Abstract
Introduction
Lung cancer (LC) is the most common cause of cancer-related deaths worldwide, and its prognosis upon metastasis remains poor. Patients with COPD face a significantly elevated LC risk, up to six times greater than those with normal lung function. We aimed to investigate LC prevalence and stage distribution among COPD outpatients. Furthermore, we aimed to outline the COPD-related variables associated with referral for LC examination.
Methods
We conducted a retrospective analysis encompassing the period from 1 January 2012 to 31 December 2018 on all outpatients with COPD and LC and individuals referred for LC examinations.
Results
Among all COPD outpatients, 2231 patients (18%) were referred for LC examinations and 565 (4.6%) were diagnosed with LC. LC patients with COPD were more likely to be stage I–II, in contrast to the non-COPD LC population (46% versus 26%, p<0.001 for all). Patients referred for LC examinations exhibited higher use of COPD-related medications, reported more severe dyspnoea (69% versus 66% with Medical Research Council dyspnoea score >2) and experienced a greater frequency of exacerbations (30% versus 24% with two or more exacerbations).
Conclusion
Our study revealed a notably high LC incidence among COPD outpatients. LC patients with COPD were diagnosed at earlier stages, and outpatients with more pronounced COPD symptoms were more inclined to undergo LC diagnostics. The overrepresentation of LC cases among COPD outpatients emphasises the importance of tailoring specific screening initiatives for this demographic.
Shareable abstract
COPD outpatients face heightened lung cancer risk and are diagnosed in earlier stages than non-COPD lung cancer patients. This emphasises the need for tailored lung cancer screening for COPD outpatients, particularly among current smokers. https://bit.ly/4c6TFMi
Introduction
Lung cancer (LC) is the second most common cancer worldwide, with over 2.2 million new cases in 2020. In Denmark, 5047 new cases were recorded in 2020, which is the 10th highest incidence rate for a country globally [1, 2]. For women, Denmark has the second highest incidence rate, surpassed only by Hungary [2]. COPD is a persistent lung condition that tends to progress over time, often proving fatal. Roughly 400 000 individuals, out of Denmark's total population of ∼5.5 million, are estimated to have obstructive lung function impairment compatible with COPD. However, only about a half of this population receives medical treatment, pointing to common underdiagnosis of the disease [3, 4]. Since 2021, COPD has been the leading cause of death in Denmark, with 3665 deaths recorded in 2022 compared to 3425 deaths caused by LC [5]. Denmark holds the unfortunate distinction of having the highest COPD-related mortality in Europe, a record maintained for several years [6].
COPD and LC share a common origin, primarily linked to cigarette smoking. While it is estimated that 85–90% of all cases of both COPD and LC result from smoking, 23% of Danish COPD outpatients were still active smokers in 2022 [7–9]. Regardless of smoking history, individuals with COPD face up to a six-fold increased risk of developing LC [10–12]. Shared pathological mechanisms include chronic inflammation, epigenetic changes, DNA damage and oxidative stress [13].
In Denmark, COPD patients typically receive care through general practitioners or specialised pulmonary outpatient clinics, depending on the severity and complexity of their condition. Central registrations of COPD are consistently collected only at the hospital level, and registrations of milder cases in general practice are not yet systematically collected [8]. When LC is suspected, either in general practice or at the hospital, patients are referred to the pulmonary fast-track diagnostic unit, undergoing comprehensive evaluations, including chest computed tomography (CT), positron emission tomography CT (PET-CT) and potentially invasive diagnostic procedures [14]. Because COPD patients are routinely monitored at a hospital level, it is plausible that LC in patients with COPD is detected at earlier stages than in those who do not have COPD. Contrarily, the overlapping symptoms of LC and COPD could also lead to a delay in LC diagnosis when deterioration in known COPD symptoms is attributed to the lung condition [15–17].
Various studies have explored the link between COPD and LC, as outlined in several systematic reviews [18, 19]. However, the majority of these studies have focused on populations from the USA, China and Japan, with only few specifically representing Northern Europe [20, 21]. Studies from Northern Europe either focus on patients diagnosed in primary care or they lack knowledge regarding the stage of LC among COPD patients. In addition, they lack data on the characteristics of COPD patients undergoing LC examinations. Gaining insight into these factors will advance our understanding of the referred COPD patient population. This information, coupled with an assessment of LC incidence and stage distribution among COPD patients, will enable us to contemplate the prospect of screening all COPD outpatients for LC. This study aims to address the existing knowledge gap by examining the incidence of and factors associated with the referral for LC diagnostics among COPD outpatients. Additionally, the study seeks to investigate the incidence and stage distribution of LC within the specific subgroup of patients with both COPD and LC.
The article is presented in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist [22].
Study design and methods
Study population
This retrospective cohort study includes data from individuals treated in the Region of Southern Denmark over a 7-year period from 1 January 2012 to 31 December 2018. Three cohorts were defined, based on data from the Danish Registry of Chronic Obstructive Pulmonary Disease (DrCOPD), the Danish Lung Cancer Registry and data on individuals examined on suspicion of LC from the regional data warehouse. Figure 1 provides a comprehensive overview of the study population, data sources and variables, along with key numbers defining the cohort.
FIGURE 1.
Data sources, study population and collected variables. All registrations originated from records within the Region of Southern Denmark during the period from 1 January 2012 to 31 December 2018. COPD cohort: all patients registered with COPD at outpatient clinics; LC examination cohort: all patients who underwent examinations on suspicion of lung cancer (LC); COPD+LC examination cohort: the overlapping population diagnosed with COPD and examined on suspicion of LC. In the figure, LC patients are represented in red, while non-LC patients are shown in grey. FEV1: forced expiratory volume in 1 s; MRC: Medical Research Council. Created with Biorender.com.
Data regarding outpatients with COPD were sourced from the DrCOPD, which is administered by the Danish Clinical Quality Programme [3]. DrCOPD was founded in 2008; however, due to initial data incompleteness and a desire to align with current clinical practice standards, we chose not to incorporate data predating 1 January 2012. It encompasses individuals aged ≥30 years, with registrations derived from The Central Person Register, the Register of Pharmaceutical Sales and the Danish National Patient Registry [23]. Registrations on COPD diagnosis originate from the Danish version of the World Health Organization International Classification of Diseases, Tenth Revision (ICD-10): COPD (J44.X) or respiratory failure (J96.X) in combination with COPD (J44.X) as a secondary diagnosis [3, 24, 25]. Details regarding smoking history, COPD-related medications, forced expiratory volume in 1 s (FEV1), Medical Research Council (MRC) dyspnoea score and the frequency of moderate exacerbations were only available for individuals registered through outpatient clinics, with no corresponding information reported during hospitalisations. Consequently, data were only included from outpatient visits and data from hospitalisations were excluded from our analysis. It should be mentioned that COPD registrations from general practice have not yet become accessible and were not part of the study.
The population referred for LC examination was derived from a previous data collection effort involving all individuals examined for LC risk during the study period [26]. These assessments took place within LC fast-track clinics, for which registrations were based on the Danish Medical Classification System known as SKS, a Danish version of the World Health Organization's ICD-10 [25, 27, 28]. The cohort was identified by either one of two SKS codes: AFB26 (indicating the initiation of examinations in the fast-track clinic) and/or DZ031B (under observation for LC) [27]. We excluded patients diagnosed with LC before the study's initiation who were referred for suspicion of relapse.
We combined the cohort of outpatients with COPD and the cohort of patients examined on suspicion of LC, labelling individuals appearing in both datasets as the “COPD+LC examination cohort”. The remaining cohorts were referred to as the “COPD cohort” and the “LC examination cohort” (figure 1).
Information regarding the diagnosis, stage and subtype of LC was obtained from the Danish Lung Cancer Registry, in which it is categorised by ICD-10 code C34 [29].
Variables
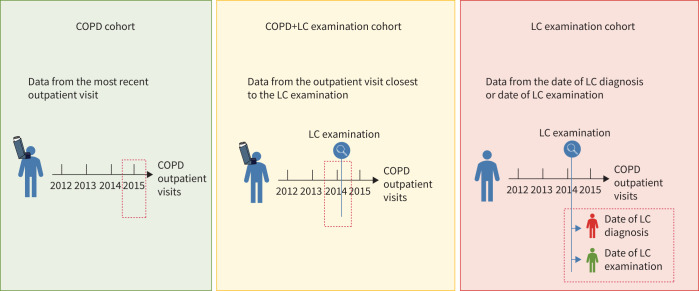
Figure 2 displays the date of registration of variables in all three cohorts. In the COPD cohort, we included the COPD-related variables registered at the most recent outpatient visit, because the data completion rate was higher in recent years. In the COPD+LC examination cohort, we included the COPD-related variables registered nearest to the LC examination, because our primary interest lay in the data closest to this event. In the LC examination cohort, LC patients were registered with their date of diagnosis, while non-LC patients were registered with the date of their initial LC examination.
FIGURE 2.
Date of registration of variables from the three cohorts. LC: lung cancer. Created with Biorender.com.
Smoking status information was categorised into four groups: non-smoker, current smoker, former smoker and unknown. Prescription medication data were collected in the following combinations: long-acting β-agonists (LABA), LABA+inhaled corticosteroids (ICS), long-acting muscarinic antagonists (LAMA), LABA+LAMA, LABA+LAMA+ICS and ICS. The variable “inhalation devices” covered all of the mentioned medicaments and was marked as present if any of these were prescribed. Each of the variables were binary, where a positive outcome (1) indicated the redemption of at least one prescription of inhalation medication during the specified year, while a negative outcome (0) signified no collection within the year. FEV1 was expressed as a percentage, excluding outliers exceeding 100% (92 out of 10 400 registrations). Furthermore, FEV1 was converted to the categorical Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades 1–4. MRC dyspnoea grade was documented on a scale from 1 to 5, with error registrations above 5 removed (153 out of 10 315 registrations). MRC grade was converted into a categorical variable, distinguishing between grade 1–2 (few symptoms) and 3–5 (many symptoms). Moderate exacerbations were recorded as whole numbers of exacerbations since the last visit, defined as moderate if they resulted in the administration of oral prednisolone and/or antibiotics. The number of exacerbations was similarly converted into grade 0–1 versus ≥2. These groupings were chosen because they align with the criteria used to differentiate between low and high risk of future exacerbations as defined by GOLD [30]. LC stages were transformed into stages I, II, III and IV and an “unknown” category. For all these variables, the reported figures reflect the number of individuals with available data.
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Danish Data Protection Agency (19/30673, 06–12–2020) and the Danish Patient Safety Authority (3–3013–3132/1, 03–30–2020). Individual consent for this retrospective analysis was waived by the competent authority.
Statistical analyses
Patient distribution is presented as percentages for categorical variables and as medians with interquartile ranges for continuous variables. To assess associations between the COPD cohort and the COPD+LC examination cohort, we employed the chi-squared test for categorical variables and the Wilcoxon signed-rank test for continuous variables. Subgroup analyses involved comparing the COPD cohort to two subsets within the COPD+LC examination cohort: those registered with COPD before LC diagnostics and those registered after LC diagnostics. In all tests, a significance level of 0.01 was used to compensate for multiple testing. All statistical analyses were conducted using STATA version 17.0 (Stata Corp LLC, College Station, TX, USA). Institutional Review Board approval was waived by the Region of Southern Denmark because all data was anonymised.
Results
Cohort distributions
Over the course of the 7-year study period, 12 351 individuals aged ≥30 years were documented with COPD from outpatient clinics in the Region of Southern Denmark. During the same timeframe, 29 477 patients underwent evaluations for suspected LC. Among these, there was an overlapping cohort comprising 2231 patients who were both diagnosed with COPD and examined for suspicion of LC. This overlapping group constituted 18% of all COPD outpatients and 8% of individuals examined for LC suspicion. All three cohorts demonstrated an increase in numbers throughout the study period (figure 3). The higher number of COPD registrations in 2018 is due to the fact that patients with multiple contacts in different years only had their latest visit registered.
FIGURE 3.
Inclusion during study period. The distribution by examination year in all three cohorts during the 7-year study period. LC: lung cancer.
Comparison of the COPD cohort and COPD+LC examination cohort
Table 1 provides a comparison of baseline demographics and COPD-related variables between the COPD cohort and the COPD+LC examination cohort. Additionally, it stratifies the COPD+LC examination cohort into LC and non-LC patients and compares these variables.
TABLE 1.
Clinical demographics and COPD-related variables in the COPD cohort and the COPD+LC examination cohort
| COPD cohort | COPD+LC examination cohort | p-value | COPD+LC examination cohort | p-value | ||
|---|---|---|---|---|---|---|
| LC | Non-LC | |||||
| Total cohort, n | 10 120 | 2231 | 565 | 1666 | ||
| Demographics | ||||||
| Total | 10 120 (100) | 2231 (100) | 565 (100) | 1666 (100) | ||
| Age, years | 71 (63–79) | 71 (65–77) | 0.415 | 72 (66–77) | 71 (64–77) | 0.145 |
| Sex | ||||||
| Male | 5006 (49.5) | 1129 (50.6) | 0.330 | 258 (45.7) | 871 (52.3) | 0.007 |
| Female | 5114 (50.5) | 1102 (49.4) | 307 (54.3) | 795 (47.7) | ||
| COPD-related medication | ||||||
| No inhalation device | 679 (6.7) | 111 (5.0) | 0.002 | 20 (3.5) | 91 (5.5) | 0.069 |
| Any inhalation devices# | 9441 (93.3) | 2120 (95.0) | 545 (96.5) | 1575 (94.5) | ||
| LABA | 1356 (13.4) | 318 (14.3) | 0.286 | 91 (16.1) | 227 (13.6) | 0.145 |
| LABA+ICS | 5807 (57.4) | 1456 (65.3) | <0.001 | 342 (60.5) | 1114 (66.9) | 0.006 |
| LAMA | 6149 (60.8) | 1556 (69.7) | <0.001 | 398 (70.4) | 1158 (69.5) | 0.676 |
| LABA+LAMA | 2471 (24.4) | 554 (24.8) | 0.680 | 135 (23.9) | 419 (25.2) | 0.550 |
| LABA+LAMA+ICS | 513 (5.1) | 78 (3.5) | 0.002 | 15 (2.7) | 63 (3.8) | 0.208 |
| ICS | 1235 (12.2) | 285 (12.8) | 0.457 | 60 (10.6) | 225 (13.5) | 0.076 |
| Smoking history | ||||||
| Total | 8632 (100) | 1743 (100) | 457 (100) | 1286 (100) | ||
| Former smoker | 5478 (63.5) | 1067 (61.2) | 0.076 | 282 (61.7) | 785 (61.0) | 0.802 |
| Never-smoker | 313 (3.6) | 42 (2.4) | 0.011 | 4 (0.9) | 38 (3.0) | 0.013 |
| Current smoker | 2841 (32.9) | 634 (36.4) | 0.005 | 171 (37.4) | 463 (36.0) | 0.589 |
| FEV1 | ||||||
| Total | 8567 (100) | 1741 (100) | 456 (100) | 1285 (100) | ||
| FEV1, % | 49 (34–64) | 47 (34–62) | 0.020 | 50 (37–65) | 46 (33–60) | <0.001 |
| MRC grade | ||||||
| Total | 8443 (100) | 1719 (100) | 450 (100) | 1269 (100) | ||
| 1–2 | 2903 (34.4) | 526 (30.6) | 0.002 | 142 (31.6) | 384 (30.3) | 0.608 |
| ≥2 | 5540 (65.6) | 1193 (69.4) | 308 (68.4) | 885 (69.7) | ||
| Number of moderate exacerbations | ||||||
| Total | 4796 (100) | 848 (100) | 188 (100) | 660 (100) | ||
| 0–1 | 3644 (76.0) | 586 (69.1) | <0.001 | 135 (71.8) | 451 (68.3) | 0.363 |
| ≥2 | 1152 (24.0) | 262 (30.9) | 53 (28.2) | 209 (31.7) | ||
Data presented as n (%) or median (interquartile range), unless otherwise indicated, with “Total” indicating the number of patients for whom data were recorded. Bold text signifies statistical significance. LC: lung cancer; LABA: long-acting β-agonists; ICS: inhaled corticosteroids; LAMA: long-acting muscarinic antagonists; FEV1: forced expiratory volume in 1 s; MRC: Medical Research Council. #: includes the registration of any of the six included inhalation medicaments.
There were no statistically significant differences observed in age and gender distribution between the COPD cohort and the COPD+LC examination cohort. Within the COPD+LC examination cohort, the LC cohort had a higher proportion of women compared to the non-LC cohort. Several medications, including LABA+ICS, LAMA and the overall registration of inhalation devices, were administered to a significantly higher proportion of patients in the COPD+LC examination cohort than in the COPD cohort. Further stratification of the COPD+LC examination cohort into LC and non-LC patients revealed that this difference was primarily driven by the non-LC cohort for LABA+ICS. Although the frequencies were small for both groups, LABA+LAMA+ICS was administered to a significantly higher proportion of patients in the COPD cohort than in the COPD+LC examination cohort (5.1% versus 3.5%, p<0.002).
The COPD cohort and the COPD+LC examination cohort demonstrated similarities in terms of smoking habits, with the exception of a slightly higher proportion of current smokers among the COPD+LC examination cohort (36.4% versus 32.9%, p=0.005). While there was no statistically significant difference between median FEV1 levels, there was a tendency for lower FEV1 among the COPD cohort (47% versus 49%, p=0.02). When converting FEV1 levels into GOLD 1–4, results between groups were significant albeit with no clinically relevant pattern (supplementary table S1).
A larger proportion of patients in the COPD+LC examination cohort had an MRC grade >2 (69% versus 66%, p=0.002), as well as ≥2 exacerbations, compared to the remaining COPD cohort (30% versus 24%, p<0.001).
Among the 2231 patients in the COPD+LC examination cohort, 1111 patients (50%) were registered with COPD before LC diagnostics. When comparing this subgroup to the COPD cohort, we identified the same associations as those presented in table 1 (supplementary table S2). The remaining 1120 patients (50%) were registered with COPD after LC diagnostics. When comparing this subgroup to the COPD cohort, no clear associations were identified (supplementary table S3). Consequently, the differences found in table 1 reflect variations between the COPD cohort and patients from the COPD+LC examination cohort who were registered with COPD before being referred for LC diagnostics.
LC distribution and stage
Of the patients undergoing LC examination, 7903 received a diagnosis of LC, among whom 565 were also diagnosed with COPD. This led to a LC incidence of 4.6% among all 12 351 COPD outpatients. The LC incidence among all patients examined on suspicion of LC was 26.8%, with no statistically significant difference between the COPD and non-COPD patients (25.3% versus 26.9%, p=0.099).
Data regarding LC stage were available for 7596 out of the 7903 LC patients (figure 4). The group of LC patients with COPD included a higher proportion of patients with stage I–II LC compared to the group of LC patients without COPD (46.2% versus 26.3%) (p<0.001 for all). The overall distribution of pathological subtypes was comparable; however, squamous cell carcinoma had higher representation among LC patients with COPD than among LC patients without COPD (25.3% and 19.6%, respectively, p<0.001) (supplementary table S4 and supplementary figure S1).
FIGURE 4.

Lung cancer (LC) stage distribution among the lung cancer patients with COPD and the group without COPD.
Discussion
Our study aimed to investigate the intersection between outpatients diagnosed with COPD and those undergoing assessment for suspected LC in the Region of Southern Denmark. Our findings reveal a substantial overlap between these two populations, with 18% of COPD outpatients being referred for LC diagnostic evaluations, resulting in a 4.6% LC diagnosis rate among outpatients with COPD.
We demonstrated that COPD outpatients referred for LC diagnostic evaluation exhibited more severe COPD symptoms than COPD outpatients not referred for such evaluations. Specifically, these patients had higher consumption of several COPD-related medicaments, reported more severe dyspnoea and experienced a greater frequency of exacerbations. These findings suggest that individuals with more pronounced COPD symptoms are more likely to undergo LC diagnostic assessments. This may be because these patients are possibly more intensely monitored for their COPD than COPD outpatients with less severe symptoms, and are therefore more likely to have imaging diagnostics such as X-ray or CT of the chest, which could lead to detection of LC. Furthermore, the presence of more severe COPD symptoms may in itself raise suspicion for the coexistence of LC, prompting further evaluation.
In addition to these findings, our study revealed that LC patients with COPD were more likely to be diagnosed at earlier stages (stage I–II) than the non-COPD LC population. This is in line with a large Canadian population-based study which revealed that underlying COPD was associated with early-stage LC detection [31]. The presence of COPD may lead to increased surveillance and earlier detection of LC, resulting in a higher proportion of early-stage diagnoses. In our study, all COPD outpatients were assessed by pulmonologists, who might have an extra focus on LC as a differential diagnosis. The pathological distribution revealed a higher rate of squamous cell carcinoma among the LC patients with COPD, which is in line with several other studies [18, 32–34].
The LC incidence among patients undergoing LC examination was 26.8%, with no significant difference between COPD and non-COPD patients (25.3% and 26.9%, p=0.099). This lack of significance might be attributable to a potential wash-out effect among COPD outpatients, where symptoms related to COPD could result in an increased number of patients being referred for LC examinations. This could also contribute to a higher rate of non-LC patients, reducing the overall LC incidence in this cohort and thereby equalising the elevated risk observed between the two categories.
The overall incidence of LC among all COPD outpatients included in our study was 4.6%. No difference was observed when focusing on patients with GOLD 1–2 COPD. Two different systematic reviews have reported a LC prevalence of 9% and 5.1%, respectively [18, 19]. This variance may be attributable to the exclusion of milder COPD cases in our study, in which only 7% of patients were graded GOLD 1. Such differences in study design pose challenges for direct comparison. Increasing the inclusion of patients with GOLD 1 COPD could potentially lead to the identification of additional LC cases, yet paradoxically result in a decrease in overall LC prevalence. This is because multiple studies have demonstrated a correlation between COPD severity and LC prevalence [12, 19, 35].
While the incidence of LC identified in this study was lower than in other studies, a rate of nearly 5% remains significantly higher than that observed in the Danish general population. In 2017, the annual risk of LC was reported to be between 0.1% and 0.3% for individuals over the age of 40 years [36]. This observed overrepresentation of LC cases among COPD outpatients highlights the importance of tailoring specific screening initiatives. Current initiatives towards the implementation of a screening programme in Denmark are based on recommended screening for individuals at high risk of LC, such as heavy smokers within a certain age interval [37]. However, our findings suggest that outpatients with COPD, regardless of smoking intensity, may also be considered for targeted screening. Another advantage of screening for LC among COPD outpatients is their expected high compliance. Compliance poses a significant challenge in LC screening [38]. Typically, COPD outpatients exhibit robust commitment to their outpatient appointments, making this patient cohort more reachable than those who are not regularly monitored by healthcare providers. Additionally, it is essential to consider further initiatives aimed at promoting smoking cessation and ensuring continuous commitment to cessation measures.
Strengths of our study include its large sample size and the use of comprehensive national registers, which allowed for the inclusion of a representative population of COPD outpatients from multiple hospital units and individuals undergoing examinations due to LC suspicion. Additionally, the use of data from a 7-year period provided a robust analysis of the study cohort. However, there are several limitations to consider. First, the study focused on a specific region in Denmark, which may limit the generalisability of the findings to other populations, where referral patterns, as well as patterns of COPD outpatient setup, may differ. Second, the study relied on registry data, which may be subject to inaccuracies or missing information. Data regarding FEV1, MRC and exacerbations were accessible for only a subset of patients from outpatient clinics, and it remains uncertain whether these missing data could potentially introduce selection bias. If a patient with severe COPD were at times less likely to undergo spirometry, and clinicians subsequently omitted MRC and exacerbation assessments, this could potentially skew the associations towards a lower degree of severity in the remaining cohorts with available data. Nevertheless, it is noteworthy that the proportion of missing cases was similar between the COPD cohort and the COPD+LC examination cohort, as well as between LC and non-LC patients within the COPD+LC examination cohort. Third, the diagnostic arrangement in outpatient clinics has the potential to introduce bias, leading to both over- and underdiagnosis of individuals with milder cases of COPD. For instance, a scenario of overdiagnosis might occur when a patient primarily consults a pulmonologist, without prior FEV1 testing. In such cases, a COPD diagnosis may be assigned without the patient meeting the diagnostic criteria, and persist for a year due to record-keeping requirements. Conversely, an instance of underdiagnosis could involve a patient with mild COPD who visits the outpatient clinic only once, and the medical staff fails to record the diagnosis. Finally, the lack of different types of data presents a limitation. For example, we lacked details about the treatment received by LC patients. This information is crucial for assessing the operability of patients and, in turn, understanding whether COPD patients with early-stage LC are suitable candidates for surgery. Advancements in operational techniques, such as segmentectomies, and curative radiation strategies provide potential curative treatment options for patients who were not previously eligible for surgery. Therefore, it is imperative that LC patients are identified in the early stages. Another category of missing data relates to information on smoking intensity, measured in pack-years. Obtaining this information would enable us to assess the eligibility of COPD outpatients for LC screening based on international criteria. Additionally, it would allow us to evaluate the number of potentially overlooked LC cases when applying these specific criteria.
Conclusion
Our study reveals a substantial overlap between outpatients with COPD and those undergoing LC examinations. Individuals with more severe COPD symptoms are more likely to undergo LC examinations, and LC patients with COPD tend to be diagnosed at earlier stages. The documented overrepresentation of LC cases among COPD outpatients emphasises the importance of tailoring specific screening initiatives for this demographic, especially among smokers. Implementing targeted screening programmes for COPD outpatients may lead to earlier detection and improved outcomes for LC in this high-risk population.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00064-2024.SUPPLEMENT (239.2KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: 1) Conception and design: M. Bang Henriksen, T.F. Hansen, L.H. Jensen, C.L. Brasen, O. Hilberg and A. Løkke; 2) administrative support: M. Bang Henriksen, T.F. Hansen, L.H. Jensen, C.L. Brasen, O. Hilberg and A. Løkke; 3) provision of study materials or patients: M. Bang Henriksen; 4) collection and assembly of data: M. Bang Henriksen; 5) data analysis and interpretation: M. Bang Henriksen, M. Borg, O. Hilberg and A. Løkke; 6) manuscript writing: all authors; 7) final approval of manuscript: all authors.
Conflict of interest: The authors have nothing to disclose.
Support statement: The project was funded by The Danish National Research Center for Lung Cancer, Danish Cancer Society (grant number R198-A14299), The Region of Southern Denmark, The University of Southern Denmark, The Dagmar Marshall Foundation and The Beckett Foundation. The funding sources did not participate in the data collection, analyses or writing of the manuscript. Funding sources were purely used for salary of the PhD student. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Danish Health Data Authority . Annual Report of New Cancer Cases. 2022. https://sundhedsdatastyrelsen.dk/da/find-tal-og-analyser/tal-og-analyser/sygdomme-og-behandlinger/kraeft/kraeft_nyetilfaelde_aarsrapport Date last accessed: 13 December 2023.
- 2.World Cancer Research Fund International . Lung Cancer Statistics. 2022. www.wcrf.org/cancer-trends/lung-cancer-statistics/ Date last accessed: 13 December 2023.
- 3.Danish Clinical Quality Program (RKKP) . Dansk register for Kronisk Obstruktiv Lungesygdom (Danish Register for COPD). 2023. www.rkkp.dk/kvalitetsdatabaser/databaser/dansk-register-for-kronisk-obstruktiv-lungesygdom/ Date last accessed: 24 April 2023. [Google Scholar]
- 4.Diab N, Gershon AS, Sin DD, et al. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 198: 1130–1139. doi: 10.1164/rccm.201804-0621CI [DOI] [PubMed] [Google Scholar]
- 5.Esundhed.dk . The Cause of Death Register. 2023. www.esundhed.dk/Emner/Hvad-doer-vi-af/Doedsaarsager Date last accessed: 13 December 2023.
- 6.Organisation for Economic Co-Operation and Development (OECD)/European Union . Health at a Glance: Europe 2018: State of Health in the EU Cycle. Paris, OECD Publishing, 2018. 10.1787/health_glance_eur-2018-en Date last accessed: 29 April 2024. [DOI]
- 7.Danish Health Authority . Patientvejledning Om Kol Kronisk Obstruktiv Lungesygdom [Patient Guidance on COPD]. 2006. www.sst.dk/∼/media/CE16657A4CF04EEB8C6DFF3CD6AA46B6.ashx Date last accessed: 13 December 2023.
- 8.Danish Clinical Quality Program (RKKP) . The Danish Register of Chronic Obstructive Pulmonary Disease. Annual Report 2022. 2023. www.sundhed.dk/content/cms/90/4690_drkol-aarsrapport-2022_offentlig.pdf Date last accessed: 13 December 2023.
- 9.World Health Organization . Lung Cancer. 2023. www.who.int/news-room/fact-sheets/detail/lung-cancer? Date last accessed: 13 December 2023.
- 10.Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer 2015; 90: 121–127. doi: 10.1016/j.lungcan.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasswa-Kintu S, Gan WQ, Man SFP, et al. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax 2005; 60: 570–575. doi: 10.1136/thx.2004.037135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009; 34: 380–386. doi: 10.1183/09031936.00144208 [DOI] [PubMed] [Google Scholar]
- 13.Parris BA, O'Farrell HE, Fong KM, et al. Chronic obstructive pulmonary disease (COPD) and lung cancer: common pathways for pathogenesis. J Thorac Dis 2019; 11: S2155–S2172. doi: 10.21037/jtd.2019.10.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danish Lung Cancer Group . Clinical Guideline. 2023. https://www.dmcg.dk/siteassets/kliniske-retningslinjer-skabeloner-og-vejledninger/kliniske-retningslinjer-opdelt-pa-dmcg/lungecancer/dlcg_visitation_diagn_stadie_v.3.0_admgodk_121223.pdf Date last accessed: 29 April 2024.
- 15.NICE . The Diagnosis and Treatment of Lung Cancer (Update). 2011. Available from: www.ncbi.nlm.nih.gov/books/NBK99021/pdf/Bookshelf_NBK99021.pdf [PubMed]
- 16.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for Asthma Management and Precention. 2023. Available from: https://goldcopd.org/2023-gold-report-2/
- 17.Cunningham Y, Wyke S, Blyth KG, et al. Lung cancer symptom appraisal among people with chronic obstructive pulmonary disease: a qualitative interview study. Psychooncology 2019; 28: 718–725. doi: 10.1002/pon.5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouronte-Roibas C, Leiro-Fernandez V, Fernandez-Villar A, et al. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett 2016; 382: 240–244. doi: 10.1016/j.canlet.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 19.Zhao G, Li X, Lei S, et al. Prevalence of lung cancer in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Front Oncol 2022; 12: 947981. doi: 10.3389/fonc.2022.947981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornum JB, Sværke C, Thomsen RW, et al. Chronic obstructive pulmonary disease and cancer risk: a Danish nationwide cohort study. Respir Med 2012; 106: 845–852. doi: 10.1016/j.rmed.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 21.Sandelin M, Mindus S, Thuresson M, et al. Factors associated with lung cancer in COPD patients. Int J Chron Obstruct Pulmon Dis 2018, 13: 1833–1839. doi: 10.2147/COPD.S162484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019; 13: S31–S34. doi: 10.4103/sja.SJA_543_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danish Clinical Quality Program (RKKP) . Dokumentation. 2023. www.rkkp.dk/kvalitetsdatabaser/databaser/dansk-register-for-kronisk-obstruktiv-lungesygdom/dokumentation Date last accessed: 29 April 2024.
- 24.Lange P, Tøttenborg SS, Sorknæs AD, et al. Danish Register of chronic obstructive pulmonary disease. Clin Epidemiol 2016; 8: 673–678. doi: 10.2147/CLEP.S99489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). 2019. https://icd.who.int/browse10/2019/en Date last accessed: 27 March 2023.
- 26.Henriksen MB, Hansen TF, Jensen LH, et al. A collection of multiregistry data on patients at high risk of lung cancer – a Danish retrospective cohort study of nearly 40,000 patients. Transl Lung Cancer Res 2023; 12: 2392–2411. doi: 10.21037/tlcr-23-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danish Health Data Authority . Classifications. 2021. https://sundhedsdatastyrelsen.dk/da/english/health_data_and_registers/classifications Date last accessed: 2 April 2023.
- 28.Danish Health Data Authority . SKS-browser. 2023. https://medinfo.dk/sks/brows.php Date last accessed: 13 December 2023.
- 29.Jakobsen E, Rasmussen TR. The Danish lung cancer registry. Clin Epidemiol 2016; 8: 537–541. doi: 10.2147/CLEP.S99458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danish Healthcare Services . Kronisk Obstruktiv Lungesygdom (KOL) [Chronic Obstructive Lung Disease]. 2023. www.sundhed.dk/sundhedsfaglig/laegehaandbogen/lunger/tilstande-og-sygdomme/obstruktive-lungesygdomme/kol/ Date last accessed: 11 August 2023.
- 31.Butler SJ, Louie A V, Sutradhar R, et al. Association between COPD and stage of lung cancer diagnosis: a population-based study. Curr Oncol 2023; 30: 6397–6410. doi: 10.3390/curroncol30070471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papi A, Casoni GL, Caramori G, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax 2004; 59: 679–681. doi: 10.1136/thx.2003.018291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennon FE, Salgia R. Mitochondrial dynamics: biology and therapy in lung cancer. Expert Opin Investig Drugs 2014; 23: 675–692. doi: 10.1517/13543784.2014.899350 [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann RF, Zarrintan S, Brandenburg SM, et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res 2013; 14: 1–13. doi: 10.1186/1465-9921-14-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mannino DM, Aguayo SM, Petty TL, et al. Low lung function and incident lung cancer in the United States: data from the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med 2003; 163: 1475–1480. doi: 10.1001/archinte.163.12.1475 [DOI] [PubMed] [Google Scholar]
- 36.Rubin KH, Haastrup PF, Nicolaisen A, et al. Developing and validating a lung cancer risk prediction model: a nationwide population-based study. Cancers (Basel) 2023; 15: 487. doi: 10.3390/cancers15020487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawson Q. NELSON trial: reduced lung-cancer mortality with volume CT screening. Lancet Respir Med 2020; 8: 236. doi: 10.1016/S2213-2600(20)30059-X [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Olivo MA, Maki KG, Choi NJ, et al. Patient adherence to screening for lung cancer in the US: a systematic review and meta-analysis. JAMA Netw Open 2020; 3: e2025102. doi: 10.1001/jamanetworkopen.2020.25102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00064-2024.SUPPLEMENT (239.2KB, pdf)