Abstract
Inflammation is a complex physiological phenomenon, which is the body's defensive response, but abnormal inflammation can have adverse effects, and many diseases are related to the inflammatory response. AMPK, as a key sensor of cellular energy status, plays a crucial role in regulating cellular energy homeostasis and glycolipid metabolism. In recent years, the anti-inflammation effect of AMPK and related signalling cascade has begun to enter everyone's field of vision – not least the impact on metabolic diseases. A great number of studies have shown that anti-inflammatory drugs work through AMPK and related pathways.
Herein, this article summarises recent advances in compounds that show anti-inflammatory effects by activating AMPK and attempts to comment on them.
Keywords: AMPK, Inflammation, SIRT, Inflammasome, NF-κB, Treatment
Graphical abstract
The Signalling Pathways Associated with AMPK Activation & Compounds’ Anti-inflammatory Effect via AMPK Activation.
1. Introduction
As a natural response to injuries, infections, and stress, inflammation is a complex physiological phenomenon that occurs in the body. Yet it remains a vital component of a number of pathological disorders. Inflammatory diseases, whether they are acute or chronic, pose a serious threat to human health [1]. Various diseases are linked to chronic inflammation, including autoimmune diseases, type 2 diabetes, and cancer. Inflammation-related diseases are now among the major causes of morbidity and mortality globally due to their increasing prevalence [2]. Hence, it is imperative to develop medications that can control inflammation in order to treat these diseases or, for an even better choice, prevent them. When there is inflammation, multiple proinflammatory cytokines are produced, and so are other inflammatory mediators [3,4]. These variables mitigate and exacerbate inflammatory responses and are implicated in the pathogenesis of numerous associated disorders. The transcription factor nuclear factor kappa-B (NF-κB) as a well-studied archetypal proinflammatory signalling pathway, strongly correlates with various proinflammatory factors [5].
1.1. AMPK overview
An AMP-activated kinase (AMPK), a heterotrimer, consists of a catalytic α- and two non-catalytic β- and γ-subunits, which bind AMP, ADP and ATP, three adenosine nucleotides [[6], [7], [8], [9], [10], [11]]. AMPK is partially directly regulated by adenylate charge. The activation of the master regulator is mediated by two different pathways, either an AMP-dependent or a Ca2+-dependent route, with liver kinase B1 (LKB1) mediating activation by the former pathway and Ca2+ calmodulin-dependent protein kinase beta (CaMKKβ) doing so by the latter [[12], [13], [14], [15]]. As a highly conserved serine/threonine kinase, it also serves as a key sensor of cellular energy status and plays an essential role in regulating cellular energy homeostasis and glycolipid metabolism. And it regulates multiple pathophysiological processes, to name a few, autophagy, protein synthesis, and apoptosis [16,17].
Among the class III histone deacetylases, sirtuin 1 (SIRT1) is the most conserved protein deacetylase that depends on nicotinamide adenine dinucleotide (NAD+). AMPK is SIRT1's mediator, mediating both upstream and downstream [18,19]. AMPK and SIRT1 play pivotal roles in energy balance as well as inflammatory response. The AMPK pathway is a negative regulator of inflammation. AMPK activation can inhibit NF-κB, inhibit the expression of inflammatory genes, and reduce inflammatory damage [20,21]. An evolutionarily conserved family of stress-responsive proteins, sestrins, is upregulated by stress conditions, such as oxidative stress, and genotoxic stress. Sestrin2 (Sesn2), an antioxidant, prevents reactive oxygen species from accumulating [22]. Researchers discovered that Sestrin2 activates AMPK both in vitro and in vivo [23]. Oxidative stress induces the formation of oxygen free radicals, which can negatively impact cells and trigger the release of inflammatory mediators, like tumour necrosis factor‐α (TNF-α), interleukin (IL)-1β, IL‐6 and nitric oxide (NO) [24].
Adding to this, AMPK reportedly is of vital importance in preventing inflammatory responses by inhibiting NLRP3 inflammasome activity. This pathway is crucial in mediating systemic inflammatory responses [25].
Research has shown that AMPK may serve as a therapeutic target for a variety of metabolic disorders. It is thought to help improve conditions like non-alcoholic fatty liver disease (NAFLD) and the progression of osteoarthritis (OA) [[26], [27], [28]]. While this paper aims to summarise the compounds that activate AMPK, directly or indirectly, it is also important to note that, AMPK activation is not the only way of regulating energy balance-related conditions [29]. Table 1 displays the effects of the compounds (Table 1). Current clinical trials of the drugs are given in Table 2.
Table 1.
Effects of the compounds.
| Compound | Model | Disease/Disorder | Targets |
|---|---|---|---|
| Melatonin | NEC mice | Necrotising enterocolitis | AMPK/SIRT1; PGC-1α; NRF1; NRF2; TFAM |
| Corosolic acid | HFD-fed mice; Th17 and Treg cells | NAFLD | AMPK/SREBPs; NF-κB/MAPK |
| Ilexgenin A | HFD-fed mice; EA.hy-926 cells | Endothelial dysfunction | LKB1; NF-κB; TXNIP/NLRP3 inflammasome |
| Cryptotanshinone | HepG2 cells | ALD | AMPK/SIRT1; ACC; NF-κB |
| Atractylenolide III | Ulcerative colitis | Ulcerative colitis | AMPK/SIRT1/PGC1α |
| Quercetin | OA rats | Osteoarthritis | AMPK/SIRT1; PGC-1α, FoxO3A; NF-κB; NRF-1, NRF-2, TFAM |
| Isovitexin | BV-2 cells; LPS-injected mice | Neuroinflammation; Depression | CaMKKβ |
| Nobiletin | LPS-induced depression rat model | Parkinson's disease; Alzheimer's disease | NLRP3 inflammasome |
| Luteolin | mouse OA model produced by DMM | Osteoarthritis | AMPK/Nrf2 |
| Hesperetin | SIRT1 knockdown 293T cells | Hepatic inflammation | AMPK/SIRT1; NF-κB; AMPK/CREB |
| HD-16 | LX-2 cells | Hepatic fibrosis | AMPK/SIRT1 |
| Resveratrol | AD model | Alzheimer's disease | AMPK/SIRT1 |
| Pterostilbene | LPS-sensitized 16HBE cells | Asthma/ | AMPK/SIRT1 |
| Magnolol | HepG2 cells; Human chondrocytes | Hyperlipidemia; NAFLD/NASH; Osteoarthritis | AMPK/SIRT3; SIRT1/AMPK/PGC-1α; NF-κB; MAPK/NF-κB/SREBP-1c |
| Honokiol | Hep-G2 cells | Tumour | AMPK/SIRT3; NF-κB |
| Dihydromyricetin | C2C12 cells | Obesity | CaMKK/AMPK; NF-κB |
| Baicalin | MCAO/R model | Neurological impairment/cerebral ischemia-reperfusion injury; Atherosclerosis | NLRP3 inflammasome; MAPK/NF-κB |
| Urolithin A | APP/PS1 mice | Memory impairment | NF-κB; TXNIP/NLRP3/IL-1β |
| Salidroside | AGEs-induced HUVECs | Lung cancer; Spinal cord injury | NF-κB |
| Stevioside | Lethal shock mouse model | Lethal shock | NF-κB; IL-6 |
| Butyric Acid | Obesity | HuR | |
| β-aminoisobutyric acid | differentiated 3 T3-L1 cells | Obesity | NF-κB; PGC-1a |
| Mangiferin | NAFLD mice | NAFLD/NASH | TXNIP |
| Curcumin | Rat knee OA model | Osteoarthritis | AMPK/PINK1/Parkin |
| Empagliflozin | PA-stimulated HepG2 cells | FAS; SREBP-1c; | |
| Ezetimibe | NAFLD/NASH | NLRP3 inflammasome |
*AMPK is not mentioned in the table unless it emphasises the related pathway and factors.
Table 2.
Current Clinical Trials of the Compounds listed in the article.
| Compound | Conditions | Phase | Period | Interventions | NCT number |
|---|---|---|---|---|---|
| Melatonin | Uveal Melanoma Uveal Melanoma, Posterior, Medium/Large Size Eye Cancer, Intraocular Melanoma |
3 | 2022-10-02 to 2031-01-01 | Drug: Melatonin | 05502900 |
| Melatonin Bioavailability | N/A | 2023-03-14 to 2023-06-30 | Dietary Supplement: dietary supplement, one tablet containing delayed-release melatonin, zinc and lemon balm | 05419466 | |
| Bioavailability | N/A | 2023-05 to 2023-07 | Dietary Supplement: Melatonin with phenyl capsaicin Dietary Supplement: Melatonin without phenyl capsaicin |
05835258 | |
| Metformin | Age-Related Macular Degeneration Macular Degeneration, Age-Related Dry Macular Degeneration Geographic Atrophy |
2 | 2016-04 to 2024-12 | Drug: Metformin | 02684578 |
| Type 2 Diabetes Mellitus | 3 | 2020-01-14 to 2021-06-03 | Drug: DBPR108 Drug: metformin hydrochloride Drug: placebo |
04218734 | |
| Empagliflozin | End Stage Renal Disease on Dialysis | 4 | 2023-03-01 to 2024-07-03 | Drug: Empagliflozin 25 mg vs Placebo Drug: Empagliflozin 10 MG |
05671991 |
| Heart Failure | 1 | 2023-03 to 2024-11 | Drug: Empagliflozin 10 mg Drug: Placebo |
05553938 | |
| Non-alcoholic Fatty Liver Disease | 4 | 2021-01-01 to 2023-05-01 | Drug: Empagliflozin 10 MG Drug: Placebo pills |
04642261 | |
| Ezetimibe | Acute Myocardial Infarction Dyslipidemias |
N/A | 2021-03-24 to 2024-04 | Drug: Ezetimibe 10 mg | 04701242 |
| Luteolin | Schizophrenia Schizoaffective Disorder |
N/A | 2022-06-13 to 2025-03 | Dietary Supplement: Luteolin Dietary Supplement: Placebo |
05204407 |
| Frontotemporal Dementia | N/A | 2019-06-01 to 2022-12-30 | Dietary Supplement: PEA-LUT Dietary Supplement: PLACEBO |
04489017 | |
| COVID | 2 | 2020-08-04 to 2022-12 | Dietary Supplement: Açaí palm berry extract – a natural product Other: Placebo |
04404218 | |
| Primary Open Angle Glaucoma | N/A | 2021-02-01 to 2023-02-28 | Dietary Supplement: GlaucoCetin Other: Placebo |
04784234 | |
| Mangiferin | Cognitive Change Stress |
N/A | 2019-11-04 to 2020-03-17 | Dietary Supplement: Zynamite® Other: Placebo |
04299217 |
| Cognitive Change | N/A | 2021-05-17 to 2022-04-14 | Dietary Supplement: Zynamite® 15 % 150 mg Dietary Supplement: Zynamite® 15 % 300 mg Dietary Supplement: Zynamite® 15 % 600 mg Dietary Supplement: Placebo |
05580146 | |
| Attention Cognition Mood |
N/A | 2022-01-04 to 2022-06-20 | Other: Mango leaf extract capsule Other: Placebo capsule |
05182450 | |
| Photoaging | N/A | 2021-05-30 to 2023-06-30 | Other: Food | 04869852 | |
| Curcumin | Palate; Wound | N/A | 2022-11-01 to 2024-04-01 | Other: curcumin gel 2 % | 05819632 |
| Acute Lymphoblastic Leukemia, Pediatric | 2 | 2021-08-22 to 2022-09 | Drug: Curcumin Dietary Supplement: Standard of Care |
05045443 | |
| Diabetes Mellitus, Type 2 Dyslipidemias Hypertension |
2 | 2023-03 to 2024-01 | Dietary Supplement: Puritans Pride Turmeric curcumin® 500 mg | 05753436 | |
| Bioavailability Gut Microbiome Safety |
1; 2 | 2023-04-01 to 2025-03-31 | Drug: Low curcumin group Drug: High curcumin group |
05774704 | |
| Dihydromyricetin | Alcohol-Related Disorders | 1 | 2022-12-01 to 2023-12-31 | Drug: Dihydromyricetin | 05623501 |
| Type 2 Diabetes Mellitus | 2 | 2019-10-30 to 2022-08-30 | Drug: Dihydromyricetin Drug: Metformin |
03606694 | |
| Insomnia Due to Anxiety and Fear | N/A | 2021-07-25 to 2022-11-03 | Dietary Supplement: DHM Dietary Supplement: Placebo |
05280561 | |
| Alcohol Drinking Alcohol Intoxication |
N/A | 2022-03-30 to 2023-05-30 | Dietary Supplement: ALCOFILTRUM | 05757089 | |
| Resveratrol | Diabetes Mellitus, Type 2 Coronary Artery Disease |
N/A | 2018-03-06 to 2023-12-31 | Dietary Supplement: Trans-resveratrol Dietary Supplement: Placebo |
03762096 |
| Dyslipidemias | 3 | 2021-05-01 to 2022-09-30 | Drug: Mega Resveratrol® capsules Drug: Mega Resveratrol® Placebo capsules |
04886297 | |
| Coronary Artery Disease Menopause Endothelial Dysfunction |
N/A | 2023-03-06 to 2026-06-05 | Dietary Supplement: Resveratrol Dietary Supplement: Placebo |
05808387 | |
| Knee Osteoarthritis | 3 | 2017-11-09 to 2022-05-12 | Drug: oral resveratrol Drug: oral placebo |
02905799 | |
| Chronic Kidney Diseases Endothelial Dysfunction |
N/A | 2019-01-01 to 2023-06-01 | Dietary Supplement: Resveratrol Other: Placebo |
03597568 | |
| Pterostilbene | Biological Availability | N/A | 2022-10-20 to 2022-11-17 | Dietary Supplement: Pterostilbene cocrystal Dietary Supplement: Pterostilbene free form |
05561075 |
| Atypical Endometrial Hyperplasia Endometrial Carcinoma |
2 | 2019-01-21 to 2023-12-07 | Drug: Megestrol Acetate Biological: Pterostilbene |
03671811 | |
| Amyotrophic Lateral Sclerosis | N/A | 2021-10-07 to 2023-10-31 | Dietary Supplement: EH301 (Nicotinamide Riboside/Pterostilbene) | 05095571 | |
| Urolithin A | Healthy Muscle Disuse Atrophy |
N/A | 2023-06-01 to 2024-09-30 | Dietary Supplement: Protein supplement Dietary Supplement: Protein supplement with Urolithin A |
05814705 |
| Ageing | N/A | 2023-01-30 to 2023-12-31 | Dietary Supplement: Softgel-containing placebo Dietary Supplement: Softgel containing 250 mg of Urolithin A (Mitopure) |
05735886 | |
| Healthy Aging Healthy Healthy Diet |
N/A | 2021-08-30 to 2023-07-31 | Dietary Supplement: Mitopure Dietary Supplement: Pomegranate Juice |
04985630 | |
| Healthy | N/A | 2021-04-21 to 2023-04-30 | Dietary Supplement: Mitopure Dietary Supplement: Placebo |
04783207 | |
| Stevioside | Healthy Human Gut Microflora |
N/A | 2022-02-15 to 2022-04-29 | Other: Sucrose Other: Steviol Glycosides |
05264636 |
| Overweight and Obesity Type 2 Diabetes Mellitus |
N/A | 2022-04-15 to 2023-07 | Other: Water Other: Glucose Other: Steviol Glycosides Other: Steviol Glycosides plus glucose Other: Rebaudioside A |
05287906 | |
| Non-Alcoholic Fatty Liver Disease | N/A | 2019-07-26 to 2023-03-01 | Other: Stevia Intervention Other: Water Intervention |
03985020 |
The data were collected from https://clinicaltrials.gov/
2. Lactone
2.1. Melatonin
Melatonin, a hormone consisting of a lactone ring fused with an indole ring, plays a vital role in regulating sleep-wake cycles and among the body's physiological processes (Fig. 1). Melatonin is produced via enzymatic reactions from the amino acid tryptophan. The antioxidant protects cells from oxidative injury and has anti-inflammatory and immunomodulatory properties.
Fig. 1.

The structure of Melatonin. Similar structures and scaffolds are highlighted in different colours. The structure highlighted in brown circle is N-ethylacetamide. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Inflammatory responses in the gut seem to be influenced by disruption of the balance between Th17 cells and Treg cells. The most common and fatal gastrointestinal disease affecting premature infants is necrotising enterocolitis (NEC). The imbalance caused by decreased Treg cells and increased Th17 cells is key to NEC. Melatonin's effects on NEC are mainly due to its ability to increase antioxidant enzyme activity and reduce oxidative stress. The melatonin treatment significantly reduces NEC incidence in humans and rats [30].
SIRT1 overexpression in vitro is associated with Th17 reductions and Treg cell differentiation. By studying SIRT1 gene expression in melatonin-treated CD4+ T cells, the researchers noticed melatonin increased SIRT1 mRNA, under Treg cell-inducing conditions, considerably greater than in vehicle-treated controls. Moreover, flow cytometry and immunoblotting showed that Ex-527, a SIRT1 antagonist, reduced the Th17 cell decline and Treg cell growth treated by melatonin. Similar to melatonin-treated neonatal mice, Ex-527 boosted STAT3 phosphorylation but decreased STAT5. Ex-527 also reduced transforming growth factor-beta (TGF-β) but increased IL-17 and IL-22 compared to melatonin. In experimental NEC, Ex-527 disrupted the Th17/Treg balance, reducing melatonin's therapeutic efficacy. Overall, the cellular inflammatory response that blocked SIRT1 activation reduced melatonin's therapeutic benefits.
Immunoblotting showed that NEC pups had lower phosphorylation of AMPK (p-AMPK) and SIRT1 than nursing groups. Melatonin increased p-AMPK and SIRT1 in NEC mice. Compound C (CC), an AMPK-specific inhibitor, negates melatonin's benefits in NEC mice. In comparison with pups treated with melatonin, CC decreased the expression of zonula occludens-1, p-AMPK, and SIRT1. CC affected intestinal melatonin secretion like Ex-527. CC prevented melatonin-treated animals from maintaining Th17/Treg cell balances, as well as melatonin-enhanced Treg and Th17 cell development in vitro. In sum, the findings demonstrate that melatonin activates AMPK, which then activates SIRT1, followed by the balance of Th17/Treg.
Ma et al. found that melatonin controls the balance between Th17 and Treg cells by activating the AMPK/SIRT1 pathway. This may be another reason why melatonin improves NEC [30].
3. Terpenoids
3.1. Corosolic acid
Corosolic acid (CA) is a pentacyclic triterpene, also known as 2α-hydroxyursolic acid, naturally occurring in various medicinal plants, including lingonberry, mignonette, loquat and so forth (Fig. 2). The compound is a common ingredient in food supplements antidiabetic agents [31]. Through AMPK-dependent autophagy, CA may protect against myocardial hypertrophy [32].
Fig. 2.
Structures of CA, IA, Cryptotanshinone and AT III. Both CA and IA are pentacyclic triterpenoids.
Currently, due to various reasons, the number of obese patients is increasing, and obesity further increases the risk of many other diseases, making it a health problem that has been widely concerned. Obesity-associated chronic inflammation is believed as a main factor of cancers, among other diseases [33].
Obesity-associated insulin resistance is caused largely by adipose tissue inflammation [34]. Insulin resistance plays a prominent role in the development and occurrence of NAFLD, particularly in patients with type 2 diabetes [34]. The research by Yang et al. claimed that modulation of AMPK activity helps suppress inflammation associated with insulin resistance [35]. The presence of hepatic steatosis, or excess fat deposits in the liver cells, is defined as NAFLD, among the most common metabolic liver diseases with increasing obesity rates. Excessive accumulation of triglycerides without heavy alcohol consumption is a feature of NAFLD, often associated with insulin resistance, metabolic syndrome, and prolonged overnutrition [[36], [37], [38]]. As NAFLD progresses, its irreversible form, non-alcoholic steatohepatitis (NASH), is involved in developing hepatocellular carcinoma (HCC) and cirrhosis. Inflammation is the main pathological component of NAFLD. Fibrosis is an essential determinant of clinical outcomes in NAFLD [39,40]. AMPK is a key regulatory enzyme for the energy balance of liver tissue cells, related to glucose and lipid metabolism in liver cells. It is a potential target for NAFLD treatment.
The CA treatment can reduce the inflammatory condition and improve the heightened oxidative stress [41]. Moreover, Yang et al.‘s research indicates that CA helps to treat high fat diet (HFD)-induced adipose tissue inflammation and adipokine expression. HFD eating causes I kappa B kinase beta (IKKβ) phosphorylation and inflammation-related gene expression in adipose tissue [42]. Oral treatment of mice with CA (20 mg/kg) reduced IKKβ phosphorylation and proinflammatory cytokine gene expressions by 76.44, 65.84, and 85.82 %, respectively, compared to HFD-fed mice. CA elevated M2 macrophage polarisation marker gene Fizz1 expression by more than ten times. CA can regulate AMPK, which inhibits inflammation and reduces insulin resistance. CA restored p-AMPK in adipose tissue reduced by HFD eating. Adipocyte basal p-AMPK was also regulated, according to the study. 10 μM of CA elevated LKB-1 and AMPK phosphorylation. The findings suggest that CA increases AMPK via LKB1 in adipocytes and tissue. HFD also causes macrophage infiltration in adipose tissue, worsening dysfunction and insulin resistance. CA inhibits macrophage invasion, thus preventing adipocyte-macrophage crosstalk [42].
In conclusion, CA is a potent anti-hyperlipidaemic and anti-hepatic steatosis agent through a mechanism involving lipogenesis and cholesterol synthesis as well as inhibition of inflammatory responses through AMPK/SREBPs and NF-κB/MAPK signalling pathways. A better understanding of the antidiabetic effects of CA and its underlying mechanisms will facilitate its application in the management of insulin resistance and diabetes [42].
3.2. Ilexgenin A
Ilexgenin A (IA) is a natural compound with a pentacyclic triterpene structure [43,44]. It is a component extracted from camellia green tea. Camellia green tea itself is used as a Chinese herbal medicine to treat hypertension and hyperlipidaemia. Both CA and IA are pentacyclic triterpenoids. Triterpenoids have various therapeutic properties, such as hypoglycaemic activity.
The endoplasmic reticulum (ER) is the primary signalling organelle that senses cellular stress, among other functions [45]. Endothelial dysfunction is the main cause of initial vascular lesions. Endothelial dysfunction is characterised by impairment of endothelium-dependent vasodilation.
In endothelial cells, palmitate stimulation induces ER stress and subsequent activation of the thioredoxin-interacting protein (TXNIP)/NLRP3 inflammasome, leading to endothelial dysfunction. The generation of IL-1β, facilitated by TXNIP, is involved in the death of β cells caused by ER stress [46]. Inhibiting TXNIP signalling protects against ER stress. The inflammasome is primarily induced by NF-κB activation signalling. IA enhances LKB1-dependent AMPK activity and ameliorates ER stress by inhibiting ROS-associated TXNIP induction. However, the knockdown of AMPK prevented these effects, indicating that AMPK is essential for its effect on suppressing ER stress. In the meantime, IA inhibits the activation of the NLRP3 inflammasome by downregulating NLRP3 and cleaving the induction of caspase-1, thereby decreasing IL-1β secretion. In addition, it inhibits inflammation and apoptosis following palmitate injury.
IA decreased ER stress and restored the loss of endothelial nitric oxide synthase (endothelial NOS or eNOS) activity in the vascular endothelium, enhancing endothelium-dependent vasodilation in rat aortas, consistent with these findings in endothelial cells. Further analysis in mice fed a high-fat diet demonstrated that oral administration of IA inhibited ER stress/NLRP3 activation decreased ROS production, and increased NO production in vascular endothelium, validating the positive effect of IA on endothelial homeostasis in vivo. These findings suggest that ER stress-associated activation of the TXNIP/NLRP3 inflammasome contributes to endothelial dysfunction and that IA ameliorates endothelial dysfunction by inhibiting ER stress and TXNIP/NLRP3 inflammasome activation and modulating AMPK endothelial dysfunction [47].
3.3. Cryptotanshinone
Cryptotanshinone (CT) is a quinone diterpene isolated from Salvia miltiorrhiza with a tetracyclic scaffold. It has been reported that CT has a vast number of biological functions, including anti-inflammation [48,49].
Activation of AMPK reduces lipogenesis and enhances fatty acid oxidation. Activation of the AMPK/SIRT1 pathway is a promising therapeutic target in alcoholic liver disease (ALD) [50,51]. AMPK activation inhibits the activation of acetyl-CoA carboxylase (ACC), which is involved in hepatic lipid synthesis and inflammation. Nagappan et al. showed that CT attenuated ethanol-induced liver injury by inhibiting lipogenesis-related genes, oxidative stress, and inflammation [52]. CT treatment has seen considerably increased phosphorylations of AMPK and ACC, reduced expression of SREBP-1c, as well as the proteins expressed in a time-dependent manner in SIRT1-HepG2 cells. An essential transcription factor, sterol regulatory element-binding protein 1c (SREBP-1c) modulates the production of triglycerides and fatty acids [53]. Peroxisome proliferation-activated receptor gamma (PPARγ) of the PPAR family, plays a crucial role in energy metabolism, adipogenesis, fatty acid oxidation, regulating mitochondrial function, etc [54,55]. The major downstream signalling molecules of AMPK involved in lipid synthesis include SREBP-1 and PPARγ.
AMPK and SIRT1 can regulate transcription factors such as nuclear factor erythroid 2‐related factor 2 (Nrf2) and NF-κB, which are involved in regulating antioxidant genes against oxidative damage and suppressing pro-inflammatory cytokines, respectively [18,56,57]. Nrf2 is an important regulator of the adaptive antioxidant response to oxidative stress in cells [58,59]. Nrf2 binds to specific DNA sequence antioxidant response elements (AREs) and stimulates the transcription of downstream target, antioxidant genes. The experimental results of Nagappan et al. found that Nrf2 may play a role in CT-mediated antioxidant activity. Importantly, inhibition of AMPK using CC prevented CT-induced increases in Nrf2 protein, suggesting that AMPK activation is involved in CT-induced Nrf2. In a nutshell, these results underline that AMPK/SIRT1 and Nrf2 signalling may also be involved in the protective effect of CT against ethanol-mediated oxidative stress.
Increased expression of hepatic cytokines mediated the progression of alcoholic hepatic steatosis in an ethanol-fed mouse model [60]. Previous studies have shown that cytokines, like TNF-α and IL-6, play crucial roles in both acute and chronic inflammation. The key transcription factor NF-κB stimulates inflammatory genes such as TNF-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1). CT reversed the ethanol-induced increase in NF-κB p65 protein levels and restored IκB levels. Consistent with the above results, CT significantly suppressed ethanol-induced inflammatory genes such as TNF-α, IL-6, and MCP-1 at the mRNA level. The compound exhibits anti-inflammatory properties by inhibiting the expression of NO, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in RAW 264.7 cells. These results suggest that CT has anti-inflammatory properties, which may help prevent the progression of alcoholic hepatic steatosis. Moreover, AMPK/SIRT activation is known to inhibit NF-κB-mediated inflammatory pathways, thereby inhibiting the expression of inflammatory cytokine genes [61]. The results demonstrated that CT significantly blocked the ethanol-induced increase in NF-κB p65 level by inducing IκB phosphorylation and suppressed the expression of inflammatory cytokine genes, including TNF-α, IL-6, and MCP-1, suggesting that the AMPK/SIRT1 pathway might also be involved in the protective effect of CT against ethanol-mediated inflammation.
Nagappan et al. for the first time, reported that CT attenuates ethanol-induced hepatic steatosis by inhibiting lipogenic genes, oxidative stress, and inflammation in chronic ethanol-fed mice and HepG2 cells [52]. The enzyme cytochrome P4502E1 (CYP2E1) is involved in the metabolism of endogenous compounds such as fatty acids and in ethanol metabolism [62,63]. Activation of AMPK/SIRT1 and Nrf2 and inhibition of CYP2E1 may be involved in the protective effect of CT against ALD. The results of this study suggest that CT may be an effective therapeutic agent for the treatment of alcohol-induced liver injury [52].
In addition, He et al. obtained seven metabolites through the biotransformation of CT by the fungus Mucor AS 3.3447, two of which had lower cytotoxic activity than their parent and had excellent anti-influenza A virus activity [64].
4. Sesquiterpene lactones
Sesquiterpene lactones are a series of sesquiterpenoids with a lactone ring. Studies by McKinnon et al. revealed the importance of the C-4-C-5 and C-2-C-3 double bonds and the C-9 acetyl group for anti-inflammatory activity, suggesting that this class of compounds has great anti-inflammatory potential [65].
4.1. Atractylenolide III
Atractylenolide III (AT III), one of the main bioactive compounds of Atractylodes macrocephala Koidz root extract, is a sesquiterpene compound with anti-inflammatory, neuroprotective and anticancer activities. Previous studies have shown that AT III inhibits the production of various pro-inflammatory factors.
Ulcerative colitis (UC) is a chronic relapsing inflammatory bowel disease (IBD). Major features of UC are epithelial damage, neutrophil infiltration, microbial translocation, and inflammatory condition [66]. Dextran sodium sulfate (DSS)-induced mice serve as an ideal mouse model for UC because of its similarity to human UC [67]. Mitochondrial dysfunction causes inflammation, oxidative stress, and barrier breakdown. Han et al. found that AT III treatment increased UC mice's colon mtDNA copy number and complex I and IV activity. AT III also boosts Tom20, the mitochondrial outer membrane protein [68].
AT III also restored colonic mitochondria-associated protein expression in UC animals, including PGC-1α, NRF-1, NRF-2, and mitochondrial transcription factor A (TFAM). Peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) is an upstream factor of the 3-phosphoinositide-dependent protein kinase-1 (PDK1). PGC-1α, the key regulator, interacts with nuclear respiratory factors (NRF-1 and NRF-2) to activate TFAM in mitochondrial DNA replication/transcription. PGC-1α is activated by AMPK and SIRT1 and deacetylated by SIRT1 in an NAD+-dependent manner [69]. AT III restored colon p-AMPK and SIRT1 levels in DSS-treated animals [70].
Han et al. found that AT III prevents UC by stimulating the AMPK/SIRT1 signalling pathway to deacetylate PGC-1α and prevent mitochondrial dysfunction. In addition, early evidence indicated that the AMPK/SIRT1/PGC1α pathway is critical for mitochondrial biogenesis and glucose metabolism in skeletal muscle [68,71].
5. Polyphenols and their derivatives
Important natural compounds with a polyphenolic structure, flavonoids, as a whole, are a well-studied group of substances. The in vitro investigations found that flavonoids reduce the production and action of many inflammatory mediators in all cell types—almost always [72]. Flavonoids can be split into a series of subgroups, including flavonols. The work of Wang et al., in 2006 pointed out that some flavonols (kaempferol, quercetin and fisetin, etc.) play a role in inhibiting inflammation and oxidant, at least in part by inhibiting NF-κB. It was also found that despite the structural similarity among various flavonols, there may be significant differences in their anti-inflammatory effects [73].
5.1. Quercetin
Quercetin, a member of the flavonoid family found in a variety of plants and a well-known one, has many beneficial properties, including anticancer and anti-inflammatory properties in various degenerative diseases (Fig. 3) [74,75].
Fig. 3.
Structures of Quercetin, Isovitexin, Nobiletin, Luteolin, Curcumin, DHM, Hesperetin, HD-16 Mangiferin and Baicalin. The reoccuring structure highlighted in red is 5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one. The structures highlighted in blue circle are (2R,3S,4R,5S)-2-methyltetrahydro-2H-pyran-3,4,5-triol or similar structures. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
OA is a common chronic joint degenerative disease in the elderly for which there is currently no cure [76]. Inflammatory stress is regarded as one of the major factors in the pathogenesis OA. According to Zhao et al.‘s work, a vital role is played by AMPK in the regulation of chondrocyte expression of PGC-1α and the FoxO family transcription factor, FoxO3A, and both inhibit NF-κΒ [77]. From Qiu et al.'s western blotting (WB), the OA rats saw an increased expression of p-AMPK, SIRT1, PGC-1α, NRF-1, NRF-2 and TFAM in their knee chondrocyte lysates. Qiu et al. have shown that quercetin significantly upregulates AMPK/SIRT1 signalling pathway genes in OA chondrocytes [78].
Despite its therapeutic features, quercetin's oral bioavailability and solubility in water are not promising. And it also has some toxic side effects [79]. Thus, it would be beneficial to conduct more research on drug design around this compound.
5.2. Isovitexin
Apigenin-6-C-β-D-glucopyranoside, also known as isovitexin, is a flavone compound found in various plant sources that displays anti-inflammatory, antioxidant, memory-enhancing, and other beneficial therapeutic effects [[80], [81], [82], [83], [84], [85]]. The compound is also capable of crossing the blood-brain barrier and remaining for a considerable period [86].
Known as clinical depression, or depression, major depressive disorder (MDD) is characterised by persistent depressive mood and loss of interest for the majority of the day. Depression is strongly linked to neuroinflammation and autophagy. Microglia are a type of macrophage that resides within the brain, protecting neurons from damage caused by neurotoxic mediators that are secreted [87]. There are two phenotypic categories: pro-inflammatory M1 microglia and anti-inflammatory M2 microglia. The latter enhances the expression of anti-inflammatory cytokines, say that may reduce neuroinflammation and neuronal damage, for example, IL-10 [88]. PPARγ coactivator-1α (PGC-1α) functions as a transcriptional coactivator through its interaction with numerous transcription factors like PPARγ. Isovitexin, by activating CaMKKβ/AMPK/PGC-1α pathway, suppresses pro-inflammatory M1 microglia and enhances anti-inflammatory M2 microglia, according to the researchers. The compound regulates the PGC-1α expression in BV2 cells via AMPK activation, and therefore, isovitexin improves LPS-induced behaviour by activating CaMKKβ and PGC-1α [89].
Consequently, isovitexin provided an effective strategy for treating inflammation-associated depressive disorders [89,90].
5.3. Nobiletin
Nobiletin is a flavonoid compound derived from citrus peel. Thanks to extensive research, it is known to boast many different effects, like anti-tumour, and anti-inflammatory effects and also preventing diseases like Parkinson's disease and Alzheimer's disease (AD) [85,91]. Autophagy negatively regulates NLRP3 inflammasome activity. AMPK plays a vital role in the regulation of autophagy. The antidepressant and anti-inflammatory effects of nobiletin were observed in a rat model of depression induced by LPS. A finding that is possibly connected to this is, both in vivo and in vitro, the compound upregulated autophagy and inhibited NLRP3 inflammasome activation. The activation of the AMPK pathway may also be a key component in nobiletin's neuroprotective activity. Overall, nobiletin could potentially be used as a natural supplement to enhance cognitive performance and improve overall mental well-being [92].
5.4. Luteolin
Luteolin is an important flavonoid found in an array of plants, to name a few, broccoli, carrots, pepper, and spinach [93,94]. It has a range of therapeutic effects, say, anti-inflammatory, antioxidant and anti-tumour effects [95]. Bronchial asthma, often referred to as asthma, a is chronic allergic airway-inflammatory disorder. Acute asthma can be alleviated by luteolin because it activates the PI3K/Akt/mTOR signalling [96]. And it also protects the human umbilical vein endothelial cells from oxidative damage via the AMPK pathway [93].
H2O2-induced decreases in cell viability, mitochondrial depolarisation and cell death remained unaffected in sh-AMPKα1 and ko-AMPKα1 primary murine chondrocytes. AMPK activation improves inflammation and redox imbalance by mediating Nrf2 signalling. Luteolin's ability to activate Nrf2 is wholly abolished when AMPK is silenced or depleted with the genetic methods. Thus, AMPK activation is required for Nrf2 activation to exert its neuroprotective effects. Notably, luteolin-induced activation of AMPK, with increased phosphorylation of AMPKα, was not affected by Nrf2 silencing or depletion [97]. These results suggest that luteolin exhibits the potential to protect chondrocytes from H2O2-induced oxidative damage by activating the AMPK pathway, which subsequently leads to an increase in the downstream Nrf2 cascade [98]. Comparing luteolin-treated mice to DMM mice revealed decreased cartilage degradation, chondrocyte loss, and OARSI score, indicating that luteolin inhibits the progression of OA. In a mouse OA model produced by DMM, oral treatment of luteolin inhibited OA progression [98]. Zhou et al. showed that luteolin activates the AMPK/Nrf2 pathway, and AMPK is a positive upstream regulator of Nrf2. Luteolin may provide us with a novel and effective idea and research direction for the treatment of OA. More of its clinical research and application is expected.
The study still has certain drawbacks, however. Its effects on mouse kidneys and livers were not studied. Furthermore, future studies on ideal dosage, timing, duration of administration, and long-term impact will provide a better understanding of its therapeutic value [98].
5.5. Dihydromyricetin
Dihydromyricetin (DHM), the main bioactive polyphenol in vine tea, has a long history of anti-inflammatory and anti-tumour properties. DHM exerts anti-inflammatory effects in rats by inhibiting NF-κB signalling in macrophages. Some DHM-related studies have reported that DHM function depends on its activation of AMPK but without direct targeting. Hou et al. used an HFD-induced obese mouse model to study inflammation-induced skeletal muscle atrophy in vivo. The results indicated that DHM counteracts inflammation-induced muscle atrophy in C2C12 cells through AMPK. Blocking AMPK upstream factors CaMKK and LKB1, respectively, further revealed that DHM resists inflammation-induced muscle atrophy in C2C12 cells through the CaMKK/AMPK pathway. The experimental results of Hou et al. showed that the effect of DHM is through CaMKK/AMPK, not LKB1. But for now, how DHM activates CaMKK is still unknown, and further research results are expected [99].
In addition, the team used DARTS to identify potential binding targets for DHM. This technique, a method for identifying potential target proteins of small molecules, allows the use of small natural molecules without any immobilisation or modification [99].
5.6. Baicalin
Baicalin is a flavonoid phytochemical extracted from the natural medicinal herb Scutellaria baicalensis, which has antioxidant and anti-inflammatory properties. It has therapeutic effects against various diseases such as tumours, pneumonia, and cardiovascular diseases.
Ras protein is involved in inflammation processes by modulating downstream pathways, for example, Raf/MEK/ERK/MAPK, followed by NF-κB activation. Mitofusin 2 (Mfn-2) may act as an inhibitor of Ras [100]. Zhang et al. demonstrated that the anti-inflammatory effect of baicalin is related to the activation of the AMPK/Mfn-2 axis for the first time [101]. Baicalin has dose-dependent effects on IL-6 and TNF-α production in RAW264.7 macrophages with Angiotensin II/oxidised LDL (Ang II/ox-LDL) treatment. AMPK/Mfn-2 may be activated by baicalin, which inhibits downstream MAPK/NF-κB signalling. Baicalin may be an effective medicine for treating atherosclerosis [101].
Ischemic stroke is among the primary causes of disability and death today [102]. In acute cerebral ischemia, a cascade of ischemic responses is almost instantly triggered, including excitotoxicity, inflammation and so forth [103]. WB and ELISA experiments showed that the Middle Cerebral Artery Occlusion Rat model (MCAO/R model) enhanced cortical neuron NLRP3, ASC, IL-1β, cleaved caspase-1 and IL-18 expression in comparison with the sham group. Baicalin dose-dependently lowered the factors. Immunofluorescence and confocal laser scanning microscopy evaluated the NLRP3 inflammasome upstream factor. Baicalin treatment significantly reduced the amount of NLRP3 fluorescence expression in the cytoplasm of damaged nerve cells in the MCAO/R group. The results suggest that baicalin acts by regulating NLRP3 inflammasomes to prevent cerebral ischemia-reperfusion injury. Additionally, subsequent studies indicated that baicalin also improved hypoxic-ischemic cortical cell survival, cerebral nerve function, and cerebral infarction volume in cerebral ischemia-reperfusion rats. When CC inhibited the AMPK pathway, cortical neuron damage worsened, and the expression of NLRP3 inflammasome-related factors was further upregulated [104].
All in all, baicalin can inhibit the NLRP3 inflammasome-dependent pyroptotic response through the AMPK signalling pathway, thereby alleviating the neurological damage caused by cerebral ischemia-reperfusion. These findings provide avenues for future clinical drug initiation and research.
5.7. Mangiferin
Mangiferin is a xanthonoid, particularly abundant in mango leaves [[105], [106], [107], [108]].
Endothelial dysfunction is closely related to cardiovascular complications in diabetic patients, and high glucose-induced endothelial dysfunction is the leading cause of initial vascular lesions [109,110]. In the study by Song et al. mangiferin effectively inhibited ER stress-related oxidative stress by attenuating IRE1α phosphorylation and reducing ROS production. In response to ER stress, TXNIP expression increases, followed by NLRP3 inflammasome activation and IL-1β secretion. Mangiferin treatment attenuated the expression of TXNIP and NLRP3 and reduced the production of IL-1β and IL-6. NLRP3 inflammasome activation is responsible for mitochondrial cell death. Elevated glucose levels result in a rise in the secretion of endothelin-1, while simultaneously causing a decrease in the generation of NO. Mangiferin restores the loss of mitochondrial membrane potential (Δψm) and inhibits caspase-3 activity, thereby protecting cells from high glucose-induced apoptosis. Furthermore, mangiferin inhibited endothelin-1 secretion and restored the loss of NO production when cells were exposed to high glucose. Mangiferin enhances AMPK phosphorylation. The AMPK inhibitor CC reduced its beneficial effects, suggesting a potential role for AMPK in its action. The findings show how mangiferin enhances endothelium homeostasis by reducing ER stress-associated TXNIP/NLRP3 inflammasome activation in endothelial cells. It suggests that mangiferin regulates endothelium homeostasis and may help treat diabetes-related cardiovascular problems [107].
Many pieces of evidence have shown that mangiferin can also serve as a potential therapeutic drug for NAFLD. A study showed that gasdermin D (GSDMD) promotes inflammation and pyroptosis in hepatocytes, accelerating the development of NAFLD. RNA-seq analysis demonstrated that mangiferin treatment upregulated genes related to energy metabolism and increased AMPK activation in mouse hepatocytes. AMPK activity was inhibited in NAFLD mice, but p-AMPKα levels saw an increase, thanks to mangiferin treatment. It also reduced markers of liver inflammation and pyroptosis, such as NLRP3, caspase-1, and IL-1β, suggesting that mangiferin suppresses NLRP3 inflammasome activation, thereby producing anti-inflammatory effects. Mangiferin treatment significantly reduced serum IL-1β levels and downregulated the transcript levels of relevant genes. Immunofluorescence assays showed that mangiferin decreased the positive staining levels of NLRP3, caspase-1, GSDMD's N-terminal domain, and IL-1β in the liver tissues of NAFLD mice, indicating the downregulation of these proteins [106].
Mangiferin, through AMPK activation and NLRP3 inflammasome inhibition, protects against NAFLD, both in vivo and in vitro. Its treatment attenuates a list of conditions, inclusive of serum glucolipid metabolism, and insulin resistance [106]. Furthermore, mangiferin was found to help with insulin resistance in HFD-induced NAFLD mice [106].
5.8. Curcumin
Turmeric is a common flavouring and colouring agent. A bisphenol compound, curcumin, also known as diferuloylmethane, is the main natural active ingredient in turmeric, one that has excellent anti-inflammatory and antibacterial abilities [111]. Yin et al.‘s work reveals the mechanism by which curcumin inhibits inflammation and suggest the potential clinical application in NLRP3-driven diseases [112].
There are several studies on attenuating OA with curcumin [113,114]. Jin et al. went further on this route. The researchers established two models: one is rat knee OA model induced by sodium monoiodoacetate and the other one OA chondrocyte model using IL-1β. GO and KEGG analyses screened 7 metabolic and AMPK signalling hub genes. A significant reduction in OA features was observed after curcumin treatment, according to the Osteoarthritis Research Society International (OARSI) and Mankin scores in animals with OA [115].
Knees of rats were stained with immunohistochemistry (IHC) to ascertain the index expressions of OA pathology and mitophagy (PINK1, Parkin, Beclin1, P62, and LC3B. OA pathology: Collagen II and MMP13.). The results of WB and qPCR showed that IL-1β and MMP 13, inflammatory markers of OA, were the only two seen to decrease by curcumin; the others all greatly increased. Curcumin activates AMPK phosphorylation in OA chondrocytes. As a result of the inhibition of AMPK, however, the vital protein involved in mitophagy was reduced remarkably. Jin et al. demonstrated the mechanism of curcumin in OA through a combination of validation experiments and network pharmacology. The chondroprotective effect of curcumin on OA is by initiating mitophagy through the AMPK/PINK1/Parkin pathway [115]. The anti-OA property of curcumin was also demonstrated through the enhancement of collagen anabolism and the reduction of inflammatory catabolism in cartilage.
In sum, it seems like it has potential against OA and inflammation in general. There is an obstacle that has to be addressed though: its low oral bioavailability made it made it yet to be promising. There are researchers trying to tackle this issue, thankfully [116,117].
5.9. Hesperetin; Hesperetin derivative-16
Hesperetin is a flavanone glycoside component of traditional Chinese medicine whose effects on liver illnesses have been extensively studied. It is an enriched form of flavanone found in citrus peels, boasting various pharmacological effects. According to Wang et al. the AMPK/SIRT1 pathway plays a crucial role in the therapeutic effects of Hesperetin. Also, they mentioned that SIRT1 is a necessary component in NF-κB inhibition. It is interesting that the compound reinforced SIRT1 expression through the AMPK/CREB pathway, in Wang's results [118]. Yet due to its low water solubility and bioavailability, its clinical applicability is restricted.
Liver fibrosis is characterised by abnormal hepatic architecture and significant deposition of extracellular matrix (ECM) proteins, such as type I and type III collagens. Without proper intervention, liver fibrosis rapidly progresses to permanent cirrhosis and cancer of the liver.
Hesperetin derivative 16 (HD-16), a hesperitin-derived monomer synthesised by Huang et al. possesses anti-inflammatory and antitumorigenic properties [119]. It is noteworthy that HD-16 showed better water-solubility, bioavailability, and potent anti-inflammatory properties in CCl4-induced acute liver injury. It has been demonstrated that HD-16 is an efficient activator of the AMPK/SIRT3 signalling pathway. SIRT3 or AMPK depletion diminished the antifibrotic action of HD-16 [120].
In hepatic fibrosis, HD-16 dramatically increased phosphorylated-AMPK expression. AMPK phosphorylation was enhanced in LX-2 cells treated with HD-16 and challenged with TGF-1. SIRT3 is a downstream target of AMPK since AMPK1 siRNA or CC may successfully suppress AMPK expression and subsequently inhibit the expression of Sirt3. Activation of the AMPK/SIRT3 signalling pathway may contribute to HD-16's antifibrotic action [120]. To conclude, HD-16 has an antifibrotic effect on liver fibrosis by activating the AMPK/SIRT3 pathway [119,120].
5.10. Resveratrol
A plant-derived phytoestrogen, resveratrol, can be extracted from various dark-coloured fruits (Fig. 4) [[121], [122], [123]]. The monomeric stilbene has multiple effects, such as anti-inflammatory response, anti-oxidation, inhibition of platelet aggregation, and anti-tumour [[122], [123], [124], [125], [126], [127], [128], [129]].
Fig. 4.
Structures of resveratrol, pterostilbene, magnolol, Honokiol, Urolithin A, salidroside and stevioside.
Among the features of AD are the increase in neuronal amyloid-β (Aβ) plaque formation, neuronal loss and neuroinflammation. Pro-inflammatory factors can be produced by activated microglia and astrocytes. These factors, in turn, increase amyloid precursor protein (APP) expression and Aβ deposition in AD models. Anti-inflammatory therapy has been suggested as a potential therapeutic strategy for AD. Resveratrol, by increasing cytosolic calcium levels and promoting CaMKKβ-dependent phosphorylation, activates AMPK. Oral resveratrol treatment crosses the blood-brain barrier and activates AMPK in the brain, followed by reducing Aβ accumulation. Vingtdeux's work laid the groundwork for further research [130].
As an inhibitor of adipogenesis and lipid accumulation, p-AMPK downregulates SREBP-1, fatty acid synthase (FAS) and PPARγ in 3T3-L1 cells, HepG2 cells and animal models expression. AMPK and SIRT1 inhibit the acetylation and translocation of NF-kB p65, which is associated with an amelioration of the inflammatory response. Furthermore, the activated AMPK signalling pathway is involved in the treatment of acne vulgaris because of its sebum-suppressive effect through the regulation of SREBP-1 [131].
Wei et al. studied the effect of resveratrol on lipogenesis, inflammation and AMPK signalling pathway in SZ95 sebocytes in vitro to study the possible mechanism of resveratrol exerting sebum suppression and anti-inflammatory effects. Resveratrol activates fibroblasts, osteoblasts SIRT1 signalling in cells and HaCaT keratinocytes to inhibit the formation of pro-inflammatory molecules, reduce p65 acetylation, and prevent oxidative stress-induced cytotoxicity [131].
The AMPK-SIRT1/p300 pathway mediates the anti-inflammatory effects of PPARγ agonists by reducing p65 acetylation. Further studies showed that resveratrol significantly suppressed peptidoglycan (PGN)-induced IL-1β and IL-6 expression at mRNA and protein levels, while CC pretreatment reversed its suppression of inflammatory cytokines. Consistent with these results, Resveratrol inhibited PGN-induced expression of acetyl-p65. However, pretreatment with the inhibitor CC increased the level of acetylated p65 compared with the combination treatment group of PGN and resveratrol. Furthermore, CC increased the level of acetyl-p65 and the release of inflammatory cytokines in SZ95 sebocytes while inhibiting the activation of SIRT1 promoted by resveratrol. CC exposure suppressed resveratrol-induced p-AMPK and SIRT1 protein expression, consistent with previous studies. AMPK may act upstream of SIRT1 in resveratrol-induced activation of AMPK and SIRT1 [131].
Taken together, the results show that resveratrol suppresses lipid synthesis and inflammatory responses in human SZ95 sebocytes in vitro, mainly mediated by activation of the AMPK signalling pathway, which may suggest that resveratrol is a potential drug candidate in acne treatment [131].
5.11. Pterostilbene
Oxidative stress cannot not be overlooked in bronchial asthma [132]. Th2 cell hyperactivation causes IgE synthesis and allergic airway inflammation in asthma [133]. Asthma severity increases with airway hyperresponsiveness, among other hallmarks of the disease.
Pterostilbene is a derivative of resveratrol and a monomeric stilbene, one that can be found in a variety of berries. Pterostilbene has improved membrane permeability and metabolic stability over resveratrol, thanks to the two methoxyl groups. This increases bioavailability and improves oral absorption and bioavailability [134].
In the study by Xu et al. pterostilbene significantly reduced goblet cell proliferation and inflammatory cell infiltration in asthmatic mice. Asthma model mice were significantly less likely to proliferate goblet cells and infiltrate inflammatory cells when treated with pterostilbene, according to the study by Xu et al. Notably, pterostilbene blocked eosinophils, lymphocytes and neutrophils. Pterostilbene boosted IFN- and decreased BALF IL-5, IL-4, and IL-13. Pterostilbene dose-dependently decreased the levels of both total IgE and IgE specifically targeting ovalbumin. Thus, pterostilbene may reduce asthmatic airway inflammation by balancing Th1/Th2 immunological responses [135].
Most importantly, pterostilbene can activate AMPK/SIRT1 and Nrf2/(heme-oxygenase-1) HO-1 signalling pathways in LPS-sensitized 16HBE cells. In a nutshell, pterostilbene alleviates oxidative stress and allergic inflammation by regulating AMPK/SIRT1 and Nrf2/HO-1 signalling pathways [136]. The study by Hseu et al. also deduced that after pterostilbene treatment, the ROS pathway was first activated, followed by the ERK and AMPK pathways in the nuclear Nrf2 activation of HaCaT cells [136]. Addedly, among the properties of pterostilbene are that it is a potent anti-melanogenic agent and that it may be used as a depigmenting agent in the preparation of topical skin-whitening products [136,137].
5.12. Magnolol; Honokiol
Magnolol is a lignin obtained from Magnolia officinalis, one of the phenolic polymers. Magnolol has strong anti-inflammatory, anti-oxidative and anti-cancer effects [138,139]. Its anti-inflammatory effects may occur through the phosphorylation of MAPK and NF-κB, whereas activation may be achieved through ACC, AKT, and AMPK activation. PPARα overexpression and SREBP-1c inhibition are highly related to these effects [140]. In conclusion, the findings of Tian et al. imply that magnolol might be a favourable candidate for the treatment of patients with NAFLD and NASH. It is noteworthy that magnolol's processes of inhibiting MAPK/NF-κB/SREBP-1c and activating AKT/AMPK/PPARα are still to be unveiled, and the discoveries could lead to novel future treatments that enhance patient outcomes [140].
Moreover, Liu et al. found that via the SIRT1/AMPK/PGC-1α pathway, magnolol can attenuate both IL-1β-induced oxidative stress and inflammation, possibly implying its non-specific protective effect, which Liu stated that it is fairly “common”. Further studies on the mechanism of the pathways related to the two are needed in the future [141].
Cytokines like IL-10 and TGF-β1 inhibit the further secretion of pro-inflammatory cytokines and reduce inflammation. Like the previous one, honokiol, which can be extracted from magnolia, is a bisphenol lignan, which is a natural product composed of a p-allylphenol and an o-allylphenol. Ether derivatives and 3,3′-diallyl derivatives also have strong growth inhibitory action on Hep-G2 cells (human hepatocellular carcinoma), suggesting that the 3′-allyl group of honokiol is implicated in tumour development. Honokiol can act as an activator of SIRT3 to enhance the activity and expression of SIRT3 [142]. Oxidative and inflammatory stress activates a good amount of NF-κB-downstream proteins associated with inflammation. Honokiol strongly inhibits NF-κB activation and pro-inflammatory cytokine release in mice exposed to LPS, suggesting that the NF-κB pathway may be implicated in its anti-septic acute lung damage effects. Honokiol reduces oxidative/nitrosative stress and inhibits cytokines (TNF-α and HMGB1), iNOS-mediated NO generation and NF-κB activation, and, further, acute lung injury and mortality. The IL-10 rise, however, remains unaffected. All in all, honokiol may be the candidate that treats septic shock disorders [143,144].
5.13. Urolithin A
Urolithins are generated by ellagitannins and ellagic acid (EA) from the microbiome of the human gut [145]. These metabolites are more quickly absorbed into the bloodstream than their precursors and contribute to the beneficial effects of ellagitannins and EA in foods and certain Chinese herbal remedies, like pomegranate and emblica [145].
A metabolite of ellagic acid produced by gut bacteria, urolithin A, has been shown to reduce neuroinflammation. Urolithin A is a possible anti-inflammatory candidate of the pancreas that activates AMPK and autophagy to suppress the TXNIP/NLRP3/IL-1β signalling pathway connected to endoplasmic reticulum stress. Using the Morris water maze to test the effect of UA on APP/PS1 mice, it turned out that UA improved the spatial memory [146].
Neuroinflammation and neuronal apoptosis may be inhibited by UA-mediated mechanisms. Gong et al. showed that UA protected cognitive function by protecting neurons from death and triggering neurogenesis through anti-inflammatory signalling in APP/PS1 mice, indicating that UA may be a therapeutic candidate for treating AD [146]. Vingtdeux's results, previously mentioned in Resveratrol section, may still be true in this case, but with UA instead. It is worth mentioning that Boakye, Xu and Gong's studies on UA all observed decreases in NF-κB levels [[146], [147], [148]].
The research showed that UA inhibits glycolipid toxicity and endoplasmic stress-related TXNIP/NLRP3/IL-1β signalling pathway through the activation of AMPK and autophagy. In addition, UA is reportedly safer than verapamil, a calcium channel blocker [149]. By preserving pancreatic beta cells, UA or the ingestion of ellagic acid-rich foods or herbs may enhance the well-being of diabetic individuals.
5.14. Salidroside
The main phenylpropanoid glycoside extract in Rhodiola rosea, a famous Chinese medicine, salidroside, is a glucoside formed from tyrosol and glucose, which has various bioactive properties [150]. Salidroside exerts anti-inflammatory effects in the airway, adipose and cardiac tissue. Salidroside has a protective effect on endothelial function [151].
Endothelial dysfunction is among the most vital risk factors for vascular disease [152]. During the inflammatory cascade, TNF-α induces cytotoxicity in endothelial cells (ECs), which leads to damage through the formation of reactive free radicals and proinflammatory molecules [153]. A significant indicator of endothelial dysfunction and inflammatory response, IL-6 also increases endothelial permeability. The cytokine IL-1β is regarded as a significant mediator of vascular inflammation. Studies by Hu et al. showed that inflammatory processes and oxidative stress in endothelial cells are inhibited by salidroside by stimulating AMPK phosphorylation and inhibiting NF-ĸB p65 and NLRP3 inflammasome activation [151].
Neuroinflammation is a major pathogenesis of secondary injury in spinal cord injury (SCI) and is considered a therapeutic target for SCI. Salidroside can improve AGEs-induced endothelial inflammation and oxidative stress in part through the AMPK/NF-κB/NLRP3 signalling pathway. The study by Wang et al. demonstrated the effect of salidroside attenuating neuroinflammation [154].
6. Others
6.1. Stevioside
Stevioside is a natural diterpene glycoside and also a food sweetener [155]. But what matters more is it can act as hypoglycaemic, antihypertensive, antifibrotic and anti-inflammatory [156].
In addition to the well-studied NF-κB, interferon regulatory factor 5(IRF5), a specific marker of inflammatory macrophages, is also responsible for regulating the secretion of pro-inflammatory cytokines [157]. IRF5 is an important pro-inflammatory transcription factor. IRF5 can be activated by LPS, followed by regulating the activation of target genes [158]. And the NF-κB pathway prolongs the crosstalk with IRF [159].
The study by Wei et al. showed stevioside enhanced IL-10 and TGF-β1 expression in LPS-treated macrophages and LPS-treated mice to some extent. In addition, stevioside also inhibited the expression of IRF5, another transcription factor that promotes the transcription of pro-inflammatory cytokines, and downregulated its levels in the nucleus. LPS induces rapid release of pro-inflammatory cytokines and mediators into the blood and causes excessive cytokine-mediated liver injury and lethal shock in d-GalN-sensitized mice. Using the said model, the team demonstrated that treatment with stevioside significantly improved survival in lethal shock animals. Moreover, the in vivo concentration of stevioside used in the study may have anti-inflammatory effects on humans—the human equivalent dose is 263.7 mg/kg, much lower than the NOADR dose—970 mg/kg. But it is worth bearing in mind that stevioside's inhibitory effect only occurred when LPS was injected before or simultaneously, not so when injected after the drug, an indication that stevioside may compete with LPS on certain targets.
In addition, the research also saw a superb enhancement in AMPK activation. And stevioside's suppressive effect on LPS-induced activation of NF-κB and IRF5 could no longer be seen, with CC pretreatment. It unveiled that enhancement may be regulating the drug's inhibition on NF-κB/IRF5 [160].
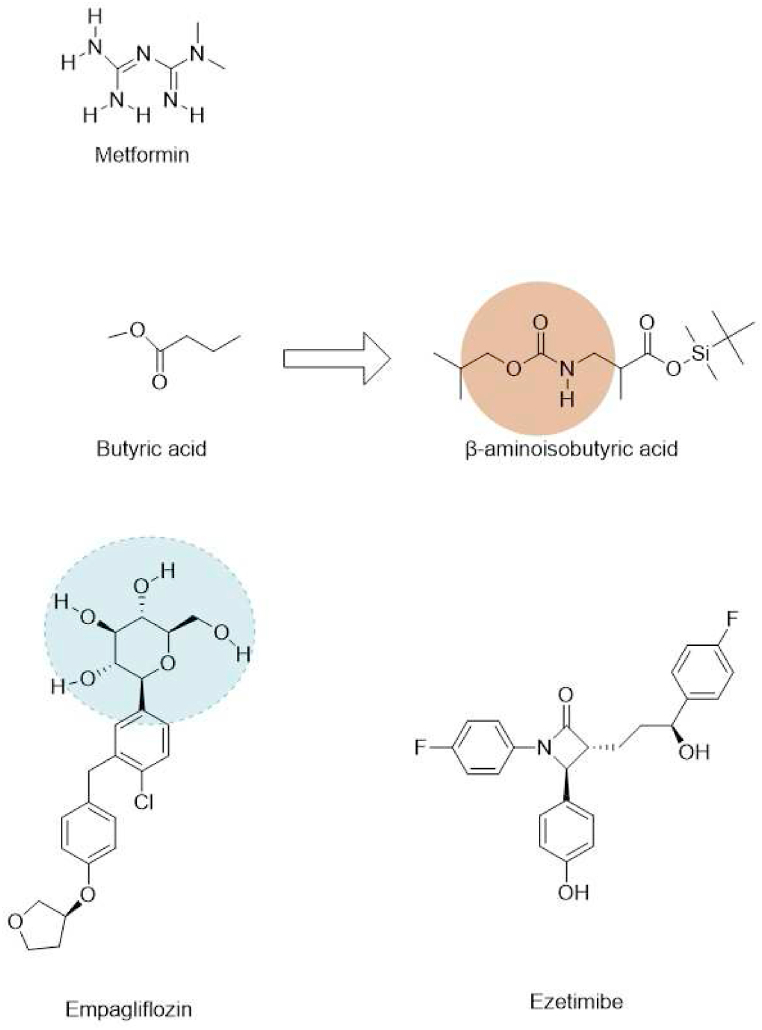
6.2. Butyric acid; β-aminoisobutyric acid
Human antigen R (HuR), an RNA-binding protein, is a major repressor in the process of adipogenesis, expressed in a variety of cells, such as intestine, fat, etc [161]. HuR exhibits both pro-inflammatory and anti-inflammatory properties, according to existing studies [162,163].
One of the saturated short-chain fatty acids, butyric acid, is the main component of fats and lipids in food and plays a crucial role in lipid metabolism (Fig. 5). In Western blot analysis, it was found that activation of AMPK causes a reduction in HuR levels. Butyric acid may be capable of inhibiting the production of lipids and alleviating inflammation by regulating HuR expression [164].
Fig. 5.
Structures of Butyric acid, BAIBA, Metformin, EMPA and Ezetimibe.
A natural thymine catabolite, β-aminoisobutyric acid (BAIBA) features beneficial effects on obesity-related metabolic diseases. According to animal experiments, the non-protein amino acid stimulates fatty acid oxidation and suppresses lipogenesis, thereby attenuating obesity [165]. In differentiated 3 T3-L1 cells, BAIBA blocked LPS-induced NF-κB and IκB phosphorylation, TNFα, and MCP-1 production. It suppressed adiponectin expression in the said cells, yet consistent with the existing studies of adiponectin [166]. BAIBA appears to boost AMPK phosphorylation without adiponectin. siRNA of AMPK abolished BAIBA's anti-inflammatory effects. And the team experimented with metformin, glimepiride, a sulfonylurea derivative that does not affect AMPK, and BAIBA, on LPS-induced pro-inflammatory cytokine secretion and glucose uptake. The three all showed positive effects to some degree. The details are worthy of note. BAIBA and metformin are more potent than 10 days of glimepiride treatment. The outcomes of metformin and glimepiride treatment of 24 h were similar to 10 days ones. In contrast, no effects of BAIBA can be seen within 24 h period, one that indicates it should be left longer to be working [165]. Overall, in differentiated 3 T3-L1 cells treated with LPS, BAIBA exhibits anti-inflammatory and anti-insulin resistance effects via the AMPK pathway. BAIBA appears to have therapeutic effects on adipose tissue as well as skeletal muscle and liver cells, as a result of these findings.
6.3. Metformin
A member of the guanidine class, metformin is a biguanide with two methyl substituents at the 1-position. Recently, it has reportedly had an anti-inflammatory effect in addition to being a hypoglycemic agent [167]. Metformin is a well-established AMPK agonist. By directly targeting mitochondrial complex I, metformin regulates AMPK phosphorylation. Metformin activates duodenal AMPK to reduce glucose production and plasma glucose levels in HFD-fed type 2 diabetes rats, and duodenal AMPK activation significantly contributed to the overall acute hypoglycaemic effect [168].
Sepsis, a deadly end-organ dysfunction, is mainly caused by an inflammatory reaction due to a cytokine storm [169]. Song et al. reported that metformin alleviated inflammation and liver damage by activating the phosphorylation of AMPK, which further inhibited the proteins of the Akt signalling pathway like HMGB1 and TNF-α in aged septic mice. Notably, they discovered the drug could reverse LPS-induced decrease of PGC-1α and the increase of PDK1 and hypoxia-inducible factor 1 α (HIF-1α). These results suggest that metformin's capability of alleviating liver injury and inflammatory responses is, perhaps, through activation of the AMPK-PGC-1α axis [170,171].
Overall, metformin may be a potential drug against sepsis. However, the researchers have only reported in vivo experiments, and no in vitro studies, so further confirmation is needed.
6.4. Empagliflozin
Sodium-glucose cotransporter two inhibitors (SGLT2is) are considered a key regulator of lipid metabolism in NAFLD treatment [172]. SGLT2 inhibitor empagliflozin (EMPA) is reported to have unparalleled benefits in diabetic patients with NAFLD or established cardiovascular disease [[173], [174], [175]]. Empagliflozin reduces triglyceride and free fatty acid (FFA) buildup by increasing energy expenditure and fatty acid oxidation [176].
Ma et al. studied the prevention of NAFLD-related inflammation by empagliflozin. In addition to its ability to inhibit inflammation by up-regulating Sesn2, empagliflozin stimulates downstream signalling, such as p-AMPK and p-mTOR and Nrf2/HO-1. Results showed that Sesn2 knockdown aggravated the inflammatory response of phosphatidic acid (PA)-stimulated hepatocytes in vitro. In contrast, in PA-stimulated HepG2 cells, Sesn2 knockdown significantly suppressed empagliflozin-mediated changes in inflammatory cytokines and AMPK/mTOR signalling, suggesting that empagliflozin-mediated inflammation is partially dependent on Sesn2 expression.
SGLT2i stimulates AMPKα, enhancing fatty acid oxidation and lowering hepatic lipid content. Thus, empagliflozin may increase fatty acid oxidation via the AMPK pathway. Circulating Sesn2 was independently related to HDL levels, suggesting that it regulates lipid metabolism [175,177,178].
HFD-induced disordered fatty acid oxidation in mice was significantly attenuated by empagliflozin treatment. As a key transcriptional regulator, PPARα is mainly involved in mitochondrial oxidation, with FFAs and fatty acid derivatives as ligands [179]. A regulator of lipid peroxides, glutathione peroxidase 4 (GPX4) converts lipid hydroperoxides to lipid alcohols, protecting cells from death. Empagliflozin increases GPX4 and PPARα to control lipid oxidation [180]. Further, Nrf2's downstream targets, say, HO-1 and GCLC, appear activated after it enters the nucleus [181]. Based on the results of Ma et al. empagliflozin inhibits oxidation by activating the Nrf2/HO-1/GCLC axis. Yet, according to the researchers, Sesn2 does not improve lipid oxidation by regulating PPARα [175].
Furthermore, empagliflozin decreased FAS, SREBP-1c, triglyceride, and FFA levels in effect but remained more remarkable than in cells with unchanged Sesn2 expression, indicating lipid metabolism reduction. In de novo lipogenesis, FAS and SREBP-1c play an essential role [182]. Additionally, adipogenesis and triglyceride and FFA levels were increased by silencing Sesn2. Instead, with Sesn2 silenced, empagliflozin cannot produce the same results. These findings indicate that empagliflozin appears to partially mitigate lipid metabolic alterations through the upregulation of Sesn2 and the downregulation of adipogenesis.
However, what remained unclear is whether empagliflozin reduces lipid metabolism and synthesis through Sesn2 or whether PPARα/AMPK depends on these changes. Thus, Sesn2 silencing in vivo models and mechanistic studies and, also, the identification of the downstream factors is needed.
These results demonstrate that empagliflozin activates the Sesn2-mediated AMPK/mTOR pathway and improves lipid accumulation in obesity-associated NAFLD. Therefore, Sesn2 may be a target for the treatment of NAFLD through its influence on the AMPK/mTOR signalling pathway, giving a direction for future research worthy of further exploration [175].
6.5. Ezetimibe
An increasing number of people are suffering from NAFLD or NASH, but there is still no FDA-approved drug for treating the two. But ezetimibe has been shown in studies to improve NAFLD [183].
Ezetimibe is a lipid-lowering drug widely used clinically. The main features that characterise NASH are impaired macro-autophagy/autophagic flux and inflammasome activation. Kim et al.'s study showed that ezetimibe's effect on alleviating hepatic steatosis, inflammation and fibrosis is autophagy pathway-dependent [184]. AMPK is essential for ezetimibe-induced therapeutic effects on steatosis and lipotoxicity via autophagy. Autophagy negatively regulates NLRP3 inflammasome activity [185]. Furthermore, inflammatory extracellular vesicles (EVs) are intercellular mediators between hepatocytes and macrophages in NASH, and ezetimibe regulates hepatocyte-macrophage communication through inflammatory EVs released by hepatocytes, thereby inhibiting inflammation.
In conclusion, ezetimibe ameliorated the beneficial effects of hepatic steatosis, inflammation and fibrosis by inducing AMPK-mediated autophagy activation. Ezetimibe is a promising therapy for steatohepatitis and fibrosis [184].
7. Discussion
An increasing number of studies have reported the role of AMPK in anti-inflammation. Abundant research evidence points to the AMPK/SIRT1 pathway. AMPK/SIRT1 activation inhibits NF-κB-mediated inflammatory pathways, thereby inhibiting the expression of inflammatory cytokine.
This article attempts to summarise the drugs with related effects. Most research results are for metabolic diseases, say, obesity-related inflammation or fatty liver. Classical drugs such as metformin, ezetimibe, and empagliflozin have anti-inflammatory effects through the AMPK pathway. Metformin is a classic hypoglycaemic agent; the latter two are traditional hypolipidemic drugs. The deepening of researchers' understanding of drugs will help guide clinical medication.
Empagliflozin, resveratrol, magnolol and other drugs have been mentioned in the research of drugs on the adjustment of PPAR. As an important transcriptional regulator, PPARα is mainly involved in mitochondrial oxidation, and its ligands include FFAs and fatty acid derivatives [61,179]. Empagliflozin regulates lipid oxidation by enhancing GPX4 and PPARα. Empagliflozin was observed to increase lipid oxidation via Sesn2 knockdown, yet, PPARα was not affected by empagliflozin treatment. This may indicate that Sesn2 does not enhance lipid oxidation through modulating PPARα. In this day and age, the development of novel approaches to target many signalling pathways is underway. There may be a more significant benefit to combining multiple drug targets and pathogenic pathways. Yet the majority of clinical trials focus on monotherapies.
Despite NASH and NAFLD long being diseases of concern, there is no approved treatment drug thus far. Natural compounds are protective not only against steatosis and inflammation but especially against fibrosis – which is a key to NAFLD treatment. In addition, we noticed that many drugs, if not most, reportedly protect endothelial cells by suppressing NLRP3 inflammasomes and activating AMPK. Hopefully, the compounds and the studies mentioned can provide new ideas for the prevention and treatment of diabetic cardiovascular complications and NAFLD.
Additionally, it is worth noting that, methodology-wise, DARTS have the advantage of directly using small natural molecules without any fixation or modification when studying to identify small molecule target proteins in natural products [99]. It might serve as a shortcut to learning other small molecules, such as those mentioned above.
This paper mentions polyphenolic compounds that can treat inflammation by activating AMPK, and their structural commonality deserves further observation. It is worth noting that the substituent at the methoxy position significantly affects the anti-inflammatory effect of curcumin, and some analogues have significant differences in their impact on inflammation. Methoxy groups may be potential structural candidates for designing drugs based on curcumin for inflammatory diseases. This also deserves further study in other medications.
Not only AMPK activation but also the inhibition of hypothalamic AMPK plays a key role in energy balance [29]. It is noteworthy that, according to López et al. the most hypothalamic AMPK activities are linked to mediating hormonal effects [186]. For example, leptin, among hormones responsible for reducing food intake, is crucial in modulating hypothalamic AMPKα2, one of the two α subunits, on homeostasis regulation within the sympathetic nervous system [187,188]. Other examples can be BMP8B, estradiol as well as metformin [[189], [190], [191], [192]]. Yet, it is also important to know that there are a great number of natural small molecule compounds modulate AMPK at the central level, say, p-coumaric acid [193]. A plant phenolic acid, p-coumaric acid, is the major constituent of Sasa quelpaertensis Nakai extract [194]. Nguyen et al.'s study suggests that p-coumaric acid exerts different effects at different levels. Treating with p-coumaric acid inhibited hypothalamic AMPK, but enhanced peripheral AMPK activation [193].
Overall, the drugs listed in this paper have various structures, many of which are natural products. Most of the natural compounds mentioned have one or more of the structures of cyclohexanol, cyclohexylidene ketone, phenol, and catechol. The classic drug for treating hyperlipidaemia, ezetimibe, also has phenol, which might be a traditional structure that activates AMPK and regulates related diseases. If we can get inspiration from the structures of these natural products, further study the structure-activity relationship, and synthesise more potent compounds, it will benefit future development. Certain compounds exhibit structural similarities, yet further investigation is required to determine which structural components constitute privileged structures with higher therapeutic potential.
CRediT authorship contribution statement
Yihua Xu: Conceptualization. Lan Bai: Conceptualization. Xinwei Yang: Visualization. Jianli Huang: Formal analysis. Jie Wang: Investigation. Xianbo Wu: Supervision, Funding acquisition. Jianyou Shi: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors of this review were supported by the National Natural Science Foundation of China (82174325, 82374415), the Sichuan Science and Technology Plan Project (23NSFSC0600), the Open Research Fund of State Key Laboratory of Southwestern Chinese Medicine Resources (SKLTCM2022019 and the study of vacuum freeze-drying technology of chuanxiong based on intelligent control algorithm neural network model).
Contributor Information
Xianbo Wu, Email: cdutcmwu@163.com.
Jianyou Shi, Email: shijianyoude@126.com.
References
- 1.Mollaei M., Abbasi A., Hassan Z.M., Pakravan N. The intrinsic and extrinsic elements regulating inflammation. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118258. [DOI] [PubMed] [Google Scholar]
- 2.Sudhakaran M., Doseff A.I. The targeted impact of flavones on obesity-induced inflammation and the potential synergistic role in cancer and the gut microbiota. Molecules. 2020;25:2477. doi: 10.3390/molecules25112477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marques-Rocha J.L., Samblas M., Milagro F.I., Bressan J., Martínez J.A., Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. Faseb. J. 2015;29:3595–3611. doi: 10.1096/fj.14-260323. [DOI] [PubMed] [Google Scholar]
- 4.Tsung A., Tohme S., Billiar T.R. High‐mobility group box‐1 in sterile inflammation. J. Intern. Med. 2014;276:425–443. doi: 10.1111/joim.12276. [DOI] [PubMed] [Google Scholar]
- 5.Chen F.E., Huang D.-B., Chen Y.-Q., Ghosh G. Crystal structure of p50/p65 heterodimer of transcription factor NF-κB bound to DNA. Nature. 1998;391:410–413. doi: 10.1038/34956. [DOI] [PubMed] [Google Scholar]
- 6.Iglesias M.A., Furler S.M., Cooney G.J., Kraegen E.W., Ye J.-M. AMP-activated protein kinase activation by AICAR increases both muscle fatty acid and glucose uptake in white muscle of insulin-resistant rats in vivo. Diabetes. 2004;53:1649–1654. doi: 10.2337/diabetes.53.7.1649. [DOI] [PubMed] [Google Scholar]
- 7.Scott J.W., Hawley S.A., Green K.A., Anis M., Stewart G., Scullion G.A., Norman D.G., Hardie D.G. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J. Clin. Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iseli T.J., Walter M., van Denderen B.J.W., Katsis F., Witters L.A., Kemp B.E., Michell B.J., Stapleton D. AMP-Activated protein kinase β subunit tethers α and γ subunits via its C-terminal sequence (186–270) J. Biol. Chem. 2005;280:13395–13400. doi: 10.1074/jbc.M412993200. [DOI] [PubMed] [Google Scholar]
- 9.Cool B., Zinker B., Chiou W., Kifle L., Cao N., Perham M., Dickinson R., Adler A., Gagne G., Iyengar R., Zhao G., Marsh K., Kym P., Jung P., Camp H.S., Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metabol. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg G.R., Kemp B.E. AMPK in health and disease. Physiol. Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 11.Xiao B., Sanders M.J., Underwood E., Heath R., V Mayer F., Carmena D., Jing C., Walker P.A., Eccleston J.F., Haire L.F., Saiu P., Howell S.A., Aasland R., Martin S.R., Carling D., Gamblin S.J. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carling D., Sanders M.J., Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. 2008;32:S55–S59. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 13.Hurley R.L., Anderson K.A., Franzone J.M., Kemp B.E., Means A.R., Witters L.A. The Ca2+/Calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 14.Oakhill J.S., Steel R., Chen Z.-P., Scott J.W., Ling N., Tam S., Kemp B.E. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. 1979. [DOI] [PubMed] [Google Scholar]
- 15.Woods A., Dickerson K., Heath R., Hong S.-P., Momcilovic M., Johnstone S.R., Carlson M., Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metabol. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon S.-M., Chandel N.S., Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price N.L., Gomes A.P., Ling A.J.Y., Duarte F.V., Martin-Montalvo A., North B.J., Agarwal B., Ye L., Ramadori G., Teodoro J.S., Hubbard B.P., Varela A.T., Davis J.G., Varamini B., Hafner A., Moaddel R., Rolo A.P., Coppari R., Palmeira C.M., de Cabo R., Baur J.A., Sinclair D.A. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metabol. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park S.J., Ahmad F., Philp A., Baar K., Williams T., Luo H., Ke H., Rehmann H., Taussig R., Brown A.L., Kim M.K., Beaven M.A., Burgin A.B., Manganiello V., Chung J.H. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148 doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao X., Zhang S., Wang L., Yu L., Zhao X., Ni H., Wang Y., Wang J., Shan C., Fu Y. Anti-inflammatory effects of neochlorogenic acid extract from mulberry leaf (morus alba L.) against LPS-stimulated inflammatory response through mediating the AMPK/Nrf2 signaling pathway in A549 cells. Molecules. 2020;25:1385. doi: 10.3390/molecules25061385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sag D., Carling D., Stout R.D., Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong L., Wang Z., Wang Z., Zhang Z. Sestrin2 as a potential target for regulating metabolic-related diseases. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.751020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budanov A.V., Karin M. p53 target genes Sestrin1 and Sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134 doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lushchak V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014;224 doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Cordero M.D., Williams M.R., Ryffel B. AMP-activated protein kinase regulation of the NLRP3 inflammasome during aging. Trends Endocrinol. Metabol. 2018;29 doi: 10.1016/j.tem.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Ding X., Jian T., Li J., Lv H., Tong B., Li J., Meng X., Ren B., Chen J. Chicoric acid ameliorates nonalcoholic fatty liver disease via the AMPK/Nrf2/NFκB signaling pathway and restores gut microbiota in high-fat-diet-fed mice. Oxid. Med. Cell. Longev. 2020;2020:1–20. doi: 10.1155/2020/9734560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan J., Cui J., Yang Z., Guo C., Cao J., Xi M., Weng Y., Yin Y., Wang Y., Wei G., Qiao B., Wen A. Neuroprotective effect of Apelin 13 on ischemic stroke by activating AMPK/GSK-3β/Nrf2 signaling. J. Neuroinflammation. 2019;16:24. doi: 10.1186/s12974-019-1406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv H., Liu Q., Wen Z., Feng H., Deng X., Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López M., Varela L., Vázquez M.J., Rodríguez-Cuenca S., González C.R., Velagapudi V.R., Morgan D.A., Schoenmakers E., Agassandian K., Lage R., De Morentin P.B.M., Tovar S., Nogueiras R., Carling D., Lelliott C., Gallego R., OreŠič M., Chatterjee K., Saha A.K., Rahmouni K., Diéguez C., Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010;16 doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma F., Hao H., Gao X., Cai Y., Zhou J., Liang P., Lv J., He Q., Shi C., Hu D., Chen B., Zhu L., Xiao X., Li S. Melatonin ameliorates necrotizing enterocolitis by preventing Th17/Treg imbalance through activation of the AMPK/SIRT1 pathway. Theranostics. 2020;10:7730–7746. doi: 10.7150/thno.45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stohs S.J., Miller H., Kaats G.R. A review of the efficacy and safety of banaba (Lagerstroemia speciosa L.) and corosolic acid. Phytother Res. 2012;26 doi: 10.1002/ptr.3664. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z.-P., Shen D., Che Y., Jin Y.-G., Wang S.-S., Wu Q.-Q., Zhou H., Meng Y.-Y., Yuan Y. Corosolic acid ameliorates cardiac hypertrophy via regulating autophagy. Biosci. Rep. 2019;39 doi: 10.1042/BSR20191860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolb R., Sutterwala F.S., Zhang W. Obesity and cancer: inflammation bridges the two. Curr. Opin. Pharmacol. 2016;29:77–89. doi: 10.1016/j.coph.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalupahana N.S., Moustaid-Moussa N., Claycombe K.J. Mol Aspects Med. 2012. Immunity as a link between obesity and insulin resistance. [DOI] [PubMed] [Google Scholar]
- 35.Yang Z., Kahn B.B., Shi H., Xue B.Z. Macrophage α1 AMP-activated protein kinase (α1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 2010;285 doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrne C.D., Targher G. NAFLD: a multisystem disease. J. Hepatol. 2015;62:S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Neuschwander-Tetri B.A. Non-alcoholic fatty liver disease. BMC Med. 2017;15:45. doi: 10.1186/s12916-017-0806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Younossi Z.M. Non-alcoholic fatty liver disease – a global public health perspective. J. Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., Mills P.R., Keach J.C., Lafferty H.D., Stahler A., Haflidadottir S., Bendtsen F. Liver fibrosis, but No other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomone F., Godos J., Zelber-Sagi S. Natural antioxidants for non-alcoholic fatty liver disease: molecular targets and clinical perspectives. Liver Int. 2016;36 doi: 10.1111/liv.12975. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi Y., Yamada K., Yoshikawa N., Nakamura K., Haginaka J., Kunitomo M. Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci. 2006;79 doi: 10.1016/j.lfs.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Yang J., Leng J., Li J.-J., Tang J., Li Y., Liu B.-L., Wen X.-D. Corosolic acid inhibits adipose tissue inflammation and ameliorates insulin resistance via AMPK activation in high-fat fed mice. Phytomedicine. 2016;23:181–190. doi: 10.1016/j.phymed.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Cui W.X., Yang J., Chen X.Q., Mao Q., Wei X.L., Wen X.D., Wang Q. Triterpenoid-rich fraction from ilex hainanensis merr. attenuates non-alcoholic fatty liver disease induced by high fat diet in rats. Am. J. Chin. Med. 2013;41 doi: 10.1142/S0192415X13500353. [DOI] [PubMed] [Google Scholar]
- 44.Cui W.X., Li J.J., Chen X.Q., Mao Q., Wei X.L., Wen X.D., Yang J., Wang Q. Ilexgenin A obtained from Ilex hainanensis Merr. Improves diet-induced non-alcoholic fatty liver disease in rats. Drug Dev. Res. 2013;74 doi: 10.1002/ddr.21066. [DOI] [Google Scholar]
- 45.Lin J.H., Walter P., Yen T.S.B. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oslowski C.M., Hara T., O'Sullivan-Murphy B., Kanekura K., Lu S., Hara M., Ishigaki S., Zhu L.J., Hayashi E., Hui S.T., Greiner D., Kaufman R.J., Bortell R., Urano F. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metabol. 2012;16 doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y., Yang J., Chen M.-H., Wang Q., Qin M.-J., Zhang T., Chen X.-Q., Liu B.-L., Wen X.-D. Ilexgenin A inhibits endoplasmic reticulum stress and ameliorates endothelial dysfunction via suppression of TXNIP/NLRP3 inflammasome activation in an AMPK dependent manner. Pharmacol. Res. 2015;99:101–115. doi: 10.1016/j.phrs.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Li X., Lian L.-H., Bai T., Wu Y.-L., Wan Y., Xie W.-X., Jin X., Nan J.-X. Cryptotanshinone inhibits LPS-induced proinflammatory mediators via TLR4 and TAK1 signaling pathway. Int. Immunopharm. 2011;11:1871–1876. doi: 10.1016/j.intimp.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 49.Tang S., Shen X.-Y., Huang H.-Q., Xu S.-W., Yu Y., Zhou C.-H., Chen S.-R., Le K., Wang Y.-H., Liu P.-Q. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW264.7 macrophages through inhibition of the NF-κB and MAPK signaling pathways. Inflammation. 2011;34:111–118. doi: 10.1007/s10753-010-9214-3. [DOI] [PubMed] [Google Scholar]
- 50.Hou X., Xu S., Maitland-Toolan K.A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T.J., Cohen R.A., Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lan F., Cacicedo J.M., Ruderman N., Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. J. Biol. Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagappan A., Kim J.-H., Jung D.Y., Jung M.H. Cryptotanshinone from the Salvia miltiorrhiza bunge attenuates ethanol-induced liver injury by activation of AMPK/SIRT1 and Nrf2 signaling pathways. Int. J. Mol. Sci. 2019;21:265. doi: 10.3390/ijms21010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujii N., Narita T., Okita N., Kobayashi M., Furuta Y., Chujo Y., Sakai M., Yamada A., Takeda K., Konishi T., Sudo Y., Shimokawa I., Higami Y. Sterol regulatory element-binding protein-1c orchestrates metabolic remodeling of white adipose tissue by caloric restriction. Aging Cell. 2017;16 doi: 10.1111/acel.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corona J.C., Duchen M.R. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med. 2016;100 doi: 10.1016/j.freeradbiomed.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Rivera J., Denner L., Dineley K.T. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav. Brain Res. 2011;216 doi: 10.1016/j.bbr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takada Y., Singh S., Aggarwal B.B. Identification of a p65 peptide that selectively inhibits NF-κ B activation induced by various inflammatory stimuli and its role in down-regulation of NF-κB-mediated gene expression and up-regulation of apoptosis. J. Biol. Chem. 2004;279:15096–15104. doi: 10.1074/jbc.M311192200. [DOI] [PubMed] [Google Scholar]
- 57.Tang L., Chen Q., Meng Z., Sun L., Zhu L., Liu J., Hu J., Ni Z., Wang X. Suppression of sirtuin-1 increases IL-6 expression by activation of the Akt pathway during allergic asthma. Cell. Physiol. Biochem. 2017;43:1950–1960. doi: 10.1159/000484119. [DOI] [PubMed] [Google Scholar]
- 58.Hou D.-X., Korenori Y., Tanigawa S., Yamada-Kato T., Nagai M., He X., He J. Dynamics of Nrf2 and Keap1 in ARE-mediated NQO1 expression by wasabi 6-(methylsulfinyl)hexyl isothiocyanate. J. Agric. Food Chem. 2011;59:11975–11982. doi: 10.1021/jf2032439. [DOI] [PubMed] [Google Scholar]
- 59.Xue D., Zhou C., Shi Y., Lu H., Xu R., He X. Nuclear transcription factor Nrf2 suppresses prostate cancer cells growth and migration through upregulating ferroportin. Oncotarget. 2016;7:78804–78812. doi: 10.18632/oncotarget.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang L.L., Wan J.B., Wang B., He C.W., Ma H., Li T.W., Kang J.X. Suppression of acute ethanol-induced hepatic steatosis by docosahexaenoic acid is associated with downregulation of stearoyl-CoA desaturase 1 and inflammatory cytokines. Prostaglandins Leukot. Essent. Fatty Acids. 2013;88 doi: 10.1016/j.plefa.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Tian R., Yang W., Xue Q., Gao L., Huo J., Ren D., Chen X. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur. J. Pharmacol. 2016;771:84–92. doi: 10.1016/j.ejphar.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 62.Butura A., Nilsson K., Morgan K., Morgan T.R., French S.W., Johansson I., Schuppe-Koistinen I., Ingelman-Sundberg M. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J. Hepatol. 2009;50 doi: 10.1016/j.jhep.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 63.Caro A.A., Cederbaum A.I. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu. Rev. Pharmacol. Toxicol. 2004;44 doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 64.He W., Li Y., Qin Y., Tong X., Song Z., Zhao Y., Wei R., Li L., Dai H., Wang W., Luo H., Ye X., Zhang L., Liu X. New cryptotanshinone derivatives with anti-influenza A virus activities obtained via biotransformation by Mucor rouxii. Appl. Microbiol. Biotechnol. 2017;101:6365–6374. doi: 10.1007/s00253-017-8351-0. [DOI] [PubMed] [Google Scholar]
- 65.McKinnon R., Binder M., Zupkó I., Afonyushkin T., Lajter I., Vasas A., de Martin R., Unger C., Dolznig H., Diaz R., Frisch R., Passreiter C.M., Krupitza G., Hohmann J., Kopp B., Bochkov V.N. Pharmacological insight into the anti-inflammatory activity of sesquiterpene lactones from Neurolaena lobata (L.) R.Br. ex Cass. Phytomedicine. 2014;21:1695–1701. doi: 10.1016/j.phymed.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 66.Baumgart D.C., Sandborn W.J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369 doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 67.Eichele D.D., Kharbanda K.K. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017;23 doi: 10.3748/wjg.v23.i33.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han J., Li W., Shi G., Huang Y., Sun X., Sun N., Jiang D. Atractylenolide III improves mitochondrial function and protects against ulcerative colitis by activating AMPK/SIRT1/PGC-1α. Mediat. Inflamm. 2022;2022:1–17. doi: 10.1155/2022/9129984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knutti D., Kralli A. PGC-1, a versatile coactivator. Trends Endocrinol. Metabol. 2001;12 doi: 10.1016/S1043-2760(01)00457-X. [DOI] [PubMed] [Google Scholar]
- 70.Zong H., Ren J.M., Young L.H., Pypaert M., Mu J., Birnbaum M.J., Shulman G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. U. S. A. 2002;99 doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song M.Y., Jung H.W., Kang S.Y., Park Y.-K. Atractylenolide III enhances energy metabolism by increasing the SIRT-1 and PGC1α expression with AMPK phosphorylation in C2C12 mouse skeletal muscle cells. Biol. Pharm. Bull. 2017;40:339–344. doi: 10.1248/bpb.b16-00853. [DOI] [PubMed] [Google Scholar]
- 72.González R., Ballester I., López-Posadas R., Suárez M.D., Zarzuelo A., Martínez-Augustin O., De Medina F.S. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011;51:331–362. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]
- 73.Wang L., Tu Y.-C., Lian T.-W., Hung J.-T., Yen J.-H., Wu M.-J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006;54:9798–9804. doi: 10.1021/jf0620719. [DOI] [PubMed] [Google Scholar]
- 74.Li J., Shi M., Ma B., Niu R., Zhang H., Kun L. Antitumor activity and safety evaluation of nanaparticle-based delivery of quercetin through intravenous administration in mice. Mater. Sci. Eng. C. 2017;77 doi: 10.1016/j.msec.2017.03.191. [DOI] [PubMed] [Google Scholar]
- 75.Ulusoy H.G., Sanlier N. A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020;60 doi: 10.1080/10408398.2019.1683810. [DOI] [PubMed] [Google Scholar]
- 76.Bai J., Zhang Y., Zheng X., Huang M., Cheng W., Shan H., Gao X., Zhang M., Sheng L., Dai J., Deng Y., Zhang H., Zhou X. LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 2020;11:763. doi: 10.1038/s41419-020-02945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao X., Petursson F., Viollet B., Lotz M., Terkeltaub R., Liu-Bryan R. Peroxisome proliferator-activated receptor γ coactivator 1α and FoxO3A mediate chondroprotection by AMP-activated protein kinase. Arthritis Rheumatol. 2014;66 doi: 10.1002/art.38791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu L., Luo Y., Chen X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018;103 doi: 10.1016/j.biopha.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Nam J.S., Sharma A.R., Nguyen L.T., Chakraborty C., Sharma G., Lee S.S. Application of bioactive quercetin in oncotherapy: from nutrition to nanomedicine. Molecules. 2016;21 doi: 10.3390/molecules21010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zielińska-Pisklak M.A., Kaliszewska D., Stolarczyk M., Kiss A.K. Activity-guided isolation, identification and quantification of biologically active isomeric compounds from folk medicinal plant Desmodium adscendens using high performance liquid chromatography with diode array detector, mass spectrometry and multidimentional nuclear magnetic resonance spectroscopy. J. Pharm. Biomed. Anal. 2014;102 doi: 10.1016/j.jpba.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 81.Khole S., Chatterjee S., Variyar P., Sharma A., Devasagayam T.P.A., Ghaskadbi S. Bioactive constituents of germinated fenugreek seeds with strong antioxidant potential. J. Funct.Foods. 2014;6 doi: 10.1016/j.jff.2013.10.016. [DOI] [Google Scholar]
- 82.Sun Y., Li H., Hu J., Li J., Fan Y.W., Liu X.R., Deng Z.Y. Qualitative and quantitative analysis of phenolics in Tetrastigma hemsleyanum and their antioxidant and antiproliferative activities. J. Agric. Food Chem. 2013;61 doi: 10.1021/jf4037547. [DOI] [PubMed] [Google Scholar]
- 83.de Oliveira D.R., Zamberlam C.R., Gaiardo R.B., Rêgo G.M., Cerutti J.M., Cavalheiro A.J., Cerutti S.M. Flavones from Erythrina falcata are modulators of fear memory. BMC Compl. Alternative Med. 2014;14 doi: 10.1186/1472-6882-14-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Awolola G.V., Koorbanally N.A., Chenia H., Shode F.O., Baijnath H. Antibacterial and anti-biofilm activity of flavonoids and triterpenes isolated from the extracts of Ficus sansibarica Warb. subsp. sansibarica (Moraceae) extracts. Afr. J. Tradit., Complementary Altern. Med. : AJTCAM / African Networks on Ethnomedicines. 2014;11 doi: 10.4314/ajtcam.v11i3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y., Peng F., Xing Z., Chen J., Peng C., Li D. Beneficial effects of natural flavonoids on neuroinflammation. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y., Zhang Y., Yang T., Li H., Guo J., Zhao Q., Xie J. Pharmacokinetics and tissue distribution study of Isovitexin in rats by HPLC-MS/MS. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2015;991 doi: 10.1016/j.jchromb.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Rawji K.S., Mishra M.K., Michaels N.J., Rivest S., Stys P.K., Yong V.W. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain. 2016;139 doi: 10.1093/brain/awv395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burke N.N., Kerr D.M., Moriarty O., Finn D.P., Roche M. Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav. Immun. 2014;42 doi: 10.1016/j.bbi.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 89.Liu B., Huang B., Hu G., He D., Li Y., Ran X., Du J., Fu S., Liu D. Isovitexin-mediated regulation of microglial polarization in lipopolysaccharide-induced neuroinflammation via activation of the CaMKKβ/AMPK-PGC-1α signaling Axis. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu B., Huang B., Hu G., He D., Li Y., Ran X., Du J., Fu S., Liu D. Corrigendum: isovitexin-mediated regulation of microglial polarization in lipopolysaccharide-induced neuroinflammation via activation of the CaMKKβ/AMPK-PGC-1α signaling Axis. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00041. (Frontiers in Immunology, (2019), 10, 10.3389/fimmu.2019.02650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakajima A., Ohizumi Y. Potential benefits of nobiletin, a citrus flavonoid, against Alzheimer's disease and Parkinson's disease. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20143380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H., Guo Y., Qiao Y., Zhang J., Jiang P. Nobiletin ameliorates NLRP3 inflammasome-mediated inflammation through promoting autophagy via the AMPK pathway. Mol. Neurobiol. 2020;57 doi: 10.1007/s12035-020-02071-5. [DOI] [PubMed] [Google Scholar]
- 93.Ou H.C., Pandey S., Hung M.Y., Huang S.H., Hsu P.T., Day C.H., Pai P., Viswanadha V.P., Kuo W.W., Huang C.Y. Luteolin: a natural flavonoid enhances the survival of HUVECs against oxidative stress by modulating AMPK/PKC pathway. Am. J. Chin. Med. 2019;47 doi: 10.1142/S0192415X19500289. [DOI] [PubMed] [Google Scholar]
- 94.Xiao C., Xia M.-L., Wang J., Zhou X.-R., Lou Y.-Y., Tang L.-H., Zhang F.-J., Yang J.-T., Qian L.-B. Luteolin attenuates cardiac ischemia/reperfusion injury in diabetic rats by modulating Nrf2 antioxidative function. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/2719252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imran M., Rauf A., Abu-Izneid T., Nadeem M., Shariati M.A., Khan I.A., Imran A., Orhan I.E., Rizwan M., Atif M., Gondal T.A., Mubarak M.S. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- 96.Wang S., Wuniqiemu T., Tang W., Teng F., Bian Q., Yi L., Qin J., Zhu X., Wei Y., Dong J. Luteolin inhibits autophagy in allergic asthma by activating PI3K/Akt/mTOR signaling and inhibiting Beclin-1-PI3KC3 complex. Int. Immunopharm. 2021;94 doi: 10.1016/j.intimp.2021.107460. [DOI] [PubMed] [Google Scholar]
- 97.Song C., Heping H., Shen Y., Jin S., Li D., Zhang A., Ren X., Wang K., Zhang L., Wang J., Shi D. AMPK/p38/Nrf2 activation as a protective feedback to restrain oxidative stress and inflammation in microglia stimulated with sodium fluoride. Chemosphere. 2020;244 doi: 10.1016/j.chemosphere.2019.125495. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Z., Zhang L., Liu Y., Huang C., Xia W., Zhou H., Zhou Z., Zhou X. Luteolin protects chondrocytes from H2O2-induced oxidative injury and attenuates osteoarthritis progression by activating AMPK-nrf2 signaling. Oxid. Med. Cell. Longev. 2022;2022:1–20. doi: 10.1155/2022/5635797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hou L., Jiang F., Huang B., Zheng W., Jiang Y., Cai G., Liu D., Hu C.Y., Wang C. Dihydromyricetin resists inflammation‐induced muscle atrophy via ryanodine receptor‐CaMKK‐AMPK signal pathway. J. Cell Mol. Med. 2021;25:9953–9971. doi: 10.1111/jcmm.16810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen K.H., Dasgupta A., Ding J., Indig F.E., Ghosh P., Longo D.L. Role of mitofusin 2 (Mfn2) in controlling cellular proliferation. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2014;28 doi: 10.1096/fj.13-230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X., Qin Y., Ruan W., Wan X., lv C., He L., Lu L., Guo X. Targeting inflammation-associated AMPK//Mfn-2/MAPKs signaling pathways by baicalein exerts anti-atherosclerotic action. Phytother Res. 2021;35 doi: 10.1002/ptr.7149. [DOI] [PubMed] [Google Scholar]
- 102.Dąbrowska-Bender M., Milewska M., Gołąbek A., Duda-Zalewska A., Staniszewska A. The impact of ischemic cerebral stroke on the quality of life of patients based on clinical, social, and psychoemotional factors. J. Stroke Cerebrovasc. Dis. 2017;26 doi: 10.1016/j.jstrokecerebrovasdis.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 103.Lakhan S.E., Kirchgessner A., Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J. Transl. Med. 2009;7 doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng W.-X., He W.-Q., Zhang Q.-R., Jia J.-X., Zhao S., Wu F.-J., Cao X.-L. Baicalin inhibits NLRP3 inflammasome activity via the AMPK signaling pathway to alleviate cerebral ischemia-reperfusion injury. Inflammation. 2021;44:2091–2105. doi: 10.1007/s10753-021-01486-z. [DOI] [PubMed] [Google Scholar]
- 105.Márquez L., García-Bueno B., Madrigal J.L.M., Leza J.C. Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur. J. Nutr. 2012;51 doi: 10.1007/s00394-011-0252-x. [DOI] [PubMed] [Google Scholar]
- 106.Yong Z., Ruiqi W., Hongji Y., Ning M., Chenzuo J., Yu Z., Zhixuan X., Qiang L., Qibing L., Weiying L., Xiaopo Z. Mangiferin ameliorates HFD-induced NAFLD through regulation of the AMPK and NLRP3 inflammasome signal pathways. J Immunol Res. 2021;2021:1–17. doi: 10.1155/2021/4084566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song J., Li J., Hou F., Wang X., Liu B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metabolism. 2015;64:428–437. doi: 10.1016/j.metabol.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Wang H.L., Li C.Y., Zhang B., De Liu Y., Lu B.M., Shi Z., An N., Zhao L.K., Zhang J.J., Bao J.K., Wang Y. Mangiferin facilitates islet regeneration and β-cell proliferation through upregulation of cell cycle and β-cell regeneration regulators. Int. J. Mol. Sci. 2014;15 doi: 10.3390/ijms15059016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Calles-Escandon J., Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr. Rev. 2001;22 doi: 10.1210/edrv.22.1.0417. [DOI] [PubMed] [Google Scholar]
- 110.Ruderman N.B., Williamson J.R., Brownlee M. Glucose and diabetic vascular disease 1. Faseb. J. 1992;6 doi: 10.1096/fasebj.6.11.1644256. [DOI] [PubMed] [Google Scholar]
- 111.Bannuru R.R., Osani M.C., Al-Eid F., Wang C. Efficacy of curcumin and Boswellia for knee osteoarthritis: systematic review and meta-analysis. Semin. Arthritis Rheum. 2018;48 doi: 10.1016/j.semarthrit.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yin H., Guo Q., Li X., Tang T., Li C., Wang H., Sun Y., Feng Q., Ma C., Gao C., Yi F., Peng J. Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J. Immunol. 2018;200:2835–2846. doi: 10.4049/jimmunol.1701495. [DOI] [PubMed] [Google Scholar]
- 113.Feng K., Ge Y., Chen Z., Li X., Liu Z., Li X., Li H., Tang T., Yang F., Wang X. Curcumin inhibits the PERK-eIF2α-CHOP pathway through promoting SIRT1 expression in oxidative stress-induced rat chondrocytes and ameliorates osteoarthritis progression in a rat model. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/8574386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Z., Leong D.J., Xu L., He Z., Wang A., Navati M., Kim S.J., Hirsh D.M., Hardin J.A., Cobelli N.J., Friedman J.M., Sun H.B. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016;18 doi: 10.1186/s13075-016-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 115.Jin Z., Chang B., Wei Y., Yang Y., Zhang H., Liu J., Piao L., Bai L. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed. Pharmacother. 2022;151 doi: 10.1016/j.biopha.2022.113092. [DOI] [PubMed] [Google Scholar]
- 116.Liu W., Zhai Y., Heng X., Che F.Y., Chen W., Sun D., Zhai G. Oral bioavailability of curcumin: problems and advancements. J. Drug Target. 2016;24 doi: 10.3109/1061186X.2016.1157883. [DOI] [PubMed] [Google Scholar]
- 117.Garg A.X., Moist L., Pannu N., Tobe S., Walsh M., Weir M. Bioavailability of oral curcumin. CMAJ (Can. Med. Assoc. J.) 2019;191 doi: 10.1503/cmaj.71719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.wei Wang S., Wang W., Sheng H., feng Bai Y., yuan Weng Y., yu Fan X., Zheng F., tian Zhu X., cai Xu Z., Zhang F. Hesperetin, a SIRT1 activator, inhibits hepatic inflammation via AMPK/CREB pathway. Int. Immunopharm. 2020;89 doi: 10.1016/j.intimp.2020.107036. [DOI] [PubMed] [Google Scholar]
- 119.Huang A.-L., Zhang Y.-L., Ding H.-W., Li B., Huang C., Meng X.-M., Li J. Design, synthesis and investigation of potential anti-inflammatory activity of O-alkyl and O-benzyl hesperetin derivatives. Int. Immunopharm. 2018;61:82–91. doi: 10.1016/j.intimp.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 120.Li J.-J., Jiang H.-C., Wang A., Bu F.-T., Jia P.-C., Zhu S., Zhu L., Huang C., Li J. Hesperetin derivative-16 attenuates CCl4-induced inflammation and liver fibrosis by activating AMPK/SIRT3 pathway. Eur. J. Pharmacol. 2022;915 doi: 10.1016/j.ejphar.2021.174530. [DOI] [PubMed] [Google Scholar]
- 121.Medina-Bolivar F., Condori J., Rimando A.M., Hubstenberger J., Shelton K., O'Keefe S.F., Bennett S., Dolan M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry. 2007;68 doi: 10.1016/j.phytochem.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 122.Rocha-González H.I., Ambriz-Tututi M., Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci. Ther. 2008;14 doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meng X., Zhou J., Zhao C.N., Gan R.Y., Bin Li H. Health benefits and molecular mechanisms of resveratrol: a narrative review. Foods. 2020;9 doi: 10.3390/foods9030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Farkhondeh T., Folgado S.L., Pourbagher-Shahri A.M., Ashrafizadeh M., Samarghandian S. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110234. [DOI] [PubMed] [Google Scholar]
- 125.Simental-Mendía L.E., Guerrero-Romero F. Effect of resveratrol supplementation on lipid profile in subjects with dyslipidemia: a randomized double-blind, placebo-controlled trial. Nutrition. 2019;58 doi: 10.1016/j.nut.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 126.Varoni E.M., Lo Faro A.F., Sharifi-Rad J., Iriti M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016;3 doi: 10.3389/fnut.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Sá Coutinho D., Pacheco M.T., Frozza R.L., Bernardi A. Anti-inflammatory effects of resveratrol: mechanistic insights. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19061812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rimando A.M., Kalt W., Magee J.B., Dewey J., Ballington J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004;52 doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 129.Chen W.M., Shaw L.H., Chang P.J., Tung S.Y., Chang T.S., Shen C.H., Hsieh Y.Y., Wei K.L. Hepatoprotective effect of resveratrol against ethanol-induced oxidative stress through induction of superoxide dismutase in vivo and in vitro. Exp. Ther. Med. 2016;11 doi: 10.3892/etm.2016.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J.E., Janle E.M., Lobo J., Ferruzzi M.G., Davies P., Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J. Biol. Chem. 2010;285 doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei Z., Chen G., Hu T., Mo X., Hou X., Cao K., Wang L., Pan Z., Wu Q., Li X., Ye F., Zouboulis C.C., Ju Q. Resveratrol ameliorates lipid accumulation and inflammation in human SZ95 sebocytes via the AMPK signaling pathways in vitro. J. Dermatol. Sci. 2021;103:156–166. doi: 10.1016/j.jdermsci.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 132.Carpagnano G.E., Scioscia G., Lacedonia D., Soccio P., Quarato C.M.I., Cotugno G., Palumbo M.G., Foschino Barbaro M.P. Searching for inflammatory and oxidative stress markers capable of clustering severe asthma. Arch. Bronconeumol. 2021;57 doi: 10.1016/j.arbres.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 133.Potaczek D.P., Miethe S., Schindler V., Alhamdan F., Garn H. Role of airway epithelial cells in the development of different asthma phenotypes. Cell. Signal. 2020;69 doi: 10.1016/j.cellsig.2019.109523. [DOI] [PubMed] [Google Scholar]
- 134.Wang C., Li L., Jiang J., Li L., Li J., Xu C., Jin S., Zhu L., Yan G. Pterostilbene inhibits FcεRI signaling through activation of the LKB1/AMPK pathway in allergic response. J. Agric. Food Chem. 2020;68:3456–3465. doi: 10.1021/acs.jafc.9b07126. [DOI] [PubMed] [Google Scholar]
- 135.Xu C., Song Y., Wang Z., Jiang J., Piao Y., Li L., Jin S., Li L., Zhu L., Yan G. Pterostilbene suppresses oxidative stress and allergic airway inflammation through AMPK/Sirt1 and Nrf2/HO‐1 pathways. Immun Inflamm Dis. 2021;9:1406–1417. doi: 10.1002/iid3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hseu Y.-C., Vudhya Gowrisankar Y., Wang L.-W., Zhang Y.-Z., Chen X.-Z., Huang P.-J., Yen H.-R., Yang H.-L. The in vitro and in vivo depigmenting activity of pterostilbene through induction of autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in keratinocytes via Nrf2-mediated antioxidant pathways. Redox Biol. 2021;44 doi: 10.1016/j.redox.2021.102007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lajis A.F.B., Ariff A.B. Discovery of new depigmenting compounds and their efficacy to treat hyperpigmentation: evidence from in vitro study. J. Cosmet. Dermatol. 2019;18 doi: 10.1111/jocd.12900. [DOI] [PubMed] [Google Scholar]
- 138.Li M., Zhang F., Wang X., Wu X., Zhang B., Zhang N., Wu W., Wang Z., Weng H., Liu S., Gao G., Mu J., Shu Y., Bao R., Cao Y., Lu J., Gu J., Zhu J., Liu Y. Magnolol inhibits growth of gallbladder cancer cells through the p53 pathway. Cancer Sci. 2015;106 doi: 10.1111/cas.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Y.H., Lin F.Y., Liu P.L., Huang Y.T., Chiu J.H., Chang Y.C., Man K.M., Hong C.Y., Ho Y.Y., Lai M.T. Antioxidative and hepatoprotective effects of magnolol on acetaminophen-induced liver damage in rats. Arch Pharm. Res. (Seoul) 2009;32 doi: 10.1007/s12272-009-1139-8. [DOI] [PubMed] [Google Scholar]
- 140.Tian Y., Feng H., Han L., Wu L., Lv H., Shen B., Li Z., Zhang Q., Liu G. Magnolol alleviates inflammatory responses and lipid accumulation by AMP-activated protein kinase-dependent peroxisome proliferator-activated receptor α activation. Front. Immunol. 2018;9:147. doi: 10.3389/fimmu.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu Z., Zhang H., Wang H., Wei L., Niu L. Magnolol alleviates IL-1β-induced dysfunction of chondrocytes through repression of SIRT1/AMPK/PGC-1α signaling pathway. J. Interferon Cytokine Res. 2020;40:145–151. doi: 10.1089/jir.2019.0139. [DOI] [PubMed] [Google Scholar]
- 142.Pillai V.B., Samant S., Sundaresan N.R., Raghuraman H., Kim G., Bonner M.Y., Arbiser J.L., Walker D.I., Jones D.P., Gius D., Gupta M.P. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat. Commun. 2015;6:6656. doi: 10.1038/ncomms7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shen J.-L., Man K.-M., Huang P.-H., Chen W.-C., Chen D.-C., Cheng Y.-W., Liu P.-L., Chou M.-C., Chen Y.-H. Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules. 2010;15:6452–6465. doi: 10.3390/molecules15096452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weng T.I., Wu H.Y., Kuo C.W., Liu S.H. Honokiol rescues sepsis-associated acute lung injury and lethality via the inhibition of oxidative stress and inflammation. Intensive Care Med. 2011;37:533–541. doi: 10.1007/s00134-010-2104-1. [DOI] [PubMed] [Google Scholar]
- 145.Shahraki A., Ebrahimi A. Binding of ellagic acid and urolithin metabolites to the CK2 protein, based on the ONIOM method and molecular docking calculations. New J. Chem. 2019;43:15983–15998. doi: 10.1039/C9NJ03508G. [DOI] [Google Scholar]
- 146.Gong Z., Huang J., Xu B., Ou Z., Zhang L., Lin X., Ye X., Kong X., Long D., Sun X., He X., Xu L., Li Q., Xuan A. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J. Neuroinflammation. 2019;16:62. doi: 10.1186/s12974-019-1450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu J., Yuan C., Wang G., Luo J., Ma H., Xu L., Mu Y., Li Y., Seeram N.P., Huang X., Li L. Urolithins attenuate LPS-induced neuroinflammation in BV2Microglia via MAPK, Akt, and NF-κB signaling pathways. J. Agric. Food Chem. 2018;66 doi: 10.1021/acs.jafc.7b03285. [DOI] [PubMed] [Google Scholar]
- 148.Boakye Y.D., Groyer L., Heiss E.H. An increased autophagic flux contributes to the anti-inflammatory potential of urolithin A in macrophages. Biochim. Biophys. Acta Gen. Subj. 2018;1862 doi: 10.1016/j.bbagen.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y., Aisker G., Dong H., Halemahebai G., Zhang Y., Tian L. Urolithin A suppresses glucolipotoxicity-induced ER stress and TXNIP/NLRP3/IL-1β inflammation signal in pancreatic β cells by regulating AMPK and autophagy. Phytomedicine. 2021;93 doi: 10.1016/j.phymed.2021.153741. [DOI] [PubMed] [Google Scholar]
- 150.Zheng T., Bian F., Chen L., Wang Q., Jin S. Beneficial effects of Rhodiola and salidroside in diabetes: potential role of AMP-activated protein kinase. Mol. Diagn. Ther. 2019;23 doi: 10.1007/s40291-019-00402-4. [DOI] [PubMed] [Google Scholar]
- 151.Hu R., Wang M., Ni S., Wang M., Liu L., You H., Wu X., Wang Y., Lu L., Wei L. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-κB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharmacol. 2020;867 doi: 10.1016/j.ejphar.2019.172797. [DOI] [PubMed] [Google Scholar]
- 152.Sabayan B., Westendorp R.G., van der Grond J., Stott D.J., Sattar N., van Osch M.J.P., van Buchem M.A., de Craen A.J.M. Markers of endothelial dysfunction and cerebral blood flow in older adults. Neurobiol. Aging. 2014;35 doi: 10.1016/j.neurobiolaging.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 153.Sun L., Kanwar Y.S. Relevance of TNF-α in the context of other inflammatory cytokines in the progression of diabetic nephropathy. Kidney Int. 2015;88 doi: 10.1038/ki.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang C., Wang Q., Lou Y., Xu J., Feng Z., Chen Y., Tang Q., Zheng G., Zhang Z., Wu Y., Tian N., Zhou Y., Xu H., Zhang X. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J. Cell Mol. Med. 2017 doi: 10.1111/jcmm.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ullah A., Munir S., Mabkhot Y., Badshah S. Bioactivity profile of the diterpene isosteviol and its derivatives. Molecules. 2019;24:678. doi: 10.3390/molecules24040678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chatsudthipong V., Muanprasat C. Stevioside and related compounds: therapeutic benefits beyond sweetness. Pharmacol. Ther. 2009;121:41–54. doi: 10.1016/j.pharmthera.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 157.Lazzari E., Jefferies C.A. IRF5-mediated signaling and implications for SLE. Clin. Immunol. 2014;153:343–352. doi: 10.1016/j.clim.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 158.Khoyratty T.E., Udalova I.A. Diverse mechanisms of IRF5 action in inflammatory responses. Int. J. Biochem. Cell Biol. 2018;99:38–42. doi: 10.1016/j.biocel.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 159.Stempel M., Chan B., Juranić Lisnić V., Krmpotić A., Hartung J., Paludan S.R., Füllbrunn N., Lemmermann N.A., Brinkmann M.M. The herpesviral antagonist m152 reveals differential activation of STING ‐dependent IRF and NF ‐κB signaling and STING ’s dual role during MCMV infection. EMBO J. 2019;38 doi: 10.15252/embj.2018100983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wei F., Zhu H., Li N., Yu C., Song Z., Wang S., Sun Y., Zheng L., Wang G., Huang Y., Bao Y., Sun L. Stevioside activates AMPK to suppress inflammation in macrophages and protects mice from LPS-induced lethal shock. Molecules. 2021;26:858. doi: 10.3390/molecules26040858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Siang D.T.C., Lim Y.C., Kyaw A.M.M., Win K.N., Chia S.Y., Degirmenci U., Hu X., Tan B.C., Walet A.C.E., Sun L., Xu D. The RNA-binding protein HuR is a negative regulator in adipogenesis. Nat. Commun. 2020;11:213. doi: 10.1038/s41467-019-14001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xiao K., Yang L., Gao X., An Y., Xie W., Jingquan G. HuR affects proliferation and apoptosis of chronic lymphocytic leukemia cells via NF- κ B pathway. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/1481572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yiakouvaki A., Dimitriou M., Karakasiliotis I., Eftychi C., Theocharis S., Kontoyiannis D.L. Myeloid cell expression of the RNA-binding protein HuR protects mice from pathologic inflammation and colorectal carcinogenesis. J. Clin. Invest. 2012;122 doi: 10.1172/JCI45021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Su M., He Y., Xue S., Yu J., Ren X., Huang N., Abdullahi R., Xu M. Butyric acid alleviated chronic intermittent hypoxia-induced lipid formation and inflammation through up-regulating HuR expression and inactivating AMPK pathways. Biosci. Rep. 2021;41 doi: 10.1042/BSR20203639. BSR20203639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jung T.W., Park H.S., Choi G.H., Kim D., Lee T. β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci. 2018;25:27. doi: 10.1186/s12929-018-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Prieto-Hontoria P.L., Fernández-Galilea M., Pérez-Matute P., Martínez J.A., Moreno-Aliaga M.J. Lipoic acid inhibits adiponectin production in 3T3-L1 adipocytes. J. Physiol. Biochem. 2013;69 doi: 10.1007/s13105-012-0230-7. [DOI] [PubMed] [Google Scholar]
- 167.Cameron A.R., Morrison V.L., Levin D., Mohan M., Forteath C., Beall C., McNeilly A.D., Balfour D.J.K., Savinko T., Wong A.K.F., Viollet B., Sakamoto K., Fagerholm S.C., Foretz M., Lang C.C., Rena G. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ. Res. 2016;119:652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Duca F.A., Côté C.D., Rasmussen B.A., Zadeh-Tahmasebi M., Rutter G.A., Filippi B.M., Lam T.K.T. Metformin activates a duodenal Ampk–dependent pathway to lower hepatic glucose production in rats. Nat. Med. 2015;21:506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.-L., Angus D.C. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Q., Tong D., Liu G., Gao J., Wang L., Xu J., Yang X., Xie Q., Huang Y., Pang J., Wang L., He Y., Zhang D., Ma Q., Lan W., Jiang J. Metformin inhibits prostate cancer progression by targeting tumor-associated inflammatory infiltration. Clin. Cancer Res. 2018;24:5622–5634. doi: 10.1158/1078-0432.CCR-18-0420. [DOI] [PubMed] [Google Scholar]
- 171.Song H., Zhang X., Zhai R., Liang H., Song G., Yuan Y., Xu Y., Yan Y., Qiu L., Sun T. Metformin attenuated sepsis-associated liver injury and inflammatory response in aged mice. Bioengineered. 2022;13:4598–4609. doi: 10.1080/21655979.2022.2036305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hsiang J.C., Wong V.W.-S. SGLT2 inhibitors in liver patients. Clin. Gastroenterol. Hepatol. 2020;18:2168–2172.e2. doi: 10.1016/j.cgh.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 173.Fitchett D., Inzucchi S.E., Cannon C.P., McGuire D.K., Scirica B.M., Johansen O.E., Sambevski S., Kaspers S., Pfarr E., George J.T., Zinman B. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation. 2019;139:1384–1395. doi: 10.1161/CIRCULATIONAHA.118.037778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cowie M.R., Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020;17:761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 175.Ma Y., Zhang G., Kuang Z., Xu Q., Ye T., Li X., Qu N., Han F., Kan C., Sun X. Empagliflozin activates Sestrin2-mediated AMPK/mTOR pathway and ameliorates lipid accumulation in obesity-related nonalcoholic fatty liver disease. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.944886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu L., Nagata N., Nagashimada M., Zhuge F., Ni Y., Chen G., Mayoux E., Kaneko S., Ota T. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–149. doi: 10.1016/j.ebiom.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hawley S.A., Ford R.J., Smith B.K., Gowans G.J., Mancini S.J., Pitt R.D., Day E.A., Salt I.P., Steinberg G.R., Hardie D.G. The Na+/Glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65:2784–2794. doi: 10.2337/db16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bai L., Sun C., Zhai H., Chen C., Hu X., Ye X., Li M., Fang Y., Yang W., Wang H., Sun S. Investigation of urinary Sestrin2 in patients with obstructive sleep apnea. Lung. 2019;197:123–129. doi: 10.1007/s00408-019-00205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mandard S., Müller M., Kersten S. Peroxisome proliferator-activated receptor a target genes. Cell. Mol. Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Forcina G.C., Dixon S.J. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics. 2019;19 doi: 10.1002/pmic.201800311. [DOI] [PubMed] [Google Scholar]
- 181.Koyani C.N., Kitz K., Rossmann C., Bernhart E., Huber E., Trummer C., Windischhofer W., Sattler W., Malle E. Activation of the MAPK/Akt/Nrf2-Egr1/HO-1-GCLc axis protects MG-63 osteosarcoma cells against 15d-PGJ2-mediated cell death. Biochem. Pharmacol. 2016;104:29–41. doi: 10.1016/j.bcp.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Obara N., Fukushima K., Ueno Y., Wakui Y., Kimura O., Tamai K., Kakazu E., Inoue J., Kondo Y., Ogawa N., Sato K., Tsuduki T., Ishida K., Shimosegawa T. Possible involvement and the mechanisms of excess trans-fatty acid consumption in severe NAFLD in mice. J. Hepatol. 2010;53:326–334. doi: 10.1016/j.jhep.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 183.Park H., Shima T., Yamaguchi K., Mitsuyoshi H., Minami M., Yasui K., Itoh Y., Yoshikawa T., Fukui M., Hasegawa G., Nakamura N., Ohta M., Obayashi H., Okanoue T. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J. Gastroenterol. 2011;46:101–107. doi: 10.1007/s00535-010-0291-8. [DOI] [PubMed] [Google Scholar]
- 184.Kim S.H., Kim G., Han D.H., Lee M., Kim I., Kim B., Kim K.H., Song Y.-M., Yoo J.E., Wang H.J., Bae S.H., Lee Y.-H., Lee B.-W., Kang E.S., Cha B.-S., Lee M.-S. Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition. Autophagy. 2017;13:1767–1781. doi: 10.1080/15548627.2017.1356977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Salminen A., Kaarniranta K., Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging. 2012;4:166–175. doi: 10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.López M. Hypothalamic AMPK: a golden target against obesity? Eur. J. Endocrinol. 2017;176 doi: 10.1530/EJE-16-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tanida M., Yamamoto N., Shibamoto T., Rahmouni K. Involvement of hypothalamic AMP-activated protein kinase in leptin-induced sympathetic nerve activation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rahmouni K., Sigmund C.D., Haynes W.G., Mark A.L. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58 doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Whittle A.J., Carobbio S., Martins L., Slawik M., Hondares E., Vázquez M.J., Morgan D., Csikasz R.I., Gallego R., Rodriguez-Cuenca S., Dale M., Virtue S., Villarroya F., Cannon B., Rahmouni K., López M., Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149 doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Martínez De Morentin P.B., González-García I., Martins L., Lage R., Fernández-Mallo D., Martínez-Sánchez N., Ruíz-Pino F., Liu J., Morgan D.A., Pinilla L., Gallego R., Saha A.K., Kalsbeek A., Fliers E., Bisschop P.H., Diéguez C., Nogueiras R., Rahmouni K., Tena-Sempere M., López M. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metabol. 2014;20 doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.López M., Tena-Sempere M. Estradiol effects on hypothalamic AMPK and BAT thermogenesis: a gateway for obesity treatment? Pharmacol. Ther. 2017;178 doi: 10.1016/j.pharmthera.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 192.Stevanovic D., Janjetovic K., Misirkic M., Vucicevic L., Sumarac-Dumanovic M., Micic D., Starcevic V., Trajkovic V. Intracerebroventricular administration of metformin inhibits ghrelin-induced hypothalamic AMP-kinase signalling and food intake. Neuroendocrinology. 2012;96 doi: 10.1159/000333963. [DOI] [PubMed] [Google Scholar]
- 193.Nguyen L.V., Nguyen K.D.A., Ma C.T., Nguyen Q.T., Nguyen H.T., Yang D.J., Le Tran T., Kim K.W., Doan K.V. P-coumaric acid enhances hypothalamic leptin signaling and glucose homeostasis in mice via differential effects on AMPK activation. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22031431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kang S.W., Il Kang S., Shin H.S., Yoon S.A., Kim J.H., Ko H.C., Kim S.J. Sasa quelpaertensis Nakai extract and its constituent p-coumaric acid inhibit adipogenesis in 3T3-L1 cells through activation of the AMPK pathway. Food Chem. Toxicol. 2013;59 doi: 10.1016/j.fct.2013.06.033. [DOI] [PubMed] [Google Scholar]