Abstract
Objective
To identify the association of magnetic resonance imaging (MRI) features with molecular subtypes of breast cancer (BC).
Materials and methods
A retrospective study was conducted on 112 invasive BC patients with preoperative breast MRI. The confirmed diagnosis and molecular subtypes of BC were based on the postoperative specimens. MRI features were collected by experienced radiologists. The association of MRI features of each subtype was compared to other molecular subtypes in univariate and multivariate logistic regression analyses.
Results
The proportions of luminal A, luminal B HER2-negative, luminal B HER2-positive, HER2-enriched, and triple-negative BC were 14.3 %, 52.7 %, 12.5 %, 10.7 %, and 9.8 %, respectively. Luminal A was associated with hypo-isointensityon T2-weighted images (OR=6.214, 95 % CI: 1.163–33.215) and non-restricted diffusion on DWI-ADC (OR=6.694, 95 % CI: 1.172–38.235). Luminal B HER2-negative was related to the presence of mass (OR=7.245, 95 % CI: 1.760–29.889) and slow/medium initial enhancement pattern (OR=3.654, 95 % CI: 1.588–8.407). There were no associations between MRI features and luminal B HER2-positive. HER2-enriched tended to present as non-mass enhancement lesions (OR=20.498, 95 % CI: 3.145–133.584) with fast uptake in the initial postcontrast phase (OR=9.788, 95 % CI: 1.689–56.740), and distortion (OR=11.471, 95 % CI: 2.250–58.493). Triple-negative were associated with unifocal (OR=7.877, 95 % CI: 1.180–52.589), hyperintensityon T2-weighted images (OR=14.496, 95 % CI: 1.303–161.328), rim-enhanced lesions (OR=18.706, 95 % CI: 1.915–182.764), and surrounding tissue edema (OR=5.768, 95 % CI: 1.040–31.987).
Conclusion
Each molecular subtype of BC has distinct features on breast MRI. These characteristics can serve as an adjunct to immunohistochemistry in diagnosing molecular subtypes, particularly in cases, where traditional methods yield equivocal results.
Keywords: MRI features, Molecular subtypes, Breast cancer
1. Introduction
Breast cancer is the most prevalent malignant tumor among women worldwide. Although the highest incidence of this malignancy occurs in developed countries, the highest mortality rates are found in developing countries [1]. The heterogeneity of breast cancer is characterized by variations in the genomic, epigenomic, transcriptomic, and proteomic profiles of cancer cells [2]. Gene expression profiling is considered the optimal method for categorizing breast cancer. However, due to the high cost of commercial multigene assays, the St. Gallen International Expert Consensus panel recommends using semiquantitative immunohistochemistry (IHC) to classify breast cancer into five molecular subtypes: luminal A, luminal B HER2-negative, luminal B HER2-positive, HER2-enriched, and triple Negative. These molecular subtypes exhibit significant differences in clinical characteristics, treatment responses, and outcomes, making them critical for individualized patient management [3], [4].
Neoadjuvant systemic therapy, also known as preoperative chemotherapy, plays an increasingly crucial role in breast cancer management. Neoadjuvant systemic therapy may be offered to reduce the extent of breast tumors, not only for inoperable, locally advanced, but also early breast cancer, leading to an increase in the likelihood of breast-conserving surgery [5], [6]. According to the latest European Society for Medical Oncology (ESMO) guideline for managing early breast cancer, the regimens used in neoadjuvant systemic therapy were chosen mainly based on the molecular subtypes from biopsy [5]. However, several studies have shown significant discrepancies in classifying molecular subtypes between core needle biopsies (CNB) and surgical resection specimens, particularly for Ki67 and HER2 markers. [7], [8], [9]. These discrepancies could be blamed on changes in tumor characteristics, intratumoral heterogeneity, and sampling and analytical errors [7]. Therefore, an in-depth preoperative understanding of the breast cancer molecular subtype, informed by not only immunohistochemical results but also imaging features, is necessary to enhance the efficacy of neoadjuvant systemic therapy [10].
Regarding the diagnosis and treatment of breast cancer, magnetic resonance imaging (MRI) has demonstrated numerous advantages over other imaging methods [11]. In the past decade, numerous studies have utilized MRI features to differentiate breast cancer subtypes. These studies have found correlations between certain MRI features and specific molecular subtypes of breast cancer. However, most research has divided molecular types into subgroups, with few studies including all five subtypes [12], [13], [14], [15], [16]. Additionally, these studies often used molecular data derived from biopsy or surgical excision specimens despite the significant discordance between these results [12], [17], [18]. This study aims to evaluate the association between the five molecular subtypes of breast cancer based on surgical excision specimens and their corresponding magnetic resonance imaging features.
2. Materials and methods
2.1. Participants
A retrospective study was conducted at the Vietnam National Cancer Hospital from March 2023 to October 2023. The study was approved by the National Cancer Hospital Clinical Research Ethics Committee (approval number 666-BVK).
We enrolled all women diagnosed with breast cancer who underwent preoperative breast MRI with five sequences (T1W nonfat-sat, T2W, STIR, diffusion-weighted imaging, and dynamic sequences) and subsequently underwent surgical management between January 2021 and January 2023. The diagnosis of breast cancer and its molecular subtypes was confirmed through postoperative pathology. Exclusion criteria included recurrent breast cancer, inadequate or incomplete MRI, and in-situ breast carcinoma, which were insufficient for molecular classification (see Flowchart 1). Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6
Fig. 1.
Flowchart.
Fig. 2.
Luminal A subtype breast cancer.
Fig. 3.
Luminal B HER2-negative breast cancer.
Fig. 4.
HER2-enriched-like breast cancer.
Fig. 5.
Triple-negative breast cancer.
Fig. 6.
Luminal B HER2-positive breast cancer characterized by both mass-like and non-mass-like lesions on MRI.
2.2. MRI protocol, pathology and immunohistochemistry data
Patients provided informed consent before undergoing MRI, according to local hospital protocol. All patients underwent 1.5 T MRI examinations in the prone position using a breast coil on GE Signa Explorer 1.5 Tesla MR unit. The breast MRI protocol included: (1) T1-weighted imaging turbo spin echo; (2) T2-weighted imaging turbo spin echo (TR = 6956 ms; TE = 110 ms, flip angle 90°; in-plane resolution 0.7 mm × 0.9 mm; 33 slices; slice thickness 4 mm); (3) T2 short tau inversion recovery (STIR) fast spin echo (TR = 4452 ms; TE = 50 ms; 33 slices; slice thickness 4 mm); (4) T1WI VIBRANT DISCO ARC dynamic sequence (TR = 8.7 ms; TE = 2.3 ms; slice thickness 1.0 mm), with precontrast and six phases after contrast administration (0.2 mL/kg, 3 mL/s); (5) Diffusion-weighted imaging (DWI) echo planar imaging with three b factors (0, 50, and 800 s/mm²)(TR = 7544 ms; TE = 90 ms; NEX 6; Matrix 192×192; FOV 280–320, 33 slices; slice thickness 5 mm). Image data were stored using the hospital's PACS system.
Regarding pathology and immunohistochemistry, the morphological and molecular subtypes of the tumors were evaluated on surgical specimens. In hospital protocol, morphological subtypes were classified according to the 2019 World Health Organization classification of breast tumors [19]. ER, PR, HER2, and Ki-67 % proliferation index status were evaluated according to the 2019 CAP/ASCO guidelines [20], [21]. Molecular subtypes were assigned according to the 2013 St. Gallen International Expert Consensus classification system based on ER, PR, HER2 status, and Ki-67 % proliferation index [22] (see Table 1).
Table 1.
Molecular subtypes of breast cancer based on the 2013 St. Gallen International Expert Consensus definition [22].
| Luminal A | Luminal B HER2(-) | Luminal B HER2(+) | HER2 enriched | Triple Negative | |
|---|---|---|---|---|---|
| ER and PR | ER+ and/or PR+ | ER+ and/or PR+ | ER+ and/or PR+ | ER- and PR- | ER- and PR- |
| HER2 | HER2- | HER2- | HER2+ | HER2+ | HER2- |
| Ki-67 % | Ki-67≤20 % | Ki-67>20 % | Any Ki-67 | Any Ki-67 | Any Ki-67 |
ER = estrogen receptor, PR = progesterone receptor, HER2 = human epidermal growth factor 2
2.3. Evaluation of images
MRI features were collected by radiologists with more than 10 years of experience in breast imaging, who were blinded to the pathological and molecular subtype classifications. If lesions were in the bilateral breast, each lesion was assessed separately with respective pathology and immunohistochemistry results. In cases with more than one lesion in the same breast, the largest was chosen to evaluate.
Breast tumors were classified as mass and non-mass enhancement. The shape (round, oval, and irregular) and margin (circumscribed, irregular, spiculated) of mass-like lessions and the distribution (focal, linear, segmental, regional, and diffuse) of non-mass-like lesions were evaluated. Other features of all lesions were assessed, including edema, multi-focal/centric, adenopathy, and distortion. The intensity of the lesions on T2W (hypointensity, isointensity or hyperintensity), DWI-ADC (restricted or non-restricted), dynamic initial phase (slow (<50 %), medium (50–100 %), fast (>100 %)), late postcontrast phase (persistent (increase more than 10 %), plateau (increase or decrease less than 10 %), wash-out (decrease more than 10 %)) were analyzed.
2.4. Statistical analysis
Statistical analyses were performed using SPSS software (version 26.0, IBM). To analyze associations between breast cancer molecular subtype and pathological data – imaging features on MRI, the Chi-square or Fisher’s exact test was used. Univariate and multivariate binary logistic regression analyses were performed to estimate the relationship between each breast cancer molecular subtype and different MRI features.
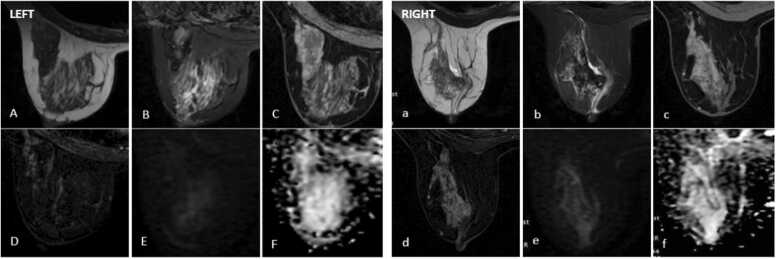
The left presents as an irregular mass lesion, hypointensity on T2W (A) and slightly hyperintensity on STIR (B), with homogeneous enhancement on dynamic sequences (C-D) and non-restricted on DWI-ADC (E-F).
The right presents as an irregular mass lesion, hyperintensity on T2W (a) and STIR (b), hypointensity on T1W non fat-sat(c) with rim enhancement on dynamic sequences (d), and non-restricted on DWI-ADC (e-f).
The left presents as a spiculated mass lesion, heterogeneous and hypointensity on T2W (A) and STIR images (B). The lesion shows heterogeneous enhancement in the initial postcontrast phase of the dynamic sequence (C) and subtraction image (D) and is restricted on DWI-ADC (E-F). Notably, a satellite nodule is also presented, pathologically confirmed to be invasive carcinoma.
The right shows another Luminal B HER2-negative breast cancer, which presents as a spiculated mass lesion, hypointensity on T2W (a) and hyperintensity on STIR image (b) with heterogeneous enhancement in the initial phase of the dynamic sequence (c) and subtraction image (d), and restricted on DWI-ADC (e-f).
The left presents as a non-mass enhancement with segmental distribution. It appears hypointense signal on T2W (A) and STIR images (B), with heterogeneous enhancement observed in the dynamic sequence (C) and subtraction image (D), and shows non-restricted diffusion on DWI-ADC (E-F).
The right presents as a non-mass enhancement with segmental distribution, with heterogeneous hypointense signal on T2W (a), and STIR images (b). The lesion shows heterogeneous enhancement in the initial phase of the dynamic sequence (c) and subtraction image (d) and is non-restricted on DWI-ADC (e-f).
The left presents as a mass lesion, heterogeneous hyperintensity on T2W and STIR sequences, rim enhancement, restricted diffusion with central necrosis and edema of surrounding parenchymal.
The right presents as a heterogeneous hyperintensity mass on T2W(a) and STIR(b), with rim enhancement in the initial phase of dynamic sequence(c) and subtraction image(d), restricted on DWI-ADC(e-f) with edema of surrounding parenchymal.
The left reveals a circumscribed, oval mass in the upper-outer quadrant of the left breast, which appears hyperintense signal on STIR and DWI(A-C) and exhibits homogeneous enhancement in the initial phase of the dynamic sequence (C). Additionally, a non-mass enhancement lesion is observed in the same breast region, hyperintensity on STIR and DWI sequences (D-F), and heterogeneous enhancement (E).
The right reveals an irregular mass in the upper-outer quadrant of the right breast, which appears hyperintense signal on STIR and DWI(a-c) with homogeneous enhancement in the initial phase of the dynamic sequence (b). Additionally, a non-mass enhancement lesion is observed in the central region of the breast, hyperintensity on STIR and DWI sequences (d-f), and heterogeneous enhancement (e).
3. Results
During the research period, 141 breast cancer patients underwent preoperative breast MRI and surgery in our institute. After excluding twenty-nine patients (two with recurrent breast cancer, twenty-five with carcinoma in situ, and two with inadequate MRI examinations), we included 112 patients with 112 lesions in the final analysis.
The mean age of the study population was 45.9 years, ranging from 26 to 88 years. Regarding morphological pathology, invasive carcinoma of no special type (NST) was the most common, accounting for 74.1 % of all cases. The majority of participants had estrogen receptor (ER) positive expression with H-scores greater than 10 (78.6 %) and high progesterone receptor (PR) expression with active percentages greater than 20 % (63.4 %). Approximately half of the participants were HER2 negative. Based on the molecular classification of the 2013 St. Gallen International Expert Consensus, the most prevalent subtype was luminal B HER2-negative (52.7 %), followed by luminal A (14.3 %), luminal B HER2-positive (12.5 %), HER2-enriched (10.7 %), and triple-negative (9.8 %) breast cancer (Table 2).
Table 2.
Baseline characteristics of participants.
| Clinical Characteristics | N | % |
|---|---|---|
| Age(mean±SD) | 45.9±9.41(26−88) | |
| <40 | 31 | 27.7 |
| ≥40 | 81 | 72.3 |
| Menstruation | ||
| Pre-menopause | 61 | 54.5 |
| Post-menopause | 51 | 45.5 |
| Histological pathology | ||
| No special type | 83 | 74.1 |
| Lobular | 11 | 9.8 |
| Mucious | 4 | 3.6 |
| Tubular | 4 | 3.6 |
| Others | 10 | 8.9 |
| Molecular Characteristic | ||
| ER expression(H-Score) | ||
| 0–10 | 24 | 21.4 |
| 11–199 | 50 | 44.7 |
| ≥200 | 38 | 33.9 |
| PR expression | ||
| <1 % | 26 | 23.2 |
| 1–19 % | 15 | 13.4 |
| ≥20 % | 71 | 63.4 |
| HER2 (Immunohistochemistry) | ||
| 0/1+ | 57 | 50.9 |
| 2+ | 29 | 25.9 |
| 3+ | 26 | 23.2 |
| Molecular subtypes | ||
| Luminal A | 16 | 14.3 |
| Luminal B, HER2- | 59 | 52.7 |
| Luminal B, HER2+ | 14 | 12.5 |
| HER2 | 12 | 10.7 |
| Triple negative | 11 | 9.8 |
Regarding imaging features, the shape or margin of mass lesions, the distribution of non-mass enhancement, and adenopathy features were not significantly related to the molecular subtypes of breast cancer. Characteristics in the late postcontrast phase were similar across all five molecular types. Additionally, no significant associations were found between MRI features and luminal B HER2-positive breast cancer (p > 0.05). Table 3
Table 3.
Imaging features on MR and molecular subtypes of breast cancer.
| Imaging features | Total n=112 | Luminal A (n=16) | Luminal B HER2- (n=59) | Luminal B HER2+ (n=14) | HER2 (n=12) | Triple Negative (n=11) |
|---|---|---|---|---|---|---|
| Type | ||||||
| Mass | 98(87.5) | 10(62.5) | 56(94.9) | 13 (92.9) | 8 (66.7) | 11(100) |
| Non-mass enhancement | 14(12.5) | 6(37.5) | 3(5.1) | 1 (7.1) | 4 (33.3) | 0 |
| p | 0.005a | 0.02b | 1.000b | 0.043b | 0.353b | |
| Shape of mass(n=98) | ||||||
| Round/Oval | 28(28.6) | 7(70) | 15(26.8) | 2(15.4) | 3(37.5) | 5(45.5) |
| Irregular | 70(71.4) | 3(30) | 41(73.2) | 11(84.6) | 5(62.5) | 6(54.5) |
| p | 1.000b | 0.822a | 0.338b | 0.685b | 0.268a | |
| Margin of mass (n=98) | ||||||
| Circumcribed | 4(4.1) | 0 | 2(3.6) | 0 | 0 | 2(18.2) |
| Irregular | 57(58.2) | 7(70) | 31(55.4) | 7(53.8) | 6(75) | 6(54.5) |
| Spiculated | 37(37.7) | 3(30) | 23(41.1) | 6(46.2) | 2(25) | 3(27.3) |
| p | 0.829b | 0.672b | 0.866b | 0.627b | 0.116b | |
| Distribution of non-mass enhancement(n=14) | ||||||
| Focal/linear | 3(21.4) | 3(5 0) | 0 | 0 | 0 | 0 |
| Segmetal/regional/ diffuse | 11(78.6) | 3(50) | 3(100) | 1(100) | 4(100) | 0 |
| p | 0.055b | 1.000b | 1.000b | 0.505b | ||
| Other features (n=112) | ||||||
| Edema | 24(21.4) | 2(12.5) | 10(16.9) | 3(21.4) | 2(16.7) | 7(63.6) |
| p | 0.515b | 0.255a | 1.000b | 1.000b | 0.002b | |
| Unifocal | 49 (43.8) | 8(50) | 25(42.4) | 4(28.6) | 3(25) | 9(81.8) |
| p | 0.786a | 0.849a | 0.261b | 0.224b | 0.010b | |
| Adenopathy | 62(55.4) | 10(62.5) | 29(49.2) | 9(64.3) | 8(66.7) | 6(64.5) |
| p | 0.596a | 0.186a | 0.572a | 0.543b | 1.000a | |
| Distortion | 19(17) | 1(6.25) | 11(18.6) | 1(7.1) | 5(41.7) | 1(9.1) |
| p | 0.3b | 0.802a | 0.458b | 0.03a | 0.687b | |
| T2W | ||||||
| Hypo/Iso-intensity | 60(53.6) | 14(87.5) | 32(54.2) | 8(57.1) | 5(41.7) | 1(9.1) |
| Hyperintensity | 52(46.4) | 2(12.5) | 27(45.8) | 6(42.9) | 7(58.3) | 10(90.9) |
| p | 0.005b | 1.000a | 1.000a | 0.542a | 0.003a | |
| DWI | ||||||
| Restricted | 104(92.9) | 12(75) | 56(94.9) | 13(92.9) | 12(100) | 11(100) |
| Non-restricted | 8(7.1) | 4(25) | 3(5.1) | 1(7.1) | 0 | 0 |
| p | 0.014b | 0.473b | 1.000b | 0.596b | 1.000b | |
| Initial phase | ||||||
| Fast | 59(52.7) | 8(50) | 24(40.7) | 9(64.3) | 10(83.3) | 8(72.7) |
| Slow/Medium | 53(47.3) | 8(50) | 35(59.3) | 5(35.7) | 2(16.7) | 3(27.3) |
| p | 1.000a | 0.008a | 0.403a | 0.032b | 0.211b | |
| Late phase | ||||||
| Type 2/3 | 103(92) | 13(81.3) | 55(93.2) | 13(92.9) | 12(100) | 10(90.9) |
| Type 1 | 9(8) | 3(18.7) | 4(6.8) | 1(7.1) | 0 | 1(9.1) |
| p | 0.118b | 0.733b | 1.000b | 0.594b | 1.000b | |
| Enhancement morphology | ||||||
| Rim enhances | 40(35.7) | 1(6.3) | 18(30.5) | 5(35.7) | 6(50) | 10(90.9) |
| Non-rim enhances | 72(64.3) | 15(93.7) | 41(69.5) | 9(64.3) | 6(50) | 1(9.1) |
| p | 0.009b | 0.242a | 1.000a | 0.343a | 0.000b | |
Values are given as no. (%); mean±standard deviation.
Chi’s square test.
Fisher’s Exact Test.
Although there were four MRI features related to luminal A in univariate regression analysis, only hypo-isointensity on T2W and non-restricted diffusion on DWI-ADC were significantly associated with this subtype about sixfold in multivariate regression analysis.
Regarding luminal B HER2-negative breast cancer, the presence of mass lesions and a slow-to-medium initial enhancement pattern increased the risk of this subtype sevenfold and threefold, respectively, compared to other molecular subtypes (OR = 7.245 and 2.863, p < 0.05).
The non-mass enhancement was more likely to be present about twentyfold in HER2-enriched breast cancers than in other subtypes. Additionally, fast uptake in the initial postcontrast phase and the presence of distortions increased the likelihood of HER2-enriched breast cancer approximately tenfold (OR = 9.788, 95 % CI: 1.689–56.740, p < 0.05) and elevenfold (OR = 11.471, 95 % CI: 2.250–58.493, p < 0.05), respectively.
For triple-negative breast cancer, MRI features such as edema of the surrounding tissue and rim enhancement significantly increased the risk, with odds ratios of 5.768 (95 % CI: 1.040–31.987, p < 0.05) and 18.706 (95 % CI: 1.915–182.764, p < 0.05), respectively. Besides, unifocal breast cancer and hyperintensity on T2W images were associated with increased risk, with odds ratios of 7.877 (95 % CI: 1.180–52.589, p < 0.05) and 14.496 (95 % CI: 1.303–161.328, p < 0.05), respectively (Table 4).
Table 4.
The association between imaging features and molecular subtypes of breast cancer in univariate and multivariate logistic regression analysis.
| Imaging features | Univariate Logistic Regression |
Multivariate Logistic Regression |
||
|---|---|---|---|---|
| OR(95 %CI) | p | OR(95 %CI) | p | |
| Luminal A | ||||
| Presence of mass | 0.152 (0.044–0.526) | 0.003 | 0.290(0.074–1.136) | 0.075 |
| Hypo/Isointensity on T2W | 7.609 (1.640–35.306) | 0.01 | 6.214(1.163–33.215) | 0.033 |
| Non-Restricted | 7.667 (1.692–34.734) | 0.008 | 6.694(1.172–38.235) | 0.032 |
| Rim enhances | 0.097 (0.012–0.768) | 0.027 | 0.215(0.025–1.870) | 0.164 |
| Luminal B HER2- | ||||
| Presence of mass | 4.889(1.283–18.630) | 0.020 | 7.245(1.760–29.889) | 0.006 |
| Slow/Medium enhanced initial phase | 2.836(1.313–6.125) | 0.008 | 3.654(1.588–8.407) | 0.002 |
| HER2 | ||||
| Presence of non-mass enhancement | 4.500(1.147–17.648) | 0.031 | 20.498(3.145–133.584) | 0.002 |
| Distortion | 4.388(1.221–15.767) | 0.023 | 11.471(2.250–58.493) | 0.003 |
| Fast enhanced initial phase | 5.204(1.085–24.964) | 0.039 | 9.788(1.689–56.740) | 0.011 |
| Triple Negative | ||||
| Edema | 8.647(2.277–32.842) | 0.002 | 5.768(1.040–31.987) | 0.045 |
| Unifocal | 6.862(1.409–33.424) | 0.017 | 7.877(1.180–52.589) | 0.033 |
| Hyperintensity on T2W | 14.048(1.732–113.957) | 0.013 | 14.496(1.303–161.328) | 0.030 |
| Rim enhances | 23.667(2.900–193.163) | 0.003 | 18.706(1.915–182.764) | 0.012 |
4. Discussion
Molecular subtypes are critical in guiding the treatment of breast cancer, influencing both metastatic and early-stage disease management. Since the introduction of the 2011 St. Gallen International Expert Consensus guidelines, molecular-based treatments have gained prominence. Inoperable or locally advanced breast cancer of the luminal subtype benefits from neoadjuvant endocrine therapy, while HER2-positive tumors respond well to dual neoadjuvant chemotherapy combined with HER2-targeted therapy [4]. Despite extensive research on MRI features to differentiate breast cancer subtypes, few studies have included all five subtypes [12], [13], [14], [15], [16]. In addition, since core needle biopsy (CNB) specimens are less reliable compared to surgical specimens for immunohistochemical (IHC) evaluation, this study classified molecular subtypes based on surgical excision specimens only [7], [8], [9]. The present study demonstrated that except for luminal B HER2-positive, each breast cancer subtype exhibited unique MRI characteristics, which could be used to predict molecular subtypes. Based on these findings, we propose that gene expression analysis could be considered to confirm the diagnosis in cases with discordance between preoperative immunohistochemical results and MRI features.
In our cohort, luminal A breast cancer, which has the best prognosis but a low pathological complete response (pCR) rate to neoadjuvant therapy, accounted for 14.3 % of participants. On MRI, 62.5 % of tumors in this subtype appeared as mass-like lesions. Although non-mass enhancement lesions were more prevalent in this group compared to others (p<0.05), multivariate analysis did not reveal a significant association with luminal A breast cancer. Our study highlighted that luminal A breast cancer was related iso/hypointense features on T2W and non-restricted diffusion, with these tumors tending to be solid without cystic formation or central necrosis. This aligns with Yuen S. et al.'s findings. The heterogeneous hypointense features on T2W indicated the luminal subtype, possibly due to exaggerated reactive tumor stroma. These tumors, compared to homogeneous hypointense ones, exhibited higher proliferation, correlating with poorer prognosis due to fibrotic focus presence [12]. Our study also revealed that luminal A breast cancer had a higher rate of non-restricted diffusion compared to other subtypes. A similar result was observed in studies by Montemezzet et al. and Martincich et al. [23], [24]. In contrast, Sou et al.'s 2019 study indicated that ER or PR-positive tumors had lower mean ADC values than other subtypes, attributed to angiogenesis inhibition and higher cellularity [14]. Variations in mean ADC values or diffusion restriction may result from differences in luminal A proportions across studies and the changing thresholds distinguishing luminal B from luminal A subtypes [4], [22]. Another contributing factor may be the inherent heterogeneity within luminal A breast cancer, as evidenced by Ciriello G. et al. [25].
Luminal B HER2-negative breast cancer was the most prevalent molecular subtype in our study, accounting for 52.7 % of cases. Compared to luminal A breast cancers, luminal B HER2-negative tumors have a poorer prognosis due to higher proliferation rates, reflected in elevated Ki67 labeling indices and lower PR scores [22]. On MRI, these tumors frequently presented as mass lesions, with a prevalence of 94.9 %, increasing the risk of luminal B HER2-negative classification sevenfold. This observation is comparable with the study of Navarro in 2017 [26]. Furthermore, tumors exhibiting slow or medium initial enhancement patterns were 3.6 times more likely to be luminal B HER2-negative breast cancer. The proportion of tumors with less than a 100 % increase in intensity during the initial postcontrast phase was higher in luminal A and B HER2-negative subtypes, at 50 % and 59.3 %, respectively. Blaschke et al. (2015) reported similar findings when comparing contrast uptake characteristics in the early postcontrast phase between luminal-like and HER2-enriched breast cancers [27]. This suggests that HER2 expression affects the kinetic features of tumors on MRI. HER2 expression correlates with vascular endothelial growth factor (VEGF) overexpression, inducing vascular permeability. Thus, lower VEGF expression in luminal B HER2-negative tumors may account for the slow or medium initial enhancement patterns observed [28].
HER2-positive breast cancer represents 10–20 % of all breast cancers and includes two subtypes: luminal B-like and HER2-enriched-like, differing in clinicopathological features and disease progression based on hormone receptor (HR) expression [6], [29]. Our analysis of MRI imaging features revealed significant differences between these subtypes. While luminal B HER2-positive breast cancer exhibited no unique imaging features due to the combination of HR+ and HER2+ characteristics, HER2-enriched-like breast cancer frequently demonstrated rapid initial phase uptake without mass lesions. These findings are consistent with studies by Constantine et al. (2012) and Navarro et al. (2017), which reported frequent non-mass enhancement in HER2-positive breast cancer [26], [30]. This supports the theory of intraductal development in this subtype, which is better assessed by MRI than other imaging modalities. Thorough evaluation of tumor spread is crucial for neoadjuvant therapy and surgical planning, particularly in breast-conserving surgery. In addition, our study identified rapid initial phase uptake as a unique MRI characteristic of HER2-enriched-like breast cancer. Blaschke et al. (2015) reported a higher volume percentage of early strong enhancement in these tumors compared to other subtypes [27]. Kawashima et al. (2014) also noted stronger initial phase enhancement in this subtype compared to luminal A breast cancer, corroborating the hypothesis of rich neovascularity in HER2-positive breast cancer related to VEGF overexpression [16], [31]. The HR and HER2 status significantly influence breast cancer behavior, prognosis, and imaging characteristics, particularly on MRI.
Triple-negative breast cancer (TNBC) generally has the poorest prognosis among the five breast cancer subtypes, as it is not amenable to endocrine therapy or HER2-targeted treatments [5], [32]. In our study, TNBC accounted for 9.8 % of cases, with all tumors presenting as mass-like lesions on MRI. Previous studies have associated round or oval shapes with TNBC [13], [30], [33]. While our study found a higher prevalence of these shapes in TNBC, the difference was not statistically significant due to the limited number of cases. However, specific imaging features of TNBC were identified, such as hyperintensity on T2W and rim enhancement, which increased the risk of TNBC and aligned with findings from several other studies [13], [34], [35]. These features suggested intratumoral necrosis, which was associated with not only TNBC but also ER-negative status of the tumor [16], [36]. Unlike HER2-enriched-like breast cancer, TNBC tended to be unifocal when evaluating disease spread. Studies by Sung et al. (2013) and Uematsu et al. (2009) reported equivalent findings [37], [38]. Pathologically, TNBC often presents as pure invasive carcinomas without transformation from ductal carcinoma in situ (DCIS) or coexistent DCIS, correlating with unifocal or multicentric imaging characteristics [39], [40]. Additionally, edema in the surrounding breast parenchyma or fat tissue increased the likelihood of TNBC. Using factors such as age, size, shape, edema presence, and infiltrative characteristics as predictors of TNBC, Costantini et al. reported an AUC of 0.699 (sensitivity, 49.4 %; specificity, 89.4 %) [30]. Despite TNBC showing unique imaging characteristics among breast cancer subtypes, several studies have noted the benign appearance of these tumors on multimodality imaging. The rates of round or oval masses in this subtype ranged from 69 % to 88 %, with circumscribed margin in 26–39 % [37], [38]. Most masses with high T2 signal intensity at MRI are benign, including apocrine metaplasia, myxoid fibroadenoma, fat necrosis, and lymph nodes [41]. Therefore, careful assessment of the internal enhancement patterns and identifying associated features, such as necrosis or peritumoral edema, should be performed to distinguish between benign tumors and TNBC.
This study has limitations, including its retrospective design and single-center setting, which may weaken the statistical power of the results. Additionally, we did not include advanced MRI sequences, such as dynamic quantitative imaging, which can provide more detailed descriptions of breast cancer angiogenesis.
5. Conclusion
Our study demonstrates that each breast cancer molecular subtype exhibits unique imaging features on MRI. These features can serve as supplementary tools to support IHC in the diagnosis of molecular subtypes.
CRediT authorship contribution statement
Van Thi Nguyen:Supervision, Methodology, Conceptualization. Duc Huu Duong: Writing – original draft, Investigation, Data curation. Quang Thai Nguyen: Formal analysis. Duy Thai Nguyen: Methodology, Investigation. Thi Linh Tran: Investigation, Formal analysis. Tra Giang Duong: Writing – review & editing, Validation.
Funding statement
Hereby, I, Duong Duc Huu, consciously assure that for the manuscript “The association of magnetic resonance imaging features with five molecular subtypes of breast cancer” the following is fulfilled: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical statement
Hereby, I, Nguyen Van Thi, consciously assure that for the manuscript “The association of magnetic resonance imaging features with five molecular subtypes of breast cancer” the following is fulfilled:
This material is the authors' own original work, which has not been previously published elsewhere.
The paper is not currently being considered for publication elsewhere.
The paper reflects the authors' own research and analysis in a truthful and complete manner.
The paper properly credits the meaningful contributions of co-authors and co-researchers.
The results are appropriately placed in the context of prior and existing research.
All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference.
All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content.
The study was approved by the National Cancer Hospital Clinical Research Ethics Committee with the number 666-BVK.
I agree with the above statements and declare that this submission follows the policies of Solid State Ionics as outlined in the Guide for Authors and in the Ethical Statement.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Guo L., Kong D., Liu J., et al. Breast cancer heterogeneity and its implication in personalized precision therapy. Exp. Hematol. Oncol. 2023;12(1):3. doi: 10.1186/s40164-022-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coates A.S., Winer E.P., Goldhirsch A., et al. Tailoring therapies--improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann. Oncol. 2015;26(8):1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldhirsch A., Wood W.C., Coates A.S., et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann. Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loibl S., Andre F., Bachelot T., et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024;35(2):159–182. doi: 10.1016/j.annonc.2023.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Jackisch C., Harbeck N., Huober J., et al. 14th St. Gallen international breast cancer conference 2015: evidence, controversies, consensus - primary therapy of early breast cancer: opinions expressed by german experts. Breast Care. 2015;10(3):211–219. doi: 10.1159/000433590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slostad J.A., Yun N.K., Schad A.E., Warrior S., Fogg L.F., Rao R. Concordance of breast cancer biomarker testing in core needle biopsy and surgical specimens: a single institution experience. Cancer Med. 2022;11(24):4954–4965. doi: 10.1002/cam4.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Wang Z., Lv Q., et al. Comparison of core needle biopsy and excision specimens for the accurate evaluation of breast cancer molecular markers: a report of 1003 cases. Pathol. Oncol. Res. 2017;23(4):769–775. doi: 10.1007/s12253-017-0187-5. [DOI] [PubMed] [Google Scholar]
- 9.Robertson S., Ronnlund C., de Boniface J., Hartman J. Re-testing of predictive biomarkers on surgical breast cancer specimens is clinically relevant. Breast Cancer Res. Treat. 2019;174(3):795–805. doi: 10.1007/s10549-018-05119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson K.S., Conant E.F., Soo M.S. Molecular subtypes of breast cancer: a review for breast radiologists. J. Breast Imaging. 2020;3(1):12–24. doi: 10.1093/jbi/wbaa110%. (J Journal of Breast Imaging) [DOI] [PubMed] [Google Scholar]
- 11.Mann R.M., Cho N., Moy L. Breast MRI: state of the art. Radiology. 2019;292(3):520–536. doi: 10.1148/radiol.2019182947. [DOI] [PubMed] [Google Scholar]
- 12.Yuen S., Monzawa S., Yanai S., et al. The association between MRI findings and breast cancer subtypes: focused on the combination patterns on diffusion-weighted and T2-weighted images. Breast Cancer. 2020;27(5):1029–1037. doi: 10.1007/s12282-020-01105-z. [DOI] [PubMed] [Google Scholar]
- 13.Huang J., Lin Q., Cui C., et al. Correlation between imaging features and molecular subtypes of breast cancer in young women (</=30 years old) Jpn J. Radio. 2020;38(11):1062–1074. doi: 10.1007/s11604-020-01001-8. [DOI] [PubMed] [Google Scholar]
- 14.Suo S., Zhang D., Cheng F., et al. Added value of mean and entropy of apparent diffusion coefficient values for evaluating histologic phenotypes of invasive ductal breast cancer with MR imaging. Eur. Radio. 2019;29(3):1425–1434. doi: 10.1007/s00330-018-5667-9. [DOI] [PubMed] [Google Scholar]
- 15.Macchini M., Ponziani M., Iamurri A.P., et al. Role of DCE-MR in predicting breast cancer subtypes. Radio. Med. 2018;123(10):753–764. doi: 10.1007/s11547-018-0908-1. [DOI] [PubMed] [Google Scholar]
- 16.Kawashima H., Inokuchi M., Furukawa H., Ikeda H., Kitamura S. Magnetic resonance imaging features of breast cancer according to intrinsic subtypes: correlations with neoadjuvant chemotherapy effects. Springerplus. 2014;3:240. doi: 10.1186/2193-1801-3-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ab Mumin N., Ramli Hamid M.T., Wong J.H.D., Rahmat K., Ng K.H. Magnetic resonance imaging phenotypes of breast cancer molecular subtypes: a systematic review. Acad. Radio. 2022;29(Suppl 1):S89–S106. doi: 10.1016/j.acra.2021.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Cho N. Imaging features of breast cancer molecular subtypes: state of the art. J. Pathol. Transl. Med. 2021;55(1):16–25. doi: 10.4132/jptm.2020.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan P.H., Ellis I., Allison K., et al. The 2019 world health organization classification of tumours of the breast. Histopathology. 2020;77(2):181–185. doi: 10.1111/his.14091. [DOI] [PubMed] [Google Scholar]
- 20.Allison K.H., Hammond M.E.H., Dowsett M., et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 2020;38(12):1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 21.Wolff A.C., Hammond M.E.H., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: american society of clinical oncology/college of American pathologists clinical practice guideline focused update. J. Clin. Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 22.Falck A.K., Ferno M., Bendahl P.O., Ryden L. St Gallen molecular subtypes in primary breast cancer and matched lymph node metastases--aspects on distribution and prognosis for patients with luminal A tumours: results from a prospective randomised trial. BMC Cancer. 2013;13:558. doi: 10.1186/1471-2407-13-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montemezzi S., Camera L., Giri M.G., et al. Is there a correlation between 3T multiparametric MRI and molecular subtypes of breast cancer? Eur. J. Radio. 2018;108:120–127. doi: 10.1016/j.ejrad.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Martincich L., Deantoni V., Bertotto I., et al. Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur. Radio. 2012;22(7):1519–1528. doi: 10.1007/s00330-012-2403-8. [DOI] [PubMed] [Google Scholar]
- 25.Ciriello G., Sinha R., Hoadley K.A., et al. The molecular diversity of Luminal A breast tumors. Breast Cancer Res. Treat. 2013;141(3):409–420. doi: 10.1007/s10549-013-2699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarro Vilar L., Alandete German S.P., Medina Garcia R., Blanc Garcia E., Camarasa Lillo N., Vilar Samper J. MR imaging findings in molecular subtypes of breast cancer according to BIRADS system. Breast J. 2017;23(4):421–428. doi: 10.1111/tbj.12756. [DOI] [PubMed] [Google Scholar]
- 27.Blaschke E., Abe H. MRI phenotype of breast cancer: kinetic assessment for molecular subtypes. J. Magn. Reson Imaging. 2015;42(4):920–924. doi: 10.1002/jmri.24884. [DOI] [PubMed] [Google Scholar]
- 28.Szabo B.K., Aspelin P., Kristoffersen Wiberg M., Tot T., Bone B. Invasive breast cancer: correlation of dynamic MR features with prognostic factors. Eur. Radio. 2003;13(11):2425–2435. doi: 10.1007/s00330-003-2000-y. [DOI] [PubMed] [Google Scholar]
- 29.Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. doi: 10.1016/S0140-6736(16)32417-5. [DOI] [PubMed] [Google Scholar]
- 30.Costantini M., Belli P., Distefano D., et al. Magnetic resonance imaging features in triple-negative breast cancer: comparison with luminal and HER2-overexpressing tumors. Clin. Breast Cancer. 2012;12(5):331–339. doi: 10.1016/j.clbc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Brown L.F., Berse B., Jackman R.W., et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum. Pathol. 1995;26(1):86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 32.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 33.Dogan B.E., Turnbull L.W. Imaging of triple-negative breast cancer. Ann. Oncol. 2012;23(Suppl 6):vi23–vi29. doi: 10.1093/annonc/mds191. [DOI] [PubMed] [Google Scholar]
- 34.Ozturk V.S., Polat Y.D., Soyder A., Tanyeri A., Karaman C.Z., Taskin F. The relationship between MRI findings and molecular subtypes in women with breast cancer. Curr. Probl. Diagn. Radiol. Nov. - 2020;49(6):417–421. doi: 10.1067/j.cpradiol.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Gigli S., Amabile M.I., David E., et al. Morphological and semiquantitative kinetic analysis on dynamic contrast enhanced MRI in triple negative breast cancer patients. Acad. Radio. 2019;26(5):620–625. doi: 10.1016/j.acra.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz A.M., Loo C.E., Wesseling J., Pijnappel R.M., Gilhuijs K.G. Association between rim enhancement of breast cancer on dynamic contrast-enhanced MRI and patient outcome: impact of subtype. Breast Cancer Res. Treat. 2014;148(3):541–551. doi: 10.1007/s10549-014-3170-9. [DOI] [PubMed] [Google Scholar]
- 37.Sung J.S., Jochelson M.S., Brennan S., et al. MR imaging features of triple-negative breast cancers. Breast J. Nov. 2013;19(6):643–649. doi: 10.1111/tbj.12182. [DOI] [PubMed] [Google Scholar]
- 38.Uematsu T., Kasami M., Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009;250(3):638–647. doi: 10.1148/radiol.2503081054. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Yu T. Clinicopathological characteristics and prognosis of triple-negative breast cancer invasive ductal carcinoma with ductal carcinoma in situ. J. Cancer Res. Clin. Oncol. 2023;149(13):11181–11191. doi: 10.1007/s00432-023-04895-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bianchini G., Balko J.M., Mayer I.A., Sanders M.E., Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016;13(11):674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westra C., Dialani V., Mehta T.S., Eisenberg R.L. Using T2-weighted sequences to more accurately characterize breast masses seen on MRI. AJR Am. J. Roentgenol. 2014;202(3):W183–W190. doi: 10.2214/AJR.13.11266. [DOI] [PubMed] [Google Scholar]