Abstract
Background
This study aimed to assess the broader environmental, healthcare and societal impacts across the entire asthma pathway from diagnosis to treatment in the UK.
Methods
A comprehensive cost-of-illness framework was developed considering the effects of the full asthma patient pathway, including greenhouse gas emissions generated from inhalers, National Health Service (NHS) costs, health-related quality of life and productivity losses. The model was based on published literature and clinical expert opinion to accurately estimate, in monetary terms, the net present value of the asthma pathway impacts for 2022–2031.
Results
The estimated net present value of the environmental, healthcare and societal impacts of the asthma pathway was £47 billion over the 2022–2031 period in the UK. Loss of disease control was a key contributor to higher greenhouse gas emissions and NHS costs. In 2022, a patient with non-severe uncontrolled asthma was estimated to incur 22% higher NHS costs than a patient with controlled asthma, while generating 0.1 t more of CO2 equivalent emissions. In the same year, the total direct impacts per patient with severe asthma were four times higher than for a patient with non-severe controlled asthma, with 0.54 t CO2 equivalent of greenhouse gas emissions. Moreover, as much as 77% of the total economic impact was driven by worsening health-related quality of life and productivity impacts occurring when patients’ symptoms were uncontrolled.
Conclusions
Uncontrolled asthma significantly impacts patients, the economy and the environment in the UK. Our results emphasise the need for a holistic approach in controlling asthma and should be carefully considered when developing policies to mitigate the overall burden of the disease.
Shareable abstract
Asthma management has far-reaching effects on the economy, environment, healthcare and society, with greater impacts seen in patients with uncontrolled disease https://bit.ly/3IMH6bD
Introduction
Asthma is a common respiratory disease characterised by chronic airway inflammation [1]. There were an estimated 262 million cases of asthma globally in 2019, with a prevalence of 27 million within Western Europe [2]; it is estimated that asthma affects as much as 12% of the UK population [3].
Despite the availability of treatment options, asthma patients may experience loss of disease control (i.e. uncontrolled asthma) associated with debilitating exacerbations that can lead to intensification of usual treatment and resource-intensive secondary care, with a detrimental impact on patients’ health-related quality of life (HRQoL) [1, 4, 5].
The impact of asthma on the environment is currently emerging as a cause of concern [6, 7]. Controller and reliever inhaler therapies, commonly involving pressurised metered-dose inhalers (pMDIs) and dry powder inhalers (DPIs) [8], have been associated with greenhouse gas (GHG) emissions, with pMDIs resulting in greater emissions than DPIs [6, 9, 10]. In a study by Janson et al. [6], CO2 equivalent (CO2e) of average usage was estimated based on device life cycle carbon footprint data for different types of DPIs and pMDIs. It was found that pMDIs had 20–30 times larger individual carbon footprints (net kg CO2e per pack) than DPIs, and the combination of the DPIs resulted in 17 kg of annual CO2e emissions per patient versus 439 kg for combined pMDIs [6]. Moreover, real-world findings revealed an overuse of reliever inhalers in patients with asthma across Europe [6]. Uncontrolled asthma may prompt an increase in the use of reliever pMDIs, which have one of the highest prescription rates in the UK [6, 7, 11, 12].
Reducing inhaler emissions has recently become a point of debate in several European countries [13]. In the UK, the National Health Service (NHS) England has set a net-zero target for 2035, aiming to reduce its carbon emissions, including emissions from inhalers at the point of usage, by 80% between 2028 and 2032 [9, 14]. Hence, national incentives have sought to reduce the GHG emissions from asthma by limiting the prescription of pMDIs or by partly switching pMDIs to DPIs [9, 14]. The pharmaceutical industry, in parallel, is investing in environmentally friendly propellants to reduce the carbon footprint of pMDI inhalers while preserving patient choice [15–19]. Hydrofluoroalkane 152a (1,1-difluoroethane) is such an aerosol propellant, resulting in an environmental impact nearly 100 times lower than currently used gases [16, 18, 20]. pMDI products formulated using these low-global warming potential (GWP) propellants are expected to be available from 2025, and then rolled-out across different therapeutic classes and geographies in the following years [15, 16].
Several studies have examined the environmental impact of asthma, focusing mainly on GHG emissions generated by treatment with inhalers or at specific steps of the asthma care pathway (e.g. at the point of inhaler switching, or emissions related to exacerbation treatment) associated with the use of rescue medication, medical services, travel costs and anaesthetic gases [6, 19, 21, 22]. However, with limited budgets available within the NHS and the increased burden of asthma, the broader impact of asthma management activities (e.g. healthcare services, increased use of reliever inhalers for uncontrolled symptoms and travel to healthcare sites) throughout the care pathway (e.g. diagnosis, maintenance treatment and asthma symptom worsening) should be considered because of their impact on GHG emissions, healthcare and societal costs. Hence, there is a need to assess the broader impact of the entire asthma pathway to fully capture and quantify its burden.
Cost of illness (CoI) studies provide a framework for the itemisation, valuation and aggregation of impacts associated with a disease, and allow a more holistic estimation of the economic burden of the disease in society [23]. The CoI accounts for the epidemiology of the disease and its effect on morbidity and mortality as well as the consequent direct, indirect and intangible costs related to premature death and disability. Precise understanding of the CoI is imperative, because it serves as a fundamental cornerstone for the development, prioritisation and efficiency of healthcare policies by guiding healthcare resource allocation [24].
In this study, a model-based CoI study was conducted to evaluate the total economic burden of asthma by estimating the environmental, healthcare and societal impacts across the entire asthma pathway particularly in the UK, identifying the key drivers of the economic burden.
Material and methods
CoI analytical approach
A CoI study was conducted to estimate the total economic burden of the asthma pathway in the UK. CoI studies allow a more extensive itemisation, valuation and aggregation of disease components and their corresponding costs and GHG emissions. Τhe key steps of the analytical approach included 1) mapping the UK asthma pathway; 2) developing an impacts framework to establish the direct and indirect environmental, healthcare and societal impacts at each stage of the asthma pathway; and 3) developing the executable economic model. For the analysis to reflect the asthma pathway in the UK as accurately as possible, literature review findings were complemented with a validation exercise by UK-based clinical experts in respiratory medicine (supplementary material, including table S3).
Asthma pathway mapping
The study considered adult patients (≥18 years) in the UK with non-severe and severe asthma. The typical asthma pathway of these patients was simplified into three distinct stages: diagnosis, maintenance treatment and uncontrolled symptoms treatment.
Impacts framework
An asthma impacts framework was developed to itemise patients and NHS activities as well as their corresponding environmental, healthcare and societal impacts for each of the three stages of the asthma pathway in the UK (supplementary table S1). These activities’ impacts were categorised into NHS costs, GHG emissions and patient travel costs, henceforth referred to as direct impacts, and were then valued in monetary terms. Indirect impacts, such as the impact of asthma on patients’ HRQoL and productivity losses, were also assessed.
CoI model
The CoI model [25] was built using Microsoft Excel to estimate the total economic burden of asthma at both a population and patient level for the period 2022–2031, presenting results for 2022 and the period 2022–2031 in net present value (NPV). Scenario analyses were conducted to explore uncertainty associated with key assumptions (supplementary section S4).
Modelling of the asthma patient population
The model analysed the most common patient journeys through the asthma pathway in a year, defined as patient-activity profiles.
The most common patient journeys were determined broadly based on five health status and healthcare activity categories: asthma severity, adherence to treatment plan, symptom control, level of uncontrolled symptoms and survival status (table 1). All possible combinations of these categories resulted in 14 patient-activity profiles, each reflecting a mix of different patient experiences within each stage of the asthma pathway.
TABLE 1.
Categories of the asthma patient population
| Population category | Description |
|---|---|
| Severity of the disease | Based on GINA guidelines [1]: 1) Non-severe asthma: collectively include mild, moderate, uncontrolled and difficult-to-treat asthma patients (the latest being a subset of uncontrolled asthma) 2) Severe asthma (4–5% of patients): lack of disease control despite optimal patient adherence to (optimised) high treatment intensity [1, 26] |
| Patient adherence to treatment plan # | 1) Suboptimal adherence: adherence to <80% of their treatment plan (subscription filling level <80%)¶ 2) Optimal adherence: adherence to at least 80% of their treatment plan (subscription filling level ≥80%)+ |
| Patient symptom control | 1) Controlled symptoms: patients who do not experience symptoms in the previous year or only have minor occurrences that do not require extensive medical intervention or affect individuals' daily lives 2) Uncontrolled symptoms: patients with worsening of symptoms or exacerbations that require medical intervention and interrupt patients’ daily lives |
| Uncontrolled symptoms stage | Patients with uncontrolled symptoms (severe and non-severe) can experience the following: 1) Mild worsening of symptoms: mild symptoms (such as increased cough, shortness of breath or night waking), resolving without treatment or requiring self-administration of a few doses of reliever medication via a reliever inhaler [1] 2) Self-managed exacerbations: severe worsening of symptoms requiring self-administration of a greater intake of a reliever medication via a reliever inhaler (may also require primary care unplanned appointments/interventions) 3) Exacerbations requiring secondary care: worsening of symptoms for which self-administration of inhaled medication is not sufficient. Depending on the severity, patients might require secondary care interventions (e.g. ambulance, hospital admission) |
| Mortality | A proportion of patients that require secondary care would also not survive the exacerbation§ |
GINA: Global Initiative for Asthma. #: defined based on available evidence, in which prescription filling is picking up the prescription by the patient in a year [27, 28]; ¶: assuming that a uniform distribution of adherence within this population results in a 40% average adherence to medication (we assumed that patients still purchase a higher proportion of medication compared to what they use, i.e. that patients purchase 60% of the medication prescribed by their treatment plan); +: assuming that a uniform distribution of adherence within this population results in a 90% average adherence; §: there is potentially a small number of patients that die of asthma without having the time to ask for medical intervention; however, these were expected to be negligible compared to the total number of asthma-related deaths.
The number of patients within each of the five categories was estimated based on published literature, proprietary and non-proprietary data, and expert opinion (supplementary table S2). This information was then used to determine the number of patients within each patient-activity profile (table 2). Patient number estimates in the model were based on the year 2021 and projected for 2022–2031 by accounting for the annual number of new asthma diagnoses (supplementary section S1.3) and for the anticipated increase in the UK asthma population using Office for National Statistics data [29].
TABLE 2.
Adult asthma population distribution for each patient-activity profile in the UK in 2021
| Asthma severity | Level of adherence to treatment | Symptoms control | Level of uncontrolled symptoms | Need for secondary care intervention | Mortality | Patients (n) |
|---|---|---|---|---|---|---|
| Non-severe asthma | Optimal adherence | Controlled | – | – | – | 844 748 |
| Uncontrolled | Worsening of symptoms | – | – | 308 710 | ||
| Having at least one exacerbation | Self-managed exacerbations | 49 101 | ||||
| Exacerbations requiring secondary care | Non-fatal | 9788 | ||||
| Fatal | 288 | |||||
| Suboptimal adherence | Controlled | – | – | – | 1 310 411 | |
| Uncontrolled | Worsening of symptoms | – | – | 724 163 | ||
| Having at least one exacerbation | Self-managed exacerbations | – | 180 435 | |||
| Having at least one exacerbation | Exacerbations requiring secondary care | Non-fatal | 35 969 | |||
| Having at least one exacerbation | Fatal | 1059 | ||||
| Severe asthma | Optimal adherence | Uncontrolled | Worsening of symptoms | – | – | 107 671 |
| Having at least one exacerbation | Self-managed exacerbations | – | 21 229 | |||
| Having at least one exacerbation | Exacerbations requiring secondary care | Non-fatal | 4232 | |||
| Having at least one exacerbation | Exacerbations requiring secondary care | Fatal | 125 | |||
| Total | 3 597 929 |
Direct and indirect impacts modelling
The asthma patient activities, along with their consequent quantifiable direct impacts, within each modelled stage of the asthma pathway are detailed in table 3 and supplementary table S4.
TABLE 3.
Direct impacts generated on NHS costs, GHG emissions and patient travel costs, and indirect impacts on HRQoL and productivity loss at work
| Asthma pathway stage | |||
|---|---|---|---|
| Diagnosis stage | Maintenance treatment | Uncontrolled asthma treatment | |
| Direct impacts # | |||
| NHS costs | HCP and tests | GP/nurse time and tests, controller inhaler, other medications | Secondary care usage (including ambulance, calls to emergency 999, hospital admissions), reliever inhaler and OCS tablets |
| GHG emissions | Running facilities, travel |
Inhaler usage, running facilities, travel | Reliever inhaler usage and OCS tablets usage |
| Patient travel costs | Travelling to and from appointments | ||
| Indirect impacts for uncontrolled asthma ¶ | |||
| HRQoL loss | Morbidity: loss of QALY due to living with asthma, non-adherence to treatment and exacerbations | Mortality: loss of QALY due to years of life lost | |
| Productivity loss (at work) | Unemployment due to inability to work | Lower hourly wage due to inability to travel long distances for better employment conditions | Higher absenteeism due to time off work related to sickness and attending appointments |
GHG: greenhouse gas; GP: general practitioner; HCP: healthcare practioner; HRQoL: health-related quality of life; NHS: National Health Service; OCS: oral corticosteroids; QALY: quality-adjusted life years. #: direct impacts were due to the main activities undertaken by patients under each asthma pathway stage. The activities could be grouped into three main categories: primary care (HCP visits for diagnosis, unplanned HCP visits and asthma reviews), secondary care (calls to 999, ambulance, accident and emergency admissions and hospitalisations (day stays and longer stays)) and pharmacological treatment (controller and reliever inhalers and add-on treatments); ¶: indirect impacts were considered only for patients with uncontrolled asthma and were assumed to be independent of the asthma pathway stage.
The modelled direct impacts in each pathway stage considered 1) GHG emissions related to operating primary care facilities (during consultations) and secondary care facilities (e.g. ambulance use), patients travelling to their appointments, and the production, usage and disposal of inhalers; 2) NHS costs attributed to NHS services (e.g. consultations, healthcare professionals (HCPs) time, ambulance usage) and from purchasing medications and conducting asthma-related clinical tests; and 3) patient costs related to travelling to and from HCP facilities.
Indirect impacts of the asthma pathway were estimated for patients with uncontrolled symptoms because patients with controlled asthma tend to have a regular daily life without significant interruptions [30]. Indirect impacts were assumed to be independent of the modelled asthma pathway stages.
Asthma has a detrimental effect on a patient's wellbeing and daily life due to uncontrolled symptoms (e.g. exacerbations), reducing the overall patient HRQoL [1]. Additionally, asthma may result in reduced life expectancy because it may lead to premature mortality among patients with moderate and severe asthma, particularly in the UK [1, 4]. There is also evidence that asthma is associated with productivity loss related to increased unemployment, absenteeism and lower hourly wages [30, 31]. Hence, indirect impacts included reductions in patients’ HRQoL and lifespan and productivity losses (table 3, supplementary table S4). Clinical experts suggest that caregiver costs are more significant in paediatric patients; hence, they were not included in the analysis due to its focus on adult patients. Preventive costs were also not included in the analysis due to lack of granularity in published evidence.
Direct and indirect impacts were estimated for each modelled patient-activity profile based on model inputs and assumptions (tables 4 and 5 and supplementary sections S2.2 and S2.3). The total expected direct and indirect impacts of the asthma pathway were then estimated by accounting for the probability of patients being defined by each modelled patient activity, and the estimated impacts for each patient-activity profile over a year.
TABLE 4.
Key assumptions applied throughout the model
| Assumptions | Value | Source/justification |
|---|---|---|
| Modelled population-related assumptions | ||
| Population considered | Adult asthma patients (≥18 years) | Limited evidence availability for paediatric patients |
| Gender split (female) | 58.51% | Indicated the % of females in the population Aged 16+ [32] |
| Annual increase in asthma prevalence | 0% | Assumption based on clinical expert opinion# |
| Average age of death for asthma patients (UK) | 72.45 years | NHS UK [33] |
| Average age of death for general population (UK) | 75 years | Faculty of Public Health [34] |
| Inflation and discount rates | ||
| Discount rate of future costs | 3.5% | HM Treasury: The Green Book [35] |
| Annual inflation rates | 2022: 2.85% 2023: 3.14% 2024: 1.86% 2025: 1.89% 2026: 2.00% 2027+: 2.00% |
HM Treasury: The Green Book [35] |
| Annual increase in tCO2e monetary value | 1.5% | HM Treasury [36] |
| Monetary value indirect impacts | ||
| Monetary value of QALY (£ per QALY) | 20 000 | |
| Monetary value of t CO2e in 2022 (£ per t CO2e) | 254.51 | HM Treasury [36] Reported values were inflated from 2020 prices to 2021 first, and future year values were estimated by applying the annual increase in t CO2e monetary value |
| Inhaler switchover targets | ||
| Year of rollout of HFA152a | 2026 | Chiesi Group [16] 2026 was used in the model as the first complete year in which HFA152a will have been rolled-out |
| Assumed reduction in pMDI emissions due to HFA152a | 90% | Chiesi Group [16, 37] |
| % of non-salbutamol inhalers prescribed being pMDI by 2023/2024 | 25% | NHS UK [14] |
| % of pMDIs to target that are switched to DPI | 10% | Assumption |
| Yearly increase in uptake of HFA152a technology above the announced commitments by manufacturers | 5% | Assumption |
DPI: dry powder inhaler; HFA: hydrofluoroalkane; pDMI: pressurised metered-dose inhaler; QALY: quality-adjusted life years; t CO2e: tonnes of CO2 equivalent. #: clinical expert opinion was elicited from a panel of UK-based respiratory physicians (supplementary material section S3).
TABLE 5.
Key model parameters and values related to population split, and unit costs (details provided in supplementary material)
| Parameter | Value |
|---|---|
| Parameters related to number of patients used as model inputs (year 2021) | |
| Total adult population diagnosed with asthma (non-severe and severe) | 3 597 929 |
| Distribution of asthma patients by severity of the condition | |
| Severe asthma population | 133 257 |
| Non-severe asthma population | 3 464 672 |
| Non-severe difficult-to-treat asthma population | 478 391 |
| Distribution of asthma patients by adherence | |
| Optimally adhering severe asthma patients | 133 257 |
| Suboptimally adhering severe asthma patients | 0 |
| Optimally adhering non-severe asthma patients | 1 212 635 |
| Suboptimally adhering non-severe asthma patients | 2 252 037 |
| Distribution of asthma patients by control of symptoms | |
| Uncontrolled non-severe asthma patients | 1 309 513 |
| Optimally adhering non-severe asthma patients with uncontrolled asthma | 367 887 |
| Suboptimally adhering non-severe asthma patients with uncontrolled asthma | 941 626 |
| Distribution of asthma patients by worsening of symptoms and exacerbation | |
| All asthma patients having at least one exacerbation | 302 226 |
| Non-severe asthma patients having at least one exacerbation | 276 641 |
| Non-severe asthma patients having at least one exacerbation (% of non-severe asthma patients) | 7.98% |
| Severe asthma patients having at least one exacerbation | 25 585 |
| Severe asthma patients having at least one exacerbation (% of severe asthma patients) | 19.2% |
| Optimally adhering non-severe asthma patients having at least one exacerbation | 59 177 |
| Optimally adhering non-severe asthma patients having at least one exacerbation (% of optimally adhering asthma patients) | 16% |
| Suboptimally adhering non-severe asthma patients having at least one exacerbation | 217 463 |
| Suboptimally adhering non-severe asthma patients having at least one exacerbation (% of suboptimally adhering asthma patients) | 23% |
| Distribution of asthma patients by worsening of symptoms and exacerbation: distributing exacerbations by patient (or patient-activity profile) | |
| Exacerbation rate of optimally adhering non-severe asthma patients having at least one exacerbation | 1.13 |
| Exacerbation rate of suboptimally adhering non-severe asthma patients having at least one exacerbation | 1.13 |
| Exacerbation rate of severe asthma patients having at least one exacerbation | 3.28 |
| Distribution of asthma patients by secondary care intervention for exacerbations | |
| Patients (non-severe and severe asthma) having an exacerbation requiring secondary care | 51 462 (10 076+37 029+4357) |
| Distribution by asthma-related mortality between patient-profile activities | |
| Asthma-related mortality (number of deaths per year) | 11 324 (1909+7016+2399) |
| Unit costs and emissions associated with primary care use | |
| Costs of HCP appointments (2021 per appointment) | |
| GP | £255 |
| Practice nurse | £44 |
| Asthma specialist | £255 |
| Emissions related to appointment | |
| GHG emissions generated by 9.22 min of contact in a GP facility | 6000 g CO2e |
| Travelling to HCP appointment (emissions and costs) | |
| Emissions related to traveling to HCP facility | 896 g CO2e |
| Patient travel cost to facility | £5.20 |
| Cost of tests to diagnose and monitor asthma | |
| Forced expiratory volume in one second from spirometry | £2.19 |
| Peak expiratory flow test | £14.50 |
| Bronchodilator reversibility test | £11.51 |
| Unit costs related to maintenance treatment | |
| Average cost of inhalers per therapeutical class and type of inhaler based on sales dataset (2021) | |
| Controller ICS DPI inhaler | £17.63 |
| Controller ICS pMDI inhaler | £9.69 |
| Controller ICS+LABA DPI inhaler | £26.83 |
| Controller ICS+LABA pMDI inhaler | £20.14 |
| Controller ICS+LABA+LAMA DPI inhaler | £29.23 |
| Controller ICS+LABA+LAMA pMDI inhaler | £22.01 |
| Reliever SABA DPI inhaler | £4.48 |
| Reliever SABA pMDI inhaler | £1.78 |
| Average GHG of inhalers per therapeutical class based on 2021 sales dataset and data on individual product emissions | |
| Controller ICS inhaler | 14 282 g CO2e |
| Controller ICS+LABA inhaler | 10 924 g CO2e |
| Controller ICS+LABA+LAMA inhaler | 8807 g CO2e |
| Reliever SABA inhaler | 18 252 g CO2e |
| Average costs per add-on medication (type), based on 2021 sales dataset | |
| Zafirlukast (LTRA) | £4.56 |
| Montelukast (LTRA) | £3.07 |
| Theophylline (theophylline) | £3.85 |
| Aminophylline (theophylline) | £2.80 |
| Bambuterol (β-2 adrenergic agonist) | £30.26 |
| Terbutaline (β-2 adrenergic agonist) | £14.06 |
| Isoprenaline (β-2 adrenergic agonist) | £0.01 |
| Ketotifen (allergy) | £8.99 |
| Omalizumab (biological treatment) | £249.29 |
| Salbutamol (salbutamol) | £33.93 |
| Mepolizumab (monoclonal antibody) | £840 |
| Benralizumab (monoclonal antibody) | £1955 |
| Prednisolone (OCS) | £79 |
| Azithromycin (antibiotics) | £108 |
| Secondary care impacts | |
| Unit costs generated by secondary services | |
| Call to 999 | £89.59 |
| Ambulance | £357.40 |
| A&E | £296.87 |
| Inpatient <1 (day case) | £497.11 |
| Inpatient >1 (average 3 days) | £1342.20 |
| Unit emissions generated by secondary services | |
| Call to 999 | NA |
| Ambulance | 75 000 g CO2e |
| A&E (per visit) | 76 000 g CO2e |
| Inpatient <1 (day case) | 125 000 g CO2e |
| Inpatient >1 (average 3 days) | 375 000 g CO2e |
| Resource utilisation related to exacerbations | |
| Call to 999 | 28% |
| Ambulance | 28% |
| A&E | 28% |
| Inpatient <1 (day case) | 14% |
| Inpatient >1 (average 3 days) | 13% |
A&E: accident and emergency; DPI: dry powder inhaler; g CO2e: grams of CO2 equivalent; GHG: greenhouse gas; GP: general practitioner; HCP: healthcare professional; ICS: inhaled corticosteroids; LABA: long-acting β-agonist; LAMA: long-acting muscarinic antagonist; LTRA: leukotriene receptor antagonists; NA: not applicable; OCS: oral corticosteroids; pMDI: pressurised metered-dose inhaler; SABA: short-acting β-agonist.
Nonmonetary impacts such as HRQoL reduction, increased mortality and GHG emissions were first estimated in natural units (e.g. quality-adjusted life years (QALY) and tonnes (t)/mega tonnes (Mt) of CO2e). To compare GHG emissions to other impacts generated by the asthma pathway, nonmonetary impacts were converted into monetary values using standard approaches (tables 4 and 5 and supplementary sections S2.3 and S2.4) [38, 36]. The monetary value per unit of GHG emissions was based on the UK Department of Businesses, Energy and Industrial Strategy carbon values (i.e. price per t CO2e) [36], while the monetary value per QALY was informed by the National Institute for Health and Care Excellence willingness-to-pay threshold lower bound (i.e. £20 000 per QALY) [39]. The total costs associated with nonmonetary impacts in a year were estimated by multiplying the monetary value per unit of impact with the number of nonmonetary impact units in that year. The monetary conversion of indirect impacts was also applied on an annual basis for the period 2022–2031 to obtain the total impacts of asthma in natural units and the 2022 NPV in monetary terms over this period.
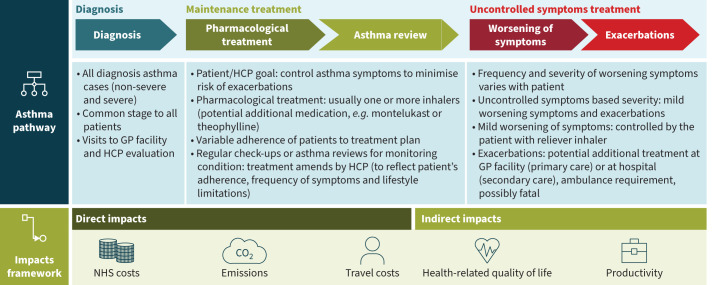
Figure 1 presents an outline of the analytical framework used to conduct the CoI analysis.
FIGURE 1.
Overview of the analytical approach of the cost-of-illness model applied. The main stages of the asthma pathway were diagnosis, maintenance treatment and uncontrolled symptoms treatment. The diagnosis stage focused only on cases of diagnosed asthma. Hence, each patient passes through the diagnosis stage, which typically involves visits to the general practitioner (GP) facility during which a healthcare professional (HCP) evaluates the patient's symptoms, medical history and lung function. Following diagnosis, the goal of the patient and HCPs in the maintenance treatment stage is to control the symptoms and minimise the risk of exacerbations and persistent airflow limitation. The HCP develops a pharmacological treatment plan that commonly includes one or more inhalers; patients adhere to the prescribed treatment to varying extents. In addition, patients undergo regular check-ups to monitor their condition, in which the HCP may amend the prescribed treatment plan. Some patients with asthma may experience uncontrolled symptoms that, based on severity, are divided into mild worsening of symptoms and exacerbations. Mild-form symptoms (e.g. increased cough or breathlessness) may be relieved by the patient using a reliever inhaler. Asthma exacerbations are acute or subacute episodes of progressively worsening of symptoms (shortness of breath, cough, wheezing and chest tightness [1]); therefore, the patient may need treatment either at the GP facility or at the hospital, sometimes requiring an ambulance. In serious cases, an exacerbation might be fatal. The direct impacts of the asthma pathway were National Health Service (NHS) costs, greenhouse gas (GHG) emissions and patient travel costs, while the indirect impacts affected health-related quality of life (HRQoL) and productivity at work. All direct impacts were generated in all three stages, but indirect impacts were assumed to be generated for all patients with uncontrolled symptoms. Direct and indirect impacts for which limited evidence were found in the literature (e.g. waste related to inhalers at the end of their use, carer burden) were not included in the asthma impacts framework.
Results
Direct impacts in all patients
GHG emissions associated with modelled asthma activities along the asthma pathway in 2022 amounted to £0.21 billion, equivalent to 0.8 Mt CO2e. Over the period of 2022–2031, the estimated total GHG emissions were 5.07 Mt CO2e, equivalent to a NPV cost of £1.2 billion. This is equal to four times the NHS costs (£319 million) for secondary care over the same period (table 6). These estimates considered a predicted 63% (equal to 0.29 Mt CO2e) reduction in GHG emissions by 2031 (table 7 and supplementary section S2.2), due to the gradual availability of pMDIs containing low-GWP propellants from 2025, which are expected to reduce total GHG emissions from pMDIs by approximately 90% [10, 19, 37].
TABLE 6.
Monetary impact of asthma pathway by stage of pathway, impact area and severity accounting for prevalence of patients by severity
| Total monetary impacts (million £) by pathway stage and impact area | Year 2022 | Total NPV (2022–2031) |
||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Controlled (non-severe) | Uncontrolled (non-severe) | Severe | Overall | Controlled (non-severe) | Uncontrolled (non-severe) | Severe | |
| NHS costs | 1027.1 | 498.1 | 368.3 | 160.6 | 9375.3 | 4568.3 | 3369.4 | 1437.6 |
| Primary care | 257.4 | 145.2 | 102.8 | 9.5 | 2377.3 | 1340.5 | 949.3 | 87.5 |
| Pharmacological treatment | 735.1 | 353.0 | 238.3 | 143.8 | 6678.7 | 3227.8 | 2168.4 | 1282.5 |
| Secondary care | 34.5 | 0.0 | 27.2 | 7.3 | 319.3 | 0.0 | 251.6 | 67.6 |
| Emissions monetary value | 205.4 | 94.5 | 92.4 | 18.5 | 1169.7 | 533.2 | 525.9 | 110.6 |
| Primary care | 9.9 | 4.6 | 3.4 | 1.9 | 89.1 | 41.6 | 30.5 | 17.0 |
| Pharmacological treatment | 193.1 | 89.6 | 87.4 | 16.2 | 1058.9 | 489.2 | 480.2 | 89.5 |
| Secondary care | 1.9 | 0.0 | 1.5 | 0.4 | 17.0 | 0.0 | 13.4 | 3.6 |
| Patient travelling | 0.5 | 0.3 | 0.2 | 0.1 | 4.7 | 2.3 | 1.7 | 0.6 |
| Patient travel costs | 11.9 | 6.1 | 4.5 | 1.4 | 110.2 | 56.0 | 41.1 | 13.0 |
| Primary care | 11.9 | 6.1 | 4.5 | 1.4 | 110.2 | 56.0 | 41.1 | 13.0 |
| Total direct impacts | 1244 . 5 | 598 . 7 | 465 . 2 | 180 . 6 | 10 655.1 | 5157 . 5 | 3936 . 3 | 1561 . 2 |
| Health-related impacts # | 1072.8 | 0.0 | 982.0 | 90.8 | 9923.0 | 0.0 | 9082.9 | 840.0 |
| Productivity impacts | 2883.0 | 0.0 | 2324.2 | 558.7 | 26 665.4 | 0.0 | 21 497.6 | 5167.8 |
| Absenteeism | 119.9 | – | 109.7 | 10.1 | 1213.0 | – | 1110.3 | 102.7 |
| Lower hourly wage | 1321.6 | – | 1059.2 | 262.4 | 13 370.4 | – | 10 715.8 | 2654.5 |
| Unemployment | 1441.5 | – | 1155.3 | 286.2 | 14 583.3 | – | 11 687.9 | 2895.3 |
| Total indirect impacts | 3955 . 8 | 0 . 0 | 3306 . 2 | 649 . 5 | 36 588.4 | 0 . 0 | 30 580.5 | 6007 . 9 |
| Total (direct and indirect) impacts | 5200 . 3 | 598 . 7 | 3771 . 5 | 830 . 1 | 47 243.5 | 5157 . 5 | 34 516.9 | 7569 . 1 |
NHS: National Health Service; NPV: net present value. #: health-related impacts include health-related quality of life and life expectancy.
TABLE 7.
Total GHG emissions generated by the asthma pathway and number of asthma patients in 2022 and over 2022–2031
| Emissions (t CO2e) by pathway stage and impact | Year 2022 | Period 2022–2031 |
|---|---|---|
| Total emissions | 807 212 | 5 069 706 |
| Primary care usage | 39 032 | 394 826 |
| HCP visits for diagnosis | 5550 | 55 504 |
| Annual reviews | 29 994 | 303 980 |
| Unplanned GP visits | 3487 | 35 342 |
| Medications (controller and reliever inhalers) | 758 717 | 4 579 019 |
| Controller inhaler | 458 178 | 2 746 442 |
| Reliever inhaler | 300 539 | 1 832 577 |
| OCS | 0 | 0 |
| Reliever inhalers in secondary care | 0.03 | 0.32 |
| Add-on medications | 0 | 0 |
| Secondary care stage | 7424 | 75 240 |
| Secondary care intervention | 7424 | 75 240 |
| Patient travelling | 2039 | 20 622 |
| Patient travel to diagnosis | 327 | 3271 |
| Patient travel to annual reviews | 1519 | 15 394 |
| Patient travel to unplanned GP visits | 193 | 1957 |
| Number of patients (millions) | ||
| Total | 3.61 | 36.57 |
| Controlled | 2.16 | 21.91 |
| Uncontrolled | 1.31 | 13.31 |
| Severe | 0.13 | 1.35 |
GHG: greenhouse gas; GP: general practitioner; HCP: healthcare professional; OCS: oral corticosteroids; t CO2e: tonnes of CO2 equivalent.
NHS costs associated with primary care, secondary care and pharmacological treatments for adult patients across the asthma pathway were estimated to be £1 billion in 2022 and £9.4 billion when accumulated throughout 2022–2031, with NHS costs as the principal driver of total direct impacts (table 6). More than £319 million in asthma pathway-related NHS costs were attributed to secondary care for managing exacerbations and unplanned visits to primary care to treat uncontrolled asthma.
Patients with non-severe asthma (controlled and uncontrolled)
Non-severe uncontrolled asthma was associated with a 28% increase per patient in the costs of direct impacts compared with controlled asthma in 2022. This increase was primarily attributed to higher NHS costs associated with secondary care, over-reliance on reliever inhalers and additional unplanned general practitioner (GP) appointments, with increases in GHG emissions contributing to a lesser extent.
Patients with non-severe uncontrolled asthma were associated on average with a 22% increase in NHS costs compared to patents with controlled asthma in 2022, accumulating to NHS costs of £3.4 billion over the period 2022–2031. Additionally, patients with non-severe uncontrolled asthma was estimated to generate, on average, 64% higher emissions than a patients with non-severe controlled asthma in 2022, resulting in an annual increase of 0.1 t CO2e in terms of emissions.
Patients with severe asthma (uncontrolled)
In 2022, the monetary value of direct impacts per patient with severe asthma (i.e. uncontrolled disease despite a maximal optimised treatment, adherence to treatment and management of contributory factors) was four times higher than those for a patient with non-severe controlled asthma (table 6). This difference was driven by an increase in NHS costs associated with secondary care and with pharmacological treatment required by patients with severe asthma. The GHG emissions per patient with severe disease were estimated to be 0.54 t CO2e in 2022, associated with a three-fold increase compared with a patient with controlled asthma, and nearly double those for a patient with non-severe uncontrolled asthma (based on table 7 and supplementary section S2.2).
Indirect impacts in all patients with uncontrolled asthma
The indirect impacts of the asthma pathway were estimated for adult patients with uncontrolled asthma. These were estimated to be £37 billion over the period 2022–2031 (table 6), resulting in indirect costs which were 3.5-times higher than the direct costs over the same period.
Costs related to productivity loss were considerably higher than costs associated with health impacts. In total, productivity losses due to uncontrolled asthma were estimated to be £2.9 billion in 2022, while costs related to HRQoL and years lost due to premature death in older asthma patients were estimated to be £1.1 billion. In patients with uncontrolled asthma, the cost of absenteeism related to sick days and absence from work related to attending HCP appointments was estimated at £0.12 billion in 2022. Again, in 2022, it was estimated that lower earnings per hour for asthma patients compared to the general population [31] contributed to total costs of £1.3 billion, while excess unemployment resulted in total costs of £1.4 billion.
Patients with non-severe asthma
Productivity loss per patient with non-severe uncontrolled asthma in 2022 was estimated at £1770, though the HRQoL loss per patient was estimated on average at £748 (equivalent to 0.0374 QALYs). The HRQoL loss per patient who experienced at least one exacerbation in a year was estimated at 0.1675 QALYs, equivalent to £3351.
Patients with severe asthma
The cost of productivity loss per patient with severe asthma was estimated to be £4180, while cost related to HRQoL reduction and premature death due to asthma was £680 (equivalent to 0.034 QALYs) in 2022, assuming that all patients with severe asthma experienced at least one exacerbation per year.
The estimated total indirect costs per asthma patient were higher in patients with severe asthma (£4860) compared to non-severe uncontrolled asthma (£2518). This difference was mainly driven by the higher productivity losses in patients with severe asthma. The costs of HRQoL loss were similar for patients with both non-severe uncontrolled asthma and severe asthma.
Direct impacts versus indirect impacts
Over the period 2022–2031, the estimated NPV of the total direct and indirect costs resulting from patients with non-severe uncontrolled asthma (£34.5 billion) was nearly seven times higher than the total costs from patients with non-severe controlled asthma (£5.2 billion) (table 6). In comparison, patients with severe asthma were estimated to incur total costs of £7.6 billion over the same period. The higher costs associated with non-severe uncontrolled asthma patients were primarily due to reductions in patient HRQoL and productivity losses (table 6).
Discussion
In this study, the total economic burden of asthma due to environmental, healthcare and societal impacts was estimated at £47 billion over the years 2022–2031. The CoI model used for this estimation reflected a simplified real-world view of the entire asthma pathway in the UK, capturing the expected economic value of both direct impacts, i.e. GHG emissions, NHS costs and patients’ travel costs, and indirect impacts of asthma, i.e. HRQoL, life expectancy and productivity losses.
Moreover, uncontrolled asthma was found to be a substantial contributor to the overall economic burden of the asthma pathway. Specifically, reductions in HRQoL and productivity losses due to uncontrolled asthma accounted for as much as 77% of the overall economic burden estimated (table 6). Nevertheless, NHS costs still represent a considerable part of all impacts (∼20%), with pharmacological treatment accounting for 16.4% of the total costs of the asthma pathway over the years 2022–2031 (based on table 6).
Within the context of asthma management, policies that advocate inhaler switching to reduce GHG emissions, such as historical policies in the UK [9, 40], may lead to loss of symptom control in patients whose disease is controlled with current treatment. A recently published literature review reported that device switch may lead to increased healthcare expenditure due to increased healthcare visits and pharmacological treatment [41]. Another literature review concluded that although evidence was limited and heterogeneous, non-consented switching may result in reduced adherence, incorrect inhaler use and increased healthcare costs, while it significantly worsened disease control [42]. While we have stressed the implications of non-clinical switching and its role in uncontrolled asthma, it is important to stress that regular treatment and adherence to inhaled corticosteroids is a key factor to maintain inflammation suppression and disease control, which correlates to a reduced environmental, economic and societal impact [43].
The present study demonstrated that patients with uncontrolled asthma have a larger impact on GHG emissions; thus, loss of disease control due to inhaler switching may eventually contribute to greater economic consequences. To explore the consequences of switching from pMDIs to DPIs for maintenance, a hypothetical cohort of 1000 asthma patients with well-controlled symptoms under current treatment was considered, assuming that switchover led to uncontrolled symptoms in 10% of these patients. This scenario resulted in a 10-fold increase in the total economic burden in 2022 (from £27 697 to £287 154) driven by reductions in HRQoL and productivity, supporting the recent withdrawal of incentives to implement such switchover policies [44].
Our findings are in line with the current policies aimed at optimising inhaler prescription to reduce GHG emissions [9], if disease control is not compromised. The analysis also accounts for pharmaceutical innovation, reflecting the future introduction of low-GWP propellants in inhalers associated with an up to 90% lower carbon footprint [10, 19].
Overall, this model, although UK-based and adopting UK treatment specifications, supports the need for a clinical focus and consideration of the broader implications of inhaler prescribing, especially in regard to environmentally centred policies that may indirectly lead to loss of disease control. Subsequently, loss of disease control could have substantial unintended consequences that extend beyond the healthcare system, to the economy and society. The results presented suggest that policies should be considered holistically and ponder the effects or implications on both the patient and the economy.
The key strength of this study is the holistic approach in analysing the entirety of the asthma pathway. This approach allowed the individual contribution of different elements to the overall economic burden of asthma to be studied from different perspectives and provided insights into the most significant drivers. Additionally, the model reflected current clinical practice (in the UK) because it was informed by recent literature, proprietary data and a panel of clinical experts in respiratory medicine. Although the analysis followed a UK societal perspective, which may limit transferability of the results and conclusions to countries where the asthma pathway varies significantly from the UK, the comprehensive methodological framework is transferable to geographies where asthma poses a serious humanistic and economic concern [2].
Nonetheless, the study has limitations. First, the analysis was restricted to adult asthma patients, precluding an understanding of the economic burden of asthma in the paediatric population. Second, the study relied on modelling and simplification of the asthma pathway and may have missed certain asthma activities that contribute to the overall economic burden of the disease. Additionally, quantitative evidence for certain components of the asthma impact, such as waste (inhalers, clinical waste, packaging), informal caregiver costs and preventive costs were not included in the analysis because limited or no evidence was identified in the literature. The GHG emissions from non-inhaler medication were also not considered. Finally, there was uncertainty around some of the model inputs due to the absence or limited availability of evidence, increasing the uncertainty describing the model outputs. Hence, assumptions and triangulation of evidence between multiple data sources were applied, with key assumptions following a conservative approach and being explored in scenario analyses. Although the number of scenario analyses conducted was limited, they demonstrated no implications in the overall conclusions (supplementary section S4). Nonetheless, inputs and key assumptions that were considered drivers of the results were validated by clinical experts.
To our knowledge, this is the first comprehensive analysis of the environmental, healthcare and societal costs along the entire asthma pathway. Our findings lay the groundwork for holistically exploring potential strategies to counteract the asthma carbon footprint, especially given the urgency to mitigate global warming. Moreover, this study could be considered upon future policy design, highlighting the need to place the patient at the centre of decision-making without compromising favourable health outcomes, which may create increased economic burden. Finally, the results shed light on areas where action can be taken to improve healthcare management, enhance patient wellbeing and overcome economic challenges associated with asthma.
Acknowledgements
We would like to acknowledge F. Sandri and S. Panigone (Chiesi Farmaceutici SpA), H. Lewis and R. Stork (Chiesi Ltd) and M. Ferrara (Frontier Economics) for their technical advice, widespread support and contribution to the study; and M. Frias (IQVIA), who contributed to the design and review of the manuscript.
Provenance: Submitted article, peer reviewed.
Author contributions: M. Orlovic contributed to the design of the study and reviewed the article. D. Tzelis drafted the article and contributed to the design of the study. I. Guerra contributed to the design and review of the article. V. Bar-Katz contributed to the design of the study, collected the data, performed the analysis and reviewed the paper. N. Woolley contributed to the design of the study, performed the analysis and reviewed the paper. H. Bray and M. Hanslot contributed to the design of the study, and the design and review of the article. O. Usmani contributed to the design and review of the article. A. Madoni contributed to conceptualisation and design of the study and reviewed the article.
Conflict of interest: D. Tzelis and I. Guerra were employees of IQVIA at the time of the development of this study and IQVIA received consulting fees from Chiesi Farmaceutici SpA to support manuscript development. V. Bar-Katz and N. Woolley were employees of Frontier Economics at the time of the development of this manuscript development, and Frontier Economics received consulting fees from Chiesi Farmaceutici SpA to support the development and execution of this study. The remaining authors have nothing to disclose.
Support statement: This study was supported by Chiesi Farmaceutici SpA, Via Palermo 26 A, 43122, Parma, Italy. Funding information for this article has been deposited with the Crossref Funder Registry.
Data availability
Non-proprietary evidence used in the current analysis is included in the supplementary material.
References
- 1.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2022. Available from: https://ginasthma.org/
- 2.Wang Z, Li Y, Gao Y, et al. Global, regional, and national burden of asthma and its attributable risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Respir Res 2023; 24: 169–181. doi: 10.1186/s12931-023-02475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.British Lung Foundation . Asthma Statistics. 2018. https://statistics.blf.org.uk/asthma Date last accessed: 8 July 2022.
- 4.Lloyd A, Price D, Brown R. The impact of asthma exacerbations on health-related quality of life in moderate to severe asthma patients in the UK. Prim Care Respir J 2007; 16: 22–27. doi: 10.3132/pcrj.2007.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol 2008; 122: 662–668. doi: 10.1016/j.jaci.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 6.Janson C, Henderson R, Lofdahl M, et al. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax 2020; 75: 82–84. doi: 10.1136/thoraxjnl-2019-213744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson A, Woodcock A. The environmental impact of inhalers for asthma: a green challenge and a golden opportunity. Br J Clin Pharmacol 2022; 88: 3016–3022. doi: 10.1111/bcp.15135 [DOI] [PubMed] [Google Scholar]
- 8.Ferguson GT, Hickey AJ, Dwivedi S. Co-suspension delivery technology in pressurized metered-dose inhalers for multi-drug dosing in the treatment of respiratory diseases. Respir Med 2018; 134: 16–23. doi: 10.1016/j.rmed.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 9.NHS England . Delivering a ‘Net Zero’ National Health Service. 2022. Available from: www.england.nhs.uk/greenernhs/wp-content/uploads/sites/51/2022/07/B1728-delivering-a-net-zero-nhs-july-2022.pdf
- 10.Panigone S, Sandri F, Ferri R, et al. Environmental impact of inhalers for respiratory diseases: decreasing the carbon footprint while preserving patient-tailored treatment. BMJ Open Respir Res 2020; 7: e000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management: treatment of asthma with short-acting bronchodilators alone is no longer recommended for adults and adolescents. Eur Respir J 2019; 53: 1901046. doi: 10.1183/13993003.01046-2019 [DOI] [PubMed] [Google Scholar]
- 12.Pernigotti D, Stonham C, Panigone S, et al. Reducing carbon footprint of inhalers: analysis of climate and clinical implications of different scenarios in five European countries. BMJ Open Respir Res 2021; 8: e001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakma MS, Usmani OS. Inhalers and the environment: pollution, plastics and policy. Pneumon 2022; 35: 26. doi: 10.18332/pne/154608 [DOI] [Google Scholar]
- 14.NHS England . Network Contract Directed Enhanced Service - Investment and Impact Fund 2022/23: Updated Guidance. 2022. Available from: www.england.nhs.uk/wp-content/uploads/2022/03/B1963-iii-Network-contract-IIF-Implementation-Guidance-September-2022.pdf
- 15.AstraZeneca . AstraZeneca's ‘Ambition Zero Carbon’ Strategy to Eliminate Emissions by 2025 and Be Carbon Negative Across the Entire Value Chain by 2030. 2020. www.astrazeneca.com/media-centre/press-releases/2020/astrazenecas-ambition-zero-carbon-strategy-to-eliminate-emissions-by-2025-and-be-carbon-negative-across-the-entire-value-chain-by-2030-22012020.html Date last accessed: 23 September 2022.
- 16.Chiesi Group . Chiesi Outlines €350 Million Investment and Announces First Carbon Minimal Pressurised Metered-Dose Inhaler (pMDI) for Asthma and COPD. 2019. www.chiesi.com/en/chiesi-outlines-350-million-investment-and-announces-first-carbon-minimal-pressurised-metered-dose-inhaler-pmdi-for-asthma-and-copd/ Date last accessed: 23 September 2022.
- 17.GSK . GSK Announces Major Renewable Energy Investment and Low Carbon Inhaler Programme Alongside Life Sciences sector Race to Zero ‘breakthrough’ at NYC Climate Week. 2021. www.gsk.com/en-gb/media/press-releases/gsk-announces-major-renewable-energy-investment-and-low-carbon-inhaler-programme-alongside-life-sciences-sector-race-to-zero-breakthrough-at-nyc-climate-week/ Date last accessed: 23 September 2022.
- 18.Corr S. HFA-152a as a Sustainable pMDI Propellant. Respir Drug Deliv 2020. Available from: https://www.zephex.com/wp-content/uploads/2020/07/Corr-2020-1.pdf [Google Scholar]
- 19.Jeswani HK, Azapagic A. Life cycle environmental impacts of inhalers. J Clean Prod 2019; 237: 117733. doi: 10.1016/j.jclepro.2019.117733 [DOI] [Google Scholar]
- 20.Recipharm . Overcoming Challenges of Environmentally Friendly Propellants. 2023. www.recipharm.com/resources/overcoming-challenges-environmentally-friendly-propellants Date last accessed: 18 November 2023.
- 21.Kponee-Shovein K, Marvel J, Ishikawa R, et al. Carbon footprint and associated costs of asthma exacerbation care among UK adults. J Med Econ 2022; 25: 524–531. doi: 10.1080/13696998.2022.2063603 [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson AJK, Braggins R, Steinbach I, et al. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open 2019; 9: e028763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol 2014; 20: 327–337. doi: 10.3350/cmh.2014.20.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice DP. Cost of illness studies: what is good about them? Inj Prev 2000; 6: 177–179. doi: 10.1136/ip.6.3.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byford S, Torgerson DJ, Raftery J. Economic note: cost of illness studies. BMJ 2000; 320: 1335. doi: 10.1136/bmj.320.7245.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asthma and Lung UK . What is Severe Asthma? 2020. www.asthma.org.uk/advice/severe-asthma/what-is-severe-asthma/#:∼:text=Severe%20asthma%20is%20the%20most,with%20high%20doses%20of%20medicines Date last updated: 30 November 2022.
- 27.Hekking PW, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 28.Ismaila A, Corriveau D, Vaillancourt J, et al. Impact of adherence to treatment with fluticasone propionate/salmeterol in asthma patients. Curr Med Res Opin 2014; 30: 1417–1425. doi: 10.1185/03007995.2014.908827 [DOI] [PubMed] [Google Scholar]
- 29.Office for National Statistics (ONS) . Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland. 2021. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland Date last accessed: 23 September 2022.
- 30.Gruffydd-Jones K, Thomas M, Roman-Rodriguez M, et al. Asthma impacts on workplace productivity in employed patients who are symptomatic despite background therapy: a multinational survey. J Asthma Allergy 2019; 12: 183–194. doi: 10.2147/JAA.S204278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glover B, Ussher K. Potential Limited: The Economic Cost of Uncontrolled Asthma. London, Demos. 2021. https://demos.co.uk/research/potential-limited-the-economic-cost-of-uncontrolled-asthma/ Date last accessed: 2022 [Google Scholar]
- 32.Asthma and Lung UK . How Many People Have Asthma in the UK? 2018. https://public.tableau.com/app/profile/asthmaandlunguk/viz/Asthmaprevalence/Asthmaprevalence Date last updated: 19 November 2022.
- 33.NHS . Mortality From Asthma: Number, by Age Group, Annual, MFP. 2022. https://digital.nhs.uk/data-and-information/publications/statistical/compendium-mortality/current/mortality-from-respiratory-diseases/mortality-from-asthma-number-by-age-group-annual-mfp Date last accessed: 4 October 2022.
- 34.Faculty of Public Health - Health Knowledge . Years of Life Lost. 2018. www.healthknowledge.org.uk/public-health-textbook/research-methods/1a-epidemiology/years-lost-life Date last accessed: 23 September 2022.
- 35.HM Treasury . The Green Book - Central Government Guidance on Appraisal and Evaluation. London, Crown Copyright, 2022. [Google Scholar]
- 36.Department for Energy Security and Net Zero . Valuation of Energy Use and Greenhouse Gas (GHG) Emissions, Supplementary Guidance to the HM Treasury Green Book on Appraisal and Evaluation in Central Government. London, Crown Copyright, 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1024054/1.Valuation_of_energy_use_and_greenhouse_gas_emissions_for_appraisal_CLEAN.pdf [Google Scholar]
- 37.Jeswani HK, Azapagic A. Environmental impacts of healthcare and pharmaceutical products: influence of product design and consumer behaviour. J Clean Prod 2020; 253: 119860. doi: 10.1016/j.jclepro.2019.119860 [DOI] [Google Scholar]
- 38.Prieto L, Sacristan JA. Problems and solutions in calculating quality-adjusted life years (QALYs). Health Qual Life Outcomes 2003; 1: 80. doi: 10.1186/1477-7525-1-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institute for Health and Care Excellence (NICE UK) . Developing NICE Guidelines: The Manual. Process and Methods. 2022. www.nice.org.uk/process/pmg20/chapter/introduction Date last accessed: August 2023; date last updated: 17 January 2024. [PubMed]
- 40.Bar-Katz V, Woolley NJ, Vitello E. POSA161 economic analysis of NHS England's policy to reduce the carbon impact of inhalers by encouraging a rapid prescribing switch from pressurised metered-dose inhalers (PMDIs) to dry powder inhalers (DPIs). Value Health 2022; 25: S65. doi: 10.1016/j.jval.2021.11.302 [DOI] [Google Scholar]
- 41.Attar-Zadeh D, Lewis H, Orlovic M. Health-care resource requirements and potential financial consequences of an environmentally driven switch in respiratory inhaler use in England. J Health Econ Outcomes Res 2021; 8: 46–54. doi: 10.36469/jheor.2021.26113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usmani OS, Bosnic-Anticevich S, Dekhuijzen R, et al. Real-world impact of nonclinical inhaler regimen switches on asthma or COPD: a systematic review. J Allergy Clin Immunol Pract 2022; 10: 2624–2637. doi: 10.1016/j.jaip.2022.05.039 [DOI] [PubMed] [Google Scholar]
- 43.Renzi-Lomholt M, Hakansson KEJ, Suppli Ulrik C. Adherence to inhaled corticosteroids in relation to quality of life and symptoms of anxiety and depression in asthma. Eur Clin Respir J 2023; 10: 2149920. doi: 10.1080/20018525.2022.2149920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert I, Wada K, Burudpakdee C, et al. The impact of a forced non-medical switch of inhaled respiratory medication among patients with asthma or chronic obstructive pulmonary disease: a patient survey on experience with switch, therapy satisfaction, and disease control. Patient Prefer Adherence 2020; 14: 1463–1475. doi: 10.2147/PPA.S242215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Non-proprietary evidence used in the current analysis is included in the supplementary material.