Abstract
Background
Evidence demonstrates that physical exercise confers several psycho-physical benefits on patients with cancer. This study aims to investigate the role of oncologists in exercise promotion.
Patients and methods
A multicenter, cross-sectional study was conducted by distributing an anonymous, self-administered questionnaire to patients with cancer. The questionnaire enclosed demographic, health, and exercise variables. The exercise-related questions included in the study used the Godin-Shephard Leisure-Time Physical Activity Questionnaire to measure the amount of physical exercise. In addition, the survey gathered information on whether exercise was discussed with patients, and whether oncologists followed the assess, advise, reinforce, and refer (AARR) process regarding exercise. The survey also asked if patients preferred that exercise be discussed during their consultations. Descriptive statistics and logistic regression were applied.
Results
With a response rate of 75%, a total of 549 patients completed the survey. Regarding the exercise discussion, 38% of patients stated that their oncologist initiated an exercise discussion, 14% started the discussion themselves, and 48% said that the issue was not considered. Overall, 35% of patients reported that the oncologist assessed their exercise level, 22% and 42% received advice or reinforcement to increase their exercise, respectively, and 10% were referred to a dedicated service. Regarding preferences, 72% of patients thought that the oncologists should initiate an exercise discussion, 2% that only patients should start the discussion, and 26% thought that the issue should not be discussed. Similarly, 74% of patients are willing to receive the exercise assessment, 59% and 75% the advice and reinforcement to increase their exercise, and 46% to be referred to an exercise service.
Conclusions
Although exercise promotion rates are low, patients are willing to receive exercise information. Dedicated strategies should be developed to support oncologists in promoting exercise to their patients.
Key words: physical exercise, oncologist’s recommendation, cancer, quality of life, physical exercise recommendation
Highlights
-
•
About 48% of patients with cancer state that exercise is never discussed with their oncologist.
-
•
Only 10% of patients are referred to dedicated exercise services.
-
•
More than 70% of patients would have an exercise discussion with their oncologist.
-
•
Tools to support oncologists in promoting exercise should be implemented.
Introduction
Over the past three decades, physical exercise has gained attention for its potential as an adjunctive strategy in the cancer setting. A considerable body of observational studies supports the association between higher levels of physical activity and lower risks of breast-, colon-, and prostate-specific mortality as well as all-cause mortality.1 In addition, emerging evidence suggests improvements in survival for patients with lung cancer,2 endometrial cancer,3 and for those undergoing immunotherapy treatment.4 Several randomized controlled trials strongly demonstrated the safety and feasibility of exercise intervention among the oncological population during anticancer treatments.5 Physical exercise has the potential to increase patients’ cardiorespiratory fitness,6 muscle strength, and mass,7 as well as to ameliorate some treatment-related side-effects, such as fatigue,8 lymphoedema,9 pain,10 peripheral neuropathy11 and improve mood by decreasing anxiety and depression levels,12 overall enhancing the quality of life.13
Different scientific societies, such as the American College of Sports Medicine (ACSM),5 the American Cancer Society (ACS),14 and the American Society of Clinical Oncology (ASCO),15 have released physical exercise guidelines dedicated to patients with cancer. Despite the growing awareness, the large majority of the oncological population remains insufficiently active.16,17 As suggested by the ASCO guidelines, an effective exercise promotion requires a team effort, in which oncologists play a key role. Indeed, the oncologist may facilitate the adoption of a healthy lifestyle by reaching a high percentage of patients and enhancing the credibility of their recommendations.15 Among the modalities by which oncologists may be proactive in exercise promotion emerges the assess, advise, and refer (AAR) process.18,19 In ∼5-min time, the oncologist may be able to assess the patient’s physical exercise, recommend them to increase or maintain their physical exercise level, and refer the insufficiently active ones to a dedicated exercise service.18,19 In addition, some authors propose incorporating the reinforcement of the importance of physical activity engagement in the assess, advise, reinforce, and refer (AARR)4 process to make the clinician recommendation more effective.
However, beyond the AARR process, some investigations have been conducted to test the impact of oncologist recommendations on patients’ physical exercise behavior. In this sense, a first randomized controlled trial on patients with breast cancer found that a 30-s recommendation led to a 30-min increase in moderate-intensity exercise compared with the control group.20 Conversely, a second study conducted on patients with breast and colorectal cancer found that the oncologist’s advice plus a motivational package was needed to improve exercise behavior, whereas the sole recommendation was insufficient to achieve significant enhancements.21 Finally, a more recent investigation, including >15 000 patients with colorectal cancer, reported that recall of discussion with the oncologist did lead to higher levels of physical activity.22 Overall, although the current data are not entirely conclusive, the available evidence suggests that it is likely that the oncologist’s advice may play a role in promoting active behavior in patients.
Although some studies have investigated the exercise promotion practice from the perspectives of oncologists and healthcare providers,23, 24, 25 to our knowledge, just two studies have explored exercise promotion by the oncologists from the patient’s perspective26,27 without deeply investigating the asses, advice, reinforce, and refer process. To fill this gap, we have designed the CONNECT study to determine (i) the percentage of patients with cancer who report being assessed, advised, or reinforced about physical exercise, and eventually referred to a dedicated exercise service/specialist; (ii) the percentage of patients who reported a physical exercise discussion with their oncologists, analyzing who initiated the discussion; (iii) the difference with other lifestyle behavioral practices promotion; (iv) the sociodemographic and medical determinants associated with the exercise discussion and the AAR process; and (v) the patients’ preferences regarding the AAR process and exercise discussion.
Materials and methods
Study design and participants
The CONNECT is a cross-sectional study in which data were anonymously collected from February 2023 to February 2024 in three cancer outpatient facilities: the Oncology Unit at the University of Verona (Italy); the Oncology Department at San Luigi Gonzaga, Orbassano (Italy); and the Oncology Unit at the IRCSS National Cancer Institute, Bari (Italy). Patients were eligible if they were aged ≥18 years, had a cancer diagnosis, and had adequate Italian language proficiency in answering the questionnaire. The recruitment procedure was similar in the three centers. On randomly selected days, patients were approached face-to-face by the staff and informed about the study. If interested in participating, patients were asked to sign the written informed consent and then received a copy of the survey to return directly. To avoid duplication, the study staff systematically asked patients if they had already filled out the questionnaire. Ethics Committee approval was obtained from the University of Verona (Prot. No. 54507), Orbassano Hospital (Prot. No. 555), and IRCSS National Cancer Institute of Bari (Prot. No. 1311). The study was designed to adhere to Good Clinical Practice principles, and the procedures were conducted following the last revision of the Declaration of Helsinki as well as the Declaration of Oviedo. The current report is compliant with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.28
Questionnaire
The self-administered questionnaire (Supplementary Material S1, Supplementary file 1 available at https://doi.org/10.1016/j.esmoop.2024.103624) was created after a literature review23,25,26,29 and revised by dedicated experts (oncologists, kinesiologists, psychologists, and representatives of patient advocacy) to develop the current version. The survey took ∼25-30 min and was composed of 38 items divided into five sections: (i) general characteristics, (ii) exercise behavior, (iii) current and preferred exercise discussion, (iv) current and preferred clinical practice regarding exercise promotion, and (v) cancer diagnosis and treatment.
General characteristics were age (years), sex (male/female), weight (kg), height (m), educational level (elementary, up to age 10-11 years/secondary, up to 14 years/secondary, up to 18-19 years/college, university/postgraduate), marital status (single/married/divorced/widowed), occupational status (retired/homemaker/part-time employed/full-time employed), and perceived economic adequacy (inadequate/barely adequate/adequate/more than adequate). Weight and height were used to obtain body mass index according to the categories proposed by the World Health Organization (WHO).
Exercise behavior was explored using the Godin-Shephard Leisure-Time Physical Activity Questionnaire (GLTEQ).30 The GLTEQ investigates the frequency of strenuous, moderate, and mild activities. The Leisure Score Index (LSI) was calculated as the sum of [vigorous × 9 metabolic equivalents (the metabolic equivalent of the task)] + (moderate × 5 metabolic equivalents). LSI ≥ 24 classifies a person as sufficiently active, that is, engaging in at least 150 min of moderate/strenuous exercise per week, as suggested by the guidelines of the ACS.14 In addition, two open-ended questions, adapted by Schmitz et al.,19 were used to explore the weekly engagement in aerobic and resistance activities. Patients who engaged in 90 min/week of aerobic exercise and at least two times per week in resistance activities were considered sufficiently active, according to the guidelines of the ACSM.5 These two methods to classify the lifestyle of patients with cancer were used to explore the impact of the different recommendations.
Current exercise discussion was investigated by asking patients ‘Was the exercise issue discussed at least once during a visit with your oncologist?’ (yes, the discussion was initiated by my oncologist/yes, the discussion was initiated by myself/no, it was not discussed).26 The preferred exercise discussion was assessed with the question, ‘Would you have liked the exercise issue to have been discussed at least once during a visit with your oncologist?’ (yes, it should be initiated by my oncologist/yes, it should be initiated only by me/no, it should not be discussed).26 To compare the current and preferred exercise discussion with other behavioral issues, the same two questions were repurposed for the following: psychological status, smoking habits, nutrition, weight control, and alcohol consumption.
Current clinical practice regarding exercise promotion was investigated by asking patients whether the oncologist has assessed their physical exercise (yes/no), advised them to increase/maintain an adequate exercise level (yes/no), reinforced the importance of physical exercise (yes/no), and referred them to a dedicated exercise service (yes/no), that is, the AARR process. The same questions were repurposed by asking patients whether they would have liked their oncologist to have assessed, advised, and reinforced the importance of physical exercise and referred them to a dedicated service.
The last section was dedicated to the self-reported medical variable, which enclosed cancer site (breast/lung/colorectal/upper gastrointestinal/head–neck/gynecological/urogenital/melanoma/other), disease status (early/advanced/metastatic/in remission or cured/unknown), date of diagnosis (month/year), type of treatment (surgery/chemotherapy/radiotherapy/hormone therapy/other), and current treatment status (about to start/ongoing/completed/not known).
Statistical analysis
Descriptive statistics are presented as mean, standard deviations, medians, and interquartile range for continuous data, and absolute counts and percentages for categorical data. A multivariable logistic regression model was applied to explore patients’ characteristics associated with current/preferred exercise discussion and current/preferred exercise practice promotion. All variables were simultaneously considered in the model without any selection procedures, and odds ratios (ORs) and their 95% confidence intervals (CIs) were reported, with an OR >1.00 indicating a higher probability of exercise discussion/exercise practice promotion. All independent continuous covariates were transformed into categorical items to allow for a straightforward interpretation of ORs. IBM SPSS Statistics (version 28.0; IBM Corporation, New York, NY) was used to carry out the analysis.
Results
A total of 732 patients were approached. Of these, 183 (25%) refused to participate and 549 (75%) completed the survey. Demographic and medical characteristics of the survey population are presented in Table 1. Patients had a mean age of 63.5 (11.3 standard deviation) years, 53.9% were male, 69.6% were married, and 56.5% were retired from employment. The most frequent cancer types were lung (40.1%), followed by upper gastrointestinal cancers (31.0%); 32.2% had metastatic disease and 77.2% were still undergoing anticancer treatments.
Table 1.
Sociodemographic characteristics of the study participants
| Variable | Values |
|---|---|
| Sex, n (%) | |
| Female | 253 (46.1) |
| Male | 296 (53.9) |
| Age (years) | |
| Median/mean | 65/63.5 |
| Interquartile range/standard deviation | 57-72/11.3 |
| Body mass index (kg/m2), n (%) | |
| <18.5 | 21 (3.8) |
| 18.5-24.9 | 264 (48.1) |
| 25.0-29.9 | 177 (32.2) |
| >29.9 | 81 (14.8) |
| Missing | 6 (1.1) |
| Education, n (%) | |
| Elementary (up to 10-11 years of age) | 62 (11.3) |
| Secondary (up to 14 years of age) | 183 (33.3) |
| Secondary (up to 18-19 years of age) | 217 (39.5) |
| College/university | 67 (12.2) |
| Postgraduate | 16 (2.9) |
| Missing | 4 (0.7) |
| Marital status, n (%) | |
| Single | 75 (13.7) |
| Married | 382 (69.6) |
| Divorced | 43 (7.8) |
| Widowed | 37 (6.7) |
| Missing | 12 (2.2) |
| Occupational status, n (%) | |
| Full-time employed | 132 (24.0) |
| Part-time employed | 36 (6.6) |
| Seeking employment. | 16 (2.9) |
| Retired | 310 (56.5) |
| Homemaker | 52 (9.5) |
| Missing | 3 (0.5) |
| Perceived income adequacy, n (%) | |
| More than adequate | 89 (16.2) |
| Adequate | 253 (46.1) |
| Barely adequate | 149 (27.1) |
| Inadequate | 40 (7.3) |
| Missing | 18 (3.3) |
| Meeting the ACSM guidelines, n (%) | |
| Yes | 49 (8.9) |
| No | 491 (89.4) |
| Missing | 9 (1.6) |
| Leisure Score Index (ACS guidelines), n (%) | |
| <24 | 505 (92.0) |
| ≥24 | 34 (6.2) |
| Missing | 10 (1.8) |
| Cancer site, n (%) | |
| Breast | 49 (8.9) |
| Lung | 220 (40.1) |
| Upper gastrointestinal area, n (%) | 170 (31.0) |
| Genitourinary | 24 (4.4) |
| Colorectal | 40 (7.3) |
| Head and neck | 12 (2.2) |
| Melanoma | 9 (1.6) |
| Gynecological | 4 (0.7) |
| Other | 12 (2.2) |
| Missing | 9 (1.6) |
| Cancer stage | |
| Metastatic | 177 (32.2) |
| Advanced | 126 (23.0) |
| Initial | 61 (11.1) |
| In remission | 81 (14.8) |
| Not known | 81 (14.8) |
| Missing | 23 (4.2) |
| Cancer treatment | |
| Surgery | 199 (36.2) |
| Chemotherapy | 360 (65.6) |
| Radiotherapy | 140 (25.5) |
| Hormonotherapy | 36 (6.6) |
| Other | 136 (24.8) |
| Treatment status, n (%) | |
| Ongoing | 424 (77.2) |
| Completed | 70 (12.7) |
| Not yet started | 19 (3.5) |
| Not known | 21 (3.8) |
| Missing | 15 (2.7) |
| Time from diagnosis | |
| Median/mean | 14/28.8 |
| Interquartile range/standard deviation | 6-36/38.8 |
ACS, American Cancer Society; ACSM, American College of Sports Medicine.
Current and preferred exercise discussion
Table 2 reports the current and preferred discussion with the oncologist about exercise and other behavioral issues. Regarding the exercise discussion, 38% of patients stated that it was initiated by the oncologist, 14% by themselves, and 47% indicated that the issue was not discussed. Compared with other behavioral issues, exercise was the second last to be discussed by the oncologist, but it was the second in terms of being initiated by the patients and the second to be not discussed. Regarding preferences, 72% of patients thought that exercise discussion should be initiated by the oncologists, 2% by the patients themselves, and 26% thought that exercise should not be discussed. With respect to other behavioral issues, exercise was listed as the fourth in terms of preference for oncologist-initiated discussion, the last in terms of being initiated by the patients themselves, and the third in thinking that it should not be discussed. Supplementary Tables S1-S6, available at https://doi.org/10.1016/j.esmoop.2024.103624 report the crossing between the current and preferred exercise and other behavioral practice promotion.
Table 2.
Current and preferred exercise and other behavioral issues discussed during the visits
| Current discussion |
Preferred discussion |
|||||
|---|---|---|---|---|---|---|
| Oncologist-initiated discussion, n (%) | Patient-initiated discussion, n (%) | Not discussed, n (%) | Oncologist should initiate discussion, n (%) | Patient should initiate discussion, n (%) | Should not be discussed, n (%) | |
| Physical exercise | 202 (38) | 76 (14) | 250 (47) | 349 (72) | 12 (2) | 124 (26) |
| Psychological issue | 232 (45) | 62 (12) | 216 (42) | 358 (75) | 46 (10) | 76 (16) |
| Tobacco smoking | 247 (49) | 15 (3) | 237 (47) | 309 (67) | 28 (6) | 126 (27) |
| Nutrition | 297 (59) | 88 (17) | 122 (24) | 422 (86) | 34 (7) | 34 (7) |
| Weight control | 345 (68) | 41 (8) | 118 (23) | 417 (85) | 34 (7) | 40 (8) |
| Alcohol consumption | 189 (37) | 19 (4) | 298 (59) | 303 (64) | 24 (5) | 143 (30) |
Current and preferred physical exercise practices promotion
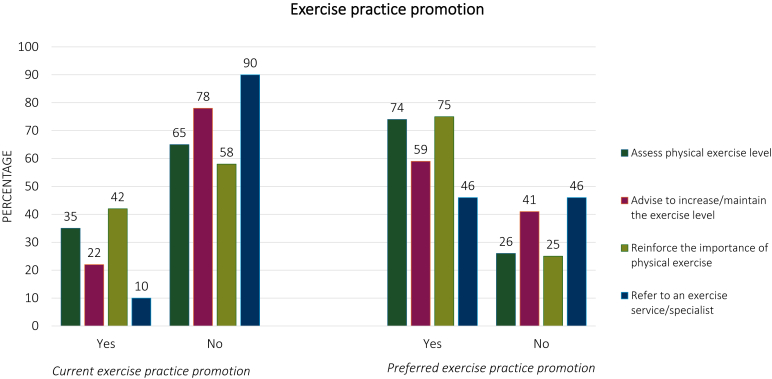
Current and preferred exercise practices promotion are set out in Figure 1. Overall, 35% of patients reported that the oncologist assessed their physical exercise level, 22% received advice to increase or maintain their exercise level, 42% stated that the oncologist reinforced the importance of being physically active, and 10% of patients were referred to an exercise service/specialist. Regarding the preferences for exercise practice promotion, 74% of patients thought that oncologists should assess their physical exercise level, 59% that they should advise patients to increase their exercise level, 75% believed that oncologists should reinforce the importance of being physically active, and 46% thought that oncologists should refer patients to a dedicated service.
Figure 1.
Current and preferred exercise practice promotion among oncologists.
Determinants associated with exercise discussion and exercise practice promotion
Table 3 displays the relation between the characteristics of patients with cancer and current and preferred exercise discussion. Compared with retired patients, those who have a part-time (OR 4.559, 95% CI 1.63-12.72; P = 0.004) and a full-time job (OR 2.024, 95% CI 1.06-3.88; P = 0.034) are more likely to report an exercise discussion with their oncologist, as well as those who had a lung cancer diagnosis (OR 3.508, 95% CI 1.36-9.05; P = 0.009), with respect to patients who have breast cancer. The engagement of patients in physical exercise was not significantly associated with the exercise discussion with their oncologist.
Table 3.
Association of current and preferred exercise discussion in patients with cancera
| Current exercise discussionb |
Preferred exercise discussionb |
|||||
|---|---|---|---|---|---|---|
| ORc | 95% CIc | P valuec | ORc | 95% CIc | P valuec | |
| Sex | ||||||
| Female (reference) | 1.000 | 1.000 | ||||
| Male | 1.562 | (0.93-2.61) | 0.089 | 1.709 | (0.97-3.01) | 0.064 |
| Age (years) | ||||||
| <65 (reference) | 1.000 | 1.000 | ||||
| ≥65 | 1.025 | (0.58-1.82) | 0.933 | 1.339 | (0.73-2.45) | 0.344 |
| Body mass index (kg/m2) | ||||||
| Underweight (reference) | 1.000 | 1.000 | ||||
| Normal weight | 1.929 | (0.57-6.51) | 0.290 | 0.804 | (0.22-2.92) | 0.741 |
| Overweight | 2.350 | (0.67-8.20) | 0.180 | 0.656 | (0.17-2.47) | 0.532 |
| Obese | 2.313 | (0.62-8.61) | 0.211 | 1.724 | (0.40-7.37) | 0.463 |
| Education | ||||||
| Up to age 14 years (reference) | 1.000 | 1.000 | ||||
| Beyond age 14 years | 1.377 | (0.86-2.20) | 0.179 | 1.778 | (1.06-2.99) | 0.030 |
| Marital status | ||||||
| Married (reference) | 1.000 | 1.000 | ||||
| Single | 0.643 | (0.33-1.25) | 0.194 | 1.151 | (0.51-2.60) | 0.735 |
| Divorced | 0.950 | (0.44-2.08) | 0.898 | 1.013 | (0.42-2.46) | 0.978 |
| Widowed | 0.759 | (0.29-1.97) | 0.572 | 0.638 | (0.23-1.75) | 0.383 |
| Occupational status | ||||||
| Retired (reference) | 1.000 | 1.000 | ||||
| Homemaker | 1.150 | (0.49-2.68) | 0.746 | 0.888 | (0.36-2.18) | 0.796 |
| Part-time | 4.559 | (1.63-12.72) | 0.004 | 17.786 | (2.18-145.03) | 0.007 |
| Full-time | 2.024 | (1.06-3.88) | 0.034 | 2.036 | (0.99-4.17) | 0.052 |
| Other | 1.438 | (0.36-5.71) | 0.606 | 6.430 | (0.70-59.01) | 0.100 |
| Income adequacy | ||||||
| Inadequate (reference) | 1.000 | 1.000 | ||||
| Adequate | 0.927 | (0.58-1.48) | 0.751 | 0.860 | (0.51-1.45) | 0.569 |
| Physical activity frequency | ||||||
| <1 time/week (reference) | 1.000 | 1.000 | ||||
| 1-2 times/week | 1.551 | (0.90-2.68) | 0.115 | 1.758 | (0.94-3.28) | 0.076 |
| >2 times/week | 1.713 | (0.99-2.94) | 0.050 | 1.059 | (0.59-1.91) | 0.848 |
| Tumor sited | ||||||
| Breast (reference) | 1.000 | 1.000 | ||||
| Lung | 3.508 | (1.36-9.05) | 0.009 | 0.623 | (0.20-1.94) | 0.414 |
| Colorectal | 2.308 | (0.74-7.25) | 0.152 | 1.190 | (0.27-5.30) | 0.820 |
| Upper gastrointestinal area | 0.982 | (0.39-2.46) | 0.969 | 0.776 | (0.25-2.37) | 0.656 |
| Urogenital | 2.322 | (0.61-8.90) | 0.219 | 0.479 | (0.10-2.30) | 0.358 |
| Other | 0.765 | (0.23-2.53) | 0.660 | 0.406 | (0.10-1.67) | 0.211 |
| Stage of disease | ||||||
| Remission (reference) | 1.000 | 1.000 | ||||
| Early | 1.135 | (0.48-2.70) | 0.775 | 1.753 | (0.62-4.96) | 0.290 |
| Advanced | 0.602 | (0.28-1.28) | 0.188 | 0.612 | (0.26-1.46) | 0.270 |
| Metastatic | 0.677 | (0.34-1.36) | 0.272 | 0.696 | (0.31-1.58) | 0.385 |
| Unknown | 0.569 | (0.25-1.28) | 0.174 | 0.546 | (0.21-1.40) | 0.207 |
| Chemotherapy | ||||||
| No (reference) | 1.000 | 1.000 | ||||
| Yes | 0.892 | (0.51-1.56) | 0.687 | 0.864 | (0.46-1.61) | 0.647 |
| Surgery | ||||||
| No (reference) | 1.000 | 1.000 | ||||
| Yes | 0.851 | (0.50-1.44) | 0.548 | 1.348 | (0.74-2.47) | 0.335 |
| Radiotherapy | ||||||
| No (reference) | 1.000 | 1.000 | ||||
| Yes | 1.209 | (0.72-2.04) | 0.475 | 1.007 | (0.56-1.81) | 0.982 |
| Hormonotherapy | ||||||
| No (reference) | 1.000 | 1.000 | ||||
| Yes | 1.071 | (0.42-2.76) | 0.888 | 2.458 | (0.67-8.98) | 0.174 |
| Other therapies | ||||||
| No (reference) | 1.000 | 1.000 | ||||
| Yes | 0.774 | (0.417-1.43) | 0.415 | 1.410 | (0.72-2.76) | 0.315 |
| Adherence to guideline | ||||||
| No (reference) | 1.000 | 1.000 | ||||
| Yes | 1.221 | (0.55-2.71) | 0.624 | 1.471 | (0.58-3.6) | 0.420 |
| Leisure Score Index | ||||||
| <24 (reference) | 1.000 | 1.000 | ||||
| ≥24 | 1.415 | (0.55-3.68) | 0.476 | 1.002 | (0.34-2.99) | 0.997 |
| Time from diagnosis | ||||||
| ≤30 m (reference) | 1.000 | 1.000 | ||||
| >30 m | 1.295 | (0.78-2.15) | 0.315 | 1.021 | (0.58-1.80) | 0.942 |
CI, confidence interval; OR, odds ratio.
Current and preferred exercise discussion classified as yes, oncologist-initiated discussion/yes, initiated by me versus no.
Each variable was mutually adjusted for the following variables if not otherwise specified: sex (male, female); age (<65 years versus ≥65 years); education (≥high school versus <lower than high school); patient’s address (outside the city, city dweller); perceived income adequacy (adequate versus nonadequate); marital status (married, unmarried, divorced, widow); occupational status (retired, stay at home, part-time employed, full-time employed, other); frequency of sweating-inducing activity (<1 time/week; 1-2 times/week; >2 times/week); adherence to guidelines (yes versus no); Leisure Score Index (<24 versus ≥24); tumor site (breast, lung, colorectal, upper gastrointestinal area, urogenital, other); stage of the disease (remission, early, advanced, metastatic, unknown); chemotherapy (yes versus no); surgery (yes versus no); radiotherapy (yes versus no); hormone therapy (yes versus no); other treatments (yes versus no); treatment status (ended, incoming, ongoing, undefined); time from diagnosis (≤30 months, >30 months).
OR and CI and P value from the logistic regression model.
Tumor sites with <10 patients are classified as ‘other’.
Regarding the preference, with respect to those who attended a secondary school up to 14 years of age, patients with a higher education level (OR 1.778, 95% CI 1.06-2.99; P = 0.030) were more likely to want a discussion about exercise with their oncologists, as well as those who have a part-time job (OR 17.786, 95% CI 2.18-145.03; P = 0.007), than those who are retired.
Determinants associated with preferred exercise discussion and exercise practice promotion
Logistic regression associating characteristics of patients with the current and preferred AARR process is shown in upplementary Tables S7-S10, available at https://doi.org/10.1016/j.esmoop.2024.103624. Among the most striking findings, current assessment, advice, and reinforcement practice are significantly associated with lung and urogenital cancer diagnosis. The current referral practice to a dedicated service/specialist is associated with an early stage of disease (OR 5.167, 95% CI 1.27-21.04; P = 0.022) and with surgical treatment (OR 2.507, 95% CI 1.05-5.99; P = 0.039), and inversely related to chemotherapy treatment (OR 0.403, 95% CI 0.17-0.97; P = 0.043). Preference to referral is related to surgical treatment (OR 1.881, 95% CI 1.14-3.11; P = 0.014) and inversely related to lung cancer diagnosis (OR 0.341, 95% CI 0.13-0.78; P = 0.025).
Discussion
The CONNECT study found that about one-half of patients with cancer reported having had an exercise discussion with their oncologist, whereas a low rate of patients have received the AARR process.
In this study, exercise was discussed with the oncologist in ∼52% of patients with cancer, and specifically, 38% of patients reported an oncologist-initiated discussion and 12% a patient-initiated discussion. Our findings are slightly lower with respect to the investigation of Martinez Aguirre-Betolaza et al.,27 who reported an overall exercise discussion in 62%, but higher compared with that of Jones et al.,26 who found that exercise discussion was initiated by oncologists for 28% of patients and by the patients themselves in 14% of cases. Conversely, whereas in the study of Jones et al.,26 82% of patients preferred that exercise discussion would initiated by the oncologist and 15% by the patients themselves, in the CONNECT study, the preferences for an oncologist- and patient-initiated exercise discussion were 71% and 2%, respectively. Only a few sociodemographics and medical variables were associated with the current and preferred exercise discussion, indicating that oncologists are not influenced by these features when discussing exercise, and similarly, the willingness of patients to exercise discussion is not impacted by their disease and sociodemographic background. Of note, patients with lung cancer were more likely to have an exercise discussion with their oncologists. This finding is likely to be related to the specific thoracic malignancies-oriented expertise of the involved centers, as well as to the presence of a dedicated exercise program in Italy for patients affected by lung cancer, supported by WALCE (Women Against Lung Cancer in Europe).31
On the one hand, the results of preference indicate that patients generally accept the exercise information and expect the oncologist to introduce the topic. On the other hand, the data regarding the current exercise discussion, particularly in comparison with other behavioral issues (such as nutrition), underline that exercise promotion is still deprioritized. With these findings and considering that engagement in physical exercise may confer clinically meaningful benefits, including a potential increase in survival,1 exercise promotion becomes crucial in clinical routine, with the oncologist being a key player.20,21
In the CONNECT study, patients reported that the oncologists assessed their physical exercise level in 35% of cases, advised their patients to increase/maintain their exercise level in 22%, reinforced the importance of exercise in 42%, and referred patients to an exercise service in only 10% of cases. Studies among oncologists and healthcare providers reported similar or slightly higher rates of assessment (63%-78%), advice (38%-72%), reinforcement (43%-80%), and referral (10%-23%).25,29,32 A certain degree of discrepancy could be related to the geographical location in which the surveys were conducted: investigations implemented in North America or Australia29,32 show higher frequencies of the AARR process, whereas, in Europe, the reported data are generally lower.25 Besides, in the CONNECT study, the oncologist practice for assessing, advising, and reinforcing has been found to be associated with lung and urogenital cancer diagnosis, whereas the referral was associated with early stage of disease, surgery, and inversely related to chemotherapy treatment. This observation suggests that oncologists may be more proactive in promoting exercise to patients affected by certain cancer types than others and that they refer to an exercise service those patients with an early stage of disease who consequently have a low burden of symptoms. However, because the literature clearly highlights the importance and the psycho-physical benefits of physical exercise across the different cancer types1,2,5,33 and stages, including those with metastatic cancer,34, 35, 36 it becomes essential to promote physical exercise in the oncological population in the wide sense of the term.
Among the possible barriers related to the low rate of the AARR process and its medical determinants, inadequate training in exercise counseling is frequently reported,25 suggesting that more education about physical exercise is needed for healthcare providers. For this purpose, the recent guidelines about survival,1 benefits, and amount of physical exercise5 and instructions to implement the AARR process19 provided by the ACSM may help train healthcare providers and offer dedicated tools to promote physical exercise among patients, especially in regions such as Europe, where the AARR appears less applied. The very low rate of referral (10%) that was observed is likely to be related to the scarcity of dedicated services, which may induce clinicians to approach rationally the available resources favoring those patients with an expected better disease trajectory. Overcoming the lack of dedicated exercise services is crucial to improving exercise promotion. For this reason and to support clinicians in the referral practice, the ASCM has launched a worldwide registry, including >1.722 exercise programs specifically designed for patients with cancer.37
Limitations of this study should be noted. Despite the effort to have a representative large sample, a possible source of error could be the selection bias because it is possible that those who have participated in the CONNECT study are mainly those patients more interested in physical exercise. Moreover, as many associations have been assessed and no multiple testing adjustments have been applied, some significant results may be due to chance. Further, in this study, information regarding patients’ conditions, such as the presence of comorbidities, as well as the patient’s lifestyle habits beyond physical exercise, was not collected. Such information could influence patients’ responses and attitudes toward recommendation prescription; future studies should clarify this point.
Conclusions
The CONNECT study highlights that the current status of exercise discussion and promotion with oncologists in clinical practice, according to the AARR process, still needs profound improvements. The good news is that patients are interested in receiving exercise information and believe the oncologist should make the first move in exercise promotion.
Acknowledgements
L.B. and A.A. are supported by grants from Associazione Pietro Casagrande ONLUS.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
Data related to this study are available from the corresponding author.
Ethics approval
All patients provided informed written consent before enrollment in the study. This study was approved by the Ethics Committee of the University of Verona (Prot. No. 54507), Orbassano Hospital (Prot. No. 555), and IRCSS National Cancer Institute of Bari (Prot. No. 1311).
Supplementary data
References
- 1.Patel A.V., Friedenreich C.M., Moore S.C., et al. American College of Sports Medicine Roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–2402. doi: 10.1249/MSS.0000000000002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloan J.A., Cheville A.L., Liu H., et al. Impact of self-reported physical activity and health promotion behaviors on lung cancer survivorship. Health Qual Life Outcomes. 2016;14:66. doi: 10.1186/s12955-016-0461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedenreich C.M., Cook L.S., Wang Q., et al. Prospective cohort study of pre- and postdiagnosis physical activity and endometrial cancer survival. J Clin Oncol. 2020;38(34):4107–4117. doi: 10.1200/JCO.20.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verheijden R.J., Cabané Ballester A., Smit K.C., et al. Physical activity and checkpoint inhibition: association with toxicity and survival. J Natl Cancer Inst. 2024;116(4):573–579. doi: 10.1093/jnci/djad245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell K.L., Winters-Stone K.M., Wiskemann J., et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott J.M., Zabor E.C., Schwitzer E., et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36(22):2297–2305. doi: 10.1200/JCO.2017.77.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koeppel M., Mathis K., Schmitz K.H., et al. Muscle hypertrophy in cancer patients and survivors via strength training. A meta-analysis and meta-regression. Crit Rev Oncol Hematol. 2021;163 doi: 10.1016/j.critrevonc.2021.103371. [DOI] [PubMed] [Google Scholar]
- 8.Mustian K.M., Alfano C.M., Heckler C., et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3(7):961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes S.C., Singh B., Reul-Hirche H., et al. The effect of exercise for the prevention and treatment of cancer-related lymphedema: a systematic review with meta-analysis. Med Sci Sports Exerc. 2022;54(8):1389–1399. doi: 10.1249/MSS.0000000000002918. [DOI] [PubMed] [Google Scholar]
- 10.Austin P.D., Lee W., Costa D.S., Ritchie A., Lovell M.R. Efficacy of aerobic and resistance exercises on cancer pain: a meta-analysis of randomised controlled trials. Heliyon. 2024;10(7) doi: 10.1016/j.heliyon.2024.e29193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo S., Han W., Wang P., Wang X., Fang X. Effects of exercise on chemotherapy-induced peripheral neuropathy in cancer patients: a systematic review and meta-analysis. J Cancer Surviv. 2023;17(2):318–331. doi: 10.1007/s11764-022-01182-3. [DOI] [PubMed] [Google Scholar]
- 12.Sun M., Liu C., Lu Y., Zhu F., Li H., Lu Q. effects of physical activity on quality of life, anxiety and depression in breast cancer survivors: a systematic review and meta-analysis. Asian Nurs Res (Korean Soc Nurs Sci) 2023;17(5):276–285. doi: 10.1016/j.anr.2023.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Buffart L.M., Kalter J., Sweegers M.G., et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Rock C.L., Thomson C.A., Sullivan K.R., et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72(3):230–262. doi: 10.3322/caac.21719. [DOI] [PubMed] [Google Scholar]
- 15.Ligibel J.A., Bohlke K., May A.M., et al. Exercise, diet, and weight management during cancer treatment: ASCO Guideline. J Clin Oncol. 2022;40(22):2491–2507. doi: 10.1200/JCO.22.00687. [DOI] [PubMed] [Google Scholar]
- 16.Avancini A., Trestini I., Tregnago D., et al. Willingness, preferences, barriers, and facilitators of a multimodal supportive care intervention including exercise, nutritional and psychological approach in patients with cancer: a cross-sectional study. J Cancer Res Clin Oncol. 2023;149(7):3435–3445. doi: 10.1007/s00432-022-04232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avancini A., Pala V., Trestini I., et al. Exercise levels and preferences in cancer patients: a cross-sectional study. Int J Environ Res Public Health. 2020;17(15):5351. doi: 10.3390/ijerph17155351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avancini A., Belluomini L., Milella M., Schena F., Novello S., Pilotto S. Drive the oncologists into exercise promotion in lung cancer. Lung Cancer. 2023;176:1–3. doi: 10.1016/j.lungcan.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz K.H., Campbell A.M., Stuiver M.M., et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–484. doi: 10.3322/caac.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones L.W., Courneya K.S., Fairey A.S., Mackey J.R. Effects of an oncologist’s recommendation to exercise on self-reported exercise behavior in newly diagnosed breast cancer survivors: a single-blind, randomized controlled trial. Ann Behav Med. 2004;28(2):105–113. doi: 10.1207/s15324796abm2802_5. [DOI] [PubMed] [Google Scholar]
- 21.Park J.H., Lee J., Oh M., et al. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: a randomized controlled trial. Cancer. 2015;121(16):2740–2748. doi: 10.1002/cncr.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher A., Williams K., Beeken R., Wardle J. Recall of physical activity advice was associated with higher levels of physical activity in colorectal cancer patients. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2014-006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alderman G., Semple S., Cesnik R., Toohey K. Health care professionals’ knowledge and attitudes toward physical activity in cancer patients: a systematic review. Semin Oncol Nurs. 2020;36(5) doi: 10.1016/j.soncn.2020.151070. [DOI] [PubMed] [Google Scholar]
- 24.Avancini A., D'Amico F., Tregnago D., et al. Nurses’ perspectives on physical activity promotion in cancer patients: a qualitative research. Eur J Oncol Nurs. 2021;55 doi: 10.1016/j.ejon.2021.102061. [DOI] [PubMed] [Google Scholar]
- 25.Pilotto S., Avancini A., Menis J., et al. Exercise in lung Cancer, the healthcare providers opinion (E.C.H.O.): results of the EORTC lung cancer Group (LCG) survey. Lung Cancer. 2022;169:94–101. doi: 10.1016/j.lungcan.2022.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Jones L.W., Courneya K.S. Exercise discussions during cancer treatment consultations. Cancer Pract. 2002;10(2):66–74. doi: 10.1046/j.1523-5394.2002.102004.x. [DOI] [PubMed] [Google Scholar]
- 27.Martinez Aguirre-Betolaza A., Dobaran Amezua A., Yagin F.H., Cacicedo J., Olasagasti-Ibargoien J., Castañeda-Babarro A. Do oncologists recommend the “Pill” of physical activity in their practice? answers from the oncologist and patients’ perspectives. Cancers (Basel) 2024;16(9):1720. doi: 10.3390/cancers16091720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(suppl 1):S31–S34. doi: 10.4103/sja.SJA_543_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ligibel J.A., Jones L.W., Brewster A.M., et al. Oncologists’ attitudes and practice of addressing diet, physical activity, and weight management with patients with cancer: findings of an ASCO survey of the oncology workforce. J Oncol Pract. 2019;15(6):e520–e528. doi: 10.1200/JOP.19.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amireault S., Godin G., Lacombe J., Sabiston C.M. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60. doi: 10.1186/s12874-015-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallone S., Carnio S., Mirandola D., et al. MA07.09 adapted physical activity (AMAti) program for lung cancer patients realized by WALCE (Women Against Lung Cancer in Europe) J Thorac Oncol. 2023;18(11) [Google Scholar]
- 32.Hardcastle S.J., Kane R., Chivers P., Hince D., Dean A., Higgs D., Cohen P.A. Knowledge, attitudes, and practice of oncologists and oncology health care providers in promoting physical activity to cancer survivors: an international survey. Support Care Cancer. 2018;26(11):3711–3719. doi: 10.1007/s00520-018-4230-1. [DOI] [PubMed] [Google Scholar]
- 33.Borsati A., Toniolo L., Trestini I., et al. Feasibility of a novel exercise program for patients with breast cancer offering different modalities and based on patient preference. Eur J Oncol Nurs. 2024;70 doi: 10.1016/j.ejon.2024.102554. [DOI] [PubMed] [Google Scholar]
- 34.Avancini A., Borsati A., Baldo E., et al. A feasibility study investigating an exercise program in metastatic cancer based on the patient-preferred delivery mode. Oncologist. 2024;29(6):e828–e836. doi: 10.1093/oncolo/oyae002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avancini A., Borsati A., Trestini I., et al. Exploring the feasibility of a combined exercise program for patients with advanced lung or pancreatic cancer. Asia Pac J Oncol Nurs. 2023;10(suppl 1) doi: 10.1016/j.apjon.2023.100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott J.M., Iyengar N.M., Nilsen T.S., et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: a randomized controlled trial. Cancer. 2018;124(12):2552–2560. doi: 10.1002/cncr.31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ACSM Exercise oncology registry. https://www.exerciseismedicine.org/eim-in-action/moving-through-cancer/ [cited 2024 23/05/2024]. Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.