Abstract
Background and objectives:
In June 2022, the mRNA COVID-19 vaccination was recommended for young children. We examined clinical characteristics and factors associated with vaccination status among vaccine-eligible young children hospitalized for acute COVID-19.
Methods:
We enrolled inpatients 8 months to <5 years of age with acute community-acquired COVID-19 across 28 US pediatric hospitals from September 20, 2022 to May 31, 2023. We assessed demographic and clinical factors, including the highest level of respiratory support, and vaccination status defined as unvaccinated, incomplete, or complete primary series [at least 2 (Moderna) or 3 (Pfizer-BioNTech) mRNA vaccine doses ≥14 days before hospitalization].
Results:
Among 597 children, 174 (29.1%) patients were admitted to the intensive care unit and 75 (12.6%) had a life-threatening illness, including 51 (8.5%) requiring invasive mechanical ventilation. Children with underlying respiratory and neurologic/neuromuscular conditions more frequently received higher respiratory support. Only 4.5% of children hospitalized for COVID-19 (n = 27) had completed their primary COVID-19 vaccination series and 7.0% (n = 42) of children initiated but did not complete their primary series. Among 528 unvaccinated children, nearly half (n = 251) were previously healthy, 3 of them required extracorporeal membrane oxygenation for acute COVID-19 and 1 died.
Conclusions:
Most young children hospitalized for acute COVID-19, including most children admitted to the intensive care unit and with life-threatening illness, had not initiated COVID-19 vaccination despite being eligible. Nearly half of these children had no underlying conditions. Of the small percentage of children who initiated a COVID-19 primary series, most had not completed it before hospitalization.
Keywords: COVID-19, vaccination, vaccine coverage, under-5 years, clinical outcomes
During the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron B.1.1.529 peak in winter 2021–2022 in the United States (US), children 6 months to younger than 5 years old comprised the highest incidence of pediatric hospitalizations attributed to acute coronavirus disease 2019 (COVID-19).1,2 On June 18, 2022, the messenger RNA (mRNA) vaccines by Pfizer-BioNTech and Moderna were recommended for use in US children ≥6 months of age.3,4 On December 9, 2022, recommendations were updated to include either a bivalent booster dose ≥2 months after completing a 2-dose series (Moderna) or to receive a bivalent dose among those completing a 3-dose series (Pfizer-BioNTech).5 Real-world evaluations among children 5–18 years of age have demonstrated Pfizer-BioNTech’s BNT162b2 vaccine to be highly effective at reducing hospitalizations and deaths attributed to COVID-19,6,7 but data are limited on the prevention of COVID-19-related hospitalization in children under 5 years of age and on pediatric vaccine effectiveness attributed to the Moderna vaccine. Real-world vaccine effectiveness against laboratory-confirmed SARS-CoV-2 infection and outpatient symptomatic COVID-19 in 3–5-year-olds has been demonstrated.8,9
In children with previous infection, vaccination induces higher neutralizing antibody titers that are cross-reactive across known SARS-CoV-2 variants, while antibodies produced from previous infection from prior variants alone do not demonstrably neutralize Omicron subvariants.10 Immunologic studies among young children from trial data have also demonstrated that two 25 μg doses of the Moderna series among children 6 months through 5 years of age, and three 3 μg doses of the Pfizer-BioNTech series among children 6 months through 4 years old, elicited noninferior immune responses compared with adolescents and young adults.11,12 Different inter-dose intervals are recommended for these vaccine series. The minimum time required to complete the initially recommended primary series s 11 weeks for Pfizer-BioNTech and 4 weeks for Moderna.13
Despite reports of severe COVID-19-related outcomes in young children and demonstrated efficacy of mRNA COVID-19 vaccination in older children and adults, data on vaccination status and clinical course of children <5 years of age hospitalized for acute COVID-19 are limited. Our objectives were to describe patient characteristics and clinical course, including the highest level of COVID-19-related respiratory support and COVID-19 vaccination status, among vaccine-eligible children <5 years of age hospitalized for COVID-19.
METHODS
Study Design and Population
We evaluated the clinical characteristics and outcomes of vaccine-eligible children 8 months to <5 years of age hospitalized through May 31, 2023, at 28 US pediatric hospitals participating in the Overcoming COVID-19 network, with acute COVID-19 as the primary reason for hospital admission.14 All patients had a positive nucleic acid amplification test or antigen test either during hospitalization or at a healthcare facility before hospitalization. In accordance with Centers for Disease Control and Prevention recommendations for COVID-19 vaccinations among children 6 months to <5 years old,3 we used 8 months as the minimum age at which a child could plausibly have completed an mRNA primary vaccination series, with 6 months being the earliest possible age at first dose, plus up to 4 weeks required to complete the 2-dose Moderna primary series, plus 14 days between time since last dose and hospitalization. Because June 21, 2022, was the initial date when vaccines became available for children under 5 years of age, we began enrollment on September 20, 2022, to account for the maximum time interval required to complete the initial recommended primary series (ie, 11 weeks for the 3-dose Pfizer-BioNTech primary series) and 2 weeks to develop immunity after the last vaccination. We excluded patients hospitalized for acute COVID-19 within the previous 60 days of the study hospital admission date and those whose vaccination status could not be verified. The surveillance protocol was reviewed by the Centers for Disease Control and Prevention and other participating institutions and was determined to be public health surveillance and not subject to informed consent requirements per 45 Code of Federal Regulations §46.101(b)(4). This report adheres to the STrengthening the Reporting of OBservational studies in Epidemiology reporting guidelines for cross-sectional studies.15
Vaccine Verification
Study personnel verified vaccination status by searching electronic medical records, viewing COVID-19 vaccination cards, and through state immunization information systems. We classified vaccination status as follows: (1) Unvaccinated (no recorded vaccination before hospitalization); (2) Initiated but incomplete primary series (not all doses received at least 14 days before hospitalization); or (3) Complete primary series (at least 2 doses of the Moderna series or at least 3 doses of the Pfizer-BioNTech series at least 14 days before hospitalization). We also calculated the time at which a primary series dose was considered overdue for children initiating the Pfizer-BioNTech or Moderna vaccination series and compared the proportion of children with an incomplete primary series between manufacturers and the timing of hospitalization relative to the last dose. We determined whether a child was overdue for a primary series dose or was hospitalized in between recommended dosing intervals.
Data were captured by chart review and through parent interviews. Patient data included demographics (age, sex, race and ethnicity, and census region), comorbidities, available clinical information on respiratory viral co-detections (where additional viral testing was performed), and clinical outcomes. Prespecified primary outcomes included: (1) the highest level of respiratory support (no or low-flow oxygen, high-flow nasal cannula, continuous positive airway pressure, bilevel positive airway pressure and invasive mechanical ventilation (IMV)) and (2) life-threatening illness, including receipt of IMV, vasopressors, or extracorporeal membrane oxygenation (ECMO) and/or death.
Statistical Analyses
Descriptive statistics included frequency (proportion) for categorical/binomial variables and median [interquartile range (IQR)] for continuous variables. Fisher exact and Wilcoxon rank-sum tests were used to compare binomial and continuous demographic and clinical variables, respectively, by age group, clinical outcomes (such as highest level of respiratory support), vaccination status (unvaccinated, initiated but incomplete primary series or completed primary series/booster dose). Patients who initiated but did not complete their primary series were further categorized by whether they were overdue for their next dose (overdue for 2nd or 3rd Pfizer-BioNTech dose or overdue for 2nd Moderna dose) or were hospitalized for COVID-19 in between recommended doses.
RESULTS
We enrolled 597 infants and young children with acute COVID-19 with verifiable documentation of vaccination status, of whom 371 (62.1%) were 8 months to <2 years of age and 226 (37.9%) were 2 to <5 years of age (Fig. 1 and Table 1). Table 1 shows the demographic and clinical characteristics of these children by age group. The largest percentage of children were enrolled in the South and the lowest number in the Northeast, compared with the West and Midwest census regions. The proportion of patients with at least one underlying condition differed by age group: among children <2 years of age, 44.7% had at least one underlying medical condition, compared with 69.9% of those 2 to <5 years old (P < 0.001). Among these children, the most common underlying conditions were respiratory or neurologic/neuromuscular. Over 80% of children (n = 499/597, 83.6%) were tested for at least 1 non-SARS-CoV-2 respiratory virus, all of whom were tested for influenza, and 490 (82.1%) were tested for respiratory syncytial virus (RSV) (Table 1). Among the 499 children with any other viral testing, 188 (37.7%) tested positive for any virus. Co-detection of influenza virus was infrequent (1.2%). RSV was co-detected in 11.4% of children <5 years old tested during the study period.
Figure 1.
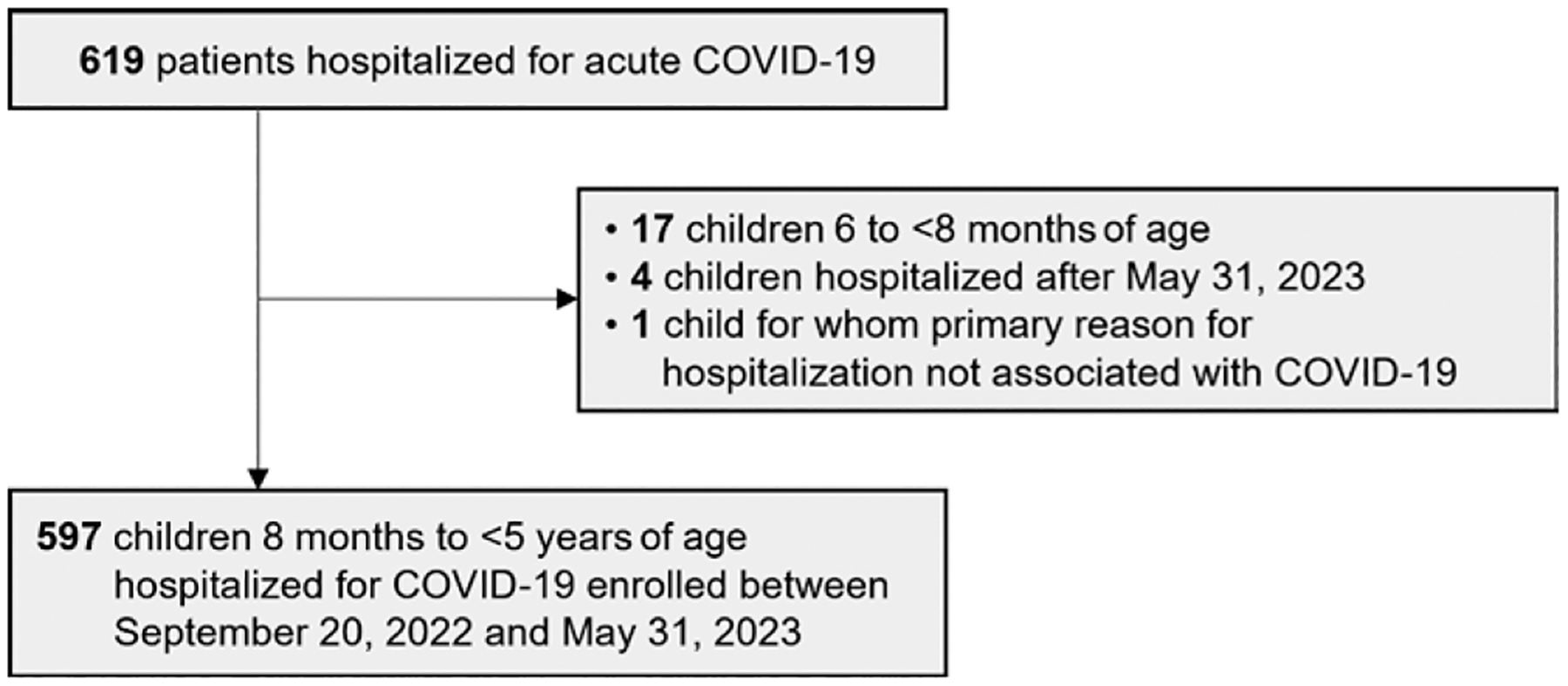
Patient enrollment and exclusion criteria among children under 5 years of age hospitalized for acute COVID-19 within the Overcoming COVID-19 network.
Table 1.
Demographic and Clinical Characteristics and Outcomes Among Children 8 Months–4 Years of Age Hospitalized with Laboratory-confirmed COVID-19—Overcoming COVID-19 Vaccine Effectiveness Network, 28 hospital sites, September 20, 2022–May 31, 2023
| Characteristic | Total (n = 597) |
8 Months to <2 Years (n = 371) |
2 Years to <5 Years (n = 226) |
P Value |
|---|---|---|---|---|
| Sex | ||||
| Male | 338 (56.6) | 211 (56.9) | 127 (56.2) | 0.87 |
| Female | 259 (43.4) | 160 (43.1) | 99 (43.8) | |
| Race/ethnicity | ||||
| White, non-Hispanic | 239 (40.0) | 160 (43.1) | 79 (35.0) | 0.01‡* |
| Black, non-Hispanic | 112 (18.8) | 62 (16.7) | 50 (22.1) | |
| Asian, non-Hispanic | 32 (5.4) | 13 (3.5) | 19 (8.4) | |
| Hispanic/Latino | 150 (25.1) | 94 (25.3) | 56 (24.8) | |
| Multiple/other, non-Hispanic | 42 (7.0) | 24 (6.5) | 18 (8.0) | |
| Unknown | 22 (3.7) | 18 (4.9) | 4 (1.8) | |
| Census region | ||||
| Northeast | 90 (15.1) | 48 (12.9) | 42 (18.6) | 0.24 |
| Midwest | 133 (22.3) | 83 (22.4) | 50 (22.1) | |
| South | 205 (34.3) | 135 (36.4) | 70 (31.0) | |
| West | 169 (28.3) | 105 (28.3) | 64 (28.3) | |
| Underlying medical conditions | ||||
| None | 273 (45.7) | 205 (55.3) | 68 (30.1) | <0.001 |
| One or more underlying medical condition | 324 (54.3) | 166 (44.7) | 158 (69.9) | |
| Multiple underlying medical conditions | 112 (18.8) | 55 (14.8) | 57 (25.2) | 0.002 |
| Cardiorespiratory | 204 (34.2) | 109 (29.4) | 95 (42.0) | 0.002 |
| Respiratory | 159 (26.6) | 80 (21.6) | 79 (35.0) | <0.001 |
| Cardiac | 77 (12.9) | 49 (13.2) | 28 (12.4) | 0.77 |
| Oncologic or immunosuppressed | 37 (6.2) | 10 (2.7) | 27 (11.9) | <0.001 |
| Non-oncologic immunosuppressive disorder | 16 (2.7) | 7 (1.9) | 9 (4.0) | 0.12 |
| Oncologic | 21 (3.5) | 3 (0.8) | 18 (8.0) | <0.001‡* |
| Neurologic/neuromuscular | 120 (20.1) | 58 (15.6) | 62 (27.4) | <0.001 |
| Endocrine* | 31 (5.2) | 15 (4.0) | 16 (7.1) | 0.10 |
| Respiratory viral co-detection† | ||||
| Any respiratory viral co-detection | 188/499 (37.7) | 108/310 (34.8) | 80/189 (42.3) | 0.09 |
| Multiple co-detections | 26/499 (5.2) | 15/310 (4.8) | 11/189 (5.8) | 0.63 |
| Influenza | 6/499 (1.2) | 2/310 (0.6) | 4/189 (2.1) | 0.21‡ |
| Respiratory syncytial virus | 56/490 (11.4) | 25/305 (8.2) | 31/185 (16.8) | 0.004 |
| Other respiratory virus§ | 137/353 (38.8) | 85/215 (39.5) | 52/138 (37.7) | 0.73 |
No children were noted to have diabetes. Obesity is not defined in children <2 years of age and height was missing for 112 (18.8%) of patients, including 44 (19.5%) of patients 2 years of age or older; there were 47 (7.9%) children in the ≥95th weight-for-age percentile.
We collected information regarding testing for the following non-SARS-CoV-2 respiratory viruses: influenza, respiratory syncytial virus (RSV), parainfluenza (types 1, 2, 3 and 4), human metapneumovirus, adenovirus or rhinovirus/enterovirus. All 499 patients were tested for influenza, 490 were tested for RSV and 353 were tested for the other viruses.
Fisher exact tests were performed where cell sizes were <5.
Other respiratory viruses include those for which we specifically collected data on the case report form: parainfluenza (types 1, 2, 3 and 4), human metapneumovirus, adenovirus and rhinovirus/enterovirus. Overall detections for these viruses were 17/353 (4.8%), 34/353 (9.6%), 24/351 (6.8%) and 82/353 (23.2%), respectively.
Clinical outcomes by age group are shown in Table 2. Median length of hospital stay was 2 days (IQR: 1–4 days) among children 8 months to <2 years and 3 days (IQR: 2–5 days) in children 2 to <5 years. Over one-third (n = 228, 38.2%) of children received respiratory support above low-flow oxygen, with high-flow nasal cannula (21.8%) being the most common, followed by IMV (8.5%) and bilevel positive airway pressure or continuous positive airway pressure (7.9%). There were 174 (29.1%) patients admitted to the intensive care unit (ICU); 75 (12.6%) had a life-threatening illness. Three patients with no underlying medical conditions required ECMO and one of these patients died. Except for longer length of stay, outcomes were similar among children 8 months to <2 years and 2 to <5 years of age.
Table 2.
Clinical Outcomes and Highest Level of Respiratory Support Among Children 8 Months–4 Years of Age Hospitalized With Laboratory-confirmed COVID-19 —Overcoming COVID-19 Vaccine Effectiveness Network, 28 hospital sites, September 20, 2022–May 31, 2023
| Hospitalization and Clinical Outcomes | Total (n = 597) |
8 Months to <2 years (n = 371) |
2 Years to <5 Years (n = 226) |
P Value* |
|---|---|---|---|---|
| Length of hospital stay, days, median (IQR) † | 3 (1–4) | 2 (1–4) | 3 (2–5) | 0.01 |
| ICU admission | 174 (29.1) | 116 (31.3) | 58 (25.7) | 0.14 |
| Life-threatening illness‡ | 75 (12.6) | 51 (13.7) | 24 (10.6) | 0.26 |
| Any respiratory support (above low-flow oxygen)§ | 228 (38.2) | 151 (40.7) | 77 (34.1) | 0.11 |
| Highest level of respiratory support | ||||
| High flow nasal cannula | 130 (21.8) | 91 (24.5) | 39 (17.3) | 0.17 |
| BiPAP/CPAP | 47 (7.9) | 27 (7.3) | 20 (8.8) | |
| Invasive mechanical ventilation | 51 (8.5) | 33 (8.9) | 18 (8.0) | |
| Vasoactive infusions | 29 (4.9) | 20 (5.4) | 9 (4.0) | 0.44 |
| ECMO | 3 (0.5) | 1 (0.3) | 2 (0.9) | 0.56* |
| In-hospital death¶ | 1 (0.2) | 0 | 1 (0.4) | 0.38* |
Fisher exact tests were performed where cell sizes were <5; Wilcoxon Rank Sum test used for continuous variables.
Length of hospital stay was determined as the days between the earliest hospital admission date (either the admission date at the study hospital or the admission date of an external hospital, if patient was transferred) and the date of hospital discharge or death.
Life-threatening illness was defined as those receiving invasive mechanical ventilation, vasoactive infusions, ECMO, or illness resulting in death.
Of 597 patients, 369 (61.8%) either received no oxygen support or low-flow oxygen support only.
The patient who died also received invasive mechanical ventilation and ECMO.
BiPAP indicates bilevel positive airway pressure; CPAP, continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation.
Figure 2 shows the demographic and clinical characteristics of children with acute COVID-19 categorized by the highest levels of respiratory support (none or low-flow oxygen only to IMV) by percentage of patients. Of 51 children requiring IMV, approximately half (25, 49.0%) were non-Hispanic White and 33 (64.7%) had underlying medical conditions (Figure 2, Supplemental Digital Content 2, http://links.lww.com/INF/F364), most commonly cardiorespiratory or neurologic/ neuromuscular disease. Of those children with no underlying medical conditions, 63.7% required no respiratory support or low-flow oxygen supplementation only; however, this declined to 48.4% and 53.3% of patients with underlying respiratory and neurologic conditions, respectively (Fig. 2). Nearly 60% of children requiring IMV who had any other viral testing had only SARS-CoV-2 detected, with no detection of a second virus (Table, Supplemental Digital Content 2, http://links.lww.com/INF/F364). Among 86 patients who required noninvasive or IMV and received additional testing, 40 (46.5%) had no viral co-detections, 46 (53.5%) had at least 1 viral co-detection and 6 (7.0%) had multiple viral co-detections (Table, Supplemental Digital Content 2, http://links.lww.com/INF/F364). Among the 3 children requiring ECMO, 2 had coinfection with human metapneumovirus, including the 1 patient who died (Table, Supplemental Digital Content 3, http://links.lww.com/INF/F365).
Figure 2.
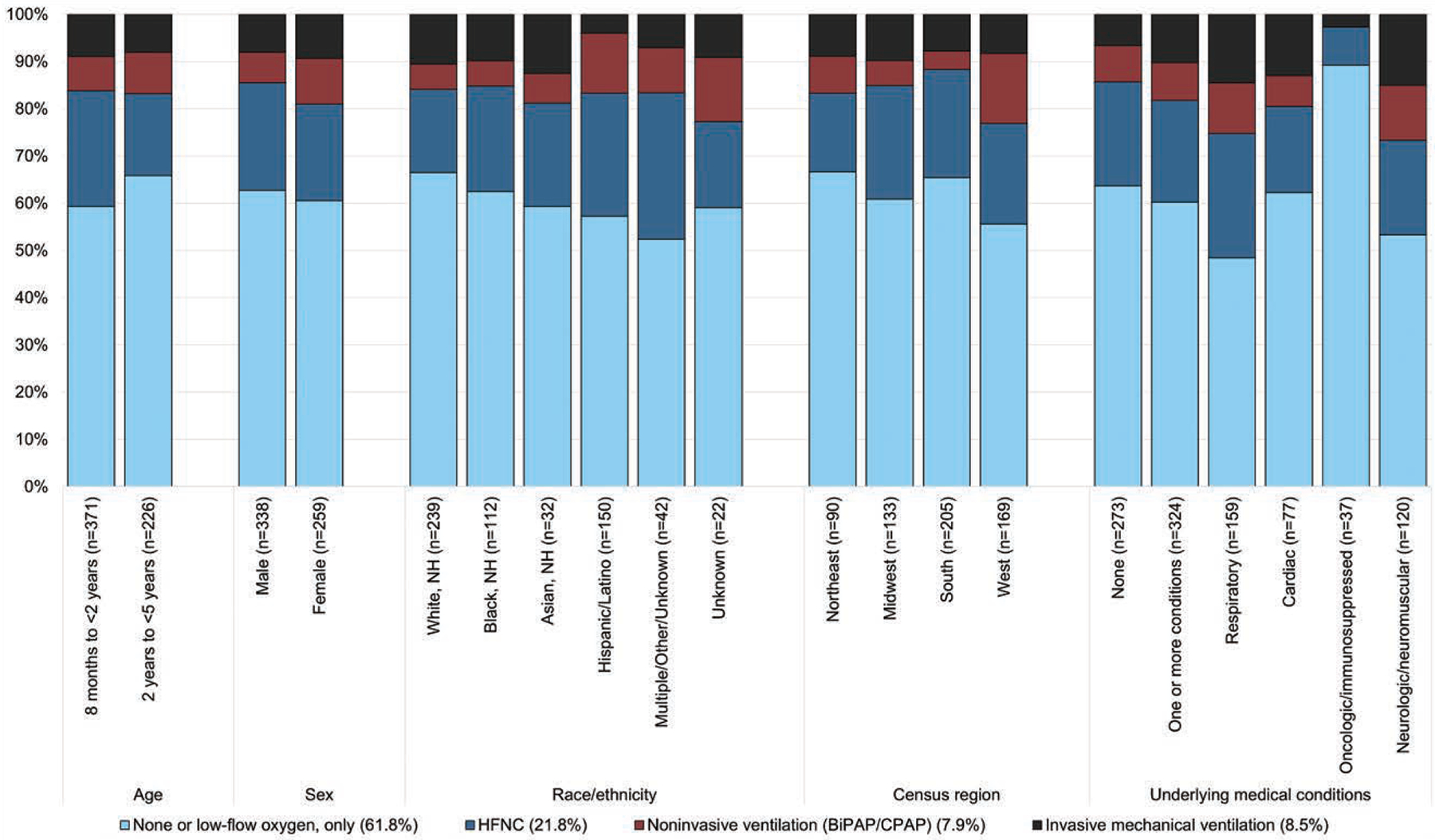
Proportion of patients hospitalized for COVID-19 requiring various levels of respiratory support, by demographic characteristic, and presence of underlying medical conditions.
Most children (n = 528, 88.4%) hospitalized with acute COVID-19 were not vaccinated against COVID-19 despite being eligible for vaccination. Only 27 (4.5%) patients had received their primary series [3 doses of Pfizer-BioNTech (n = 8) or 2 doses of Moderna (n = 19)]. White non-Hispanic patients comprised most patients who completed the primary series (n = 20, 74.1%). Completion of a primary mRNA vaccination series was low across all regions but was highest in the Northeast (11/90, 12.2%) and lowest in the South (3/205, 1.5%). Most children with critical illness were unvaccinated or had not completed their primary series, including 164/174 (94.3%) children with COVID-19 who were admitted to the ICU, 45/51 (88.2%) who received IMV, and 69/75 (92.0%) with life-threatening COVID-19. This included all 3 patients who required ECMO (1 died) and all 29 patients who required vasopressors (Table 3). All 6 patients who completed their primary series and were hospitalized with a life-threatening illness had at least 1 underlying respiratory or neurologic condition, including 4 patients who had both (Table, Supplemental Digital Content 4, http://links.lww.com/INF/F366).
Table 3.
Demographic and Clinical Characteristics and Outcomes Among Children 8 Months - 4 Years of Age Hospitalized with Laboratory-confirmed COVID-19, by Vaccination Status*
| Completed Primary Series ≥14 Days Prior to Hospitalization | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Total N |
Unvaccinated n (%) |
Initiated But Incomplete Primary Series n (%) |
Primary Series or Booster† n (%) |
Pfizer-BioNTech | Moderna |
| All patients | 597 | 528 (88.4) | 42 (7.0) | 27 (4.5) | 8 | 19 |
| Age group | ||||||
| 8 months to <2 years | 371 | 330 (88.9) | 27 (7.3) | 14 (3.8) | 4 | 10 |
| 2 years to <5 years | 226 | 198 (87.6) | 15 (6.6) | 13 (5.7) | 4 | 9 |
| Sex | ||||||
| Male | 338 | 296 (87.6) | 30 (8.9) | 12 (3.6) | 5 | 7 |
| Female | 259 | 232 (89.6) | 12 (4.6) | 15 (5.8) | 3 | 12 |
| Race/ethnicity | ||||||
| White, non-Hispanic | 239 | 199 (83.3) | 20 (8.4) | 20 (8.3) | 6 | 14 |
| Black, non-Hispanic | 112 | 108 (96.4) | 3 (2.7) | 1 (0.9) | 0 | 1 |
| Hispanic/Latino | 150 | 136 (90.7) | 13 (8.7) | 1 (0.7) | 0 | 1 |
| Multiple/Other & Unknown‡ | 96 | 85 (88.5) | 6 (6.3) | 5 (5.2) | 2 | 3 |
| Census region | ||||||
| Northeast | 90 | 74 (82.2) | 5 (5.6) | 11 (12.2) | 3 | 8 |
| Midwest | 133 | 116 (87.2) | 10 (7.5) | 7 (5.3) | 3 | 4 |
| South | 205 | 193 (94.1) | 9 (4.4) | 3 (1.5) | 0 | 3 |
| West | 169 | 145 (85.8) | 18 (10.7) | 6 (3.6) | 2 | 4 |
| Underlying medical conditions§ | ||||||
| None | 273 | 251 (91.9) | 13 (4.8) | 9 (3.3) | 2 | 7 |
| One or more underlying medical condition | 324 | 277 (85.5) | 29 (9.0) | 18 (5.5) | 6 | 12 |
| Multiple underlying medical conditions | 112 | 94 (83.9) | 9 (8.0) | 9 (8.0) | 3 | 6 |
| Cardiorespiratory | 204 | 173 (84.8) | 17 (8.3) | 14 (6.9) | 3 | 11 |
| Respiratory | 159 | 132 (83.0) | 14 (8.8) | 13 (8.2) | 3 | 10 |
| Cardiac | 77 | 71 (92.2) | 3 (3.9) | 3 (3.9) | 1 | 2 |
| Neurologic/neuromuscular | 120 | 104 (86.7) | 8 (6.7) | 8 (6.7) | 3 | 5 |
| Hospitalization outcome | ||||||
| Length of hospital stay, days, median (IQR)¶ | 597 | 2 (1 – 4) | 3 (2 – 5) | 3 (1 – 4) | 4 (3 – 4) | 2 (1 – 5) |
| ICU admission | 174 | 156 (89.7) | 8 (4.6) | 10 (5.7) | 4 | 6 |
| Life-threatening illnessǁ | 75 | 66 (88.0) | 3 (4.0) | 6 (8.0) | 3 | 3 |
| Any respiratory support | 228 | 197 (86.4) | 19 (8.3) | 12 (5.3) | 5 | 7 |
| Highest level of respiratory support | ||||||
| High flow nasal cannula | 130 | 116 (89.2) | 9 (6.9) | 5 (3.8) | 2 | 3 |
| BiPAP/CPAP | 47 | 38 (80.9) | 8 (17.0) | 1 (2.1) | 0 | 1 |
| Invasive mechanical ventilation | 51 | 43 (84.3) | 2 (3.9) | 6 (11.8) | 3 | 3 |
| Vasoactive infusions | 29 | 27 (93.1) | 2 (6.9) | 0 | 0 | 0 |
| ECMO | 3 | 3 (100.0) | 0 | 0 | 0 | 0 |
| In-hospital death | 1 | 1 (100.0) | 0 | 0 | 0 | 0 |
Vaccination status is computed as row percentages to portray vaccination coverage among each subgroup.
Includes two patients who received an mRNA booster dose. The remaining 25 patients had completed a primary series only.
Given limited sample size, 32 patients of Asian descent and 22 patients with unknown race/ethnicity were combined into this group.
There were 31 patients diagnosed with an endocrine disorder, and none were noted to have diabetes (Type 1 or Type 2).
Length of hospital stay was determined as the days between the earliest hospital admission date (either the admission date at the study hospital or the admission date of an external hospital, if patient was transferred) and the date of hospital discharge.
Life-threatening illness was defined as those receiving invasive mechanical ventilation, vasoactive infusions, ECMO, or illness resulting in death.
Some patients initiated but did not complete the COVID-19 primary vaccination series before hospitalization (n = 42, 7.0%). Of these, 33 were overdue for a vaccine dose at the time of hospitalization while 9 were hospitalized between doses but were not overdue for their next dose (Figure 3). Overdue doses were common among those who initiated the Pfizer-BioNTech primary series [n = 27 (81.8%)] compared with those who initiated the Moderna primary series [n = 6 (18.2%)]. Likewise, among the 9 nonoverdue patients who were hospitalized in between primary series doses, 8 initiated the Pfizer-BioNTech primary series.
Figure 3.
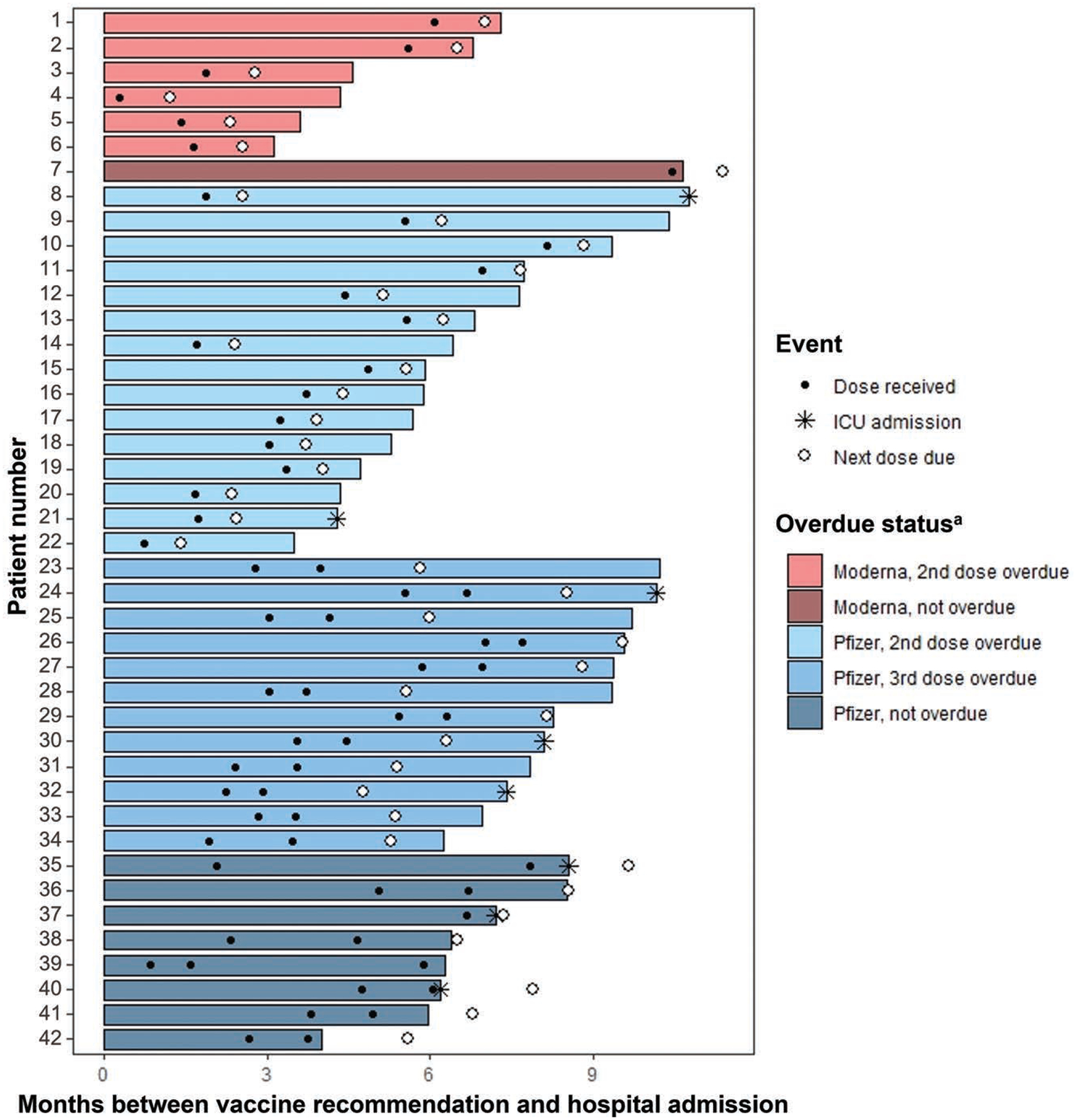
Timing of hospitalization relative to vaccination course among 42 pediatric patients hospitalized for COVID-19 with an incomplete vaccine series.
DISCUSSION
Across 28 centers in 21 states from September 20, 2022 to May 31, 2023, we identified 597 children between 8 months and <5 years of age hospitalized for COVID-19. Nearly half were previously healthy with no underlying conditions. Over a quarter of these hospitalized children required ICU admission, and life-threatening COVID-19 occurred in over 1 in 10. All 3 of the children who required ECMO for acute COVID-19 were previously healthy, and none of these children nor the 29 children requiring vasopressors were vaccinated, and 1 died. Vaccine coverage in this cohort was very low despite being eligible, including among those developing severe and life-threatening disease. Furthermore, over 60% of those hospitalized for acute COVID-19 who had initiated vaccination had not completed their primary series prior to hospitalization. Given the low overall vaccination coverage among these patients, it is imperative to understand the underlying reasons for such limited vaccine uptake in this age group.
Vaccine efficacy has primarily been ascertained through immunogenicity studies that were not designed to assess protection against severe clinical outcomes, which occurred rarely among these trial cohorts. Our platform is designed specifically to enroll hospitalized patients; however, fewer than 5% of children hospitalized for acute COVID-19 and approximately 7% of SARS-CoV-2 negative patients enrolled through this same network had completed the primary COVID-19 vaccination series before admission, precluding real-world evaluation of vaccine effectiveness against severe COVID-19 illness. This low coverage stands in contrast to influenza vaccine uptake in this age group. In previous reports of influenza vaccine effectiveness among children <5 years of age hospitalized for acute respiratory infection within the New Vaccine Surveillance Network and the Pediatric Intensive Care Influenza Network, between 30% and 40% of children <9 years of age hospitalized for influenza and 50%–70% of hospitalized influenza negative controls had received a seasonal influenza vaccine.16–18 Notably, over the first 4 months following the rollout of the Pfizer-BioNTech and Moderna monovalent primary series in this age group, US real-world vaccine effectiveness against outpatient symptomatic SARS-CoV-2 infection among children 3–5 years old was demonstrated to be 31% and 36% effective, respectively.8 In Singapore, vaccine effectiveness against outpatient symptomatic SARS-CoV-2 infection among infection-naïve children 1–4 years of age was 63%.9 Vaccine effectiveness should be continuously monitored, given the recent incorporation of doses targeting the Omicron subvariant XBB.1.5, into all primary mRNA vaccination series.
This investigation highlights the need to improve COVID-19 vaccination coverage and awareness of severe COVID-19 illness in young children. Despite an observed decrease in the proportion of hospitalized children <5 years of age requiring ICU admission during the period of Omicron predominance, similar proportions are still requiring IMV.19 In this analysis, those children with underlying respiratory or neurologic conditions developed more severe COVID-19, requiring higher levels of respiratory support. Although children with these conditions are known to be at higher risk for COVID-19-related complications,20 only a few were vaccinated. Among the small percentage of children who were fully vaccinated, 70% were non-Hispanic White, and the highest proportion of children completing their primary series reside in the Northeast census region. Disparities in vaccination coverage by race and ethnicity and geographic region comport with previous findings in this age group, likewise finding disproportionately higher vaccine coverage among non-Hispanic White children and those residing in the Northeast.21
Low vaccine coverage in this age group may indicate logistical barriers to provision of COVID-19 vaccine to very young children, which include (1) challenges in vaccine distribution (where doses may be available only in pediatric hospitals or large pediatric practices), (2) reliance on pediatricians and hospitals over pharmacies to administer doses to very young or medically complex children and (3) the relative complexity of the Pfizer-BioNTech primary series (three doses, with different inter-dose intervals), which may reduce parental compliance with the primary series schedule. Reflecting this, we noted that a higher proportion of patients initiated but did not complete the Pfizer-BioNTech primary series compared with those who initiated the Moderna primary series. While nearly 70% of those who completed the primary series initiated the Moderna series, over 80% of children who did not complete their primary series initiated the Pfizer-BioNTech primary series. The longer timeframe required to complete the 3-dose Pfizer-BioNTech primary series may also allow for more opportunities for infection during inter-dose intervals. In addition, caregiver perceptions of COVID-19 severity and vaccine safety may predict COVID-19 vaccination intention for children <5 years. In the early months of eligibility in this age group, there was substantial evidence of parental hesitancy and low vaccine confidence.22 Despite this, pre- and early post-marketing monitoring for severe adverse events indicated that COVID-19 vaccination was overwhelmingly safe and effective among children 6 months to <5 years of age, with adverse events rarely reported.11,12,23,24 Of note, 73% of US children have completed their routine pediatric vaccines, all consisting of multidose series, and 17% have initiated but not completed one or more of their routine series. While these figures highlight a need for promotion and programmatic optimization to improve routine pediatric vaccine uptake, COVID-19 vaccination coverage in young children remains far below coverage of routine pediatric vaccination.25 Expanded efforts to address gaps in vaccine access and additional caregiver outreach may help promote adherence to COVID-19 vaccination schedules.
Strengths of this investigation include representative enrollment of hospitalized COVID-19 patients by site, wide geographic breadth of included sites covering 28 pediatric hospitals in 21 states, rigorous vaccine verification procedures, and timing of patient enrollment that aligned with vaccine eligibility in this age group. This investigation, however, was subject to several limitations. First, even with simultaneous enrollment of a separate population of hospitalized SARS-CoV-2-negative patients, vaccine coverage in this population was too low to evaluate vaccine effectiveness. Second, we did not universally test all patients for other respiratory viruses; rather, clinical data on viral co-detections were obtained solely through medical records abstraction. Influenza affected relatively few patients, but it is possible that RSV, human metapneumovirus or other respiratory viral co-detections influenced disease severity. Third, we could not reliably differentiate between patients who received low-flow oxygen supplementation compared with those who received no oxygen support, as low-flow oxygen supplementation is frequently transient and challenging to ascertain through retrospective medical record abstraction. Finally, patients were enrolled largely from tertiary referral pediatric centers, so while participating sites represented geographic breadth, the findings from this investigation may not be generalizable to all patients 8 months to 5 years hospitalized for severe COVID-19 illness in the community.
Despite eligibility, most children hospitalized for COVID-19, including most children with underlying medical conditions, had not initiated their COVID-19 mRNA primary vaccination series before their hospitalization. Of the children who had initiated vaccination, particularly those initiating the Pfizer-BioNTech primary series, most had not completed their primary series by the time they were hospitalized. Our investigation shows that some young children, including those who are otherwise previously healthy, are at risk of developing severe COVID-19. Strategies to reduce barriers to vaccine access among young children, as well as caregiver education, are vital components of efforts to expand coverage and promote completion of all recommended COVID-19 mRNA vaccine doses.
Supplementary Material
Acknowledgments
This study was funded by the US Centers for Disease Control and Prevention
Footnotes
The authors have no conflicts of interest to disclose.
Overcoming COVID-19 Investigator (List of Collaborators, Supplemental Digital Content 1, http://links.lww.com/INF/F363).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Marks KJ, Whitaker M, Agathis NT, et al. Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19—COVIDNET, 14 states, March 2020–February 2022. Morb Mortal Wkly Rep. 2022;71:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Laboratory-Confirmed COVID-19-Associated Hospitalizations. COVID-NET. Published 2023. Available at: https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html
- 3.Fleming-Dutra KE, Wallace M, Moulia DL, et al. Interim recommendations of the advisory committee on immunization practices for use of Moderna and Pfizer-BioNTech COVID-19 vaccines in children aged 6 months–5 years—United States, June 2022. MMWR Morb Mortal Wkly Rep. 2022;71:859–868. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Moderna and Pfizer-BioNTech COVID-19 Vaccines for Children Down to 6 Months of Age. 2022. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-covid-19-vaccines-children
- 5.U.S. Centers for Disease Control and Prevention. CDC Expands Updated COVID-19 Vaccines to Include Children Ages 6 Months through 5 Years. Media Statement. Published 2022. Available at: https://www.cdc.gov/media/releases/2022/s1209-covid-vaccine.html. Accessed June 13, 2023.
- 6.Price A, Olson S, Newhams M, et al. BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med. 2022;386:1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson S, Newhams M, Halasa N, et al. Effectiveness of BNT162b2 vaccine against critical COVID-19 in adolescents. N Engl J Med. 2022;386:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming-Dutra KE, Ciesla AA, Roper LE, et al. Preliminary estimates of effectiveness of monovalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection among children aged 3–5 years—increasing community access to testing program, United States, July 2022–February 2023. MMWR Morb Mortal Wkly Rep. 2023;72:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wee LE, Tang N, Gce A, et al. Effectiveness of monovalent mRNA vaccines against omicron XBB infection in Singaporean children younger than 5 years. JAMA Pediatr. 2023;177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang J, Novak T, Hecker J, et al. Cross-reactive immunity against the SARS-CoV-2 omicron variant is low in pediatric patients with prior COVID-19 or MIS-C. Nat Commun. 2022;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson EJ, Creech CB, Berthaud V, et al. ; KidCOVE Study Group. Evaluation of mRNA-1273 vaccine in children 6 months to 5 years of age. N Engl J Med. 2022;387:1673–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muñoz FM, Sher LD, Sabharwal C, et al. ; C4591007 Clinical Trial Group. Evaluation of BNT162b2 Covid-19 vaccine in children younger than 5 years of age. N Engl J Med. 2023;388:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. Published 2023. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html. Accessed June 13, 2023.
- 14.Randolph AG, Bembea MM, Cheifetz IM, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Pediatric Acute Lung Injury and Sepsis Investigators (PALISI): evolution of an investigator-initiated research network. Pediatr Crit Care Med. 2022;23:1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 16.Sahni LC, Naioti EA, Olson SM, et al. Sustained within-season vaccine effectiveness against influenza-associated hospitalization in children: evidence from the new vaccine surveillance network, 2015–2016 through 2019–2020. Clin Infect Dis. 2023;76:e1031–e1039. [DOI] [PubMed] [Google Scholar]
- 17.Campbell AP, Ogokeh C, Weinberg GA, et al. ; New Vaccine Surveillance Network (NVSN). Effect of vaccination on preventing influenza-associated hospitalizations among children during a severe season associated with B/Victoria viruses, 2019–2020. Clin Infect Dis. 2021;73:e947–e954. [DOI] [PubMed] [Google Scholar]
- 18.Olson SM, Newhams MM, Halasa NB, et al. ; Pediatric Intensive Care Influenza Investigators. Vaccine effectiveness against life-threatening influenza illness in US children. Clin Infect Dis. 2022;75:230–238. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Almeida FJ, Baillie JK, et al. International pediatric COVID-19 severity over the course of the pandemic. JAMA Pediatr. 2023;177:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodruff RC, Campbell AP, Taylor CA, et al. Risk factors for severe COVID-19 in children. Pediatrics. 2022;149:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy BP, Fast HE, Zell E, et al. COVID-19 vaccination coverage and demographic characteristics of infants and children aged 6 months–4 years—United States, June 20–December 31, 2022. MMWR Morb Mortal Wkly Rep. 2023;72:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellithorpe ME, Aladé F, Adams RB, et al. Looking ahead: caregivers’ COVID-19 vaccination intention for children 5 years old and younger using the health belief model. Vaccine. 2022;40:1404–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goddard K, Donahue JG, Lewis N, et al. Safety of COVID-19 mRNA vaccination among young children in the vaccine safety datalink. Pediatrics. 2023;152:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hause AM, Marquez P, Zhang B, et al. COVID-19 mRNA vaccine safety among children aged 6 months to 5 years—United States, June 18, 2022-July 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michels SY, Niccolai LM, Hadler JL, et al. Failure to complete multidose vaccine series in early childhood. Pediatrics. 2023;152:e2022059844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


