Abstract
Background
Coronary slow flow (CSF) is a microvascular disease characterized by delayed opacification of the epicardial coronary arteries during angiography. The main pathogenesis of CSF is endothelial dysfunction caused by diffuse atherosclerosis. Dyslipidemia is one of the primary factors raising the risk of atherosclerosis. Compared to conventional lipid profiles, non-traditional lipid profiles more accurately reflect dyslipidemic status. In this work, we compared the non-high density lipoprotein-cholesterol (HDL-C)/HDL-C ratio (NHHR) with other conventional and non-conventional lipid profiles in order to determine its impact on CSF.
Methods
A total of 9112 subjects who underwent coronary angiography were screened retrospectively, of whom 130 subjects with CSF and 130 subjects with normal CF were included. Multivariate regression analysis was used to identify independent predictors of CSF. Additionally, in order to predict CSF, the diagnostic accuracies of NHHR and other non-traditional lipid profiles were examined.
Results
There were significantly higher non-traditional lipid profiles in the CSF group (all p < 0.001). Compared to other non-traditional lipid profiles, NHHR had a stronger association with thrombolysis in myocardial infarction frame count (r = 0.3593, p < 0.0001). In addition to NHHR, non-HDL-C, Castelli’s risk index-II, atherogenic index of plasma, plasma glucose, dyslipidemia, smoking, and body mass index were identified as independent predictors of CSF. The ability of NHHR to detect CSF was superior to other non-traditional lipid profiles (area under the curve: 0.785; confidence interval: 0.730-0.840; p < 0.001).
Conclusions
NHHR was found to be a potent and reliable predictor of CSF. This indicates that NHHR can be used as a reliable biomarker for risk stratification of CSF.
Keywords: Coronary slow flow, Non-HDL-C/HDL-C ratio, Non-traditional lipid markers
Abbreviations
AIP, Atherogenic index of plasma
ARB, Angiotensin receptor blockers
BMI, Body mass index
BP, Blood pressure
CAD, Coronary artery disease
CCB, Calcium channel blocker
CI, Confidence interval
CRI-I, Castelli’s risk index-I
CRI-II, Castelli’s risk index-II
CSF, Coronary slow flow
DM, Diabetes mellitus
e-GFR, Estimated glomerular filtration rate
HDL-C, High-density lipoprotein cholesterol
LAD, Left anterior descending coronary artery
LCI, Lipoprotein combined index
LCX, Left circumflex coronary artery
LDL-C, Low-density lipoprotein cholesterol
LVEF, Left ventricular ejection fraction
MPV, Mean platelet volume
NCF, Normal coronary flow
NHHR, Non-HDL-C/HDL-C ratio
OR, Odds ratio
RCA, Right coronary artery
RDW, Red cell distribution width
ROC, Receiver operating characteristic
TC, Total cholesterol
TFC, TIMI frame count
TG, Triglyceride
TIMI, Thrombolysis in myocardial infarction
T2DM, Type 2 diabetes mellitus
INTRODUCTION
The epicardial coronary arteries do not have severe mechanical stenosis, and perfusion of the distal vasculature is delayed by forward flow, according to the definition of the coronary slow flow (CSF) phenomenon.1 There is mounting evidence from intravascular ultrasound imaging data that CSF is a diffuse form of atherosclerosis affecting the epicardial coronary arteries as well as the microvascular system.2,3 Considerable interactions between morphological and functional alterations in the epicardial coronary arteries and microvascular circulation have been demonstrated, and they can be seen as two distinct but related manifestations of the same atherosclerotic process.4,5 In the early stage of the atherosclerotic process, luminal irregularity is not observed in coronary angiography despite the presence of atherosclerotic plaques due to positive coronary remodeling.6 Endothelial dysfunction, which occurs before obstructive lesions reduce coronary blood flow, is an early sign of atherosclerosis.7,8 Even if diffuse subclinical coronary atherosclerosis does not cause stenotic lesions, it causes functional and structural microvascular changes that may cause ischemia by lowering the myocardial perfusion pressure along the epicardial coronary artery.9,10 Coronary microvascular dysfunction also induces atherosclerotic plaque development in epicardial coronary arteries by slowing coronary blood flow and reducing wall shear stress.11,12 It has been shown that microvascular resistance, which is an indicator of the deterioration of microvascular circulation, is also increased in CSF.13 In addition, those who have slower blood flow in their epicardial coronary arteries have been demonstrated to be more likely to develop obstructive coronary artery disease (CAD) and have negative cardiovascular clinical outcomes.14,15 Taken together, these data suggest that CSF may represent different stages of endothelial dysfunction and the development of coronary atherosclerosis in epicardial coronary arteries and coronary microcirculation. Therefore, CSF may be an early microvascular component of CAD development. In this context, implementing risk stratification by determining the indicators of CSF development in these patients would appear to be beneficial in both primary and secondary prevention.
The development of atherosclerotic plaques is significantly influenced by atherogenic dyslipidemia, which comprises elevated blood triglycerides (TGs), apolipoprotein B, tiny, dense low-density lipoprotein (LDL-C) particles, and decreased high density lipoprotein-cholesterol (HDL-C) particles.16 In addition to contributing significantly to the etiopathogenesis of coronary endothelial and microvascular dysfunction, atherogenic dyslipidemia causes atherosclerosis.17 While oxidized LDL particles and TGs cause endothelial dysfunction with a proatherogenic effect, HDL particles show antiatherogenic effects and exert a protective effect on the endothelium.16,17 Studies have shown that formulations obtained by combining these traditional lipid profiles (TGs, LDL-C, HDL-C) in various fractions [non-HDL-C, non-HDL-C/HDL-C ratio (NHHR) and Castelli’s risk indexes I and II (CRI-I, CRI-II), atherogenic index of plasma (AIP), lipoprotein combined index (LCI)] show a stronger correlation with cardiovascular diseases and have better predictive and diagnostic ability for cardiovascular events, and thus they may more accurately depict the equilibrium between proatherogenic and antiatherogenic lipid profiles.18-23
This study focuses on CSF, a particular microvascular condition marked by delayed distal vasculature perfusion without severe epicardial coronary artery stenosis. The novelty lies in the investigation of the relationship between non-traditional lipid profiles, particularly NHHR, and CSF. Few studies have investigated the comparative effect of NHHR and other traditional and non-traditional lipid profiles on CSF. Clarifying the correlation between CSF and lipid profiles will help guide further research into the prognosis and management of individuals affected by this entity. For this purpose, we aimed to investigate the possible effects of NHHR and other non-traditional and traditional lipid profiles on CSF in order to improve risk prediction and implement primary and secondary preventive measures in patients with CSF.
METHODS
Study population
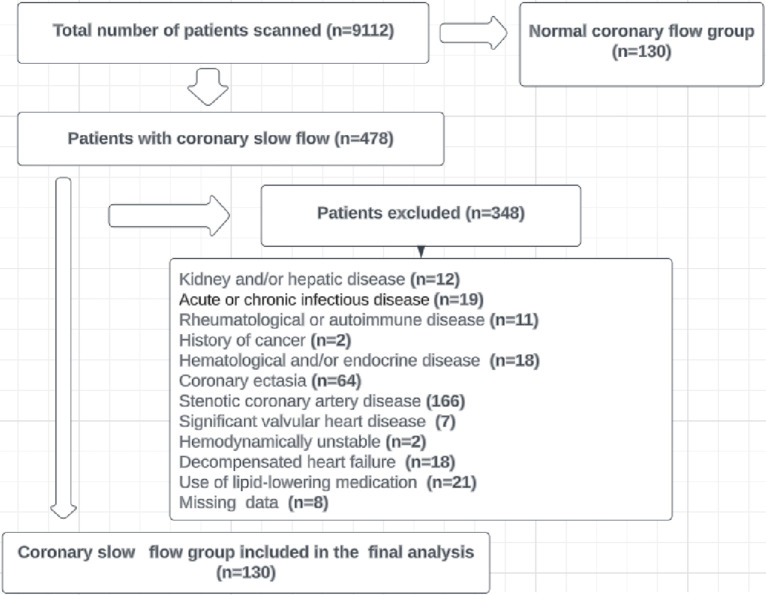
In this retrospective cross-sectional and observational study, the angiographic results of 9112 individuals who underwent coronary angiography between January 2020 and April 2023 were reviewed. Of these patients, 478 had CSF in their coronary angiography results. After applying the exclusion criteria, 130 patients were included in the study, along with 130 consecutive subjects with normal coronary flow (NCF) and normal coronary arteries as the control group (Figure 1). Those with advanced liver and/or kidney failure, acute or chronic infectious diseases, rheumatological or autoimmune diseases, history of cancer, hematological and/or endocrine diseases including anemia, CAD, including CAD with > 50% stenosis, acute coronary syndrome, history of coronary intervention and coronary ectasia, significant valvular heart disease, hemodynamically unstable, decompensated heart failure, taking lipid-lowering medications, and patients with incomplete data were excluded from the study. The local ethics committee approved the study, which was carried out in conformity with the Declaration of Helsinki.
Figure 1.
Flowchart of study population inclusion.
Data gathering and laboratory evaluations
From the hospital’s patient database, we gathered information on the participants’ demographics, clinical and comorbid features, and laboratory results. Laboratory parameters obtained from peripheral venous blood of the participants after at least 8 hours of fasting were used for data analysis. HDL-C, total cholesterol (TC), LDL-C, and triglyceride (TG) levels were measured using the direct enzymatic colorimetric method with a Roche Diagnostics Cobas analyzer Cobas 6000, c501 module (Roche, Mannheim, Germany). The estimated glomerular filtration rate (e-GFR) was calculated using a formula devised by the Chronic Kidney Disease Epidemiology Collaboration.
Calculation of non-traditional lipid profiles
HDL-C was subtracted from TC to determine non-HDL-C values. The atherogenic coefficient, also known as the NHHR, was calculated by dividing non-HDL-C by HDL-C. Log10(TG/HDL-C) was used to compute the AIP. The formula LCI = TC * TG * LDL-C/HDL-C was used to calculate the LCI. CRI-I was calculated as TC/HDL-C, and CRI-II as LDL-C/HDL-C.
Clinical definitions and measurements
The definition of hypertension was previously diagnosed hypertension or at least two office blood pressure (BP) measurements with a repeat systolic BP of ≥ 140 mmHg and/or a diastolic BP of ≥ 90 mmHg. A history of taking cholesterol-lowering medications or the presence of one of the following four lipid profile measures was considered to indicate dyslipidemia: (1) TGs > 150 mg/dl, (2) TC > 200 mg/dl, (3) LDL-C > 130 mg/dl, (4) HDL-C > 40 mg/dl in men and > 50 mg/dl in women. Hemoglobin A1c ≥ 6.5%, anti-diabetic medication use, or fasting blood glucose ≥ 126 mg/dL were all considered indicators of diabetes mellitus (DM). The body mass index (BMI) was calculated by dividing body weight (kg) by height squared (m2). e-GFR < 60 ml/min was defined as renal failure. During hospitalization, the left ventricular ejection fraction (LVEF) was assessed by two skilled cardiologists using echocardiography and the modified Simpson method (Philips Epiq 7 equipment, Andover, MA, USA).
Thrombolysis in myocardial infarction (TIMI) frame count (TFC) and coronary angiography assessments
All coronary angiographic interventions were percutaneously performed by experienced invasive cardiologists using the standard Judkins technique (Philips Allura Xper FD10 cardiovascular X-ray system). Iopromide was used as a radiocontrast agent in coronary angiography (Omnipaque; GE Healthcare, Cork, Ireland). Epicardial coronary arteries were imaged at 30 frames per second (fps) by angling from the right and left oblique projections and in the cranial and caudal planes. For visualization of the lumen during coronary angiography, intracoronary contrast medium was administered manually, with at least 10 ml for the left system and at least 6 ml for the right system. All angiographic images were digitally recorded in accordance with Digital Imaging and Communications in Medicine standards for quantitative analysis. At least two skilled invasive cardiologists who were not aware of the patients’ demographic and medical features evaluated angiographic TIMI frame counts (TFCs) to categorize the study participants according to coronary flow. TFCs were measured in order to quantitatively analyze the flow in the coronary arteries, as previously described by Gibson et al.24 The first frame was considered to be the one in which antegrade filling resulted in lumen opacification of greater than 70%. The point where the dye opacification reached the distal landmark of the coronary arteries was defined as the final frame. The left circumflex artery’s (LCX) most distal bifurcation of the obtuse marginal branch, the right coronary artery’s (RCA) first lateral branch of the posterolateral artery, and the left anterior descending artery’s (LAD) distal bifurcation (also known as a "whale tail" or "mustache" or "hay fork") were all identified as the distal landmarks. The corrected LAD TFC (cTFC) was obtained by dividing the frame counts of the LAD by a coefficient of 1.7 to maintain the proportional balance between the epicardial coronary arteries, because the LAD has a longer course than the LCX and RCA. The TFCs of three arteries were summed and divided by three to obtain the mean TFC. According to the recommendations of Gibson et al., coronary flows with a TFC greater than 27 were considered CSF.24 The intraobserver and interobserver coefficients of variance were 4.5% and 8%, respectively.
Statistical analysis
The statistical analyses were carried out using SPSS 26.0 (SPSS Inc, Chicago, IL, USA) software. The analytical process encompassed various techniques to comprehensively explore the data. To ascertain if the distribution of continuous variables was normal, the Kolmogorov-Smirnov test was utilized. This test scrutinizes whether the data follows a Gaussian distribution. For continuous variables, two descriptive statistical methods were used: 1) mean ± standard deviation; and 2) median with the interquartile range, which provides a robust description for non-normally distributed data. Continuous variables were compared using two distinct methods depending on the data distribution: 1) Student’s t-test for normally distributed data; and 2) Mann-Whitney U test for non-normally distributed data. Categorical variables were presented as percentages and raw counts. To compare differences between categorical variables, two statistical tests were applied: 1) the chi-square (χ2) test for larger sample sizes; and 2) Fisher’s exact test for smaller sample sizes, ensuring robust analysis. Relationships between NHHR and other non-traditional lipid profiles with TFC were explored using the Spearman rank correlation test. This test captures monotonic relationships and offers valuable insights into associations between variables. To identify independent predictors of CSF, both univariate and multivariate logistic regression analyses were conducted. Variables that exhibited significance between the CSF and NCF groups were included in the initial univariate regression analysis. Variables that remained statistically significant (p < 0.05) in the univariate regression analysis were included in the multivariate regression models. Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were then calculated to quantify the strength and precision of the associations. The Hosmer-Lemeshow test was used to assess the goodness-of-fit of the logistic regression models, ensuring that they accurately represented the data. Receiver operating characteristic (ROC) curve analysis was done to see how well the independent predictors could distinguish between CSF and other conditions. This graphical tool provides insights into the trade-off between sensitivity and specificity. The optimal cut-off value for NHHR for the detection and prediction of CSF was derived from the point of maximum sensitivity and specificity based on Youden’s J index. This precision-driven approach ensured the best balance between true positive and true negative predictions. To gauge the predictive performance and discriminative power of NHHR and other non-traditional lipid profiles (non-HDL-C, AIP, LCI, CRI-I, CRI-II) concerning CSF, the DeLong test was utilized. This statistical test allows for rigorous comparisons of the predictive abilities of different variables. A significance level of p < 0.05 was adopted for all statistical tests, denoting statistical significance.
RESULTS
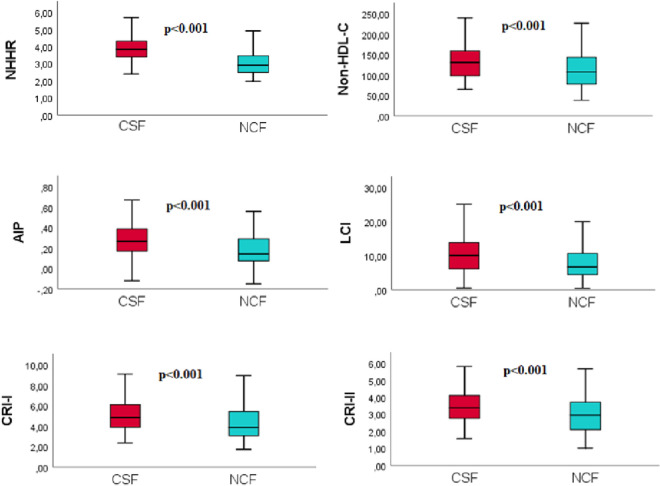
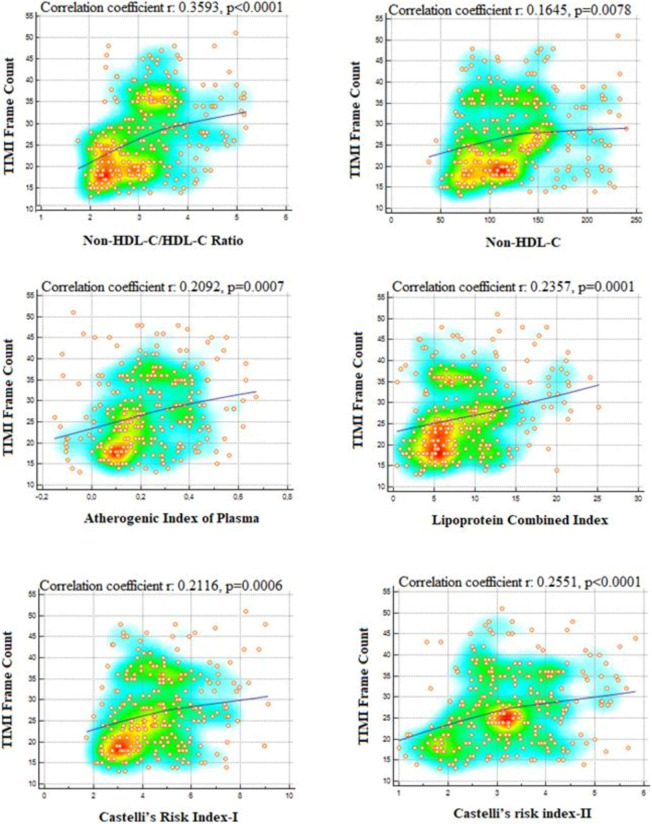
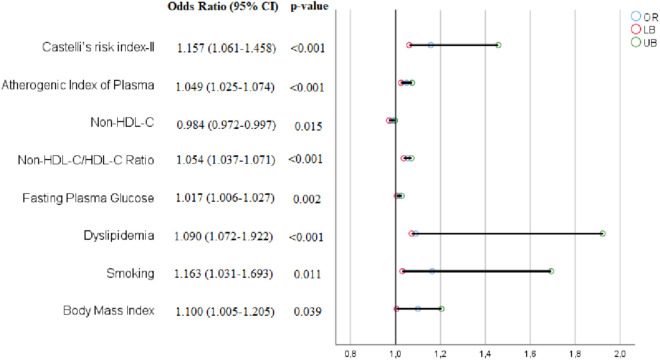
A total of 130 consecutively selected patients with CSF and 130 consecutively selected subjects with NCF as the control group were included in this study. The baseline demographic, clinical, and laboratory data of both groups are shown in Table 1. In the CSF group, the prevalence rates of male gender, diabetes mellitus, smoking, and dyslipidemia were substantially greater compared to the NCF group (all p < 0.05). Also, the CSF group had a higher BMI. There were no significant differences in hemodynamic characteristics, including BP, heart rate, LVEF, and pre-procedural medicines between the two groups (all p > 0.05). Compared to the NCF group, the fasting plasma glucose, uric acid, and CRP levels were all noticeably higher, but albumin level was lower in the CSF group (all p < 0.05). Regarding traditional lipid profiles, the HDL-C level was lower and TG, TC, and LDL-C levels were all significantly higher in the CSF group than in the NCF group (all p < 0.05). Regarding non-traditional lipid profiles, the non-HDL-C, NHHR, AIP, LCI, CRI-I, and CRI-II values were significantly higher in the CSF group than in the NCF group (all p < 0.001) (Figure 2). Table 2 shows the angiographical information of the study population. CSF was observed in the LAD in 69.6%, LCX in 59.3%, and RCA in 61.6% of the study population. In addition, CSF was observed in 1 vessel in 37.7%, in 2 vessels in 33.1%, and in 3 vessels in 29.2% of the study population. As expected, the TFCs of coronary arteries in the CSF group were significantly higher than those in the NCF group (all p < 0.001). NHHR (r: 0.3593, p < 0.0001), non-HDL-C (r: 0.1645, p = 0.0078), AIP (r: 0.2029, p = 0.0007), LCI (r: 0.2537, p = 0.00019), CRI-I (r: 0.2116, p = 0.0006) and CRI-II (r: 0.2551, p < 0.0001) were positively correlated with TFC. However, the strongest correlation was with NHHR (Figure 3). Univariate and multivariate logistic regression analyzes were performed to identify independent predictors of CSF. After adjustment for traditional confounders, NHHR (OR: 1.054, CI: 1.037-1.071, p < 0.001), non-HDL-C (OR: 0.984, CI: 0.972-0.997, p = 0.015), AIP (OR: 1.049, CI: 1.025-1.074, p < 0.001), CRI-II (OR: 1.157, CI: 1.061-1.458, p < 0.001), dyslipidemia (OR: 1.090, CI: 1.072-1.922, p < 0.001), smoking (OR: 1.163, CI: 1.031-1.693, p = 0.011) and BMI (OR: 1.100, CI: 1.005-1.205, p = 0.039) remained significantly and independently associated with CSF (Table 3, Table 4, Figure 4). ROC curve analysis showed no significant difference between the traditional lipid profiles TG, TC, HDL-C and LDL-C in terms of their CSF discriminating capability (Figure 5). In addition, NHHR predicted the presence of CSF with 74% sensitivity and 72% specificity at the optimal cut-off value of 3.3 (area under the curve: 0.785; 95% CI: 0.730-0.840; p < 0.001) (Figure 6). Furthermore, when the ability of non-traditional lipid profiles to detect and predict CSF was pairwise compared with ROC analysis, the ability of NHHR to discriminate CSF was superior to all other non-traditional lipid profiles (p < 0.05, for all comparisons) (Figure 6). Taken together, these results indicated that NHHR, which is an indicator of atherogenic dyslipidemia and may be involved in the pathophysiology of CSF, a type of atherosclerotic vascular disease, could probably better reflect the proatherogenic and antiatherogenic balance between the measured lipid profiles.
Table 1. Basic demographic, clinical characteristics, and laboratory findings of the study population.
| Variables | Normal coronary flow (n = 130) | Coronary slow flow (n = 130) | p value |
| Demographics and medical history | |||
| Age, years | 57.3 ± 12.7 | 58. 0 ± 13.0 | 0.676 |
| Gender-male (n, %) | 77 (59.2) | 94 (72.3) | 0.026 |
| BMI, kg/m2 | 26.7 ± 4.2 | 27.8 ± 3.9 | 0.034 |
| Diabetes mellitus (n, %) | 23 (8.8) | 37 (14.2) | 0.039 |
| Hypertension (n, %) | 66 (50.8) | 62 (47.7) | 0.620 |
| Dyslipidemia (n, %) | 78 (60.0) | 50 (38.5) | 0.001 |
| Smoking (n, %) | 21 (16.2) | 38 (29.2) | 0.012 |
| Hemodynamic properties | |||
| Systolic blood pressure, mmHg | 146 ± 19 | 144 ± 18 | 0.363 |
| Diastolic blood pressure, mmHg | 79 ± 15 | 80 ± 14 | 0.506 |
| Heart rate, bpm | 81 ± 7 | 82 ± 7 | 0.135 |
| LVEF (%) | 56 ± 6 | 57 ± 6 | 0.556 |
| Pre-procedural medications (%) | |||
| Antiplatelet | 22 (16.9) | 18 (13.8) | 0.492 |
| β-blocker | 18 (13.8) | 28 (21.5) | 0.104 |
| CCB | 17 (13.1) | 22 (16.9) | 0.385 |
| ACEI/ARB | 36 (27.79 | 39 (30.0) | 0.681 |
| Laboratory results | |||
| FPG (mg/dL) | 115 ± 32 | 126 ± 39 | 0.018 |
| Creatinine (mg/dL) | 0.79 ± 0.14 | 0.78 ± 0.19 | 0.531 |
| Uric acid (mg/dL) | 5.4 ± 0.7 | 5.6 ± 0.5 | 0.019 |
| Albumin (mg/dL) | 3.2 ± 1.0 | 2.9 ± 0.9 | 0.018 |
| CRP (mg/dL) | 0.40 (0.10-0.74) | 0.50 (0.21-1.26) | 0.011 |
| eGFR (ml/min) | 92 ± 19 | 94 ± 19 | 0.390 |
| WBC (×1000/mm3) | 9.2 ± 3.7 | 9.9 ± 4.1 | 0.154 |
| Lymphocyte (×1000/mm3) | 2.1 ± 0.8 | 2.0 ± 0.7 | 0.091 |
| Monocytes (×1000/mm3) | 0.60 (0.50-0.90) | 0.70 (0.50-0.98) | 0.127 |
| Neutrophil (×1000/mm3) | 6.5 ± 2.5 | 7.1 ± 3.0 | 0.057 |
| RDW, fL | 12.6 ± 1.5 | 13.0 ± 1.6 | 0.051 |
| MPV, fL | 8.1 ± 1.6 | 8.4 ± 1.2 | 0.117 |
| Hemoglobin (mg/dL) | 14.2 ± 1.9 | 13.8 ± 1.7 | 0.084 |
| Hematocrit (%) | 42.8 ± 4.9 | 41.8 ± 5.6 | 0.129 |
| Platelet count (×1000/mm3) | 259 (224-303) | 278 (223-332) | 0.114 |
| Traditional lipid profiles | |||
| Triglyceride (mg/dL) | 166 (117-220) | 177 (141-227) | 0.027 |
| TC (mg/dL) | 146 (113-179) | 156 (188-129) | 0.019 |
| HDL-C (mg/dL) | 38 (36-42) | 37 (35-41) | 0.009 |
| LDL-C (mg/dL) | 109 (73-133) | 116 (97-139) | 0.030 |
| Non-traditional lipid profiles | |||
| Non-HDL-C (mg/dL) | 107 (78-144) | 131 (97-159) | < 0.001 |
| NHHR | 2.90 (2.48-3.45) | 3.83 (3.38-4.32) | < 0.001 |
| AIP | 0.14 (0.07-0.29) | 0.26 (0.16-0.39) | < 0.001 |
| LCI (×10-4) | 6.7 (4.4-10.7) | 10.0 (6.1-13.9) | < 0.001 |
| Castelli’s risk index-I | 3.9 (3.0-5.5) | 4.8 (3.8-6.0) | < 0.001 |
| Castelli’s risk index-II | 3.0 (2.0-3.8) | 3.3 (2.6-4.1) | < 0.001 |
Values are mean ± SD, n (%), or median (interquartile range) unless otherwise stated.
ACEI, angiotensin-converting enzyme inhibitors; AIP, atherogenic index of plasma; ARB, angiotensin receptor blockers; BMI, body mass index; CCB, calcium channel blocker; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LCI, lipoprotein combined index; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MPV, mean platelet volume; NHHR, non-HDL-C/HDL-C ratio; RDW, red cell distribution width; TC, total cholesterol; WBC, white blood cell.
Figure 2.
Level differences of non-traditional lipid profiles between coronary slow flow group and normal coronary flow. AIP, atherogenic index of plasma; CRI-I, Castelli’s risk index I; CRI-II, Castelli’s risk index II; CSF, coronary slow flow; LCI, lipoprotein combined index; NCF, normal coronary flow; NHHR, non-HDL-C/HDL-C ratio.
Table 2. Angiographic data of study population.
| Normal coronary flow (n = 130) | Coronary slow flow (n = 130) | p value | |
| Coronary arteries with slow flow | |||
| LAD (%) | 69.6 | ||
| LCX (%) | 59.3 | ||
| RCA (%) | 61.6 | ||
| Number of coronary arteries with slow flow | |||
| One-vessel, n (%) | 49 (37.7) | ||
| Two-vessel, n (%) | 43 (33.1) | ||
| Three-vessel, n (%) | 38 (29.2) | ||
| TIMI frame count | |||
| Corrected LAD | 20.2 ± 4.1 | 35.9 ± 6.4 | < 0.001 |
| LCX | 20.4 ± 4.2 | 33.9 ± 4.5 | < 0.001 |
| RCA | 19.5 ± 3.2 | 32.5 ± 6.1 | < 0.001 |
| Mean TFC | 20.0 ± 3.8 | 34.5 ± 6.1 | < 0.001 |
Values are mean ± SD or (%), unless otherwise stated.
LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; TIMI, thrombolysis in myocardial infarction.
Figure 3.
Representation of the relationship between thrombolysis in myocardial infarction (TIMI) frame count and non-traditional lipid profiles in scatter diagram with heat map; non-HDL-C/HDL-C ratio shows a stronger positive correlation with TIMI frame count than other non-traditional lipid profiles [correlation coefficient [r = 0.3593, p < 0.0001)]. HDL-C, high-density lipoprotein cholesterol.
Table 3. Univariable logistic regression analysis for identifying independent predictors of coronary slow flow.
| Variables | Univariable analyses | |
| OR (95% CI) | p value | |
| Gender, male | 1.797 (1.069-3.022) | 0.027 |
| Body mass index | 1.067 (1.004-1.134) | 0.036 |
| Diabetes mellitus | 1.851 (1.026-3.339) | 0.041 |
| Dyslipidemia | 2.400 (1.459-3.949) | 0.001 |
| Smoking | 2.144 (1.176-3.910) | 0.013 |
| Fasting plasma glucose | 1.008 (1.001-1.015) | 0.019 |
| Uric acid | 1.579 (1.068-2.335) | 0.022 |
| Albumin | 0.741 (0.576-0.952) | 0.019 |
| CRP | 1.348 (1.041-1.744) | 0.023 |
| Triglyceride | 1.002 (1.000-1.005) | 0.106 |
| Total cholesterol | 1.004 (0.999-1.009) | 0.153 |
| HDL-C | 0.939 (0.889-0.993) | 0.026 |
| LDL-C | 1.007 (1.000-1.015) | 0.048 |
| Non-HDL-C | 1.010 (1.004-1.015) | 0.001 |
| Non-HDL-C/HDL-C ratio | 1.031 (1.022-1.041) | < 0.001 |
| Atherogenic index of plasma | 1.040 (1.023-1.057) | < 0.001 |
| Lipoprotein combined index | 1.146 (1.084-1.211) | < 0.001 |
| Castelli’s risk index-I | 1.375 (1.167-1.622) | < 0.001 |
| Castelli’s risk index-II | 1.767 (1.360-2.296) | < 0.001 |
CI, confidence interval; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio.
Table 4. Multivariate logistic regression analysis of coronary slow flow risk with non-traditional lipid profiles.
| Variables | Model 1 | Model 2 | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| NHHR | 1.058 (1.040-1.076) | < 0.001 | 1.054 (1.037-1.071) | < 0.001 |
| Non-HDL-C | 0.968 (0.949-0.989) | 0.002 | 0.984 (0.972-0.997) | 0.015 |
| AIP | 1.040 (1.018-1.063) | < 0.001 | 1.049 (1.025-1.074) | < 0.001 |
| LCI | 1.035 (0.935-1.146) | 0.502 | - | - |
| CRI-I | 1.511 (0.929-2.456) | 0.096 | - | - |
| CRI-II | 1.074 (1.386-2.102) | < 0.001 | 1.157 (1.061-1.458) | < 0.001 |
Odds ratio (OR) and 95% confidence intervals (CI) were obtained by the multivariate logistic regression model: Model 1: After adjustment the gender, body mass index, dyslipidemia, diabetes mellitus and smoking; Model 2: Model 1 + further adjusted for fasting plasma glucose, uric acid, albumin and CRP.
AIP, atherogenic index of plasma; CRI-I, Castelli’s risk index-I; CRI-II, Castelli’s risk index-II; HDL-C, high-density lipoprotein cholesterol; LCI, lipoprotein combined index; NHHR, non-HDL-C/HDL-C ratio.
Figure 4.
Forest plot of independent predictors of coronary slow flow detected in multivariate analysis. CI, confidence interval; HDL-C, high-density lipoprotein cholesterol.
Figure 5.
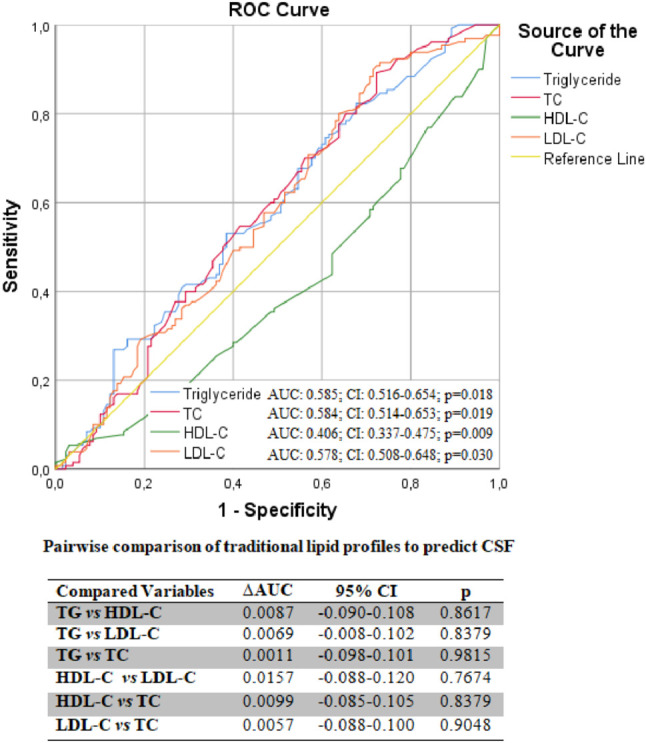
Pairwise comparison of traditional lipid profiles with ROC analysis to predict CSF. There is no significant difference between the CSF separability performances of traditional lipid profiles. AUC, area under the curve; CI, confidence interval; CSF, coronary slow flow; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ROC, receiver operating characteristic; TC, total cholesterol; TG, triglyceride; ΔAUC, differences between areas under the curve.
Figure 6.
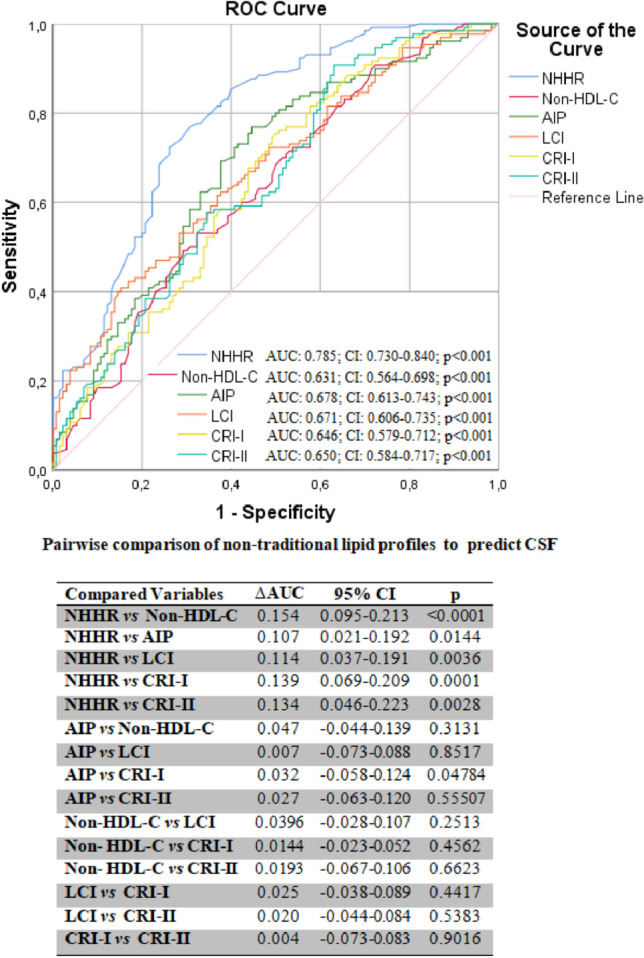
The non-HDL-C/HDL-C ratio (NHHR) predicts the CSF with 74% sensitivity and 72% specificity at the best cut-off value (3.3). When non-traditional lipid profiles were pairwise compared with ROC analysis to predict the CSF, the NHHR was superior to all other non-traditional lipid profiles in predicting CSF. AIP, atherogenic index of plasma; AUC, area under the curve; CI, confidence interval; CRI-I, Castelli’s risk index-I; CRI-II, Castelli’s risk index-II; CSF, coronary slow flow; HDL-C, high-density lipoprotein cholesterol; LCI, lipoprotein combined index; ROC, receiver operating characteristic; TC, total cholesterol; ΔAUC, differences between areas under the curve.
DISCUSSION
In this study, we investigated the correlations between CSF, a type of atherosclerotic microvascular disease, and traditional and nontraditional lipid profiles. Our results emphasize the role of atherogenic dyslipidemia, which includes increased TG, small, dense LDL-C particle, and decreased HDL-C particle levels, in the development of CSF. The results further showed how combining traditional lipid profiles in different formulations (e.g., NHHR, AIP) may provide a deeper understanding of the risk factors for CSF. The key conclusion is that high NHHR levels are highly and independently linked with the presence of CSF. In addition, we found that NHHR showed a stronger positive correlation with TFC, which is a quantitative analysis of CSF, compared to other non-traditional lipid profiles. Furthermore, NHHR had better predictive power for CSF than non-traditional and single lipid measurements.
CSF is defined as slowing of forward flow in the epicardial coronary arteries without any flow-limiting causes such as macroscopic spasm, dissection, significant luminal stenosis or thrombus. Although the exact etiology of CSF is still unknown, there is mounting evidence that subclinical atherosclerosis, which causes endothelial and microvascular insufficiency, is the fundamental underlying cause of this entity.1-3 Atherogenic dyslipidemia, which is known to occur when the ratio of pro- to anti-atherogenic lipid particles shifts in the atherogenic direction, is one of the major risk factors for atherosclerosis.7,8,25 Ding et al. demonstrated that individuals with CSF had considerably greater levels of the dyslipidemic enzyme lipoprotein-associated phospholipase A2 than those with NCF.26 In addition, Cin et al. observed extensive atherosclerotic plaques in the microvascular bed and epicardial coronary arteries that did not cause negative vascular remodeling in subjects with CSF detected in their epicardial coronary arteries on intravascular ultrasonography.2 Similar findings were reported by Pekdemir et al. in patients with CSF in epicardial coronary arteries, where they discovered diffuse intimal thickening and calcification along the coronary artery lumen.3 Additionally, Signori et al. demonstrated that individuals with CSF had compromised endothelial functioning, as evidenced by elevated TG and low HDL-C values.27 These findings support the role of the atherosclerotic process in CSF, since statins have been demonstrated to enhance coronary microvascular performance in patients with CSF.28,29 These studies suggest that CSF represents a type of endothelial dysfunction and microvascular CAD associated with atherogenic dyslipidemia before negative remodeling.
Many clinical studies have shown that non-traditional atherogenic lipid profiles such as AIP, LCI, non-HDL-C, CRI-I, CRI-II and NHHR obtained by combining traditional lipid profiles, TG, HDL, LDL, and TC particles in various fractions have superior predictive value to single lipid parameters in many cardiovascular diseases, possibly because they better reflect the pro-atherogenic and anti-atherogenic balance.18-23 Aciksari et al. reported that the TG/HDL-C ratio was a reliable indicator of CSF.30 Kaplangoray et al. showed a close relationship between TG-glucose index and CSF.31
In our study, although NHHR, non-HDL-C, AIP and CRI-II were found to be independent predictors of CSF, the correlation of NHHR with CSF was higher than for the other non-traditional lipid profiles, and the ability of NHHR to discriminate CSF was superior to the other non-traditional lipid profiles. Non-HDL-C contains many atherogenic lipid particles such as lipoprotein(a), intermediate-density lipoprotein cholesterol, very-low-density lipoprotein cholesterol and LDL-C, indicating that it carries a higher atherogenic load than LDL-C.32 Increasing evidence has shown that non-HDL-C is a stronger predictor of the development of microvascular and endothelial dysfunction due to atherosclerosis than LDL-C, and therefore it is recommended as a primary treatment target by current guidelines.33-35 Wang et al. reported that non-HDL-C levels in people with type 2 diabetes were associated with CRP levels, and that they were accurate predictors of vascular inflammation.36 Another study found that patients with type 2 diabetes who did not have non-HDL-C as a treatment goal had a considerably higher risk of microvascular events.37 In addition, non-HDL-C was more strongly related with subclinical atherosclerosis than any other conventional lipid profile in the study by Orakzai et al.38 Karasek et al. found a strong correlation between ApoB and non-HDL-C, and that correlations of ApoB and non-HDL-C with hemostatic endothelial markers such as von Willebrand factor, plasminogen activator inhibitor-1 and carotid intima-media thickness, a morphological marker of atherosclerotic vascular disease were very similar.39 Non-HDL-C levels have been found to be positively associated with proinflammatory macrophages CD-14, CD-16, and CD-36 in visceral adipose tissue.40 For predicting future cardiovascular risk in children with type 1 diabetes, Proda et al. hypothesized that non-HDL-C may be more accurate than hsCRP.41 Kanda et al. reported that a non-HDL-C level ≥ 100 mg/dL was an independent risk factor for the development of new lesions in patients using high-dose statins after a percutaneous coronary intervention.42 Non-HDL-C has been shown to be a robust predictor of CVD, and particularly coronary events, in both men and women with diabetes.43
HDL-C is the body’s primary anti-atherogenic lipid particle, in contrast to the powerful atherogenic characteristics of non-HDL-C.44 Combining the atherogenic properties of non-HDL-C and the antiatherogenic properties of HDL-C in a single parameter may better reflect the lipid balance in the body through a synergistic interaction, and it has been reported that the ability of the resulting formulation (NHHR) to predict cardiovascular events associated with atherogenic dyslipidemia may be superior to single lipid parameters. Mao et al. reported that patients with acute coronary syndrome and high NHHR levels had considerably worse cardiovascular prognoses and more severe coronary artery disease.18 Wang et al. demonstrated a substantial correlation between NHHR and carotid plaque stability.19 In postmenopausal women, Iannuzzi et al. found a substantial correlation between NHHR and both carotid atherosclerosis and very-low-density lipoprotein levels.45 In addition, Han et al. found that high NHHR levels were linked to an elevated incidence of type 2 diabetes mellitus (T2DM).46 Wang et al. observed that NHHR was closely related to the emergence of nonalcoholic steatohepatitis, which is recognized to be a risk factor for cardiovascular issues and T2DM.47 NHRR was demonstrated to be a useful marker of the incidence of CAD in subjects with chronic renal disease in a study by Lamprea-Montealegre et al.48 Kim et al. suggested that NHRR may be a more accurate measure for detecting metabolic syndrome and insulin resistance than the apoB/apoA1 ratio.49 Reynoso-Villalpando et al. also reported that NHHR could predict metabolic syndrome better than its components alone in T2DM subjects.50
Taken together, these results suggest that NHHR is significantly and strongly associated with CAD and its predisposing comorbid diseases. In this context, considering that CSF is a type of diffuse subclinical atherosclerotic vascular disease, the superiority of the relationship between NHHR and CSF over other traditional and non-traditional lipid profiles can be attributed to its broad and powerful influence on endothelial and microvascular functions.
Limitations
Some limitations to our investigation should be noted. First, our research was retrospective and observational. Second, a small number of people participated in our study. Third, because of the retrospective design, invasive imaging results such as intravascular ultrasonography or optical coherence tomography, which provide better information about subclinical diffuse atherosclerosis, and measurement results that can better evaluate endothelial function such as flow-mediated dilatation and pulse wave analysis were not available. Fourth, as this is a cross-sectional study, a definitive causal relationship between NHHR and CSF cannot be concluded. Fifth, despite the fact that known confounders were incorporated into the regression analysis to identify the independent predictors of CSF, selection bias and the impact of unaccounted confounders on study outcomes cannot be completely ruled out due to the retrospective study design. Sixth, because there was insufficient information, ApoB and small dense LDL-C levels, which can more accurately reflect the total load of atherogenic particles than LDL-C, were also excluded from the analysis. Additionally, data on the study population that could have affected lipid profiles, such as diet and exercise, could not be recorded due to the retrospective design. Finally, the short- and long-term clinical results of the study population were unavailable. To validate the findings of our investigation, prospective randomized controlled trials involving larger cohorts are required.
Investigating how these markers interact with each other in the context of CSF is essential. A more in-depth analysis of these interactions could provide a more comprehensive understanding of the pathophysiological mechanisms underlying CSF. Additionally, the identification of potential therapeutic interventions derived from our findings is a valuable area for further research. We believe that considering lifestyle modifications, pharmacological interventions, or other treatments aimed at modulating the identified biochemical markers could be a promising avenue for improving outcomes in individuals at risk of CSF.
New knowledge gained
• Non-traditional lipid profiles have a higher predictive value than single lipid profiles, especially in cardiovascular diseases.
• CSF is a microvascular disease in which diffuse subclinical atherosclerosis plays a fundamental role in the pathogenesis.
• The power of the non-HDL-C/HDL-C ratio to predict CSF was superior to other non-traditional lipid profiles and self-containing lipid profiles.
• The non-HDL-C/HDL-C ratio can be used as a primary therapeutic target in patients with CSF.
CONCLUSIONS
Our results showed that NHHR is a more potent and independent predictor of CSF than other non-traditional lipid profiles. Therefore, NHHR may be a useful biomarker both in shedding light on the pathophysiology of CSF and in predicting the presence of CSF. Additionally, NHHR may be an inexpensive and readily accessible biomarker that can be used as a treatment target for risk assessment of CSF patients and enable long-term follow-up of these patients in clinical practice. As a result, this study emphasizes the importance of NHHR as a potential biomarker for risk stratification of patients with CSF compared to other non-traditional lipid profiles. It also identifies NHHR as a particularly significant predictor of CSF.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
Acknowledgments
All the authors would like to thank all colleagues contributed to this study.
FUNDING
There is no funding for this study.
REFERENCES
- 1.Chalikias G, Tziakas D. Slow coronary flow: pathophysiology, clinical implications, and therapeutic management. Angiology. 2021;72:808–818. doi: 10.1177/00033197211004390. [DOI] [PubMed] [Google Scholar]
- 2.Cin VG, Pekdemir H, Camsar A, et al. Diffuse intimal thickening of coronary arteries in slow coronary flow. Jpn Heart J. 2003;44:907–919. doi: 10.1536/jhj.44.907. [DOI] [PubMed] [Google Scholar]
- 3.Pekdemir H, Cin VG, Ciçek D, et al. Slow coronary flow may be a sign of diffuse atherosclerosis. Contribution of FFR and IVUS. Acta Cardiol. 2004;59:127–133. doi: 10.2143/AC.59.2.2005166. [DOI] [PubMed] [Google Scholar]
- 4.Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suda A, Takahashi J, Hao K, et al. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol. 2019;74:2350–2360. doi: 10.1016/j.jacc.2019.08.1056. [DOI] [PubMed] [Google Scholar]
- 6.Varnava AM, Davies MJ. Relation between coronary artery remodelling (compensatory dilatation) and stenosis in human native coronary arteries. Heart. 2001;86:207–211. doi: 10.1136/heart.86.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 8.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 9.Sechtem U, Brown D, Godo S, et al. Coronary microvascular dysfunction in stable ischaemic heart disease (non-obstructive coronary artery disease and obstructive coronary artery disease). Cardiovasc Res. 2020;116:771–786. doi: 10.1093/cvr/cvaa005. [DOI] [PubMed] [Google Scholar]
- 10.De Bruyne B, Hersbach F, Pijls NH, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but "Normal" coronary angiography. Circulation. 2001;104:2401–2406. doi: 10.1161/hc4501.099316. [DOI] [PubMed] [Google Scholar]
- 11.Hung OY, Molony D, Corban MT, et al. Comprehensive assessment of coronary plaque progression with advanced intravascular imaging, physiological measures, and wall shear stress: a pilot double-blinded randomized controlled clinical trial of nebivolol versus atenolol in nonobstructive coronary artery disease. J Am Heart Assoc. 2016;5:e002764. doi: 10.1161/JAHA.115.002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siasos G, Sara JD, Zaromytidou M, et al. Local low shear stress and endothelial dysfunction in patients with nonobstructive coronary atherosclerosis. J Am Coll Cardiol. 2018;71:2092–2102. doi: 10.1016/j.jacc.2018.02.073. [DOI] [PubMed] [Google Scholar]
- 13.Fineschi M, Bravi A, Gori T. The "slow coronary flow" phenomenon: evidence of preserved coronary flow reserve despite increased resting microvascular resistances. Int J Cardiol. 2008;127:358–361. doi: 10.1016/j.ijcard.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Sadr-Ameli MA, Saedi S, Saedi T, et al. Coronary slow flow: benign or ominous? Anatol J Cardiol. 2015;15:531–535. doi: 10.5152/akd.2014.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Shen H, Gao F, et al. Clinical profile and outcome in patients with coronary slow flow phenomenon. Cardiol Res Pract. 2019;2019:9168153. doi: 10.1155/2019/9168153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponte-Negretti CI, Isea-Perez JE, Lorenzatti AJ, et al. Atherogenic dyslipidemia in Latin America: prevalence, causes and treatment: expert’s position paper made by The Latin American Academy for the Study of Lipids (ALALIP) endorsed by the Inter-American Society of Cardiology (IASC), the South American Society of Cardiology (SSC), the Pan-American College of Endothelium (PACE), and the International Atherosclerosis Society (IAS). Int J Cardiol. 2017;243:516–522. doi: 10.1016/j.ijcard.2017.05.059. [DOI] [PubMed] [Google Scholar]
- 17.Padró T, Vilahur G, Badimon L. Dyslipidemias and microcirculation. Curr Pharm Des. 2018;24:2921–2926. doi: 10.2174/1381612824666180702154129. [DOI] [PubMed] [Google Scholar]
- 18.Mao Q, Zhao J, Zhao X. Association of non-HDL-C-to-HDL-C ratio with coronary lesions and its prognostic performance in first-onset NSTEMI. Biomark Med. 2023;17:29–39. doi: 10.2217/bmm-2022-0548. [DOI] [PubMed] [Google Scholar]
- 19.Wang A, Li Y, Zhou L, et al. Non-HDL-C/HDL-C ratio is associated with carotid plaque stability in general population: a cross-sectional study. Front Neurol. 2022;13:875134. doi: 10.3389/fneur.2022.875134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toprak K. Atherogenic index of plasma is an independent risk factor for contrast induced nephropathy in patients with non-ST elevation myocardial infarction. Angiology. 2023;74:427–434. doi: 10.1177/00033197221110723. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Z, Wang H, Hou Q, et al. Non-traditional lipid parameters as potential predictors of carotid plaque vulnerability and stenosis in patients with acute ischemic stroke. Neurol Sci. 2023;44:835–843. doi: 10.1007/s10072-022-06472-3. [DOI] [PubMed] [Google Scholar]
- 22.Millán J, Pintó X, Muñoz A, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J, Wang A, Wang Y, et al. Non-traditional lipid parameters as potential predictors of asymptomatic intracranial arterial stenosis. Front Neurol. 2021;12:679415. doi: 10.3389/fneur.2021.679415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 25.Jin JL, Cao YX, Wu LG, et al. Atherogenic dyslipidaemia and cardiovascular events in patients with diabetes or pre-diabetes and stable coronary artery disease: a prospective, cohort study. BMJ Open. 2021;11:e037340. doi: 10.1136/bmjopen-2020-037340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding YD, Pei YQ, Wang R, et al. Increased plasma lipoprotein-associated phospholipase A2 levels are associated with coronary slow flow. BMC Cardiovasc Disord. 2020;20:248. doi: 10.1186/s12872-020-01463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Signori LU, Quadros AS, Sbruzzi G, et al. Endothelial function in patients with slow coronary flow and normal coronary angiography. Clinics (Sao Paulo) 2012;67:677–680. doi: 10.6061/clinics/2012(06)22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caliskan M, Erdogan D, Gullu H, et al. Effects of atorvastatin on coronary flow reserve in patients with slow coronary flow. Clin Cardiol. 2007;30:475–479. doi: 10.1002/clc.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan Y, Yang SS, Yu JB, et al. Atorvastatin use and coronary flow reserve in patients with coronary slow flow. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:143–146. [PubMed] [Google Scholar]
- 30.Aciksari G, Cetinkal G, Kocak M, et al. The relationship between triglyceride/high-density lipoprotein cholesterol ratio and coronary slow-flow phenomenon. Int J Cardiovasc Imaging. 2022;38:5–13. doi: 10.1007/s10554-021-02387-w. [DOI] [PubMed] [Google Scholar]
- 31.Kaplangoray M, Toprak K, Başanalan F, et al. Investigation of the relationship between triglycerides-glucose index and coronary slow flow: a retrospective case-control study. Arq Bras Cardiol. 2023;120:e20220679. doi: 10.36660/abc.20220679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enkhmaa B, Prakash N, Berglund L. Non-HDL-C levels and residual cardiovascular risk: do population-specific precision approaches offer any advantages? Atherosclerosis. 2018;274:230–231. doi: 10.1016/j.atherosclerosis.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roever L, Biondi-Zoccai G, Chagas AC. Non-HDL-C vs. LDL-C in predicting the severity of coronary atherosclerosis. Heart Lung Circ. 2016;25:953–954. doi: 10.1016/j.hlc.2016.06.790. [DOI] [PubMed] [Google Scholar]
- 34.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337–345. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 35.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 36.Wang CY, Chang TC. Non-HDL cholesterol level is reliable to be an early predictor for vascular inflammation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:4762–4767. doi: 10.1210/jc.2004-0820. [DOI] [PubMed] [Google Scholar]
- 37.Toth PP, Simko RJ, Palli SR, et al. The impact of serum lipids on risk for microangiopathy in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2012;11:109. doi: 10.1186/1475-2840-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orakzai SH, Nasir K, Blaha M, et al. Non-HDL cholesterol is strongly associated with coronary artery calcification in asymptomatic individuals. Atherosclerosis. 2009;202:289–295. doi: 10.1016/j.atherosclerosis.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Karasek D, Vaverkova H, Cibickova L, et al. Apolipoprotein B vs non-high-density lipoprotein cholesterol: association with endothelial hemostatic markers and carotid intima-media thickness. J Clin Lipidol. 2017;11:442–449. doi: 10.1016/j.jacl.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Poledne R, Kralova Lesna I, Kralova A, et al. The relationship between non-HDL cholesterol and macrophage phenotypes in human adipose tissue. J Lipid Res. 2016;57:1899–1905. doi: 10.1194/jlr.P068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prado MM, Carrizo T, Abregú AV, et al. Non-HDL-cholesterol and C-reactive protein in children and adolescents with type 1 diabetes. J Pediatr Endocrinol Metab. 2017;30:285–288. doi: 10.1515/jpem-2016-0307. [DOI] [PubMed] [Google Scholar]
- 42.Kanda D, Miyata M, Ikeda Y, et al. The priority of non-HDL-C assessment to predict new lesions among stable angina patients with strong statins. J Atheroscler Thromb. 2022;29:894–905. doi: 10.5551/jat.62908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu W, Resnick HE, Jablonski KA, et al. Non-HDL cholesterol as a predictor of cardiovascular disease in type 2 diabetes: the strong heart study. Diabetes Care. 2003;26:16–23. doi: 10.2337/diacare.26.1.16. [DOI] [PubMed] [Google Scholar]
- 44.Bruckert E, Hansel B. HDL-C is a powerful lipid predictor of cardiovascular diseases. Int J Clin Pract. 2007;61:1905–1913. doi: 10.1111/j.1742-1241.2007.01509.x. [DOI] [PubMed] [Google Scholar]
- 45.Iannuzzi A, Giallauria F, Gentile M, et al. Association between non-HDL-C/HDL-C ratio and carotid intima-media thickness in post-menopausal women. J Clin Med. 2021;11:78. doi: 10.3390/jcm11010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han M, Li Q, Qie R, et al. Association of non-HDL-C/HDL-C ratio and its dynamic changes with incident type 2 diabetes mellitus: The Rural Chinese Cohort Study. J Diabetes Complications. 2020;34:107712. doi: 10.1016/j.jdiacomp.2020.107712. [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Wang L, Wang Z, et al. Higher non-HDL-cholesterol to HDL-cholesterol ratio linked with increased nonalcoholic steatohepatitis. Lipids Health Dis. 2018;17:67. doi: 10.1186/s12944-018-0720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamprea-Montealegre JA, Sharrett AR, Matsushita K, et al. Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: the ARIC study. Atherosclerosis. 2014;234:42–46. doi: 10.1016/j.atherosclerosis.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Kim SW, Jee JH, Kim HJ, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168:2678–2683. doi: 10.1016/j.ijcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 50.Reynoso-Villalpando GL, Sevillano-Collantes C, Valle Y, et al. ApoB/ApoA1 ratio and non-HDL-cholesterol/HDL-cholesterol ratio are associated to metabolic syndrome in patients with type 2 diabetes mellitus subjects and to ischemic cardiomyopathy in diabetic women. Endocrinol Diabetes Nutr (Engl Ed) 2019;66:502–511. doi: 10.1016/j.endinu.2019.03.019. [DOI] [PubMed] [Google Scholar]