5 peptide and bioactive glass (BG) and obtained dense remineralization layer both in vitro and in vivo [176]. Therefore, as a new biomimetic synthetic material with good biocompatibility, polypeptide hydrogels have great potential to be explored for enamel remineralization.
5.2.7. Synthetic DNA hydrogel
As a common cellular molecule, DNA has good biocompatibility. Using molecular biology, a large number of specific DNA sequences can be synthesized in a simple and quick manner. Among DNA-based materials, DNA hydrogels, comprising 3D networks of DNA polymeric chains, have received considerable attention as a new class of polymeric materials, particularly as biomaterials, showing great potential for a wide range of promising applications [[177], [178], [179]].
DNA, a naturally negatively charged macromolecule, has the potential to attract Ca2+ to form HAP crystals. Therefore, it has been used in the study of enamel in recent years [180]. Moreover, DNA is not easily degraded by nucleases after specific binding to HAP crystals, which ensures stability of the DNA template during mineralization [181]. Stiff DNA–HAP composites can be fabricated by mineralization on a DNA template [178]. Although DNA hydrogels have great potential for enamel remineralization, further studies are needed to verify their feasibility.
5.3. Hybrid hydrogel
Hybrid hydrogels are formed by cross-linking two or more polymers, combining the advantages of each component to obtain hydrogels with superior properties [182]. Hybrid hydrogels contain multiple polymer network systems, including double network hydrogels, triple network hydrogels [183], quadruple network hydrogels, and quintuple network hydrogels (5 × N) [184]. The most representative multinetwork hydrogel is a double-network hydrogel. Double-network hydrogels consist of two polymer network systems that can form an interpenetrating network by interacting with each other; these are called interpenetrating network hydrogels (IPN hydrogels). A particular feature of IPN hydrogels, characterized by high resistance to wear and high fracture strength, is the preparation of a densely cross-linked ionic hydrogel, with the second network being a neutral loosely cross-linked network [185].
Natural hydrogels with excellent biocompatibility and biodegradability have attracted attention because of their potential as biomaterials. However, their poor mechanical properties limit their applications. IPN hydrogels have been shown to have significantly improved mechanical properties. Natural polymer-based IPN hydrogels can integrate the advantages of each component, thus expanding the properties of the hydrogels and improving their mechanical properties of natural polymer-based hydrogels [186]. Agarose can control the mineralization of HAP crystals; however, its mechanical properties are poor, and its affinity for Ca ions is relatively low. Silk fibroin (SF) networks were introduced into Carboxylated Agarose (CA) hydrogels to enhance the mechanical properties of the hydrogels, and the β-sheet conformation of the gelling SF molecules allowed for the ordered alignment of the active sites, which was beneficial for controlling the orientation and size of cristals [187]. The CA/SF hydrogel can be used as a template for HAP crystal mineralization, and its degradation rate depends on the content of β-sheet conformation [188]. Therefore, the degradation rate of the hydrogel can be regulated by controlling the fibroin content. In general, the CA/SF hydrogel has great potential for applications in enamel remineralization. Gelatin has good elasticity, and agar can control the morphology of HAP crystals, alginate can provide more Ca2+-binding sites, which we have discussed above. Combining two materials can prepare materials that integrate their strengths. IPN hydrogels based on gelatin/agar and gelatin/alginate improve the performance of gelatin hydrogels, enhance their HAP-forming ability, and control the growth of rod-shaped HAP [189,190]. Although IPN hydrogels of natural polymers can achieve the complementary advantages of the two components, such as Ca2+-binding activity, crystal morphology, and controlled degradation rate, it is still not easy to improve the inherent shortcomings of natural polymer hydrogels and their poor mechanical properties, even when enhanced by IPN networks. However, its high swelling and poor mechanical properties are still unsatisfactory [189].
Synthetic polymer-based IPN hydrogels are different from natural polymers limited by natural factors, and they can be designed to have a variety of properties according to the needs of researchers; thus, synthetic polymer IPN hydrogels have broad application prospects. PAA-PAM IPN hydrogels with mutual interlacing, that is the formation of additional physical junctions and lower swelling properties, have higher mechanical strength than PAM hydrogels [140]. Because of its amide group, the chemical activity of acrylamide differs from that of AA with carboxyl groups. PAM hydrogels can control the morphology of the apatite crystals, too [143,144]. The complexing ability of PAM with metal ions enables Ca2+ to be attracted to it; as a surfactant, PAM has a good affinity for hydroxide ions and PO43− groups in solution and further promotes the synthesis of HAP [144]. As mentioned above, the rich carboxyl groups of PAA can form a large number of nucleation sites, and the hydroxyl groups in PVA can be cross-linked with PAA through hydrogen bonding to form PAA/PVA hydrogels with a high cross-linking density. PAA/PVA hydrogels can synthesize HAP crystals by combining the advantages of each component as a template, and they have a lower swelling rate and higher mechanical strength than single-network hydrogels with each component [191,192]. In addition, synthetic polymer-based IPN hydrogels can be designed to control the mineralization orientation of HAP crystals. Kazuki et al. [193] carried out HAP mineralization in stretched tough IPN hydrogels; anisotropic mineralization of HAP occurred in stretched hydrogels, and the C-axis of mineralized HAP aligned along the stretching direction. Despite the vast diversity of synthetic polymer-based hydrogels, a wide variety of polymers can be synthesized according to the imagination of researchers, and polymer hydrogels with higher mechanical properties than natural polymers can be fabricated. However, the potential toxicity of synthetic polymer-based hydrogels caused by the crosslinking agents, photoinitiators, and processing conditions cannot be ignored.
In IPN hydrogels formed using synthetic and natural polymers, the good biocompatibility and biodegradability of natural polymers can be combined with the rich and controllable properties of synthetic polymers to obtain hydrogels with more abundant and better properties. PVA/CS hydrogels formed by the physical crosslinking of PVA and CS can be used as templates for HAP crystal mineralization; not only is the mineralization template with better mechanical properties obtained, but physical crosslinking can also avoid the toxicity problems caused by crosslinking agents [193]. In addition, the PVA/Col hydrogel formed by physical cross-linking of PVA and Col can also be controlled in the mechanical properties and degradation rate of the hydrogel template by adjusting the proportion of its components [194]. In polyethylene (ethylene glycol) diacrylate (PEGDA)/methacrylated poly (γ-glutamic acid) (mPGA) hydrogels, dense mineral deposits were observed in the pores of the hydrogels. Therefore, highly crosslinked synthetic natural polymer IPN hydrogels can be designed as ideal templates for HAP crystal mineralization [195]. The polymer chains in IPN hydrogels formed by synthetic natural polymers can still stabilize ACP [196].
IPN hydrogels are special polymer networks that are designed to improve one or more properties of their components. Compared to single-network hydrogels, IPN hydrogels can be prepared in a more compact manner with stronger mechanical properties and more controllable properties. However, the opposite disadvantage is the decrease in hydrogel porosity caused by the increase in crosslinking density and the resulting decrease in plasticity and other properties. Therefore, the advantages and disadvantages of adding a second network should be fully considered according to the design requirements of IPN hydrogels (Table 2).
Table 2.
Summarizes the hydrogels used for enamel remineralization.
| Hydrogels | Methods | Results | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Geltin hydrogel | Both in vitro and vivo. Gelatin gel which contained phosphate, fluoride and calcium ions were placed onto the tooth enamel surface in vitro. In human oral, the demineralized tooth area was treated with the pretreatment calcium-containing solution. The calcium-gelatin film was positioned into the splint and covered with the phosphate/fluoride-containing gel. Directly after the gels were combined, the splint was positioned into the mouth. To prevent saliva flowing under the splint and dissolving the gels, the splint was sealed. |
Enamel-like layer was visible on the surface of the treated samples; EDX analysis demonstrated a penetration depth of fluoride of 4.10 ± 3.32 μm in enamel |
|
|
[[106], [107], [108]] |
| In vitro. Double-layered gelatin hydrogel was added to the top of etched enamel, calcium-containing gelatin hydrogel and ion-free gelatin hydrogel in sequence from bottom to up. Phosphate solution was added onto the top. | A well-aligned enamel-like structure with an elastic modulus of 52.14 ± 8.48 and nanohardness of 0.73 ± 0.23 GPa was obtained. |
|
|
[100] | |
| Agarose hydrogel | In vitro. Agarose hydrogels covered on etched enamel slice in the presence of 500 ppm fluoride. | The generated tissue had enamel prism-like layers containing well-defined hexagonal hydroxyapatite crystals. The elastic modulus and nanohardness of the regenerated enamel were 89.46 ± 11.82 and 3.04 ± 0.75 GPa, respectively |
|
|
[31] |
| In vitro. Agarose hydrogel was applied on the etched enamel and then they were placed into phosphate solution. | Prismatic enamel configurations became hidden by mineral depositions, But the mineralization is not uniform. |
|
The remineralization layer was incomplete compaction and remineralization was not uniform. (2) The mechanical properties of remineralization layer are low. |
[123] | |
| In vitro. EMD-CaCl2 agarose hydrogel was applied on the etched enamel and were incubated in a phosphate solution containing. fluoride. | SEM observed enamel prism-like crystals formed on the enamel. They had typical apatite hexagonal structures. XRD confirmed that they were fluorinated hydroxyapatite. The elastic modulus and nanohardness of the regenerated enamel were 78.45 ± 14.01 and 2.15 ± 0.61 GPa, respectively. |
|
|
[53] | |
| CS hydrogel | CS-AMEL hydrogel was applied on the etched tooth slice and then dry the hydrogel-covered tooth slice, Transfer the tooth slice to a beaker containing 30 ml of artificial saliva solution at 37 °C. | An enamel-like layer with a thickness of 15 μm was formed on the etched enamel surface. The newly-grown layer was made of highly ordered arrays of crystals with a diameter of ∼50 nm, growing preferentially along the c-axis, perpendicular to the surface. XRD analysis confirmed that the newly-grown layer was composed of apatite crystals. The elastic modulus and nanohardness of the regenerated enamel were about 30 and 1.0 GPa, respectively. |
|
|
[54] |
| LRAP–CS hydrogel was applied to demineralized enamel for several days, followed by drying, and then tooth slices were immersed in artificial saliva at 37 °C. | A dense mineralized layer consisting of highly organized enamel-like apatite crystals was formed. There was a marked improvement in the surface hardness after treatment of the demineralized sample with almost 87 % recovery of the hardness value to that of sound enamel sections. |
|
|
[83] | |
| CS-AMEL hydrogel was applied to demineralized enamel, and then two pH-cycling systems were designed to simulate the daily cariogenic challenge as well as the nocturnal PH conditions in the oral cavity. | CS-AMEL repaired the artificial incipient caries by re-growing oriented crystals and reducing the depth of the lesions by up to 70 % in the pH-cycling systems |
|
|
[131] | |
| CS-EMD hydrogel was applied to etched enamel for followed by drying and immersed in artificial saliva. | SEM-EDX, HRTEM-SAED, FTIR and micro-Raman findings indicated formation of carbonate-substituted HAP, B-type, with c-axis orientation and hardness of 2.48 GPa were recorded. |
|
|
[128] | |
| CS hydrogel was daubed to etched enamel then the samples were soaked in supersaturated calcification solution at 37 °C. | SEM showed a continuous structure of columns crystal with size of 10–40 nm and parallel direction inside, as same as the crystal array in the top of enamel rod. The elastic modulus and nanohardness of the regenerated enamel were 119.74 (Knoop hardness). |
|
The hardness of remineralization layer do not meet the level of natural healthy enamel | [197] | |
| PVP/ACP hydrogel | Electrospun ACP/PVP mat was added to the wet etched enamel surface with artificial saliva solution containing 300 ppm sodium fluoride to produce a hydrogel. | A contiguous overlayer of crystalline fluoridated hydroxyapatite was produced. |
|
ACPs tend to form spherical agglomerations in the spun fibres. | [198] |
| TS@NaF hydrogel | Both in vitro and vivo. TS@NaF hydrogel was applied to etched enamel and placed in artificial saliva at 37 °C. In vivo, TS@NaF hydrogels were applied orally twice a day followed by postoperative fasting from water and food for 2 h. |
SEM showed a compact remineralization was obtained, The XRD spectra of all groups showed peaks of hydroxyapatite. |
|
Potential systemic and topical toxicity caused by rapid F− release. | [199] |
| P11-4 hydrogel | Both in vitro and vivo, P11-4 hydrogel was applied to deminieralized enamel and placed in remineralization buffer in vitro. In human oral, Lyophilised P11−4 was rehydrated with 0.05 ml of sterile water and a single drop of the resulting solution immediately applied directly to the etched enamel surface. Moisture control was ensured until the P11−4 solution was no longer visible (approximately 2 min). |
μCT showed significant remineralization on the surface of enamel. A dense mineralized layer consisting of highly organized enamel-like apatite crystals was formed. |
|
|
[171,200,201] |
| BG-QP5 hydrogel | Both in vitro and vivo, BG-QP5 hydrogel was applied to etched bovine enamel blocks and placed in artificial saliva at 37 °C. Hydrogels were applied on the enamel of rats by using a brush for 5 min three times daily for 5 weeks in vivo. |
SEM showed the enamel surface was covered by a dense mineralized layer. with almost no pores. |
|
BG-QP5 hydrogel is complicated to prepare. | [176] |
| Chitosan–Agarose hydrogel | In vitro. CS–Agarose hydrogel covered on etched enamel slices in artificial saliva with presence of 300 ppm fluoride. | SEM showed hierarchical HAP structure was formed. An analogous Ca/P ratio (1.64) to natural tooth enamel and microhardness recovery of 77.4 % of the enamel-like layer are obtained. |
|
The hardness of remineralization layer do not meet the level of natural healthy enamel. | [30] |
6. Conclusions and future prospects
We summarized the hydrogels commonly used in enamel remineralization. Natural hydrogels have good biocompatibility and controllable degradability, but relatively low mechanical strength, which limits their application of natural hydrogels in the enamel remineralization. In contrast, synthetic polymer hydrogels exhibit superior mechanical properties and can be designed according to the requirements of a wide variety of structures and functions. However, many synthetic polymers exhibit poor degradability and are potentially toxic. Thus, IPN hydrogels could be used to solve these problems. As the advantages of each component can be combined into IPN hydrogels, researchers can design and synthesize IPN hydrogels with excellent properties according to their requirements. However, the design and preparation process of IPN hydrogels are complex, complicated and costly, and the preparation of IPN hydrogels with biocompatibility, biodegradability and bioactivity is attractive, but still challenging.
Therefore, the use of hydrogels for enamel remineralization still faces many challenges, including: 1) Synthesis of HAP crystals with similar structure and properties to natural enamel; 2) Having good mechanical properties to resist the mechanical force in the oral environment; 3) A controllable degradation rate that matches enamel regeneration; and 4) good biocompatibility. Although this is still a challenging task, the synthesis of IPN hydrogels constitutes a promising direction for enamel remineralization, which is expected to receive much attention in the future.
Data availability
Data availability is not applicable to this article as no new data were created or analyzed in this study.
CRediT authorship contribution statement
Jiayi Liao: Writing – original draft, Conceptualization. Junhong Qiu: Methodology. Yanfang Lin: Methodology. Zhihua Li: Writing – review & editing, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jiayi Liao, Email: 413009220002@email.ncu.edu.cn.
Junhong Qiu, Email: Qiujunhong.Ida@email.ncu.edu.cn.
Yanfang Lin, Email: 413009220003@email.ncu.edu.cn.
Zhihua Li, Email: lwlq323@163.com.
References
- 1.Ruan Q., Moradian-Oldak J. Amelogenin and enamel biomimetics. J. Mater. Chem. B. 2015;3:3112–3129. doi: 10.1039/C5TB00163C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Besnard C., Marie A., Sasidharan S., Buček P., Walker J.M., Parker J.E., Spink M.C., Harper R.A., Marathe S., Wanelik K., Moxham T.E.J., Salvati E., Ignatyev K., Kłosowski M.M., Shelton R.M., Landini G., Korsunsky A.M. Multi-resolution Correlative Ultrastructural and chemical analysis of carious enamel by Scanning microscopy and tomographic imaging. ACS Appl. Mater. Interfaces. 2023;15:37259–37273. doi: 10.1021/acsami.3c08031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alghadeer A., Hanson-Drury S., Patni A.P., Ehnes D.D., Zhao Y.T., Li Z., Phal A., Vincent T., Lim Y.C., O'Day D., Spurrell C.H., Gogate A.A., Zhang H., Devi A., Wang Y., Starita L., Doherty D., Glass I.A., Shendure J., Freedman B.S., Baker D., Regier M.C., Mathieu J., Ruohola-Baker H. Single-cell census of human tooth development enables generation of human enamel. Dev. Cell. 2023;58:2163–2180.e2169. doi: 10.1016/j.devcel.2023.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor D.L., in het Panhuis M. Self-healing hydrogels. Adv. Mater. 2016;28:9060–9093. doi: 10.1002/adma.201601613. [DOI] [PubMed] [Google Scholar]
- 5.Liu X., Gao M., Chen J., Guo S., Zhu W., Bai L., Zhai W., Du H., Wu H., Yan C., Shi Y., Gu J., Qi H.J., Zhou K. Recent advances in stimuli-responsive shape-morphing hydrogels. Adv. Funct. Mater. 2022;32 [Google Scholar]
- 6.Vera D., García-Díaz M., Torras N., Álvarez M., Villa R., Martinez E. Engineering tissue barrier models on hydrogel microfluidic platforms. ACS Appl. Mater. Interfaces. 2021;13:13920–13933. doi: 10.1021/acsami.0c21573. [DOI] [PubMed] [Google Scholar]
- 7.Xie L., Wei H., Kou L., Ren L., Zhou J. Antibiotic drug release behavior of poly (vinyl alcohol)/sodium alginate hydrogels. Mater. Werkst. 2020;51:850–855. [Google Scholar]
- 8.Yuan T., Shao Y., Zhou X., Liu Q., Zhu Z., Zhou B., Dong Y., Stephanopoulos N., Gui S., Yan H., Liu D. Highly permeable DNA supramolecular hydrogel promotes neurogenesis and functional recovery after completely transected spinal cord injury. Adv. Mater. 2021;33 doi: 10.1002/adma.202102428. [DOI] [PubMed] [Google Scholar]
- 9.Ye S., Wei B., Zeng L. Advances on hydrogels for oral science research. Gels. 2022;8:302. doi: 10.3390/gels8050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayala-Ham A., López-Gutierrez J., Bermúdez M., Aguilar-Medina M., Sarmiento-Sánchez J.I., López-Camarillo C., Sanchez-Schmitz G., Ramos-Payan R. Hydrogel-based scaffolds in oral tissue engineering. Frontiers in Materials. 2021;8 [Google Scholar]
- 11.Wichterle O., Lím D. Hydrophilic gels for biological use. Nature. 1960;185:117–118. [Google Scholar]
- 12.Slaughter B.V., Khurshid S.S., Fisher O.Z., Khademhosseini A., Peppas N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashir S., Hina M., Iqbal J., Rajpar A.H., Mujtaba M.A., Alghamdi N.A., Wageh S., Ramesh K., Ramesh S. Fundamental concepts of hydrogels: synthesis, properties, and their applications. Polymers. 2020;12:2702. doi: 10.3390/polym12112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gyles D.A., Castro L.D., Silva J.O.C., Ribeiro-Costa R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017;88:373–392. [Google Scholar]
- 15.Lau H.K., Kiick K.L. Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules. 2015;16:28–42. doi: 10.1021/bm501361c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omar J., Ponsford D., Dreiss C.A., Lee T.-C., Loh X.J. Supramolecular hydrogels: design strategies and contemporary biomedical applications. Chem. Asian J. 2022;17 doi: 10.1002/asia.202200081. [DOI] [PubMed] [Google Scholar]
- 17.Li S., Wang W., Li W., Xie M., Deng C., Sun X., Wang C., Liu Y., Shi G., Xu Y., Ma X., Wang J. Fabrication of thermoresponsive hydrogel scaffolds with engineered microscale vasculatures. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 18.Han Z., Wang P., Mao G., Yin T., Zhong D., Yiming B., Hu X., Jia Z., Nian G., Qu S., Yang W. Dual pH-responsive hydrogel actuator for lipophilic drug delivery. ACS Appl. Mater. Interfaces. 2020;12:12010–12017. doi: 10.1021/acsami.9b21713. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Scheiger J.M., Levkin P.A. Design and applications of photoresponsive hydrogels. Adv. Mater. 2019;31 doi: 10.1002/adma.201807333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z., Li Y., Chen C., Cheng Y. Magnetic-responsive hydrogels: from strategic design to biomedical applications. J. Contr. Release. 2021;335:541–556. doi: 10.1016/j.jconrel.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Wang H., Luo Z., Wang Y., He T., Yang C., Ren C., Ma L., Gong C., Li X., Yang Z. enzyme-catalyzed formation of supramolecular hydrogels as promising vaccine adjuvants. Adv. Funct. Mater. 2016;26:1822–1829. [Google Scholar]
- 22.Xu Q., Zhang Z., Xiao C., He C., Chen X. Injectable polypeptide hydrogel as biomimetic scaffolds with tunable bioactivity and controllable cell adhesion. Biomacromolecules. 2017;18:1411–1418. doi: 10.1021/acs.biomac.7b00142. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., Wang M., Xu T., Zhang X., Lin C., Gao W., Xu H., Lei B., Mao C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65–76. doi: 10.7150/thno.29766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqui Z., Sarkar B., Kim K.-K., Kadincesme N., Paul R., Kumar A., Kobayashi Y., Roy A., Choudhury M., Yang J., Shimizu E., Kumar V.A. Angiogenic hydrogels for dental pulp revascularization. Acta Biomater. 2021;126:109–118. doi: 10.1016/j.actbio.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park J., Jeon J., Kim B., Lee M.S., Park S., Lim J., Yi J., Lee H., Yang H.S., Lee J.Y. Electrically conductive hydrogel nerve guidance conduits for peripheral nerve regeneration. Adv. Funct. Mater. 2020;30 [Google Scholar]
- 26.Wang Y., Cao X., Ma M., Lu W., Zhang B., Guo Y. A GelMA-PEGDA-nHA composite hydrogel for bone tissue engineering. Materials. 2020;13:3735. doi: 10.3390/ma13173735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandya M., Diekwisch T.G.H. Enamel biomimetics—fiction or future of dentistry. Int. J. Oral Sci. 2019;11:8. doi: 10.1038/s41368-018-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda M.J., Shimodaira T., Ogaeri T., Shinohara Y., Hata K., Ueda M. A novel culture system for porcine odontogenic epithelial cells using a feeder layer. Arch. Oral Biol. 2006;51:282–290. doi: 10.1016/j.archoralbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Klein O.D., Duverger O., Shaw W., Lacruz R.S., Joester D., Moradian-Oldak J., Pugach M.K., Wright J.T., Millar S.E., Kulkarni A.B., Bartlett J.D., Diekwisch T.G.H., DenBesten P., Simmer J.P. Meeting report: a hard look at the state of enamel research. Int. J. Oral Sci. 2017;9 doi: 10.1038/ijos.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muşat V., Anghel E.M., Zaharia A., Atkinson I., Mocioiu O.C., Buşilă M., Alexandru P. A chitosan–agarose polysaccharide-based hydrogel for biomimetic remineralization of dental enamel. Biomolecules. 2021;11:1137. doi: 10.3390/biom11081137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Y., Mei M.L., Li Q.-L., Lo E.C.M., Chu C.H. Agarose hydrogel biomimetic mineralization model for the regeneration of enamel prismlike tissue. ACS Appl. Mater. Interfaces. 2014;6:410–420. doi: 10.1021/am4044823. [DOI] [PubMed] [Google Scholar]
- 32.Lacruz R.S., Habelitz S., Wright J.T., Paine M.L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 2017;97:939–993. doi: 10.1152/physrev.00030.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habelitz S., Bai Y. Mechanisms of enamel mineralization guided by amelogenin nanoribbons. J. Dent. Res. 2021;100:1434–1443. doi: 10.1177/00220345211012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lijima M., Moriwaki Y., Wen H.B., Fincham A.G., Moradian-Oldak J. Elongated growth of octacalcium phosphate crystals in recombinant amelogenin gels under controlled ionic flow. J. Dent. Res. 2002;81:69–73. doi: 10.1177/002203450208100115. [DOI] [PubMed] [Google Scholar]
- 35.Wen H.B., Moradian-Oldak J., Fincham A.G. Dose-dependent modulation of octacalcium phosphate crystal habit by amelogenins. J. Dent. Res. 2000;79:1902–1906. doi: 10.1177/00220345000790111501. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J., Wang J., Ma C., Lu J. Hydroxyapatite Formation coexists with amyloid-like self-assembly of human amelogenin. Int. J. Mol. Sci. 2020;21:2946. doi: 10.3390/ijms21082946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandya M., Lin T., Li L., Allen M.J., Jin T., Luan X., Diekwisch T.G.H. Posttranslational amelogenin processing and changes in matrix assembly during enamel development. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.C. Robinson, S.J. Brookes, W.A. Bonass, R.C. Shore, J. Kirkham, Enamel Maturation, Ciba Foundation Symposium 205 ‐ Dental Enamel, pp. 156-174. . [DOI] [PubMed]
- 39.Willershausen B., Köttgen C., Ernst C.P. The influence of restorative materials on marginal gingiva. Eur. J. Med. Res. 2001;6:433–439. [PubMed] [Google Scholar]
- 40.Gupta S.K., Saxena P., Pant V.A., Pant A.B. Release and toxicity of dental resin composite. Toxicol. Int. 2012;19:225–234. doi: 10.4103/0971-6580.103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garhammer P., Schmalz G., Hiller K.A., Reitinger T., Stolz W. Patients with local adverse effects from dental alloys: frequency, complaints, symptoms. allergy, Clinical oral investigations. 2001;5:240–249. doi: 10.1007/s007840100127. [DOI] [PubMed] [Google Scholar]
- 42.Al-Wahadni A., Mansour Y., Khader Y. Periodontal response to all-ceramic crowns (IPS Empress) in general practice. Int. J. Dent. Hyg. 2006;4:41–46. doi: 10.1111/j.1601-5037.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 43.Luo Q., Ren T., Lei Z., Huang Y., Huang Y., Xu D., Wan C., Guo X., Wu Y. Non-toxic chitosan-based hydrogel with strong adsorption and sensitive detection abilities for tetracycline. Chem. Eng. J. 2022;427 [Google Scholar]
- 44.Das N. Preparation methods and properties of hydrogel: a review. Int. J. Pharm. Pharmaceut. Sci. 2013;5:112–117. [Google Scholar]
- 45.Oh H.-N., Yoo D., Park S., Lee S., Kim W.-K. Developmental neurotoxicity induced by glutaraldehyde in neuron/astrocyte co-cultured cells and zebrafish. Ecotoxicol. Environ. Saf. 2022;242 doi: 10.1016/j.ecoenv.2022.113891. [DOI] [PubMed] [Google Scholar]
- 46.Jimtaisong A., Saewan N. Plant-derived polyphenols as potential cross-linking agents for methylcellulose-chitosan biocomposites. Solid State Phenom. 2018;283:140–146. [Google Scholar]
- 47.Garnica-Palafox I.M., Sánchez-Arévalo F.M. Influence of natural and synthetic crosslinking reagents on the structural and mechanical properties of chitosan-based hybrid hydrogels. Carbohydr. Polym. 2016;151:1073–1081. doi: 10.1016/j.carbpol.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 48.Draye J.-P., Delaey B., Van de Voorde A., Van Den Bulcke A., De Reu B., Schacht E. In vitro and in vivo biocompatibility of dextran dialdehyde cross-linked gelatin hydrogel films. Biomaterials. 1998;19:1677–1687. doi: 10.1016/s0142-9612(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 49.Baldino L., Concilio S., Cardea S., De Marco I., Reverchon E. Complete glutaraldehyde elimination during chitosan hydrogel drying by SC-CO2 processing. J. Supercrit. Fluids. 2015;103:70–76. [Google Scholar]
- 50.Gao L., Gan H., Meng Z., Gu R., Wu Z., Zhang L., Zhu X., Sun W., Li J., Zheng Y., Dou G. Effects of genipin cross-linking of chitosan hydrogels on cellular adhesion and viability. Colloids Surf. B Biointerfaces. 2014;117:398–405. doi: 10.1016/j.colsurfb.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Bi S., Wang P., Hu S., Li S., Pang J., Zhou Z., Sun G., Huang L., Cheng X., Xing S., Chen X. Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair. Carbohydr. Polym. 2019;224 doi: 10.1016/j.carbpol.2019.115176. [DOI] [PubMed] [Google Scholar]
- 52.Yuan L., Wu Y., Gu Q.-s., El-Hamshary H., El-Newehy M., Mo X. Injectable photo crosslinked enhanced double-network hydrogels from modified sodium alginate and gelatin. Int. J. Biol. Macromol. 2017;96:569–577. doi: 10.1016/j.ijbiomac.2016.12.058. [DOI] [PubMed] [Google Scholar]
- 53.Cao Y., Mei M.L., Li Q.-L., Lo E.C.M., Chu C.H. Enamel prism-like tissue regeneration using enamel matrix derivative. J. Dent. 2014;42:1535–1542. doi: 10.1016/j.jdent.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Au - Ruan Q., Au - Moradian-Oldak J. Development of amelogenin-chitosan hydrogel for in vitro enamel regrowth with a dense interface. JoVE. 2014 doi: 10.3791/51606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W., Gibson C.W., Abrams W.R., Andrews D.W., DenBesten P.K. Reduced hydrolysis of amelogenin may result in X-linked amelogenesis imperfecta. Matrix Biol. 2001;19:755–760. doi: 10.1016/s0945-053x(00)00121-9. [DOI] [PubMed] [Google Scholar]
- 56.Gadhia K., McDonald S., Arkutu N., Malik K. Amelogenesis imperfecta: an introduction. Br. Dent. J. 2012;212:377–379. doi: 10.1038/sj.bdj.2012.314. [DOI] [PubMed] [Google Scholar]
- 57.Jeon O., Bouhadir K.H., Mansour J.M., Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30:2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 58.Zeng Z., Mo X.-m., He C., Morsi Y., El-Hamshary H., El-Newehy M. An in situ forming tissue adhesive based on poly(ethylene glycol)-dimethacrylate and thiolated chitosan through the Michael reaction. J. Mater. Chem. B. 2016;4:5585–5592. doi: 10.1039/c6tb01475e. [DOI] [PubMed] [Google Scholar]
- 59.Wade R.J., Bassin E.J., Rodell C.B., Burdick J.A. Protease-degradable electrospun fibrous hydrogels. Nat. Commun. 2015;6:6639. doi: 10.1038/ncomms7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prajapati S., Tao J., Ruan Q., De Yoreo J.J., Moradian-Oldak J. Matrix metalloproteinase-20 mediates dental enamel biomineralization by preventing protein occlusion inside apatite crystals. Biomaterials. 2016;75:260–270. doi: 10.1016/j.biomaterials.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prajapati S., Ruan Q., Mukherjee K., Nutt S., Moradian-Oldak J. The presence of MMP-20 reinforces biomimetic enamel regrowth. J. Dent. Res. 2018;97:84–90. doi: 10.1177/0022034517728504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oyen M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014;59:44–59. [Google Scholar]
- 63.Zhang X.N., Wang Y.J., Sun S., Hou L., Wu P., Wu Z.L., Zheng Q. A tough and stiff hydrogel with tunable water content and mechanical properties based on the synergistic effect of hydrogen bonding and hydrophobic interaction. Macromolecules. 2018;51:8136–8146. [Google Scholar]
- 64.Lee K.Y., Rowley J.A., Eiselt P., Moy E.M., Bouhadir K.H., Mooney D.J. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules. 2000;33:4291–4294. [Google Scholar]
- 65.Zhang D., Duan J., Wang D., Ge S. Effect of preparation methods on mechanical properties of PVA/HA composite hydrogel. JBE. 2010;7:235–243. [Google Scholar]
- 66.Huang X., Li J., Luo J., Gao Q., Mao A., Li J. Research progress on double-network hydrogels. Mater. Today Commun. 2021;29 [Google Scholar]
- 67.Wu J., Pan Z., Zhao Z.-Y., Wang M.-H., Dong L., Gao H.-L., Liu C.-Y., Zhou P., Chen L., Shi C.-J., Zhang Z.-Y., Yang C., Yu S.-H., Zou D.-H. Anti-swelling, robust, and adhesive extracellular matrix-mimicking hydrogel used as intraoral dressing. Adv. Mater. 2022;34 doi: 10.1002/adma.202200115. [DOI] [PubMed] [Google Scholar]
- 68.Zaragoza J., Fukuoka S., Kraus M., Thomin J., Asuri P. Exploring the role of nanoparticles in enhancing mechanical properties of hydrogel nanocomposites. Nanomaterials. 2018;8:882. doi: 10.3390/nano8110882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han M., Li Q.-L., Cao Y., Fang H., Xia R., Zhang Z.-H. In vivo remineralization of dentin using an agarose hydrogel biomimetic mineralization system. Sci. Rep. 2017;7 doi: 10.1038/srep41955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu M., Wang L., Zhang W., Ganss B. An evolutionarily conserved subdomain in amelotin promotes amorphous calcium phosphate-to-hydroxyapatite phase transition. Cryst. Growth Des. 2019;19:2104–2113. [Google Scholar]
- 71.Lotsari A., Rajasekharan A.K., Halvarsson M., Andersson M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018;9:4170. doi: 10.1038/s41467-018-06570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le Norcy E., Kwak S.-Y., Wiedemann-Bidlack F.B., Beniash E., Yamakoshi Y., Simmer J.P., Margolis H.C. Potential role of the amelogenin N-terminus in the regulation of calcium phosphate formation in vitro. Cells Tissues Organs. 2011;194:188–193. doi: 10.1159/000324827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gelli R., Ridi F., Baglioni P. The importance of being amorphous: calcium and magnesium phosphates in the human body. Adv. Colloid Interface Sci. 2019;269:219–235. doi: 10.1016/j.cis.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 74.Boskey A.L., Posner A.S. Conversion of amorphous calcium phosphate to microcrystalline hydroxyapatite. A pH-dependent, solution-mediated, solid-solid conversion. J. Phys. Chem. 1973;77:2313–2317. [Google Scholar]
- 75.Bussola Tovani C., Gloter A., Azaïs T., Selmane M., Ramos A.P., Nassif N. Formation of stable strontium-rich amorphous calcium phosphate: possible effects on bone mineral. Acta Biomater. 2019;92:315–324. doi: 10.1016/j.actbio.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 76.Gelli R., Briccolani-Bandini L., Pagliai M., Cardini G., Ridi F., Baglioni P. Exploring the effect of Mg2+ substitution on amorphous calcium phosphate nanoparticles. J. Colloid Interface Sci. 2022;606:444–453. doi: 10.1016/j.jcis.2021.08.033. [DOI] [PubMed] [Google Scholar]
- 77.Sinusaite L., Kareiva A., Zarkov A. Thermally induced crystallization and phase evolution of amorphous calcium phosphate substituted with divalent cations having different sizes. Cryst. Growth Des. 2021;21:1242–1248. [Google Scholar]
- 78.Schweikle M., Bjørnøy S.H., van Helvoort A.T.J., Haugen H.J., Sikorski P., Tiainen H. Stabilisation of amorphous calcium phosphate in polyethylene glycol hydrogels. Acta Biomater. 2019;90:132–145. doi: 10.1016/j.actbio.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 79.Root M.J. Inhibition of the amorphous calcium phosphate phase transformation reaction by polyphosphates and metal ions. Calcif. Tissue Int. 1990;47:112–116. doi: 10.1007/BF02555994. [DOI] [PubMed] [Google Scholar]
- 80.Yasar O.F., Liao W.-C., Stevensson B., Edén M. Structural role and spatial distribution of carbonate ions in amorphous calcium phosphate. J. Phys. Chem. C. 2021;125:4675–4693. [Google Scholar]
- 81.Chen Y., Gu W., Pan H., Jiang S., Tang R. Stabilizing amorphous calcium phosphate phase by citrate adsorption. CrystEngComm. 2014;16:1864–1867. [Google Scholar]
- 82.Kwak S.Y., Litman A., Margolis H.C., Yamakoshi Y., Simmer J.P. Biomimetic enamel regeneration mediated by leucine-rich amelogenin peptide. J. Dent. Res. 2017;96:524–530. doi: 10.1177/0022034516688659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukherjee K., Ruan Q., Liberman D., White S.N., Moradian-Oldak J. Repairing human tooth enamel with leucine-rich amelogenin peptide–chitosan hydrogel. J. Mater. Res. 2016;31:556–563. [Google Scholar]
- 84.Wang Z., Zhou Z., Fan J., Zhang L., Zhang Z., Wu Z., Shi Y., Zheng H., Zhang Z., Tang R., Fu B. Hydroxypropylmethylcellulose as a film and hydrogel carrier for ACP nanoprecursors to deliver biomimetic mineralization. J. Nanobiotechnol. 2021;19:385. doi: 10.1186/s12951-021-01133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan Y., Wen Z.T., Liao S., Lallier T., Hagan J.L., Twomley J.T., Zhang J.-F., Sun Z., Xu X. Novel amelogenin-releasing hydrogel for remineralization of enamel artificial caries. J. Bioact. Compat Polym. 2012;27:585–603. doi: 10.1177/0883911512458050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukherjee K., Chakraborty A., Sandhu G., Naim S., Bauza Nowotny E., Moradian-Oldak J. Amelogenin peptide-chitosan hydrogel for biomimetic enamel regrowth. Frontiers in Dental Medicine. 2021;2 doi: 10.3389/fdmed.2021.697544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shoulders M.D., Raines R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antoine E.E., Vlachos P.P., Rylander M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: characterization of mechanics, structure, and transport. Tissue Eng., Part B. 2014;20:683–696. doi: 10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Landis W.J., Jacquet R. Association of calcium and phosphate ions with collagen in the mineralization of vertebrate tissues. Calcif. Tissue Int. 2013;93:329–337. doi: 10.1007/s00223-013-9725-7. [DOI] [PubMed] [Google Scholar]
- 90.Zhang W., Liao S.S., Cui F.Z. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem. Mater. 2003;15:3221–3226. [Google Scholar]
- 91.Wang Y., Azaïs T., Robin M., Vallée A., Catania C., Legriel P., Pehau-Arnaudet G., Babonneau F., Giraud-Guille M.-M., Nassif N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012;11:724–733. doi: 10.1038/nmat3362. [DOI] [PubMed] [Google Scholar]
- 92.Chen L., Wu C., Chen S., Zhang Y., Liu A., Ding J., Wei D., Guo Z., Sun J., Fan H. Biomimetic mineralizable collagen hydrogels for dynamic bone matrix formation to promote osteogenesis. J. Mater. Chem. B. 2020;8:3064–3075. doi: 10.1039/c9tb02633a. [DOI] [PubMed] [Google Scholar]
- 93.Ikeda Y., Neshatian M., Holcroft J., Ganss B. The enamel protein ODAM promotes mineralization in a collagen matrix. Connect. Tissue Res. 2018;59:62–66. doi: 10.1080/03008207.2017.1408603. [DOI] [PubMed] [Google Scholar]
- 94.Ikeda Y., Holcroft J., Ikeda E., Ganss B. Amelotin promotes mineralization and adhesion in collagen-based systems. Cell. Mol. Bioeng. 2022;15:245–254. doi: 10.1007/s12195-022-00722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alipal J., Mohd Pu'ad N.A.S., Lee T.C., Nayan N.H.M., Sahari N., Basri H., Idris M.I., Abdullah H.Z. A review of gelatin: properties, sources, process, applications, and commercialisation. Mater. Today: Proc. 2021;42:240–250. [Google Scholar]
- 96.Liu D., Nikoo M., Boran G., Zhou P., Regenstein J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015;6:527–557. doi: 10.1146/annurev-food-031414-111800. [DOI] [PubMed] [Google Scholar]
- 97.Azami M., Moosavifar M.J., Baheiraei N., Moztarzadeh F., Ai J. Preparation of a biomimetic nanocomposite scaffold for bone tissue engineering via mineralization of gelatin hydrogel and study of mineral transformation in simulated body fluid. J. Biomed. Mater. Res. 2012;100A:1347–1355. doi: 10.1002/jbm.a.34074. [DOI] [PubMed] [Google Scholar]
- 98.Asgari B., Azami M., Amiri A., Imani Fooladi A.A., Nourani M.R. Bone scaffold biomimetics based on gelatin hydrogel mineralization, journal of biomimetics. Biomaterials and Tissue Engineering. 2013;17:59–69. [Google Scholar]
- 99.Busch S., Dolhaine H., DuChesne A., Heinz S., Hochrein O., Laeri F., Podebrad O., Vietze U., Weiland T., Kniep R. Biomimetic morphogenesis of fluorapatite-gelatin composites: fractal growth, the question of intrinsic electric fields, core/shell assemblies, hollow spheres and reorganization of denatured collagen. Eur. J. Inorg. Chem. 1999;1999:1643–1653. [Google Scholar]
- 100.Chen Z., Miao Z., Zhang P., Xiao H., Liu H., Ding C., Tan H., Li J. Bioinspired enamel-like oriented minerals on general surfaces: towards improved mechanical properties. J. Mater. Chem. B. 2019;7:5237–5244. doi: 10.1039/c9tb00676a. [DOI] [PubMed] [Google Scholar]
- 101.Busch S. Regeneration of human tooth enamel. Angew. Chem. Int. Ed. 2004;43:1428–1431. doi: 10.1002/anie.200352183. [DOI] [PubMed] [Google Scholar]
- 102.Ye Q., Zhang Y., Dai K., Chen X., Read H.M., Zeng L., Hang F. Three dimensional printed bioglass/gelatin/alginate composite scaffolds with promoted mechanical strength, biomineralization, cell responses and osteogenesis. J. Mater. Sci. Mater. Med. 2020;31:77. doi: 10.1007/s10856-020-06413-6. [DOI] [PubMed] [Google Scholar]
- 103.Miao F., Liu T., Zhang X., Wang X., Wei Y., Hu Y., Lian X., Zhao L., Chen W., Huang D. Engineered bone tissues using biomineralized gelatin methacryloyl/sodium alginate hydrogels. Journal of Biomaterials Science. 2022;33:137–154. doi: 10.1080/09205063.2021.1980360. Polymer Edition. [DOI] [PubMed] [Google Scholar]
- 104.Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou L., Tan G., Tan Y., Wang H., Liao J., Ning C. Biomimetic mineralization of anionic gelatin hydrogels: effect of degree of methacrylation. RSC Adv. 2014;4:21997–22008. [Google Scholar]
- 106.Guentsch A., Fahmy M.D., Wehrle C., Nietzsche S., Popp J., Watts D.C., Kranz S., Krafft C., Sigusch B.W. Effect of biomimetic mineralization on enamel and dentin: a Raman and EDX analysis. Dent. Mater. 2019;35:1300–1307. doi: 10.1016/j.dental.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guentsch A., Busch S., Seidler K., Kraft U., Nietzsche S., Preshaw P.M., Chromik J.N., Glockmann E., Jandt K.D., Sigusch B.W. Biomimetic mineralization: effects on human enamel in vivo. Adv. Eng. Mater. 2010;12:B571–B576. [Google Scholar]
- 108.Guentsch A., Seidler K., Nietzsche S., Hefti A.F., Preshaw P.M., Watts D.C., Jandt K.D., Sigusch B.W. Biomimetic mineralization: long-term observations in patients with dentin sensitivity. Dent. Mater. 2012;28:457–464. doi: 10.1016/j.dental.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 109.Highley C.B., Prestwich G.D., Burdick J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016;40:35–40. doi: 10.1016/j.copbio.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 110.Burdick J.A., Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Z., Zhou H., Wang X., Sang L., Wang C., Ma J., Li X. Controlled mineralization by extracellular matrix: monodisperse, colloidally stable calcium phosphate-hyaluronan hybrid nanospheres. Chem. Commun. 2010;46:1278–1280. doi: 10.1039/b918835e. [DOI] [PubMed] [Google Scholar]
- 112.Yang X., Akhtar S., Rubino S., Leifer K., Hilborn J., Ossipov D. Direct ″click″ synthesis of hybrid bisphosphonate–hyaluronic acid hydrogel in aqueous solution for biomineralization. Chem. Mater. 2012;24:1690–1697. [Google Scholar]
- 113.A combination of biphasic calcium phosphate scaffold with hyaluronic acid-gelatin hydrogel as a new tool for bone regeneration. Tissue Eng. 2014;20:1993–2004. doi: 10.1089/ten.tea.2013.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ondeck M.G., Engler A.J. Mechanical characterization of a dynamic and tunable methacrylated hyaluronic acid hydrogel. J. Biomech. Eng. 2016;138 doi: 10.1115/1.4032429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Spearman B.S., Agrawal N.K., Rubiano A., Simmons C.S., Mobini S., Schmidt C.E. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. 2020;108:279–291. doi: 10.1002/jbm.a.36814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hahn S.K., Park J.K., Tomimatsu T., Shimoboji T. Synthesis and degradation test of hyaluronic acid hydrogels. Int. J. Biol. Macromol. 2007;40:374–380. doi: 10.1016/j.ijbiomac.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 117.Augst A.D., Kong H.J., Mooney D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 118.Lee C.S.D., Moyer H.R., Gittens I R.A., Williams J.K., Boskey A.L., Boyan B.D., Schwartz Z. Regulating in vivo calcification of alginate microbeads. Biomaterials. 2010;31:4926–4934. doi: 10.1016/j.biomaterials.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suneetha M., Kim H.J., Han S.S. Bone-like apatite formation in phosphate-crosslinked sodium alginate-based hydrogels. Mater. Lett. 2022;328 doi: 10.1016/j.ijbiomac.2023.128364. [DOI] [PubMed] [Google Scholar]
- 120.Strasser V., Matijaković N., Mihelj Josipović T., Kontrec J., Lyons D.M., Kralj D., Dutour Sikirić M. Factors affecting calcium phosphate mineralization within bulk alginate hydrogels. J. Polym. Res. 2019;26:262. [Google Scholar]
- 121.Mohabatpour F., Yazdanpanah Z., Papagerakis S., Chen X., Papagerakis P. Self-crosslinkable oxidized alginate-carboxymethyl chitosan hydrogels as an injectable cell carrier for in vitro dental enamel regeneration. J. Funct. Biomater. 2022;13:71. doi: 10.3390/jfb13020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mohabatpour F., Duan X., Yazdanpanah Z., Tabil X.L., Lobanova L., Zhu N., Papagerakis S., Chen X., Papagerakis P. Bioprinting of alginate-carboxymethyl chitosan scaffolds for enamel tissue engineering in vitro. Biofabrication. 2023;15 doi: 10.1088/1758-5090/acab35. [DOI] [PubMed] [Google Scholar]
- 123.El Moshy S., Abbass M.M.S., El-Motayam A.M. Biomimetic remineralization of acid etched enamel using agarose hydrogel model. F1000Research. 2018;7:1476. doi: 10.12688/f1000research.16050.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cao C.Y., Li Q.-L. Scanning electron microscopic analysis of using agarose hydrogel microenvironment to create enamel prism-like tissue on dentine surface. J. Dent. 2016;55:54–60. doi: 10.1016/j.jdent.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 125.Fu J., Yang F., Guo Z. The chitosan hydrogels: from structure to function. New J. Chem. 2018;42:17162–17180. [Google Scholar]
- 126.Pellá M.C.G., Lima-Tenório M.K., Tenório-Neto E.T., Guilherme M.R., Muniz E.C., Rubira A.F. Chitosan-based hydrogels: from preparation to biomedical applications. Carbohydr. Polym. 2018;196:233–245. doi: 10.1016/j.carbpol.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 127.Racine L., Texier I., Auzély-Velty R. Chitosan-based hydrogels: recent design concepts to tailor properties and functions. Polym. Int. 2017;66:981–998. [Google Scholar]
- 128.Zaharia A., Muşat V., Anghel E.M., Atkinson I., Mocioiu O.-C., Buşilă M., Pleşcan V.G. Biomimetic chitosan-hydroxyapatite hybrid biocoatings for enamel remineralization. Ceram. Int. 2017;43:11390–11402. [Google Scholar]
- 129.Wang Z., Su J., Ali A., Yang W., Zhang R., Li Y., Zhang L., Li J. Chitosan and carboxymethyl chitosan mimic biomineralization and promote microbially induced calcium precipitation. Carbohydr. Polym. 2022;287 doi: 10.1016/j.carbpol.2022.119335. [DOI] [PubMed] [Google Scholar]
- 130.Sogias I.A., Khutoryanskiy V.V., Williams A.C. Exploring the factors affecting the solubility of chitosan in water. Macromol. Chem. Phys. 2010;211:426–433. [Google Scholar]
- 131.Ruan Q., Liberman D., Bapat R., Chandrababu K.B., Phark J.H., Moradian-Oldak J. Efficacy of amelogenin-chitosan hydrogel in biomimetic repair of human enamel in pH-cycling systems. Journal of biomedical engineering and informatics. 2016;2:119–128. doi: 10.5430/jbei.v2n1p119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ren Q., Ding L., Li Z., Wang X., Wang K., Han S., Li W., Zhou X., Zhang L. Chitosan hydrogel containing amelogenin-derived peptide: inhibition of cariogenic bacteria and promotion of remineralization of initial caries lesions. Arch. Oral Biol. 2019;100:42–48. doi: 10.1016/j.archoralbio.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 133.Jiang S., Cao Y., Li S., Pang Y., Sun Z. Dual function of poly (acrylic acid) on controlling amorphous mediated hydroxyapatite crystallization. J. Cryst. Growth. 2021;557 [Google Scholar]
- 134.Jiang S., Cao Y., Li S., Pang Y., Sun Z. Dual function of poly(acrylic acid) on controlling amorphous mediated hydroxyapatite crystallization. J. Cryst. Growth. 2021;557 [Google Scholar]
- 135.Yokoi T., Kawashita M., Ohtsuki C. Biomimetic mineralization of calcium phosphates in polymeric hydrogels containing carboxyl groups. Journal of Asian Ceramic Societies. 2013;1:155–162. [Google Scholar]
- 136.Shen X., Tong H., Zhu Z., Wan P., Hu J. A novel approach of homogenous inorganic/organic composites through in situ precipitation in poly-acrylic acid gel. Mater. Lett. 2007;61:629–634. [Google Scholar]
- 137.Yang C., Yin T., Suo Z. Polyacrylamide hydrogels. I. Network imperfection. J. Mech. Phys. Solid. 2019;131:43–55. [Google Scholar]
- 138.Cohen Y., Ramon O., Kopelman I.J., Mizrahi S. Characterization of inhomogeneous polyacrylamide hydrogels. J. Polym. Sci. B Polym. Phys. 1992;30:1055–1067. [Google Scholar]
- 139.Das P., Akkus O., Azad A.-M. Optimization of the mineral content in polymeric gels: the effect of calcium to phosphate molar ratio. J. Cryst. Growth. 2005;280:587–593. [Google Scholar]
- 140.Xu Z., Qian G., Feng M. Using polyacrylamide to control particle size and synthesize porous nano hydroxyapatite. Results Phys. 2020;16 [Google Scholar]
- 141.Yokoi T., Kawashita M., Ohtsuki C. Effects of polymer concentration on the morphology of calcium phosphate crystals formed in polyacrylamide hydrogels. J. Cryst. Growth. 2013;383:166–171. [Google Scholar]
- 142.Yokoi T., Kawashita M., Kikuta K., Ohtsuki C. Biomimetic mineralization of calcium phosphate crystals in polyacrylamide hydrogel: effect of concentrations of calcium and phosphate ions on crystalline phases and morphology. Mater. Sci. Eng. C. 2010;30:154–159. [Google Scholar]
- 143.Kumar A., Mishra R., Reinwald Y., Bhat S. Cryogels: freezing unveiled by thawing. Mater. Today. 2010;13:42–44. [Google Scholar]
- 144.Zhu X., Zhang Y., Deng J., Luo X. Effect of glycerol on the properties of the cross-linked polyvinyl alcohol hydrogel beads. ChemistrySelect. 2018;3:467–470. [Google Scholar]
- 145.Nho Y.C., Park K.R. Preparation and properties of PVA/PVP hydrogels containing chitosan by radiation. J. Appl. Polym. Sci. 2002;85:1787–1794. [Google Scholar]
- 146.Wang M., Bai J., Shao K., Tang W., Zhao X., Lin D., Huang S., Chen C., Ding Z., Ye J. Poly(vinyl alcohol) hydrogels: the old and new functional materials. International Journal of Polymer Science. 2021;2021 [Google Scholar]
- 147.Timofejeva A., D'Este M., Loca D. Calcium phosphate/polyvinyl alcohol composite hydrogels: a review on the freeze-thawing synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017;95:547–565. [Google Scholar]
- 148.Tetsushi T., Akio K., Mitsuru A. Hydroxyapatite Formation on/in poly(vinyl alcohol) hydrogel matrices using a novel alternate soaking process. Chem. Lett. 1998;27:711–712. [Google Scholar]
- 149.Nayar S., Pramanick A.K., Sharma B.K., Das G., Ravi Kumar B., Sinha A. Biomimetically synthesized polymer-hydroxyapatite sheet like nano-composite. J. Mater. Sci. Mater. Med. 2008;19:301–304. doi: 10.1007/s10856-007-3129-z. [DOI] [PubMed] [Google Scholar]
- 150.Poursamar S.A., Azami M., Mozafari M. Controllable synthesis and characterization of porous polyvinyl alcohol/hydroxyapatite nanocomposite scaffolds via an in situ colloidal technique. Colloids Surf. B Biointerfaces. 2011;84:310–316. doi: 10.1016/j.colsurfb.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 151.Taguchi T., Shiraogawa M., Kishida A., Akashi M. A study on hydroxyapatite formation on/in the hydroxyl groups-bearing nonionic hydrogels. Journal of Biomaterials Science. 1999;10:19–32. doi: 10.1163/156856299x00252. Polymer Edition. [DOI] [PubMed] [Google Scholar]
- 152.Kaneko T., Ogomi D., Mitsugi R., Serizawa T., Akashi M. Mechanically drawn hydrogels uniaxially orient hydroxyapatite crystals and cell extension. Chem. Mater. 2004;16:5596–5601. [Google Scholar]
- 153.D’souza A.A., Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expet Opin. Drug Deliv. 2016;13:1257–1275. doi: 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- 154.Qiu C., Xiao X., Liu R. Biomimetic synthesis of spherical nano-hydroxyapatite in the presence of polyethylene glycol. Ceram. Int. 2008;34:1747–1751. [Google Scholar]
- 155.Zhang Y., Dong Y. Effect of surfactant on morphology of hydroxyapatite, synthesis and reactivity in inorganic, metal-organic. Nano-Metal Chemistry. 2015;45:411–414. [Google Scholar]
- 156.Zuo G., Wei X., Sun H., Liu S., Zong P., Zeng X., Shen Y. Morphology controlled synthesis of nano-hydroxyapatite using polyethylene glycol as a template. J. Alloys Compd. 2017;692:693–697. [Google Scholar]
- 157.Dhanalakshmi C., Vijayalakshmi L., Narayanan V. Synthesis and preliminary characterization of polyethylene glycol (PEG)/hydroxyapatite (HAp) nanocomposite for biomedical applications. Int. J. Phys. Sci. 2012;7:2093–2101. [Google Scholar]
- 158.Wylon K., Dölle S., Worm M. Polyethylene glycol as a cause of anaphylaxis, Allergy. Asthma & Clinical Immunology. 2016;12:67. doi: 10.1186/s13223-016-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ogueri K.S., Ivirico J.L.E., Nair L.S., Allcock H.R., Laurencin C.T. Biodegradable polyphosphazene-based blends for regenerative engineering. Regenerative Engineering and Translational Medicine. 2017;3:15–31. doi: 10.1007/s40883-016-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schacht E., Vandorpe J., Dejardin S., Lemmouchi Y., Seymour L. Biomedical applications of degradable polyphosphazenes. Biotechnol. Bioeng. 1996;52:102–108. doi: 10.1002/(SICI)1097-0290(19961005)52:1<102::AID-BIT10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 161.Nukavarapu S.P., Kumbar S.G., Brown J.L., Krogman N.R., Weikel A.L., Hindenlang M.D., Nair L.S., Allcock H.R., Laurencin C.T. Polyphosphazene/nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules. 2008;9:1818–1825. doi: 10.1021/bm800031t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Modzelewski T., Hotham I., Allcock H.R. Deposition of calcium hydroxyapatite on negatively charged polyphosphazene surfaces. J. Appl. Polym. Sci. 2015;132 [Google Scholar]
- 163.Allcock H.R., Pucher S.R., Scopelianos A.G. Poly[(amino acid ester)phosphazenes]: synthesis, crystallinity, and hydrolytic sensitivity in solution and the solid state. Macromolecules. 1994;27:1071–1075. [Google Scholar]
- 164.Allcock H.R., Morozowich N.L. Bioerodible polyphosphazenes and their medical potential. Polym. Chem. 2012;3:578–590. [Google Scholar]
- 165.Li L., Mao C., Wang J., Xu X., Pan H., Deng Y., Gu X., Tang R. Bio-Inspired enamel repair via glu-directed assembly of apatite nanoparticles: an approach to biomaterials with optimal characteristics. Adv. Mater. 2011;23:4695–4701. doi: 10.1002/adma.201102773. [DOI] [PubMed] [Google Scholar]
- 166.Mondal S., Das S., Nandi A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter. 2020;16:1404–1454. doi: 10.1039/c9sm02127b. [DOI] [PubMed] [Google Scholar]
- 167.Cai L., Liu S., Guo J., Jia Y.-G. Polypeptide-based self-healing hydrogels: design and biomedical applications. Acta Biomater. 2020;113:84–100. doi: 10.1016/j.actbio.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 168.Park S.-B., Sung M.-H., Uyama H., Han D.K. Poly(glutamic acid): production, composites, and medical applications of the next-generation biopolymer. Prog. Polym. Sci. 2021;113 [Google Scholar]
- 169.Zeng J., Yang S., Yu H., Xu Z., Quan X., Zhou J. Simulation insight into the synergic role of citrate and polyaspartic peptide in biomineralization. Langmuir. 2021;37:3410–3419. doi: 10.1021/acs.langmuir.0c03626. [DOI] [PubMed] [Google Scholar]
- 170.Bonchev A., Vasileva R., Dyulgerova E., Yantcheva S. Self-assembling peptide P11-4: a biomimetic agent for enamel remineralization. Int. J. Pept. Res. Therapeut. 2021;27:899–907. [Google Scholar]
- 171.Kind L., Stevanovic S., Wuttig S., Wimberger S., Hofer J., Müller B., Pieles U. Biomimetic remineralization of carious lesions by self-assembling peptide. J. Dent. Res. 2017;96:790–797. doi: 10.1177/0022034517698419. [DOI] [PubMed] [Google Scholar]
- 172.Alkilzy M., Tarabaih A., Santamaria R.M., Splieth C.H. Self-assembling peptide P11-4 and fluoride for regenerating enamel. J. Dent. Res. 2018;97:148–154. doi: 10.1177/0022034517730531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bröseler F., Tietmann C., Bommer C., Drechsel T., Heinzel-Gutenbrunner M., Jepsen S. Randomised clinical trial investigating self-assembling peptide P11-4 in the treatment of early caries. Clin. Oral Invest. 2020;24:123–132. doi: 10.1007/s00784-019-02901-4. [DOI] [PubMed] [Google Scholar]
- 174.Wierichs R.J., Kogel J., Lausch J., Esteves-Oliveira M., Meyer-Lueckel H. Effects of self-assembling peptide P11-4, fluorides, and caries infiltration on artificial enamel caries lesions in vitro. Caries Res. 2017;51:451–459. doi: 10.1159/000477215. [DOI] [PubMed] [Google Scholar]
- 175.Aggeli A., Bell M., Carrick L.M., Fishwick C.W.G., Harding R., Mawer P.J., Radford S.E., Strong A.E., Boden N. pH as a trigger of peptide β-sheet self-assembly and reversible switching between nematic and isotropic phases. J. Am. Chem. Soc. 2003;125:9619–9628. doi: 10.1021/ja021047i. [DOI] [PubMed] [Google Scholar]
- 176.Liu Z., Lu J., Chen X., Xiu P., Zhang Y., Lv X., Jiang X., Wang K., Zhang L. A novel amelogenesis-inspired hydrogel composite for the remineralization of enamel non-cavitated lesions. J. Mater. Chem. B. 2022;10:10150–10161. doi: 10.1039/d2tb01711c. [DOI] [PubMed] [Google Scholar]
- 177.Bertran O., del Valle L.J., Revilla-López G., Chaves G., Cardús L., Casas M.T., Casanovas J., Turon P., Puiggalí J., Alemán C. Mineralization of DNA into nanoparticles of hydroxyapatite. Dalton Trans. 2014;43:317–327. doi: 10.1039/c3dt52112e. [DOI] [PubMed] [Google Scholar]
- 178.Zhou Y., Deng J., Zhang Y., Li C., Wei Z., Shen J., Li J., Wang F., Han B., Chen D., Fan C., Zhang H., Liu K., Wei Y. Engineering DNA-guided hydroxyapatite bulk materials with high stiffness and outstanding antimicrobial ability for dental inlay applications. Adv. Mater. 2022;34 doi: 10.1002/adma.202202180. [DOI] [PubMed] [Google Scholar]
- 179.Li F., Tang J., Geng J., Luo D., Yang D. Polymeric DNA hydrogel: design, synthesis and applications. Prog. Polym. Sci. 2019;98 [Google Scholar]
- 180.Athanasiadou D., Carneiro K.M.M. DNA nanostructures as templates for biomineralization. Nat. Rev. Chem. 2021;5:93–108. doi: 10.1038/s41570-020-00242-5. [DOI] [PubMed] [Google Scholar]
- 181.Brundin M., Figdor D., Sundqvist G., Sjögren U. DNA binding to hydroxyapatite: a potential mechanism for preservation of microbial DNA. J. Endod. 2013;39:211–216. doi: 10.1016/j.joen.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 182.Vasile C., Pamfil D., Stoleru E., Baican M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules. 2020;25:1539. doi: 10.3390/molecules25071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li J., Li H., Wu C., Zhang W. PVA-AAm-AG multi-network hydrogel with high mechanical strength and cell adhesion. Polymer. 2022;247 [Google Scholar]
- 184.Panteli P.A., Patrickios C.S. Complex hydrogels based on multiply interpenetrated polymer networks: enhancement of mechanical properties via network multiplicity and monomer concentration. Macromolecules. 2018;51:7533–7545. [Google Scholar]
- 185.Dragan E.S. Design and applications of interpenetrating polymer network hydrogels. A review, Chemical Engineering Journal. 2014;243:572–590. [Google Scholar]
- 186.Suo H., Zhang D., Yin J., Qian J., Wu Z.L., Fu J. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C. 2018;92:612–620. doi: 10.1016/j.msec.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 187.Hu J.-X., Ran J.-B., Chen S., Jiang P., Shen X.-Y., Tong H. Carboxylated agarose (CA)-Silk fibroin (SF) dual confluent matrices containing oriented hydroxyapatite (HA) crystals: biomimetic organic/inorganic composites for tibia repair. Biomacromolecules. 2016;17:2437–2447. doi: 10.1021/acs.biomac.6b00587. [DOI] [PubMed] [Google Scholar]
- 188.Horan R.L., Antle K., Collette A.L., Wang Y., Huang J., Moreau J.E., Volloch V., Kaplan D.L., Altman G.H. In vitro degradation of silk fibroin. Biomaterials. 2005;26:3385–3393. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 189.Mehta P., Kaith B.S. A Novel approach for the morphology controlled synthesis of rod-shaped nano-hydroxyapatite using semi-IPN and IPN as a template. Int. J. Biol. Macromol. 2018;107:312–321. doi: 10.1016/j.ijbiomac.2017.08.164. [DOI] [PubMed] [Google Scholar]
- 190.Stancu I.-C., Dragusin D.M., Vasile E., Trusca R., Antoniac I., Vasilescu D.S. Porous calcium alginate–gelatin interpenetrated matrix and its biomineralization potential. J. Mater. Sci. Mater. Med. 2011;22:451–460. doi: 10.1007/s10856-011-4233-7. [DOI] [PubMed] [Google Scholar]
- 191.Simeonov M.S., Apostolov A.A., Vassileva E.D. In situ calcium phosphate deposition in hydrogels of poly(acrylic acid)–polyacrylamide interpenetrating polymer networks. RSC Adv. 2016;6:16274–16284. [Google Scholar]
- 192.Iwatsubo T., Kishi R., Miura T., Ohzono T., Yamaguchi T. Formation of hydroxyapatite skeletal materials from hydrogel matrices via artificial biomineralization. J. Phys. Chem. B. 2015;119:8793–8799. doi: 10.1021/acs.jpcb.5b03181. [DOI] [PubMed] [Google Scholar]
- 193.Fukao K., Nonoyama T., Kiyama R., Furusawa K., Kurokawa T., Nakajima T., Gong J.P. Anisotropic growth of hydroxyapatite in stretched double network hydrogel. ACS Nano. 2017;11:12103–12110. doi: 10.1021/acsnano.7b04942. [DOI] [PubMed] [Google Scholar]
- 194.Wang M., Li J., Li W., Du Z., Qin S. Preparation and characterization of novel poly (vinyl alcohol)/collagen double-network hydrogels. Int. J. Biol. Macromol. 2018;118:41–48. doi: 10.1016/j.ijbiomac.2018.05.200. [DOI] [PubMed] [Google Scholar]
- 195.Yang Q., Song F., Zou X., Liao L. Preparation and mineralization of a biocompatible double network hydrogel. Journal of Biomaterials Science. 2017;28:431–443. doi: 10.1080/09205063.2017.1279044. Polymer Edition. [DOI] [PubMed] [Google Scholar]
- 196.Zhong C., Chu C.C. Biomimetic mineralization of acid polysaccharide-based hydrogels: towards porous 3-dimensional bone-like biocomposites. J. Mater. Chem. 2012;22:6080–6087. [Google Scholar]
- 197.Tian K., Peng M., Fei W., Liao C.H., Ren X.H. Induced synthesis of hydroxyapatite by chitosan for enamel remineralization. Adv. Mater. Res. 2012;530:40–45. [Google Scholar]
- 198.Fletcher J., Walsh D., Fowler C.E., Mann S. Electrospun mats of PVP/ACP nanofibres for remineralization of enamel tooth surfaces. CrystEngComm. 2011;13:3692–3697. [Google Scholar]
- 199.Zhen L., Liang K., Luo J., Ke X., Tao S., Zhang M., Yuan H., He L., Bidlack F.B., Yang J., Li J. Mussel-Inspired hydrogels for fluoride delivery and caries prevention. J. Dent. Res. 2022;101:1597–1605. doi: 10.1177/00220345221114783. [DOI] [PubMed] [Google Scholar]
- 200.Sindhura V., Uloopi K.S., Vinay C., Chandrasekhar R. Evaluation of enamel remineralizing potential of self-assembling peptide P11-4 on artificially induced enamel lesions in vitro. J. Indian Soc. Pedod. Prev. Dent. 2018;36 doi: 10.4103/JISPPD.JISPPD_255_18. [DOI] [PubMed] [Google Scholar]
- 201.Brunton P.A., Davies R.P.W., Burke J.L., Smith A., Aggeli A., Brookes S.J., Kirkham J. Treatment of early caries lesions using biomimetic self-assembling peptides – a clinical safety trial. Br. Dent. J. 2013;215 doi: 10.1038/sj.bdj.2013.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study.