Abstract
Nasopharyngeal carcinoma (NPC), which is closely associated with the Epstein-Barr virus (EBV), is a highly metastatic malignant tumor. An important activity in tumor invasion and metastasis is that of the 92-kDa type IV collagenase or gelatinase, matrix metalloproteinase 9 (MMP-9), which mediates the degradation of the basement membrane and extracellular matrix. The expression of MMP-9 has been shown to be enhanced by the EBV oncoprotein, latent membrane protein 1 (LMP-1). LMP-1, which is expressed in NPC, has two essential signaling domains within the carboxy terminus, termed C-terminal activation regions 1 (CTAR-1) and CTAR-2. This study reveals that either signaling domain can activate the MMP-9 promoter and induce MMP-9 activity; however, LMP-1 deletion mutants lacking either CTAR-1 or CTAR-2 had a decreased ability to induce MMP-9 expression. The deletion of both activation regions completely abolished the induction of MMP-9 activity, while the cotransfection of both the CTAR-1 and CTAR-2 deletion mutants restored MMP-9 activity to levels produced by wild-type LMP-1. The NF-κB and activator protein 1 (AP-1) binding sites in the MMP-9 promoter were essential for the activation of MMP-9 gene expression by both CTAR-1 and CTAR-2. The induction of MMP-9 expression by LMP-1 and both CTAR-1 and CTAR-2 mutants was blocked by the overexpression of IκB. The tumor necrosis factor receptor-associated factor (TRAF) pathway also contributed to the activation of the MMP-9 promoter as shown by the use of TRAF-2 and TRAF-3 dominant-negative constructs. These data indicate that the activation of both the NF-κB and AP-1 pathways by LMP-1, CTAR-1, and CTAR-2 is necessary for the activation of MMP-9 expression. In NPC, LMP-1 may contribute to invasiveness and metastasis through the induction of MMP-9 transcription and enzymatic activity.
Epstein-Barr virus (EBV), a ubiquitous human gamma herpesvirus, is associated with several malignant tumors such as endemic Burkitt’s lymphoma, Hodgkin’s disease, and nasopharyngeal carcinoma (NPC) (23, 46, 49, 60). EBV establishes a latent infection in human B lymphocytes, and infection in vitro results in immortalization (25). Latent membrane protein 1 (LMP-1) is considered the principal oncoprotein of EBV and is essential for lymphocyte immortalization (21). LMP-1 expression has also been detected in rare examples of preinvasive NPC lesions, suggesting that LMP-1 expression is an important contributor to the development of NPC (44). LMP-1 is an integral membrane protein consisting of 386 amino acids (aa). Six transmembrane spanning regions (162 aa) connect a short N-terminal cytoplasmic domain (24 aa) with a long C-terminal cytoplasmic domain (200 aa) (10). Mutational analysis has identified two activation domains in the C terminus of LMP-1: C-terminal activation region 1 (CTAR-1) (residues 187 to 231) and CTAR-2 (residues 351 to 386) (14, 37). LMP-1 associates with the tumor necrosis factor receptor family-associated factors (TRAFs) through a TRAF interaction domain within CTAR-1 (8, 30, 34, 35). TRAF-2 is of particular interest as it mediates the activation of the transcriptional factor NF-κB, following interaction with LMP-1 (8, 22, 34, 48). TRAF-1 and TRAF-3 strongly associate with CTAR-1 and modulate the activation of NF-κB (6, 34, 36, 50). CTAR-2 is a stronger activator of NF-κB than CTAR-1 in reporter assays (11, 14, 37) and has recently been shown to interact with the tumor necrosis factor receptor adaptor protein TRADD (19). Several studies have indicated that LMP-1 activates the c-Jun N-terminal kinase (JNK) pathway through CTAR-2 but not CTAR-1 (9, 12, 24).
NPC is a highly metastatic and invasive malignant tumor in which the EBV genes encoding LMP-1, LMP-2A and -2B, and EBNA-1 are expressed. Essential steps in the process of tumor invasion and metastasis include the degradation of the extracellular matrix (ECM) and basement membrane (BM). The invasion of the BM by tumor cells is thought to be one of the critical steps in metastasis, which includes sequential multistep processes (26, 40). Many proteolytic enzymes degrade components of the ECM and BM (39, 45). Among these, the matrix metalloproteinases (MMPs) are attractive candidates for enzymes required for tumor metastasis. The MMPs contain a zinc ion at their active sites and can degrade native collagens and other ECM components (27, 31). The MMP family includes four types of collagenase (MMP-1, -8, -13, and -18), three types of stromelysin (MMP-3, -10, and -11), and the 72- and 92-kDa type IV gelatinases or collagenases (MMP-2 and MMP-9) (18). Several membrane-type MMPs that activate pro-MMP-2 to activate MMP2 have also been identified recently (51, 58). MMP activity is tightly regulated by the following steps: (i) control of gene transcription, (ii) activation of the latent form of the enzyme to its active form by eliminating an N-terminal peptide, and (iii) regulation by endogenous proteins known as tissue inhibitors of metalloproteinases (7, 32). As type IV collagen is one of the integral components of BM, the uncontrolled expression of two type IV collagenases, MMP-2 and MMP-9, is believed to play a critical role in the invasion of BM by tumor cells (28). MMP-2 and MMP-9 are often expressed by tumor cells, but their expression is not always coordinated with that of MMP-1 and MMP-3 (52). The release of MMP-2 and/or MMP-9 has been associated with metastasis in a variety of model systems (2, 13, 55–57). The expression of MMP-2 and that of MMP-9 are not necessarily linked, which suggests an independent expression pattern for both proteinases (42). The promoters for MMP-2 and MMP-9 differ markedly, with the MMP-9 promoter having several putative activator protein 1 (AP-1) and NF-κB binding sites not found in the MMP-2 promoter (15, 16, 53). Pro-MMP-2 is activated to MMP-2 by membrane-type MMPs (51, 58); however, activators of MMP-9 expression or activity have not been reported.
We have recently shown that the expression of MMP-9, but not that of MMP-2, was induced by the EBV oncoprotein LMP-1 (59). This study reveals that both LMP-1 activation domains, CTAR-1 and CTAR-2, contribute to the full activation of the MMP-9 promoter and the induction of MMP-9 enzymatic activity through the activation of the NF-κB and AP-1 transcription factors.
MATERIALS AND METHODS
Cell lines.
C33A epithelial cells, derived from a human cervical carcinoma, were grown at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Sigma) and antibiotics.
Plasmids.
The LMP-1 open reading frame was subcloned downstream of the cytomegalovirus (CMV) immediate-early promoter into the EcoRI site of pcDNA3. A series of 5′ flanking sequences of MMP-9 were inserted upstream of the chloramphenicol acetyltransferase (CAT) reporter gene as described previously (52). The CMV immediate-early promoter-driven IκB expression plasmid was obtained from Albert Baldwin (54). Constructs TRAF-2 dominant negative (DN), containing aa 98 to 501, and TRAF-3DN, containing aa 345 to 568, were cloned into the pSG5 vector, which contains the simian virus 40 early promoter and intron sequences from the rabbit β-globin gene (Stratagene). All the LMP-1 mutants were cloned into the EcoRI site of the pcDNA3 expression vector and have been previously described (34, 36).
Transient transfection and conditioned media.
The transfection of C33A cells was carried out with 5 × 105 cells per 60-mm-diameter dish with the use of Lipofectamine (GIBCO/BRL) following the manufacturer’s protocol. Five micrograms of appropriate reporter and effector plasmids were transfected. Transfected cells were cultured in DMEM with 10% fetal bovine serum overnight and then in a serum-free medium (OPTI-MEM I; GIBCO/BRL) without antibiotics for 5 h at 37°C. Transfection efficiency was monitored by cotransfection with a β-galactosidase reporter construct.
Western blot analysis.
C33A cells were harvested 48 h after transfection with FLAG-LMP mutants. Whole cell extracts were prepared by washing cells once in cold phosphate-buffered saline solution and then lysing them in 500 μl of lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 0.5% Nonidet P-40, 5 mM dithiothreitol, 0.2 mM Na orthovanadate, 100 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 5 μg of leupeptin per ml) with repeated freezing and thawing. The supernatant fluid was clarified by centrifugation and was stored at −80°C until use. After sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, the proteins were transferred to NitroPlus membranes (Micron Separations Inc.) with the Hoefer semidry transfer apparatus. Nonspecific reactivity was blocked by incubation overnight in Tris-buffered saline solution containing 0.1% Tween 20 and 5% nonfat dried milk. The membrane was then incubated with a primary antibody to FLAG protein (Santa Cruz Biotechnology, Inc.; 1:200 dilution [34]). A secondary antibody (1:2,000 dilution) was used to detect the bound primary antibody. The reactive protein was detected by enhanced chemiluminescence (Amersham).
CAT reporter assay.
CAT assays were performed with extracts of C33A cells after transient transfection. The construction of the MMP-9 promoter reporter series has been described previously (52). Cells were incubated 48 h after transfection in DMEM with 10% fetal bovine serum and antibiotics and then harvested; acetylated [14C]chloramphenicol was quantitated with a PhosphorImager (Molecular Dynamics). The data were evaluated by comparison with the transfection efficiency of β-galactosidase.
Gelatin zymography.
MMP-2 and MMP-9 were assayed for gelatinolytic activity by means of gelatin zymography as reported previously (59). The conditioned medium was mixed with an SDS sample buffer (50 mM Tris-HCl [pH 6.8], 10% glycerol, 1% SDS, 0.01% bromophenol blue) in the absence of a reducing agent to denature MMPs and to dissociate any complexes with tissue inhibitors of metalloproteinases. The mixture was then incubated at 37°C for 20 min, and SDS-polyacrylamide gel electrophoresis (containing gelatin at a final concentration of 0.1%) was performed. After electrophoresis, the gel was rinsed in 2.5% Triton X-100 for 1 h and then incubated for 24 h at 37°C in a solution containing 50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 10 mM CaCl2, and 0.02% NaN3. The MMPs were identified following staining of the gel in 0.1% Coomassie blue R250 (Sigma) dissolved in 40% methanol–10% acetic acid and destaining in the same solution without Coomassie blue. Gelatinolytic activity was visualized as a clear band against a dark background of stained gelatin. This is the most sensitive method for the identification of MMP-2 and MMP-9. MMP-2 is detected by the clear band appearing at 72 kDa, and MMP-9 is detected by the one at 92 kDa (18, 32, 51, 58).
Electrophoretic mobility shift assay (EMSA).
Nuclear extracts were prepared from C33A cells transfected with LMP-1 and its mutant-expressing plasmids (43). Synthetic oligonucleotides used for probes were identical to the NF-κB or AP-1 nuclear factor binding sequences in the promoter region of MMP-9 (52). The oligonucleotides were labeled with [α-32P]CTP with the use of Klenow DNA polymerase. The unlabeled oligonucleotides were used for competition. Nuclear extracts were incubated in a buffer containing 12 mM HEPES, 12% glycerol, 4 mM Tris-HCl (pH 7.9), 1 mM EDTA, and 3 μg of poly(dI-dC) with the probe labeled at a rate of 50,000 cpm. The mixture was analyzed on a 4.8% polyacrylamide gel in 0.5× TBE buffer (90 mM Tris–64.6 mM boric acid–2.5 mM EDTA, pH 8.3).
RESULTS
LMP-1 deletion mutants and polypeptides.
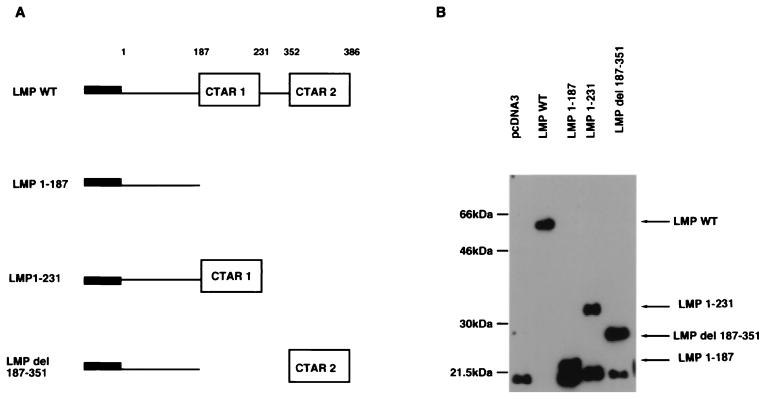
The panel of mutants used in this study is shown schematically in Fig. 1A (14, 37). These constructs were cloned into the pcDNA3 vector (Invitrogen), and a FLAG epitope was inserted at the amino terminus to facilitate the detection of protein expression. The expression of proteins of expected size from LMP-1 and the mutated constructs was verified by transfecting each plasmid into EBV-negative C33A cells and assaying by Western blotting with the FLAG antibody. As illustrated in Fig. 1B, all mutant LMP-1 polypeptides were expressed.
FIG. 1.
(A) Schematic representation of wild-type (WT) and mutant LMP-1 proteins. LMP-1 consists of a 23-aa N-terminal cytoplasmic domain, six hydrophobic transmembrane domains, and a 200-aa C-terminal cytoplasmic domain, in which two regions important for NF-κB activation and phenotypic changes have been identified, CTAR-1 (residues 187 to 231) and CTAR-2 (residues 352 to 386). TRAFs interact with CTAR-1 but not with CTAR-2. LMP 1-187 and LMP 1-231 mutants contain stop codons following amino acids 187 and 231, respectively. The LMP 1-187 mutant has the entire carboxy-terminal domain deleted, while LMP 1-231 has only CTAR-1 and LMP del 187-351 has only CTAR-2. Each plasmid contains a FLAG expression sequence in the amino acid terminus. (B) Immunoblot analysis of FLAG–LMP-1 wild-type (WT) and mutant proteins. The FLAG–LMP-1 mutant proteins are identified after transfection into C33A cells.
MMP-9 activity in cells transfected with LMP-1 deletion mutants.
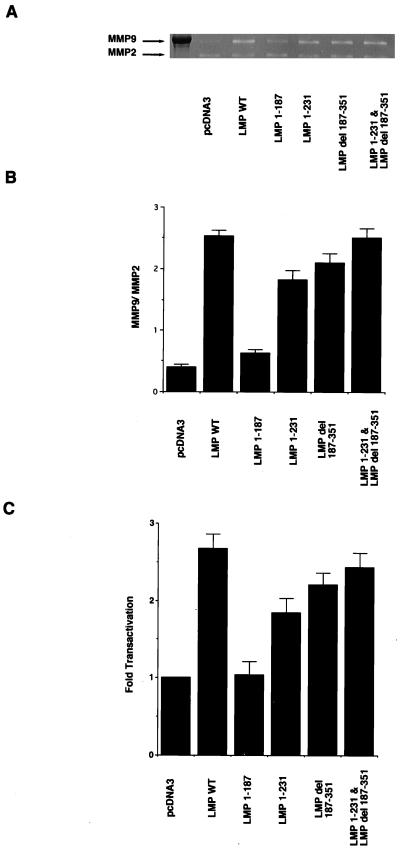
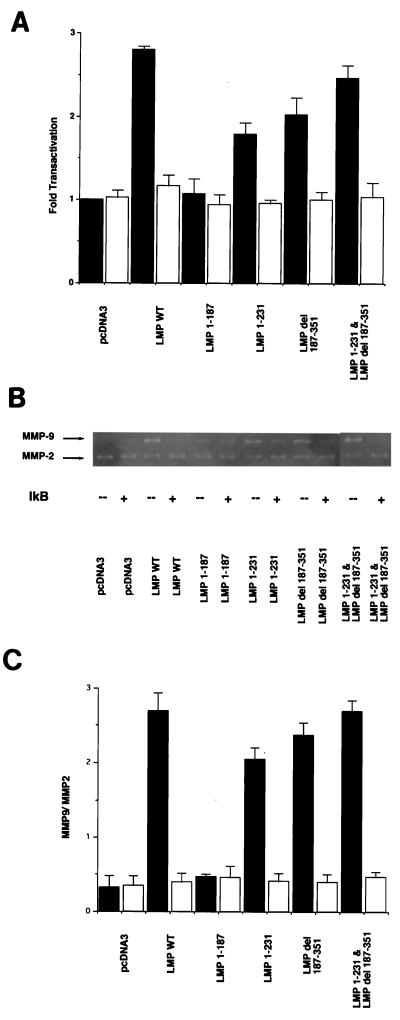
Analysis of MMP gelatinolytic activity revealed an increase in MMP-9 activity in C33A cells transfected with wild-type LMP-1 (Fig. 2A). The ratio of MMP-9/MMP-2 activity, as measured by reverse imaging and densitometric analysis (5), indicated that LMP-1 enhanced activity approximately sixfold above that of the control (Fig. 2B). The LMP-1 mutant lacking the entire carboxy terminus (LMP 1-187) was completely unable to induce MMP-9 activity. The mutants that retained either CTAR-1 (LMP 1-231) or CTAR-2 (LMP del 187-351) had reduced levels of MMP-9 gelatinolytic activity with 66 and 84% of the activity of LMP1, respectively. Interestingly, the coexpression of the deletion mutants LMP 1-231 and LMP del 187-351 restored full activity, suggesting that both domains contribute additively to MMP-9 activation. The levels of the 72-kDa MMP-2 activity were unchanged by LMP-1 or any of the mutants (Fig. 2A).
FIG. 2.
MMP-9 and MMP-2 activity in C33A cells transfected with LMP-1 and deletion mutants. (A) Gelatin zymography. Samples of C33A cells were prepared as described in Materials and Methods. WT, wild type. (B) Ratio of MMP-9/MMP-2 activity as measured by reverse imaging the data from gelatin zymography and densitometric analysis. Solid bars represent the ratio of each LMP-1 and deletion mutant. The mean values and standard deviations (error bars) are the results of five experiments. (C) CAT assays. The effector plasmids were cotransfected with full-length MMP-9 promoter CAT. The data were compared with results from assays of transfection efficiency of β-galactosidase, and activities were given relative to the activity of pcDNA3, which was defined as 1. The mean values and standard deviations (error bars) represented were obtained from five experiments.
Analyses of the MMP-9 promoter revealed that LMP-1 activated the transcription of MMP-9 expression construct with a 2.6-fold increase over the vector control. LMP 1-187 did not transactivate the MMP-9 promoter. The deletion mutants LMP 1-231 and LMP del 187-351 had reduced transactivation, with LMP 1-231 and LMP del 187-351 retaining 50 and 72% of LMP1 transactivation, respectively (Fig. 2C). These data indicated a close correlation between the direct measurement of MMP-9 enzyme activity and the transactivation of the MMP-9 promoter. These experiments also show that either activation region of LMP-1, CTAR-1 or CTAR-2, can activate the MMP-9 promoter and induce MMP-9 activity; however, both domains are required for maximal activity.
Identification of LMP-1-induced transcription factors that activate the MMP-9 promoter.
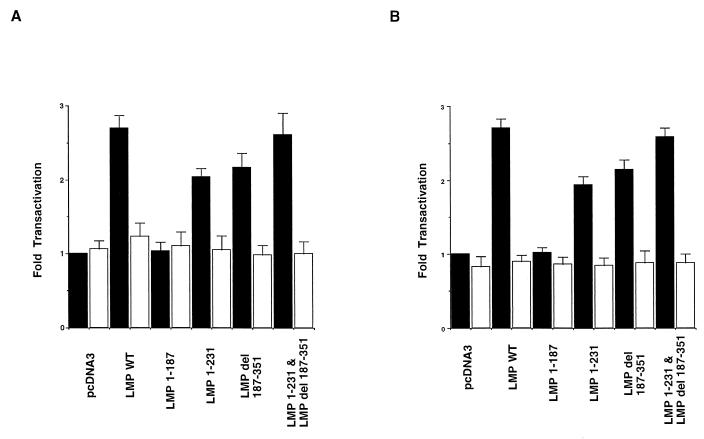
MMP activity is regulated by the control of gene transcription and also by posttranslational control. The MMP-9 promoter is primarily regulated by NF-κB, AP-1, and, to a lesser extent, secretory protein 1 (7, 32, 59). To determine the contribution of the CTAR-1 and CTAR-2 domains to the activation of NF-κB and AP-1 in the MMP-9 promoter, MMP-9 promoter constructs containing point mutations in the NF-κB or AP-1 sites were cotransfected with LMP-1 or the LMP-1 mutants. The mutation of the NF-κB site slightly increased the basal activity of the promoter by approximately 5% (Fig. 3A), while the mutation of the AP-1 site reduced basal activity by 16% (Fig. 3B). The mutation of the NF-κB site (Fig. 3A) or the AP-1 site (Fig. 3B) abolished transactivation by LMP-1 and both of the LMP-1 mutants. These data indicate that both the NF-κB and AP-1 binding sites are necessary for the activation of the MMP-9 promoter and that both LMP-1 activation regions mediate transactivation through NF-κB and AP-1.
FIG. 3.
Mutational analysis of the cis elements required for LMP-1 and deletion mutants induced MMP-9 promoter activity. (A) Solid and open bars represent CAT activity of the wild-type (WT) MMP-9 promoter and the mutated NF-κB binding site in the MMP-9 promoter in C33A cells. The data were compared with the transfection efficiency of β-galactosidase, and activities were given relative to the activity of pcDNA3 with wild-type MMP-9 promoter, which was defined as 1. The mean values and standard deviations (error bars) are the results of five experiments. (B) Solid and open bars represent CAT activities of wild-type (WT) MMP-9 promoter and the mutated AP-1 binding site in the MMP-9 promoter, respectively, in C33A cells. The data were evaluated by the same method as that described for panel A.
As reported previously, LMP-1 induces nuclear factors that bind to NF-κB and AP-1 sequences in the MMP-9 promoter region (59). Through the interaction of the TRAFs with CTAR-1 and that of TRADD with CTAR-2, LMP-1 activates NF-κB inducing kinase and JNK (1, 9, 24). Previous studies have indicated that CTAR-2 is the more potent activator of NF-κB (11, 14, 37) and that only CTAR-2 can activate JNK (9, 24). However, NF-κB activation by both domains is partially inhibited by a dominant-negative deletion mutant of TRAF-2, TRAF-2DN, suggesting that TRAF-2 is a common mediator for NF-κB activation (22).
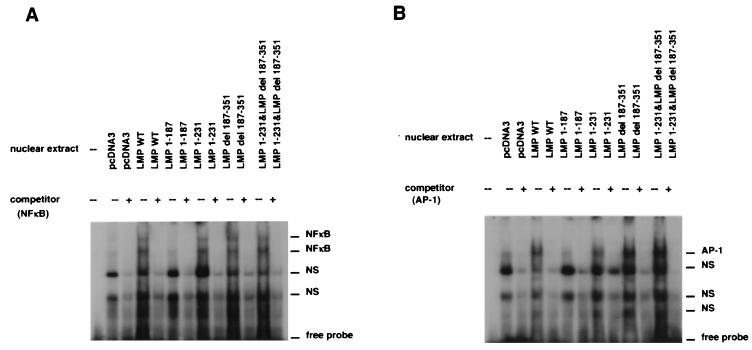
As the mutational analysis of the MMP-9 promoter indicated that both domains activated transcription through both the NF-κB and AP-1 sites, it was important to identify the nuclear factors that bind to these sites. With the NF-κB sequence in the MMP-9 promoter used as the probe in an EMSA, NF-κB activity was detected in C33A cells transfected with LMP-1, LMP 1-231 (CTAR-1 only), LMP del 187-351 (CTAR-2 only), and LMP 1-231 combined with LMP del 187-351 but not with LMP 1-187 (Fig. 4A). Two predominant shifted bands were detected with all constructs. These data confirmed that NF-κB is efficiently activated by both CTAR-1 and CTAR-2 (36).
FIG. 4.
Induction of NF-κB (A) and AP-1 (B) DNA-binding activity in cells transfected with wild-type (WT) LMP-1 and deletion mutants. Nuclear extracts from C33A cells transfected with LMP-1 mutants were mixed with either NF-κB or AP-1 32P-labeled probes. Excesses of nonlabeled NF-κB and AP-1 probes (×100) were used as competitors (NFκB, 5′-GATCGGGTTGCCCCAGTGGAATTCCCCAGCCTT-3′; AP-1, 5′-GATCTTCTAGACCGGATGAGTCATAGCTG-3′). Underlined letters indicate binding sequences in the promoter of the MMP-9 gene. NS, nonspecific binding.
In agreement with the promoter mutational analyses, an increase in binding to the AP-1 sequence was also detected with the same set of constructs (Fig. 4B). Previous studies have indicated that only CTAR-2 activates JNK, and these data also show that a greater amount of AP-1, detected by EMSA, is induced by CTAR-2 (9, 24). However, both LMP-1 activation regions activated AP-1 binding to the MMP-9 promoter and required this site for transactivation (Fig. 3).
Regulation of the MMP-9 promoter through TRAF signaling.
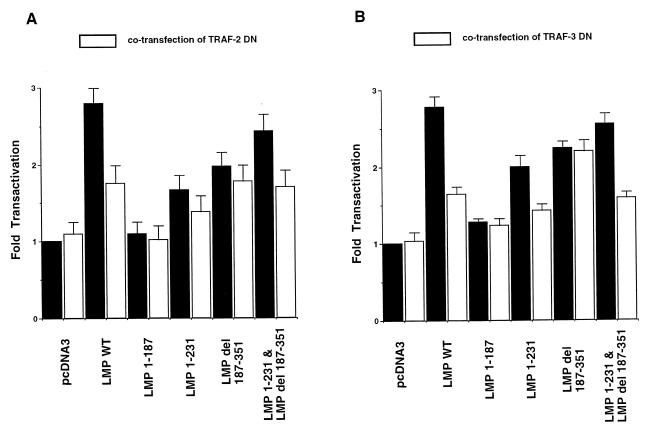
In order to determine the involvement of TRAFs in mediating MMP-9 activation induced by the LMP-1 mutants, C33A cells were transiently cotransfected with LMP-1 mutants and plasmids expressing dominant-negative forms of TRAF-2 (TRAF-2DN) or TRAF-3 (TRAF-3DN). The expression of TRAF-2DN has previously been shown to partially inhibit signaling from both CTAR-1 and CTAR-2 (22). In this study, TRAF-2DN reduced the activation by LMP-1 by 58%. TRAF2-DN reduced LMP 1-231 by approximately 43% and LMP del 187-351 by 20%. TRAF-2DN also reduced MMP-9 promoter transactivation by the combined mutants (LMP 1-231 plus LMP del 187-351) by 50% (Fig. 5A).
FIG. 5.
The effect of transient expression of TRAF-2DN or TRAF-3DN on MMP-9 transcriptional activity induced by wild-type (WT) LMP-1 or deletion mutants. Solid bars represent the CAT activity of LMP-1 and deletion mutants, while open bars represent the CAT activity when cells were cotransfected with TRAF2-DN (A) and TRAF-3DN (B). The data were compared with the transfection efficiency as determined by β-galactosidase assay, and activities were given relative to the activity of pcDNA3 without TRAF-DN, which was defined as 1. The mean values and standard deviations (error bars) are the results of five experiments.
Previous studies have shown that the activation of NF-κB by CTAR-1 but not CTAR-2 is inhibited by the expression of TRAF-3DN (6, 22, 34). In this study, TRAF-3DN reduced the transactivation of the MMP-9 promoter induced by LMP-1, LMP 1-231, and the reconstruction plasmids by approximately 60% but did not affect transactivation by LMP del 187-351 (Fig. 5B). These data indicate that signaling from CTAR-1 is mediated through both TRAF-2 and TRAF-3, that TRAF2 contributes to signaling from CTAR-2, and that both TRAF-2 and TRAF-3 contribute to the activation of the MMP-9 promoter.
IκB inhibits MMP-9 expression.
The activation of NF-κB and NF-κB binding is necessary for the activation of the MMP-9 promoter (6, 22, 30, 34, 48). Therefore, the inhibitory effect of a constitutively activated form of the NF-κB repressor, IκB (17), on the induction of MMP-9 expression by the LMP mutants was determined. In assays performed with the CAT reporter construct, the expression of IκB abolished the induction of the MMP-9 promoter by LMP-1 and the LMP-1 mutants (Fig. 6A). Analysis of MMP-9 activity detected by gelatin zymography also indicated that the cotransfection of the IκB plasmid with the LMP mutants repressed MMP-9 gelatinolytic activity but did not affect the activity of MMP-2 (Fig. 6B and C). The activation of MMP-9 assessed by gelatin zymography correlated with the activation of the MMP-9 promoter. These data confirm that the activation of NF-κB is necessary to activate the MMP-9 promoter.
FIG. 6.
IκB inhibits the enhancement of MMP-9 expression by LMP-1 or deletion mutants. (A) CAT assays. The effector plasmids were cotransfected with wild-type MMP-9 promoter CAT. Solid bars represent CAT activity of wild-type (WT) LMP-1 or deletion mutants in the absence of the IκB effector plasmid. Open bars represent CAT activity in the presence of the IκB effector plasmid. The data were compared with the transfection efficiency of β-galactosidase, and activities were given relative to the activity of pcDNA3 without the IκB effector plasmid, which was defined as 1. The mean values and standard deviations (error bars) are the results of five experiments. (B) Gelatin zymography. MMP-9 and MMP-2 activity in C33A cells transfected with wild-type (WT) LMP-1 or mutants in the presence (+) or absence (−) of the IκB effector plasmid. (C) Ratio of MMP9/MMP2 activity as shown in Fig. 2B. Solid bars represent the ratio of wild-type (WT) LMP-1 or deletion mutants in the absence of IκB, while open bars show the ratio in the presence of IκB. The data shown are the mean values and standard deviations (error bars) of five independent experiments.
DISCUSSION
LMP-1 is essential for the transformation of B lymphocytes and has profound effects on cellular gene expression (4, 38). In addition LMP-1 can activate the type IV collagenase MMP-9, which is implicated in tumor invasion of BM (59). Whether this effect is unique among the oncogenic viruses is unknown. The two activation domains of LMP-1, CTAR-1 and CTAR-2, both activate NF-κB yet also have distinct properties (14, 34, 37). CTAR-1, which interacts with TRAFs, induces the expression of the epidermal growth factor receptor through a pathway distinct from NF-κB activation, as epidermal growth factor receptor expression is not induced by CTAR-2 (34). In contrast, CTAR-2 has a greater ability to activate NF-κB in reporter gene assays (14, 34, 37) and is thought to be responsible for JNK activation by LMP-1 (9, 24). As LMP-1 also induces the expression of MMP-9, which is known to be regulated by NF-κB and AP-1 (59), it was of interest to determine the contribution of CTAR-1 and CTAR-2 to this transactivation. The data presented here reveal that both CTAR-1 and CTAR-2 of LMP-1 can activate the MMP-9 promoter and induce MMP-9 activity and that the domains have an additive effect for transactivation. These results suggest that the complete activation of the MMP-9 promoter by LMP-1 requires both CTAR-1 and CTAR-2, which can be present on separate molecules. It is likely that the oligomerization of LMP-1, mediated by the transmembrane domain, results in complexes that contain both signaling domains, albeit on separate molecules.
Although previous studies have suggested that JNK activation is mediated through CTAR-2 (9, 24), in this study both CTAR-1 and CTAR-2 induced AP-1, as detected by EMSA, with CTAR-2 inducing a greater amount. CTAR-1 interacts with TRAF-2 (8, 30, 34, 35), CTAR-2 interacts with TRADD, which binds TRAF-2 (19), and both domains are partially inhibited by TRAF-2DN. These results suggest that TRAF-2 signaling is a common pathway arising from these two domains. TRAF-2 has previously been shown to activate both NF-κB and JNK through distinct pathways (1, 20, 29, 41, 47). Thus, it is not surprising that both CTAR-1 and CTAR-2 would activate both NF-κB and AP-1, as revealed by these studies of the MMP-9 promoter. The previous studies of JNK activation have analyzed JNK activity on a glutathione S-transferase–Jun substrate in the presence of overexpressed JNK-1 (9, 24, 29, 38). The data presented here detect activated AP-1 on an authentic AP-1 site in the MMP-9 promoter. This activity may reflect the activation of distinct JNK kinases in vivo or indicate that CTAR-1 activates AP-1 through some other indirect mechanism.
The data also indicate that both NF-κB and AP-1 are essential for the activation of the MMP-9 promoter. The effects are not additive; thus, the mutation of either the NF-κB or AP-1 site eliminates the transactivation of the promoter by LMP-1. The complete inhibition of promoter activity by the constitutive active form of IκB also indicates that NF-κB binding is essential for MMP-9 promoter activity. These data suggest that both the NF-κB and AP-1 sites must be occupied to initiate transcription.
As NPC is a highly metastatic tumor with frequent expression of LMP-1 (3), the activation of MMP-9 may be an important contributing factor to pathogenesis. The data presented here reveal that NF-κB and AP-1 are both essential for this activation. Thus, agents that specifically inhibit NF-κB activation or JNK activation may be effective in preventing the metastasis of NPC (33). These findings suggest that the biologic phenotype of tumors associated with EBV latency types in which LMP-1 is expressed may include a potential for metastasis.
ACKNOWLEDGMENTS
We thank Luwen Zhang for helpful discussions, Albert Baldwin for the CMV-IκB plasmid, and Elliott Kieff for the TRAF-3DN construct.
This work was supported in part by a grant-in-aid from the Ministry of Education, Science and Culture of Japan and by grants from the National Institutes of Health (CA19014 to J.S.P. and N.R.-T. and CA32979 to N.R.-T.).
REFERENCES
- 1.Akiba H, Nakano H, Nishikawa S, Shindo M, Kobata T, Atsuta M, Morimoto C, Ware C F, Malinin N L, Wallach D, Yagita H, Okumura K. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF-2, TRAF-5, and NF-kappaB-inducing kinase. J Biol Chem. 1998;273:13353–13358. doi: 10.1074/jbc.273.21.13353. [DOI] [PubMed] [Google Scholar]
- 2.Bernhard E J, Gruber S T, Muschel R J. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci USA. 1994;91:4293–4297. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks L, Yao Q Y, Rickinson A B, Young L S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA-1, LMP-1, and LMP-2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M L, Tsai C N, Liang C L, Shu C H, Huang C R, Sulitzeanu D, Liu S T, Chang Y S. Cloning and characterization of the latent membrane protein (LMP) of a specific Epstein-Barr virus variant derived from the nasopharyngeal carcinoma in the Taiwanese population. Oncogene. 1992;7:2131–2140. [PubMed] [Google Scholar]
- 5.Chow D. Image densitometric analysis. In: Chow D, editor. NIH Image 1.54: using image for densitometric analysis of 1-D gels. Bethesda, Md: National Institutes of Health; 1994. pp. 1–23. [Google Scholar]
- 6.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kieff E, Mosialos G. Association of TRAF-1, TRAF-2, and TRAF-3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-kappaB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docherty A J, O’Connell J, Crabbe T, Angal S, Murphy G. The matrix metalloproteinases and their natural inhibitors: prospects for treating degenerative tissue diseases. Trends Biotechnol. 1992;10:200–207. doi: 10.1016/0167-7799(92)90214-g. [DOI] [PubMed] [Google Scholar]
- 8.Eliopoulos A G, Stack M, Dawson C W, Kaye K M, Hodgkin L, Sihota S, Rowe M, Young L S. Epstein-Barr virus LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene. 1997;14:2899–2916. doi: 10.1038/sj.onc.1201258. [DOI] [PubMed] [Google Scholar]
- 9.Eliopoulos A G, Young L S. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP-1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 10.Fennewald S, van Santen V, Kieff E. Nucleotide sequence of an mRNA transcribed in latent growth-transforming virus infection indicates that it may encode a membrane protein. J Virol. 1984;51:411–419. doi: 10.1128/jvi.51.2.411-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floettmann J E, Rowe M. Epstein-Barr virus latent membrane protein-1 (LMP-1) C-terminus activation region 2 (CTAR-2) maps to the far C-terminus and requires oligomerisation for NF-kappaB activation. Oncogene. 1997;15:1851–1858. doi: 10.1038/sj.onc.1201359. [DOI] [PubMed] [Google Scholar]
- 12.Hatzivassiliou E, Miller W E, Raab-Traub N, Kieff E, Mosialos G. A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-kappa B, and stress-activated protein kinase. J Immunol. 1998;160:1116–1121. [PubMed] [Google Scholar]
- 13.Hua J, Muschel R J. Inhibition of matrix metalloproteinase 9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996;56:5279–5284. [PubMed] [Google Scholar]
- 14.Huen D S, Henderson S A, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP-1) mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- 15.Huhtala P, Chow L T, Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990;265:11077–11082. [PubMed] [Google Scholar]
- 16.Huhtala P, Tuuttila A, Chow L T, Lohi J, Keski-Oja J, Tryggvason K. Complete structure of the human gene for 92-kDa type IV collagenase. Divergent regulation of expression for the 92- and 72-kilodalton enzyme genes in HT-1080 cells. J Biol Chem. 1991;266:16485–16490. [PubMed] [Google Scholar]
- 17.Ito C Y, Kazantsev A G, Baldwin A S., Jr Three NF-kappa B sites in the I kappa B-alpha promoter are required for induction of gene expression by TNF alpha. Nucleic Acids Res. 1994;22:3787–3792. doi: 10.1093/nar/22.18.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh Y. Degradation of extracellular matrix in cancer; activation of MMPs promotes cancer cell invasion and metastasis. Cell Technol. 1998;17:523–533. [Google Scholar]
- 19.Izumi K M, Kieff E D. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karin M, Delhase M. JNK or IKK, AP-1 or NF-kappaB, which are the targets for MEK kinase 1 action? Proc Natl Acad Sci USA. 1998;95:9067–9069. doi: 10.1073/pnas.95.16.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye K M, Izumi K M, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaye K M, Devergne O, Harada J N, Izumi K M, Yalamanchili R, Kieff E, Mosialos G. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-kappa B activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc Natl Acad Sci USA. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2343–2396. [Google Scholar]
- 24.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein G. Epstein-Barr virus strategy in normal and neoplastic B cells. Cell. 1994;77:791–793. doi: 10.1016/0092-8674(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 26.Liotta L A, Rao C N, Wewer U N. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- 27.Liotta L A, Steeg P S, Stetler-Stevenson W G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 28.Liotta L A. Tumor invasion and metastases—role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 29.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 30.Marsters S A, Ayres T M, Skubatch M, Gray C L, Rothe M, Ashkenzaki A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem. 1997;272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 31.Matrisian L M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 32.Matrisian L M. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 33.Mayo M W, Wang C Y, Cogswell P C, Roger-Graham K S, Lowe S W, Der C J, Baldwin A S., Jr Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 34.Miller W E, Mosialos G, Kieff E, Raab-Traub N. Epstein-Barr virus LMP1 induction of epidermal growth factor receptor is mediated through a TRAF signaling pathway distinct from NF-κB activation. J Virol. 1997;71:586–594. doi: 10.1128/jvi.71.1.586-594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller W E, Cheshire J L, Baldwin A S, Jr, Raab-Traub N. The NPC derived C15 LMP1 protein confers enhanced activation of NF-κB and induction of the EGFR in epithelial cells. Oncogene. 1998;16:1869–1877. doi: 10.1038/sj.onc.1201696. [DOI] [PubMed] [Google Scholar]
- 36.Miller W E, Cheshire J L, Raab-Traub N. Interaction of tumor necrosis factor receptor-associated factor signaling proteins with the latent membrane protein 1 PXQXT motif is essential for induction of epidermal growth factor receptor expression. Mol Cell Biol. 1998;18:2835–2844. doi: 10.1128/mcb.18.5.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell T, Sugden B. Stimulation of NF-κB-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J Virol. 1995;69:2968–2976. doi: 10.1128/jvi.69.5.2968-2976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moorthy R K, Thorley-Lawson D A. All three domains of the Epstein-Barr virus-encoded latent membrane protein LMP-1 are required for transformation of Rat-1 fibroblasts. J Virol. 1993;67:1638–1646. doi: 10.1128/jvi.67.3.1638-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moscatelli D, Rifkin D B. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. Biochim Biophys Acta. 1988;948:67–85. doi: 10.1016/0304-419x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima M, Welch D, Belloni P N, Nicholson G L. Degradation of basement membrane type IV collagen and lung subendothelial matrix by rat mammary adenocarcinoma cell clones of differing metastatic potentials. Cancer Res. 1987;47:4869–4876. [PubMed] [Google Scholar]
- 41.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 42.Netzel-Arnett S, Sang Q X, Moore W G, Navre M, Birkedal-Hansen H, Van Wart H E. Comparative sequence specificities of human 72- and 92-kDa gelatinases (type IV collagenases) and PUMP (matrilysin) Biochemistry. 1993;32:6427–6432. doi: 10.1021/bi00076a016. [DOI] [PubMed] [Google Scholar]
- 43.Osborn L, Kunkel S, Nabel G J. Tumor necrosis factor α and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kB. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- 45.Pauli B U, Schwartz D E, Thonar E J, Kuettner K E. Tumor invasion and host extracellular matrix. Cancer Metastasis Rev. 1983;2:129–152. doi: 10.1007/BF00048966. [DOI] [PubMed] [Google Scholar]
- 46.Raab-Traub N, Flynn K, Pearson G, Huang A, Levine P, Lanier A, Pagano J. The differentiated form of nasopharyngeal carcinoma contains Epstein-Barr virus DNA. Int J Cancer. 1987;39:25–29. doi: 10.1002/ijc.2910390106. [DOI] [PubMed] [Google Scholar]
- 47.Reinhard C, Shamoon B, Shyamala V, Williams L T. Tumor necrosis factor α-induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J. 1997;16:1080–1092. doi: 10.1093/emboj/16.5.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Relaix F, Wei X J, Wu X, Sassoon D A. Peg3/Pw1 is an imprinted gene involved in the TNF-NFkappaB signal transduction pathway. Nat Genet. 1998;18:287–291. doi: 10.1038/ng0398-287. [DOI] [PubMed] [Google Scholar]
- 49.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2397–2446. [Google Scholar]
- 50.Sandberg M, Hammerschmidt W, Sugden B. Characterization of LMP-1’s association with TRAF-1, TRAF-2, and TRAF-3. J Virol. 1997;71:4649–4656. doi: 10.1128/jvi.71.6.4649-4656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 52.Sato H, Kida Y, Mai M, Endo Y, Sasaki T, Tanaka J, Seiki M. Expression of genes encoding type IV collagen-degrading metalloproteinases and tissue inhibitors of metalloproteinases in various human tumor cells. Oncogene. 1992;7:77–83. [PubMed] [Google Scholar]
- 53.Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8:395–405. [PubMed] [Google Scholar]
- 54.Scheinman R I, Cogswell P C, Lofquist A K, Baldwin A S., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 55.Sehgal G, Hua J, Bernhard E J, Sehgal I, Thompson T C, Muschel R J. Requirement for matrix metalloproteinase-9 (gelatinase B) expression in metastasis by murine prostate carcinoma. Am J Pathol. 1998;152:591–596. [PMC free article] [PubMed] [Google Scholar]
- 56.Stearns M E, Fudge K, Garcia F, Wang M. IL-10 inhibition of human prostate PC-3 ML cell metastases in SCID mice: IL-10 stimulation of TIMP-1 and inhibition of MMP-2/MMP-9 expression. Invasion Metastasis. 1997;17:62–74. [PubMed] [Google Scholar]
- 57.Sugihara Y, Shimada H, Seeger R C, Laug W E, DeClerck Y A. Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: contribution of stromal cells to their production and correlation with metastasis. Cancer Res. 1998;58:2209–2216. [PubMed] [Google Scholar]
- 58.Takino T, Sato H, Shinagawa A, Seiki M. Identification of the second membrane-type matrix metalloproteinase (MT-MMP-2) gene from a human placenta cDNA library. MT-MMPs form a unique membrane-type subclass in the MMP family. J Biol Chem. 1995;270:23013–23020. doi: 10.1074/jbc.270.39.23013. [DOI] [PubMed] [Google Scholar]
- 59.Yoshizaki T, Sato H, Furukawa M, Pagano J S. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci USA. 1998;95:3621–3626. doi: 10.1073/pnas.95.7.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young L, Alfieri C, Hennessy K, Evans H, O’Hara C, Anderson K C, Ritz J, Shapiro R S, Rickinson A, Kieff E, Cohen J I. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–1085. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]