Abstract
INTRODUCTION
Gait ability is often cited by stroke survivors. Robot-assisted gait training (RAGT) can help stroke patients with lower limb motor impairment regain motor coordination.
EVIDENCE ACQUISITION
PubMed, Cochrane Library, Embase were systematically searched until September 2023, to identify randomized controlled trials presenting: stroke survivors as participants; RAGT as intervention; conventional rehabilitation as a comparator; gait assessment, through scales or quantitative parameters, as outcome measures.
EVIDENCE SYNTHESIS
Twenty-seven publications involving 1167 patients met the inclusion criteria. Meta-analysis showed no significant differences in speed, cadence, spatial symmetry, and changes in joint mobility angles between the RAGT group and the control group. In addition, RAGT was associated with changes in affected side step length (SMD=0.02, 95% CI: 0.01, 0.03; P<0.0001), temporal symmetry (SMD=-0.38, 95% CI: -0.6, -0.16; P=0.0006], Six-Minute Walk Test (SMD=25.14, 95% CI: 10.19, 40.09; P=0.0010] and Functional Ambulation Categories (SMD=0.32, 95% CI: 0.01, 0.63; P=0.04). According to the PEDro scale, 19 (70.4%) studies were of high quality and eight were of moderate quality (29.6%).
CONCLUSIONS
Taken together, the review synthesis showed that RAGT might have a potential role in the recovery of walking dysfunction after stroke. However, its superiority over conventional rehabilitation requires further research. Additionally, it may provide unexpected benefits that the effects of RAGT with different types or treatment protocols were further compared.
Key words: Stroke, Walking, Gait, Meta-analysis
Introduction
Stroke is the second leading cause of death, and about 60% of stroke patients have walking dysfunction.1, 2 Six months after stroke, 40% of patients who had regained partial walking ability had difficulty walking in unsupported conditions, and the rest had trouble walking in the community.3, 4 Besides, walking independently and safely is the most frequently cited goal of stroke survivors.5 Obviously, improving walking ability is also a key goal in stroke recovery.6, 7
Early input of the correct physiological gait pattern is conducive to the recovery of gait.8 As a safe, intensive and task-specific repetitive training mode, Robot-assisted Gait Training (RAGT) can help stroke patients with lower limb motor impairment regain motor coordination. RAGT not only provides high-intensity and long-duration training but also helps to reduce the workload of therapists.
Robotic devices for limb rehabilitation fall into two main categories: Exoskeletons and End-Effector robots.9 According to the support they apply, it is further divided into Treadmill-based RAGT (T-RAGT) and Overground RAGT (O-RAGT).10 Typically, T-RAGT is used in conjunction with a body-weight support system (BWS).11 A number of studies have pointed out the effectiveness of RAGT in improving walking function.12-15 And a 2022 meta-analysis16 suggests that further randomized controlled trials comparing the efficacy of RAGT with conventional physical therapy are still warranted. As research has progressed, the scientific evidence for the benefits of RAGT may have been updated. Therefore, updating the review is indispensable. Additionally, few studies have focused on the effects of RAGT on temporal and spatial parameters.
In light of these considerations, to update the efficacy of RAGT and explore its effects on kinematic parameters, this study conducted a systematic review and meta-analysis of all extant studies on stroke.
Evidence acquisition
This systematic review and meta-analysis followed the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).17 And it has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42023459950).
Study selection
The principle of PICOS (population, intervention, comparison, outcome, study) was adopted to retrieve and screen articles in this study. And the inclusion criteria were as follows: 1) population: adult stroke patients diagnosed with lower limb motor dysfunction according to clinical guidelines; 2) intervention: experimental group received RAGT by exoskeleton robots or end-effectors; 3) comparison: control group received conventional training or treadmill walking training; 4) outcome: the primary outcomes were walking speed and step length, and the secondary outcomes were parameters (temporal, spatial, and temporal-spatial) and clinical scales associated with walking function in stroke; 5) study: randomized controlled study. Moreover, exclusion criteria were as follows: 1) study protocols; 2) conference summaries; 3) studies that could not isolate the efficacy of RAGT; 4) non-English literature.
Retrieval strategy
Up to September 2023, studies in PubMed, Cochrane Library and Embase were retrieved based on the PICOS principle. Medical Subject Headings (MeSH) and keywords were used to search, such as stroke*[MeSH], apoplexy [MeSH], Exoskeleton [MeSH], Robotics [MeSH], End-effector* [Title/Abstract] “gait parameter*” [Title/Abstract], walking [MeSH], etc. Accordingly, the detailed retrieval strategies are available in Supplementary Digital Material 1 (Supplementary Text File 1).
Data extraction
Two researchers (S.S. and D.Y.) conducted literature screening, data extraction and cross-verification independently. Any disagreements were resolved by discussion or sent to a third researcher (W.Y.) to judge until a consensus was reached. What is more, Endnote X9 was used to do literature management, read the titles and abstracts, eliminate obviously irrelevant literature and record the reasons and quantities. And if the literature contained multiple subgroups, the data matching the subgroups of this study were extracted. Also, we tried to contact the original author to supplement when there existed a lack of information in literature.
To summarize the effects of RAGT on walking function in stroke patients, the following data were extracted from the included studies: 1) basic information: first author, year of publication, country, etc.; 2) basic characteristics of the subjects: sample size, age, gender, stroke onset time, stroke location, etc.; 3) intervention protocols and treatment courses; 4) key elements of bias risk assessment; 5) outcome indicators.
Quality assessment
The methodological quality of the literature was assessed by the physiotherapy evidence database scale (PEDro)18, 19 and the Cochrane risk bias assessment tool.20 Two researchers (S.S. and D.Y.) conducted the quality assessment independently. And if the results were different, they discussed and negotiated with the third researcher (W.Y.) until a consensus was reached. PEDro scale has 11 assessment items such as randomization, blinding of participants and assessors, dropout rates, etc. Moreover, the score of 7-10 is classified as high quality literature, 5-6 as medium quality literature, and ≤4 as low quality literature.21
Statistical analysis
Two statistical software, RevMan 5.4 and Stata15, were used for meta-analysis.
Effect size
The mean and standard deviation value were combined to calculate the mean difference due to the outcome indicators are continuous variables with the same unit. And 95%CI are given for each effect size. The median and quartile values of the included studies were converted to mean or standard deviation according to the formula and then combined for analysis.22, 23
Heterogeneity
I2 statistic was used for evaluation. Its value represents small (25% or lower), medium or large (75% or higher) heterogeneity.24 A I2 threshold of 50% was set to evaluate heterogeneity across studies. If I2≥50%, it indicated the application of a random effect model for data analysis, otherwise, the fixed effects model was employed. To identify the sources of heterogeneity, subgroup analyses were conducted based on post-stroke time (acute phase [≤6 months], subacute phase and chronic phase [>6 months]) or robot training type (O-RAGT and T-RAGT).
Sensitivity analysis
Stata/SE was used to conduct a meta-analysis after removing individual studies successively, and evaluate the differences between the eliminated results and the original combined results.
Publication bias was directly judged by drawing funnel plot.
Evidence synthesis
Study selection
A total of 5965 studies (1892 from PubMed, 950 from Cochrane Library and 3123 from Embase) were retrieved using the above retrieval strategies. Duplicate literature was eliminated and simultaneously the remaining literature was screened. Only 27 studies25-51 which met our stringent criteria were finally included. A total of 1167 patients were included in the study cohort (607 in the experimental group and 560 in the control group) and their basic information is shown in Supplementary Digital Material 2 (Supplementary Table I). In addition, Figure 1 shows the literature screening process and results in detail. Only two studies28, 35 combined RAGT with Conventional gait training (CGT) while others were single RAGT. Moreover, in 85% of the included studies, treatment cycles were greater than or equal to four weeks, with a single treatment duration ranging from twenty minutes to one hour.
Figure 1.
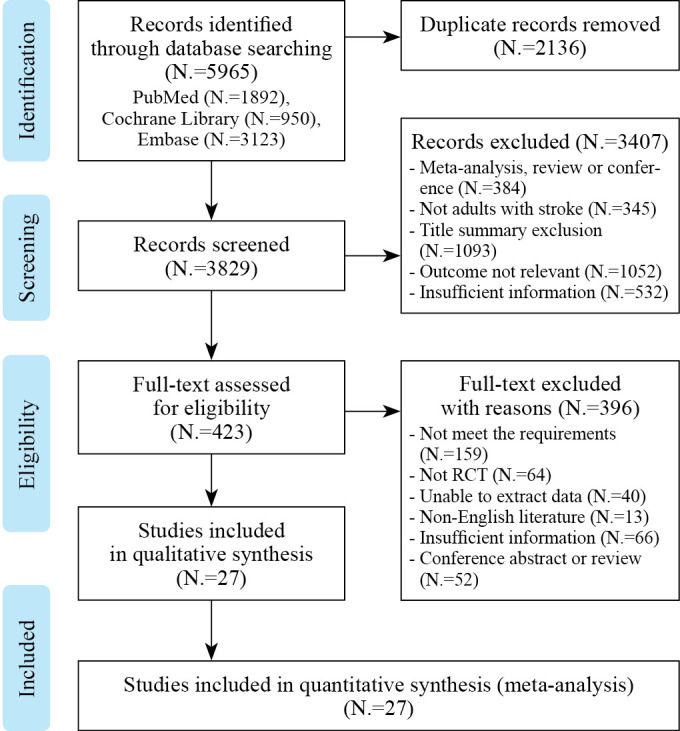
—Literature screening process and results.
Risk of bias
Figure 2 and Table I display the risk of bias in the included studies.25-51 Among them, eight studies25, 26, 31, 40, 41, 43, 44, 51 not explicitly stated whether blind method was used, one study33 did not use blind method, 15 studies27-30, 32, 35, 37-39, 45-50 used single blind method, one study36 used double blind method, and two studies34, 42 used triple blind method. As for random sequence generation, only three studies32, 34, 40 have unclear random sequence generation methods, and the rest have clear descriptions. Meanwhile, nine studies26, 27, 31-33, 40, 43, 44, 49 did not mention allocation hiding, while the rest had detailed descriptions. Moreover, data were completely reported in all studies. The majority of studies had no other risk of bias or were unclear, and only one study39 was defined as high risk due to the availability of Research and development funding. According to the PEDro score, there were 8 medium-quality and 19 high-quality studies.
Figure 2.
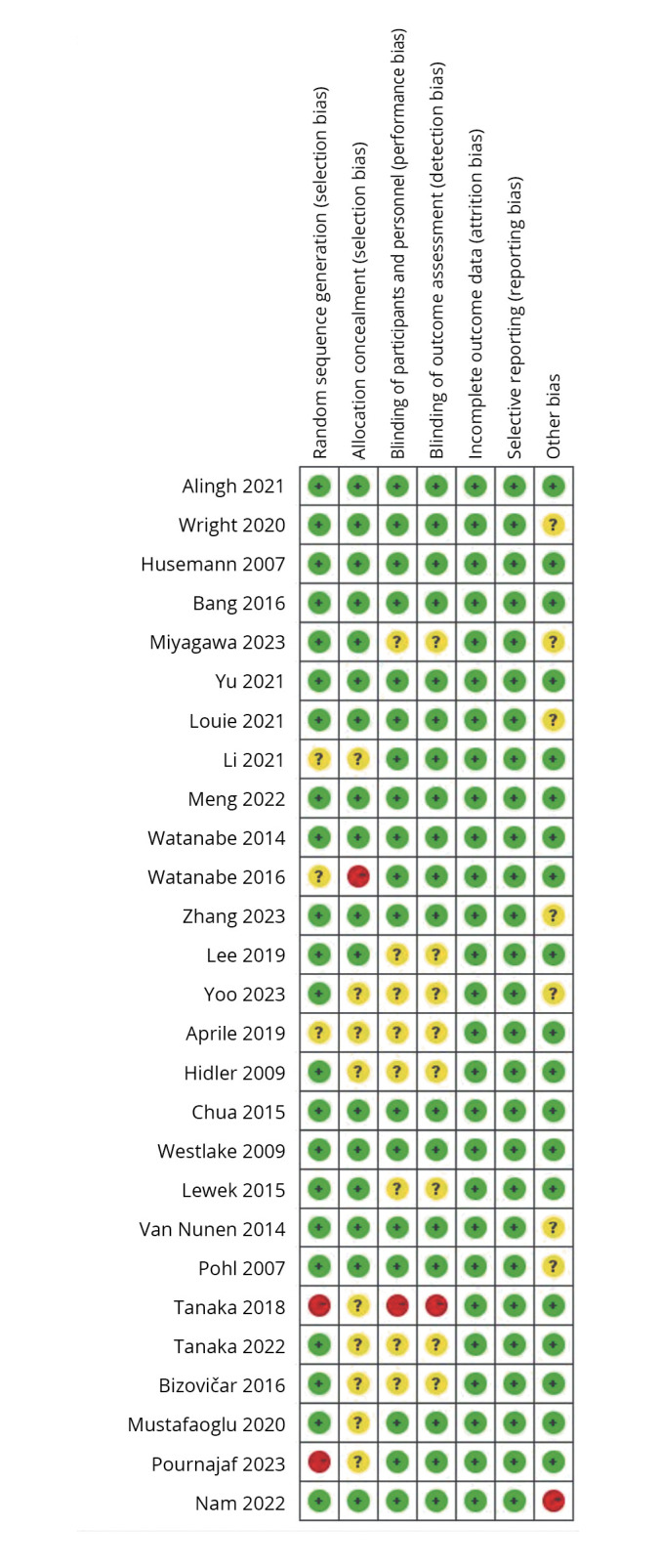
—Cochrane bias risk score.
Table I. —Summary of PEDro score.25-51.
| Inclusion study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alingh 28 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Wright 29 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Husemann 35 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Bang 36 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 9 | |
| Miyagawa 41 | √ | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Yu 48 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Louie 38 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Li 32 | √ | √ | √ | √ | √ | √ | √ | 6 | ||||
| Meng 30 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Watanabe 42 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | 10 |
| Watanabe 34 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Zhang 50 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Lee 51 | √ | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Yoo 26 | √ | √ | √ | √ | √ | √ | √ | 6 | ||||
| Aprile 40 | √ | √ | √ | √ | √ | √ | 5 | |||||
| Hidler 43 | √ | √ | √ | √ | √ | √ | √ | 6 | ||||
| Chua 37 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Westlake 45 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Lewek 25 | √ | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Van Nunen 46 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Pohl 47 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Tanaka 31 | √ | √ | √ | √ | √ | √ | √ | 6 | ||||
| Bizovičar 44 | √ | √ | √ | √ | √ | √ | √ | 6 | ||||
| Mustafaoglu 27 | √ | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Pournajaf 49 | √ | √ | √ | √ | √ | √ | √ | 6 | ||||
| Tanaka 33 | √ | √ | √ | √ | √ | √ | 5 | |||||
| Nam39 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 8 |
1: eligibility criteria; 2: randomly allocated; 3: assigning concealment; 4: similar at baseline; 5: blinding of all subjects; 6: blinding of all therapists; 7: blinding of all assessors; 8: measures of at least one key outcome; 9: intention to treat; 10: comparison between groups; 11: point measures and measures of variability.
Results of individual studies
Walking speed
Thirteen studies were included, involving 509 subjects with lower limb dysfunction after stroke. Due to the heterogeneity test results (P<0.00001, I2=94%), a random effect model analysis was used. And as shown in Figure 3A, the results indicated that there was no significant statistical difference between the experimental group and the control group (SMD=0.06, 95% CI: -0.02, 0.14; P=0.14). Sensitivity analysis found that the results showed satisfactory robustness, as shown in Figure 4A.
Figure 3.
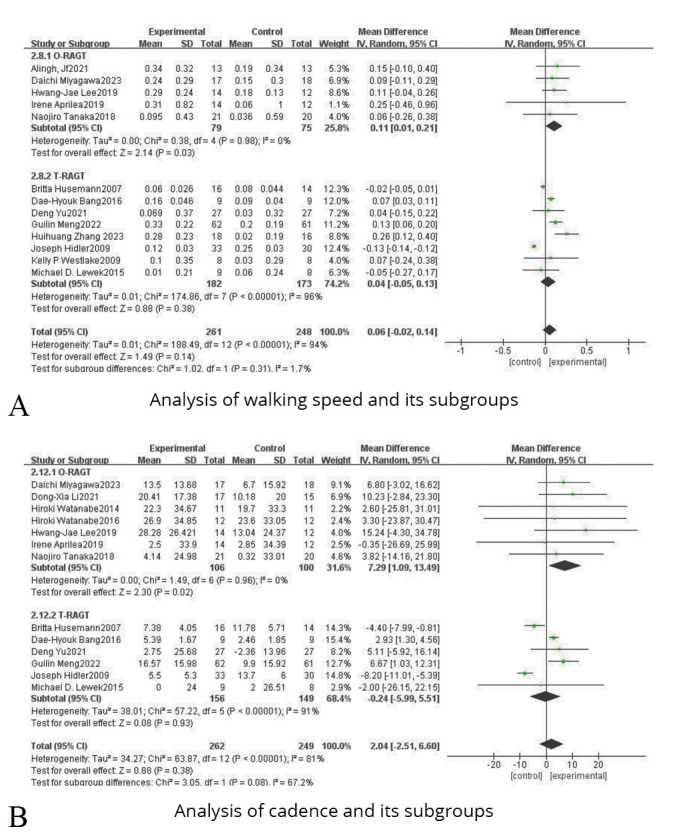
—Forest plots for the analysis of walking speed, cadence and their subgroups.
Figure 4.
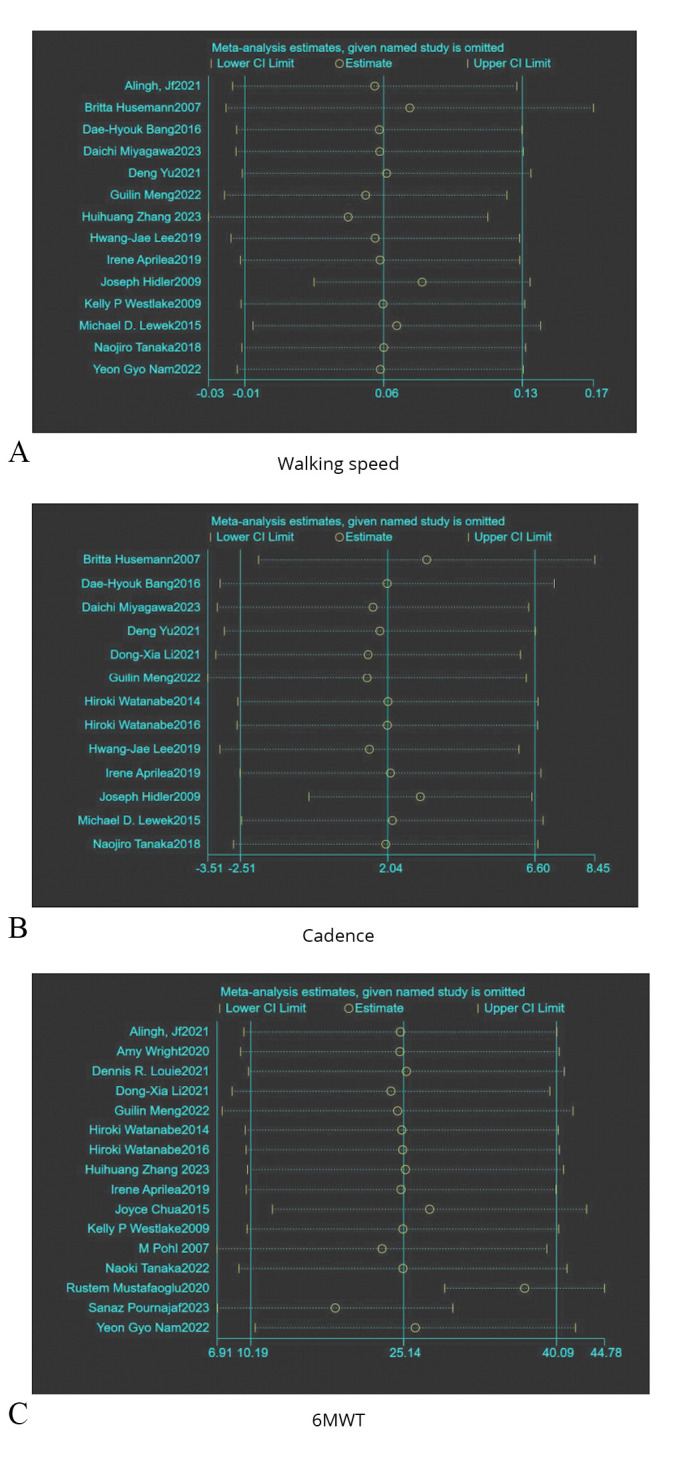
—Summary of sensitivity analysis plots.
Subgroup analysis of robot training type indicated that O-RAGT (SMD=0.11, 95% CI: 0.01, 0.21; P=0.03) showed a higher effect size than T-RAGT (SMD=0.04, 95% CI: -0.05, 0.13; P=0.38), as shown in Figure 3A.
Cadence
Thirteen studies were included, including 511 patients with lower limb dysfunction after stroke. Due to the heterogeneity test results (P<0.00001, I2=81%), a random effect model analysis was used. And as shown in Figure 3B, the results reflected that there was no significant statistical difference between the experimental group and the control group (SMD=2.04, 95% CI: -2.51, 6.60; P=0.38). Sensitivity analysis pointed out that the results showed satisfactory robustness after removing Joseph,43 as shown in Figure 4B.
Subgroup analysis of robot training type indicated that O-RAGT (SMD=7.29, 95% CI: 1.09, 13.49; P=0.02] showed a higher effect size than T-RAGT (SMD=-0.24, 95% CI: -5.99, 5.51; P=0.93, as shown in Figure 3B.
Affected side step length
Six studies were included, involving 266 patients with lower limb dysfunction after stroke. On account of the heterogeneity test results (P=0.38, I2=6%), a fixed effect model analysis was used. And as shown in Figure 5A, the results indicated that there was a statistical difference between the experimental group and the control group (SMD=0.02, 95% CI: 0.01, 0.03; P<0.0001).
Figure 5.
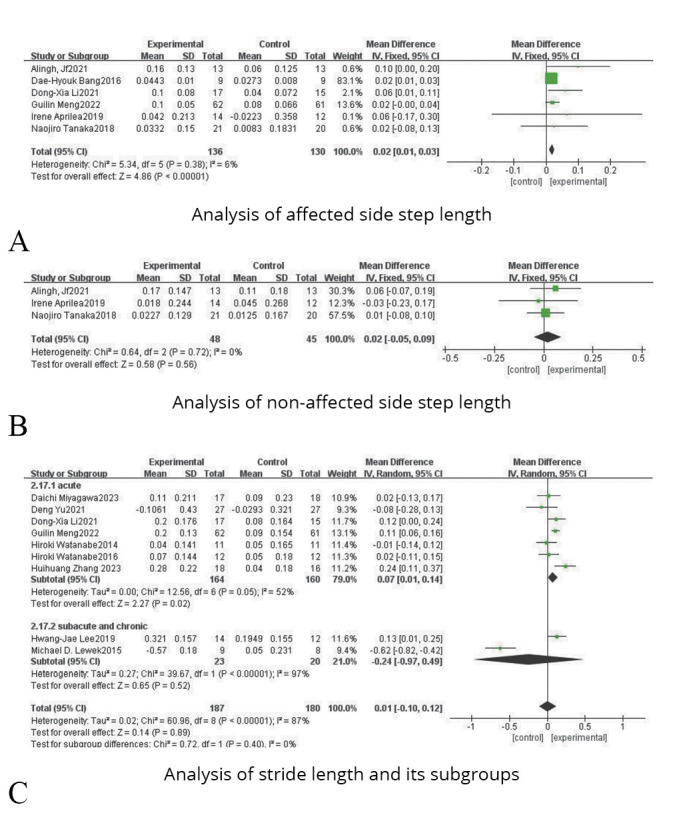
—Forest plots for the analysis of affected side step length, non-affected side step length, stride length and their subgroups.
Non-affected side step length
Three studies were included, involving 93 patients with lower limb dysfunction after stroke. On account of the heterogeneity test results (P=0.72, I2=0%), a fixed effect model analysis was used. And as shown in Figure 5B, the results indicated that there was no statistical difference between the experimental group and the control group (SMD=0.02, 95% CI: -0.05, 0.09; P=0.56).
Symmetry
It can be divided into spatial symmetry and temporal symmetry.
Regarding spatial symmetry, four studies were included, involving 119 patients with lower limb dysfunction after stroke. Due to the heterogeneity test results (P=0.12, I2=49%), a fixed effect model analysis was used. And as shown in Figure 6A, the results indicated that there was no statistical difference between the experimental group and the control group (SMD=0.00, 95% CI: -0.10, 0.11; P=0.98).
Figure 6.
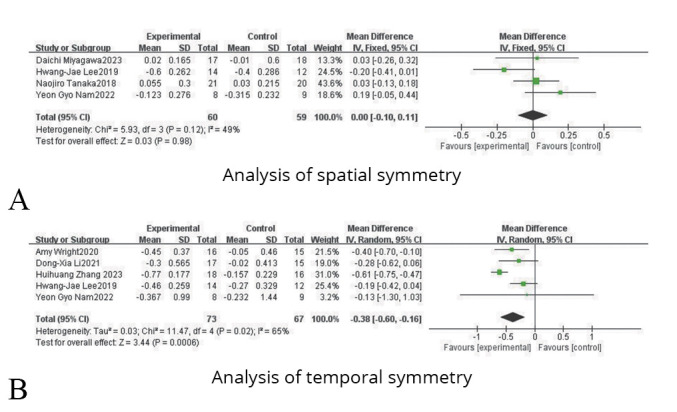
—Forest plots for the analysis of symmetry.
Regarding temporal symmetry, five studies were included, involving 140 patients with lower limb dysfunction after stroke. On account of the heterogeneity test results (P=0.02, I2=65%), a random effect model analysis was used. And as shown in Figure 6B, the results indicated that there was a statistical difference between the experimental group and the control group (SMD=-0.38, 95% CI: -0.6, -0.16; P=0.0006).
Six-Minute Walk Test (6MWT)
Sixteen studies were included, involving 813 patients with lower limb dysfunction after stroke. Due to the heterogeneity test results (P=0.0010, I2=60%), a random effect model analysis was used. And as shown in Figure 7A, the results indicated that there was a statistical difference between the experimental group and the control group (SMD=25.14, 95% CI: 10.19, 40.09; P=0.0010). Sensitivity analysis reminded that the results showed satisfactory robustness after eliminating Rustem27 and Sanaz,49 as shown in Figure 4C.
Figure 7.
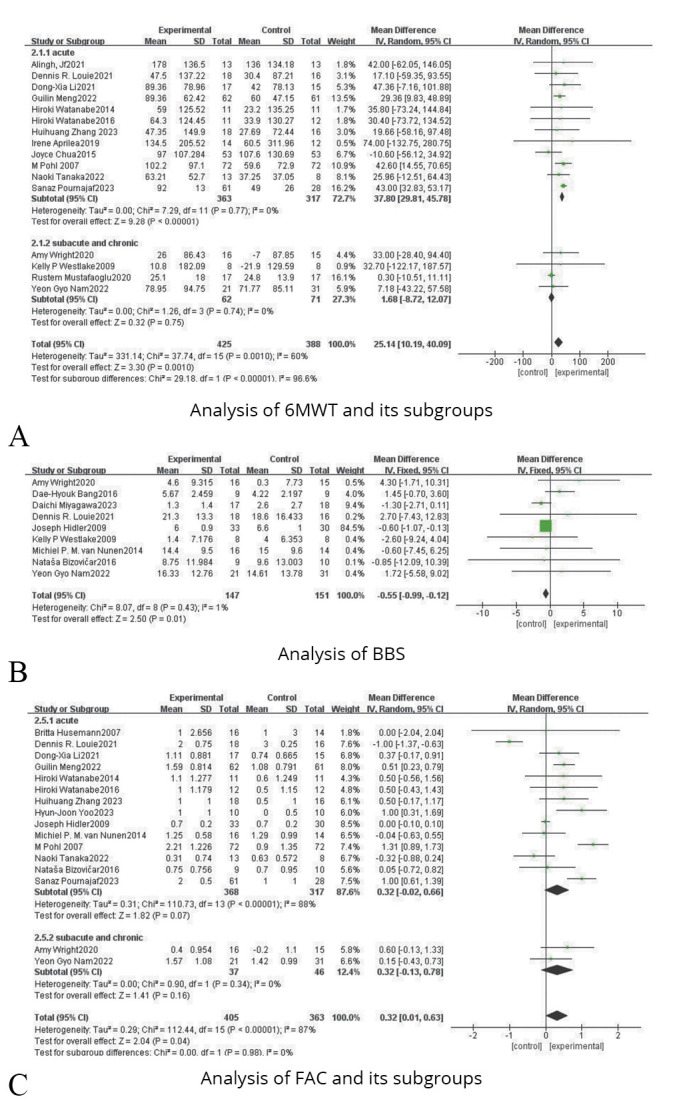
—Forest plots for the analysis of 6MWT, BBS, FAC and their subgroups.
Subgroup analysis of stroke onset time reflected that acute phase (SMD=37.80, 95% CI: 39.81, 45.78; P<0.00001) showed a better effect size than subacute or chronic phase (SMD=1.68, 95% CI: -8.72, 12.07; P=0.75), as shown in Figure 7A.
Berg Balance Scale (BBS)
Nine studies were included, including 298 patients with lower limb dysfunction after stroke. Due to the heterogeneity test results (P=0.43, I2=1%), a fixed effect model analysis was used. And as shown in Figure 7B, the results indicated that there was a statistical difference between the experimental group and the control group (SMD=-0.55, 95% CI: -0.99, -0.12; P=0.01).
Functional Ambulation Categories (FAC)
Sixteen studies were included, involving 768 patients with lower limb dysfunction after stroke. Due to the heterogeneity test results (P<0.00001, I2=87%), a random effect model analysis was used. And as shown in Figure 7C, the results indicated that there was a statistical difference between the experimental group and the control group (SMD=0.32, 95% CI: 0.01, 0.63; P=0.04).
Subgroup analysis of stroke onset time indicated that acute phase (SMD=0.32, 95% CI: -0.02, 0.66; P=0.07) showed a higher effect size than subacute or chronic phase (SMD=0.32, 95% CI: -0.13, 0.78; P=0.16), as shown in Figure 7C.
Angle of joint motion
It can be categorized into three aspects: affected hip, affected knee and affected ankle.
In terms of changes in the affected hip motion, four studies were included, involving 200 patients with lower limb dysfunction after stroke. Due to the heterogeneity test results (P=0.24, I2=29%), a fixed effect model analysis was used. And as shown in Figure 8A, the results indicated that there was no statistical difference between the experimental group and the control group (SMD=0.34; 95% CI: -1.62, 2.29; P=0.74).
Figure 8.
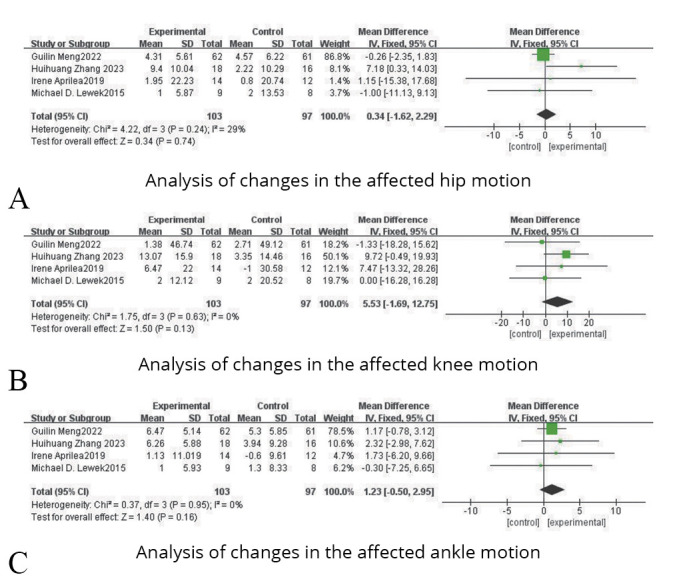
—Forest plots for the analysis of changes in the angle of joint motion.
In terms of changes in the affected knee motion, four studies were included, involving 200 patients with lower limb dysfunction after stroke. Due to the heterogeneity test results (P=0.63, I2=0%), a fixed effect model analysis was used. And as shown in Figure 8B, the results indicated that there was no statistical difference between the experimental group and the control group (SMD=5.53, 95% CI: -1.69, 12.75; P=0.13).
In terms of changes in the affected ankle motion, four studies were included, involving 200 patients with lower limb dysfunction after stroke. Due to the heterogeneity test results (P=0.95, I2=0%), a fixed effect model analysis was used. And as shown in Figure 8C, the results indicated that there was no statistical difference between the experimental group and the control group (SMD=1.23, 95% CI: -0.50, 2.95; P=0.16).
Stride length
Nine studies were included, including 367 patients with lower limb dysfunction after stroke. Due to the heterogeneity test results (P<0.00001, I2=87%), a random effect model analysis was used. And as shown in Figure 5C, the results indicated that there was no statistical difference between the experimental group and the control group (SMD=0.01, 95% CI: -0.10, 0.12; P=0.89).
Subgroup analysis of stroke onset time reflected that acute phase (SMD=0.07, 95% CI: 0.01, 0.14; P=0.02) showed a better effect size than subacute or chronic phase (SMD=-0.24, 95% CI: -0.97, 0.49; P=0.52), as shown in Figure 5C.
Publication bias
Most of the included studies used walking speed and 6MWT as outcome indicators. Furthermore, funnel plots (Figure 9) showed that there was less publication bias in both of them.
Figure 9.
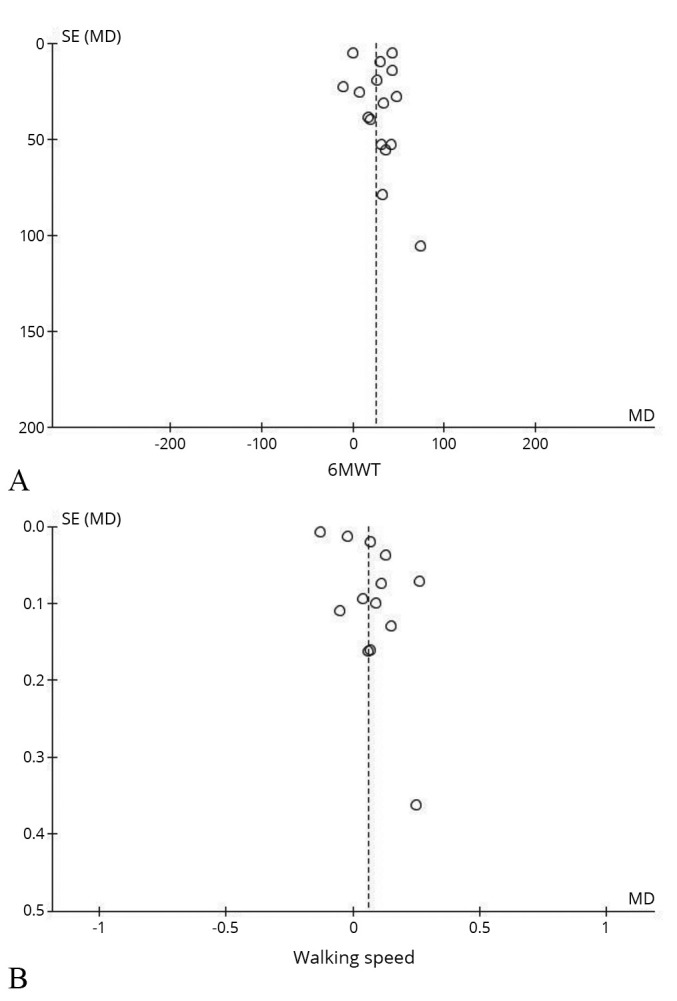
—Funnel plots.
Discussion
To investigate the specific effects of RAGT, researchers in randomized controlled trials compared O-RAGT or T-RAGT with CGT. Although RAGT requires less involvement from therapists, physicians, etc., it may be an improvement over traditional therapy. Our study found that most of the results were robust except for the three studies27, 43, 49 involved in two indicators, cadence and 6MWT. Random allocation and allocation concealment emerged high risk or unclear may be the main reasons for the unrobust merger results. In addition, our study showed that RAGT was associated with the changes of the affected side step length, temporal symmetry, 6MWT and FAC which had potential clinical benefits for patients. But compared with the control group, the experimental group had no significant advantages in walking speed, cadence, spatial symmetry, balance, angle of joint motion changes and stride length. RAGT for post-stroke walking dysfunction is a growing area of research, with about 60% of the studies in this study published in 2018 or later.
Although the studies included in this review used slightly different instruments to measure physical function, they all included walking speed in their measurements. Walking speed, a prognosticator of survival and disability in the elderly, was associated with a 12% lower risk of death for every 0.1 m/s increase over at least five years of follow-up. However, this study found that RAGT and non-RAGT had similar improvements in speed which seems surprising. Although there is no obvious correlation between RAGT and speed, most studies36, 52-54 believe that RAGT has a positive effect on the improvement of step speed. Tedla et al.55 reported that there was no significant difference between RAGT and CGT on speed improvement, which is consistent with the results of another meta-analysis10 and ours. Moreover, the results of our meta-analysis indicated that the experimental group and the control group had similar improvements in cadence. Nevertheless, its change does not directly and accurately reflect the improvement of motion patterns. Thus, it can be better understood by combining and considering the results of changes in other indicators.
Compared with speed and cadence, symmetry can better reflect the degree of injury, compensatory mechanism and recovery of stroke patients.56 It has been noted that RAGT has greater improvement in temporal and spatial parameters than CGT.33, 57 However, few studies have shown a significant association between RAGT and improvements in motor symmetry. Our results extend this evidence by showing that RAGT improves temporal symmetry but not spatial symmetry. Heidi et al.10 came to a similar conclusion in this regard. As the operating mode of RAGT is to set movement parameters in advance, patients may obtain a better physiological movement pattern that is closer to normal. Furthermore, self-driven training and training with properly afferent feedback can stimulate changes in motor cortex excitability.58 Thus more stable and rhythmic peripheral input may be the main reason for the improvement of temporal symmetry. And RAGT may promote the output of central motor control through the input of peripheral stimuli that mimic physiological gait patterns. Besides, intensive and repetitive assisted walking in coordination with voluntary motion may enhance motor relearning through neuroplasticity.
Spatial symmetry has more stringent requirements on balance, muscle strength, motor control and other aspects. Therefore, it is perhaps not surprising that RAGT does not show a significant correlation with it. Additionally, there was a significant difference in the improvement of affected side step length while there was not in the improvement of non-affected side step length. This suggests that the contribution of step length to spatial symmetry may be limited. And the changes in the improvement of the angle of joint motion were not significantly different between groups, which seems to confirm the limitations of RAGT in improving spatial symmetry. Yet, the limited sample size and the inconsistent quality of the evidence (including 1 medium-quality study40 and 3 high-quality studies25, 30, 50) impacted the stringency of the conclusion. Moreover, joint motion requires the cooperation of peripheral sensation, central control, muscle function and other aspects. Strong muscle function supports strong joint movement. In other words, muscle function may take precedence over joint performance. Therefore, it is necessary to further explore the relationship between muscle function and spatial symmetry. That is to explore the interaction between dynamics and kinematics. Notably, electromyography is a reliable approach.
A good endurance level plays a prominent role in improving walking ability after stroke. 6MWT is an important test to assess walking endurance which reflects functional compensatory ability for daily physical activity.59, 60 And few studies have focused on the endurance improvement of stroke patients by RAGT. Delightfully our study noticed this and indicated that RAGT had a better performance in improving endurance than the control group. Compared to CGT, RAGT can provide safer (with BWS) and higher-intensity (reaching running speed) training to promote the cardiorespiratory function of patients. Also, in this meta-analysis, RAGT significantly improved the FAC score, suggesting that it has advantages in enhancing the ability to walk on the ground and stairs independently. This finding is consistent with the results of a randomized controlled trial by Yeung et al.,61 which found that RAGT can reduce functional gait dependence and promote motor recovery. The ability of stroke patients to walk independently in the community was associated with increased walking speed.62 Although our study found no statistical difference in speed, its effect size is still worthy of recognition.
In summary, we confirmed that RAGT has positive effect on walking dysfunction after stroke. Proprioceptive input plays an important role in neuroplasticity. The interaction of proprioception, superficial senses and multisensory afferents on neuroplasticity could be the focus of further study.
Robot training type
Subgroup analysis revealed that O-RAGT had a better effect size than T-RAGT in terms of speed improvement. Compared to T-RAGT, which is attached to a fixed exoskeleton, O-RAGT has no restrictions on treadmill and allows walking training to be complete in a more realistic environment.16 Hence, patients treated with O-RAGT may exhibit greater autonomy of motivation. At the same time, due to the absence of BWS, O-RAGT may provide a stronger pressure sensation from the mechanoreceptors under foot soles than T-RAGT. Additionally, stronger sensory feedback is beneficial to stimulate plastic changes in gait patterns.63 Only when proprioceptive feedback was provided, cortical-muscular coherence increased with a predominant information flow from the sensorimotor cortex to the muscles.64 Thus future studies focused on exploring the impacts of different RAGTs on gait patterns could enhance the understanding of neuroplasticity. Moreover, the use of NIR and EEG may be able to contribute to this understanding.
In terms of cadence, subgroup analysis revealed a significantly better effect size in the O-RAGT group than T-RAGT. This finding corroborates that patients in the O-RAGT group may demonstrated a stronger self-drive in treatment. Yet, for FAC enhancement, the effect size was slightly better in the T-RAGT group. Only two31, 49 of the included studies in the O-RAGT group utilized BWS whereas all of the studies included in the T-RAGT group used BWS. And BWS can facilitate easier implementation of RAGT for patients with poorer functional status. And there is also more room for the improvement of functional independence among them. Consequently, it makes sense to have such a change.
Post-stroke time
After subgroup analysis, the heterogeneity decreased significantly, indicating that stroke onset time is probably the source of heterogeneity in 6MWT. Meanwhile, there exists a statistical difference between subgroups. The improvement of endurance was better in the acute stage than in the non-acute stage. In fact, the first six months of stroke are considered the golden period of treatment, so the superiority of functional improvement during this period is naturally self-evident. Although FAC and stride length did not have a statistical difference between subgroups, the differences in effect size could also reflect the superiority of early recovery from stroke to some extent.
Strengths of the study
One of the strengths of this study lies in the extensive database search that was conducted. This methodology not only amplifies the comprehensiveness of the review but also augments the likelihood of identifying a diverse range of studies, thereby offering a more holistic view of the current research landscape. Furthermore, this meta-study confirms the efficacy of RAGT in the treatment of walking dysfunction after stroke and supplements the literature on the effects of RAGT on kinematic parameters. Also, to our knowledge, this study fills the gap of differences in efficacy between different RAGTs. This effort could potentially foster a deeper understanding and facilitate advancements in the treatment of RAGT, providing a theoretical foundation for further research and discussions in this field, as well as informing clinical decision-making.
Limitations of the study
The main limitation of this study is the lack of a meta-analysis of different RAGT treatment protocols and systems. The operation of some RAGT systems like HAL requires specially trained personnel. Although instrumental measurements largely make the results more objective, differences in protocols and systems are still inconsistent factors that may give rise to some heterogeneity. Additionally, some studies may cause potential bias due to the difficulty of implementing blind methods. It inevitably casts a shadow on the overall findings. While this does not invalidate our results, it does necessitate a more cautious and discerning interpretation.
Conclusions
According to the available data, RAGT plays a role in the improvement of walking dysfunction after stroke. Also, RAGT does perform better in some kinematic indexes compared with non-RAGT training. O-RAGT may be superior to T-RAGT. However, whether it is superior to CGT needs to be demonstrated in further studies. These conclusions should be viewed with caution in light of the recognized shortcomings of the existing studies. Further large-scale multi-center studies are urgently needed which compare different treatment regimens and RAGT devices.
Supplementary Digital Material 1
Supplementary Text File 1
Search strategy for PubMed
Supplementary Digital Material 2
Supplementary Table I
Descriptive analysis of the included clinical studies.
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.Callegari B, Garcez DR, Júnior AT, Almeida AD, Candeira SR, do Nascimento NI, et al. Gait patterns in ischemic and hemorrhagic post-stroke patients with delayed access to physiotherapy. Hong Kong Physiother J 2021;41:77–87. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34177196&dopt=Abstract 10.1142/S1013702521500074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez-Fernández A, Lobo-Prat J, Font-Llagunes JM. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J Neuroeng Rehabil 2021;18:22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33526065&dopt=Abstract 10.1186/s12984-021-00815-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wevers L, van de Port I, Vermue M, Mead G, Kwakkel G. Effects of task-oriented circuit class training on walking competency after stroke: a systematic review. Stroke 2009;40:2450–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19461035&dopt=Abstract 10.1161/STROKEAHA.108.541946 [DOI] [PubMed] [Google Scholar]
- 4.Verma R, Arya KN, Sharma P, Garg RK. Understanding gait control in post-stroke: implications for management. J Bodyw Mov Ther 2012;16:14–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22196422&dopt=Abstract 10.1016/j.jbmt.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 5.Lord SE, Rochester L. Measurement of community ambulation after stroke: current status and future developments. Stroke 2005;36:1457–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15947265&dopt=Abstract 10.1161/01.STR.0000170698.20376.2e [DOI] [PubMed] [Google Scholar]
- 6.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil 2005;86:1552–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16084807&dopt=Abstract 10.1016/j.apmr.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 7.van Herk IE, Arendzen JH, Rispens P. Ten-metre walk, with or without a turn?. Clin Rehabil 1998;12:30–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9549023&dopt=Abstract 10.1191/026921598667081596 [DOI] [PubMed] [Google Scholar]
- 8.Kim K, Lee S, Lee K. Effects of Progressive Body Weight Support Treadmill Forward and Backward Walking Training on Stroke Patients’ Affected Side Lower Extremity’s Walking Ability. J Phys Ther Sci 2014;26:1923–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25540499&dopt=Abstract 10.1589/jpts.26.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrò RS, Sorrentino G, Cassio A, Mazzoli D, Andrenelli E, Bizzarini E, et al. Italian Consensus Conference on Robotics in Neurorehabilitation (CICERONE) . Robotic-assisted gait rehabilitation following stroke: a systematic review of current guidelines and practical clinical recommendations. Eur J Phys Rehabil Med 2021;57:460–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33947828&dopt=Abstract 10.23736/S1973-9087.21.06887-8 [DOI] [PubMed] [Google Scholar]
- 10.Nedergård H, Arumugam A, Sandlund M, Bråndal A, Häger CK. Effect of robotic-assisted gait training on objective biomechanical measures of gait in persons post-stroke: a systematic review and meta-analysis. J Neuroeng Rehabil 2021;18:64. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33863345&dopt=Abstract 10.1186/s12984-021-00857-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morone G, Paolucci S, Cherubini A, De Angelis D, Venturiero V, Coiro P, et al. Robot-assisted gait training for stroke patients: current state of the art and perspectives of robotics. Neuropsychiatr Dis Treat 2017;13:1303–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28553117&dopt=Abstract 10.2147/NDT.S114102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hramov AE, Grubov V, Badarin A, Maksimenko VA, Pisarchik AN. Functional Near-Infrared Spectroscopy for the Classification of Motor-Related Brain Activity on the Sensor-Level. Sensors (Basel) 2020;20:2362. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32326270&dopt=Abstract 10.3390/s20082362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrò RS, Cacciola A, Bertè F, Manuli A, Leo A, Bramanti A, et al. Robotic gait rehabilitation and substitution devices in neurological disorders: where are we now? Neurol Sci 2016;37:503–14. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26781943&dopt=Abstract 10.1007/s10072-016-2474-4 [DOI] [PubMed] [Google Scholar]
- 14.Jarrassé N, Proietti T, Crocher V, Robertson J, Sahbani A, Morel G, et al. Robotic exoskeletons: a perspective for the rehabilitation of arm coordination in stroke patients. Front Hum Neurosci 2014;8:947. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25520638&dopt=Abstract 10.3389/fnhum.2014.00947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimaldi G, Manto M. Functional impacts of exoskeleton-based rehabilitation in chronic stroke: multi-joint versus single-joint robotic training. J Neuroeng Rehabil 2013;10:113. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24354518&dopt=Abstract 10.1186/1743-0003-10-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calafiore D, Negrini F, Tottoli N, Ferraro F, Ozyemisci-Taskiran O, de Sire A. Efficacy of robotic exoskeleton for gait rehabilitation in patients with subacute stroke: a systematic review. Eur J Phys Rehabil Med 2022;58:1–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34247470&dopt=Abstract 10.23736/S1973-9087.21.06846-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen W, Fan X, Wang L, Zhang Y. Traditional Chinese Medicine for Post-Stroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Front Pharmacol 2022;13:816333. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35237166&dopt=Abstract 10.3389/fphar.2022.816333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cha HG, Kim MK. Effects of strengthening exercise integrated repetitive transcranial magnetic stimulation on motor function recovery in subacute stroke patients: A randomized controlled trial. Technol Health Care 2017;25:521–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28106573&dopt=Abstract 10.3233/THC-171294 [DOI] [PubMed] [Google Scholar]
- 19.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003;83:713–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12882612&dopt=Abstract 10.1093/ptj/83.8.713 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 64; 2023 [Internet]. Available from: https://training.cochrane.org/handbook [cited 2024, Apr 9].
- 21.Lemes ÍR, Ferreira PH, Linares SN, Machado AF, Pastre CM, Netto J. Resistance training reduces systolic blood pressure in metabolic syndrome: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 2016;50:1438–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26964146&dopt=Abstract 10.1136/bjsports-2015-094715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–805. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27683581&dopt=Abstract 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 23.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25524443&dopt=Abstract 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12111919&dopt=Abstract 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 25.Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby TG. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther 2009;89:829–39. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19520734&dopt=Abstract 10.2522/ptj.20080180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo HJ, Bae CR, Jeong H, Ko MH, Kang YK, Pyun SB. Clinical efficacy of overground powered exoskeleton for gait training in patients with subacute stroke: A randomized controlled pilot trial. Medicine (Baltimore) 2023;102:e32761. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36705351&dopt=Abstract 10.1097/MD.0000000000032761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mustafaoglu R, Erhan B, Yeldan I, Gunduz B, Tarakci E. Does robot-assisted gait training improve mobility, activities of daily living and quality of life in stroke? A single-blinded, randomized controlled trial. Acta Neurol Belg 2020;120:335–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31989505&dopt=Abstract 10.1007/s13760-020-01276-8 [DOI] [PubMed] [Google Scholar]
- 28.Alingh JF, Fleerkotte BM, Groen BE, Rietman JS, Weerdesteyn V, van Asseldonk EH, et al. Effect of assist-as-needed robotic gait training on the gait pattern post stroke: a randomized controlled trial. J Neuroeng Rehabil 2021;18:26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33546733&dopt=Abstract 10.1186/s12984-020-00800-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright A, Stone K, Martinelli L, Fryer S, Smith G, Lambrick D, et al. Effect of combined home-based, overground robotic-assisted gait training and usual physiotherapy on clinical functional outcomes in people with chronic stroke: A randomized controlled trial. Clin Rehabil 2021;35:882–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33356519&dopt=Abstract 10.1177/0269215520984133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng G, Ma X, Chen P, Xu S, Li M, Zhao Y, et al. Effect of early integrated robot-assisted gait training on motor and balance in patients with acute ischemic stroke: a single-blinded randomized controlled trial. Ther Adv Neurol Disord 2022;15:17562864221123195. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36147622&dopt=Abstract 10.1177/17562864221123195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka N, Ebihara K, Ebata Y, Yano H. Effect of gait rehabilitation with a footpad-type locomotion interface on gait ability in subacute stroke patients. NeuroRehabilitation 2022;50:401–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35068423&dopt=Abstract 10.3233/NRE-210317 [DOI] [PubMed] [Google Scholar]
- 32.Li DX, Zha FB, Long JJ, Liu F, Cao J, Wang YL. Effect of Robot Assisted Gait Training on Motor and Walking Function in Patients with Subacute Stroke: A Random Controlled Study. J Stroke Cerebrovasc Dis 2021;30:105807. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33895428&dopt=Abstract 10.1016/j.jstrokecerebrovasdis.2021.105807 [DOI] [PubMed] [Google Scholar]
- 33.Tanaka N, Matsushita S, Sonoda Y, Maruta Y, Fujitaka Y, Sato M, et al. Effect of Stride Management Assist Gait Training for Poststroke Hemiplegia: A Single Center, Open-Label, Randomized Controlled Trial. J Stroke Cerebrovasc Dis 2019;28:477–86. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30420315&dopt=Abstract 10.1016/j.jstrokecerebrovasdis.2018.10.025 [DOI] [PubMed] [Google Scholar]
- 34.Watanabe H, Goto R, Tanaka N, Matsumura A, Yanagi H. Effects of gait training using the Hybrid Assistive Limb® in recovery-phase stroke patients: A 2-month follow-up, randomized, controlled study. NeuroRehabilitation 2017;40:363–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28222558&dopt=Abstract 10.3233/NRE-161424 [DOI] [PubMed] [Google Scholar]
- 35.Husemann B, Müller F, Krewer C, Heller S, Koenig E. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke 2007;38:349–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17204680&dopt=Abstract 10.1161/01.STR.0000254607.48765.cb [DOI] [PubMed] [Google Scholar]
- 36.Bang DH, Shin WS. Effects of robot-assisted gait training on spatiotemporal gait parameters and balance in patients with chronic stroke: A randomized controlled pilot trial. NeuroRehabilitation 2016;38:343–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27061162&dopt=Abstract 10.3233/NRE-161325 [DOI] [PubMed] [Google Scholar]
- 37.Chua J, Culpan J, Menon E. Efficacy of an Electromechanical Gait Trainer Poststroke in Singapore: A Randomized Controlled Trial. Arch Phys Med Rehabil 2016;97:683–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26802969&dopt=Abstract 10.1016/j.apmr.2015.12.025 [DOI] [PubMed] [Google Scholar]
- 38.Louie DR, Mortenson WB, Durocher M, Schneeberg A, Teasell R, Yao J, et al. Efficacy of an exoskeleton-based physical therapy program for non-ambulatory patients during subacute stroke rehabilitation: a randomized controlled trial. J Neuroeng Rehabil 2021;18:149. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34629104&dopt=Abstract 10.1186/s12984-021-00942-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nam YG, Ko MJ, Bok SK, Paik NJ, Lim CY, Lee JW, et al. Efficacy of electromechanical-assisted gait training on clinical walking function and gait symmetry after brain injury of stroke: a randomized controlled trial. Sci Rep 2022;12:6880. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35477986&dopt=Abstract 10.1038/s41598-022-10889-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aprile I, Iacovelli C, Goffredo M, Cruciani A, Galli M, Simbolotti C, et al. Efficacy of end-effector Robot-Assisted Gait Training in subacute stroke patients: clinical and gait outcomes from a pilot bi-centre study. NeuroRehabilitation 2019;45:201–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31498139&dopt=Abstract 10.3233/NRE-192778 [DOI] [PubMed] [Google Scholar]
- 41.Miyagawa D, Matsushima A, Maruyama Y, Mizukami N, Tetsuya M, Hashimoto M, et al. Gait training with a wearable powered robot during stroke rehabilitation: a randomized parallel-group trial. J Neuroeng Rehabil 2023;20:54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37118743&dopt=Abstract 10.1186/s12984-023-01168-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe H, Tanaka N, Inuta T, Saitou H, Yanagi H. Locomotion improvement using a hybrid assistive limb in recovery phase stroke patients: a randomized controlled pilot study. Arch Phys Med Rehabil 2014;95:2006–12. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25010538&dopt=Abstract 10.1016/j.apmr.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 43.Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair 2009;23:5–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19109447&dopt=Abstract 10.1177/1545968308326632 [DOI] [PubMed] [Google Scholar]
- 44.Bizovičar N, Matjačić Z, Stanonik I, Goljar N. Overground gait training using a motorized assistive device in patients with severe disabilities after stroke. Int J Rehabil Res 2017;40:46–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27779500&dopt=Abstract 10.1097/MRR.0000000000000199 [DOI] [PubMed] [Google Scholar]
- 45.Westlake KP, Patten C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J Neuroeng Rehabil 2009;6:18. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19523207&dopt=Abstract 10.1186/1743-0003-6-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Nunen MP, Gerrits KH, Konijnenbelt M, Janssen TW, de Haan A. Recovery of walking ability using a robotic device in subacute stroke patients: a randomized controlled study. Disabil Rehabil Assist Technol 2015;10:141–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24611590&dopt=Abstract 10.3109/17483107.2013.873489 [DOI] [PubMed] [Google Scholar]
- 47.Pohl M, Werner C, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, et al. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: a single-blind, randomized multicentre trial (DEutsche GAngtrainerStudie, DEGAS). Clin Rehabil 2007;21:17–27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17213237&dopt=Abstract 10.1177/0269215506071281 [DOI] [PubMed] [Google Scholar]
- 48.Yu D, Yang Z, Lei L, Chaoming N, Ming W. Robot-Assisted Gait Training Plan for Patients in Poststroke Recovery Period: A Single Blind Randomized Controlled Trial. BioMed Res Int 2021;2021:5820304. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34497851&dopt=Abstract 10.1155/2021/5820304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pournajaf S, Calabrò RS, Naro A, Goffredo M, Aprile I, Tamburella F, et al. ; TreadStroke Group. Robotic versus Conventional Overground Gait Training in Subacute Stroke Survivors: A Multicenter Controlled Clinical Trial. J Clin Med 2023;12:439. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36675371&dopt=Abstract 10.3390/jcm12020439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Li X, Gong Y, Wu J, Chen J, Chen W, et al. Three-Dimensional Gait Analysis and sEMG Measures for Robotic-Assisted Gait Training in Subacute Stroke: A Randomized Controlled Trial. BioMed Res Int 2023;2023:7563802. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37082189&dopt=Abstract 10.1155/2023/7563802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HJ, Lee SH, Seo K, Lee M, Chang WH, Choi BO, et al. Training for walking efficiency with a wearable hip-assist robot in patients with stroke a pilot randomized controlled trial. Stroke 2019;50:3545–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31623545&dopt=Abstract 10.1161/STROKEAHA.119.025950 [DOI] [PubMed] [Google Scholar]
- 52.Nam YG, Lee JW, Park JW, Lee HJ, Nam KY, Park JH, et al. Effects of Electromechanical Exoskeleton-Assisted Gait Training on Walking Ability of Stroke Patients: A Randomized Controlled Trial. Arch Phys Med Rehabil 2019;100:26–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30055163&dopt=Abstract 10.1016/j.apmr.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 53.Moucheboeuf G, Griffier R, Gasq D, Glize B, Bouyer L, Dehail P, et al. Effects of robotic gait training after stroke: A meta-analysis. Ann Phys Rehabil Med 2020;63:518–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32229177&dopt=Abstract 10.1016/j.rehab.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 54.Schröder J, Truijen S, Van Criekinge T, Saeys W. Feasibility and effectiveness of repetitive gait training early after stroke: A systematic review and meta-analysis. J Rehabil Med 2019;51:78–88. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30516821&dopt=Abstract 10.2340/16501977-2505 [DOI] [PubMed] [Google Scholar]
- 55.Tedla JS, Dixit S, Gular K, Abohashrh M. Robotic-Assisted Gait Training Effect on Function and Gait Speed in Subacute and Chronic Stroke Population: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur Neurol 2019;81:103–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31167193&dopt=Abstract 10.1159/000500747 [DOI] [PubMed] [Google Scholar]
- 56.Allen JL, Kautz SA, Neptune RR. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait Posture 2011;33:538–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21316240&dopt=Abstract 10.1016/j.gaitpost.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calabrò RS, Naro A, Russo M, Bramanti P, Carioti L, Balletta T, et al. Shaping neuroplasticity by using powered exoskeletons in patients with stroke: a randomized clinical trial. J Neuroeng Rehabil 2018;15:35. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29695280&dopt=Abstract 10.1186/s12984-018-0377-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehrholz J, Thomas S, Werner C, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev 2017;5:CD006185. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28488268&dopt=Abstract [DOI] [PMC free article] [PubMed]
- 59.Ng SS, Tsang WW, Cheung TH, Chung JS, To FP, Yu PC. Walkway length, but not turning direction, determines the six-minute walk test distance in individuals with stroke. Arch Phys Med Rehabil 2011;92:806–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21530729&dopt=Abstract 10.1016/j.apmr.2010.10.033 [DOI] [PubMed] [Google Scholar]
- 60.Agarwala P, Salzman SH. Six-Minute Walk Test: Clinical Role, Technique, Coding, and Reimbursement. Chest 2020;157:603–11. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31689414&dopt=Abstract 10.1016/j.chest.2019.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeung LF, Ockenfeld C, Pang MK, Wai HW, Soo OY, Li SW, et al. Randomized controlled trial of robot-assisted gait training with dorsiflexion assistance on chronic stroke patients wearing ankle-foot-orthosis. J Neuroeng Rehabil 2018;15:51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29914523&dopt=Abstract 10.1186/s12984-018-0394-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 1995;26:982–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7762050&dopt=Abstract 10.1161/01.STR.26.6.982 [DOI] [PubMed] [Google Scholar]
- 63.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res 2008;51:S225–39. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18230848&dopt=Abstract 10.1044/1092-4388(2008/018) [DOI] [PubMed] [Google Scholar]
- 64.Khademi F, Naros G, Nicksirat A, Kraus D, Gharabaghi A. Rewiring cortico-muscular control in the healthy and post-stroke human brain with proprioceptive beta-band neurofeedback. J Neurosci 2022;42:6861–77. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35940874&dopt=Abstract 10.1523/JNEUROSCI.1530-20.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text File 1
Search strategy for PubMed
Supplementary Table I
Descriptive analysis of the included clinical studies.


