Abstract
Many cardiology associations endorse the role of the cardiopulmonary exercise test (CPET) to define the severity of impairment of functional capacity in individuals with heart failure with reduced ejection fraction (HFrEF) and when evaluating the need for advanced therapies for these patients. The focus of the CPET within the cardiology community has been on peak volume of oxygen uptake (VO2). However, several CPET variables are associated with outcomes in individuals with and without chronic disease and can inform clinical decisions in individuals with HFrEF. In this manuscript, we will review the normal cardiopulmonary response to a graded exercise test and review current guideline recommendations relative to CPET in patients with HFrEF.
Keywords: Cardiology, Exercise testing, Oxygen uptake, Risk assessment
Introduction
Many cardiology associations endorse the role of the cardiopulmonary exercise test (CPET) to define the severity of impairment of functional capacity in individuals with heart failure with reduced ejection fraction (HFrEF) and when evaluating the need for advanced therapies in these patients [1–5]. Starting with the seminal study by Mancini et al. [6], the focus of the CPET within the cardiology community has been on peak volume of oxygen uptake (VO2). However, several CPET variables (e.g., ventilatory efficiency, oxygen pulse, and breathing reserve) are associated with outcomes in individuals with and without chronic disease and can inform clinical decisions in individuals with HFrEF. In this manuscript, we review the normal cardiopulmonary response to a graded exercise test and current guideline recommendations relative to CPET in patients with HFrEF.
Overview of cardiopulmonary exercise testing
During a CPET, expired gas from the patient is continuously measured by a metabolic cart while the patient exercises at increasing intensities (e.g., graded exercise test). The test is typically performed on a treadmill or leg (cycle) ergometer. An exercise protocol is selected to allow a patient to start at a very low exercise intensity and progresses until they reach their maximum tolerance or a clinical sign or symptom is noted indicating exercise should be stopped. An exercise protocol is typically selected with the goal exercise duration of about 10 min. There are several excellent resources that extensively cover exercise testing procedures, data collection, and averaging methodology available [7–9].
Nearly all metabolic carts approved for clinical use measure expired gas on a breath-by-breath frequency. The data is then reported in intervals of 10–30 s. The primary measurements made by the metabolic cart are volume of expired gas, respiratory rate, and concentration of oxygen and carbon dioxide. From these measurements and data on the temperature, humidity, and barometric pressure of the laboratory, various CPET variables are derived including VO2, volume of carbon dioxide output (VCO2), and minute ventilation (VE). Calibration and maintenance of the metabolic cart per manufacture recommendations, along with appropriately trained staff, are critical to valid and repeatable CPET data [10]. With appropriately trained staff and quality assurance procedures [11], the coefficient of variation of various CPET variables among patients with HFrEF is 4–10% [12]. While there are no formal accreditation requirements for CPET laboratories, there are some minimal standards for software considerations, system maintenance, and quality control that are important [8, 9]. However, minimal standards for staff training are lacking. Additional noteworthy items are provided in Table 1.
Table 1.
Additional noteworthy items when considering a cardiopulmonary exercise test (CPET)
| • Peak VO2 is ~ 10% higher when a test is performed on a treadmill compared to an upright leg (cycle) ergometer |
| • A CPET cannot be performed on patients who require supplemental oxygen without additional equipment that might not be available in your local laboratory |
| • A CPET requires a sign/symptom-limited maximal effort by the patient |
| • It is critical that CPET staff are trained in the normal and anticipated pathologic response of CPET variables |
| • On some metabolic carts, the respiratory exchange ratio (RER) is labelled the respiratory quotient (RQ) |
| • Performing a CPET with invasive monitoring of arterial/pulmonary pressures along with blood gases provides measured oxygen uptake for the calculation of the Fick-derived cardiac output |
Cardiopulmonary exercise test response
During acute exercise, skeletal muscle cells meet the energy demands of work through aerobic and anaerobic metabolic pathways. As the exercise workload increases during a CPET, additional muscle fibers are recruited. As a result, VO2, VCO2, and VE increase with increasing exercise intensity. An example of a normal individual during a CPET using the Bruce treadmill protocol is shown in Fig. 1. As shown in Fig. 1A, during early exercise, VO2 and VCO2 increase at a similar rate. As exercise intensity progresses, an increasing number of muscle cells augment the production of adenosine triphosphate (ATP) from aerobic metabolism through anaerobic glycolysis which results in increasing concentrations of blood lactate. The resultant metabolic acidosis is buffered by bicarbonate and can be observed in the increasing rate of VCO2 output. The respiratory centers of the autonomic nervous system respond to increasing hydrogen ion concentration by increasing VE [10]. This is shown in the near linear response of VCO2 and VE (Fig. 1B). The slope of the change in VE relative to change in VCO2 is called the VE/VCO2 slope (aka, ventilatory efficiency).
Fig. 1.
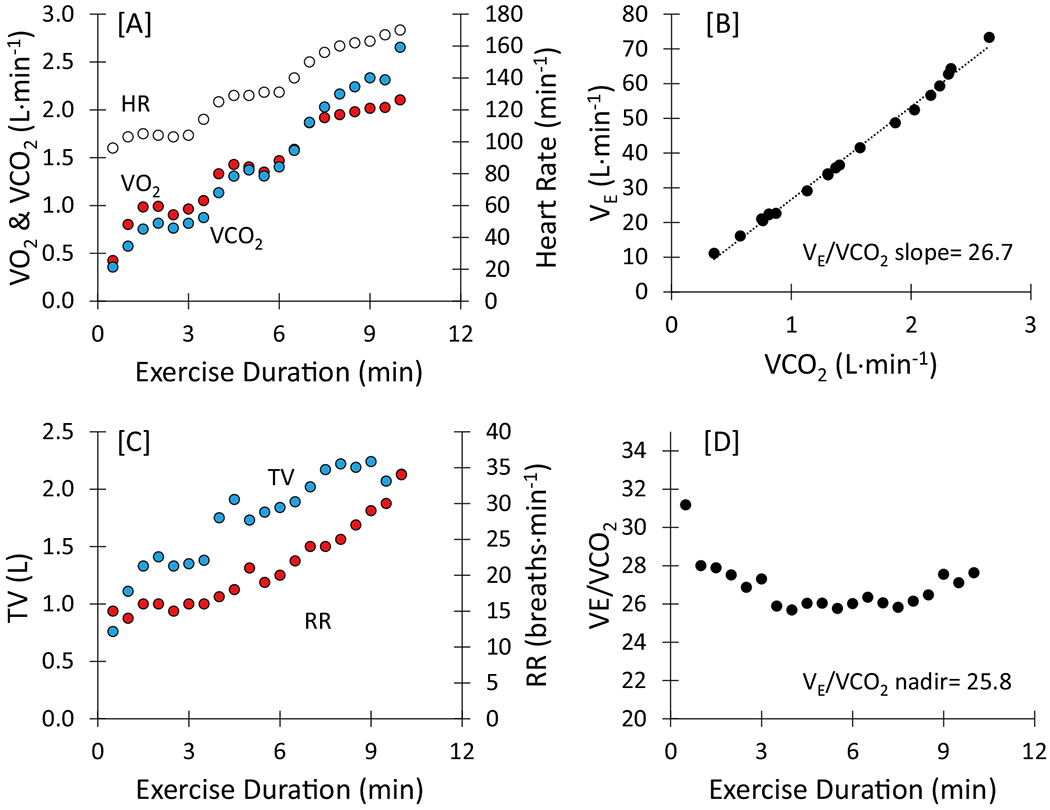
Exercise response of an apparently healthy individual who performed the Bruce treadmill protocol to maximum capacity. HR, heart rate; RR, respiratory rate; TV, tidal volume; VCO2, volume of carbon dioxide output; VE, minute ventilation; VO2, volume of oxygen uptake
In the simplest sense, the ability to increase VO2 to meet the metabolic oxygen demand is dependent on the capacity to transport and utilize oxygen. This is described in the Fick equation that is rearranged to solve for VO2 (see Fig. 2, Eqs. 1a and 1b). In this equation, the transport and utilization of oxygen are conditional on cardiac output and the arterial-mixed venous oxygen content difference, respectively. Increases in cardiac output are dependent on increases in heart rate and stroke volume [10]. And increases in arterial-mixed venous oxygen content difference are dependent on the diffusing capacity of the lungs and skeletal muscle, oxygen capacity of arterial blood, distribution of blood to the lungs and skeletal muscle, oxidative capacity of skeletal muscle, and oxyhemoglobin dissociation [10]. Factors that hinder the transportation and utilization of oxygen will limit the individual’s ability to increase VO2 and result in exercise intolerance.
Fig. 2.
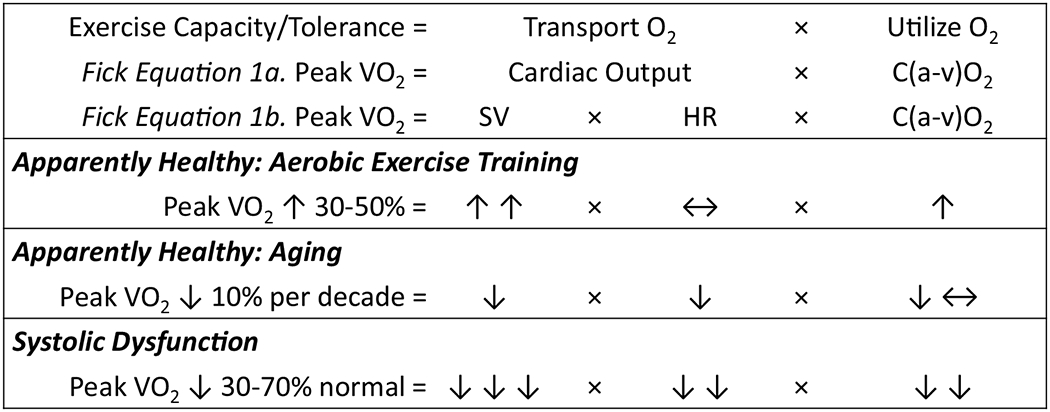
Factors contributing to the ability to transport and utilize oxygen in apparently healthy individuals and those with systolic dysfunction. C(a-v)O2, arterial-mixed venous oxygen content difference; HR, heart rate; SV, stroke volume; VO2, volume of oxygen uptake. Upward arrow (↑), increase; downward arrow (↓), decrease; left right arrow (↔), no-to-minimal change
The response of factors represented in the Fick equation and their collective effect on exercise capacity in healthy individuals and those with systolic dysfunction is shown in Fig. 2. As shown in Fig. 2, increases in peak VO2 are seen in healthy individuals after an aerobic exercise training program due to the ability to increase both stroke volume and the extraction of oxygen by skeletal muscle. Normal aging results in reductions in peak stroke volume and heart rate. Patients with systolic dysfunction have a peak VO2 that is as much as 70% below age-matched norms largely due to a reduced ability to increase stroke volume and heart rate, and a reduced capacity to metabolize oxygen by skeletal muscle. In addition to low peak stroke volume, chronotropic incompetence, and skeletal muscle abnormalities, additional limiters include endothelial dysfunction, pulmonary hypertension, right ventricular dysfunction, and anemia [10]. Because of the multiple factors that contribute to exercise intolerance in patients with HFrEF, resting ejection fraction is not an accurate predictor of peak VO2 (Fig. 3).
Fig. 3.
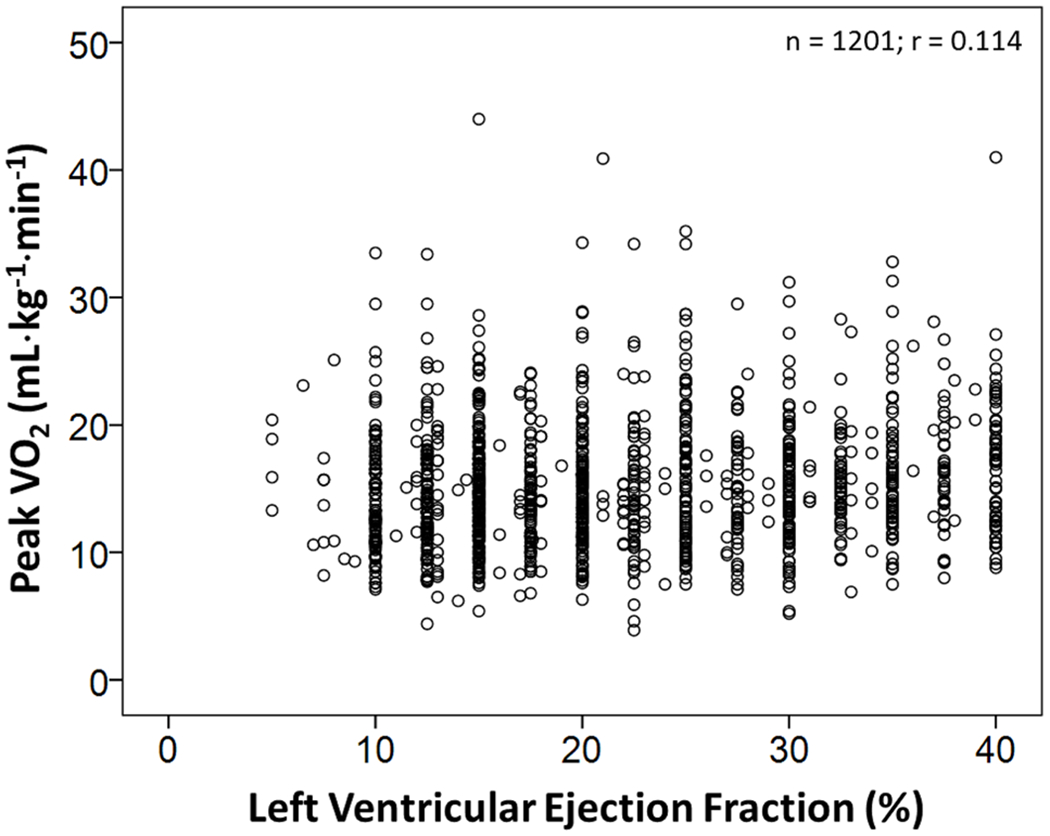
Scatter plot of resting left ventricular ejection fraction and peak oxygen uptake (VO2) in patients with heart failure with reduced ejection fraction tested at Henry Ford Hospital
A submaximal exercise test would result in an underestimated exercise capacity. Therefore, the goal of a CPET should be a sign/symptom-limited effort by the patient. The respiratory exchange ratio (RER; VCO2/VO2) provides an estimate of the degree of cardiometabolic stress. As a measure of whole-body gas exchange, RER is affected by ventilatory patterns and the composition of metabolites being consumed which changes during a CPET. During steady-state conditions (e.g., rest), RER is equivalent to the respiratory quotient, which is the analogous measure at the cellular level. RER is typically 0.85–0.95 at rest but can be > 1 if the patient is overbreathing or when oscillatory ventilation is present. During early exercise, RER will remain steady at 0.75–0.90 (or decline to < 0.90 if > 1 at rest) and then gradually increase up to values as high as ~ 1.3. Various peak RER criteria have been used to define a maximal cardiometabolic stress [13]. The International Society for Heart Lung Transplantation (ISHLT) defines a submaximal stress at RER ≤ 1.05 [1, 13].
During exercise, normal individuals typically have ample pulmonary reserve despite reaching their peak VO2 [14]. In other words, in individuals without high levels of aerobic training, the lungs typically do not impose a limitation on exercise capacity [15]. If a patient is limited from a pulmonary standpoint, they usually have one of the following limitations: a mechanical ventilatory limitation, a diffusion abnormality, or a gas exchange abnormality.
A mechanical ventilatory limitation is typically identified based on an individual’s ventilatory reserve (aka, breathing reserve) at peak exercise. Ventilatory reserve is the percent difference between an individual’s peak VE and their maximal ventilatory capacity. Maximal ventilatory capacity during exercise is estimated by measuring the maximal voluntary ventilation (MVV) or estimating MVV from the forced expiratory volume in 1 s (FEV1) [9, 10]. The latter is defined as estimated MVV = FEV1 × k, where k is a value of 35 to 40 [9, 10, 16, 17] which is a hypothetical maximal respiratory rate.
In the normal ventilatory response during a CPET, initial increases in VE are due to increases in tidal volume. Tidal volume continues to increase through about 60–70% of exercise capacity, and additional increases in VE are due to increases in respiratory rate (see Fig. 1C). At maximal effort, VE typically reaches 70 to 85% MVV (≥ 15% breathing reserve) in healthy individuals [9, 10]. If a patient’s VE at peak exercise is > 85% MVV (< 15% breathing reserve), they do not have adequate pulmonary reserve to continue to exercise and are mechanical ventilatory limited [18, 19].
Not all patients with a mechanical ventilatory limitation will have a breathing reserve < 15% during CPET; there may be more subtle abnormalities. A healthy individual should be able to augment tidal volume during exercise to 2–3 times their resting value [18–20]. In addition, they should be able to achieve a maximal tidal volume that is approximately 50–60% of their resting vital capacity [21]. Finally, the normal response for respiratory rate does not typically exceed 50 breaths.min−1 even at peak exercise [10]. Rapid, shallow breathing is an inefficient means to exchange gas, and a high respiratory rate (with a likely corresponding limitation in tidal volume) can also be an example of a mechanical ventilatory limitation.
A mechanical ventilatory limitation can also be defined by dynamic hyperinflation. When patients begin to exercise, they will have a corresponding increase in their tidal volume and respiratory rates secondary to increases in ventilatory demand. If expiratory time during exercise is insufficient to return to baseline end-expiratory lung volume, patients will begin to trap gas. This process is referred to as dynamic hyperinflation [22]. As exercise continues, dynamic hyperinflation can lead to mechanical inefficiency and reduction in tidal volume and prevent necessary augmentation of VE [22]. This process will ultimately accelerate a ventilatory limitation [22]. Dynamic hyperinflation is shown in Fig. 4. Note the decreasing tidal volume despite increasing workload. While it is feasible patients with HFrEF can develop air trapping and dynamic hyperinflation during an exercise test, it is typically the result of other comorbid conditions, especially reactive airway disease, asthma, or chronic obstructive pulmonary disease.
Fig. 4.
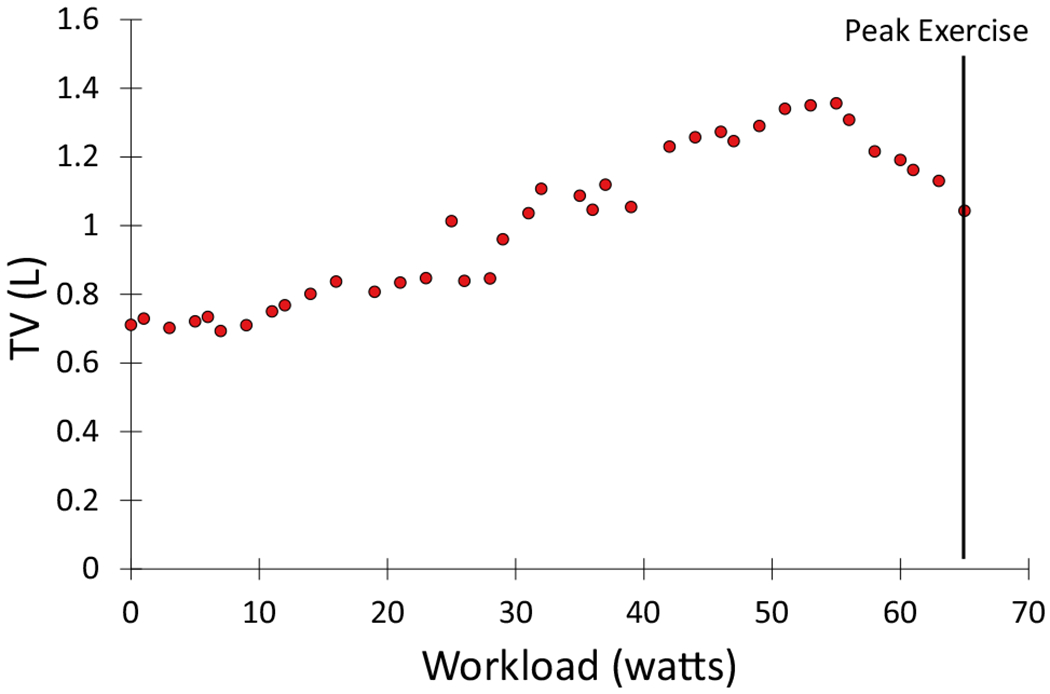
Example of dynamic hyperinflation during exercise as demonstrated by the decreasing tidal volume (TV) despite increasing work
Oxygen desaturation during exercise can be a clue to underlying pulmonary disease; however, it is rare to have a corresponding reduction in peak VO2 unless a patient has significant arterial hypoxemia [23]. As such, exercise capacity is typically not exclusively limited by a diffusion abnormality unless a patient has a significant arterial desaturation < 89% [24]. Using pulse oximetry, a technically significant drop in oxygen saturation during exercise is ≥ 5% from rest and should be confirmed with arterial blood gases [9].
Ventilatory efficiency and respiration are governed by the ventilatory demand equation (aka, modified alveolar equation; see Eq. 1) [9, 10, 25]. As shown in the rearranged version of this equation, VE/VCO2 is directly related to ventilatory dead space (VD/VT) and inversely related to PaCO2 [9, 10]. An elevated VE/VCO2 indicates an increased ventilatory requirement to eliminate a given amount of CO2 due to inefficient gas exchange [9, 10] or a hyperventilatory response to exercise secondary to extrapulmonary mechanisms [25]. Many disease processes can lead to pathological elevations in VE/VCO2. This includes interstitial lung disease [26], chronic obstructive pulmonary disease [27, 28], pulmonary hypertension [29], congestive heart failure [30], and neuromuscular diseases [31]. However, differentiating pulmonary diseases causing gas exchange abnormalities from underlying cardiovascular processes by exclusively using the VE/VCO2 requires arterial blood gases. During a CPET, VE/VCO2 gradually declines with increasing exercise intensity until 60–80% of exercise capacity after which it begins to increase due to compensatory increases in VE (see Fig. 1D). The nadir of VE/VCO2 is the best estimate of increases in dead space ventilation [32]. In the absence of a hyperventilatory response to exercise (with a corresponding decrease in PaCO2), a VE/VCO2 nadir that is greater than normal for age suggests an increase in dead space ventilation [10, 32]. In healthy individuals, VE/VCO2 increases with age and is higher in women (see Fig. 5) [32]. Based on data from Sun et al. [32], a VE/VCO2 nadir ≥ 35 is above the upper limit of normal for men and women across all age groups and might serve as a convenient “rule of thumb.”
| (1) |
where VCO2 is the volume of carbon dioxide to be exhaled, PaCO2 is the partial pressure of CO2 in arterial blood, VD is the physiologic dead space, and VT is the tidal volume.
Fig. 5.
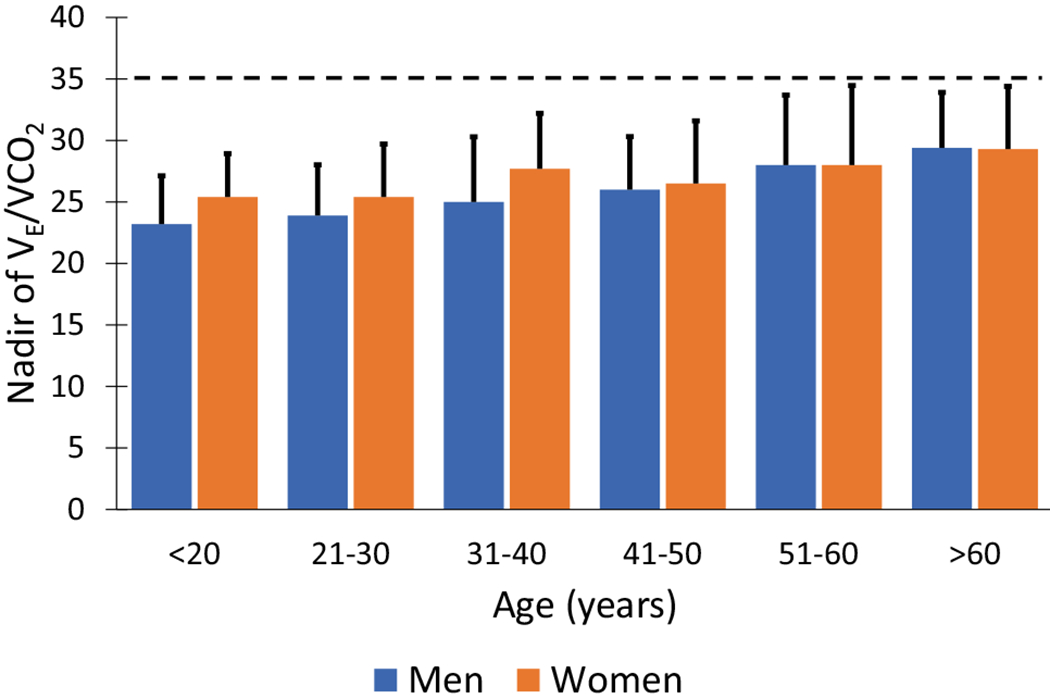
Nadir of VE/VCO2 in men and women. Bars represent the mean, and the error bars are the upper limit of normal (i.e., 1.96 × standard deviation). Horizontal dashed line represents “rule of thumb” upper limit of normal for men and women. Data adapted from Sun et al. [32]
It is important to note that VE/VCO2 (aka, VE/VCO2 ratio) can be calculated at any time point during a CPET with the nadir being the best representation of increased dead space ventilation. However, in the HFrEF literature, the slope of the change in VE versus change in VCO2 (VE/VCO2 slope) during exercise is reported more often than VE/VCO2. Although the VE/VCO2 slope is calculated using linear regression from the start of exercise up to anaerobic threshold (e.g., the onset of exercise-induced metabolic acidosis) or to the end of exercise, the relationship between change in VE and change in VCO2 is hyperbolic and the nadir of VE/VCO2 is similar to the VE/VCO2 slope (see Fig. 1B, D).
Over the past 30 years, there have been many studies that support a strong and independent relationship between various CPET variables and outcomes in patients with HFrEF. Among these, the study by Brawner et al. [33] is one of the few to assess the prognostic value of a majority of proposed CPET variables in a side-by-side comparison. Among patients with HFrEF (n = 1201), they evaluated the relationship of 36 CPET variables to risk for a composite outcome of all-cause mortality, left ventricular assist device implantation, or cardiac transplantation [33]. After adjustment for age, sex, ejection fraction, and beta-adrenergic blockade, all but 5 variables were related to the composite outcome [33]. The rank order of the variables that were significantly associated with the composite outcome is shown in Table 2.
Table 2.
Relative ranking of cardiopulmonary exercise test variables based on and their associationa to a composite outcome of death, left ventricular assist device implant, or heart transplant in patients with heart failure with reduced ejection fraction
| Ranking | Variable | Wald X2 | P |
|---|---|---|---|
| 1 | % predicted maximum VO2 | 203 | < 0.001 |
| 2 | VE/VCO2 slope | 201 | < 0.001 |
| 3 | Peak oxygen uptake efficiency (OUE) ratio | 184 | < 0.001 |
| 4 | Peak VE/VCO2 | 179 | < 0.001 |
| 5 | VE/VCO2 slope/peak VO2 | 168 | < 0.001 |
| 6 | Peak VO2 (mL·min−1) | 166 | < 0.001 |
| 7 | Peak VO2 (mL·kg−1·min−1) | 161 | < 0.001 |
| 8 | Oxygen uptake efficiency slope (OUES) | 154 | < 0.001 |
| 9 | Circulatory powerb | 143 | < 0.001 |
| 10 | Ventilatory powerc | 140 | < 0.001 |
| 11 | Peak end-tidal CO2 | 111 | < 0.001 |
| 12 | Peak O2 pulse | 97 | < 0.001 |
| 13 | Peak rate-pressure product | 92 | < 0.001 |
| 14 | Peak heart rate | 66 | < 0.001 |
| 15 | Heart rate reserve (peak-rest) | 61 | < 0.001 |
| 16 | Peak systolic blood pressure | 60 | < 0.001 |
| 17 | % predicted maximum heart rate | 52 | < 0.001 |
| 18 | Chronotropic index | 51 | < 0.001 |
| 19 | Peak ventilation | 47 | < 0.001 |
| 20 | Systolic blood pressure reserve (peak-rest) | 42 | < 0.001 |
| 21 | Peak mean arterial blood pressure | 40 | < 0.001 |
| 22 | Peak mean arterial blood pressure reserve (peak-rest) | 22 | < 0.001 |
| 23 | Test limited by shortness of breath | 16 | < 0.001 |
| 24 | Rest SBP < 100 mmHg | 16 | < 0.001 |
| 25 | Exercise oscillatory ventilation | 13 | < 0.001 |
| 26 | Heart rate recovery at 2 min < 22 beats | 9 | 0.002 |
| 27 | Heart rate recovery at 1 min < 12 beats | 7 | 0.009 |
| 28 | Peak respiratory exchange ratio < 1 | 5 | 0.021 |
Adapted from Brawner et al. [33]
Based on a Cox regression analysis adjusted for age, sex, ejection fraction, and beta-adrenergic blockade therapy
Circulatory power = peak VO2 (mL·kg−1·min−1) × peak systolic blood pressure
Ventilatory power = peak systolic blood pressure/VE/VCO2 slope
The Metabolic Exercise Test Data Combined with Cardiac and Kidney Indexes (MECKI) is a prospective, multisite study (13 sites in Italy) to develop a model to predict cardiovascular death or heart transplant that incorporates CPET data along with other clinical data (e.g., renal function, ejection fraction) in patients with HFrEF (n = 2716) [34]. From a list of 19 CPET variables that were considered in a stepwise selection method, percent-predicted maximum peak VO2 and VE/VCO2 slope were the only CPET variables retained in the final model along with hemoglobin, serum sodium, left ventricular ejection fraction, and glomerular filtration rate [34]. Among the many studies of HFrEF and prognosis, peak VO2 (in mL.kg−1.min−1 or % predicted) and VE/VCO2 slope are consistently the strongest prognostic variables. An oscillatory response of ventilation (e.g., exercise oscillatory ventilation) is also frequently reported as a strong predictor.
Overview of guideline statements on CPET
Professional associations from the USA and Europe advocate the role of CPET to define advanced HF and to guide listing for advanced therapies in patients with HF [1–3, 5]. CPET recommendations from relevant guideline statements are shown in Table 3. The most detailed recommendations are from the ISHLT [1, 13] in which they provide advice for several patient characteristics (see Table 4). Criteria for peak VO2 from the ISHLT is conditional on the presence of beta-adrenergic blockade which is based on the mortality benefit of beta-adrenergic blockade with no change in peak VO2 [13].
Table 3.
Recommendations relevant to cardiopulmonary exercise testing from guideline statements on patients with heart failure (HF)
| Organization | CPET recommendations | Comment |
|---|---|---|
| American Heart Association, American College of Cardiology, and Heart Failure Society of America [4] | None; references ISHLT | In selected ambulatory patients with HF, CPET is recommended to determine appropriateness of advanced treatments (e.g., LVAD, heart transplant) |
| European Society of Cardiology [5] | Peak VO2 < 12 mL.kg−1.min−1 and/or peak VO2 < 50% predicted | Criteria for advanced HF; potentially eligible for LVAD |
| Heart Failure Association of the European Society of Cardiology [3] | Peak VO2 < 12–14 mL.kg−1.min−1 and references ISHLT | Criterion for advanced HF |
| Heart Failure Society of America [2] | Peak VO2 < 14 mL.kg−1.min−1 or peak VO2 < 50% predicted | Indicators of advanced HF triggers and consideration for referral for evaluation of advanced therapies |
| International Society of Health and Lung Transplantation (ISHLT) [1, 13] | Peak VO2 < 12 mL.kg−1.min−1 (< 14 if no beta-blocker), peak VO2 < 50% predicted, or VE/VCO2 slope > 35 | Criteria for listing patients for heart transplantation |
LVAD left ventricular assist device, VCO2 volume of carbon dioxide exhaled, VO2 volume of oxygen uptake, VE minute ventilation, ISHLT International Society for Heart Lung Transplantation
Table 4.
Listing criteria for heart transplantation based on cardiopulmonary exercise test data from the International Society for Heart Lung Transplantation
| Condition | Criteria |
|---|---|
| Beta-adrenergic blockade | Peak VO2 ≤ 12 mL·kg−1·min−1 |
| No beta-adrenergic blockade | Peak VO2 ≤ 14 mL·kg−1·min−1 |
| < 50 years and women | In addition to peak VO2 ≤ 12 (or 14) mL·kg−1·min−1, consider peak VO2 < 50% predicted |
| Peak respiratory exchange ratio < 1.05 | VE/VCO2 slope > 35 may be considered |
| Obese (body mass index > 30 kg·m−2) | Consider lean body mass–adjusted peak VO2 < 19 mL·kg−1·min−1 |
VE minute ventilation, VCO2 volume of carbon dioxide exhaled, VO2 volume of oxygen uptake. Adapted from Mehra et al. [1]
The ISHLT recommends using a percent of predicted maximum VO2 < 50% in addition to peak VO2 < 12 (or 14) mL·kg−1·min−1 in patients < 50 years and women (Table 4) [1, 13]. Several equations to predict normal maximum VO2 have been published that are based on sex, age, height, and/or weight [35]. While the ISHLT does not recommend which VO2 prediction equation should be used, it is important to note that there are clinically meaningful differences in the identification of patients with predicted VO2 < 50% based on the equation used [35]. Observations by the authors suggest that the most frequently used equations in studies of CPET in patients with HF are those from Wasserman and Hansen [10]. Other authors have also recommended using the equations by Wasserman and Hansen [36]. It is also important to note that the Wasserman-Hansen equations solve for a predicted normal maximum VO2 in L·min−1. A patient who is obese may have a peak VO2 in L·min−1 that is near normal, but a low peak VO2 when indexed to their body mass (i.e., mL·kg−1·min−1).
The ISHLT recommends using a VE/VCO2 slope > 35 when the peak VO2 < 12 (or 14) mL·kg−1·min−1 but the peak RER < 1.05, suggesting a submaximal cardiometabolic stress (Table 4) [1, 13]. While there are many studies to support using VE/VCO2 slope to risk stratify patients with HF, it is unclear if the risk associated with a VE/VCO2 slope > 35 is similar to a peak VO2 < 12 (or 14) mL·kg−1·min−1. It is also unknown if a VE/VCO2 slope > 35 represents a similar risk in patients with and without beta-adrenergic blockade, between men and women, and across age groups.
To address some of this knowledge gap, Ehrman et al. [36] reported the 1- and 3-year survival rates free from all-cause death, left ventricular assist device implant, or cardiac transplant among 1085 patients with HFrEF (33% women; 79% on beta-adrenergic blockade). As shown in Fig. 6, the % predicted VO2 and VE/VCO2 slope associated with an event-free survival that exceeded the 1-year cardiac transplant survival rate were similar to the thresholds recommended by the ISHLT, but peak VO2 (in mL·kg−1·min−1) was higher. In addition, the survival rate between men and women over a median of 5.7 years was not significantly different for predicted VO2 < 50% but was significantly different for peak VO2 < 12 mL· kg−1min−1 and VE/VCO2 slope ≥ 36 [37]. More work is needed to refine the CPET criteria recommended by the ISHLT.
Fig. 6.
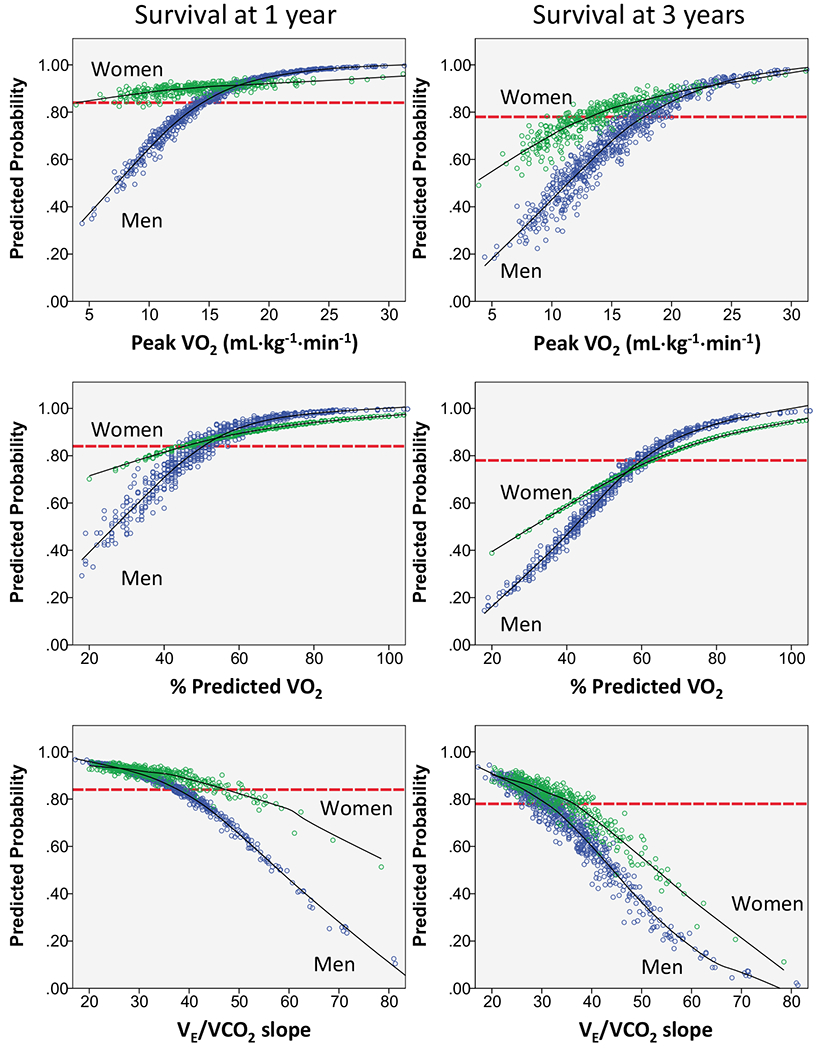
Logistic regression scatterplots for a 1- and 3-year composite outcome for each cardiopulmonary exercise test variable. The probability of survival free from death, left ventricular assist device implant, or cardiac transplant is depicted using logistic regression for the composite outcome at 1 and 3 years for peak VO2, percent-predicted peak VO2, and VE/VCO2 slope. Each individual’s predicted survival probability is plotted, and a line of best fit is drawn for both men (blue dots) and women (green dots). Perspective horizontal dashed lines are placed on the graphs to depict the 1- (84%) and 3-year (78%) North American cardiac transplant survival rate. VE/VCO2 slope, slope of minute ventilation relative to carbon dioxide exhaled; VO2, peak oxygen uptake. Figure from Ehrman et al. [37]. Reprinted with permission from Elsevier
Finally, the ISHLT recommends calculating the lean body mass–adjusted peak VO2 in patients who are obese (Table 4) [1, 13]. However, determining lean body mass requires additional equipment and expertise that is likely not available in most CPET laboratories or cardiology clinics.
Conclusions
In this manuscript, we reviewed the normal cardiopulmonary response to a graded exercise test and current guideline recommendations relative to CPET in patients with HFrEF. We just touched the surface of the data available from a CPET. For more in-depth reading, we suggest the 2003 review by the American Thoracic Society [9] or the text by Sietsema et al. [10]. CPET provides unique information on the physiological response to exercise, is useful in patients with HFrEF to determine the degree to which cardiac pathology limits their exercise capacity, and it provides valuable prognostic information.
Funding
CA Brawner: Dr. Brawner is supported by the following grants from the National Institutes of Health: 1R01AG077179-01 and R33HL143099.
Footnotes
Ethical approval Not applicable. The article type is a review.
Competing interests CA Brawner: Dr. Brawner has served as a Project Director for exercise testing core laboratory service with service agreements with Actelion Pharmaceuticals, Bristol Myers Squibb, Medpace, and Labcorp. Payment for these services is made to Henry Ford Health System. MH Lazar: No conflicts to disclose.
Availability of data and materials
Not applicable. The article type is a review.
References
- 1.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EA, Zuckermann A (2016) The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 35(1):1–23. 10.1016/j.healun.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 2.Fang JC, Ewald GA, Allen LA, Butler J, Canary CA, Colvin-Adams M, Dickinson MG, Levy P, Stough WG, Sweitzer NK, Teerlink JR, Whellan DJ, Albert NM, Krishnamani R, Rich MW, Walsh MN, Bonnell MR, Carson PE, Chan MC, Dries DL, Hernandez AF, Hershberger RE, Katz SD, Moore S, Rodgers JE, Rogers JG, Vest AR, Givertz MM (2015) Advanced (stage D) heart failure: a statement from the Heart Failure Society of America guidelines committee. J Card Fail 21(6):519–534. 10.1016/j.cardfail.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 3.Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge-Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hulsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska-Migaj E, McDonagh T, Seferovic P, Ruschitzka F (2018) Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 20(11):1505–1535. 10.1002/ejhf.1236 [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW (2022) AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 145(18):e895–e1032. 10.1161/cir.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 5.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A (2021) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42(36):3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 6.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR (1991) Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 83(3):778–786. 10.1161/01.cir.83.3.778 [DOI] [PubMed] [Google Scholar]
- 7.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA (2013) American Heart Association Exercise CR, CoNPA Prevention Committee of the Council on Clinical Cardiology, CoC Metabolism, N Stroke, E Council on, and Prevention (2013) Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 128(8):873–934. 10.1161/CIR.0b013e31829b5b44 [DOI] [PubMed] [Google Scholar]
- 8.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV (2010) American Heart Association Exercise CR, C Prevention Committee of the Council on Clinical, E Council on, Prevention, D Council on Peripheral Vascular, C Interdisciplinary Council on Quality of, and R Outcomes (2010) Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122(2):191–225. 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society and American College of Chest Physicians (2003) ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 167(2):211–77. 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 10.Sietsema KE, Sue DY, Stringer WW, Rossiter HB, Ward SA (2020) Wasserman & Whipp’s principles of exercise testing and interpretation: including pathophysiology and clinical applications. Lippincott Williams & Wilkins. [Google Scholar]
- 11.Brawner CA, Ehrman JK, AldredH Schairer JR, Keteyian SJ (2008) Quality assurance and cardiopulmonary exercise testing in clinical trials. J Card Fail 14(4):283–289. 10.1016/j.cardfail.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 12.Keteyian SJ, Brawner CA, Ehrman JK, Ivanhoe R, Boehmer JP, Abraham WT (2010) Reproducibility of peak oxygen uptake and other cardiopulmonary exercise parameters: implications for clinical trials and clinical practice. Chest 138(4):950–955. 10.1378/chest.09-2624 [DOI] [PubMed] [Google Scholar]
- 13.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, Mohacsi P, Augustine S, Aaronson K, Barr M (2006) Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates–2006. J Heart Lung Transplant 25(9):1024–1042. 10.1016/j.healun.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 14.Wasserman K, Whipp BJ (1975) Exercise physiology in health and disease. Am Rev Respir Dis 112(2):219–49. 10.1164/arrd.1975.112.2.219 [DOI] [PubMed] [Google Scholar]
- 15.Peters CM, Dempsey JA, Hopkins SR, Sheel AW (2023) Is the lung built for exercise? Med Sci Sports Exerc, Advances and unresolved questions. 10.1249/mss.0000000000003255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell SC (1982) A comparison of the maximum voluntary ventilation with the forced expiratory volume in one second: an assessment of subject cooperation. J Occup Med 24(7):531–533 [PubMed] [Google Scholar]
- 17.Dillard TA, Hnatiuk OW, McCumber TR (1993) Maximum voluntary ventilation. Spirometric determinants in chronic obstructive pulmonary disease patients and normal subjects. Am Rev Respir Dis 147(4):870–5. 10.1164/ajrccm/147.4.870 [DOI] [PubMed] [Google Scholar]
- 18.Hansen JE, Sue DY, Wasserman K (1984) Predicted values for clinical exercise testing. Am Rev Respir Dis 129(2 Pt 2):S49–55. 10.1164/arrd.1984.129.2P2.S49 [DOI] [PubMed] [Google Scholar]
- 19.Sue DY, Hansen JE (1984) Normal values in adults during exercise testing. Clin Chest Med 5(1):89—98. [PubMed] [Google Scholar]
- 20.Hansen JE, Casaburi R, Cooper DM, Wasserman K (1988) Oxygen uptake as related to work rate increment during cycle ergometer exercise. Eur J Appl Physiol Occup Physiol 57(2):140–145. 10.1007/BF00640653 [DOI] [PubMed] [Google Scholar]
- 21.Spiro SG, Juniper E, Bowman P, Edwards RH (1974) An increasing work rate test for assessing the physiological strain of submaximal exercise. Clin Sci Mol Med 46(2):191–206. 10.1042/cs0460191 [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell DE, Laveneziana P (2006) The clinical importance of dynamic lung hyperinflation in COPD. COPD 3(4):219–32. 10.1080/15412550600977478 [DOI] [PubMed] [Google Scholar]
- 23.Ulrich S, Schneider SR, Bloch KE (2017) Effect of hypoxia and hyperoxia on exercise performance in healthy individuals and in patients with pulmonary hypertension: a systematic review. J Appl Physiol 123(6):1657–1670. 10.1152/japplphysiol.00186.2017 [DOI] [PubMed] [Google Scholar]
- 24.Dempsey JA, Wagner PD (1999) Exercise-induced arterial hypoxemia. J Appl Phys 87(6):1997–2006. 10.1152/jappl.1999.87.6.1997 [DOI] [PubMed] [Google Scholar]
- 25.Woods PR, Olson TP, Frantz RP, Johnson BD (2010) Causes of breathing inefficiency during exercise in heart failure. J Card Fail 16(10):835–842. 10.1016/j.cardfail.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barratt SL, Davis R, Sharp C, Pauling JD (2020) The prognostic value of cardiopulmonary exercise testing in interstitial lung disease: a systematic review. ERJ Open Res 6(3). 10.1183/23120541.00027-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neder JA, Berton DC, Arbex FF, Alencar MC, Rocha A, Sperandio PA, Palange P, O’Donnell DE (2017) Physiological and clinical relevance of exercise ventilatory efficiency in COPD. Eur Respir J 49(3):1602036. 10.1183/13993003.02036-2016 [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell DE, Elbehairy AF, Faisal A, Webb KA, Neder JA, Mahler DA (2016) Exertional dyspnoea in COPD: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev 25(141):333–347. 10.1183/16000617.0054-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reybrouck T, Mertens L, Schulze-Neick I, Austenat I, Eyskens B, Dumoulin M, Gewillig M (1998) Ventilatory inefficiency for carbon dioxide during exercise in patients with pulmonary hypertension. Clin Physiol 18(4):337–344. 10.1046/j.1365-2281.1998.00109.x [DOI] [PubMed] [Google Scholar]
- 30.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ (2000) Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J 21(2):154–161. 10.1053/euhj.1999.1863 [DOI] [PubMed] [Google Scholar]
- 31.Riley MS, Nicholls DP, Cooper CB (2017) Cardiopulmonary exercise testing and metabolic myopathies. Ann Am Thorac Soc 14(Supplement_1):S129–S139. 10.1513/AnnalsATS.201701-014FR [DOI] [PubMed] [Google Scholar]
- 32.Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K (2002) Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med 166(11):1443–1448. 10.1164/rccm.2202033 [DOI] [PubMed] [Google Scholar]
- 33.Brawner CA, Shafiq A, Aldred HA, Ehrman JK, Leifer ES, Selektor Y, Tita C, Velez M, Williams CT, Schairer JR, Lanfear DE, Keteyian SJ (2015) Comprehensive analysis of cardiopulmonary exercise testing and mortality in patients with systolic heart failure: the Henry Ford Hospital cardiopulmonary exercise testing (FIT-CPX) project. J Card Fail 21(9):710–718. 10.1016/j.cardfail.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 34.Agostoni P, Corra U, Cattadori G, Veglia F, La Gioia R, Scardovi AB, Emdin M, Metra M , Sinagra G, Limongelli G, Raimondo R, Re F, Guazzi M, Belardinelli R, Parati G, Magri D, Fiorentini C, Mezzani A, Salvioni E, Scrutinio D, Ricci R, Bettari L, Lenarda A Di, Pastormerlo LE, Pacileo G, Vaninetti R, Apostolo A, Iorio A, Paolillo S, Palermo P, Contini M, Confalonieri M, Giannuzzi P, Passantino A, Cas LD, Piepoli MF, Passino C, Group MSR (2013) Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multiparametric approach to heart failure prognosis. Int J Cardiol 167(6):2710–2718. 10.1016/j.ijcard.2012.06.113 [DOI] [PubMed] [Google Scholar]
- 35.Brawner CA, Ehrman JK, Shafiq A, Saval MA, Russell SD, Lanfear DE, Keteyian SJ (2018) Challenges with percent predicted maximal VO2 in patients with heart failure. Med Sci Sports Exerc 50(2):204–210. 10.1249/mss.0000000000001431 [DOI] [PubMed] [Google Scholar]
- 36.Gargiulo P, Olla S, Boiti C, Contini M, Perrone-Filardi P, Agostoni P (2014) Predicted values of exercise capacity in heart failure: where we are, where to go. Heart Fail Rev 19(5):645–653. 10.1007/s10741-013-9403-x [DOI] [PubMed] [Google Scholar]
- 37.Ehrman JK, Brawner CA, Shafiq A, Lanfear DE, Saval M, Keteyian SJ (2018) Cardiopulmonary exercise measures of men and women with HFREF differ in their relationship to prognosis: the Henry Ford Hospital cardiopulmonary exercise testing (FIT-CPX) project. J Card Fail 24(4):227–233. 10.1016/j.cardfail.2018.02.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable. The article type is a review.


